Abstract
Objectives:
Previous analyses of cataract in radiation-exposed populations have assessed relative risk; radiogenic excess additive risk, arguably of more public health importance, has not been estimated. Previous analysis of a large prospective cohort of US radiologic technologists quantified excess relative risk of cataract in relation to occupational radiation dose. We aim to assess excess additive risks of cataract.
Methods:
We estimated excess additive risks of cataract/cataract surgery in US radiologic technologists using generalised additive models in relation to occupational radiation exposure, and assessed risk modification by a priori-selected cataract risk factors (diabetes, body-mass index, smoking, race, sex, birth-year, ultraviolet-B radiation exposure).
Results:
There were 11,345 cataract diagnoses and 5440 of cataract surgery during 832,462 and 888,402 person years of follow-up, respectively. Cumulative occupational radiation exposure was associated with self-reported cataract, but not with cataract surgery, with excess additive risk/104 person-year Gy =94 (95% CI 47, 143, p<0.001) and =13 (95% CI <0, 57, p=0.551), respectively. There was marked (p<0.001) variation of excess additive risk by age and by diabetes status, with risk higher among persons ≥75 years and diabetics. There were indications of elevated risk among those with higher ultraviolet-B radiation (p=0.045), Caucasians (p=0.056) and among those with higher levels of cigarette smoking (p=0.062). Elevated additive risk was observed for occupational radiation eye lens doses <100 mGy (p=0.004) with no dose-response curvature (p=0.903).
Conclusions:
The elevated additive risks associated with low-dose radiation, if confirmed elsewhere, have important public health and clinical implications for radiation workers as well as regulatory measures.
Keywords: cataract, ionising radiation, additive risk, diabetes, ultraviolet radiation, cigarette smoking
Introduction
Cataract is one of the major morbidities in the US population, with overall prevalence of ~24% above age 40 years but increasing to ~71% above age 75 1. High radiation doses of 1 Gy or more can induce cataract 2, and accumulating evidence from follow-up studies of the Japanese atomic bomb survivors 3–5 and worker populations 6–12 suggests that cataracts may be induced by cumulative lower doses, of the order of 100–250 mGy. A recent study of cataract in the US radiologic technologists (USRT) demonstrated cataract risk associated with cumulative ionising radiation exposure below 100 mGy 13. Use of diagnostic computed tomography (CT) in the US is substantial with about 18% of visits to the emergency room associated with use of CT 14. Among persons receiving one or more CTs it is estimated that about 15% will receive cumulative effective radiation doses of 100 mGy or more 15.
All previous studies including our studies have assessed radiogenic relative risk of cataract, and in particular in the only two studies that have examined potential modification of effect by known and suspected non-radiation risk factors (e.g., the Japanese atomic bomb survivors and the USRT) have done so in relation to measures of excess relative risk (ERR) 4 13. To the best of our knowledge radiogenic excess additive risk (EAR) (i.e. the number of cases per person year and per unit of dose) has not previously been assessed for cataract, although additive risks have been assessed for radiation and cancer risks 16. The ERR is the proportional increase in risk over the risk in the unexposed, while the EAR is the additional risk above that among the unexposed. The EAR is arguably more important in assessing the impact of an exposure on public health than ERR, by providing an estimate of the number of cases that could potentially be prevented by reducing this exposure.
The objective of this study is to provide the first evaluation of estimated excess additive risk of cataract in relation to highly fractionated low-dose and low dose-rate radiation exposure. We assessed additive risks of self-reported questionnaire-derived history of cataracts or cataract surgery in the USRT cohort in relation to estimated cumulative absorbed dose to the eye lens from occupational radiation exposures. We examined the degree to which excess additive radiation risk was modified by a broad range of known and suspected risk factors for cataract. We report additive risk at various levels of estimated occupational radiation eye lens dose.
Data and Methods
Population and follow-up
The USRT study has been previously described 13. Four questionnaires (administered during 1983–2014) collected information on health outcomes, work history, and other variables 13. Since data on cataract were only elicited in the second through fourth questionnaires, we only included responders to the second or third questionnaire and at least one subsequent questionnaire for follow-up of these ocular endpoints. Exclusions are as previously used, so that we censored follow-up after a diagnosis of cancer (other than non-melanoma skin cancer [NMSC]) because of the potential for radiotherapy. After various exclusions (for history of radiotherapy on the first or second surveys, inconsistent questionnaire responses for cataract or cataract surgery, or reporting cataract or cataract surgery at the first questionnaire responded to), all as in the previous analysis 13 (and also as detailed in Appendix A Figures A1 and A2), there were a total of 67,246 technologists eligible for study of cataract and 67,709 eligible for study of cataract surgery; 13 a further 3894 technologists were excluded with trivial follow-up (i.e., with date of exit from follow-up = date of entry to follow-up), resulting in an analysis cohort for cataract history of 63,352 persons. A similar process, but excluding also 3088 technologists with trivial follow-up resulted in a cohort of 64,621 persons informative for cataract surgery. Among these exclusions were 4502 persons with prevalent cataract history at baseline questionnaire, and 82 cataract surgeries at baseline questionnaire. Follow-up began at the first survey in which lack of cataract was reported, and ended at the earlier of the date the last questionnaire answered, or the date of the first assessed cataract. There was no information elicited on the questionnaires about type of cataract. Additional details are given in our earlier paper 13.
