Abstract
V-domain immunoglobulin suppressor of T cell activation (VISTA) is an inhibitory immune checkpoint molecule that is broadly expressed on lymphoid and myeloid cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Near-infrared photoimmunotherapy (NIR-PIT) is a cancer treatment that utilizes an antibody-photoabsorber (IRDye 700DX NHS ester) conjugate to selectively kill target cells after the local application of NIR light. Depletion of VISTA-expressing cells in the tumor microenvironment (TME) using NIR-PIT could enhance anti-tumor immune responses by removing immune suppressive cells. The purpose of this study was to evaluate the anti-tumor efficacy of VISTA-targeted NIR-PIT using two murine tumor models, MC38-luc and LL2-luc. VISTA was expressed on T cells including Tregs and MDSCs in the TME of these tumors. In contrast, CD45 − cells, including cancer cells, did not express VISTA. VISTA-targeted NIR-PIT depleted VISTA-expressing cells ex vivo. In vivo VISTA-targeted NIR-PIT inhibited tumor progression and prolonged survival in both models. After VISTA-targeted NIR-PIT, augmented CD8 + T cell and dendritic cell activation were observed in regional lymph nodes. In conclusion, VISTA-targeted NIR-PIT can effectively treat tumors by decreasing VISTA-expressing immune suppressor cells in the TME. Local depletion of VISTA-expressing cells in the tumor bed using NIR-PIT is a promising new cancer immunotherapy for treating various types of tumors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03205-5.
Keywords: Near-infrared photoimmunotherapy (NIR-PIT), V-domain immunoglobulin suppressor of T cell activation (VISTA), Regulatory T cell (Treg), Myeloid-derived suppressor cell (MDSC)
Introduction
Near-infrared photoimmunotherapy (NIR-PIT) is a novel cancer therapy that employs antibody-photoabsorber conjugates (APCs) that target cells in the tumor and/or tumor microenvironment (TME) [1, 2]. The injected APCs bind to the target cells over time (usually in less than 24 h), then are activated by irradiation with NIR light. NIR-PIT kills the target cells rapidly and selectively [3]. NIR-PIT was originally developed to target antigens on cancer cell membranes, which has proven to be successful in the clinic. For instance, in Japan, the first APC targeting epidermal growth factor receptor (EGFR; ASP-1929, Akalux®, Rakuten Medical Inc., San Diego, CA, USA) has been approved as a clinical agent for the treatment of advanced head and neck cancers. However, non-cancer cells in the TME can also be a target of NIR-PIT. In fact, in experiments using immunocompetent mice, NIR-PITs targeting CD25 and cytotoxic T lymphocyte associated protein 4 (CTLA4), which are highly expressed on regulatory T cells (Tregs), resulted in the elimination of Tregs and activation of tumor immunity, causing an anti-tumor effect [4–6]. However, there are other immunosuppressive cells besides Tregs that could interfere with the immune response. Therefore, we speculated that targeting not only Tregs but also other immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs), could produce better anti-tumor effects.
V-domain immunoglobulin suppressor of T cell activation (VISTA), a member of the B7 family, is a novel immunomodulatory receptor that maintains the quiescent state of T cells and myeloid cells and has been reported to be abundantly expressed in human and mouse myeloid compartments, especially in MDSCs and Tregs of tumor-infiltrating lymphocytes (TILs) [7, 8]. Although VISTA is mainly expressed in immune cells in the TME, it has also been reported to be expressed in tumor cells of lung, kidney, colon, endometrial, and ovarian cancers in humans [9–12]. However, the expression of VISTA in mouse cancer cell lines is considered rare [13]. A previous study using syngeneic mice showed that frequent administration of an anti-VISTA-monoclonal antibody (mAb) decreased MDSCs and Tregs, resulting in tumor suppression [8]. This data have led to clinical trials using anti-VISTA antibodies as a checkpoint inhibitor for solid tumors [14]. Using IRDye 700DX NHS ester (IR700)-conjugated anti-VISTA-mAb as an APC, this study investigates whether VISTA-targeted NIR-PIT can suppress tumor growth by decreasing immune suppressor cells in the TME of immunocompetent mice.
