Abstract
Introduction
Granulocyte colony-stimulating factor (G-CSF), including pegfilgrastim, increases the peripheral blood leukocyte count and is widely used in clinical practice in combination with cytotoxic chemotherapy. The most frequent side effects of G-CSF are pain and fever; aortitis, in contrast, is a rare and serious side effect.
Case Presentation
A 73-year-old man with small-cell lung cancer was treated with a full dose of a combination of carboplatin/etoposide/durvalumab and pegfilgrastim. The patient developed fever and right ear pain 12 days after pegfilgrastim administration and was diagnosed with aortitis by contrast-enhanced computed tomography 5 days later. Because the patient had already been administered the immune checkpoint inhibitor and had a history of hepatitis B, the patient was followed up without corticosteroid administration, and the patient’s symptoms resolved spontaneously.
Conclusion
In situations where immunosuppression should be avoided, we believe that follow-up without corticosteroids for G-CSF-induced aortitis is a promising option.
Keywords: Pegfilgrastim, Aortitis, Lung cancer, Hepatitis B virus, Immune checkpoint inhibitors
Introduction
Granulocyte colony-stimulating factor (G-CSF) preparations promote the differentiation and proliferation of neutrophil progenitor cells, the release of mature neutrophils from the bone marrow, and neutrophil hyperfunctional effects [1]. Therefore, they have been used to treat febrile neutropenia (FN) caused by cytotoxic chemotherapy in various carcinomas. Polyethylene glycol (PEG)-G-CSF (pegfilgrastim) also has an extended half-life by binding PEG and can prevent neutropenia when administered after cytotoxic chemotherapy. It is widely used clinically for FN prophylaxis when using regimens with a high frequency of FN or during cytotoxic chemotherapy for certain carcinomas [1]. However, G-CSF has been reported to cause mild side effects such as myalgia and fever, and serious but rare side effects such as aortitis [2]. In recent years, reports of G-CSF-induced aortitis have increased, especially in the fields of breast and obstetric/gynecologic cancers, and many cases of systemic corticosteroid therapy have been reported [3]. However, there are some situations in which we hesitate to administer corticosteroids because of their immunosuppressive profile, such as in a patient with a history of hepatitis B because of reactivation or a patient treated with immune checkpoint inhibitors (ICIs) because of corticosteroids counteracting the antitumor effect. Here, we report a case of small-cell lung cancer (SCLC) in a patient with a history of hepatitis B and C who developed aortitis after PEG-G-CSF administration using a regimen of ICIs that resolved without treatment with corticosteroids. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000534931).
Case Presentation
A 73-year-old man with abnormal chest radiographic findings on an annual examination at his workplace was admitted to our hospital. His medical history included atrial fibrillation, cerebral infarction, benign prostatic hyperplasia, and hepatitis B/C, and he took bisoprolol fumarate, edoxaban tosilate hydrate, and silodosin. He currently smoked (pack-years from age 20–73 years) and had no allergies. He was in good health, with a score of 0 on the Eastern Cooperative Oncology Group Performance Status Scale. Chest radiography revealed nodular lesions in the right lung area, and chest computed tomography (CT) revealed nodular lesions in the right upper lung and enlarged mediastinal lymph nodes. Positron emission tomography-CT showed a strong accumulation of fluorodeoxyglucose in the primary lesion and mediastinal lymph nodes. Contrast-enhanced (CE) magnetic resonance imaging of the head revealed no evidence of brain metastases. We performed bronchoscopy and diagnosed the patient with SCLC (extended disease by the Japanese guidelines of 2021, cT3N3M0, stage 3C according to the 8th edition of the Union for International Cancer Control TNM Classification of Malignant Tumors).