Occupational ionising radiation dosimetry
A historical dose reconstruction was undertaken to estimate cumulative annual radiation absorbed doses to the eye lens from occupational exposure for each radiologic technologist 13 17 up to December 31st 1997. Doses were lagged by 5 years. Additional details are given elsewhere. 13
UVB cumulative radiant exposure
As discussed elsewhere18 cataract is thought to result from oxidative stress in the eye lens 19, part of which is likely due to cumulative ultraviolet A (UVA) exposure 20. UVA and UVB are highly correlated so it is sufficient for our purposes, as an adjusting variable, to use UVB. A standard UVB exposure metric is cumulative UVB radiant exposure (in units of J m−2), which is proportional to cumulative solar UVB energy deposition on a surface over a period of time. This measure of cumulative ambient UVB exposure is recommended by the Commission Internationale de l’Eclairage (CIE) (International Commission on Illumination) 21. We estimated this measure from National Aeronautics Space Administration (NASA) Total Ozone Mapping Spectrometer (TOMS) satellite-based UVB data22, linkage of which with the USRT we now describe.
On the third questionnaire in the USRT study cohort, city and state of longest residential location for five age periods (age <13, 13–19, 20–39, 40–64, 64+) was collected. The TOMS database (http://toms.gsfc.nasa.gov) maintained by NASA, provides a daily estimate of ambient UVB exposure, measured as the irradiance at 305 nm in mW m−2 nm−1 23, in a 1° longitude x 1° latitude grid. Using residential location for the five age periods, ambient UVB irradiance was calculated at each point over the lifetime. The scaling of this measure to provide cumulative UVB radiant exposure used the method previously described 18.
Statistical methods
A person year table was generated for the dataset using the variables listed in Appendix Table A1. Risks for cataract were assessed using a generalized additive model 24 in which the expected number of cases in cell i with associated group (for some modifying variable) m of the tabulation is assumed to be Poisson distributed with mean:
| (1) |
where was the number of person years of follow-up and is the mean time-varying cumulative 5-year lagged eye-lens absorbed dose in Gy, were the lifestyle (e.g., cigarette smoking history), environmental (e.g., ultraviolet B (UVB), and medical risk factors (e.g., diabetes, obesity), was the excess additive risk coefficient (EAR) per unit eye-lens absorbed dose (Gy) for group m, and were coefficients adjusting for other risk factors. The term allowed for departures from linearity in the dose response. The baseline (zero dose) group is the group without radiation exposure. The model was fitted via Poisson maximum likelihood 25, using Epicure 26. The baseline (zero-dose) models, given by the terms inside the first exp[] in expression (1) were chosen using the forward stepwise algorithm, using a p-value for selection of p=0.05 25. For cataract surgery this procedure was modified slightly, resulting in the indicated terms for [body mass index (BMI)]3, BMI4, BMI5 being dropped from the model because of instability in certain fits. Similar models were used to adjust for baseline risk in Figures 1 and 2, using for Figure 2 factor terms rather than continuous loglinear adjustments for age, and dropping the adjustment for year of birth, as this led to instability. Cumulative UVB radiant exposure was treated as time varying, and lagged by 5 years, as previously 13. Because UVB radiation can only be estimated for the subset of persons who answered the third questionnaire, an indicator for UVB missing data was also included in the baseline risk model. The test of heterogeneity conditional on the marginal totals, used in Appendix A Table A2, employed a generalisation of Fisher’s exact test, and was evaluated via 107 simulation using the algorithm of Patefield27 in R28.
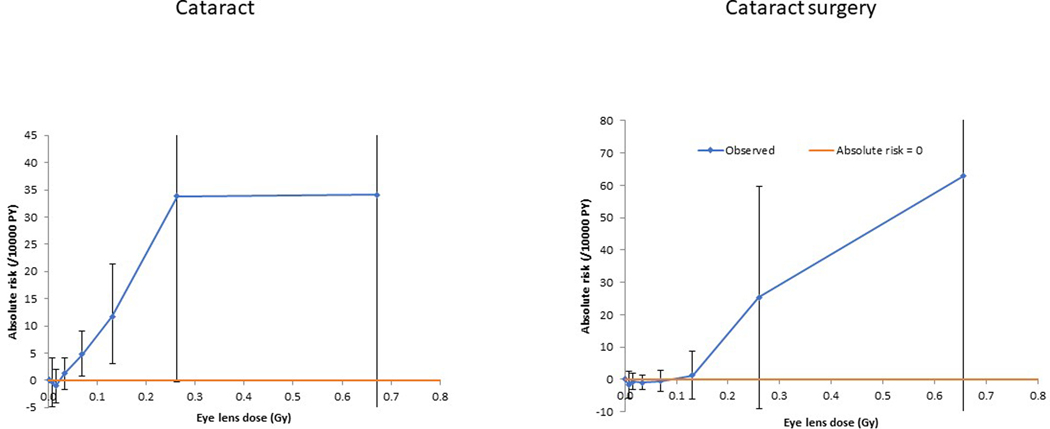
Figure 1. Adjusted additive excess risk as a function of occupational dose, for self- reported history of diagnosis of cataract (plot a) and cataract surgery (plot b) (+95% CI).a.
aRisks for cataract are evaluated using a model with factor terms in the background (zero-dose) model for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, [birth date], [birth date]2, [birth date]3, (time varying) cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, BMI3, BMI4, BMI5, year (of follow-up), year2, year3, year4, year5, year6. For cataract surgery the indicated model uses adjustments for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, ln[age]4, [birth date], [birth date]2, (time varying) cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, year (of follow-up), year2, year3, year4, year5, year6.