Materials and methods
Reagents
The water-soluble, silica-phthalocyanine derivative IRDye 700DX NHS ester (IR700) was obtained from LI-COR Biosciences (Lincoln, NE). An anti-mouse VISTA mAb (clone 13F3) was purchased from Bio X Cell (West Lebanon, NH, USA). All other chemicals were of reagent grade.
Synthesis of IR700-conjugated anti-VISTA mAb
Conjugation of dye with mAb was performed according to a previous report [15]. In brief, anti-VISTA mAb (1.0 mg, 6.7 nmol) was incubated with IR700 NHS ester (65.1 μg, 33.3 nmol, 10 mmol/L in DMSO) in 0.1 mol/L Na2HPO4 (pH 8.5) at room temperature for 1 h. The mixture was purified with a Sephadex G25 column (PD-10; GE Healthcare, Piscataway, NJ, USA). The protein concentration was determined by measuring the absorption with UV–Vis (8453 Value System; Agilent Technologies, Santa Clara, CA, USA). We abbreviate IR700 conjugated to anti-VISTA mAb as anti-VISTA-IR700. The success of conjugation was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with a 4–20% gradient polyacrylamide gel (Life Technologies, Gaithersburg, MD, USA). Non-conjugated antibody was used for control. After electrophoresis at 80 V for 2 h, the gel was imaged with a Pearl Imager (LI-COR Biosciences) using the 700 nm fluorescence channel. The gel was then stained with Colloidal Blue to compare the molecular weight of the conjugated antibody to that of the non-conjugated antibody.
Cell culture
MC38 cells (murine colon cancer) were generously provided by Dr. Thomas Waldmann, NIH. MC38 cells stably expressed luciferase via stable transduction with RediFect Red-Fluc lentivirus from PerkinElmer (Waltham, MA, USA) per manufacturer recommendations. Luciferase-expressing MC38 cancer cell lines, abbreviated as MC38-luc, were used in this study. LL2-luc (lung cancer) cells were purchased from Imanis Life Sciences (Rochester, MN, USA). EL4 cells (lymphoma) were purchased from ATCC (Manassas, VA, USA). MC38-luc and LL2-luc cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). EL4 cells were cultured in DMEM medium (ATCC) supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin (Thermo Fisher Scientific). Cells were maintained in culture for no more than 30 passages and tested for mycoplasma.
VISTA expression on cell lines in vitro
Anti-VISTA mAb (13F3) was conjugated with Alexa Fluor 647 NHS Ester (Alexa647, Thermo Fisher Scientific). The conjugation was performed with the same method used in IR700 conjugation. Alexa647-conjugated anti-VISTA mAb is abbreviated as anti-VISTA-Alexa647. One million EL4, MC38-luc, or LL2-luc cells were incubated with the anti-VISTA-Alexa647 or a control-Alexa647 (Alexa647-conjugated hamster IgG, clone HTK888, BioLegend, San Diego, CA, USA) and LIVE/DEAD Fixable Dead Cell Stain (Thermo Fisher Scientific) for 1 h at 4 °C. The stained cells were analyzed with FACSLyric (BD Biosciences, San Jose, CA, USA) and FlowJo software (BD Biosciences).
Microscopic observation of NIR-PIT
To detect the effect of VISTA-targeted NIR-PIT, microscopic observation was performed (IX81; Olympus America, Inc.). EL4 cells were used for this experiment. Anti-VISTA-IR700 at 10 μg/mL was added to the cell suspension with staining buffer (PBS containing no calcium or magnesium with 1% FBS) and incubated for 1 h at 4 °C. After washing, cells were re-suspended with staining buffer. Then, cells were seeded on a glass slide overlaid with a cover glass. NIR laser-light (690 nm, 150 mW/cm2) using an ML7710 laser system (Modulight, Tampere, Finland) was applied at 50 J/cm2. Serial transmitted light differential interference contrast (DIC) images were acquired.
