Abstract
Objective:
High-grade serous ovarian cancer, the most frequent type of ovarian cancer, has a poor prognosis and novel treatments are needed for patients with platinum resistant/refractory disease. New therapeutic strategies targeting cell cycle checkpoints, including CHK1 inhibition with prexasertib, may help improve clinical response and overcome resistance.
Methods:
Patients with ovarian cancer (N=169) were assigned to 4 cohorts as part of the Phase 2 multicenter trial (NCT03414047): Cohort 1: platinum resistant, BRCA-wildtype with ≥3 lines prior therapy; Cohort 2: platinum resistant BRCA-wildtype with <3 lines prior therapy; Cohort 3: platinum resistant, BRCA-mutated with prior PARP inhibitor therapy; Cohort 4: platinum refractory, BRCA-mutated, or BRCA-wildtype with any number of prior therapy lines. The primary endpoint was objective response rate (ORR) and secondary endpoints included disease control rate (DCR), and safety. DNA from tumor biopsies was sequenced to identify biomarkers.
Results:
The ORR in platinum resistant patients (Cohorts 1–3) was 12.1%, and 6.9% in platinum refractory patients. In platinum resistant patients, DCR was 37.1%, and consistent across cohorts. In platinum refractory patients, DCR was 31.0%. Consistent with the prexasertib mechanism of action, the most common treatment related adverse events of all grades included thrombocytopenia, neutropenia, fatigue, nausea, and anemia.
Conclusions:
Prexasertib demonstrated durable single agent activity in a subset of patients with recurrent ovarian cancer regardless of clinical characteristics, BRCA status, or prior therapies, including PARPi. There was no obvious correlation with genomic alterations in responders vs non-responders, emphasizing the need for alternative biomarker approaches for responder identification.
Keywords: checkpoint kinase inhibitor, ovarian cancer, platinum resistant, platinum refractory
Introduction
Despite recent therapeutic advances, patients with platinum resistant or refractory high-grade serous ovarian cancer (HGSOC) have a poor prognosis and need novel treatments. Surgery and platinum-based therapies with or without maintenance therapy are standards of care for the initial treatment of HGSOC, as well as fallopian tube and primary peritoneal cancers. However, ≥75% of patients relapse after first line therapy and need subsequent treatment [1]. Patients who are refractory to initial treatment (primary platinum refractory patients) or relapse/progress within 6 months of the most recent platinum-based therapy (platinum resistant patients), are commonly treated with single agent chemotherapy with or without an anti-angiogenic agent such as bevacizumab [2,3]. A greater response rate is observed with bevacizumab and chemotherapy (27.3% vs 11.8% for chemotherapy alone) [3]. Although response may be obtained in multiple lines of therapy, patients with recurrent HGSOC eventually succumb to their disease due to treatment resistance.
Homologous recombination (HR) DNA repair is commonly defective in HGSOC; poly (ADP-ribose) polymerase (PARP) inhibitors have emerged as effective targeted therapy in HGSOC patients with defects in HR such as BRCA1 and BRCA2 mutations [1]. However, resistance to PARP inhibitors develops due to reversion mutations in the HR pathway, stabilization of DNA replication forks, or other mechanisms [4].
Prexasertib (LY2606368, ACR-368) is a checkpoint kinase 1 (CHK1) ATP-competitive kinase inhibitor [5]. As monotherapy, prexasertib leverages the role of CHK1 in regulating unperturbed cell-cycle progression, resulting in DNA damage dependent upon factors controlling replication initiation. Consequently, CHK1 inhibition by prexasertib promotes firing of late replication origins, leading to replication stress and stalled replication forks that degenerate into double-strand DNA breaks. HR is the predominant pathway for repair of double-strand DNA breaks in S-phase cells, and approximately half of HGSOCs have HR pathway defects [6, 7]. Thus, HR defects may confer an increased sensitivity to prexasertib. Mutually exclusive with loss-of-function mutations in BRCA1 and BRCA2, CCNE1 (which encodes cyclin E1) is amplified in approximately 20% of HGSOCs [8]. Overexpression of cyclin E induces replication stress and DNA damage that activates HR [9]. Cyclin E overexpression in HGSOC is associated with chemotherapy resistance and poorer survival [10]. CHK1 inhibition by prexasertib in CCNE1-amplified ovarian cancers may leverage the role of CHK1 in the DNA damage response and diminish the ability of cells to tolerate high levels of replication stress induced by cyclin E overexpression.
In nonclinical models of HGSOC, prexasertib demonstrated compelling monotherapy activity, including in in vitro models with or without BRCA1 mutations, CCNE1 amplification, and increased levels of replication stress [11–13]. Preclinically, prexasertib also exhibits monotherapy activity in HR repair-deficient and proficient models as well as tumor models with de novo or acquired PARP inhibitor resistance [13]. Additionally, organoid cultures with replication fork instability derived from HGSOC patients were more sensitive to prexasertib than models with stable forks [14]. Favorable preliminary clinical activity of prexasertib was observed in a single-center, investigator-initiated, Phase 2, proof-of-concept study in patients with BRCA-wildtype, platinum resistant or refractory HGSOC [15]. An exploratory analysis of patients with tumors harboring CCNE1 amplification, overexpression, or both, suggested a potential benefit of prexasertib in this subgroup of patients. The safety profile observed in HGSOC patients aligned with Phase 1 data of patients with solid tumors [11–13]; mechanism-based reversible myelosuppression, consistent with observations of other DDR targeted agents [16,17], was the most common AE associated with prexasertib. To further characterize the efficacy and safety of prexasertib in HGSOC, with a focus on defining the clinical characteristic associated with response and resistance, the current multi-cohort study was initiated in platinum resistant, BRCA-wildtype patients who progressed on ≥3 (Cohort 1) or <3 lines (Cohort 2) of prior therapy; platinum resistant BRCA-mutated patients who progressed on prior PARP therapy (Cohort 3); and platinum refractory BRCA-wildtype or BRCA-mutated patients with prior therapy (Cohort 4).
Methods
Study design
The study was a multicenter, nonrandomized, open-label, parallel cohort, Phase 2 study of prexasertib in patients with HGSOC, primary peritoneal, or fallopian tube cancer; hereafter all types are referred to as HGSOC (NCT03414047). Patients with recurrent disease were assigned to 4 independent cohorts based on clinical characteristics and prior treatment. Cohort 1 included platinum resistant patients (progression within 6 months of last dose), BRCA-wildtype with ≥3 prior therapy lines; Cohort 2: platinum resistant BRCA-wildtype who received <3 lines of prior therapy; Cohort 3: platinum resistant, BRCA-mutated with no restriction on number of lines of prior therapy, but who were previously treated with a PARP inhibitor; and Cohort 4: primary platinum refractory, BRCA-mutated or BRCA-wildtype (defined as disease recurrence within 4 weeks from the initial platinum containing regimen) with no restrictions on number of lines of prior therapy. Per protocol, patients were classified as either being BRCA-negative or BRCA-positive. Patients were BRCA-negative if there was absence of any deleterious or suspected deleterious BRCA mutations based on blood or tumor samples performed as part of standard clinical care prior to study entry. Alternatively, patients were BRCA-positive if such mutations were detected. Patients with variants of unknown significance were considered BRCA-negative. For clarity and consistency with convention, throughout this manuscript BRCA-negative and BRCA-positive patients are referred to as BRCA-wildtype and BRCA-mutated, respectively.
The primary study objective was to assess the overall response rate (ORR) for each cohort. Secondary objectives included evaluating other efficacy endpoints (listed below) and safety of prexasertib. Additionally, a relationship between biomarkers and clinical outcomes was assessed as an exploratory objective (Supplementary Figure 1).
