Abstract
Background and Aims
Gastroesophageal reflux disease (GERD) is a prevalent gastrointestinal disorder that may complicate conditions such as obstructive airway disease. Our group has identified predictive biomarkers of GERD in particulate exposed first responders with obstructive airway disease. In addition, GERD diagnosis and treatment is costly and invasive. In light of these clinical concerns, we aimed to systematically review studies identifying noninvasive, multiOmic, and multicompartmental biomarkers of GERD.
Methods
A systematic review of PubMed and Embase was performed using keywords focusing on reflux disease and biomarkers and registered with PROSPERO. We included original human studies in English, articles focusing on noninvasive biomarkers of GERD published after December 31, 2009. GERD subtypes (non-erosive reflux disease and erosive esophagitis) and related conditions (Barrett’s Esophagus [BE] and Esophageal Adenocarcinoma). Predictive measures were synthesized and risk of bias assessed (Newcastle-Ottawa Scale).
Results
Initial search identified n = 238 studies and n = 13 articles remained after applying inclusion/exclusion criteria. Salivary pepsin was the most studied biomarker with significant sensitivity and specificity for GERD. Serum assessment showed elevated levels of Tumor Necrosis Factor- alpha in both GERD and Barrett’s. Exhaled breath volatile sulfur compounds and acetic acid were associated with GERD. Oral Microbiome: Models with Lautropia, Streptococcus, and Bacteroidetes showed the greatest discrimination between BE and controls vs Lautropia; ROCAUC 0.94 (95% confidence interval; 0.85–1.00).
Conclusion
Prior studies identified significant multiOmic, multicompartmental noninvasive biomarker risks for GERD and BE. However, studies have a high risk of bias and the reliability and accuracy of the biomarkers identified are greatly limited, which further highlights the need to discover and validate clinically relevant noninvasive biomarkers of GERD.
Keywords: Systematic Review, Biomarkers, GERD, Reflux Disease, Barrett's esophagus
Background
Gastroesophageal reflux disease (GERD) is a highly prevalent disorder with an incidence of 5/1000 person-years and costs > $9–10 billion/year.1, 2, 3, 4, 5 GERD diminishes health-related quality of life (QoL), productivity, accounts for about 5% of outpatient visits, and is an independent risk factor of the metaplastic changes of Barrett’s Esophagus (BE).3,6, 7, 8 Refractory reflux, which is defined as a partial or complete lack of response to twice daily proton pump inhibitors (PPIs) was associated with both anxiety and depression and once weekly episodes of GERD was detrimental to QoL.8, 9, 10, 11
Heterogeneous presentation of GERD, including cough, heartburn, and/or regurgitation, can make diagnosis challenging. Diagnosis can be made clinically as per the Montreal consensus or it can be definitive as per the Porto and Lyon consensus statements.12, 13, 14, 15, 16, 17, 18, 19, 20 In the recently published American College of Gastroenterology guidelines, GERD is objectively defined by the presence of characteristic mucosal injury seen and/or an abnormal reflux monitoring study.15 Conclusive evidence of GERD includes endoscopic findings of erosive esophagitis (ERD) Los Angeles grade C or D, a stricture or Barrett’s esophagus, and an esophageal acid exposure time > 6% on a pH or pH impedance study in at least one full day of recording.12, 13, 14, 19
However, the main methods of reflux testing are invasive and expensive. They include a wireless telemetry capsule attached to the esophageal mucosa during endoscopy and trans-nasal catheter-based testing.21,22 Further invasive measures are required for GERD symptoms refractory to empiric PPI therapy, such as high resolution manometry and reflux monitoring.15,23 Prior to use of these invasive diagnostic modalities, noncompliance to PPI should always be ruled out. Moreover, studies show that the correlation between symptoms, laryngoscopic findings, and other objective testing such as pH-impedance testing is low.24,25
Several studies have recently focused on the utility and potentially improved diagnosis of GERD using noninvasive biomarkers such as pepsin.26,27 In the context of these prior works and clinical need, we developed our systematic review which focuses on noninvasive biomarkers of reflux disease and severity.
Methods
Review Strategy
Our systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.28,29 Our Population, Intervention, Control, Outcome question was “In adult patients with diagnosed GERD (P), we performed a systematic review to identify (I) the noninvasive multiOmic and multicompartmental biomarkers of reflux disease (O)". Given the design of our systematic review, no comparison control (C) was needed.
PubMed and Embase were searched on February 2, 2022 as per the protocol of our systematic review were registered on PROSPERO (2022-CRD42022301543) and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=301543.
Search Terms
Databases were searched for the following: (Gastric Acid Reflux OR Gastric Acid Reflux Disease OR Gastro-Esophageal Reflux Disease OR Gastro Esophageal Reflux Disease OR Gastro-Esophageal Reflux Diseases OR Gastro-oesophageal Reflux OR Gastro oesophageal Reflux OR Gastroesophageal Reflux Disease OR GERD OR Reflux, Gastroesophageal OR Esophageal Reflux OR Gastro-Esophageal Reflux OR Gastro Esophageal Reflux) AND (Biological Marker OR Biologic Marker OR Biological Markers OR Biologic Markers OR Biomarker OR Immune Markers OR Immunologic Markers OR Immune Marker OR Immunologic Marker OR Serum Markers OR Serum Marker OR Surrogate Endpoints OR Surrogate End Point OR Surrogate End Points OR Surrogate Endpoint OR Clinical Markers OR Clinical Marker OR Viral Markers OR Viral Marker OR Biochemical Marker OR Biochemical Markers OR Laboratory Markers OR Laboratory Marker OR Surrogate Markers OR Surrogate Marker).
Reference-List Screening was also Used
For this review, we have defined reflux disease to include GERD and two of its main phenotypes: nonerosive reflux disease with no evidence of mucosal injury and ERD.30 To maximize studies of noninvasive biomarkers of GERD, we included studies with GERD and/or GERD patients with BE and esophageal adenocarcinoma.
Study Criteria
Studies were included if they (1) were of noninvasive biomarkers of GERD in blood, serum, saliva, or exhaled breath in diagnosed adult (clinically or endoscopically) reflux patients; (2) evaluated diagnostic tests (assessed sensitivity, specificity, positive/negative predictive values, risk, and/or accuracy of these biomarkers); (3) were written in English; and (4) published after December 31, 2009.
Studies were excluded if they (1) were not original research; (2) not written in English; (3) published before January 1, 2010; (4) exclusively used any nonhuman subjects or in vitro studies; (5) were conducted in a pediatric population; (6) focused on biomarkers in biopsied specimens; or (7) involved invasive tissue sampling and immunohistochemistry.