In vitro NIR-PIT against cancer cell lines
The efficacy of VISTA-targeted NIR-PIT was evaluated with three cell lines. EL4 cells were seeded at 2 × 105 per well in quadruplicate onto a 12-well plate and incubated in fresh culture medium containing 10 µg/mL of anti-VISTA-IR700 for 1 h at 37 °C. The cell suspension was transferred to a FACS tube, washed with PBS, and phenol red-free medium was added. NIR laser-light (690 nm, 150 mW/cm2) using an ML7710 laser system was applied at 0, 5, 10, 25 and 50 J/cm2. MC38-luc and LL2 cells were seeded at 2 × 105 per well in quadruplicate onto 12-well plates in 2 mL of medium and incubated for 24 h. The cells were then incubated with fresh culture medium containing 10 µg/mL of anti-VISTA-IR700 for 1 h at 37 °C. After washing with PBS, phenol red-free medium was added. NIR laser-light (690 nm, 150 mW/cm2) using an ML7710 laser system was applied at 50 J/cm2. Immediately after NIR-PIT, the cytotoxic effects of NIR-PIT with anti-VISTA-IR700 were determined by propidium iodide (PI, Life Technologies, Carlsbad, CA, USA) staining as follows. Cells were stained with 1 μg/mL of PI; the percentage of cells stained with PI was analyzed by FACSLyric and FlowJo software.
VISTA expression ex vivo
To evaluate the ex vivo VISTA expression, established untreated tumors were harvested when they reached a volume of approximately 200 mm3. Single-cell suspensions from tumor samples were prepared using the following protocol: Whole tumors were minced and incubated in media containing collagenase type IV (1 mg/mL, Worthington Biochemical, Lakewood, NJ, USA) and DNaseI (20 μg/mL, Millipore Sigma, Burlington, MA, USA) at 37 °C for 1 h. Tumors were then gently dissociated and filtered with 70 μm cell strainers (Corning, Corning, NY, USA). 3.0 × 106 cells were stained and data for 3.0 × 105 cells were collected for each tumor. The cells were stained with the following antibodies: anti-CD3ε (clone 145-2C11) and anti-Ly6G (clone 1A8) were obtained from Biolegend; anti-CD45 (clone 30-F11), anti-CD8α (clone 53–6.7), anti-Foxp3 (clone FJK-16s), anti-CD4 (clone RM4-5), CD11b (clone M1/70), and Ly6C (clone HK1.4) were obtained from eBioscience. To assess surface VISTA expression, the cells were stained with anti-VISTA-Alexa647 or control-Alexa647 before permeabilization. Foxp3 was stained after fixation and permeabilization using the Foxp3 Transcription Factor Staining Buffer Set (eBioscience). Dead cells were removed from analysis based on fsc, ssc, and staining with Fixable Viability Dye (eBioscience). The stained cells were analyzed with FACSLyric, and the data were analyzed with FlowJo software.
Ex vivo NIR-PIT
Single-cell suspensions from MC38-luc tumors were prepared using the method described above. One million cells were incubated with or without 10 µg/mL of anti-VISTA-IR700 for 1 h at 37 °C. After washing the cells with PBS, NIR light (690 nm, 150 mW/cm2) was applied at 0, 20, or 50 J/cm2 (n = 4). After 1 h, the live cell percentage of each cell population was analyzed with a flow cytometer after staining.