This study was conducted in accordance with the International Conference on Harmonization requirements for Good Clinical Practice and with the consensus ethics principles derived from the International Ethics Guidelines, outlined in the Declaration of Helsinki and Council for International Organizations of Medical Sciences [18].
Eligibility
Women who had histologically or cytologically verified ovarian, primary peritoneal, or fallopian tube cancer of high-grade serous histology were included in the study. Patients ≥18 years of age with recurrent platinum resistant (progressed within 6 months after completion of platinum-based chemotherapy) or platinum refractory (progressed during or within 4 weeks after last dose of first line platinum-based chemotherapy) disease, Eastern Cooperative Oncology Group performance score of 0 or 1 [19], having a minimum of 1 measurable lesion as per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria [20], had discontinued all prior cancer treatments, had adequate organ function, had documented BRCA test results in either tumor or blood (Cohorts 1–3) prior to study treatment, and were willing to undergo a mandatory pretreatment tumor biopsy, were included in the study. Study exclusion criteria included patients who had received all of the following at any time in the platinum-resistant setting: gemcitabine, PEGylated liposomal doxorubicin, and paclitaxel (patients receiving only 1 or 2 of the agents for platinum resistant disease were not excluded) and/or prior radiotherapy to the whole pelvis, had known central nervous system disease, had a serious cardiac condition, and who had participated in any study involving a CHK1 inhibitor. All patients provided written informed consent prior to study enrollment.
Intervention
Eligible patients received 105 mg/m2 intravenous infusion of prexasertib every 2 weeks (days 1 and 15) in a 4-week cycle, until radiographically confirmed progression of disease, unacceptable AEs, or patient/physician request. The doses administered were determined by the patients’ body surface area at the beginning of each cycle. Dose reductions to 80 mg/m2 (first dose reduction) or 60 mg/m2 (second dose reduction) were required for Grade 3/4 non-hematologic AEs and febrile neutropenia occurring with prophylactic GCSF. If a third dose reduction was required, the patient was discontinued from treatment.
Study assessments
Assessments and endpoints
Tumor assessments were performed at baseline and thereafter, every 8 weeks (±1 week) up to 1 year, followed by every 12 weeks (±1 week) throughout the study period. Primary efficacy outcome evaluated the ORR, defined as the proportion of patients who achieved confirmed best overall response (BOR) of partial response (PR) and complete response (CR) as per RECIST version 1.1 as assessed by the investigator. Secondary efficacy outcomes included estimating disease control rate (DCR; proportion of patients who achieve a BOR of CR, PR or stable disease [SD]) where SD is ≥4 months, duration of response (DoR; time from the date of first objective response until the first date of radiographic documentation of progression or date of death), progression-free survival (PFS; time from enrollment until the first radiographic documentation of progression or death), overall survival (OS;), and CA-125 response (≥50% reduction from a pretreatment sample, confirmed and maintained for ≥28 days).
Common Terminology Criteria for Adverse Events version 4.0 [21] grading of adverse events (AEs) was performed by the investigator throughout the study. Furthermore, laboratory assessments including CA-125 and plasma biomarker samples were collected ≤3 days prior to the day of dosing. Post-treatment CA-125 samples were collected until the patient had documented progression, started another therapy, or study completion. Correlations between biomarkers in tumor tissue and clinical outcomes were assessed.
Biomarker analysis
Formalin-fixed, paraffin-embedded (FFPE) tumor tissue from a study-mandated tumor biopsy was collected at baseline from patients. Archived FFPE tumor tissue was also collected if available and utilized for testing if the corresponding baseline biopsy was unavailable or not evaluable. Genomic profiling to detect base substitutions, short insertions and deletions, and copy number alterations in 324 cancer-related genes, and select rearrangements was performed using the FoundationOne® CDx hybrid-capture next-generation sequencing (NGS) assay at Foundation Medicine (Cambridge, MA, USA). The analysis of genomic data focused on aberrations with known or likely functional consequences as annotated by Foundation Medicine.
Evaluation of cyclin E1 expression via immunohistochemistry was performed at the Eli Lilly and Company Clinical Diagnostics Laboratory (Indianapolis, IN, USA) using anti-cyclin E1 (clone EP435E) antibody (Abcam, Cambridge, UK) on the Dako Omnis platform using EnVision FLEX reagents (Agilent, Santa Clara, CA, USA) including the target retrieval solution at low pH for antigen retrieval. Slides were counterstained with hematoxylin. The intensity of the immunohistochemical label was scored based on a 4-point scale (0–3; negative, weak, medium, and strong staining) and the percent of positive tumor cells was determined manually. H-scores were calculated using the formula 1 x (% of 1+ cells) + 2 x (% of 2+ cells) + 3 x (% or 3+ cells) [22].
Statistical considerations
The study enrolled patients based on a Bayesian adaptive design with the potential to close specific cohorts early based on the interim analysis results. Approximately 20 patients were initially enrolled to each cohort, followed by a futility analysis for each respective cohort. The decision to close a cohort was made if results suggested the posterior probability of ORR exceeding the prespecified threshold of activity was below 20%. In Cohorts 1–3, a cohort could be stopped early if posterior probability of ORR >25% was below 20%; in Cohort 4, the threshold of ORR was chosen to be 15%. Up to 180 patients could be enrolled in the study across the cohorts. The Bayesian rule was monitored on an ongoing basis for each cohort after the first interim analysis, and the number of patients in a cohort was not fixed but based on the interim analysis rules. As cohorts were halted, new eligible patients were allocated to the enrolling cohorts. All safety and efficacy analyses were based on patients who received at least 1 dose of prexasertib, and the exploratory biomarker analysis was performed on the subset of those patients with valid assay results.
Results
Patient disposition
Overall, 172 patients were enrolled, of which 169 (98.3%) were nonrandomly assigned to cohorts and received ≥1 dose of the study drug (Cohort 1: n=53, Cohort 2: n=46, Cohort 3: n=41, Cohort 4: n=29). At the time of final database lock (February 2021), all patients had discontinued study treatment, with most patients discontinuing because of progressive disease (PD) (n=136; 79.1%). The other reasons for discontinuation included withdrawal of subject (n=13; 7.6%), physician decision (n=8; 4.7%), death (n=7; 4.1%), and AE (n=5; 2.9%).
Demographic and baseline characteristics
The demographic and baseline characteristics were comparable across cohorts. Patients had an overall median age of 59 years. The majority of patients had ovarian cancer (n=139; 82.2%) and most were diagnosed at stage III/IV (n=152; 89.9%). The median time from diagnosis was longer in Cohorts 1 and 3 (1554 and 1803 days, respectively) compared with Cohorts 2 and 4 (526 and 446, respectively). Demographic and baseline characteristics by cohort are in Table 1.
Table 1.