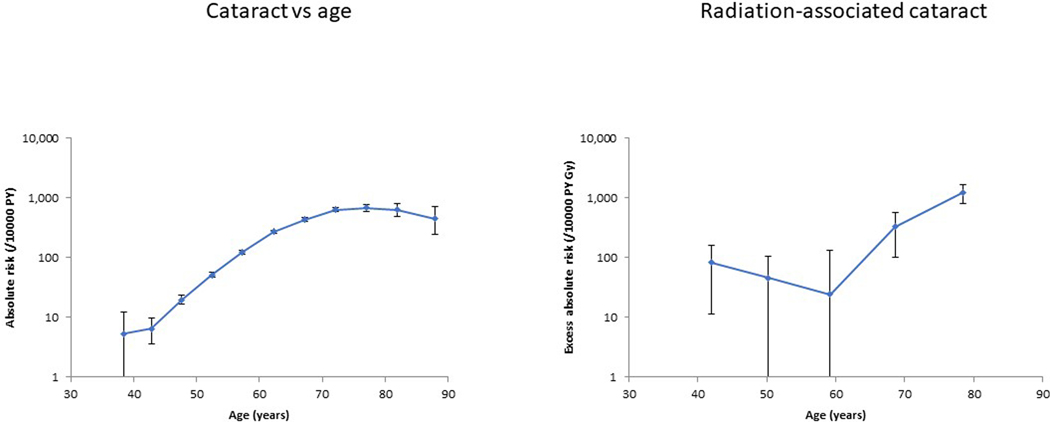
Figure 2. Adjusted additive risk as a function of age (plot a), and radiation associated excess risk (per 104 person year Gy) (plot b) as a function of age, for self-reported history of diagnosis of cataract (+95% CI).
aRisks are evaluated using a model with factor terms in the background (zero-dose) model for age, sex, diabetes status, smoking status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for cumulative UVR radiant exposure (including missingness), baseline BMI (including missingness), BMI2, BMI3, BMI4, BMI5, year, year2, year3, year4, year5, year6.
Ethical approval
This study has been approved annually by the National Cancer Institute Special Studies Institutional Review Board and by the University of Minnesota Institutional Review Board.
Data sharing
The data and all code used for the analysis are available on application to the lead author (MPL) or the co-Principal Investigators (CMK, MSL).
Competing interests
All authors declare: no support from any organisation for the submitted work except the NCI Intramural Research Program; no financial relationships with any other organisations that might have an interest in the submitted work; and no other relationships or activities that could appear to have influenced the submitted work.
Results
There were 11,345 technologists with self-reported cataract among 63,352 eligible persons with 832,462 person years of follow up; for cataract surgery there were 5440 cases among 64,621 persons with 888,402 person years of follow-up (data not shown) (Table 1). There were pronounced, and generally highly statistically significant (p<0.001) differences between the groups determined by whether or not they developed cataract or cataract surgery for a number of baseline characteristics (Appendix Table A2); only for ethnic group was this heterogeneity not statistically significant. Appendix Table A2 demonstrates that those in each subcohort who had cataract surgery as well as a reported history of cataract were more likely to be male, older at start of follow-up, born earlier in time, had a history of diabetes, currently or formerly smoked cigarettes and had higher cumulative UVB than those who reported a history of cataract only but no cataract surgery. Occupational radiation exposure was strongly associated with self-reported cataract, with an excess additive risk of 94.21 / 104 person-year Gy (95% confidence intervals (CI) 46.67 to 142.9, p<0.001), although there was no such significant association for cataract surgery, with an excess additive risk of 12.98 / 104 person-year Gy (95% confidence intervals (CI) −26.82 to 56.91, p=0.551) (Table 1, Figure 1). We observed pronounced (p<0.001) variation of radiogenic excess additive risk by attained age (Table 1, Figure 2), and by diabetes status (both p<0.001) (Table 1), with risk higher among older persons (age ≥75 years) and among those with diabetes; there were similar variations of excess additive risk of cataract surgery by attained age (p=0.007) and by diabetes status (p<0.001) (Table 1). There was weaker evidence (p=0.045) of heterogeneity of radiation risk between groups characterized according to cumulative UVB radiant exposure, with additive radiation risk increasing among those with higher cumulative UVB, although there were weak indications of non-convergence. Table 1 also shows that there was weaker evidence of heterogeneity by racial group (p=0.056) and by levels of cigarette smoking (p=0.062) and by sex (p=0.080), with risk higher among whites, among those with higher levels of cigarette smoking and for males, but with the first two of these factors there were indications of non-convergence.
Table 1.