The patient was treated with a full dose of a combination of carboplatin/etoposide/durvalumab (Dur) and PEG-G-CSF, with an emphasis on dose intensity. A fourth dose of the coronavirus vaccine (elasomeran and davesomeran) was administered 10 days after PEG-G-CSF administration. On the 12th day after PEG-G-CSF administration, he developed a fever of 38°C and right ear pain, which did not improve, and was seen off appointment on the 17th day. Blood tests showed markedly elevated inflammatory response with a white blood cell count of 15,600/μL, C-reactive protein of 33.9 mg/dL, and interleukin 6 (IL-6) level of 114 pg/mL (Table 1). However, physical examination and simple CT of the entire trunk and head failed to detect the source of the fever or cause of the elevated inflammatory response (Fig. 1a). Although the patient did not develop FN, the possibility of a bacterial infection could not be ruled out; therefore, the patient was immediately hospitalized, and ampicillin/sulbactam was administered. CE-CT of the entire trunk performed the next day showed increased adipose tissue density around the descending thoracic aorta from the aortic arch, suggesting aortitis (Fig. 1b). The patient tested negative for anti-neutrophil cytoplasmic antibodies and other autoantibodies. He had a history of hepatitis B/C virus (HBV/HCV) infection, had a tendency toward fever resolution since admission, and had been treated with ICIs. Therefore, he was followed up carefully with antipyretic analgesics alone without systemic corticosteroid treatment. On the 20th day, the patient’s body temperature improved to 36°C, right ear pain and inflammatory response gradually improved, and contrast CT on the 33rd day showed improvement in periaortic inflammatory findings (Fig. 1c, 2). Serum IL-6 level diminished to 8.7 pg/mL at that time. As the possibility of immune-related adverse events remained, carboplatin/etoposide was reduced to an 80% dose, and Dur was discontinued during the second course of chemotherapy. The patient’s condition progressed uneventfully, except for a slight decrease in the white blood cell count. Dose-reduced carboplatin/etoposide was continued at 4-week intervals, and the patient’s progress was good, with no signs of aortitis flare-up.
Table 1.
Blood and biochemical test date performed on admission
| TP | 7 | g/dL |
| Alb | 3.2 | g/dL |
| T-Bil | 0.7 | mg/dL |
| AST | 18 | U/L |
| ALT | 15 | U/L |
| ALP | 300 | U/L |
| γ-GTP | 78 | U/L |
| LDH | 190 | U/L |
| CK | 168 | U/L |
| BUN | 19 | mg/dL |
| Cre | 1.03 | mg/dL |
| eGFR | 54.8 | mL/min/1.73 m2 |
| UA | 4.8 | mg/dL |
| Na | 138 | mmol/L |
| K | 3.8 | mmol/L |
| Cl | 101 | mmol/L |
| Ca | 8.7 | mg/dL |
| S-Glu | 99 | mg/dL |
| CRP | 33.9 | mg/dL |
| PCT | 0.31 | ng/mL |
| WBC | 15,600 | /μL |
| Ne | 78.6 | % |
| Hb | 11.5 | g/dL |
| PLT | 334,000 | /μL |
| Ferritin | 1,537 | ng/mL |
| U-RBC | Negative | |
| U-WBC | Negative | |
| IgG | 1,118 | mg/dL |
| IgA | 472 | mg/dL |
| IgM | 31 | mg/dL |
| IgE | 1,639 | U/mL |
| C3 | 186 | mg/dL |
| C4 | 42.9 | mg/dL |
| CH50 | 67 | U/mL |
| ESR (60 min) | 111 | mm |
| PT-INR | 1.21 | |
| APTT | 30.4 | sec |
| Fib | 1,104 | mg/dL |
| D-dimer | 1.42 | μg/mL |
| HIV antibody | Negative | |
| IgG4 | 46.2 | mg/dL |
| RF | <5 | IU/mL |
| PR3-ANCA | <1.0 | |
| MPO-ANCA | <1.0 | |
| anti-GBM ab | <2.0 | |
| ANA | 40 | times |
| IL-6 | 114 | pg/mL |
| sIL-2R | 1,250 | U/mL |
| HBs ag | Negative | |
| HBs ab | Positive | |
| HBc ab | Positive | |
| HBV-DNA | Negative | |
| HCV ab | Positive | |
| HCV-RNA | Negative |
Blood and biochemical test results on the day of admission. Markedly elevated inflammatory markers (CRP, WBC, ferritin, ESR, and fibrinogen) were observed, but all autoantibodies such as ANCA were negative.