In vivo NIR-PIT experiments
All animal experiments were approved by the local Animal Care and Use Committee. Six- to 8- week-old female C57BL/6 mice (strain #000664) were purchased from The Jackson Laboratory. For MC38-luc and LL2-luc, we established tumors by subcutaneously injecting 1.0 × 106 cells in the right caudal flank area. During NIR-PIT and fluorescence/bioluminescence imaging (BLI), mice were anesthetized with 2–3% isoflurane inhalation and/or intraperitoneal injection of 1 mg sodium pentobarbital (Nembutal Sodium Solution, Ovation Pharmaceuticals Inc.). The hair above the tumor site was removed for NIR light irradiation and imaging. IR700 fluorescence images of the dorsal surface of mice were acquired before and after NIR-PIT using the 700 nm fluorescence channel of Pearl Imager (LI-COR Biosciences). Pearl Cam Software (LI-COR Biosciences) was used to analyze the fluorescence of the regions of interest (ROIs) on the tumor. The acute effects of treatment were evaluated by BLI, in which 200 µL of 15 mg/mL D-luciferin was administered intraperitoneally and luciferase activity was analyzed using Photon Imager (Biospace Laboratory, Nesles la Vallée, France) and M3 Vision Software (Biospace Laboratory). The ROIs were set to cover the entire tumor. To calculate the tumor volume, the greatest longitudinal diameter (length) and the greatest transverse diameter (width) were measured using a caliper. Tumor volume was calculated as follows: tumor volume = length × width2 × 0.5. Tumor size was measured three times a week until the tumor volume reached 2,000 mm3 or the length reached 2 cm, after which the mice were euthanized by carbon dioxide inhalation.
To evaluate the efficacy of VISTA-targeted NIR-PIT for MC38-luc or LL2-luc tumors, tumor-bearing mice were randomized into three groups: (1) no treatment (Control); (2) anti-VISTA-IR700 APC i.v., no NIR light irradiation (APC iv); (3) anti-VISTA-IR700 i.v., NIR light irradiation (NIR-PIT). 100 μg anti-VISTA-IR700 was injected 6 days after inoculating cancer cells, and NIR light (690 nm, 150 mW/cm2, 50 J/cm2) was administered 7 days after inoculating cancer cells. To assess the immune reaction in the regional lymph nodes, the nodes were harvested 2 days after NIR-PIT for MC38-luc tumors. The cells were stained with the following antibodies: anti-CD3ε (clone 145-2C11) anti-CD25 (clone PC61), anti-CD86 (clone GL-1), anti-F4/80 (clone BM8), and anti-I-A/I-E (clone M5/114.15.2) were obtained from Biolegend; anti-CD45 (clone 30-F11), anti-CD8α (clone 53–6.7), anti-CD69 (clone H1.2F3), anti-CD40 (clone 1C10), and anti-CD80 (clone 16-10A1) were obtained from eBioscience. Dead cells were removed from analysis based on fsc, ssc, and staining with Fixable Viability Dye (eBioscience). The stained cells were evaluated with FACSLyric, and the data were analyzed with FlowJo software.
Histologic analysis
One hour after NIR-PIT, tumors were harvested, formalin fixed and paraffin-embedded, and thinly sectioned. Following standard hematoxylin and eosin (H–E) staining, bright-light photomicrographs were obtained using Mantra Quantitative Pathology Workstation (Akoya Biosciences).
Statistical analysis
Data are expressed as means ± SEM unless otherwise indicated. Statistical analysis was performed with GraphPad Prism (GraphPad Software, La Jolla, CA, USA). For a two-group comparison with one-time measurement, Mann–Whitney test was used. For multiple-group comparison with one-time measurement, a one-way analysis of variance (ANOVA) followed by Tukey’s test was used. For comparison of tumor volumes, a two-way repeated measures ANOVA followed by Tukey’s test was used. P values less than 0.05 were considered significant.
Results
APC synthesis of anti-VISTA-IR700
We verified the conjugation of IR700 to anti-VISTA mAb using SDS-PAGE. Anti-VISTA-IR700 and non-conjugated anti-VISTA mAb had approximately the same molecular weight, and IR700 fluorescence was only observed with anti-VISTA-IR700 (Fig. 1a).
Fig. 1.