Baseline and demographic characteristics
| Parameter | Cohort 1: Platinum resistant, BRCA-wildtype with ≥3 prior lines of therapy N=53 | Cohort 2: Platinum resistant, BRCA-wildtype with <3 prior lines of therapy N=46 | Cohort 3: Platinum resistant, BRCA-mutated with prior PARP inhibitor therapy N=41 | Cohort 4: Platinum refractory with any number of prior lines of therapy N=29 | All cohorts N=169 |
|---|---|---|---|---|---|
| Age, mean (SD) | 61.3 (9.4) | 62.3 (9.4) | 59.5 (8.6) | 59.0 (13.4) | 60.8 (10.0) |
| Race, n (%) | |||||
| Asian | 6 (11.3) | 8 (17.4) | 4 (9.8) | 3 (10.3) | 21 (12.4) |
| African American | 0 (0.0) | 1 (2.2) | 0 (0.0) | 1 (3.4) | 2 (1.2) |
| Native Hawaiian or Pacific Islander | 1 (1.9) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 2 (1.2) |
| White | 46 (86.8) | 36 (78.3) | 37 (90.2) | 25 (86.2) | 144 (85.2) |
| Initial pathological diagnosis*, n (%) | |||||
| Fallopian tube cancer | 4 (7.5) | 3 (6.5) | 1 (2.4) | 3 (10.3) | 11 (6.5) |
| Ovarian cancer | 43 (81.1) | 37 (80.4) | 37(90.2) | 22 (75.9) | 139 (82.2) |
| Primary peritoneal carcinoma | 6 (11.3) | 6 (13.0) | 3 (7.3) | 4 (13.8) | 19 (11.2) |
| Median time since primary diagnosis*, days(min-max)a Prior therapies | 1554 (506–4649) | 526 (247–2580) | 1803 (780–4572) | 446 (103–3648) | 1052 (103–4649) |
| Surgery | 51 (96.2) | 44 (95.7) | 41 (100.0) | 26 (89.7) | 162 (95.9) |
| Radiotherapy | 5 (9.4) | 1 (2.2) | 6 (14.6) | 2 (6.9) | 14 (8.3) |
| Systemic therapy | 53 (100.0) | 46 (100.0) | 41 (100.0) | 29 (100.0) | 169 (100.0) |
| Median prior systemic regimens | 4.0 (3.0,5.0) | 2.0 (1.0,2.0) | 5.0 (4.0,6.0) | 2.0 (1.0,3.0) | 3.0 (2.0,5.0) |
The total ‘N’ varied (N=138); Cohort 1 n=41; Cohort 2 n=39; Cohort 3 n=32; Cohort 4 n=26.
Duration of disease is the time from date of initial pathological diagnosis to date of first exposure to treatment
Abbreviation: BRCA, breast cancer gene; ECOG, Eastern Cooperative Oncology Group; SD, Stable disease.
Efficacy
Primary efficacy
The ORR in platinum resistant patients (Cohorts 1–3) was 12.1% (Cohort 1: 11.3%; Cohort 2: 13.0%; Cohort 3: 12.2%) and in platinum refractory patients (Cohort 4) was 6.9%. Across all cohorts, a total of 87 (51.5%) patients had SD with 42 (24.9%) patients maintaining SD for ≥4 months (Table 2 and Figure 1). Interestingly, the response rate was comparable between Cohort 3 (patients with BRCA-mutated tumors that had received prior PARP inhibitor therapy) and Cohorts 1 and 2 (BRCA-wildtype patients with or without prior PARP inhibitor therapy).
Table 2.
Efficacy results of prexasertib in patients with ovarian, fallopian, and peritoneal cancer
| Parameter | Cohort 1: Platinum resistant, BRCA-wildtype with ≥3 prior lines of therapy N=53 | Cohort 2: Platinum resistant, BRCA-wildtype with <3 prior lines of therapy N=46 | Cohort 3: Platinum resistant, BRCA-mutated with prior PARP inhibitor therapy N=41 | Cohort 4: Platinum refractory with any number of prior lines of therapy N=29 |
|---|---|---|---|---|
| Best overall response, n (%) (95% CI) | ||||
| CR | 0 (0.0) (0.0, 6.7) | 1 (2.2) (0.1, 11.5) | 0 (0.0) (0.0, 8.6) | 0 (0.0) (0.0, 11.9) |
| PR | 6 (11.3) (4.3, 23.0) | 5 (10.9) (3.6, 23.6) | 5 (12.2) (4.1, 26.2) | 2 (6.9) (0.8, 22.8) |
| SD | 33 (62.3) (47.9, 75.2) | 24 (52.2) (36.9, 67.1) | 17 (41.5) (26.3, 57.9) | 13 (44.8) (26.4, 64.3) |
| PD | 9 (17.0) (8.1,29.8) | 15 (32.6) (19.5,48.0) | 15 (36.6) (22.1,53.1) | 11 (37.9) (20.7,57.7) |
| ORR (CR/PR), n (%) (95% CI) | 6 (11.3) (4.3, 23.0) | 6 (13.0) (4.9, 26.3) | 5 (12.2) (4.1, 26.2) | 2 (6.9) (0.8, 22.8) |
| DCR (CR+PR+Stable disease persisting≥4 months), n (%) (95% CI) | 24 (45.3) (31.6, 59.6) | 15 (32.6) (19.5, 48.0) | 13 (31.7) (18.1,48.1) | 9 (31. 0) (15.3, 50.8) |
| DoR, median months (95% CI); patients censored (%)a | 8.6 (5.6, NE); 0 (0.0) | 3.8 (2.8, NE); 1 (16.7) | 5.6 (3.7, NE); 0 (0.0) | 5.3 (5.1, NE); 0 (0.0) |
| PFS, median months (95% CI); patients censored (%) | 3.9 (3.7, 5.7); 6 (11.3) | 3.7 (3.1, 4.7); 4 (8.7) | 3.6 (1.9, 3.9); 4 (9.8) | 3.7 (1.8, 4.7); 4 (13.8) |
| OS, median months (95% CI); Patients censored (%) | 13.0 (7.5, 19.3); 18 (34.0) | 14.3 (11.8, 16.5); 12 (26.1) | 11.1 (7.2, 16.4); 12 (29.3) | 8.2 (6.2, 11.9); 4 (13.8) |
The total ‘N’ varied (N=19); Cohort 1 n=6; Cohort 2 n=6; Cohort 3 n=5; Cohort 4 n=2
Abbreviations: BRCA, breast cancer gene; CI, confidence interval; CR, complete response; DCR, disease control rate; DoR, duration of response; N = number of patients in the population; n = number of patients in the specified category; NE = not evaluable; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease
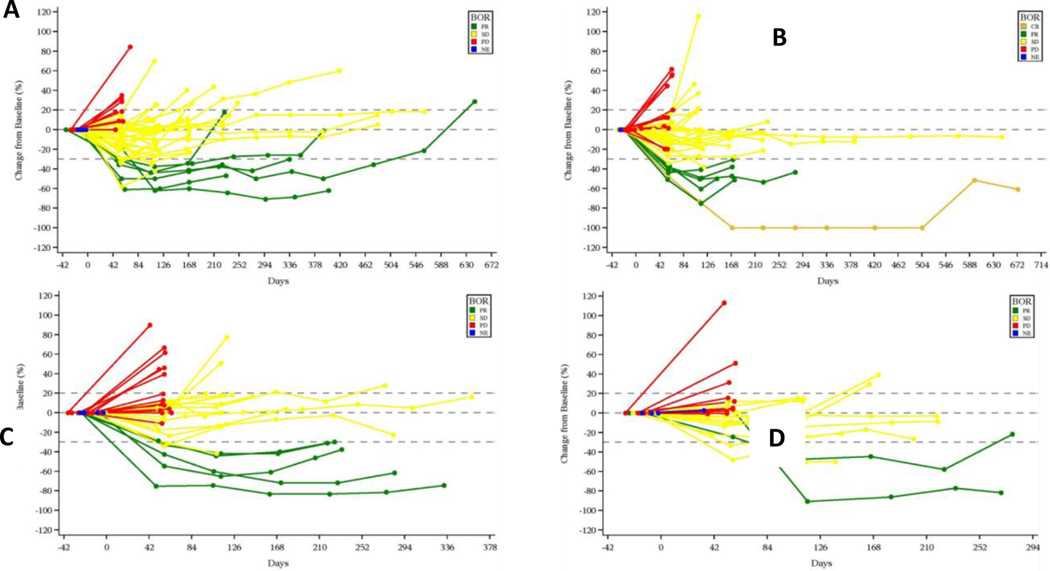
FIGURE 1.