TP, total protein; Alb, albumin; T-bil, total-bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase; CK, creatine kinase; BUN, blood urea nitrogen; Cre, creatinine; eGFR, estimated glomerular filtration rate; UA, uric acid; S-Glu, serum-glucose; CRP, C-reactive protein; PCT, procalcitonin; WBC, white blood cells; Ne, neutrophil; Hb, hemoglobin; PLT, platelet; U-RBC, urine red blood cells; U-WBC, urine white blood cells; IgG,immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; IgE, immunoglobulin E; C3, complement 3; C4, complement 4; CH50, 50% hemolytic complement activity; ESR, erythrocyte sedimentation rate; PT-INR, international normalized ratio of prothrombin time; APTT, activated partial thromboplastin; Fib, fibrinogen; HIV, human immunodeficiency virus; IgG4, immunoglobulin G4; RF, rheumatoid factor; PR3-ANCA, proteinase 3 anti-neutrophil cytoplasmic antibody; MPO-ANCA, myeloperoxidase anti-neutrophil cytoplasmic antibody; anti-GBM ab, anti-glomerular basement membrane antibody; ANA, anti-nuclear antibody; IL-6, interleukin 6; sIL-2R, soluble interleukin-2 receptor; HBs ag, hepatitis B surface antigen; HBs ab, hepatitis B surface antibody; HBc ab, hepatitis B core antibody; HBV-DNA, hepatitis B virus-deoxyribonucleic acid; HCV ab, hepatitis C virus antibody; HCV-RNA, hepatitis C virus-ribonucleic acid.
Fig. 1.
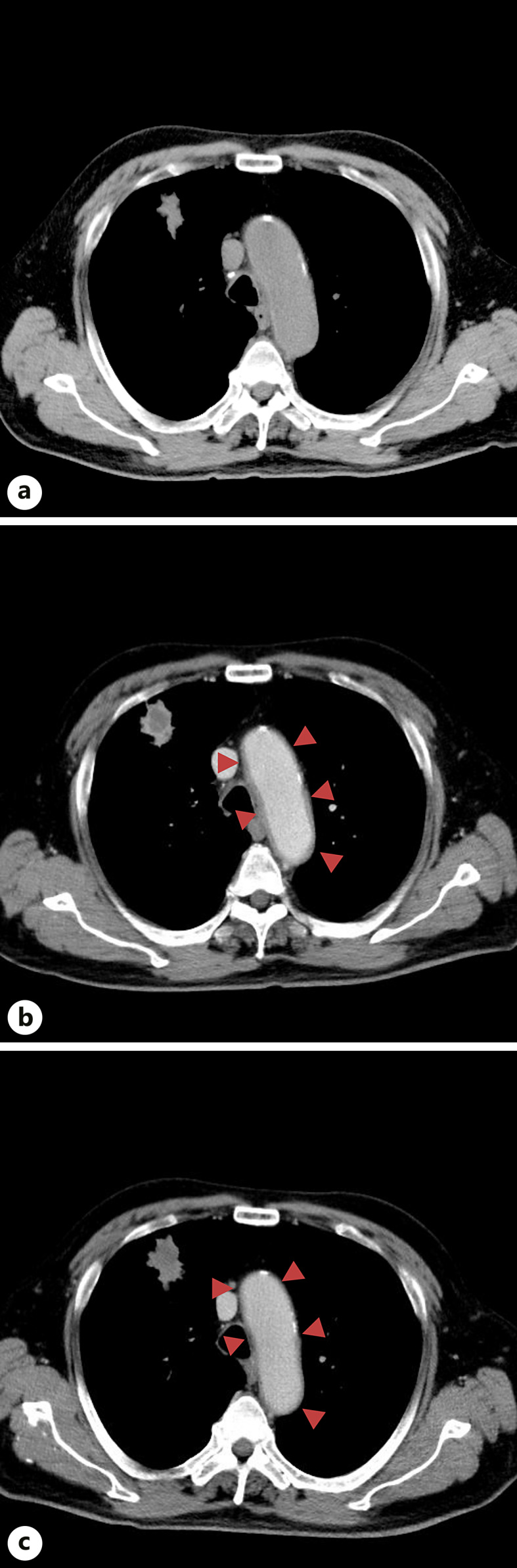
Computed tomography (CT) images. a A chest plane CT image taken on the 17th day after PEG-G-CSF administration. This image does not show an increased soft periaortic shadow. b A chest contrast-enhanced (CE)-CT scan obtained on the 18th day of PEG-G-CSF administration. This image shows an increased soft periaortic shadow suggestive of aortitis. c The chest CE-CT scan performed on the 33rd day of PEG-G-CSF administration. As indicated by the red triangle in the figures, the periaortic soft shadow shrunk compared with that shown in b.
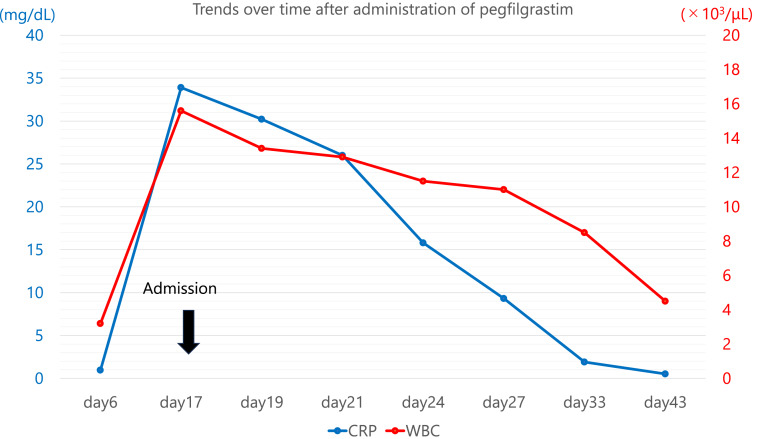
Fig. 2.
