Abstract
A 38-year-old male with recently diagnosed HIV and gonorrhea presented with umbilicated facial lesions and blepharoconjunctivitis of the right eye. Polymerase chain reaction test was performed of the skin were positive for Monkeypox (MPX). The patients’ ocular symptoms improved with acyclovir, azithromycin, gemifloxacin, and tecovirimat after 3 weeks of treatment. The incidence of MPX has been on the rise in 2022, and this case represents a unique presentation and an addition to the pool of data pertinent to diagnosis and treatment of MPX and its ocular manifestations. Due to the MPX reemergence, it is imperative for ophthalmologists to keep MPX on the differential for patients presenting with blepharoconjunctivitis.
Keywords: Monkeypox, Conjunctivitis, Eye discharge, Case report
Introduction
Monkeypox (MPX), a zoonotic disease caused by an orthopoxyvirus, has recently reemerged raising concern worldwide. Although there is a wide array of symptoms and presentations, it most often presents as a smallpox-like disease (Bunge). The predominant signs in MPX are skin rash, fever, adenopathy, headache, and myalgias. We present a rare case of PCR-confirmed MPX in an HIV positive patient who presented with blepharoconjunctivitis and umbilicated cutaneous facial lesions. The 38-year-old African American male presented to the emergency department with right eye pain, redness, yellow discharge, blurry vision, photophobia, and periorbital swelling for 3 days. The patient also reported discharge from his penis and burning with urination. Additionally, the patient disclosed that his male sexual partner had a fever a few days prior to the onset of his symptoms. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000533914).
Case Report
Exam was notable for fever, painful cervical adenopathy, blepharoconjunctivitis, and pedunculated and umbilicated lesions on his face (Fig. 1). His initial laboratory work came back as positive for HIV and gonorrhea. He denied any recent travel or contact with confirmed MPX cases. An MPX swab from the lesions of his rash was sent to the health department which takes several days to process. He was preemptively started on acyclovir 800 mg 5x daily, azithromycin 2 g PO, and gemifloxacin 320 mg PO to treat his various sexually transmitted diseases. He was also started on moxifloxacin eye drops in the right eye four times daily for the eye discharge and swelling. He was told to follow up in the ophthalmology clinic and with his primary care physician the next day.
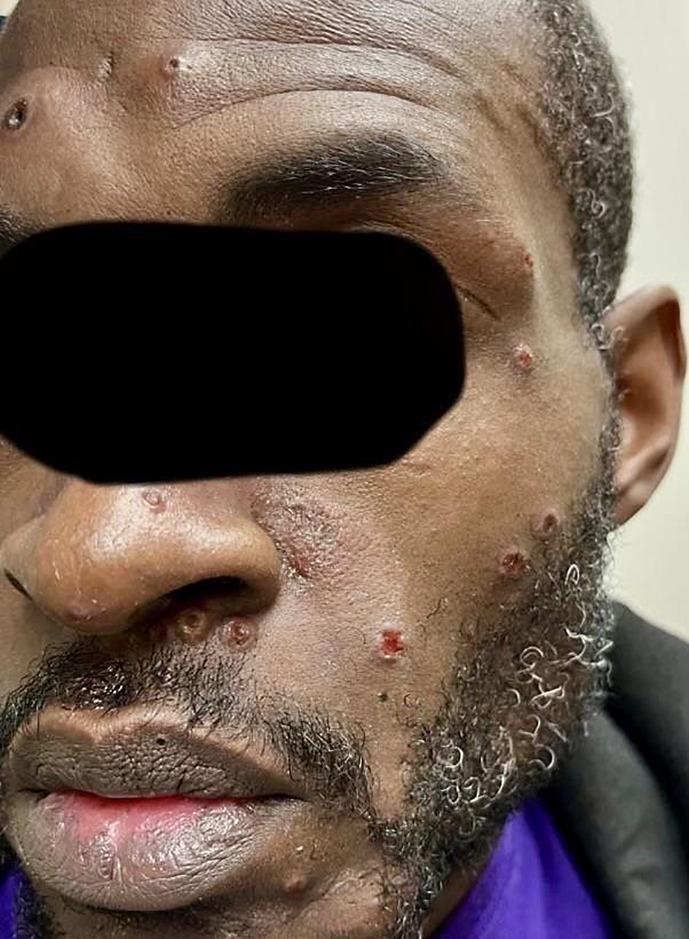
Fig. 1.
