Abstract
Several nucleotide analogues have been approved for use in treating hepatitis B virus (HBV) infection. Long-term exposure to therapy leads to the emergence of mutations within the HBV DNA polymerase gene, resulting in drug resistance, a major factor contributing to therapy failure. Chronic HBV patients from the Khyber Pakhtunkhwa province, Pakistan, who had completed 6 months of therapy participated in this study. Samples were collected from 60 patients. In this study, the entire reverse transcriptase domain of the HBV polymerase gene was amplified using nested polymerase chain reaction and sequenced. Drug-resistant mutations were detected in nine (22.5%) patients. All of these patients had lamivudine-resistant mutations (rtM204V + L180M), while seven individuals (17.5%) had both lamivudine- plus entecavir-resistant mutations (L180M + M204V + S202G). N236T, a mutation that gives rise to tenofovir and adefovir resistance, was observed in two (5%) patients. T184A, a partial drug-resistant mutation to entecavir, was found in five (12.5%) patients. Furthermore, other genotypic variants (100%) and vaccine escape mutations (5%) were additionally observed. Moreover, pN459Y (35%), pN131D (20%), pL231S (20%), pP130Q (17.5%), pS189Q (12.5%), pP161S (5%), pH160P (2.5%), pT322S (2.5%), and pA223S (2.5%) mutations in the polymerase gene, as well as sA166V (17.5%), sQ181K (12.5%), sV184R (7.5%), sA17E (5%), sP153S/K (5%), sW156C (5%), sC76Y (2.5%), and S132F (2.5%) mutations in the small surface gene, were identified for the first time in this study. Phylogenetic analysis showed that genotype D was predominant amongst the HBV carriers. Subtype D1 was found in most patients, while two patients were subtype D9. These novel findings may contribute to the body of knowledge and have clinical significance for treating and curing HBV infections in Pakistan.
Keywords: hepatitis B virus, polymerase gene, surface gene, genotypes, mutations, phylogenetic analysis
1. Introduction
The hepatitis B virus (HBV) belongs to the Hepadnaviridae family. HBV is a DNA-containing virus with a diameter of 42 nm and a small circular partially double-stranded genome of about 3.2 kilo base pair [1,2]. The genome contain four partially overlapping open reading frames (ORFs). These ORFs encode seven proteins, including polymerase protein encoded by the P gene (longest ORF), core and e antigen encoded by the C gene, S gene encoding large, middle, and small surface antigen proteins and the X gene encoding the X protein [3]. The polymerase gene (P) has four domains, N-terminal (aa 1–177), spacer (aa 178–346), reverse transcriptase (RT) (aa 347–690) and the RNaseH domain (aa 691–843). The RT domain is involved in the replication of the HBV genome and is a target for developing antiviral HBV therapy [4,5].
The RT domain is further divided into seven subdomains (A-G). Domain A (rt 75–91) is involved in coordinating incoming triphosphate moiety of dNTP and making a portion of the dNTP binding pocket. Domain B (rt 163–189) forms a helix with a loop that helps to attach primers to the template. Subdomain C (rt 200–210) has a YMDD (tyrosine-methionine-aspartate-aspartate) motif in its active site. Domain D (rt 230–241) and E (rt 247–257) are attached with deoxynucleoside triphosphate (dNTPs) and form part of the template binding site. Domain F (rt 37–47) and G (rt 26–36) are present before domain A and take part in the interaction of dNTP with the template [3,6].
In Pakistan more than 9 million people live with this infection, and its prevalence is increasing daily along with HCV [7,8]. The prevalence of HBsAg (hepatitis B surface antigen) in the entire population is 2.5% [8]. Five nucleotide analogues are currently prescribed in Pakistan to prevent disease progression and viral replication: lamivudine, adefovir, entecavir, telbivudine, and tenofovir [9]. Due to the lack of proofreading activity in the viral polymerase, resistance mutations arise against every nucleotide analogue used to treat the infection, which mostly occurs in the RT domain of the P gene [10,11]. These resistance mutations pose one of the biggest challenges in treating HBV infection, as they decrease the binding efficacy of drugs with RT by affecting the structures. In addition, the RT domain partially overlaps with the small surface protein (HBsAg) of the virus; therefore, RT mutations may also affect HBsAg [10].
The current study aims to investigate the emergence of drug resistance mutations in patients (from the Khyber Pakhtunkhwa province, Pakistan) who have been treated with nucleotide analogues, so that the common genotypes can be identified.
2. Materials and Methods
2.1. Sample Collection
A total of 60 patients were included in the study. Of these patients, 20 recovered from the infection and were declared PCR negative. The recovered patients were excluded from the study, while the remaining 40 PCR-positive patients were enrolled from 2016 to 2017. These patients had elevated alanine aminotransferase (ALT) and aspartate aminotransferase levels (AST) despite undergoing nucleotide analogue therapy. These 40 individuals were non-responder patients (patients who received their first therapy yet were still PCR positive). Amongst the study cohort, 11 patients received lamivudine and tenofovir, 6 received lamivudine and entecavir, 9 received entecavir, 8 tenofovir/telbivudine, while 6 patients were treated with adefovir for at least six months. The patients strictly followed drug adherence. All the patients strictly followed AASLD (American Association for the Study of Liver Diseases) 2018 hepatitis B guidelines [12].
2.2. Viral DNA Isolation
Viral DNA was isolated from 150 µL of serum using the instant viral RNA/DNA kit from Analytic Jena (Thuringia (Jena), Germany), per the manufacturer’s protocol. Each sample’s final eluate containing viral DNA was stored at −20 °C.