Excess additive risk (EAR) of self-reported cataract and cataract surgery per 104 person year Gy (and 95% CI) in relation to estimated occupational radiation eye lens dose, and by potential modification to the association by various lifestyle and environmental risk factors.a
| Cataract | Cataract surgery | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Cases | EAR / 104 PY Gy (95% CI) | p-value for heterogeneity | Cases | EAR / 104 PY Gy (95% CI) | p-value for heterogeneity |
| Total | 11,345 | 94.21 (46.67, 142.9) | <0.001b | 5440 | 12.98 (−26.82c, 56.91) | 0.551b |
|
| ||||||
| Sex | ||||||
| Males | 2416 | 144.3 (70.17, 224.3) | 0.080 | 1239 | 49.20d (−17.86c, 116.3c) | 0.016d |
| Female | 8929 | 77.45 (27.43, 129.1) | 4201 | -1.56d (−44.14c, 41.02c) | ||
|
| ||||||
| Racial/ethnic group | ||||||
| White | 10,808 | 98.94d (52.66c, 145.2c) | 0.056d | 5182 | 14.61d (−25.65c, 54.87c) | 0.534d |
| Black | 299 | -12.58d (−152.7c, 127.6c) | 140 | -37.96d (−193.9c, 109.5) | ||
| Other | 238 | 7.62d (−143.5c, 158.7c ) | 118 | -11.25d (−168.7c, 146.2c) | ||
|
| ||||||
| Age attained (years) | ||||||
| < 45 | 88 | 59.57 (−2.46, 128.8) | <0.001 | 28 | -12.59d (−87.25c, 62.07c) | 0.007d |
| 45–54 | 1383 | 81.26 (25.56, 139.5) | 468 | 12.34d (−32.94c, 57.61c) | ||
| 55–64 | 5134 | 117.4 (15.87, 222.1) | 1724 | 16.12d (−54.54c, 86.78c) | ||
| 65–74 | 3944 | 523.3 (301.4, 750.8) | 2383 | 85.89d (−70.49c, 242.3c) | ||
| ≥ 75 | 796 | 1447.0 (1002.0, 1915.0) | 837 | 150.6d (−124.8c, 426.0c) | ||
|
| ||||||
| Birth year | ||||||
| <1920 | 72 | 183.8 (−457.1c, 954.7) | 0.777 | 22 | 670.6e (110.5, 1442.0) | 0.007e |
| 1920–1929 | 732 | 264.0 (−23.31, 568.5) | 426 | 199.7e (−65.27, 490.3) | ||
| 1930–1939 | 2901 | 85.77 (−50.08, 237.4) | 1972 | 240.8e (79.21, 408.9) | ||
| 1940–1949 | 5063 | 102.4 (38.64, 170.1) | 2212 | 23.27e (−36.63, 89.54) | ||
| ≥ 1950 | 2577 | 87.81 (33.35, 144.9) | 808 | -3.36e (−45.29c, 45.37) | ||
|
| ||||||
| Baseline body mass index (BMI)(kg m−2) | ||||||
| Unknown | 240 | 298.0e (66.07, 592.6) | 0.494e | 130 | 83.58e (−156.7c, 388.3) | 0.607e |
| < 18.5 (underweight) | 116 | 72.39e (−23.81, 336.7) | 54 | 54.59e (−179.7c, 407.3) | ||
| 18.5–24.9 (normal) | 5029 | 83.42e (29.76, 139.4) | 2306 | -5.19e (−51.22c, 45.45) | ||
| 25.0–29.9 (overweight) | 3822 | 97.71e (34.41, 166.2) | 1940 | 20.13e (−38.67c, 85.59) | ||
| ≥ 30 (obese) | 2138 | 108.0e (27.20, 197.9) | 1010 | 63.86e (−21.06c, 157.3) | ||
|
| ||||||
| Baseline diabetes | ||||||
| No diabetes/unknown diabetes | 10,770 | 110.2 (63.58, 157.9) | <0.001 | 5123 | 12.49 (−26.42c, 55.37) | <0.001 |
| Diabetes | 575 | 1725.0 (1227.0, 2270.0) | 317 | 1412.0 (968.4, 1902.0) | ||
|
| ||||||
| Baseline smoking status | ||||||
| Unknown smoking status | 93 | 73.06 (−235.6c, 469.5) | 0.194 | 54 | -1.68e (−295.4c, 346.6) | 0.528e |
| Never smoked | 5784 | 77.10 (25.75, 130.9) | 2665 | -1.84e (−46.49c, 50.27) | ||
| Former smoker | 3968 | 99.46 (34.89, 170.0) | 2008 | 12.69e (−50.09c, 82.61) | ||
| Current smoker | 1500 | 185.8 (85.83, 295.6) | 713 | 71.53e (−27.90c, 174.2) | ||
|
| ||||||
| Baseline smoking quantity (cigarettes/day) | ||||||
| Smoking unknown/never smoked | 6092 | 80.19e (28.85, 133.8) | 0.062e | 2828 | 12.27d (−32.97c, 57.50c) | 0.761d |
| 0–9 | 875 | 25.92e (−70.89c, 139.7) | 429 | 6.72d (−98.10c, 111.5c) | ||
| 10–19 | 1679 | 91.53e (16.01, 177.5) | 828 | 15.84d (−64.04c, 95.73c) | ||
| 20–29 | 1874 | 135.4e (47.78, 231.9) | 921 | 9.29d (−71.54c, 90.12c) | ||
| 30–39 | 466 | 251.4e (83.18, 452.9) | 241 | 28.61d (−140.6c, 197.8c) | ||
| 40–49 | 273 | 139.5e (−99.42c, 430.5) | 146 | 44.11d (−201.1c, 289.3c) | ||
| 50–59 | 44 | 1080.0e (283.8, 2254.0) | 25 | 131.4d (−583.1c, 846.0c) | ||
| ≥60 | 42 | 15.79e (−571.4c, 863.7) | 22 | -60.08d (−637.1c, 516.9c) | ||
|
| ||||||
| Baseline age stopped smoking (years) | ||||||
| Smoking unknown/never smoked | 6087 | 81.16e (30.00, 134.7) | 0.162e | 2834 | 12.77d (−32.33c, 57.87c) | 0.932d |
| 0–19 | 48 | 20.47e (−304.7c, 580.0) | 13 | -3.68d (−296.2c, 288.8c) | ||
| 20–29 | 985 | 24.88e (−57.39c, 112.5) | 414 | 2.46d (−84.46c, 89.38c) | ||
| 30–39 | 1252 | 147.3e (58.40, 247.0) | 574 | 13.97d (−67.93c, 95.87c) | ||
| 40–49 | 1524 | 177.4e (83.98, 279.7) | 725 | 20.54d (−67.73c, 108.8c) | ||
| 50–59 | 1114 | 117.0e (−43.32, 297.5) | 671 | 33.06d (−146.6c, 212.7c) | ||
| ≥60 | 3 35 | 49.23e (−321.2c, 440.7) | 209 | 29.77d (−389.5c, 449.1c) | ||
|
| ||||||
| Cumulative UVB radiant exposure (time varying) | ||||||
| UVB unknown | 1559 | 10.02e (−63.69c, 91.87) | 0.045e | 854 | 31.67 (−57.12c, 136.5) | 0.103 |
| < 0.075 MJ cm−2 | 9216 | 111.0e (61.09, 162.2) | 4141 | 8.55 (−32.38c, 53.60) | ||
| 0.075–0.10 MJ cm−2 | 565 | 435.9e (55.17, 851.9) | 438 | 295.8 (13.97, 621.5) | ||
| ≥0.10 MJ cm−2 | 5 | 333.8e (−1064.0, 3728.0) | 7 | 1749.0 (−377.4, 5767.0) | ||
CI, confidence interval. EAR, excess additive risk. PY, person years.