Data Extraction
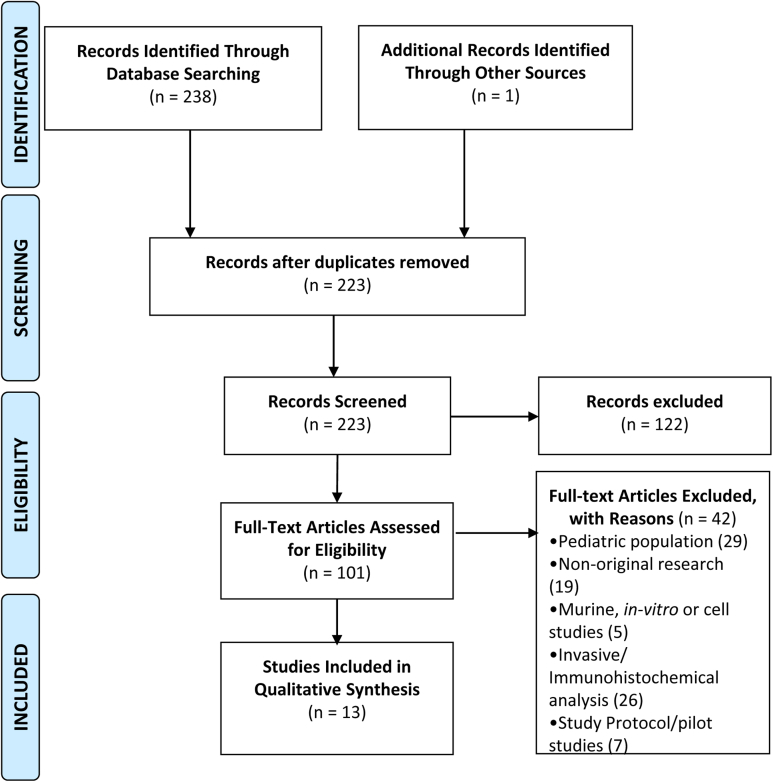
Articles were reviewed and data regarding study design, patient characteristics, sample size, tools used, severity, and prevalence of reflux disease were extracted. Results from each database search were filtered for human subjects, English language, publication date, and imported into (EndNote X9). The references were then screened for duplicates using RefWorks (ProQuest LLC). Original research papers were reviewed (title, abstract, and full text) to ascertain eligibility. We examined references cited in the relevant articles. All results were screened by M.S.F. and S.P. and further independently evaluated by A.N. Disagreements were resolved by consensus. Details as per 2020 PRISMA are found in Figure 1 and resultant manuscripts that meet these criteria are detailed (Supplemental Tables 1-6).31
Figure 1.
Risk of Bias Assessment
Inherent biases (selection, detection, performance, and reporting) were addressed through the study design/search algorithm. Selection bias was addressed by having predetermined inclusion and exclusion criteria and distinct definitions. Detection and performance bias was addressed by having at least two rounds of screening individually performed by M.F. and S.P. Reporting bias was minimized by using PubMed and Embase search filters for peer-reviewed published articles of human subjects written in English and removing duplicates. The Newcastle-Ottawa Scale,32 a domain-based approach, was used to assess the degree of bias as in prior studies.33 Low-risk studies reflected were concordant in all domains (green); studies with at least one unclear or high-risk domain were considered as unclear or high risk of bias studies (yellow or red), respectively (Supplemental Tables 7A-B).
Results
Literature Search
A total of 238 studies were identified from PubMed, Embase, and reference-list screening (Figure 1). After application of selection criteria, 223 research arrticles were assessed for inclusion. Exclusion criteria were met by n = 208 (Supplemental Table 5). Finally, n = 13 original research articles were considered eligible. Data from screening and extraction are available (Supplemental Tables 1-6).34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48
Risk of bias using Newcastle-Ottawa Scale was performed in case-cohort (n = 5) and case-control (n = 8) studies. Among the case-cohort studies, n = 3 had a high risk and n = 2 had a low risk of bias. Similarly, for case-control studies, n = 7 had a high risk and n = 1 had a low risk of bias (Supplemental Table 7A-B).
Study Characteristics by Manuscript
The populations of patients studied include GERD (n = 13), BE (n = 5), and Esophageal Adenocarcinoma (EAC) (n = 2). The biomarkers were found in specimens such as saliva (n = 6), serum (n = 4), exhaled breath (n = 2), and exfoliated tongue cells (n = 1) (Table 1).
Table 1.
Study Characteristics (N = 13)
| Studya | Country | Population/Design | Study size (N) | Specimen/Assay | Biomarker/End Points |
Significant findings | |
|---|---|---|---|---|---|---|---|
| 1 | Yuskel 201234 | US | GERD, Prospective blinded case-control | GERD N = 58, controls N = 51 | Saliva/ Lateral flow device | Pepsin/Positive peptest prevalence | Pepsin test with rapid LFD has acceptable test characteristics in patients with GERD and may replace the need for EGD and pH testing |
| 2 | Bor 201935 | Turkey | GERD, observational case-cohort study | GERD N = 10 | Saliva/ Lateral flow device | Pepsin/Positive peptest prevalence | Pepsin test showed a PPV of 69% to detect MII-pH reflux eventb |
| 3 | Wang 201936 | China | GERD, multicenter case control study | Control N = 323, GERD N = 709, •NERD N = 488, •ERD N = 221 |
Saliva/ Lateral flow device | Pepsin/Predictive values | Results across all centers showed pepsin-positive sensitivity 85% Sensitivity of pepsin for NERD and ERD subgroups were 86% and 84%, respectively. |
| 4 | Guo 202027 | China | ΦRefractory GERD, observational case-cohort study | Conclusive GERD N = 48; against GERD N = 24; inconclusive GERD N = 58 | Saliva/ELISA | Pepsin/pepsin concentration | Pepsin test had a moderate diagnostic value for PPI refractory conclusive GERD patients |
| 5 | Buas 201740 | US | GERD/BE, observational case-cohort study | SRD: GERD N = 100, BE N = 62 | Serum/LC-MS | Multiple serum metabolites/FC serum metabolites |
SRD: Nine metabolites were elevated in BE (FC > 1) (Creatine; homocysteine; 3-nitrotyrosine Hydroxyproline/amino levulinate; Arginine; tyrosine; Sorbitol; linoleic acid; ornithine) |
| 6 | Haider 201841 | US | GERD/BE, WTC-FDNY cohort, observational prospective case-cohort study | Source cohort GERD N = 915, BE N = 106, controls N = 637 Biomarker cohort GERD N = 153, BE N = 20, controls N = 112 |
Serum- Luminex 200IS | TNF-α C-peptide Fractalkine IP-10 /Mean serum Biomarker level, odds ratio |
C- peptide(pg/mL): GERD (791.2) vs control (550.7) TNF-α(pg/mL): GERD (4.7) vs BE (5.7) vs control (4.3) Fractalkine (pg/mL): BE (101.4) vs control (70.6) IP-10(pg/mL): BE (323.2) vs control (236.60); |