2.3. Detection of Viral DNA
The viral load was determined using real-time PCR (Rotor-Gene® 3000). The standard Analytik Jena kit (Jena, Germany) was used as per the manufacturer instructions. The quantitative PCR conditions were as follows: initial temperature 95 °C for 4 min, 45 cycles of denaturation at 95 °C for 0.15 min followed by elongation at 57 °C for 1 min. The lower limit of detection of the quantitative HBV DNA assay is 210 copies/mL.
2.4. Polymerase Gene Amplification
The polymerase gene covering the reverse transcriptase domain was amplified using the nested PCR protocol with different sets of primers (Table 1) [13]. The reverse transcriptase domain (~1200 bp) was amplified in 3 overlapping fragments using the first-round product as a template. A 25 µL reaction mixture was prepared using 12.5 µL of ready-to-use master mix (Bioran life sciences, catalog. No 101605, Römerberg, Germany), 1.0 µL of each forward and reverse primers, 5 µL of template DNA, and 5.5 µL of PCR grade water. For the first round of PCR, the initial denaturation was set at 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 53 °C for 30 s and extension at 72 °C for 2 min and final elongation at 72 °C for 15 min. For the second round of PCR, the same master mix and PCR conditions were applied; however, different primer sets were used, and the first round PCR product template was also different. A negative control was used for each PCR run. Ten second round PCR product microliters from each sample were analyzed by 2% agarose gel electrophoresis and visualized using a gel documentation system. The PCR product was purified and then sequenced ABI BigDye® Terminator v3.1 chemistry (Table 1).
Table 1.
List of primers used in current study.
| Primers | Sequence (5′ → 3′) | Direction | Nucleotide |
|---|---|---|---|
| 1st Round PCR | |||
| Outer forward | TTTCACCTCTGCCTAATCATCT | Forward | 1823 |
| Outer reverse | CAGACCAATTTATGCCTACAGCCT | Reverse | 1801 |
| 2nd Round PCR | |||
| Forward (F1) | GGTCACCATATTCTTGGGAAC | Forward | 2821 |
| Reverse (R1) | TGAGAGAAGTCCACCACGAGT | Reverse | 272 |
| Forward (F2) | CTAGGACCCCTGCTCGTGTT | Forward | 179 |
| Reverse (R2) | CGAACCACTGAACAAATGGCACT | Reverse | 704 |
| Forward (F3) | GTATTCCCATCCCATCATCCTG | Forward | 599 |
| Reverse (R3) | GCTAGGAGTTCCGCAGTATGG | Reverse | 1286 |
2.5. HBV Genotyping and Phylogenetic Analysis of HBV Isolates
The genotypes of the study samples were determined using a hepatitis B virus genotyping tool (https://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi, accessed on 30 January 2023). For the phylogenetic analysis, purified samples were sent to the Beijing Genomics Institute Mainland China (BGI) (https://en.genomics.cn/ (accessed on 30 January 2023)), and the chromatograms were evaluated through Chromas software 2.6.6 (http://technelysium.com.au/wp/chromas/ (accessed on 20 February 2023)) to refine the sequence. The sequences were submitted to National Center for Biotechnology Information (NCBI) database in FASTA format for accession number. Phylogenetic analysis was conducted using Molecular Evolutionary Genetic Analysis Version X (MEGA X) software (http://www.megasoftware.net (accessed on 25 February 2023)) [14,15]. For the purposes of analysis, we generated two datasets, one having our current study sequence with all HBV genotype (A-J) reference sequences, to classify our sequence similarity with genotypes of HBV. Another dataset has our sequences and sub-genotypes sequence of HBV to show classification of our sequence with HBV sub-genotype. The phylogenetic tree was constructed using Maximum Likelihood techniques with 1000 bootstrap values as the default configuration [16]. The trees were annotated in the Interactive Tree Of Life (iTOL) version v5 (accessed 30 September 2023) [17].
2.6. Mutation Analysis
Mutations were analyzed by aligning our sequences with HBV reference sequences belonging to different genotypes (genotype A, AF090842.1, X51970.1 and LC519823.1, Genotype B, LC365289.1, D00329.1 and AB073846.1, genotype C, X04615.1, AB014381.1 and LC365290.1, genotype D, FJ904424.1, X65259.1 and M32138.1, genotype E, X75657.1, AB032431.1 and LC513657.1, genotype F, X69798.1 and AB036910.1, genotype G, AB625343.1, AF160501.1 and AB064310.1 and genotype H, AY090454.1 and AY090457.1) and sub-genotypes (AB104712.1 D1 Egypt, AB126581.1 D1 Russia, AB222712.1 D1 Uzbekistan, EU594396.1 D1 Kazakhstan, Y07587.1 D1 Germany, AY721612.1 D1 Turkey, AB222713.1 D1 Uzbekistan, AY161157.1 D1 India, X02496.1 D1 Latvia, AY945307.1 D1 India, AF121240.1 D1 Turkey, AY721607.1 D1 Turkey, AY945307.1 D1 India, AY741797.1 D1 Iran, AB246347.1 D1 India, AB188244.1 D1 Uzbekistan, AB246348.1 D1 USA, AF280817.1 D1 China, FJ904424.1 D1, AY721605.1 D1, AB267090.1 D2, Z35716.1 D2, AB210822.1 D2, AB109475.1 D2, JN664919.1 D9 India, AB555496.1 D10, AY233294.1 D3, Y233293.1 D3, AY233292.1 D3, AB048701.1 D4, AB033559.1 D4, AB033558.1 D5, DQ315779.1 D5, AB493845.1 D6, AB493846.1 D6, AB493848.1 D6, FJ904436.1 D7, FJ904405.1 D7, FJ904439.1 D7, FN594770.1 D8, FN594769.1 D8, JN664942.1 D9, and JN664919.1 D9), using Snap gene 3.2.1 software. Additionally, sequences were also submitted to an international repository for hepatitis B virus strain data and were subsequently confirmed by geno2pheno analysis (https://www.geno2pheno.org, accessed on 30 January 2023) by using default setting.