Cutaneous pedunculated and umbilicated lesions on the face.
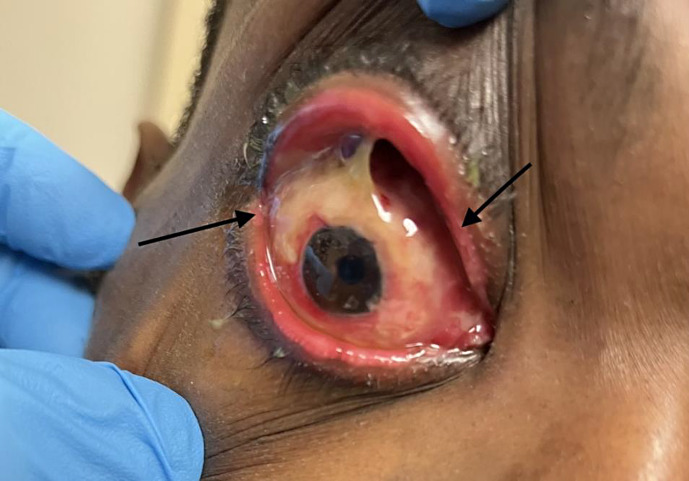
Upon presentation to the ophthalmology clinic the following day, the patient’s symptoms were unchanged. His visual acuity was 20/400 in his right eye and 20/20 in his left eye. Intraocular pressure was 14 mm Hg in both eyes. External exam was significant for multiple cutaneous pedunculated and umbilicated lesions on his face. He had erythematous, ulcerated, sharply demarcated soft tissue involvement at the upper and lower lid margins. Slit lamp exam of his right eye revealed significant periorbital edema and erythema (Fig. 2). Furthermore, a papillary and follicular reaction of the upper and lower eyelids with copious conjunctival white discharge and chemosis was noted. Corneal exam demonstrated punctate epithelial erosions. The anterior chamber was deep and formed with no inflammatory reaction. Examination of the posterior segment was unremarkable. A culture of the conjunctival discharge was taken which did not result in any growth after 1 week. Samples of the affected eye were also sent for polymerase chain reaction (PCR) testing, but a laboratory error resulted in an invalid sample.
Fig. 2.
Right eye. Significant periorbital edema, erythema, and blepharoconjunctivitis.
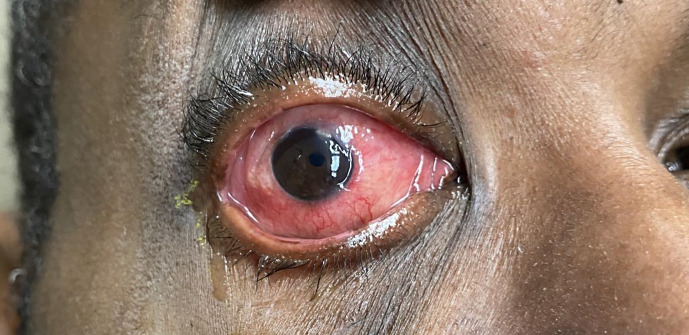
At the 1-week follow-up visit, the real-time PCR test from the facial skin lesions were positive for MPX and the patient was subsequently started on tecovirimat (TPOXX) 600 mg twice daily. The CDC and local health department were both notified of the positive MPX PCR result. A few days later, he reported his skin lesions and periorbital edema had improved although he continued to experience blurry vision, irritation, and discharge (Fig. 3). He was started on trifluridine five times daily to treat any concomitant herpetic infection.
Fig. 3.
Right eye. Skin lesions and periorbital edema improved.