Conjugation of IR700 to anti-VISTA-mAb and evaluation of in vitro NIR-PIT. a Evaluation of anti-VISTA-IR700 by SDS-PAGE (left: Colloidal Blue staining, right: 700 nm fluorescence). Uncondugated anti-VISTA-mAb was used as a control. b The binding of anti-VISTA-Alexa647 was tested on EL4 cells, analyzed by flow cytometry. c EL4 cells were incubated with anti-VISTA-IR700 for 1 h and irradiated with NIR light. Bleb formations are indicated by yellow arrows. Scale bar = 10 µm. d In vitro VISTA-targeted NIR-PIT against EL4 cells. EL4 cells were incubated with anti-VISTA-IR700 and irradiated with NIR light at various doses. (n = 4; ****, P < 0.0001; vs. untreated control; one-way ANOVA followed by Dunnett's test). e The binding of anti-VISTA-Alexa647 was tested on MC38-luc cells, analyzed by flow cytometry. f MC38-luc cells were incubated with anti-VISTA-IR700 and irradiated with NIR light in 50 J/cm2 (n = 4; ns, not significant; Mann–Whitney test). g The binding of anti-VISTA-Alexa647 was tested on LL2-luc cells, analyzed by flow cytometry. h LL2-luc cells were incubated with anti-VISTA-IR700 and irradiated with NIR light in 50 J/cm2 (n = 4; ns, not significant; Mann–Whitney test). Each value represents mean ± SEM of independent experiment.
VISTA expression on cell lines and In vitro NIR-PIT
We employed EL4 cells as the VISTA-expressing model. VISTA was expressed on the surface of EL4 cells. (Fig. 1b). We tested the cytotoxic effects of VISTA-targeted NIR-PIT on EL4 cells in vitro. After incubation of EL4 cells with anti-VISTA-IR700 for 1 h, NIR light induced cell swelling, bleb formation, and vesicle rupture, indicating necrotic cell death, typical of NIR-PIT responses (Fig. 1c). The cytotoxic effects of VISTA-targeted NIR-PIT on EL4 cells were quantitatively assessed by PI staining. PI positivity showed an increase in cell membrane damage in a light dose-dependent manner (Fig. 1d). As we aim to perform NIR-PIT on non-cancer cells in TME, a non-VISTA expressing cancer cell line is an important negative control. We observed no VISTA expression in MC38-luc and LL2-luc cell lines, and VISTA-targeted NIR-PIT against these cells did not show cytotoxicity (Fig. 1e–h). Therefore, MC38-luc or LL2-luc cells were used in the following experiments as tumor models.