2.7. Statistical Analysis
GraphPad prism 7.0 and statistical package for social sciences (SPSS) 29.0.10 were used for statistical analysis. Results were expressed as mean ± standard deviation.
3. Results
Demographic characteristics of study patients are listed in Table 2 (Supporting Information File: Table S1: Viral Load of all the study cohort). Of the 40 patients, 26 (65%) were male, and 14 (35%) were female. Their ages ranged from 10 to 65 years.
Table 2.
Demographic characteristics of study patients.
| Gender | Age Range | Therapy (No of Patients) | ALT | AST | Viral Load IU/mL |
|---|---|---|---|---|---|
| Female 14 (35%) Male 26 (65%) |
10–65 | Entecavir (09) Lamivudine + tenofovir (11) Lamivudine + entecavir (6) Tenofovir (08) Adefovir (06) |
Mean ± SD 73.77 ± 10.43 Median 73.12 Range 56–95 |
Mean 71.03 ± 9.10 Median 70.52 Range 52–93 |
3.0 × 104 to 5.6 × 107 |
Abbreviation: SD: standard deviation, ALT: Alanine transaminase, AST: Asparagine transaminase.
3.1. Genotyping and Phylogenetic Analysis
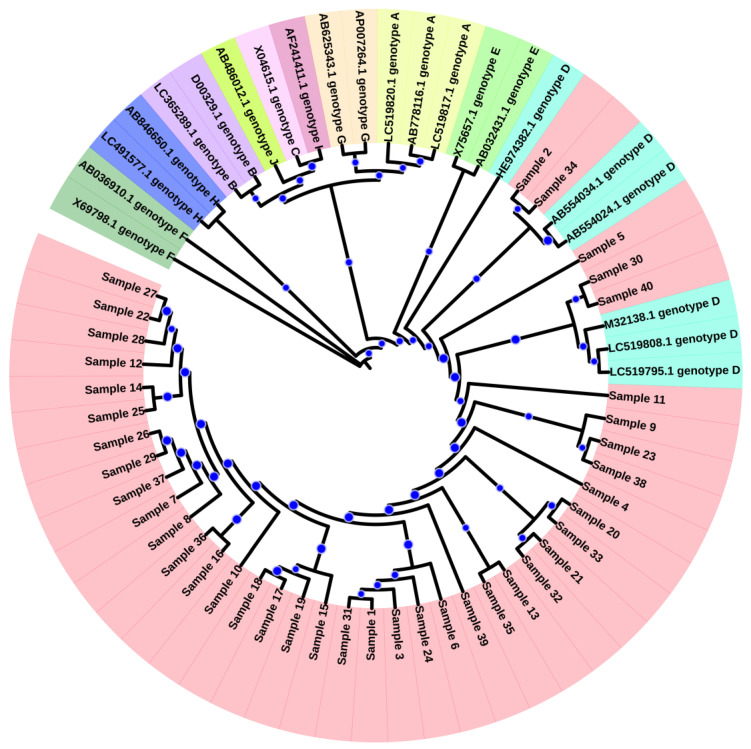
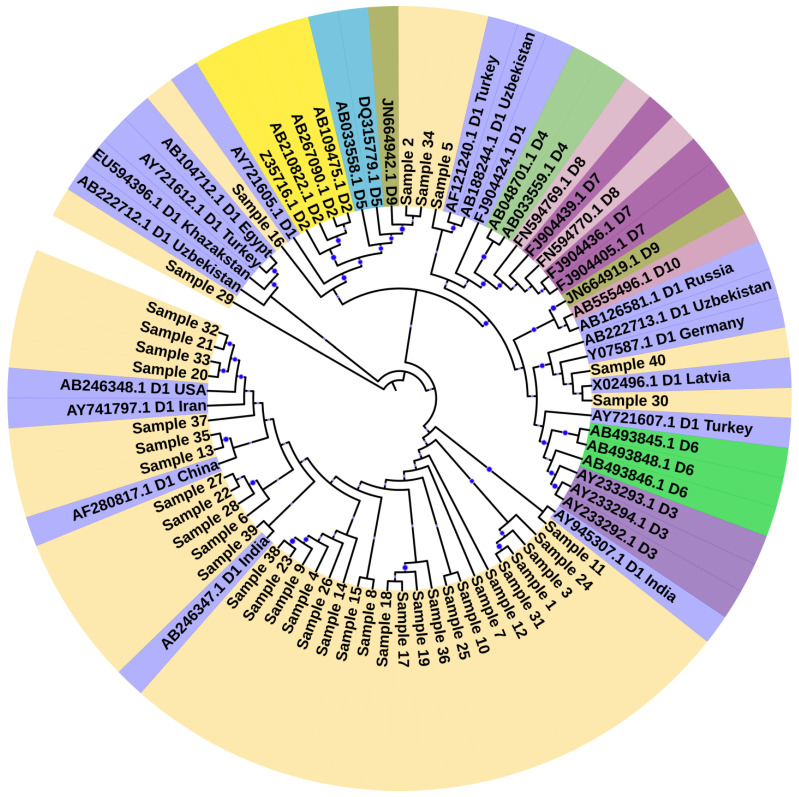
Sequences were submitted to the National Center for Biotechnology Information (NCBI) gene bank with accession numbers (MK213855-MK213894) (Supporting Information File: Table S2: Sequences generated from current study). All samples were classified as genotype D through the NCBI genotyping tool (https://www.ncbi.nlm.nih.gov/projects/genotyping/view.cgi?db=2, accessed on 30 January 2023). For phylogenetic analysis, representative reference sequences for each genotype were downloaded from the NCBI genotyping tool with accession numbers LC519823.1 (Bangladesh), AF090842.1 (Belgium), X51970.1 (Germany), LC365289.1 (Japan), D00329.1 (Japan), AB073846.1 (Asia), LC365290.1 (Japan), X04615.1 (Japan), AB014381.1 (Japan), X65259.1 (Italy), M32138.1 (France), FJ904424.1 (Tunisia), X75657.1 (Sweden), AB032431.1, X69798.1 (Germany), AB036910.1 (India), LC513657.1 (Japan), AF160501.1 (Korea), AB064310.1 (Japan), AB625343.1 (Mexico), AY090454.1 (Nicaragua), and AY090457.1 (Japan). Sequences were aligned and a phylogenetic tree was constructed using neighbor-joining method (bootstrap analysis of 500 replicates using MEGA X) (Figure 1). Based on the dendrogram analysis, our current study sequences were clustered with genotype D (Figure 1). This finding showed that HBV genotype D is the predominant genotype in Pakistan. For the determination of sub-genotypes, reference sequences were downloaded from NCBI, and a phylogenetic tree was constructed using MEGA X, which showed that the majority of the samples had genotype D1, while 2 samples were clustered with subtype D9 as shown in Figure 2.
Figure 1.