At his 2-week visit, his symptoms overall improved; however, conjunctival redness and irritation persisted. The patient was subsequently then lost to follow-up.
Discussion
The reemergence of MPX has raised both concern and interest from the medical and scientific community. The impact of its reemergence continues to grow and does not spare those practicing ophthalmology throughout the USA. As such, we feel it is important for ophthalmologists to further discuss and continue to learn about this pathology so they may identify, treat, and ultimately prevent the spread of MPX.
The smallpox virus has been shown to be excreted in tears during conjunctival infection [1] which suggests that the MPX virus can be spread through conjunctival secretions. Furthermore, the MPX virus has been theorized to be spread by fomites, creating the possibility of ocular infection through autoinoculation [1]. The ocular manifestations of MPX are less documented in comparison to its systemic and dermatologic findings. A review of MPX positive cases in the endemic area of the Democratic Republic of Congo (DRC) revealed conjunctivitis in nearly 23% of cases [2], while other studies report the incidence of conjunctivitis and blepharitis to be as high as 30% in unvaccinated individuals [3]. Other conjunctival manifestations include focal “pock” lesions on the conjunctiva itself, with reports from the 1980–1985 breakout in Zaire in the DRC, estimating 17% of unvaccinated and 13% of vaccinated suffered from focal lesions on conjunctiva or eyelids. That same study found corneal ulceration occurred in 4% of unvaccinated individuals and 1% of those who were vaccinated [4]. Other literature summarizes a wider range of possible ocular manifestations, including photophobia, frontal headache, and pre-auricular lymphadenopathy [5]. These summaries mostly highlight data from endemic regions in the DRC, providing a baseline for the ophthalmic manifestations of this virus but not providing information specific to the current 2022 outbreak. In fact, conjunctivitis has only been reported in 4.7% of cases as of October 2022 in the USA, highlighting the possible differences between this outbreak and endemic MPX [6]. Cash-Goldwasser et al. [7] and colleagues performed a case series highlighting 5 patients from the 2022 outbreak with ocular manifestations of MPX including itchiness, redness, pain, periorbital swelling, conjunctivitis, conjunctival ulcer, corneal ulcer, keratitis, and decreased visual acuity [7]. In addition to conjunctivitis, our patient demonstrated significant periorbital edema and copious white discharge, symptoms that have not been reported in the current literature. Our patients’ symptoms add to our current knowledge of MPXV ocular manifestations.
Current demographics and clinical data show that roughly 32% of MPX cases are black or African American individuals, 31% Hispanic or Latino, and 29% white. Most commonly affecting men aged 31–35 [8]. Men who have sex with men are at particularly increased risk. Clinical characteristics of those affected varies based on severity of disease; however, some common clinical characteristics include firm, well circumscribed, and often umbilicated lesions, most commonly in anogenital or mouth region and may involve multiple or single lesions. The rash is not always widespread and will progress through several stages before scabbing and desquamating. Of note, the lesions will typically develop and progress simultaneously. Other systemic symptoms include a prodrome of fever, malaise, and myalgias, rectal pain or bleeding, and respiratory symptoms [9].
Data throughout the outbreak suggested that those with HIV are at a higher risk of contracting MPX. From May to July of 2022, 38% of new MPX patients were HIV-positive individuals. Of this group, nearly 82% were found to have HIV viral loads of less than 200 copies/mL, suggesting even those with proper viral suppression were at increased risk. Patients with HIV experienced worse clinical outcomes, including a higher hospitalization for patients with HIV compared to non-HIV-positive MPX patients [10].