Spider plot of tumor size change from baseline A. Cohort 1: Platinum resistant, BRCA-wildtype with ≥3 prior lines of therapy; B. Cohort 2: Platinum resistant, BRCA-wildtype with <3 prior lines of therapy; C. Cohort 3: Platinum resistant, BRCA-mutated with prior PARP inhibitor therapy; D. Cohort 4: Platinum refractory with any number of prior lines of therapy
Abbreviations: BOR, best overall response; CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
However, in Cohort 1 and 2, response to prexasertib was more likely in patients with no prior PARP treatment versus prior PARP treatment (Cohorts 1 [14% vs. 0%] and 2 [13.6% vs. 0.0%]) (Table S1). The ORR was comparable for patients with or without prior anti-angiogenic treatment in Cohorts 1 (13.3% vs. 8.7%) and 2 (15.0% vs. 11.5%); response to prexasertib was more likely in patients with prior anti-angiogenic therapy in Cohort 3 (17.6% vs. 8.3%) and Cohort 4 (11.8% vs. 0%) (Table S1).
Secondary efficacy
In platinum resistant patients, the DCR was 37.1% (Cohort 1: 45.3%; Cohort 2: 32.6%; Cohort 3: 31.7%. In platinum refractory patients, the DCR was 31.0%. Patients with BRCA-wildtype, platinum resistant cancer with ≥3 lines of prior therapy (Cohort 1) showed the highest DCR of 45.3%, respectively (Table 2).
The overall median DoR for 19 patients with an objective response was 5.6 months (95% CI: 3.9, 7.6). The DoR for patients in Cohort 1 (8.6 months [95% CI: 5.6, NE]) trended to be greater than for the other cohorts. The DoR for the 2 platinum refractory patients who experienced a PR was 5.1 and 5.6 months, respectively. The overall median PFS and OS were 3.7 months (95% CI: 1.8, 4.7) and 8.2 months (95% CI: 6.2,11.9), respectively. Of note, PFS was similar in patients with or without prior anti-angiogenic therapy (3.7 months [95% CI: 3.6, 4.7] vs. 3.7 months [95% CI: 3.4, 4.7]) and PARP inhibitor treatment (3.5 months [95% CI: 1.9, 3.8] vs. 3.8 months [95% CI: 3.7, 5.3]). Patients with BRCA-wildtype, platinum resistant cancer with <3 lines of prior therapy (Cohort 2) tended to show the highest median OS (14.3 months [95% CI: 11.8, 16.5]) (Table 2).
Safety
The most common treatment-emergent AEs (TEAEs), regardless of relatedness to study drug included fatigue, nausea, thrombocytopenia, neutropenia, and abdominal pain (Supplementary Table S2), whereas the most common study treatment-related adverse events (TRAE) included thrombocytopenia, neutropenia, fatigue, nausea, and anemia (Table 3). TEAE ≥Grade 3 events were observed in 123 (72.8%) patients, and TRAEs ≥Grade 3 occurred in 98 (58.0%) patients. Reversible neutropenia was the most common ≥Grade 3 TEAE observed occurring in 37.9% of patients. A total of 70.4% of patients were administered granulocyte colony stimulating factor (GCSF), of which 62.7% patients were given GCSF prophylactically. Of patients, 30.8% and 39.6% required dose reduction and dose delay respectively, due to AEs. Serious AEs (SAEs) were reported in 43.8% of patients, with 27.2% assessed to be study treatment related. Febrile neutropenia was the most common SAE, experienced by 10.1% of patients (Supplementary Table S3). A total of 5 (2.9%) patients discontinued study treatment due to an AE, all of which discontinued due to an AE deemed by the investigator to be related to study treatment (large intestine perforation, blood creatinine increased, febrile neutropenia, neutropenia, and pneumonia). Seven (4.1%) deaths occurred on therapy during the study, 9 (5.3%) deaths occurred within 30 days of treatment discontinuation; however, only 1 death (sepsis) was deemed to be related to study treatment.
Table 3.
Treatment-related adverse events related to study treatment occurring in >20% of the population
| Parameter | Cohort 1: Platinum resistant, BRCA-wildtype with ≥3 prior lines of therapy N=53 | Cohort 2: Platinum resistant, BRCA-wildtype with <3 prior lines of therapy N=46 | Cohort 3: Platinum resistant, BRCA-mutated with prior PARP inhibitor therapy N=41 | Cohort 4: Platinum refractory with any number of prior lines of therapy N=29 | All cohorts N=169 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | G3/4/5* | All | G3/4/5 | All | G3/4/5 | All | G3/4/5 | All | G3/4/5 | |
| Thrombocytopenia | 24 (45.3) | 12 (22.6) | 20 (43.5) | 6 (13.0) | 19 (46.3) | 8 (19.5) | 11 (37.9) | 4 (13.8) | 74 (43.8) | 30 (17.8) |
| Neutropenia | 23 (43.4) | 19 (35.8) | 20 (43.5) | 16 (34.8) | 17 (41.5) | 17 (41.5) | 12 (41.4) | 12 (41.4) | 72 (42.6) | 64 (37.9) |
| Fatigue | 25 (47.2) | 4 (7.5) | 17 (37.0) | 0 (0.0) | 17 (41.5) | 2 (4.9) | 12 (41.4) | 4 (13.8) | 71 (42.0) | 10 (5.9) |
| Nausea | 17 (32.1) | 1 (1.9) | 20 (43.5) | 1 (2.2) | 11 (26.8) | 1 (2.4) | 13 (44.8) | 0 (0.0) | 61 (36.1) | 3 (1.8) |
| Anemia | 16 (30.2) | 7 (13.2) | 12 (26.1) | 1 (2.2) | 13 (31.7) | 5 (12.2) | 12 (41.4) | 5 (17.2) | 53 (31.4) | 18 (10.7) |
| Vomiting | 14 (26.4) | 2 (3.8) | 12 (26.1) | 2 (4.3) | 9 (22.0) | 2 (4.9) | 10 (34.5) | 1 (3.4) | 45 (26.6) | 7 (4.1) |
| Diarrhea | 11 (20.8) | 0 (0.0) | 13 (28.3) | 0 (0.0) | 4 (9.8) | 0 (0.0) | 6 (20.7) | 1 (3.4) | 34 (20.1) | 1 (0.6) |
Abbreviations: BRCA, breast cancer gene; G, grade.
The only Grade 5 AE was one patient in Cohort 1 with sepsis.