Line graph of trends in white blood cell (WBC) counts and C-reactive protein (CRP). The blue line indicates the trend in CRP levels, and the red line indicates the trend in WBC counts. Both CRP level and WBC count were abnormally high on the 17th day after PEG-G-CSF administration. However, they continued to decrease without corticosteroid administration and were almost normalized on the 33rd day of PEG-G-CSF administration.
Discussion
We encountered a rare case of aortitis following treatment with PEG-G-CSF. In the lung cancer field, Sato et al. [4] reported a case of G-CSF-induced aortitis with subsequent Stanford B-type aortic dissection. However, including this case, it should be noted that none of the previously reported cases of PEG-G-CSF-induced aortitis was fatal. The present case is typical of PEG-G-CSF-induced aortitis in the following respects. First, fever and right ear pain developed on day 12 after PEG-G-CSF treatment. Second, the diagnosis of aortitis was confirmed by CE-CT performed after admission, which illustrated the typical findings of aortitis. Finally, the patient’s symptoms disappeared on the 20th day without corticosteroids.
In the present case, we successfully detected findings suggestive of PEG-G-CSF-induced aortitis. CE-CT was the diagnostic method used in most cases, and soft-tissue thickening was observed around the aorta and other arteries. The median onset of aortitis is 8 days after G-CSF administration, and the majority of the initial symptoms are fever and pain; this makes it difficult to distinguish G-CSF-induced aortitis from FN because the timing and symptoms of aortitis are also typical of FN. G-CSF-induced aortitis may be the main cause of fever of unknown origin, which seemed to improve with antibiotics. In such cases, the fever seemed to improve spontaneously with antibiotics, as in this case. Based on previous reports and the present case, we believe that when a patient develops pyrexia of unknown origin with a prominent inflammatory response after G-CSF administration, CE-CT should be performed to diagnose aortitis [3]. Clinicians should be aware that aortitis is a rare but serious adverse effect of G-CSF therapy.