Phylogenetic Tree Representing Clustering of Study Samples with Genotype D. A maximum likelihood method and Tamura-Nei model were used to generate a phylogenetic tree representing the HBV sequences found in 40 HBV-positive patients. The bootstrap value was shown with blue dots. Nodes with 70% confidence were included and the lower were removed. The HBV sequences were aligned with different HBV genotype reference sequences using ClustalW. This analysis involved 62 nucleotide sequences. There was a total of 5131 positions in the final dataset. Evolutionary analyses were conducted using MEGA X. Light pink color represent patient samples. The genotypes are as follows: Genotype A (light yellow), Genotype B (light purple), Genotype C (light orange), Genotype D (Cyan), Genotype E (light green), Genotype F (Green), Genotype G (green) and Genotype H (blue). Each sequence’s accession numbers and genotype were mentioned in the phylogenetic tree. The clustering indicates that genotype D is the most common genotype in our study cohort.
Figure 2.
Phylogenetic tree identifying the HBV sub-genotypes in the study cohort. Evolutionary history was inferred by using the Maximum Likelihood method and Tamura-Nei model. This analysis involved 81 nucleotide sequences. The bootstrap value was shown with blue dots. Nodes with 70% confidence were included and the lower were removed. Patient samples are represented by light yellow color. Each sequence’s accession numbers, sub-genotype and country of origin were mentioned in the phylogenetic tree. The clustering indicates that sub-genotype D1 is the most common genotype in our study cohort. Two patient samples clustering with sub-genotype D9 showed the appearance of novel subtype in Pakistan. The evolutionary analyses were conducted in MEGA X.
3.2. Drug Resistance Mutation
In both the reverse transcriptase/surface protein overlapping areas, there are a lot of drug-resistant mutations found. Therefore, we analyzed the replacement of the same nucleotide, at the expense of overlapping the frames of reading the replacement in both the enzyme and the surface protein. The present study found resistance mutations in 9 of the 40 patients (22.5%). Of the nine patients, all had lamivudine resistance mutations (rtM204V + L180M), while seven patients (17.5%) had both lamivudine plus entecavir resistance mutations (L180M + M204V + S202G). The N236T mutation gives rise to partial resistance to tenofovir and adefovir resistance. These mutations were observed in 2 of the 40 (5%) patients. Partial resistance mutations to entecavir T184A were found in 5 of the 40 (12.5%) patients, respectively. Compensatory mutations such as V173M (n = 2/40) (5%), V191G (n = 2/40) (5%), Q215P (n = 5/40) (12.5%) and N238T (n = 3/40) (7.5%) were also observed in these patients (Table 3).
Table 3.
Drug resistance mutations observed in the study cohort.