Treatment guidelines for ocular MPX have not been completely established; however, currently the CDC recommends tecorvirmat initiation for eligible patients with severe MPX infection, including those with ocular manifestations [6]. Tecorvirmat is a drug approved for treatment of smallpox and has shown efficacy in animal models in the treatment of MPX, allowing its approval for use in current outbreak [11]. It should be noted that tecorvirmat is not FDA approved for the treatment of MPX. Tecovirimat is currently approved for use in eligible patients (those with severe disease or in anatomic areas that might result in serious sequel) under CDCs expanded access Investigational New Drug protocol [12]. Topical ocular treatments vary, in a recent MPXV case series, patients were initiated on a regimen of both tecovirimat and topical trifluridine with complete resolution of ocular symptoms in 4 out of 5 patients [11]. In these cases, topical trifluridine was initiated at first sign of ocular involvement. Tecorvirmat was initiated as an oral dose after confirmation of positive PCR for MPXV and was upgraded to IV tecovirimat in the cases with worsening ocular symptoms or new/worsening lesions despite oral tecovirimat. Other treatment approaches have been taken. One case report details an MPX virus-positive patient with eyelid margin ulcer lesions, conjunctival infiltrative lesions, and conjunctival thickening [1]. This patient was treated with systemic tecovirimat 600 mg every 12 h, IV acyclovir every 8 h, and topical ocular regimen of chlorhexidine, 0.2%, ganciclovir 0.15%, moxifloxacin, and povidone iodine 1%, each 5 times daily. Two weeks after initiation of the initial treatment, topical Fluorometholone was started four times a day. The conjunctival lesions subsequently resolved after 4 weeks. The introduction of steroid agents should come with hesitation as steroids can blunt the immune response allowing for viral replication. This holds true for the Poxviridae family as one case study reported that the introduction of steroid drops to control inflammation in a patient with cowpox ocular infection resulted in prolonged infection and severe corneal damage [13]. Another consideration for physicians is the addition of topical antibiotics to prevent bacterial superinfection. The addition of antibiotics is currently used on a case-by-case basis, with no reported adverse effects in patients prescribed the additional antibiotics to their regimen. Because our patient’s ocular PCR swabs were not able to confirm MPX, it is difficult to completely rule out a sole bacterial ocular infection which led to the improvement with topical antibiotics. Our patient was treated successfully with acyclovir 800 mg 5 times daily, tecovirimat (TPOXX) 600 mg twice a day, topical moxifloxacin 4 times a day, and topical trifluridine 5 times a day which was tolerated well and showed improvement of symptoms. Of note, our patient was treated prophylactically for HSV with both oral and topical anti-HSV medications despite a negative HSV cell culture. The justification for this usage is that Herpes is known to masquerade as benign cutaneous conditions [14]. Additionally, it is known that a negative cell culture cannot reliably rule out HSV, and treatment of possible atypical presentations should be at the discretion of the physician [15]. Finally, the choice of trifluridine was initiated as it has been shown in vitro to be effective against other viruses of the same family as MPX [16]. This treatment mirrors aspects of both treatment regimens discussed above and helps support the efficacy of the regimens. Unfortunately, our patient was lost to follow-up. Overall, MPX is a sight threatening condition that requires close ophthalmologic follow-up, if treated appropriately, the condition typically responds well.
Beginning in May of 2022, the CDC began tracking the MPX outbreak in the USA. According to their data, the total number of cases as of June 2023 in the US has reached nearly 30,468 [12]. Prior to 2022, only one outbreak has ever occurred in the USA. 47 presumed cases of MPX were reported due to contact with infected pet prairie dogs in a 2003 outbreak [12]. That outbreak was the first-time human MPX, reported outside of Africa. Taking this into consideration, the current 2022 outbreak and cases like ours are constantly adding to a small pool of data regarding our understanding of the current ophthalmic manifestations of this disease. Given the status of this epidemic and emerging ophthalmic cases in literature, it is critical for ophthalmologists to be familiar with the ocular manifestations of MPX virus so they may properly recognize it and treat patients.
Conclusion
It is important to have MPX on the differential in a patient who comes into an ophthalmic office with umbilicated skin lesions and conjunctivitis.