VISTA expression in TME and ex vivo effects of VISTA-targeted NIR-PIT
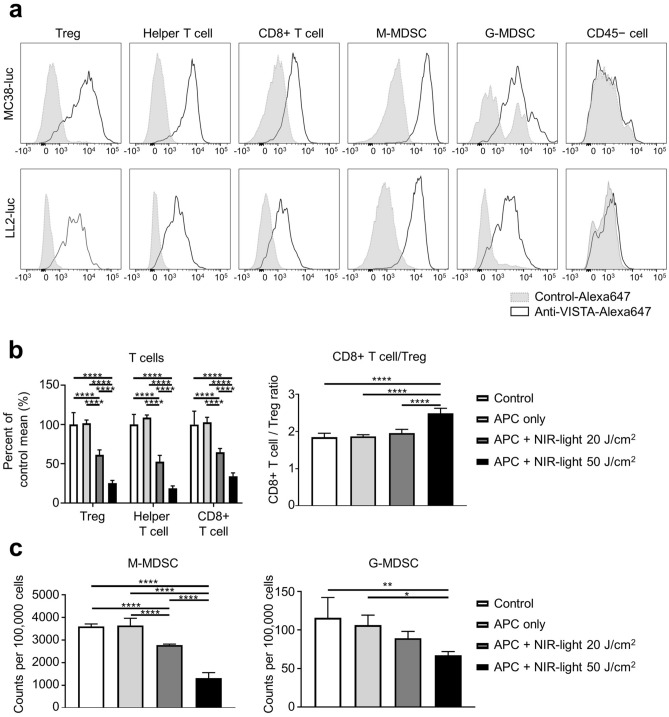
We tested the surface expression of VISTA in cells of the TME to predict which cell populations would be killed by VISTA-targeted NIR-PIT. Flow cytometry results showed that VISTA was expressed on T cells and MDSCs, but not on the surface of CD45-negative cells, including cancer cells (both MC38-luc and LL/2-luc tumors). (Fig. 2a). Next, to verify whether VISTA-targeted NIR-PIT can decrease T cells and MDSCs, we performed NIR-PIT ex vivo. Without NIR light, there was no significant difference between control and APC only (Fig. 2b and c). With APC and NIR light, the count of live T cells and MDSCs decreased in a light dose-dependent manner (Fig. 2b and c). The CD8 + T cells/Tregs ratio, which is considered to be an indicator of robust immune activity, was higher in the 50 J/cm2 NIR light irradiation group than in the other groups (Fig. 2b). The results showed that VISTA-targeted NIR-PIT decreased both CD8 + T cells and non-Treg CD4 + T cells along with immunosuppressive cells such as Treg and MDSC. However, higher CD8 + T cells/Tregs ratio at 50 J/cm2 NIR light suggested that Tregs were more affected than CD8 + T cells.
Fig. 2.
VISTA expression in the tumor microenvironment and ex vivo VISTA-targeted NIR-PIT. Immune cell populations were analyzed by flow cytometry. CD45 + CD3 + CD4 + Foxp3 + cells were defined as Tregs, CD45 + CD3 + CD4 + Foxp3 − cells were defined as helper T cells, CD45 + CD3 + CD8 + cells were defined as CD8 + T cells, CD45 + CD11b + Ly6C + Ly6G − cells were defined as monocytic MDSCs (M-MDSCs), CD45 + CD11b + Ly6C − Ly6G + cells were defined as granulocytic MDSCs (G-MDSCs). a VISTA expression in T lymphocytes (Treg, helper T cell, CD8 + T cell), MDSCs (M-MDSC, G-MDSC), and CD45 − cells populations in MC38-luc and LL2-luc tumors were analyzed by flow cytometry. Histograms for surface VISTA expression and isotype control are shown. b T lymphocyte cells after ex vivo VISTA-targeted NIR-PIT against MC38-luc tumors (n = 4; ****, P < 0.0001; one-way ANOVA followed by Dunnett's test). c MDSCs after ex vivo NIR-PIT against MC38-luc tumors (n = 4; ****, P < 0.0001; One-way ANOVA followed by Dunnett's test)
In vivo VISTA-targeted NIR-PIT in two tumor models