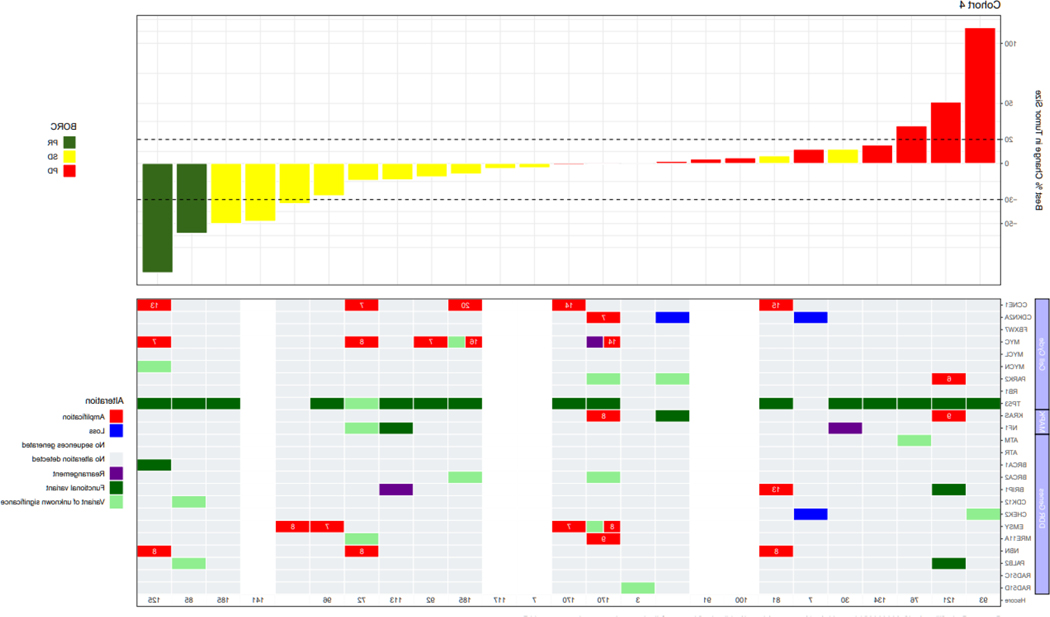
Biomarker analysis
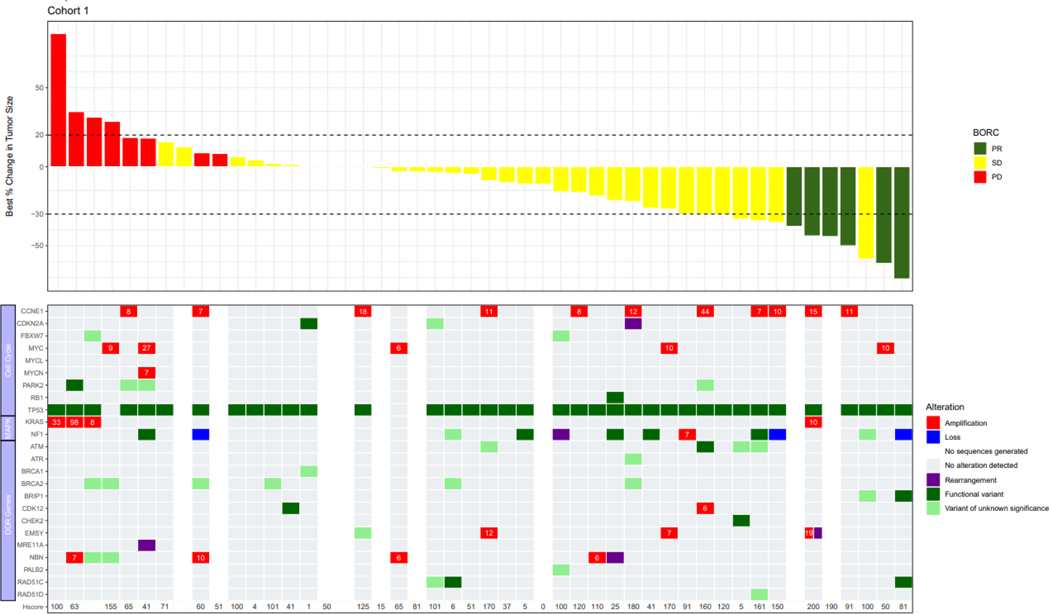
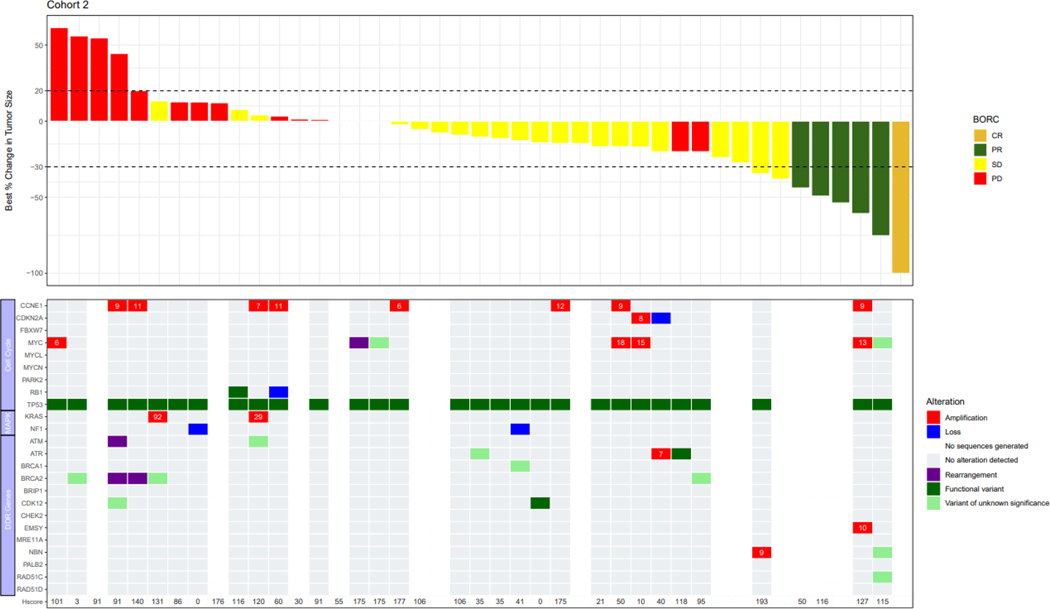
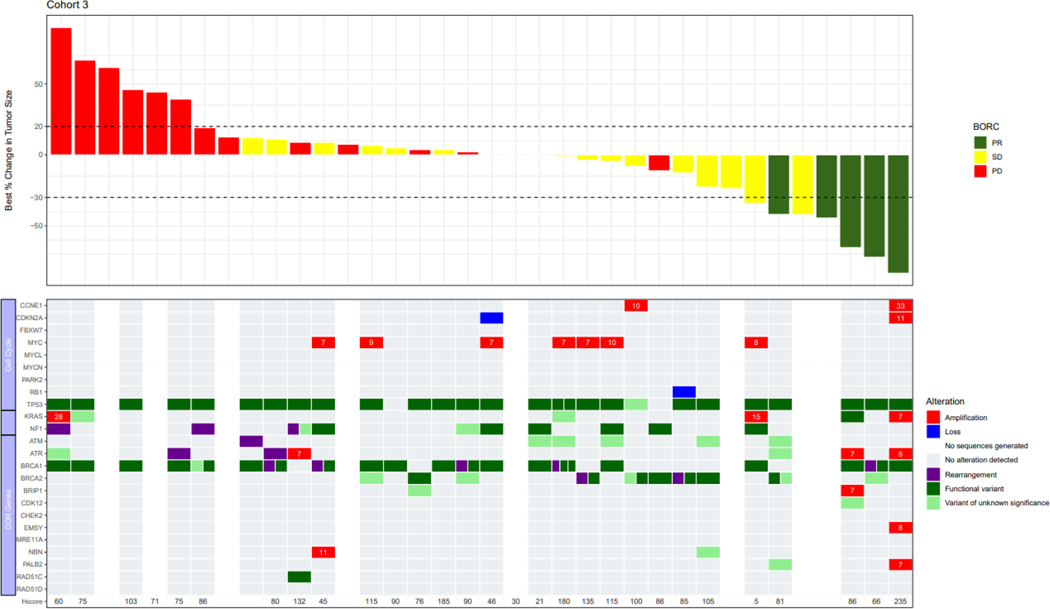
Genomic profiles consisting of selected genes involved with cell cycle regulation or the DNA damage response were integrated with objective response data in Figure 2. Documented BRCA results from prior testing used to meet eligibility requirements were concordant with BRCA status by central testing for all but 3 patients. Two patients in BRCA-wildtype Cohort 2 were BRCA-negative by prior blood-based testing, but harbored BRCA2 rearrangements by tissue-based central testing (Figure 2B). One patient enrolled to BRCA-mutated Cohort 3 lacked a BRCA mutation by central testing.