| S. No | Drug Resistance Mutations | Therapy Used | Small Surface Gena Mutation |
|---|---|---|---|
| 1 | L180M, S202G, M204V | Lamivudine + entecavir | sI195M, sS193L |
| 2 | L180M, S202G, M204V | Lamivudine + entecavir | sI195M, A166V/T |
| 3 | L180M, S202G, M204V | Entecavir | sI195M |
| 4 | Y135S, V173M, L180M, S202G, M204V, N248H | entecavir | sI195M |
| 5 | S202G, M204V, L180M | Lamivudine+ entecavir | sI195M, A166V/T, Q129H (Vaccine escape mutation), Q181K/R |
| 6 | rtM204V + L180M, S202G | Entecavir | Not detected |
| 7 | rtM204V + L180M | Lamivudine + entecavir | sI195M, Q181K/R |
| 8 | rtM204V + L180M, V173L/M | Lamivudine + entecavir | sI195M, Q181K/R |
| 9 | rtM204V + L180M | Lamivudine + entecavir | sI195M |
| 10 | T184A | Entecavir | Not detected |
| 11 | A194V | Tenofovir | Not detected |
| 12 | Not detected | Tenofovir | Not detected |
| 13 | Q215P, V191G | Lamivudine + Tenofovir | P203S/R, P142L (vaccine escape mutation), W156C, P203R |
| 14 | N236T | Adefovir | Not detected |
| 15 | N238H/T | Tenofovir | Not detected |
| 16 | Not detected | Adefovir | Not detected |
| 17 | Not detected | Lamivudine + tenofovir | P153S, W156C, P203R |
| 18 | T184A | Entecavir | Not detected |
| 19 | Not detected | Lamivudine + tenofovir | A166V/T |
| 20 | Not detected | Tenofovir | Not detected |
| 21 | T184A | Entecavir | Not detected |
| 22 | Not detected | Tenofovir | Not detected |
| 23 | V191G | Lamivudine + tenofovir | A166V/T, V184R, A17E |
| 24 | Not detected | Tenofovir | Not detected |
| 25 | Not detected | Entecavir | Not detected |
| 26 | Not detected | Adefovir | Not detected |
| 27 | Not detected | Lamivudine + tenofovir | P153S |
| 28 | N236T | Adefovir | S193L, S132F, V184R |
| 29 | Not detected | Lamivudine + tenofovir | A166V/T, Q181K/R |
| 30 | Not detected | Tenofovir | Not detected |
| 31 | Not detected | entecavir | P203R, C76Y, Q129H (vaccine escape mutation), P142L, Q181K/R |
| 32 | T184A | Entecavir | Not detected |
| 33 | Not detected | Lamivudine + tenofovir | Not detected |
| 34 | Not detected | Lamivudine + tenofovir | Not detected |
| 35 | Not detected | Tenofovir | Not detected |
| 36 | Not detected | Lamivudine + tenofovir | A166V/T, V184R |
| 37 | Not detected | Adefovir | Not detected |
| 38 | Not detected | Lamivudine + tenofovir | Not detected |
| 39 | N238H/T | Adefovir | Not detected |
| 40 | Not detected | Lamivudine + tenofovir | A166V/T, A17E |
3.3. Genotypic Variants
Genotypic variants refer to variations in any amino acid. Genotypic variants in the reverse transcriptase domain are shown in Table 4. The commonly detected genotypic variants were Y135S in 40 (100%) patients, followed by N248H in 37 (92.5%), N459Y in 14 (35%), D263E in 13 (32.5%), H124Y, V278I, I266R in 9 (22.5%), N131D in 8 (20%), S189Q in 5 (12.5%), V190M in 3 (7.5%), C262S in 4 (10%), Y257H in 4 (10%), P161S in 2 (5%), V253I in 2 (5%), L145M in 2 (5%) and E271D in 2 (5%) patients. Contrastingly, other variants, including A223S, L231S/W, H160P and T322S, were also observed (2.5% each) but in different patients.
Table 4.
Genotypic variants observed in the reverse transcriptase domain in the study cohort.
| Amino Acid Substitution | Frequency | Percentage % |
|---|---|---|
| N248H | 37 | 92.5 |
| N459Y | 14 | 35 |
| N131D | 8 | 20 |
| P130Q | 7 | 17.5 |
| S189Q | 5 | 12.5 |
| Y257H | 4 | 10 |
| C262S | 4 | 10 |
| V190M | 3 | 7.5 |
| L145M | 2 | 5 |
| P161S | 2 | 5 |
| V253I | 2 | 5 |
| E271D | 2 | 5 |
| A223S | 1 | 2.5 |
| L231S/W | 1 | 2.5 |
| T322S | 1 | 2.5 |
3.4. Other Polymerase Gene Mutation
Beside the reverse transcriptase domain, mutations were also observed in parts of the polymerase gene as shown in Table 5. The most predominant mutations were pS709L/R and pC256, which were found in 10 samples (25%), followed by pE718K in 5 (12.5%), pM699I in 3 (7.5%), and pT707P in 2 (5%) samples, while pR705P and pL712 were found in 1 (2.5%) sample. Furthermore, pN459Y (35%), pN131D (20%), pL231S (20%), pP130Q (17.5%), pS189Q (12.5%), pP161S (5%), pT322S (2.5%), pH160P (2.5%), and pA223S (2.5%), in polymerase gene were newly identified mutations.
Table 5.
Polymerase gene mutations in the current study cohort.
| Other Polymerase Gene Mutation | Frequency | Percentage % |
|---|---|---|
| S709L/R | 10 | 25 |
| C256 | 10 | 25 |
| E718K | 5 | 12.5 |
| M699I | 3 | 7.5 |
| T707P | 2 | 5 |
| L712 | 1 | 2.5 |
3.5. Small Surface Gene Mutation
Table 3 shows the small surface gene amino acid substitutions in the patients. Other mutations that were not reported previously and were observed in the present study are sA17E (5%), sC76Y (2.5%), s132F (2.5%), sP153S/K (5%), sW156C (5%), sA166V (17.5%), sQ181K (12.5%), and sV184R (2.5%).
4. Discussion
The hepatitis B virus is divided into ten genotypes; however, the distribution of these genotypes varies globally. Genotypes C and D are the most prevalent in Pakistan and are often associated with severe liver disease and poor patient response to the appropriate antiviral therapy. The current study identified that genotype D is dominant in the Khyber Pakhtunkhwa province population. These results are consistent with previous studies [18,19,20]. Presently, different types of antiviral drugs are used for the treatment of HBV patients, including pegylated interferon (Peg-IFN), entecavir (ETV) andtenofovir disoproxil fumarate (TDF). Although, this drugs has been recommended as a first line therapy and showed significant potential, but upto some extent, side effects have also been noted [21]. Due to problems with drug resistance, telbivudine, adefovir, and lamivudine have become nearly useless. The appearance of drug resistance will lead to hepatitis flare, and hepatic decompensation, which is life threatening [22]. Other problems associated with these therapies are the cost and long-term treatment duration.