Corticosteroids play a key role in the treatment of inflammatory diseases because of their anti-inflammatory and immunosuppressive effects. However, their side effects vary, and they are often difficult to manage appropriately. One side effect of corticosteroids of particular note is the reactivation of HBV, which can lead to fulminant hepatitis and death [5]. Patients with prior HBV infection should be carefully monitored for symptoms, such as fatigue and liver enzyme trends, during corticosteroid therapy. Another adverse effect of corticosteroids is that they counteract the antitumor effects of ICIs. Although it is still not frequently used in the breast and obstetrics/gynecology fields, ICIs are already commonly used in lung cancer, and ICIs and G-CSF are often used together. Since ICIs activate autologous immune cells to achieve their antitumor effect, it is believed that immunosuppression by corticosteroids inevitably attenuates their effect [6]. Therefore, treatment with ICIs should be avoided for patients who receive corticosteroid therapy, or the target population for treatment with ICIs should be limited to patients whose dose of corticosteroids is less than 10 mg/day of the prednisone equivalent [7]. Since the patient in the present case was treated with ICIs and had a history of hepatitis B, we were concerned about the reactivation of HBV and counteracting the effect of ICIs. In addition, his symptoms tended to resolve; therefore, we did not administer corticosteroids and carefully monitored his progression.
The aortitis improved spontaneously in the patient, as reported previously [3]. Approximately 60% of patients with G-CSF-induced aortitis initiated corticosteroids, but there was no significant difference in the time to symptom improvement with corticosteroids; the mean times were 15.7 versus 16.0 days for patients treated with corticosteroids versus those who were not, respectively [3]. Therefore, corticosteroids may be unnecessary for the treatment of G-CSF-induced aortitis, especially in cases in which the side effects of corticosteroids may have serious consequences. Although the initiation of corticosteroids may be considered for patients with G-CSF-induced aortitis within several days from the onset, it may be unnecessary thereafter.
Both in the present case and in prior reports, a notable surge followed by a subsequent decline in IL-6 has been observed [4]. In Takayasu’s arteritis and giant cell arteritis, which exhibit similarities to G-CSF-induced aortitis, serum IL-6 levels have been reported to serve as indicators of disease activity [8]. It has been established that G-CSF triggers neutrophil differentiation and proliferation along with the production of inflammatory cytokines such as IL-6 [9]. Additionally, research has illuminated the secretion of IL-6 by vascular wall cells in giant cell arteritis patients, suggesting that IL-6 produced by certain factors might incite aortitis [10]. Consequently, in the present case, it is conceivable that G-CSF-induced IL-6 overproduction, thereby precipitating aortitis. If the condition escalates, it could lead to aortic aneurysm or aortic dissection, hence underscoring the significance of anti-inflammatory interventions involving corticosteroids or tocilizumab. Nevertheless, there exists a paucity of reports regarding G-CSF-induced aortitis progressing to aortic dissection. This implies that in scenarios such as the one at hand, where immunosuppression is undesired, some cases might ameliorate through vigilant monitoring without resorting to anti-inflammatory therapies [3].
A limitation of this report is the possibility that aortitis was caused by ICIs or the coronavirus vaccine, rather than PEG-G-CSF. Although a case of ICI-induced aortitis has been reported, we considered this possibility less likely because of the later onset of immune-related adverse events (median time, 3 months) and the typical course of G-CSF-induced aortitis [11, 12]. Although we cannot rule out the possibility that it was caused by a coronavirus vaccine, we believe that the aortitis was caused by PEG-G-CSF. This is because it closely resembles that caused by PEG-G-CSF [3], while there have been no reports of a coronavirus vaccine.