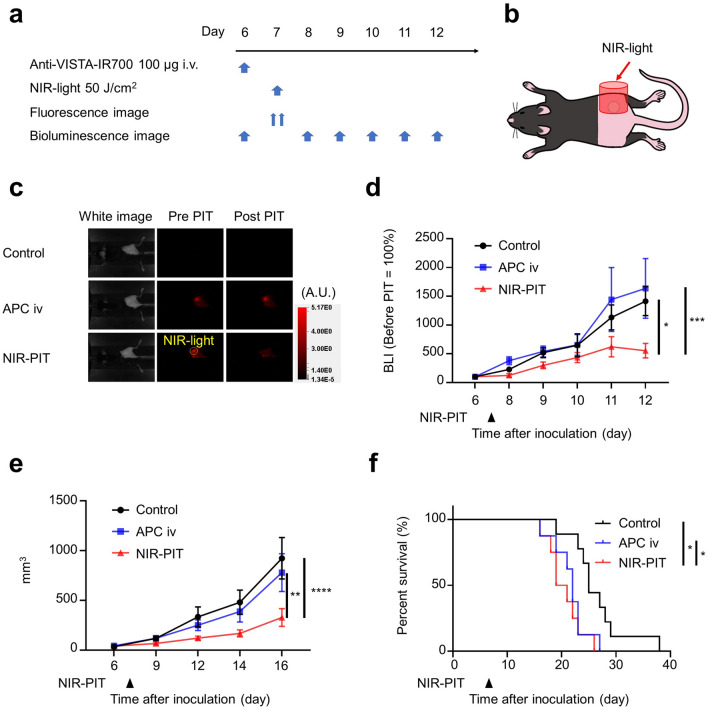
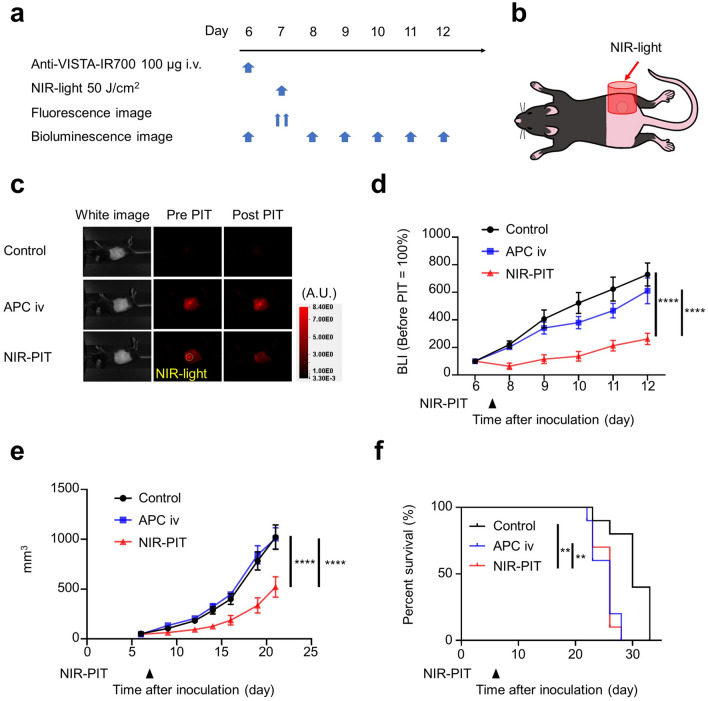
To confirm the anti-tumor effect of VISTA-targeted NIR-PIT, we conducted in vivo experiments with MC38-luc and LL2-luc tumors. The NIR-PIT regimen and imaging protocol are depicted in Figs. 3a and 4a. One day after injection of anti-VISTA-IR700, the tumors were exposed to 50 J/cm2 of NIR light (Figs. 3b and 4b). After NIR light irradiation, the fluorescence in the tumor area decayed to the same level as background in NIR-PIT groups in both tumor models (Figs. 3c and 4c), suggesting that a sufficient amount of light was given to photobleach the IR700 dye, an indicator of dye activation. The decrease in BLI signal, indicating killing of cancer cells, was more significant in the NIR-PIT group than in the other groups on day 12 in both tumor models (Figs. 3d and 4d). One hour after NIR-PIT, H-E staining showed no histological change in the tumor tissue (Supplemental Fig. 1). This result suggested that VISTA-targeted NIR-PIT did not directly damage the tumor cells but induced anti-tumor effect via the host immune response. Post-treatment tumor volumes of the NIR-PIT groups were significantly smaller than untreated and APC iv only groups on day 16 in MC38-luc tumors and on day 22 in LL2-luc tumors (Figs. 3e and 4e), and survival was significantly prolonged in the NIR-PIT group in both tumor models (Figs. 3f and 4f). Thus, VISTA-targeted NIR-PIT targeting non-cancer cells showed an anti-tumor effect in both models.
Fig. 3.
VISTA-targeted NIR-PIT suppressed tumor progression of MC38-luc tumors. a VISTA-targeted NIR-PIT regimen. Anti-VISTA-IR700 was administered through the tail vein, and NIR light irradiation was performed the following day. b Diagram of NIR light irradiation. NIR light was irradiated locally only on the tumor. c Fluorescent imaging before and after NIR-PIT in MC38-luc tumor-bearing mouse. d Luciferase activity calculated from BLI before (day 6) and after (days 8–12) NIR-PIT in MC38-luc tumor-bearing mice (n = 10; two-way ANOVA followed by Tukey’s test; *, P < 0.05; ***, P < 0.001; vs control group). e Tumor volume curves (n = 10; two-way ANOVA followed by Tukey’s test; **, P < 0.01; ****, P < 0.0001). f Survival curves (n = 10; log-rank test with Bonferroni correction; *, P < 0.05)
Fig. 4.