| 7 | Kim 201042 | South Korea | NERD/ERD, observational case-cohort study | NERD N = 89, ERD N = 80 | Exhaled breath/ Halimeter | Halitosis /VSC ppb levels | Erosive mucosal changes were strongly associated with VSC levels |
| 8 | Dryahina 201443 | Czech Republic | GERD, case-control study | GERD N = 22, controls N = 24 | Exhaled breath / SIFT-MS | Acetic acid /End expiratory conc. | Breath acetic acid is a useful biomarker of GERD and other conditions lowering pH of airway lining. |
| 9 | Snider 201844 | US | BE (93.8% had GERD), prospective case-control | BE N = 32c, control N = 17 | Saliva/ PCR | Oral microbiome /alpha and beta diversity | Oral microbiome in BE was markedly altered and distinguished BE |
| 10 | Yan 201645 | China | GERD, case-control study | GERD N = 166, Control N = 72 |
Exfoliated tongue cell coatings/ rt-PCR | miR-203/miR-203 expression | miR-203 was significantly downregulated in GERD patients compared to healthy controls |
| 11 | Maddalo 201846 | Italy | Phase III prospective case-control study | Case (N = 231) Controls N = 107) |
Serum- ELISA | SCCA-IgM) | Patients with levels above cut off have a 33 times higher relative risk of developing BE or EAC. |
| 12 | Dosedelova 202047 | Czech Republic | GERD, Case-control study |
GERD N = 20, Control N = 12 |
Saliva/ capillary electrophoresis w/ capacitively coupled contactless conductivity detector | Salivary bicarbonate, phosphate, inorganic anions (CL-, NO2-, NO3-, SO4-, SCN-) organic anions (acetate, butyrate | Bicarbonate levels were significantly elevated in saliva of GERD patients |
| 13 | Thrift 201448 | Australia | BE (models 1,2,3 included GERD frequency and duration in the questionnaire), case-control study | Definitive BE N = 141 Control N = 138 |
Serum/ electrochemiluminescence assay | Multibiomarker risk score, association with BE risk (odds ratio) | Multibiomarker score improved discrimination between BE and controls compared with using only GERD frequency and duration |
Abbreviations: US, United States; UK, United Kingdom; GERD, Gastroesophageal Reflux Disease; NERD, Non-Erosive Reflux Disease; ERD, Erosive Reflux Disease; BE, Barrett’s Esophagus; EAC/EA, Esophageal Adenocarcinoma; WTC, World Trade Center; FDNY, Fire Department of New York; SRD, Study of Reflux Disease; ELISA, Enzyme-Linked Immunosorbent Assay; LC-MS, Liquid Chromatography Mass Spectrometry; SIFT-MS, Selected Ion Flow Tube Mass Spectrometry; rt-PCR, reverse transcriptase-Polymerase Chain Reaction; PPV, Positive Predictive Value; NPV, Negative Predictive Value; FC, Fold Change; VSC, Volatile Sulfur Compounds; ppb, parts per billion; mi-RNA, microRNA; SCCA-IgM, Squamous Cell Carcinoma Antigen-Immunoglobulin M Complex; ROCAUC, Area Under the Receiver Operator Curve; TNF-α, Tumor Necrosis Factor-Alpha; IP-10, Interferon gamma-induced protein-10; IL, Interleukin; RR, Relative Risk; OR, Odds Ratio; CI, Confidence Interval.
Φ Conclusive GERD: LA, grade C and D erosive Esophagitis (n = 4), Long segment Barrett’s Mucosa (n = 1), Peptic strictures or Acid Exposure Time (AET) > 6% (n = 44). Evidence against GERD, group (n = 24): erosive esophagitis, AET < 4%, 40 reflux episodes, Reflux hypersensitivity (RH) (n = 10) or functional heart burn (FH) (n = 14).
Inconclusive GERD (n = 58): LA, grade A and B ERD (n = 42), AET, between 4% and 6% (n = 24), positive reflux symptom association (n = 22), reflux episode > 80 (n = 16), Low mean nocturnal baseline impedance (MNBI) (n = 33) and low postreflux swallow-induced peristaltic wave index (PSPWI) (n = 58).
First Author Year (Ref).
At least one positive pepsin result was seen in 16 patients irrespective of GERD and LPR (80% both).
Includes BE, without dysplasia N = 16, BE, with low grade dysplasia (LGD) N = 6, N = 5, Esophageal adenocarcinoma (EAC) N = 5.
Pepsin, A Classic Biomarker of Reflux Disease
N = 5 of the 13 studies used pepsin as a biomarker. Peptest, which detects salivary pepsin as low as 16 ng/mL using a lateral flow device, was used in n = 4 studies.34, 35, 36, 37 One study used Enzyme-Linked Immunosorbent Assay with a minimum detectable level of 0.93 ng/mL to quantify salivary pepsin (Table 1).38
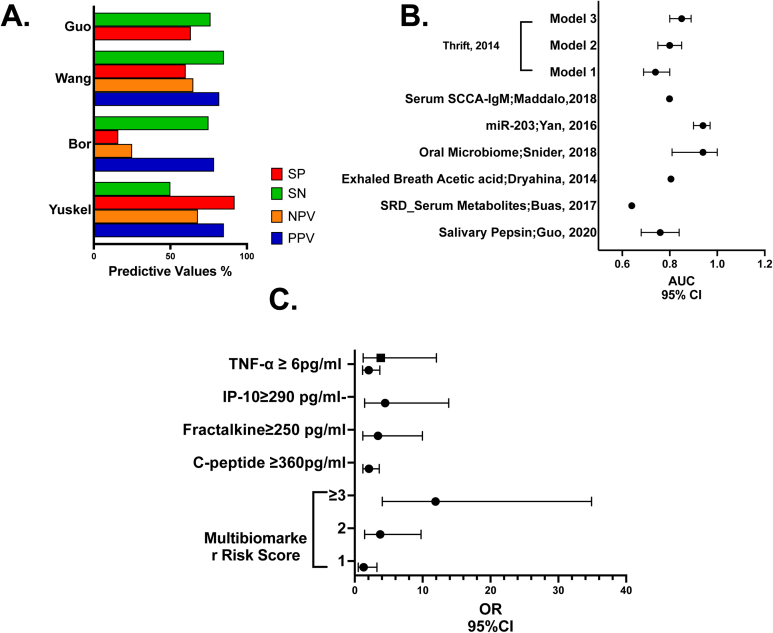
In a prospective blinded case-control study, a cut-off of 50 ng/mL of pepsin was evaluated using the area under receiver operating curve (ROCAUC) analysis from 52 gastric samples and 54 sterile water samples.34 Patients with GERD underwent endoscopy and wireless 48-hour pH monitoring. This study yielded a positive pepsin test prevalence of 22% (GERD) vs 12% (controls). There was a stepwise increase in the prevalence of positive pepsin with 24% positive with heartburn-only symptoms, 43% with abnormal pH, and 55% with endoscopic esophagitis. The positive and negative predictive values were calculated based on the disease definition of esophagitis and/or abnormal pH monitoring (Table 2) (Figure 2A).34 Sensitivity ranged from 50% to 85%, with specificity ranging from 60% to 100%.34, 35, 36, 37,49,50
Table 2.