In the current study, we investigated the drug-resistant mutations in 40 non-responder patients (patients who have received their first therapy yet are still PCR positive) in the reverse transcriptase domain/small surface gene protein overlapping region of HBV in a Pakistani population. Due to the lack of proofreading activity, the HBV reverse transcriptase domain of polymerase, encoded by the biggest ORF in the genome, introduces random mutations into the HBV genome at a rate of about 10−4 to 10−7 mutations per site per year, making it highly error-prone [23,24].
Among these patients, drug resistance mutations were detected only in 9 of the 40 patients. Mutations that give rise to lamivudine resistance (rtM204V and rtL180M) were frequently detected, followed by entecavir resistance mutations (rtL180M + rtM204V + rtS202G). The rtS202G mutation emerged only when the lamivudine resistance substitution (rtM204V/I and rtL180M) was present [25]. Mutation rtN236T, responsible for adefovir and partial resistance to tenofovir [10,26,27], was observed in two patients in the current study. Recently, an in vitro study was conducted, which shows that rtA194V is associated with resistance to tenofovir, which was found in one of our patients [28].
We found that the rtY135S mutation was found in all the investigated patients, followed by rtN248H (92.5%). Similar results were also obtained [29]. However, they observed the presence of the rtN248H mutation in all the samples they had studied. The rtN248H mutation has recently been reported for its role in adefovir resistance [10,30], while the rtY135S mutation was found in treatment-resistant strains; however, its role in drug resistance is not clear [31]. However, rtA181T is responsible for lamivudine and adefovir resistance [32,33], which is not reported here. These results agree with previous studies from Islamabad (Pakistan) that did not observe the rtA181T mutations [29]. Another study reported by Liu et al. (2009) from Chinese patients with chronic HBV also supports our findings [34]. Other compensatory mutations, such as N238T (7.5%), V173M (5%), V191G (2.5%), and Q215P (5%), were also observed in this study, which is in agreement with the results of a study reported in 2018 [35].
Due to the overlapping nature of the HBV genome, mutations in the RT domain and small surface genes may occur simultaneously. The sI195M mutation is due to the rtM204V mutation, which causes resistance to lamivudine and telbivudine. Similarly, the sS193L mutation is due to the rtS202G mutation, which causes resistance to entecavir [36]. In the surface gene, a highly immunogenic region is a determinant region that spans 101–160 amino acids. The mutations in or around this region may lead to viral immune escape. In the present study, sS193L, sQ129H, sI195H, s132F, and sP142L mutations were detected. These mutations have also been previously reported [35,37,38]. These mutations played a significant role in the virus immune escape, and these variations have largely been justified by factors associated with host immunity, such as HBV-specific T- and/or B-cell production, and antigen presentation failure. Additionally, viral determinants, such as the HBV genotypes and their evolving variants, have played a significant role in contributing to these variations [39,40]. A study reported that surface antigen causes dysfunction of myeloid dendritic cells, which serve as a possible immune escape mechanism in HBV [39].
Furthermore, other mutations, such as A223S (2.5%), N131D (20%), L231S (20%), S189Q (12.5%), L145M (5%), H160P (2.5%), P161S (5%), N459Y (35%), T322S (2.5%), and P130Q (17.5%) in the P gene, as well as sA166V (17.5%), sQ181K (12.5%), sA17E (5%), sP153S/K (5%), sW156C (5%), sV184R (7.5%), and sC76Y (2.5%) in the S gene, were also detected. The role of these novel mutations in therapy resistance and disease progression is not fully understood. Hence, further investigations are required.
5. Conclusions
In conclusion, based on our phylogenetic analysis, genotype D is found to be the most prevalent genotype in the Khyber Pakhtunkhwa province, Pakistan, with subtype D1 being the most occurring sub-genotype. Analysis of the polymerase gene in the hepatitis B virus revealed interesting information regarding drug resistance mutations, which may be instrumental in devising appropriate therapy. Due to the overlapping of the surface and polymerase gene frames, small surface gene mutations are also detected, which can be helpful in further studies examining the correlation between amino acid substitutions and the pathogenicity of the hepatitis B virus. The small surface gene mutations were also observed, but the exact role in the virus’s immune escape and the disease’s progression is yet to be elucidated completely. It is important that these mutations are monitored during the immunosuppressive phase in the patients to observe the role in immune escape. Additionally, novel mutations found in both surface and polymerase genes need exploration. These mutations, potentially influencing disease severity and progression, warrant further investigation.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R350), King Saud University, Riyadh, Saudi Arabia. The author acknowledged the Centre of Biotechnology and Microbiology for permitting us to use resources for experimental persistence and analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11112622/s1, Table S1: Viral Load of all the study cohort, Table S2: Sequences generated from current study. 40 sequences submitted to NCBI GenBank along with submission id and relevant URLs. Table S3 samples in which no drug resistance mutations were detected.