Risks for cataract are evaluated using a linear model [expression (1) with γm = 0] with factor terms in the underlying background (zero-dose) model for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, [birth date], [birth date]2, [birth date]3, (time varying) cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, BMI3, BMI4, BMI5, year (of follow-up), year2, year3, year4, year5, year6. For cataract surgery the indicated model uses adjustments for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, ln[age]4, [birth date], [birth date]2, (time varying) cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, year (of follow-up), year2, year3, year4, year5, year6.
p-value of improvement in fit over null model (with no trend in dose), assessed via likelihood ratio test.
Wald-based confidence intervals.
Non-convergence.
Weak indications of non-convergence.
Table 2 demonstrates that there was elevated excess additive risk for cumulative dose under 200 mGy with an excess additive risk of 88.09 / 104 person-year Gy (95% CI 39.12, 138.1, p<0.001), and under 100 mGy with an excess additive risk of 81.61 / 104 person-year Gy (95% CI 25.10, 139.2, p=0.004), although not under 50 mGy (p=0.157). Cataract surgery risks were non-significantly negative for all the restricted dose ranges considered (Table 2). Table 3 reveals that there was no pronounced curvature in the dose response, either for cataract (p=0.903) or for cataract surgery (p=0.400).
Table 2.
Excess additive risk (EAR) of self-reported cataract and cataract surgery per 104 person year Gy (and 95% CI), in relation to level of estimated occupational radiation eye lens dose.a
| Level of estimated eye-lens dose | Self-reported cases in person-year table | EAR / PY Gy x 104 (95% CI) | p-value (trend) |
|---|---|---|---|
| Cataract | |||
|
| |||
| Unrestricted | 11,345 | 94.21 (46.67, 142.9) | <0.001 |
| <200 mGy | 10,737 | 88.09 (39.12, 138.1) | <0.001 |
| <100 mGy | 8,659 | 81.61 (25.10, 139.2) | 0.004 |
| <50 mGy | 4,914 | 65.10 (−25.54, 153.8) | 0.157 |
|
| |||
| Cataract surgery | |||
|
| |||
| Unrestricted | 5,440 | 12.98 (−26.82b, 56.91) | 0.551 |
| <200 mGy | 5,148 | −0.95 (−21.99b, 45.09) | 0.944 |
| <100 mGy | 4,133 | −8.13c (−33.20b, 22.16) | 0.568c |
| <50 mGy | 2,508 | −1.78d (−5.31b, 2.04) | 0.313d |
Risks are evaluated using a linear model [expression (1) with γm = 0] with factor terms in the underlying background (zero-dose) model for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, [birth date], [birth date]2, [birth date]3, (time varying) cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, BMI3, BMI4, BMI5, year (of follow-up), year2, year3, year4, year5, year6. For cataract surgery the indicated model uses adjustments for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, ln[age]4, [birth date], [birth date]2, (time varying) cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, year (of follow-up), year2, year3, year4, year5, year6.
Wald-based confidence intervals.
Weak indications of non-convergence.
Non-convergence.
Table 3.
Evidence for curvature in dose-response for the excess additive risk (EAR) of self-reported cataract and cataract surgery in relation to occupational eye lens radiation dose, via fit of linear-exponential model (as given by expression (1)).a
| Model | Linear coefficient EAR / PY Gy x 104 (95% CI) | Exponential coefficient/Gy (95% CI) | p-value (trend) |
|---|---|---|---|
| Cataract | |||
|
| |||
| Linear model | 94.21 (46.67, 142.9) | <0.001b | |
| Linear-exponential model | 92.57 (43.47, 156.1) | 0.18 (−5.14, 1.90) | 0.903c |
|
| |||
| Cataract surgery | |||
|
| |||
| Linear model | 12.98 (−26.82d, 56.91) | 0.551b | |
| Linear-exponential model | 16.62e (−18.95d, 54.34) | 2.05e (−1.28d, 5.38d) | 0.400c e |
Risks are evaluated using a linear-exponential model [expression (1)] in dose with factor terms in the underlying background (zero-dose) model for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, [birth date], [birth date]2, [birth date]3, cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, BMI3, BMI4, BMI5, year, year2, year3, year4, year5, year6. For cataract surgery the indicated model uses adjustments for sex, baseline diabetes status, baseline smoking status, baseline numbers of cigarettes per day, baseline age at stopped smoking, and continuously adjusted for ln[age], ln[age]2, ln[age]3, ln[age]4, [birth date], [birth date]2, (time varying) cumulative UVB radiant exposure (including missingness), baseline BMI (including missingness), BMI2, year (of follow-up), year2, year3, year4, year5, year6.
p-value of improvement in fit over null model (with no trend in dose), assessed via likelihood ratio test.
p-value of improvement in fit over linear model, assessed via likelihood ratio test.