Predictive Values of Biomarkers
| Study | Biomarker | ROCAUC (95% CI) | Cutoffa | Sn % |
Sp % |
PPV % |
NPV % |
OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Yuskel 201234 | Salivary pepsin | - | 50 ng/mL | 50 | 92 | 85 | 68 | - | ||
| Bor 201935 | Salivary pepsin | - | 16 ng/mL | 75 | - | 78.6 | - | - | ||
| Wang 201936 | Salivary pepsin | - | 75 ng/mL | 85 | 60 | 82 | 65 | - | ||
| Guo 202027 | Salivary pepsin | 0.76 (0.68–0.84) | 4.21 ng/mL | 76.36 | 63.41 | - | - | - | ||
| Buas 201740 | Multiple serum metabolites | SRD 0.64 | - | - | - | - | - | - | ||
| Haider 201841 | TNF-α C-peptide Fractalkine IP-10 |
C-peptide ≥ 360 pg/mL | - | - | - | - | GERD vs control: 2.08 (1.20–3.61) |
|||
| TNF-α ≥ 6 pg/mL | GERD vs control: 2.06 (1.15–3.70); |
|||||||||
| BE vs control: 3.84 (1.23–12.03) |
||||||||||
| Fractalkine ≥ 250 pg/mL | BE vs control: 3.42 (1.18–9.96) |
|||||||||
| IP-10 ≥ 290 pg/mL | BE vs control: 4.47 (1.45–13.84) |
|||||||||
| Kim 201042 | Halitosis | - | - | - | - | - | - | |||
| Dryahina 201443 | Exhaled breath acetic acid | 0.805 | - | - | - | - | - | |||
| Snider 201844 | Changes in oral microbiome | 0.94 (0.81–1.00) | 96.9 | 88.2 | - | - | ||||
| Yan 201645 | miR-203 | 0.94 (0.90–0.7) | - | 91.7 | 87.3 | - | - | |||
| Maddalo 201846 | Serum SCCA-IGM | 0.799 | 56.6 AU/mL | 91.5 | 75.4 | 85.8 | 84.4 | |||
| Dosedelova 202047 | Salivary bicarbonate, phosphate, inorganic anions (CL-, NO2-, NO3-, SO4-, SCN-) organic anions (acetate, butyrate) | - | - | - | - | - | - | |||
| Thrift 201448 |
Model 1 0.74 (0.69–0.80) |
- | - | - | - | - | ||||
| Model 2 0.80 (0.75–0.85) |
- | - | - | - | - | - | ||||
| IL-12p70, IL-6, IL-8, IL-10 leptin |
Model 3 0.85 (0.80–0.89) |
IL-12p70 0.77 pg/mL | - | - | - | - | Multibiomarker risk score | 1 | 1.30 (0.51–3.27) | |
| IL-6 0.18 pg/mL | ||||||||||
| IL-8 0.1 pg/mL | ||||||||||
| IL-10 7.5 pg/mL | 2 | 3.75 (1.44–9.78) | ||||||||
| Leptin 43 pg/mL | ≥ 3 | 11.9 (4.06–34.9) | ||||||||
ABBREVIATIONS: SRD, Study of Reflux Disease; SN, Sensitivity; SP, Specificity; PPV, Positive Predictive Value; NPV, Negative Predictive Value; ppb, parts per billion; mi-miRNA, microRNA; SCCA-IgM, Squamous Cell Carcinoma Antigen-Immunoglobulin M Complex; ROCAUC, Area Under the Receiver Operator Curve; TNF-α, Tumor Necrosis Factor-Alpha; IP-10, Interferon gamma-induced protein-10; IL, Interleukin; RR, Relative Risk; OR, Odds Ratio; CI, Confidence Interval.
Cutoff determined as per individual manuscript methods.
Figure 2.
Synthesis of biomarker outcomes. (A) Predictive values of pepsin. (B) Area under the receiver operator characteristic curve (ROCAUC) of biomarkers. (C) Odds ratio (OR) of biomarkers.
In another study, GERD had a similar positive Peptest prevalence of 80%.35 A multicenter case-control study enrolled 1032 participants with GERD who received endoscopy and a Peptest.36 The overall sensitivity of Peptest was 85% and 60% specificity. The authors acknowledge that there was poor selection of controls in some centers resulting in specificity as low as 37%–40% and suggest room for improvement by investigating the impact of smoking history.
Furthermore, Guo et al assayed salivary pepsin levels in patients with refractory GERD, using Enzyme-Linked Immunosorbent Assay.38 Subjects were categorized into three groups (conclusive, inconclusive, and evidence against GERD) as per the Lyon consensus based on upper endoscopic findings, 24-h multichannel intraluminal impedance-pH monitoring (24-h pH-MII), and high resolution manometry. This study noted that salivary pepsin concentrations were significantly different among patients with conclusive GERD (8.2 ng/ml), inconclusive GERD (4.0 ng/ml), and evidence against GERD (2.4 ng/ml) (P < .001) (Table 1, Table 2 and Figure 2A).27
Serum metabolites and Other Biomarkers of GERD, BE, and Esophageal Adenocarcinoma
To predict the risk of EA in patients with GERD, BE, an observational case-cohort study explored the metabolome in serum. In the study of reflux disease (n = 162), Table 1, nine metabolites were elevated in the BE vs GERD comparison group (study of reflux disease) (ROCAUC 0.64; P < .05) (Table 2 and Figure 2B).40
Similarly, in a study on the World Trade Center (WTC) particulate-exposed firefighters with obstructive airway disease (OAD), serum biomarkers were assessed. The study showed that serum TNF-α ≥ 6 pg/mL predicted both GERD and BE, C-peptide ≥ 360 pg/mL predicted GERD, Fractalkine ≥ 250 pg/mL, and IP-10 ≥ 290 pg/mL predicted BE. These biomarkers sampled prior to disease presentation showed strong predictive abilities (Table 2 and Figure 2C).41
Exhaled Breath Biomarkers to Distinguish Subtypes of GERD
In a case-cohort study, a Halimeter was used to measure volatile sulfur compounds in exhaled breath. Erosive mucosal changes were strongly associated with volatile sulfur compounds levels with mean Halimeter ppb levels of 191.85 vs 136.43 (P = .042) in ERD vs nonerosive reflux disease (Table 1).