Author Contributions
Conceptualization, M.G. and I.U.R.; methodology, I.U.R. and A.U.; software, M.G., M.A.K., J.A. and S.B.; validation, H.Y. and I.U.R.; formal analysis, M.A.K. and S.B.; investigation, M.G. and I.U.R.; resources, A.A. and I.U.R.; data curation, J.A.; writing—original draft preparation, M.G. and A.U.; writing—review and editing, J.A. and I.U.R.; visualization, S.B. and M.G.; supervision, I.U.R. and J.A.; project administration, I.U.R. and A.U.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the ethical committee of The Center of Biotechnology and Microbiology, University of Peshawar, and all experiments were performed in accordance with the HELSINKI guidelines and regulations. Informed consent was obtained from every patient involved in this study who was above the age of 18. Additionally, informed consent was obtained from the parents or legal guardians of patients who were less than 18 years of age.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are fully available and can be found within the manuscript or in the Supporting Information file. There are no legal restrictions in this regard. Additionally, the datasets generated and/or analyzed during the current study are available in the NCBI repository (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 30 January 2023) and access number has been provided in the Supplementary File (Table S2).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Researchers Supporting Project Number (RSP2023R350), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dos Santos M.I.M.A., Pacheco S.R., Stocker A., Schinoni M.I., Paraná R., Reis M.G., Silva L.K. Mutations associated with drug resistance and prevalence of vaccine escape mutations in patients with chronic hepatitis B infection. J. Med. Virol. 2017;89:1811–1816. doi: 10.1002/jmv.24853. [DOI] [PubMed] [Google Scholar]
- 2.Chuang Y.C., Tsai K.N., Ou J.H.J. Pathogenicity and virulence of Hepatitis B virus. Virulence. 2022;13:258–296. doi: 10.1080/21505594.2022.2028483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sadeq D.W., Taleb S.A., Zaied R.E., Fahad S.M., Smatti M.K., Rizeq B.R., Al Thani A.A., Yassine H.M., Nasrallah G.K. Hepatitis B virus molecular epidemiology, host-virus interaction, coinfection, and laboratory diagnosis in the MENA region: An update. Pathogens. 2019;8:63. doi: 10.3390/pathogens8020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu J. Hepatitis B Virus in Human Diseases. Springer; Berlin/Heidelberg, Germany: 2016. Hepatitis B virus virology and replication; pp. 1–34. [Google Scholar]
- 5.Pollack J.R., Ganem D. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J. Virol. 1994;68:5579–5587. doi: 10.1128/jvi.68.9.5579-5587.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das K., Xiong X., Yang H., Westland C.E., Gibbs C.S., Sarafianos S.G., Arnold E. Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine (3TC) and emtricitabine (FTC) J. Virol. 2001;75:4771–4779. doi: 10.1128/JVI.75.10.4771-4779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M.A., Khan S.A., Hamayun M., Ali M., Idrees M. Sequence variability of HCV 3a isolates based on core gene in patients from Lahore, Pakistan. Future Virol. 2019;14:641–653. doi: 10.2217/fvl-2019-0086. [DOI] [Google Scholar]
- 8.Ali M., Idrees M., Ali L., Hussain A., Rehman I.U., Saleem S., Afzal S., Butt S. Hepatitis B virus in Pakistan: A systematic review of prevalence, risk factors, awareness status and genotypes. Virol. J. 2011;8:102. doi: 10.1186/1743-422X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi H., Bile K.M., Jooma R., Alam S.E., Afrid H.U.R. Prevalence of hepatitis B and C viral infections in Pakistan: Findings of a national survey appealing for effective prevention and control measures. EMHJ-East. Mediterr. Health J. 2010;16:15–23. doi: 10.26719/2010.16.Supp.15. [DOI] [PubMed] [Google Scholar]
- 10.Choi Y.M., Lee S.Y., Kim B.J. Naturally occurring hepatitis B virus reverse transcriptase mutations related to potential antiviral drug resistance and liver disease progression. World J. Gastroenterol. 2018;24:1708. doi: 10.3748/wjg.v24.i16.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartholomeusz A., Locarnini S. Hepatitis B virus mutations associated with antiviral therapy. J. Med. Virol. 2006;78:S52–S55. doi: 10.1002/jmv.20608. [DOI] [PubMed] [Google Scholar]
- 12.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.-M., Hwang J.P., Jonas M.M., Brown R.S., Jr., Bzowej N.H., Wong J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran S., Zhai X., Thai H., Campo D.S., Xia G., Ganova-Raeva L.M., Drobeniuc J., Khudyakov Y.E. Evaluation of intra-host variants of the entire hepatitis B virus genome. PLoS ONE. 2011;6:e25232. doi: 10.1371/journal.pone.0025232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 17.Letunic I., Bork P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali L., Idrees M., Ali M., Rehman I.U., Hussain A., Afzal S., Butt S., Saleem S., Munir S., Badar S. An overview of treatment response rates to various anti-viral drugs in Pakistani Hepatitis B Virus infected patients. Virol. J. 2011;8:20. doi: 10.1186/1743-422X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam M.M., Zaidi S.Z., Shaukat S., Sharif S., Angez M., Naeem A., Saleha S., Butt J.A., Malik S.A. Common Genotypes of Hepatitis B virus prevalent in Injecting drug abusers (addicts) of North West Frontier Province of Pakistan. Virol. J. 2007;4:63. doi: 10.1186/1743-422X-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmood M., Anwar M.A., Khanum A., Zaman N., Raza A. Distribution and clinical significance of hepatitis B virus genotypes in Pakistan. BMC Gastroenterol. 