FIGURE 2:
Correlation with alterations in DNA damage response genes and cell cycle regulators; Cohort 1: Platinum resistant, BRCA-wildtype with ≥3 prior lines of therapy; Cohort 2: Platinum resistant, BRCA-wildtype with <3 prior lines of therapy; Cohort 3: Platinum resistant, BRCA-mutated with prior PARP inhibitor therapy; Cohort 4: Platinum refractory with any number of prior lines of therapy. The copy number is indicated in the cells for genes with amplification.
Abbreviations: BRCA, breast cancer gene.
Notes: cyclin E1 H-score is also included; Several patients had a >30% decrease in target lesions, but the PRs were not confirmed and therefore per RECISIT 1.1 the confirmed best overall response is SD. Similarly, several patients had <20% increase in target lesions but met another criterion for progressive disease (eg. detection of a new lesion, or unequivocal progression of a non-target lesion).
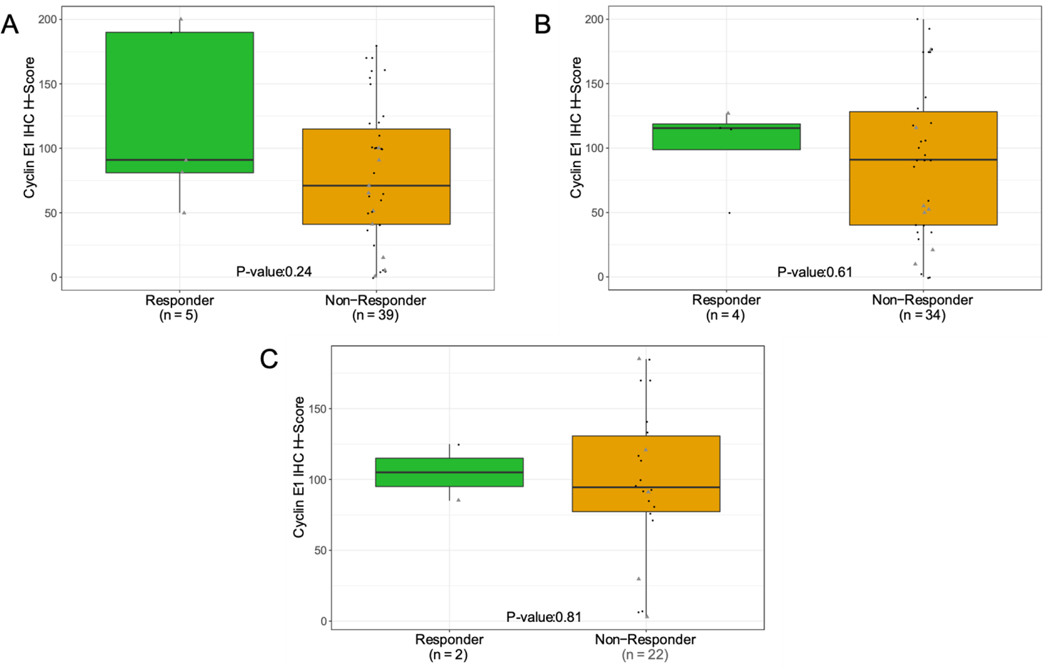
TP53 was the most frequently mutated gene in the overall population (93%). CCNE1 amplification, defined as a copy number ≥6, occurred in 29% of patients enrolled to BRCA-wildtype Cohorts 1 and 2, and 26% of patients in Cohort 4. The response rate was not significantly different by Fisher’s exact test in patients with CCNE1-amplified tumors as compared to non-amplified tumors across all cohorts (19.2% vs. 7.4%, p=0.13), and in Cohorts 1 (18.2% vs. 7.1%, p=0.56), 2 (12.5% vs. 4.5%, p=0.47) and 4 (20% vs. 5.9%, p=0.41). Across all cohorts, median PFS was 3.7 months in all patients regardless of CCNE1-amplification status. H-scores for cyclin E1 expression did not significantly differ in patients who experienced PR compared to those with SD or PD (Figure 2).
Discussion
In this Phase 2 multi-cohort study, treatment with prexasertib resulted in responses in 12.1% of platinum resistant HGSOC patients (Cohorts 1–3) and a corresponding PFS of 3.7 months. The response rates and median PFS observed with prexasertib are similar to that observed with single agent chemotherapy in patients with platinum resistant HGSOC [23–27]. Notably, the prexasertib response rates were 14.3% (7.6, 23.6) in patients who had received prior anti-angiogenic therapy. Of note, although ORR observed in both treatment groups were limited, a significant proportion of responses were durable (mDoR = 5.6 months). The DCR with prexasertib suggests clinical benefit was achieved in a subset of patients. Non-hematologic toxicity with prexasertib was generally Grade 1 and 2 and compares favorably to toxicities observed with standard chemotherapy agents used in the treatment of ovarian cancer. Consistent with prior studies, prexasertib was associated with reversible mechanism based hematologic toxicity. Grade 3/4 neutropenia was observed in 38% of patients, which is lower than that reported for previous studies with prexasertib [12,28,29]. This may be due to the assessment schedule and the higher than usual rate of prophylactic GCSF use in this study. Serious febrile neutropenia was reported in 10.1% of patients but was manageable with GCSF administration given either prophylactically or to treat the neutropenia.
A unique element of this study was the use of a Bayesian adaptative design to monitor the ongoing efficacy and allow early termination of a cohort based on a prespecified futility rule. This approach was implemented based on the results of a pilot study where activity was observed across a wide spectrum of clinical characteristics and prior treatments. Notably, in that study, 6/19 (32% [95% CI 13–57]) heavily pretreated platinum resistant or refractory HGSOC patients achieved an objective response [15]. Since in the pilot study activity was observed in heavily pretreated patients, Cohorts 1 and 2 of this study segregated patients on the number of prior therapy lines. Similarly, the pilot study suggested differing outcomes for patients with and without BRCA mutations [30], which provided the justification for Cohort 3. Cohort 4 included platinum-refractory patients to explore the clinical benefit of prexasertib in high unmet medical need patients. The cohort size was not fixed, such that based on interim analysis rules, the cohorts meeting prespecified rules could be expanded while allowing those with minimal activity to close. However, since the activity was consistent across cohorts and clinical characteristics, including the time from initial diagnosis, these were not a driver of prexasertib activity, and the cohort size remained relatively balanced. An exception was Cohort 4 which was smaller and enrolled women with primary platinum-refractory disease, occurring in up to 25% of the total patient population. The Bayesian adaptive design was well-suited for this study and is a reasonable consideration for studies with agents where the optimal population has not yet been defined.