Another case-control study compared the end expiratory concentrations of acetic acid in the exhaled breath condensate (EBC) of GERD patients with controls. Median acetic acid concentration was significantly higher in GERD compared to controls (85 ppbv vs 48 ppbv; p = 6 × 10-5) (Table 1). Acetic acid as a diagnostic biomarker for GERD had an ROCAUC of 0.805 (Table 2 and Figure 2B).43
Oral Microbiome: A Screening Biomarker of BE
Reflux-related conditions including BE alter the esophageal microbiome.51,52 The oral microbiome was compared in a case-control study in BE patients (n = 32) and controls (n = 17). Among controls, n = 10 (59%) had GERD and n = 6 (35%) were on PPI. Alpha diversity was no different from BE patients to controls (mean Shannon index: BE 2.73 vs controls 2.89; P = .10). At the phylum level, there was a significant increase in the relative abundance of Firmicutes (27.1 vs 14.6 %; P = .005) and a significantly decreased Proteobacteria (23.8 vs 34.5%; P = .02) in BE vs controls, respectively. Other notable differences included an increased relative abundance of Streptococcus, Veillonella, and Enterobacteriaceae in BE and several taxa (Neisseria, Lautropia, and Corynebacterium) in controls. Models with Lautropia, Streptococcus, and Bacteroidetes showed the greatest discrimination between BE and controls vs Lautropia alone; ROCAUC 0.94 (95% confidence interval [CI] 0.85–1.00; P = .04) (Figure 2B and Table 2).44
Additional Biomarkers
One study determined microRNA (miRNA) expression (miR-143, -145, -192, -194, -203, and -205) on exfoliated tongue cells across a discovery cohort (GERD n = 24, control n = 24).45 Validated results showed significantly downregulated miR-203 in GERD (n = 142) as compared to healthy controls (n = 48), P < .0001; ROCAUC 0.94 (95% CI: 0.90–0.97), with a sensitivity of 91.7% and a specificity of 87.3% (Figure 2B and Table 2).
The role of immunocomplexed squamous cell carcinoma antigen (SCCA-IgM) as a screening biomarker of BE and EAC, was determined in a phase III cancer screening biomarker development study (n = 213).46 Median serum SCCA-IgM levels were higher in BE 90 U/mL (95% CI: 89.31–131.55; P < .0001) and EAC 76 U/mL (95% CI: 56.63–178.87; P < .0001) as compared to controls which included GERD patients 36.6 U/mL (95% CI: 40.32–68.28) and blood donors 41.5 U/mL (95% CI: 49.37–91.33) with a relative risk 33 (95% CI: 12.66–89.46). BE patients with long segment or dysplastic BE had SCCA-IgM levels significantly higher than those with short nondysplastic BE (P = .035) (Table 2).
Salivary anion levels were measured in GERD (n = 20) vs healthy controls (n = 12) using background electrolyte with capillary electrophoresis.47 They found a significant difference in mean bicarbonate concentration in GERD vs controls (8.1 mM vs 5.7 mM; P = .004). No difference was reported in the mean concentrations of phosphate (6.4 mM vs 5.7 mM; P = .272) (Table 1).
Multibiomarker Model of BE Risk
A case-control study of 279 subjects compared the accuracy of three risk prediction models that used demographic and clinical variables (Table 1).48 Model 1 included GERD frequency and duration; Model 2 included GERD frequency, duration, age, sex, race, waist-to-hip ratio, and H-pylori status; and Model 3 included all variables in model 2 and used multiple serum biomarkers (IL-12p70, IL6, IL8, IL10, and leptin). The addition of risk scores associated with BE and multibiomarker risk scores improved the AUC compared to Model 1 significantly (ROCAUC of 0.85 [95% CI 0.80–0.89; P = .01]).
Discussion
GERD a highly prevalent disease which affects 20% of the US population53 and has a heterogeneous biomarker profile. GERD diminishes QoL, productivity, and accounts for about 5% of outpatient visits. Furthermore, refractory reflux is associated with anxiety, depression, and even once weekly episodes of GERD were detrimental to QoL.8, 9, 10
Therefore, GERD diagnosis and management is highly relevant to the health and wellbeing of a significant portion of our patient population. While GERD can be a clinical diagnosis (ie, symptomatic heartburn and regurgitation) as per the Montreal consensus statement both invasive and noninvasive biomarkers are often used.54 GERD detection with endoscopy, ambulatory pH testing, and other invasive testing poses a rare but potential risk and contributes to a higher economic burden.4 Sensitivity of endoscopy may be limited.55 Conversely, those with endoscopic evidence of reflux may be completely asymptomatic.56,57 Invasive adjunctive testing to aid in disease detection pose rare but real medical risk, contribute to a higher economic burden, but little benefit is gained as the methods are plagued with poor sensitivity.4 Sensitivity of endoscopy is limited because many patients with GERD do not have mucosal injury.55 Conversely, those with endoscopic evidence of reflux may be completely asymptomatic.56,57 Therefore, optimizing biomarkers and specifically enriching for noninvasive biomarkers of GERD were the focus of our review.