Statement of Ethics
Our research complies with the internationally accepted standards for research practice and reporting. Written informed consent was obtained from the patient to publish for publication of the details of their medical case and any accompanying images. IRB approval is not required for this study in accordance with local and national guidelines for brief case reports. The authors provide the journal consent to publish this article.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Jeff Bloom, Michael Parise, Omar Saeed, Cameron Holicki, and Brian Mihok attest that they meet the current ICMJE criteria for authorship and agree to be accountable for all aspects of the work. Jeff Bloom provided substantial contributions to the conception of the work, drafted the work, and interpreted the work. Omar Saeed and Mike revised the work critically for important intellectual content and interpreted the work. Cameron Holicki and Brian Mihok contributed to the acquisition of the work, revised the work for important intellectual content, and provided final approval of the version to be published.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Ly-Yang F, Miranda-Sánchez A, Burgos-Blasco B, Fernández-Vigo JI, Gegúndez-Fernández JA, Díaz-Valle D. Conjunctivitis in an individual with monkeypox. JAMA Ophthalmol. 2022;140(10):1022–4. [DOI] [PubMed] [Google Scholar]
- 2. Hughes C, McCollum A, Pukuta E, Karhemere S, Nguete B, Shongo Lushima R, et al. Ocular complications associated with acute monkeypox virus infection, DRC. Int J Infect Dis. 2014;21:276–7. [Google Scholar]
- 3. Damon IK. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(Suppl 4):D54–59. [DOI] [PubMed] [Google Scholar]
- 4. Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293–8. [DOI] [PubMed] [Google Scholar]
- 5. Abdelaal A, Serhan HA, Mahmoud MA, Rodriguez-Morales AJ, Sah R. Ophthalmic manifestations of monkeypox virus. Eye. 2023;37:383–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention . Interim clinical considerations for management of ocular mpox. Centers for Disease Control and Prevention. Published October 7, 2022. Available from: https://www.cdc.gov/poxvirus/monkeypox/clinicians/ocular-infection.html [accessed January, 31 2023]. [Google Scholar]
- 7. Cash-Goldwasser S, Labuda SM, McCormick DW, Rao AK, McCollum AM, Petersen BW, et al. Ocular monkeypox: United States, July–September 2022. MMWR Morb Mortal Wkly Rep. 2022;71(42):1343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. “MPOX cases by age and gender, race/ethnicity, and symptoms.” Centers for Disease Control and Prevention; 2023. Available from: www.cdc.gov/poxvirus/mpox/response/2022/demographics.html. [Google Scholar]
- 9. “Clinical recognition.” Centers for Disease Control and Prevention; 2023. Available from: www.cdc.gov/poxvirus/mpox/clinicians/clinical-recognition.html. [Google Scholar]
- 10. Curran KG, Eberly K, Russell OO, Snyder RE, Phillips EK, Tang EC, et al. HIV and sexually transmitted infections among persons with monkeypox: eight U.S. Jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(36):1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sherwat A, Brooks JT, Birnkrant D, Kim P. Tecovirimat and the treatment of monkeypox: past, present, and future considerations. N Engl J Med. 2022;387(7):579–81. [DOI] [PubMed] [Google Scholar]
- 12. “2022 outbreak cases and data.” Centers for Disease Control and Prevention; 2023. Available from: www.cdc.gov/poxvirus/mpox/response/2022/index.html. [Google Scholar]
- 13. Graef S, Kurth A, Auw-Haedrich C, Plange N, Kern WV, Nitsche A, et al. Clinicopathological findings in persistent corneal cowpox infection. JAMA Ophthalmol. 2013;131(8):1089–91. [DOI] [PubMed] [Google Scholar]
- 14. Hoyt B, Bhawan J. Histological spectrum of cutaneous Herpes infections. Am J Dermatopathol. 2014;36(8):609–19. [DOI] [PubMed] [Google Scholar]
- 15. Kowalski RP, Thompson PP, Cronin TH. Cell culture isolation can miss the laboratory diagnosis of HSV ocular infection. Int J Ophthalmol. 2010;3(2):164–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shamim AM, Padhi BK, Satapathy P, Veeramachaneni SD, Chatterjee C, Tripathy S, et al. The use of antivirals in the treatment of human monkeypox outbreaks: a systematic review. Int J Infect Dis. 2023;127:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.