The response rate with prexasertib was consistent across a variety of clinical characteristics, including line of prior therapy, BRCA status, and prior anti-angiogenic or PARP inhibitor treatments. A subset of patients across all cohorts achieved durable objective responses, suggesting a potential underlying molecular mechanism. As a result, an exploratory biomarker analysis was conducted. HGSOC is characterized by genomic instability associated with nearly universal mutation of TP53 and a high prevalence of HR deficiency (HRD) [2,8,31]. Mutually exclusive with BRCA mutations, frequent amplification of CCNE1 also promotes genomic instability by generating replication stress [32]. Tumors with defective G1/S checkpoint signaling due to TP53 loss and high replication stress are heavily dependent on ATR/CHK1-mediated replication fork stabilization, G2/M phase arrest, and DNA repair, potentially rendering them more sensitive to prexasertib. Consistent with this hypothesis, approximately two thirds of BRCA-wildtype HGSOC patients with cyclin E1-overexpressing tumors derived clinical benefit from prexasertib in the pilot Phase 2 study [15]. In the current study, 29% of tumor samples from BRCA-wildtype patients in Cohorts 1 and 2 and 26% of patients in Cohort 4 harbored CCNE1 amplifications. Higher response rates were observed in patients with CCNE1-amplified tumors, but there were no statistically significant associations. Similarly, median cyclin E1 H-scores were numerically higher in responders compared to non-responders, but differences were not significant. Conclusions are limited by small sample sizes as well as a lack of established cutoffs for defining high-level CCNE1 amplification or cyclin E1 overexpression. Whether prexasertib treatment exacerbates endogenous replication stress to a level sufficient to induce replication catastrophe and cell death in an individual tumor likely depends on multiple factors. For example, downregulation of FAM122A expression stabilizes WEE1 and reduces replication stress [33]. Tumors with compensatory low expression of FAM122A may be more tolerant of intrinsic oncogene-induced replication stress and resistant to prexasertib. Methods to directly assess baseline levels of replication stress in the clinical setting may benefit the development of CHK1 inhibitors [33]. Additional factors such as levels of cyclin B1/CDK1 activity and expression of DNA repair pathway genes may also impact the sensitivity of BRCA-wildtype tumors to prexasertib [34]. In the current study, response rates were similar in BRCA-mutated and wildtype cohorts, suggesting HRD status is not a determinant of prexasertib sensitivity. However, BRCA-mutated patients enrolled to Cohort 3 were required to have received prior PARP inhibitor therapy, and mechanisms of resistance to PARP inhibitors include restoration of HR function and replication fork stability, which could result in cross resistance to prexasertib [35]. Nonetheless, objective responses to prexasertib were still observed in 12.2% of Cohort 3, suggesting that prexasertib monotherapy may be beneficial in PARP inhibitor resistant tumors [13]. However, in the small group of BRCA-wild type patients from Cohorts 1, 2 who also received prior PARP inhibitor, no response to prexasertib was observed. These data may suggest PARP inhibitor resistance in BRCA-wild type patients will likely not benefit from prexasertib monotherapy. This biomarker analysis, which primarily focused on genomic alterations and cyclin E1 expression, did not identify any potential predictive biomarkers for prexasertib response, suggesting alternative approaches, including proteomics-based biomarkers, may need to be explored to understand which patients will benefit from prexasertib treatment.
Sequential monotherapy has been used in patients with platinum resistant HGSOC. However, recent advances with anti-angiogenic therapy, targeted agents, and immunotherapy lay the groundwork for considering combination therapies in advanced lines of therapy [2]. Specifically, there is strong rationale to combine prexasertib with either PD-L1 [36] or PARP inhibitors [14,36–39]. The safety of prexasertib and a PD-L1 inhibitor was characterized in a Phase 1 study where preliminary activity was observed in patients with CCNE1-amplified HGSOC with evidence of cytotoxic T-cell activation [40]. Similarly, a Phase 1 combination study demonstrated the feasibility of combining prexasertib with olaparib and preliminary clinical activity in patients harboring BRCA mutations with HGSOC who had previously progressed on a PARP inhibitor [41]. These studies provide preliminary data to support combination therapy as another approach to build on and extend the clinical benefit of prexasertib observed in this study.
Conclusion
Prexasertib demonstrated durable single agent activity in a subset of patients with recurrent HGSOC regardless of clinical characteristics or prior therapy. The safety profile was consistent with previous reports with transient and reversible mechanism-based neutropenia being the most common treatment related adverse effect. This study did not identify any potential predictive biomarkers for prexasertib response albeit with small sizes, suggesting alternative biomarker approaches or combination therapies may be needed to extend the activity of prexasertib in patients with recurrent HGSOC.
Supplementary Material
Figure 3.
Association with cyclin E1 H-score and ORR; A. Cohort 1: Platinum resistant, BRCA-wildtype with ≥3 prior lines of therapy; B. Cohort 2: Platinum resistant, BRCA-wildtype with <3 prior lines of therapy; C. Cohort 4: Platinum refractory, BRCA-mutatedor BRCA-wildtype with any number of lines of prior therapy. Subjects with a BOR of NE were excluded. P−values were determined using Student’s t−test. Archival biopsy (grey triangle) was defined as any tissue that was collected more than 28 days before the treatment start date.
Highlights.
Prexasertib, a checkpoint kinase 1 (CHK1) inhibitor, was assessed in this multi-cohort study in platinum resistant or platinum refractory high grade serous ovarian cancer patients (HGSOC).
Prexasertib demonstrated durable single agent activity in a subset of patients with recurrent HGSOC regardless of clinical characteristics, BRCA status, or prior therapies including PARP inhibitors.
The most common treatment related adverse events included neutropenia, thrombocytopenia, fatigue, nausea, and anemia.
This study did not identify any potential predictive biomarkers for prexasertib response, suggesting alternative approaches to biomarker identification may need to be explored.
Acknowledgements
We thank our patients and caregivers for their participation in this study, the study investigators and their staff, and the Study JTJN clinical trial team. The authors would also like to thank Darryl Ballard and Janet Grondin of the Eli Lilly and Company Clinical Diagnostics Laboratory for their expertise in performing the immunohistochemistry assay development, validation, and staining runs; Abby Jeske and Arantxa Uruñuela for providing clinical trial support; Elaine Jennings for her writing and editorial contributions; and Meena Ravuri for providing support in addressing peer review comments from the journal with contributions from the authors.