Pepsin was the most commonly studied noninvasive biomarker of GERD in our systematic review. Recent American College of Gastroenterology guidelines have raised concerns about pepsin testing due to poor diagnostic reliability and its inability to distinguish between patients with extraesophageal symptoms.15,22 However, based on the finding of papers highlighted in our manuscript, pepsin likely has some diagnostic utility that needs to be further studied in targeted subpopulations, additional compartments such as EBC, or in a multivariate biomarker model.58
EBC is a window into the aerodigestive compartment and is composed of droplets of airway lining fluid. It has emerged as a target for noninvasive biomarkers of GERD. Specifically, it has the potential to address unmet medical needs by expanding the portfolio of noninvasive assays to diagnose erosive changes and multiple coexisting pathological mechanisms of GERD.59 Compounds identified in EBC include volatile sulfur compounds, histamine, adenosine, ammonia, and leukotrienes.60 The identified compounds are of biologically plausible since histamine stimulates cells in the stomach lining to produce hydrochloric acid. H2-blockers a common treatment of GERD competes with histamine for H2-receptors on the stomach’s parietal cells and thereby depresses the production of hydrochloric acid.61 Similarly, adenosine has been found to regulate acetylcholine release, which stimulates the proton pumps.62
The host-gut microbiome interaction has been an area of active investigation in several gastrointestinal disorders and is particularly of interest in light of the clinical relevance of EBC highlighted above.63 Gut microbiota are linked to the regulation of the innate immune system and have been linked to markers found in EBC.59,64, 65, 66, 67 Alteration in the microbiota and bacterial products can result in the activation of pathways involved in inflammation. Changes in esophageal microbiota can lead to the production of large amounts of bacterial components like lipopolysaccharide which can delay gastric emptying via COX1/2 and predispose to GERD. The role of Lautropia (which was one of the organisms identified as predictive of BE) is analogous to that of Clostridia in the colon and a decrease in bacterial load leads to the proliferation of other proinflammatory bacteria. In another study, a decrease in the concentration of Lautropia was seen in patients with periodontitis and successful treatment resulted in a subsequent increase in lautropia.68 This taxonomic difference was used by Snider et al. using oral swabs and 16S rRNA gene sequencing in BE patients with GERD.44,69
Biomarkers of GERD and BE in the FDNY WTC Exposed Cohort
A prime example of the importance of the gut/lung axis was found in the WTC-exposed first responder cohort.70 WTC-particulate matter exposure is associated with OAD, GERD, and BE.71, 72, 73 WTC-exposed firefighters with OAD had a three times higher risk of developing GERD.74 Approximately 44% of WTC responders developed GERD symptoms by 2005, which is 8.2 times its pre-9/11 prevalence.75 We identified serum biomarkers of GERD and BE in a cohort of nonsmoking firefighters with WTC exposure.41,76 Greater odds of developing GERD were associated with elevated TNF-α and C-peptide, whereas BE was associated with TNF-α, Fractalkine, and IP-10 (Table 2).41
Systematic Reviews by their Very Nature are Subject to Limitations and Inherent Biases
The heterogeneity of baseline characteristics, diagnostic criteria, standards for comparison of MII-pH testing, manometry, 24-hour reflux monitoring, questionnaires, endoscopy, and lack of validation limits the interpretability. This underlines the importance of developing diagnostic biomarkers of GERD and Barrett’s and invites future studies for developing standardized methods of diagnosis. Although several studies that were a focus of our review used Pepsin as a biomarker, current guidelines do not recommend pepsin testing for evaluation of patients with reflux and limit further clinical interpretation.15 While our review focused on noninvasive biomarkers of reflux disease, we understand that optimal diagnostic testing would be an integration of invasive and noninvasive biomarkers. The studies included in this review did not distinguish between refractory GERD and refractory GERD symptoms.57 Finally, risk of bias was high in most studies included in this review. This adds additional importance to all future work, in that the clinically relevant noninvasive biomarkers of GERD and associated conditions are needed.
Conclusion
Studies identified include multiOmic, multicompartmental noninvasive biomarkers of GERD. This further highlights the fact that several pathways are biologically active in GERD. However, due to study limitations and variable controls, further validation studies are warranted to ascertain the reliability and accuracy of these biomarkers.
Future Plans
Our future work will focus on validating the previously discovered biomarkers of WTC-aerodigestive disease in longitudinally phenotyped WTC-exposed cohorts. We will also develop novel, noninvasive disease phenotyping of premalignant diseases such as BE and identify potential targeted therapeutics to improve care; ClinicalTrials.gov #NCT05216133.
Acknowledgments
Author Contributions
M.S.F.: Conceptualization, Data curation, Formal analysis, and Writing–original draft; S.P.: Data curation, Formal analysis, Validation, and Writing–original draft; G.C.: Formal analysis and Writing–original draft; U.J. and Y.L.: Formal analysis, Validation, and Writing–original draft; M.L.: Validation and Writing–original draft; S.K.: Supervision and Writing–original draft; G.G., F.F., and A.R.K.: Writing–original draft; A.N.: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, and Writing–original draft.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: Centers for Disease Control/National Institute for Occupational Safety and Health (CDC/NIOSH) U01-[OH012069, OH011300, OH011855]; National Institutes of Health (NIH): National Heart Lung and Blood Institute (NHLBI) R01HL119326; National Institute of Environmental Health Science (NIEHS) R01ES032808; National Center for Advancing Translational Sciences (NCATS) KL2TR001446; and Stony Wold-Herbert Fund. The funding agencies did not participate in the study design; collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: All data produced in the present work are contained in the manuscript.
Reporting Guidelines: PRISMA and SAGER.
Preprint: medRxiv 2022.06.20.22276215; doi: https://doi.org/10.1101/2022.06.20.22276215
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.gastha.2023.01.014.
Supplementary data
References
- 1.Dent J., El-Serag H.B., Wallander M.A., et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savarino E., Bredenoord A.J., Fox M., et al. Advances in the physiological assessment and diagnosis of GERD. Nature Rev Gastroenterol & Hepatol. 2017;14:665. doi: 10.1038/nrgastro.2017.130. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen N.J., Hansen R.A., Morgan D.R., et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 4.Richter J.E., Rubenstein J.H. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267–276. doi: 10.1053/j.gastro.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis D.O., Rymer J.A., Slaughter J.C., et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108:905–911. doi: 10.1038/ajg.2013.69. [DOI] [PubMed] [Google Scholar]