Wald-based confidence intervals.
Weak indications of non-convergence.
Discussion
We have documented pronounced excess additive risk of cataract (but not of cataract surgery), with particularly marked excess risk among those with diabetes and in old age (age ≥75 years). Risks remain significantly elevated at dose under 100 mGy, the conventional level determining low dose exposure 29 30. To the best of our knowledge this is the first study to estimate excess additive risk of cataract in relation to low-dose radiation exposure. Therefore, we are unable to directly compare our findings with those from other radiation-exposed populations. However, in the previous cataract analysis of the USRT cohort excess relative risks were compatible with most other exposed groups 13.
Excess additive risk is arguably more important in assessing the impact of an exposure on public health than relative risk, as it expresses the additive level of excess risk that a person may be subject to, and the degree of burden on society in relation to provision of health services. In particular, it expresses the excess additive radiation risk in relation to the medical and lifestyle risk factors that may modify risk. We have shown that persons who have diabetes, who therefore have an elevated baseline risk of cataract, are at even higher risk of a radiogenic cataract. Our study suggests that about 5 excess cases of cataract would be expected among 1000 persons receiving 50 mGy (close the mean level of exposure of 56 mGy in this cohort 13) and followed over 10 years (close to the mean 13.1 year follow-up (Table 1)). This suggests that special protective measures may be warranted for persons with diabetes who undergo radiation exposure.
Table 1 and Figure 2 demonstrate that the increase of radiogenic excess additive risk with age is not generally as fast as the increase of the underlying background (zero dose) cataract risk with age. This explains therefore the strongly decreasing modification of radiogenic excess relative risk with increasing age observed in the previous analysis 13. The findings in relation to diabetes are more striking. We previously reported a marked reduction in excess relative risk among those with diabetes 13, whereas we report here a radiogenic excess additive risk among those with diabetes that is markedly elevated, nearly twenty times that among those without this disease (Table 1). This may be explained by the fact that the background (zero dose) cataract risk is much higher among those with diabetes than those without 13.
Strengths of the present study include the large population with low (mostly <100 mGy) cumulative protracted radiation doses and prospective cohort design. We utilised a comprehensive occupational dosimetry with estimated absorbed doses specific to the eye lens 13. Although a substantial proportion of the estimated cumulative occupational dose is derived from questionnaires 17, the dosimetry has been extensively validated, in particular via chromosome aberrations assayed via fluorescence in situ hybridisation (FISH) 31. The large number of covariates evaluated was another strength, and the comprehensive work history, demographic, lifestyle and medical factors evaluated facilitated analysis of modifying effects of these variables on the radiation dose response.
Our study had several limitations, particularly the self-report and lack of clinical validation of cataracts. However, the population of radiologic technologists reported here is medically literate, so that self-reports of cataract and other medical conditions such as diabetes should be reasonably reliable. All analyses adjust for age, which should largely eliminate the major risk factor for tendency to mis-recall diagnostic information. A related weakness is lack of information on cataract subtype. No information was collected on ocular trauma, which is a well-known risk factor for cataract 32, although relatively rare 33 and thus unlikely to substantially confound the trend with occupational dose. As with many occupational studies, cohort members had to survive to answer the second questionnaire and be free of cataract at that point. Such selection will not necessarily bias our analysis, since everyone had to survive to answer a questionnaire, and all risk was assessed conditional on that. Follow-up was censored at the date of the last informative questionnaire answered; it is plausible that such censoring was non-informative for cataract.
Of some concern is the discrepancy between the findings for cataract history and cataract surgery, where risks for latter were somewhat lower and generally not significant (Table 1); treating these as independent samples (which they are not) the risks for cataract surgery were significantly lower (p=0.014) than the risks for cataract history (Table 1, Figure 1). This contrasts with the situation for relative excess risk considered in the previous analysis 13, where the difference in risks between cataract surgery and cataract (again treating them as independent samples, which they are not) was not significant (p=0.366). The rather lower background risks for cataract surgery (by about a factor of about 2 [=11345/5440 (Table 1)]) combined with the approximately twofold lower relative risks for this endpoint (=0.69/0.34)13 will mean that one would expect about fourfold lower additive risks, not far removed (especially if uncertainties are allowed for) from the sevenfold lower risks (Table 1 [94.21/12.98]) that we observed. There are differences between those who reported a history of cataract surgery and a history of cataract versus those who reported a history of cataract but no surgery for a number of baseline characteristics; the former were more likely to be male, older at start of follow-up, born earlier in time, had a history of diabetes, currently or formerly smoked cigarettes and had higher cumulative UVB (Appendix Table A2). However, we do not regard these as necessarily explaining the differences between the radiation risks for cataract and cataract surgery. Although the baseline characteristics may exhibit heterogeneity of this sort, nevertheless very similar variations in radiation risk by these baseline characteristics are seen for cataract and cataract surgery (Table 1). As with the previous analysis of the present cohort using relative risk models 13, a very similar pattern of significant ERR for cataract and non-significant ERR for cataract surgery has also been reported in the Russian Mayak nuclear workers 9 34. Indeed among the various groups that have been studied for radiation-associated cataract, a significant ERR for cataract surgery has been reported only in the Japanese atomic bomb survivors4 12 35, possibly a consequence of differences in exposure (dose rate) or mean follow-up period from the present cohort or the Mayak workers35. While some of the USRT subjects may harbour vision impairing cataracts, it is likely that only a proportion of these will further receive cataract surgery, depending on various factors which may include not only the cataract size, severity, and location within the eye, but also socioeconomic, medical-cost and health consciousness, visual acuity in the opposite eye, the nature of the work or vocational activities affecting the need for visual acuity, and amount of ultraviolet exposure 35 36. Therefore, there is no reason to suppose that cataract surgery prevalence will be simply proportional to cataract prevalence. Even in developed countries not all vision impairing cataracts are treated with cataract surgery 37.