2016;16:104. doi: 10.1186/s12876-016-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien R.N., Liaw Y.F. Current trend in antiviral therapy for chronic hepatitis B. Viruses. 2022;14:434. doi: 10.3390/v14020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liaw Y.-F., Chien R.-N., Yeh C.-T., Tsai S.-L., Chu C.-M. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567–572. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- 23.Girones R., Miller R.H. Mutation rate of the hepadnavirus genome. Virology. 1989;170:595–597. doi: 10.1016/0042-6822(89)90455-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim J.H., Park Y.K., Park E.S., Kim K.H. Molecular diagnosis and treatment of drug-resistant hepatitis B virus. World J. Gastroenterol. 2014;20:5708–5720. doi: 10.3748/wjg.v20.i19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doğan M., Müderrisoğlu C., Fincancı M., Ceylan B., Özdemir G.E., Polat H. kronik hepatit b’de lamivudin direnci ve lamivudin direnci gelişimi üzerine etkili faktörler. Evaluation. 2007;39:44. [Google Scholar]
- 26.Tenney D.J., Rose R.E., Baldick C.J., Pokornowski K.A., Eggers B.J., Fang J., Wichroski M.J., Xu D., Yang J., Wilber R.B., et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 27.Geipel A., Glebe D., Will H., Gerlich W.H. Hepatitis B virus rtI233V mutation and resistance to adefovir. N. Engl. J. Med. 2014;370:1667–1668. doi: 10.1056/NEJMc1400292. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Chen X., Wei M., Zhang C., Xu T., Liu L., Xu Z. Potential resistant mutations within HBV reverse transcriptase sequences in nucleos (t) ide analogues-experienced patients with hepatitis B virus infection. Sci. Rep. 2019;9:8078. doi: 10.1038/s41598-019-44604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmood M., Anwar M.A. Analysis of resistant mutations in reverse transcriptase domain of hepatitis B virus from patients from Islamabad, Pakistan. J. Unexplor. Med. Data. 2017;2:60–64. doi: 10.20517/2572-8180.2017.13. [DOI] [Google Scholar]
- 30.Marrone A., Zampino R., Karayannis P., Cirillo G., Cesaro G., Guerrera B., Ricciotti R., del Giudice E.M., Utili R., Adinolfi L.E., et al. Clinical reactivation during lamivudine treatment correlates with mutations in the precore/core promoter and polymerase regions of hepatitis B virus in patients with anti-hepatitis B e-positive chronic hepatitis. Aliment. Pharmacol. Ther. 2005;22:707–714. doi: 10.1111/j.1365-2036.2005.02653.x. [DOI] [PubMed] [Google Scholar]
- 31.Qin B., Pei R., He T., Huang Z., Pan G., Tu C., Lu M., Chen X. Polymerase mutations rtN238R, rtT240Y and rtN248H of hepatitis B virus decrease susceptibility to adefovir. Chin. Sci. Bull. 2013;58:1760–1766. doi: 10.1007/s11434-013-5770-x. [DOI] [Google Scholar]
- 32.Amini-Bavil-Olyaee S., Herbers U., Sheldon J., Luedde T., Trautwein C., Tacke F. The rtA194T polymerase mutation impacts viral replication and susceptibility to tenofovir in hepatitis B e antigen–positive and hepatitis B e antigen–negative hepatitis B virus strains. Hepatology. 2009;49:1158–1165. doi: 10.1002/hep.22790. [DOI] [PubMed] [Google Scholar]
- 33.Chen C.H., Wang J.H., Lu S.N., Hu T.H., Hung C.H., Chang M.H., Changchien C.S., Lee C.M. Treatment response and evolution of HBV resistance during lamivudine plus adefovir or entecavir therapy in patients with adefovir-resistant mutants. Antivir. Ther. 2012;17:701–709. doi: 10.3851/IMP2074. [DOI] [PubMed] [Google Scholar]
- 34.Yan L., Wang C.M., Cheng J., Liang Z.L., Zhong Y.W., Ren X.Q., Xu Z.H., Zoulim F., Xu D.P. Hepatitis B virus in tenofovir-naive Chinese patients with chronic hepatitis B contains no mutation of rtA194T conferring a reduced tenofovir susceptibility. Chin. Med. J. Engl. 2009;122:1585–1586. [PubMed] [Google Scholar]
- 35.Özgüler M., Sayan M. Could resistant and escape variants of hepatitis B virus be a problem in the future? Future Virol. 2018;13:171–179. doi: 10.2217/fvl-2017-0144. [DOI] [Google Scholar]
- 36.Romanò L., Paladini S., Galli C., Raimondo G., Pollicino T., Zanetti A.R. Hepatitis B vaccination: Are escape mutant viruses a matter of concern? Hum. Vaccines Immunother. 2015;11:53–57. doi: 10.4161/hv.34306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamontagne R.J., Bagga S., Bouchard M.J. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163. doi: 10.20517/2394-5079.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang M., Ma S., Hu X. Cellular immune responses in patients with hepatitis B surface antigen seroclearance induced by antiviral therapy. Virol. J. 2011;8:69. doi: 10.1186/1743-422X-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Den Brouw M.L., Binda R.S., van Roosmalen M.H., Protzer U., Janssen H.L.A., van der Molen R.G., Woltman A.M. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: A possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280–289. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamadalnil Y.M., Bakheit S. Hepatitis B virus_surface gene mutations and their clinical implications. Sudan J. Med. Sci. 2017;12:101–113. doi: 10.18502/sjms.v12i2.920. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are fully available and can be found within the manuscript or in the Supporting Information file. There are no legal restrictions in this regard. Additionally, the datasets generated and/or analyzed during the current study are available in the NCBI repository (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 30 January 2023) and access number has been provided in the Supplementary File (Table S2).