Conclusion
We encountered a rare case of aortitis following the administration of PEG-G-CSF to prevent FN, after a combination of ICI and chemotherapy for SCLC. When we diagnose a patient with G-CSF-induced aortitis, especially in situations such as preexisting HBV infection or after administration of ICIs, which should avoid an immunosuppressive state, corticosteroid administration should be avoided, and careful observation should be made with antipyretic analgesics alone.
Acknowledgments
We would like to thank Editage (https://www.editage.com) for their help with English language editing.
Statement of Ethics
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. This study protocol was reviewed and the need for approval was waived by the Ethical Review Board of National Hospital Organization Kyoto Medical Center.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported in part by a grant from the National Hospital Organization’s fiduciary funds (for English editing).
Author Contributions
Takanori Ito and Osamu Kanai treated the patient, contributed to the conception of this manuscript, and wrote the draft. Zentaro Saito, Takuma Imakita, Issei Oi, Kohei Fujita, Hiromasa Tachibana, and Tadashi Mio contributed to the conception and revised the manuscript. All authors have checked and agreed for publication of final version of the manuscript.
Funding Statement
This study was supported in part by a grant from the National Hospital Organization’s fiduciary funds (for English editing).
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author [O.K.] upon reasonable request.
Supplementary Material
References
- 1. D’Souza A, Jaiyesimi I, Trainor L, Venuturumili P. Granulocyte colony-stimulating factor administration: adverse events. Transfus Med Rev. 2008;22(4):280–90. [DOI] [PubMed] [Google Scholar]
- 2. Oshima Y, Takahashi S, Tani K, Tojo A. Granulocyte colony-stimulating factor-associated aortitis in the Japanese adverse drug event report database. Cytokine. 2019;119:47–51. [DOI] [PubMed] [Google Scholar]
- 3. Hoshina H, Takei H. Granulocyte-colony stimulating factor-associated aortitis in cancer: a systematic literature review. Cancer Treat Res Commun. 2021;29:100454. [DOI] [PubMed] [Google Scholar]
- 4. Sato Y, Kaji S, Ueda H, Tomii K. Thoracic aortitis and aortic dissection following pegfilgrastim administration. Eur J Cardio Thorac Surg. 2017;52(5):993–4. [DOI] [PubMed] [Google Scholar]
- 5. Du W, Zheng Z, Han S, Ma S, Chen S. HBV reactivation in an occult HBV infection patient treated with prednisone for nephrotic syndrome: case report and literature review. BMC Infect Dis. 2013;13(1):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–8. [DOI] [PubMed] [Google Scholar]
- 8. Unizony S, Stone JH, Stone JR. New treatment strategies in large-vessel vasculitis. Curr Opin Rheumatol. 2013;25(1):3–9. [DOI] [PubMed] [Google Scholar]
- 9. Pajkrt D, Manten A, van der Poll T, Tiel-van Buul MM, Jansen J, Wouter ten Cate J, et al. Modulation of cytokine release and neutrophil function by granulocyte colony-stimulating factor during endotoxemia in humans. Blood. 1997;90(4):1415–24. [PubMed] [Google Scholar]
- 10. Emilie D, Liozon E, Crevon MC, Lavignac C, Portier A, Liozon F, et al. Production of interleukin 6 by granulomas of giant cell arteritis. Hum Immunol. 1994;39(1):17–24. [DOI] [PubMed] [Google Scholar]
- 11. Bloomer CH, Annabathula RV, Aggarwal V, Upadhya B, Lycan TW. A case report of immune checkpoint inhibitor-induced aortitis treated with tocilizumab. Case Reports Immunol. 2022;2022:7971169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors: a systematic review. Clin Rheumatol. 2018;37(9):2579–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author [O.K.] upon reasonable request.