In summary, we found a notable increase in excess additive risk of cataract associated with low dose and low dose-rate occupational protracted radiation exposure (e.g., eye-lens absorbed dose mostly <100 mGy at <5 mGy/h). There was a particularly large excess additive risk among those with diabetes and among those ages ≥75 years. The implications for those with diabetes who may experience radiation exposure may be clinically important to consider. The additive risks of cataract that we report have not been assessed in any other study, but the variation in relative risks previously reported 13 are comparable with those in other populations evaluated for radiation exposure response. In particular, our findings, if confirmed, have important implications for clinical screening and public health along with consideration of radiation protection and regulatory measures, particularly for physicians performing fluoroscopically-guided interventional procedures who have been shown to potentially receive eye-lens doses well over the 100 mGy 38 39. Future studies should assess excess additive risk of cataracts along with excess relative risks in other radiation-exposed occupational groups with clinically-ascertained diagnosis of cataract by cataract type, medical record validation of cataract severity/opacity, well-validated dosimetry and high-quality data on relevant lifestyle, environmental, and medical risk factors, to ascertain if our findings are confirmed that cataract is inducible by low doses of radiation (at <100 mGy).
Supplementary Material
Key messages.
What is already known about this subject?
Previous analyses of cataract in radiation-exposed populations have assessed relative risk, with emerging evidence of risk at low dose (<100 mGy).
Radiogenic excess additive cataract risk may be of more importance than relative risk for assessing impact on public health.
What are the new findings?
We observed markedly elevated excess additive risks of cataract in relation to occupational radiation dose in the US Radiologic Technologists, with excess risk below 100 mGy; there was no elevation in risk of cataract surgery.
Risk was very much higher among persons aged >75 y and among diabetics.
How might this impact on policy or clinical practice in the foreseeable future?
The elevated additive risks associated with low-dose radiation have important public health and clinical implications for radiation workers as well as worker protection and regulatory measures, also for those exposed to diagnostic medical exposures such as computed tomography.
Those with diabetes and radiation exposed should be subject to increased surveillance for cataract.
Acknowledgements:
The authors would like to thank the Associate Editor and the two referees for their detailed and helpful comments. The authors thank the radiologic technologists who participated in the study, Dr Jerry Reid of the American Registry of Radiologic Technologists for continued support, and Diane Kampa and Allison Iwan of the University of Minnesota for study management and data collection.
Funding:
This work was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. The views expressed herein by the authors are independent of all funding agencies.
References
- 1.Klein BEK, Klein R, Lee KE. Incidence of age-related cataract: the Beaver Dam Eye Study. Arch. Ophthalmol 1998;116:219–225. [DOI] [PubMed] [Google Scholar]
- 2.Edwards AA, Lloyd DC. Risks from ionising radiation: deterministic effects. J.Radiol.Prot 1998;18:175–183. [DOI] [PubMed] [Google Scholar]
- 3.Minamoto A, Taniguchi H, Yoshitani N et al. Cataract in atomic bomb survivors. Int.J.Radiat.Biol 2004;80:339–345. [DOI] [PubMed] [Google Scholar]
- 4.Neriishi K, Nakashima E, Akahoshi M et al. Radiation dose and cataract surgery incidence in atomic bomb survivors, 1986–2005. Radiology 2012;265:167–174. [DOI] [PubMed] [Google Scholar]
- 5.Neriishi K, Nakashima E, Minamoto A et al. Postoperative cataract cases among atomic bomb survivors: radiation dose response and threshold. Radiat.Res 2007;168:404–408. [DOI] [PubMed] [Google Scholar]
- 6.Worgul BV, Kundiyev YI, Sergiyenko NM et al. Cataracts among Chernobyl clean-up workers: implications regarding permissible eye exposures. Radiat.Res 2007;167:233–243. [DOI] [PubMed] [Google Scholar]
- 7.Chylack LT Jr., Peterson LE, Feiveson AH et al. NASA study of cataract in astronauts (NASCA). Report 1: Cross-sectional study of the relationship of exposure to space radiation and risk of lens opacity. Radiat.Res 2009;172:10–20. [DOI] [PubMed] [Google Scholar]
- 8.Chylack LT, Jr., Feiveson AH, Peterson LE et al. NASCA report 2: Longitudinal study of relationship of exposure to space radiation and risk of lens opacity. Radiat.Res 2012;178:25–32. [DOI] [PubMed] [Google Scholar]
- 9.Azizova TV, Hamada N, Grigoryeva ES, Bragin EV. Risk of various types of cataracts in a cohort of Mayak workers following chronic occupational exposure to ionizing radiation. Eur J Epidemiol 2018;33:1193–1204. [DOI] [PubMed] [Google Scholar]
- 10.Ainsbury EA, Bouffler SD, Dörr W et al. Radiation cataractogenesis: a review of recent studies. Radiat.Res 2009;172:1–9. [DOI] [PubMed] [Google Scholar]
- 11.Hammer GP, Scheidemann-Wesp U, Samkange-Zeeb F, Wicke H, Neriishi K, Blettner M. Occupational exposure to low doses of ionizing radiation and cataract development: a systematic literature review and perspectives on future studies. Radiat Environ Biophys 2013;52:303–19. [DOI] [PubMed] [Google Scholar]