- 6.Lagergren J., Bergstrom R., Lindgren A., et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 7.Mody R., Eisenberg D., Hou L., et al. Comparison of health care resource utilization and costs among patients with GERD on once-daily or twice-daily proton pump inhibitor therapy. Clinicoecon Outcomes Res. 2013;5:161–169. doi: 10.2147/CEOR.S41189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang S.H., Ryu H.S., Choi S.C., et al. Psychological factors influence the gastroesophageal reflux disease (GERD) and their effect on quality of life among firefighters in South Korea. Int J Occup Environ Health. 2016;22:315–320. doi: 10.1080/10773525.2016.1235675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronkainen J., Aro P., Storskrubb T., et al. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population - the Kalixanda study. Aliment Pharm Ther. 2006;23:1725–1733. doi: 10.1111/j.1365-2036.2006.02952.x. [DOI] [PubMed] [Google Scholar]
- 10.Jansson C., Nordenstedt H., Wallander M.A., et al. Severe gastro-oesophageal reflux symptoms in relation to anxiety, depression and coping in a population-based study. Aliment Pharmacol Ther. 2007;26:683–691. doi: 10.1111/j.1365-2036.2007.03411.x. [DOI] [PubMed] [Google Scholar]
- 11.Fass R. Proton-pump inhibitor therapy in patients with gastro-oesophageal reflux disease: putative mechanisms of failure. Drugs. 2007;67:1521–1530. doi: 10.2165/00003495-200767110-00001. [DOI] [PubMed] [Google Scholar]
- 12.Gyawali C.P., Kahrilas P.J., Savarino E., et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman S., Gyawali C.P., Savarino E., et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017;29:1–15. doi: 10.1111/nmo.13067. [DOI] [PubMed] [Google Scholar]
- 14.Gyawali C.P., Roman S., Bredenoord A.J., et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: Conclusions from an international consensus group. Neurogastroenterol Motil. 2017;29:e13104. doi: 10.1111/nmo.13104. [DOI] [PubMed] [Google Scholar]
- 15.Katz P.O., Dunbar K.B., Schnoll-Sussman F.H., et al. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Official journal of the American College of Gastroenterology | ACG. 2022;117:27–56. doi: 10.14309/ajg.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannini E.G., Zentilin P., Dulbecco P., et al. Management strategy for patients with gastroesophageal reflux disease: a comparison between empirical treatment with esomeprazole and endoscopy-oriented treatment.The. American journal of gastroenterology. 2008;103:267–275. doi: 10.1111/j.1572-0241.2007.01659.x. [DOI] [PubMed] [Google Scholar]
- 17.Vakil N., van Zanten S.V., Kahrilas P., et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 18.Numans M.E., Lau J., de Wit N.J., et al. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease. Ann Intern Med. 2004;140:518–527. doi: 10.7326/0003-4819-140-7-200404060-00011. [DOI] [PubMed] [Google Scholar]
- 19.Savarino E., Savarino E., Bredenoord A.J., et al. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665–676. doi: 10.1038/nrgastro.2017.130. [DOI] [PubMed] [Google Scholar]
- 20.Bytzer P., Jones R., Vakil N., et al. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:1360–1366. doi: 10.1016/j.cgh.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Kessels S.J.M., Newton S.S., Morona J.K., et al. Safety and Efficacy of wireless pH monitoring in patients suspected of gastroesophageal reflux disease: a systematic review. J Clin Gastroenterol. 2017;51:777–788. doi: 10.1097/MCG.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 22.Gyawali C.P., Carlson D.A., Chen J.W., et al. ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Am J Gastroenterol. 2020;115:1412–1428. doi: 10.14309/ajg.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoikes N., Drapekin J., Kushnir V., et al. The value of multiple rapid swallows during preoperative esophageal manometry before laparoscopic antireflux surgery. Surg Endosc. 2012;26:3401–3407. doi: 10.1007/s00464-012-2350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechien J.R., Schindler A., De Marrez, et al. Instruments evaluating the clinical findings of laryngopharyngeal reflux: A systematic review. Laryngoscope. 2019;129:720–736. doi: 10.1002/lary.27537. [DOI] [PubMed] [Google Scholar]
- 25.Branski R.C., Bhattacharyya N., Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope. 2002;112:1019–1024. doi: 10.1097/00005537-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Kia L., Pandolfino J.E., Kahrilas P.J. Biomarkers of reflux disease. Clin Gastroenterol Hepatol. 2016;14(6):790–797. doi: 10.1016/j.cgh.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Z., Wu H., Jiang J., et al. Pepsin in saliva as a diagnostic marker for gastroesophageal reflux disease: a meta-analysis. Med Sci Monitor. 2018;24:9509–9516. doi: 10.12659/MSM.913978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 29.Shamseer L., Moher D., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 30.Long J.D., Orlando R.C. Nonerosive reflux disease. Minerva Gastroenterol Dietol. 2007;53:127–141. [PubMed] [Google Scholar]
- 31.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells G.A., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Available from: [cited 2023 Feb 27]
- 33.Li H., Boakye D., Chen X., et al. Association of body mass index with risk of early-onset colorectal cancer: systematic review and meta-analysis. Am Coll Gastroenterol. 2021:116. doi: 10.14309/ajg.0000000000001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saritas Yuksel E., Hong S.K.S., Strugala V., et al. Rapid salivary pepsin test: Blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope. 2012;122(6):1312–1316. doi: 10.1002/lary.23252. [DOI] [PubMed] [Google Scholar]
- 35.Bor S., Capanoglu D., Vardar R., et al. Validation of peptestTM in patients with gastro-esophageal reflux disease and laryngopharyngeal reflux undergoing impedance testing. J Gastrointestin Liver Dis. 2019;28:383–387. doi: 10.15403/jgld-335. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Yang C.Q., Chen Y.X., et al. Validation in China of a non-invasive salivary pepsin biomarker containing two unique human pepsin monoclonal antibodies to diagnose gastroesophageal reflux disease. J Digest Dis. 2019;20(6):278–287. doi: 10.1111/1751-2980.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dettmar P.W., Watson M., McGlashan J., et al. A Multicentre Study in UK Voice Clinics Evaluating the Non-invasive Reflux Diagnostic Peptest in LPR Patients. SN Comp Clin Med. 2020;2(1):57–65. [Google Scholar]
- 38.Guo Z., Wu Y., Li L., et al. The role of salivary pepsin in the diagnosis of gastroesophageal reflux disease (GERD) evaluated using high-resolution manometry and 24-hour multichannel intraluminal impedance-pH monitoring. Med Sci Monitor. 2020:26. doi: 10.12659/MSM.927381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Corso E., Baroni S., Salonna G., et al. Impact of bile acids on the severity of laryngo-pharyngeal reflux. Clin Otolaryngol. 2021;46:189–195. doi: 10.1111/coa.13643. [DOI] [PubMed] [Google Scholar]
- 40.Buas M.F., Gu H., Djukovic D., et al. Candidate serum metabolite biomarkers for differentiating gastroesophageal reflux disease, Barrett’s esophagus, and high-grade dysplasia/esophageal adenocarcinoma. Metabolomics. 2017;13(3) doi: 10.1007/s11306-016-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haider S.H., Kwon S., Lam R., et al. Predictive Biomarkers of Gastroesophageal Reflux Disease and Barrett’s Esophagus in World Trade Center Exposed Firefighters: a 15 Year Longitudinal Study. Sci Rep. 2018;8(1):3106. doi: 10.1038/s41598-018-21334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.G., Kim Y.J., Yoo S.H., et al. Halimeter ppb levels as the predictor of erosive gastroesophageal reflux disease. Gut Liver. 2010;4(3):320–325. doi: 10.5009/gnl.2010.4.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dryahina K., Pospisilova V., Sovova K., et al. Exhaled breath concentrations of acetic acid vapour in gastro-esophageal reflux disease. J Breath Res. 2014;8(3) doi: 10.1088/1752-7155/8/3/037109. [DOI] [PubMed] [Google Scholar]