Footnotes
Conflict of Interest
Nicoletta Colombo: Consultant/advisor: Roche, PharmaMar, AstraZeneca, Clovis Oncology, MSD, GlaxoSmithKline, Tesaro, Pfizer, BIOCAD, Immunogen, Mersana, Eisai, Oncxerna. Promotional ppeaker: AstraZeneca, Tesaro, Novartis, Clovis, MSD, GlaxoSmithKline, Eisai. Investigator/researcher: AstraZeneca, PharmaMar, Roche. Nonfinancial interests: Steering Committee member for ESMO Clinical Guidelines, Chair Scientific Committee ACTO onlus.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C. High-grade serous ovarian cancer: basic sciences, clinical and therapeutic standpoints. Int J Mol Sci. 2019;20(4):952. doi: 10.3390/ijms20040952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leung SOA, Konstantinopoulos PA. Advances in the treatment of platinum resistant epithelial ovarian cancer: an update on standard and experimental therapies. Expert Opin Investig Drugs. 2021;30(7):695–707. doi: 10.1080/13543784.2021.1939305 [DOI] [PubMed] [Google Scholar]
- [3].Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489 [DOI] [PubMed] [Google Scholar]
- [4].Bitler BG, Watson ZL, Wheeler LJ, Behbakht K. PARP inhibitors: Clinical utility and possibilities of overcoming resistance. Gynecologic Oncology. 2017;147(3):695–704. doi: 10.1016/j.ygyno.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Evangelisti G, Barra F, Moioli M, Sala P, Stigliani S, Gustavino C, et al. Prexasertib: an investigational checkpoint kinase inhibitor for the treatment of high-grade serous ovarian cancer. Expert Opin Investig Drugs. 2020;29(8):779–792. doi: 10.1080/13543784.2020.1783238 [DOI] [PubMed] [Google Scholar]
- [6].Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5(11):1137–1154. doi: 10.1080/13543784.2020.1783238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–120. doi: 10.1038/nrc.2015.21 [DOI] [PubMed] [Google Scholar]
- [8].Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci U S A. 2013;110(48):19489–11494. doi: 10.1073/pnas.1314302110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jones RM, Mortusewicz O, Afzal I, Lorvellec M, Garcia P, Helleday T, et al. Increased replication initiation and conflicts with transcription underlie Cyclin E-induced replication stress. Oncogene. 2013;32(32):3744–3753. doi: 10.1038/onc.2012.387 [DOI] [PubMed] [Google Scholar]
- [10].Petersen S, Wilson AJ, Hirst J, Roby KF, Fadare O, Crispens MA, et al. CCNE1 and BRD4 co-amplification in high-grade serous ovarian cancer is associated with poor clinical outcomes. Gynecol Oncol. 2020;157(2):405–410. doi: 10.1016/j.ygyno.2020.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Iyer S, Zhang S, Yucel S, Horn H, Smith SG, Reinhardt F, et al. Genetically defined syngeneic mouse models of ovarian cancer as tools for the discovery of combination immunotherapy. Cancer Discov. 2021;11(2):384–407. doi: 10.1158/2159-8290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McNeely Samuel C, Burke Teresa F DS, Barnard Darlene S, Marshall Mark S, Bence Aimee K, Beckmann Richard P. Abstract A108: LY2606368, a second generation Chk1 inhibitor, inhibits growth of ovarian carcinoma xenografts either as monotherapy or in combination with standard-of-care agents. AACR-NCI-EORTC International Conference: Molecular Targets and Cancer. 2011. [Google Scholar]
- [13].Parmar K, Kochupurakkal BS, Lazaro JB, Wang ZC, Palakurthi S, Kirschmeier PT, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res. 2019;25(20):6127–6140. doi: 10.1158/1078-0432.CCR-19-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hill SJ, Decker B, Roberts EA, Horowitz NS, Muto MG, Worley MJ Jr., et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8(11):1404–1421. doi: 10.1158/2159-8290.CD-18-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee J-M, Nair J, Zimmer A, Lipkowitz S, Annunziata CM, Merino MJ, et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. The Lancet Oncol. 2018;19(2):207–215. doi: 10.1016/S1470-2045(18)30009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Biechonski S, Yassin M, Milyavsky M. DNA-damage response in hematopoietic stem cells: an evolutionary trade-off between blood regeneration and leukemia suppression. Carcinogenesis. 2017;38(4):367–377. doi: 10.1093/carcin/bgx002 [DOI] [PubMed] [Google Scholar]
- [17].Jessen BA, Lee L, Koudriakova T, Haines M, Lundgren K, Price S, et al. Peripheral white blood cell toxicity induced by broad spectrum cyclin-dependent kinase inhibitors. J Appl Toxicol. 2007;27(2):133–142. doi: 10.1002/jat.1177 [DOI] [PubMed] [Google Scholar]
- [18].World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- [19].Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- [20].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- [21].U.S. Department of health and human services NIoH, National Cancer Institute of Canada Clinical Trials, Group. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf. Accessed 13 November, 2020.
- [22].Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13(1):33–42. doi: 10.1016/S1470-2045(11)70318-7 [DOI] [PubMed] [Google Scholar]
- [23].Davis A, Tinker AV, Friedlander M. “Platinum resistant” ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol. 2014;133(3):624–631. doi: 10.1016/j.ygyno.2014.02.038 [DOI] [PubMed] [Google Scholar]
- [24].Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26(6):890–896. doi: 10.1200/JCO.2007.13.6606 [DOI] [PubMed] [Google Scholar]
- [25].Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19(14):3312–3322. doi: 10.1200/JCO.2001.19.14.3312 [DOI] [PubMed] [Google Scholar]
- [26].Gynecologic Oncology G, Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;101(3):436–440. doi: 10.1016/j.ygyno.2005.10.036 [DOI] [PubMed] [Google Scholar]
- [27].Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25(19):2811–2818. doi: 10.1200/JCO.2006.09.6735 [DOI] [PubMed] [Google Scholar]
- [28].Byers LA, Navarro A, Schaefer E, Johnson M, Ozguroglu M, Han JY, et al. A phase II trial of prexasertib (LY2606368) in patients with extensive-stage small-cell lung cancer. Clin Lung Cancer. 2021;S1525–7304(21)00089–9. doi: 10.1016/j.cllc.2021.04.005 [DOI] [PubMed] [Google Scholar]
- [29].Hong DS, Moore K, Patel M, Grant SC, Burris HA 3rd, William WN Jr., et al. Evaluation of prexasertib, a checkpoint kinase 1 inhibitor, in a phase Ib study of patients with squamous cell carcinoma. Clin Cancer Res. 2018;24(14):3263–3272. doi: 10.1158/1078-0432.CCR-17-3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lampert EJ, An D, McCoy A, Kohn EC, Annunziata CM, Trewhitt K, et al. Prexasertib, a cell cycle checkpoint kinase 1 inhibitor, in BRCA mutant recurrent high-grade serous ovarian cancer (HGSOC): A proof-of-concept single arm phase II study. J Clin Oncol. 2020;38(15_suppl):6038. doi: 10.1200/JCO.2020.38.15_suppl.6038 [DOI] [Google Scholar]
- [31].Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karst AM, Jones PM, Vena N, Ligon AH, Liu JF, Hirsch MS, et al. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014;74(4):1141–1152. doi: 10.1158/0008-5472.CAN-13-2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li F, Kozono D, Deraska P, Branigan T, Dunn C, Zheng XF, et al. CHK1 inhibitor blocks phosphorylation of FAM122A and promotes replication stress. Mol Cell. 2020;80(3):410–22.e6. doi: 10.1016/j.molcel.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nair J, Huang TT, Murai J, Haynes B, Steeg PS, Pommier Y, et al. Resistance to the CHK1 inhibitor prexasertib involves functionally distinct CHK1 activities in BRCA wild-type ovarian cancer. Oncogene. 2020;39(33):5520–5535. doi: 10.1038/s41388-020-1383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dias MP, Moser SC, Ganesan S, Jonkers J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat Rev Clin Oncol. 2021. doi: 10.1038/s41571-021-00532-x [DOI] [PubMed] [Google Scholar]
- [36].Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9(5):646–661. doi: 10.1158/2159-8290.CD-18-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brill E, Yokoyama T, Nair J, Yu M, Ahn YR, Lee JM. Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer. Oncotarget. 2017;8(67):111026–111040. doi: 10.18632/oncotarget.22195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cho HY, Kim YB, Park WH, No JH. Enhanced efficacy of combined therapy with checkpoint kinase 1 inhibitor and rucaparib via regulation of rad51 expression in BRCA wild-type epithelial ovarian cancer cells. Cancer Res Treat. 2021;53(3):819–828. doi: 10.4143/crt.2020.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sen T, Tong P, Stewart CA, Cristea S, Valliani A, Shames DS, et al. CHK1 inhibition in small-cell lung cancer produces single-agent activity in biomarker-defined disease subsets and combination activity with cisplatin or olaparib. Cancer Res. 2017;77(14):3870–3884. doi: 10.1158/0008-5472.CAN-16-3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Do KT, Manuszak C, Thrash E, Giobbie-Hurder A, Hu J, Kelland S, et al. Immune modulating activity of the CHK1 inhibitor prexasertib and anti-PD-L1 antibody LY3300054 in patients with high-grade serous ovarian cancer and other solid tumors. Cancer Immunol Immunother. 2021;70(10):2991:3000. doi: 10.1007/s00262-021-02910-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Do KT, Kochupurakkal B, Kelland S, de Jonge A, Hedglin J, Powers A, et al. Phase 1 Combination Study of the CHK1 Inhibitor Prexasertib and the PARP Inhibitor Olaparib in High-grade Serous Ovarian Cancer and Other Solid Tumors. Clin Cancer Res. 2021;27(17):4710–4716. doi: 10.1158/1078-0432.CCR-21-1279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.