- 12.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys 2013;52:435–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little MP, Kitahara CM, Cahoon EK et al. Occupational radiation exposure and risk of cataract incidence in a cohort of US radiologic technologists. European Journal of Epidemiology 2018;33:1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellolio MF, Heien HC, Sangaralingham LR et al. Increased Computed Tomography Utilization in the Emergency Department and Its Association with Hospital Admission. West J Emerg Med 2017;18:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodickson A, Baeyens PF, Andriole KP et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009;251:175–84. [DOI] [PubMed] [Google Scholar]
- 16.Grant EJ, Brenner A, Sugiyama H et al. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiat Res 2017;187:513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon SL, Preston DL, Linet MS et al. Radiation organ doses received in a nationwide cohort of U.S. radiologic technologists: methods and findings. Radiat. Res 2014;182:507–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little MP, Tatalovich Z, Linet MS, Fang M, Kendall GM, Kimlin MG. Improving assessment of lifetime solar ultraviolet radiation exposure in epidemiologic studies: comparison of ultraviolet exposure assessment methods in a nationwide U.S. occupational cohort. Photochem. Photobiol 2018;94:1297–1307. [DOI] [PubMed] [Google Scholar]
- 19.Linetsky M, Raghavan CT, Johar K et al. UVA light-excited kynurenines oxidize ascorbate and modify lens proteins through the formation of advanced glycation end products: implications for human lens aging and cataract formation. J Biol Chem 2014;289:17111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sliney DH. Estimating the solar ultraviolet radiation exposure to an intraocular lens implant. J Cataract Refract Surg 1987;13:296–301. [DOI] [PubMed] [Google Scholar]
- 21.Sliney DH, International Commission on Illumination (CIE). Radiometric quantities and units used in photobiology and photochemistry: recommendations of the Commission Internationale de L’Eclairage (International Commission on Illumination). Photochem Photobiol 2007;83:425–32. [DOI] [PubMed] [Google Scholar]
- 22.National Aeronautics and Space Administration (NASA). Total Ozone Spectrometer Data Product: Erythemal UV Exposure., Greenbelt, MD.: Goddard Space Flight Center; 2004. [Google Scholar]
- 23.Diffey BL. Sources and measurement of ultraviolet radiation. Methods 2002;28:4–13. [DOI] [PubMed] [Google Scholar]
- 24.Hastie TJ, Tibshirani RJ. Generalized additive models. Boca Raton, FL: Chapman & Hall/CRC, 1990. [Google Scholar]
- 25.McCullagh P, Nelder JA. Generalized linear models. 2nd edition Monographs on statistics and applied probability 37, Boca Raton, FL: Chapman and Hall/CRC; 1989;1–526. [Google Scholar]
- 26.Risk Sciences International. Epicure version 2.0.1.0. 55 Metcalfe, K1P 6L5, Canada: Risk Sciences International, 2015. [Google Scholar]
- 27.Patefield WM. Algorithm AS 159: An Efficient Method of Generating Random R × C Tables with Given Row and Column Totals. J. Royal Statist. Soc. Series C (Appl. Statist.) 1981;30:91–97. [Google Scholar]
- 28.R Project version 3.6.1. R: A language and environment for statistical computing. version 3.6.1 https://www.r-project.org. Vienna, Austria: R Foundation for Statistical Computing, 2019.
- 29.Wakeford R, Tawn EJ. The meaning of low dose and low dose-rate. J. Radiol. Prot 2010;30:1–3. [DOI] [PubMed] [Google Scholar]
- 30.International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007;37:1–332. [DOI] [PubMed] [Google Scholar]
- 31.Little MP, Kwon D, Doi K et al. Association of chromosome translocation rate with low dose occupational radiation exposures in U.S. radiologic technologists. Radiat. Res 2014;182:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiol Rev 1995;17:336–46. [DOI] [PubMed] [Google Scholar]
- 33.Rafnsson V, Olafsdottir E, Hrafnkelsson J, Sasaki H, Arnarsson A, Jonasson F. Cosmic radiation increases the risk of nuclear cataract in airline pilots: a population-based case-control study. Arch.Ophthalmol 2005;123:1102–1105. [DOI] [PubMed] [Google Scholar]
- 34.Azizova TV, Hamada N, Bragin EV, Bannikova MV, Grigoryeva ES. Risk of cataract removal surgery in Mayak PA workers occupationally exposed to ionizing radiation over prolonged periods. Radiat Environ Biophys 2019;58:139–149. [DOI] [PubMed] [Google Scholar]
- 35.Shore RE. Radiation and cataract risk: Impact of recent epidemiologic studies on ICRP judgments. Mutat Res 2016;770:231–237. [DOI] [PubMed] [Google Scholar]
- 36.Rahmani B, Tielsch JM, Katz J et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology 1996;103:1721–6. [DOI] [PubMed] [Google Scholar]
- 37.Michon JJ, Lau J, Chan WS, Ellwein LB. Prevalence of visual impairment, blindness, and cataract surgery in the Hong Kong elderly. Br J Ophthalmol 2002;86:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob S, Donadille L, Maccia C et al. Eye lens radiation exposure to interventional cardiologists: a retrospective assessment of cumulative doses. Radiat Prot Dosimetry 2013;153:282–93. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor U, Walsh C, Gallagher A et al. Occupational radiation dose to eyes from interventional radiology procedures in light of the new eye lens dose limit from the International Commission on Radiological Protection. Br J Radiol 2015;88:20140627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.