- 44.Snider E.J., Compres G., Freedberg D.E., et al. Barrett’s esophagus is associated with a distinct oral microbiome. Clin Transl Gastroenterol. 2018;9:135. doi: 10.1038/s41424-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan X., Zhu S., Zhang H. MiR-203 expression in exfoliated cells of tongue Coating Represents a sensitive and specific biomarker of gastroesophageal reflux disease. Gastroenterol Res Pract. 2016;2016:2349453. doi: 10.1155/2016/2349453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maddalo G., Fassan M., Cardin R., et al. Squamous cellular carcinoma antigen serum determination as a biomarker of Barrett esophagus and esophageal cancer: a phase III study. J Clin Gastroenterol. 2018;52:401–406. doi: 10.1097/MCG.0000000000000790. [DOI] [PubMed] [Google Scholar]
- 47.Dosedelova V., Durc P., Dolina J., et al. Analysis of bicarbonate, phosphate and other anions in saliva by capillary electrophoresis with capacitively coupled contactless conductivity detection in diagnostics of gastroesophageal reflux disease. Electrophoresis. 2020;41(1–2):116–122. doi: 10.1002/elps.201900319. [DOI] [PubMed] [Google Scholar]
- 48.Thrift A.P., Garcia J.M., El-Serag H.B. A multibiomarker risk score Helps predict risk for Barrett'sEsophagus. Clin Gastroenterol Hepatol. 2014;12(8):1267–1271. doi: 10.1016/j.cgh.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du X., et al. The diagnostic value of pepsin detection in saliva for gastro-esophageal reflux disease: a preliminary study from China. Bmc Gastroenterol. 2017;17:107. doi: 10.1186/s12876-017-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du X., Wang F., Hu Z.W., et al. The diagnostic value of pepsin detection in saliva for gastro-esophageal reflux disease: a preliminary study from China. BMC Gastroenterol. 2017:17. doi: 10.1186/s12876-017-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amir I., Konikoff F.M., Oppenheim M., et al. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2014;16:2905–2914. doi: 10.1111/1462-2920.12285. [DOI] [PubMed] [Google Scholar]
- 52.Yang L., Lu X., Nossa C.W., et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Commisso A., Lim F. Lifestyle Modifications in adults and Older adults with chronic gastroesophageal reflux disease (GERD) Crit Care Nurs Q. 2019;42:64–74. doi: 10.1097/CNQ.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 54.Vakil N., van Zanten, Kahrilas P., et al. The Montreal definition and classification of gastroesophageal reflux disease: A global, evidence-based consensus paper. Z Gastroenterol. 2007;45:1125–1140. doi: 10.1055/s-2007-963633. [DOI] [PubMed] [Google Scholar]
- 55.Shaw M.J., Talley N.J., Beebe T.J., et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:52–57. doi: 10.1111/j.1572-0241.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 56.Lim S.W., Lee J.H., Kim J.H., et al. Management of asymptomatic erosive esophagitis: an e-Mail survey of physician’s opinions. Gut Liver. 2013;7:290–294. doi: 10.5009/gnl.2013.7.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu C.L. Silent gastroesophageal reflux disease. J Neurogastroenterol Motil. 2012;18:236–238. doi: 10.5056/jnm.2012.18.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee A.L., Button B.M., Denehy L., et al. Exhaled breath condensate pepsin: potential noninvasive test for gastroesophageal reflux in COPD and bronchiectasis. Respir Care. 2015;60:244–250. doi: 10.4187/respcare.03570. [DOI] [PubMed] [Google Scholar]
- 59.Thompson D.G., O'Brien J.D., Hardie J.M. Influence of the oropharyngeal microflora on the measurement of exhaled breath hydrogen. Gastroenterology. 1986;91:853–860. doi: 10.1016/0016-5085(86)90686-4. [DOI] [PubMed] [Google Scholar]
- 60.Horvath I., Hunt J., Barnes P.J., et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 61.International Foundation for Gastrointestinal Disorders H2 Blockers – Indications, Effectiveness and Longterm Use. www.iffgd.org
- 62.Arin R.M., Gorostidi A., Navarro-Imaz H., et al. Adenosine direct and indirect actions on gastric acid secretion. Front Physiol. 2017;8:737. doi: 10.3389/fphys.2017.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smolinska A., Tedjo D.I., Blanchet L., et al. Volatile metabolites in breath strongly correlate with gut microbiome in CD patients. Anal Chim Acta. 2018;1025:1–11. doi: 10.1016/j.aca.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 64.Clarke T.B. Early innate Immunity to bacterial Infection in the lung is regulated Systemically by the Commensal microbiota via Nod-like receptor Ligands. Infect Immun. 2014;82:4596–4606. doi: 10.1128/IAI.02212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segal L.N., Rom W.N., Weiden M.D. Lung microbiome for clinicians. New discoveries about bugs in healthy and diseased lungs. Ann Am Thorac Soc. 2014;11:108–116. doi: 10.1513/AnnalsATS.201310-339FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonnell M.J., Hunt E.B., Ward C., et al. Current therapies for gastro-oesophageal reflux in the setting of chronic lung disease: state of the art review. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00190-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlottmann F., Andolfi C., Herbella F.A., et al. GERD presence and size of hiatal hernia influence clinical presentation, esophageal function, reflux profile, and degree of mucosal injury. Am Surg. 2018;84:978–982. [PubMed] [Google Scholar]
- 68.Colombo A.P., Boches S.K., Cotton S.L., et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–1432. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lv J., Guo L., Liu J.J., et al. Alteration of the esophageal microbiota in Barrett’s esophagus and esophageal adenocarcinoma. World J Gastroenterol. 2019;25:2149–2161. doi: 10.3748/wjg.v25.i18.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haider S.H., Veerappan A., Crowley G., et al. Multiomics of world trade center particulate matter-induced persistent airway hyperreactivity. Role of receptor for advanced glycation end products. Am J Respir Cell Mol Biol. 2020;63:219–233. doi: 10.1165/rcmb.2019-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prezant D.J., Weiden M., Banauch G.I., et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. New Engl J Med. 2002;347:806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 72.de la Hoz R.E., Christie J., Teamer J.A., et al. Reflux symptoms and disorders and pulmonary disease in former World Trade Center rescue and recovery workers and volunteers. J Occupat Environ Med. 2008;50:1351–1354. doi: 10.1097/JOM.0b013e3181845f9b. [DOI] [PubMed] [Google Scholar]
- 73.Li J., Brackbill R.M., Stellman S.D., et al. Gastroesophageal reflux symptoms and comorbid asthma and posttraumatic stress disorder following the 9/11 terrorist attacks on World Trade Center in New York City. Am J Gastroenterol. 2011;106:1933–1941. doi: 10.1038/ajg.2011.300. [DOI] [PubMed] [Google Scholar]
- 74.Liu X., et al. The effect of world trade center exposure on the timing of diagnoses of obstructive airway disease, chronic Rhinosinusitis, and gastroesophageal reflux disease. Front Public Health. 2017;5:2. doi: 10.3389/fpubh.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X., Yip J., Zeig-Owens R., et al. The effect of World Trade Center exposure on the timing of diagnoses of obstructive airway disease, chronic rhinosinusitis, and gastroesophageal reflux disease. Front Public Health. 2017;5:2. doi: 10.3389/fpubh.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haider S.H., Lee A.K., Caraher E.J., et al. Aerodigestive continuum: GERD and Barrett’s esophagus in World Trade Center exposed firefighters. Eur Resp J. 2016;48:PA4301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.