VISTA-targeted NIR-PIT suppressed tumor progression of LL2-luc tumors. a VISTA-targeted NIR-PIT regimen. Anti-VISTA-IR700 was administered through the tail vein, and NIR light irradiation was performed the following day. b Diagram of NIR light irradiation. NIR light was irradiated locally only on the tumor. c Fluorescent imaging before and after NIR-PIT in LL2-luc tumor-bearing mouse. d Luciferase activity calculated from BLI before (day 6) and after (days 8–12) NIR-PIT in LL2-luc tumor-bearing mice (n = 10; two-way ANOVA followed by Tukey’s test; ****, P < 0.0001; vs control group). e Tumor volume curves (n = 10; two-way ANOVA followed by Tukey’s test; ****, P < 0.0001). f Survival curves (n = 10; log-rank test with Bonferroni correction; **, P < 0.01)
Activation of CD8 + T cells and dendritic cells (DCs) by VISTA-targeted NIR-PIT
We investigated the state of immune activation after VISTA-targeted NIR-PIT to determine if removing suppressor cells from the TME resulted in anti-tumor immune activation. Two days after VISTA-targeted NIR-PIT, regional lymph nodes were analyzed by flow cytometry. First, we tested expression of DC activation markers CD80, CD86, and CD40. The relative fluorescence intensity (RFI) of CD80 on DCs relative to the control group was higher in the NIR-PIT group than in the other groups (Fig. 5a), whereas there was no significant change in CD86 and CD40 expression. Next, we tested CD8 + T cell activation markers CD69 and CD25. CD69 + /CD8 + T cell ratios in the NIR-PIT group were higher than in other groups (Fig. 5b), whereas CD25 expression was unchanged. These results suggested that anti-tumor immunity was activated by reducing the number of Tregs and MDSCs in the TME following VISTA-targeted NIR-PIT.
Fig. 5.
Immune cell response after the VISTA-targeted NIR-PIT. The regional lymphoid nodes were harvested 2 days after VISTA-targeted NIR-PIT and FACS analysis was performed. Cell phenotypes were defined based on the antigen expressions as the following: CD45 + F4/80 − CD11c + I-A/I-E + = DC cell, CD45 + CD3 + CD8 + = CD8 + T cell. a The expression of activation markers on DCs (n = 5–6; one-way ANOVA followed by Tukey’s test; ****, P < 0.0001; ns, not significant). b The expression of activation markers on CD8 + T cells (n = 5–6; one-way ANOVA followed by Tukey’s test; **, P < 0.01; ns, not significant)
Discussion
VISTA-targeted NIR-PIT activated anti-tumor immunity by decreasing Tregs and MDSCs from the TME. This resulted in tumor shrinkage and prolonged survival in NIR-PIT treated animals. Further, we showed that VISTA was expressed on T cells including Tregs, and MDSCs, but not on CD45 − cells including cancer cells in the TME. Ex-vivo VISTA-targeted NIR-PIT decreased the number of VISTA-expressing T cells and MDSCs in the TME.
A higher CD8 + T cells/Tregs ratio in the TME is an indicator of a robust anti-tumor immune response and portends a better prognosis [6–18]. VISTA-targeted NIR-PIT resulted in an increase in the CD8 + T cells/Tregs ratio. This was likely because VISTA-targeted NIR-PIT at 50 J/cm2 decreased Tregs more robustly than CD8 + T cells, and created a more favorable CD8 + T cell-dominant TME that can augment immune activation, suppress tumor growth, and prolong survival.
NIR-PIT has been shown to be effective with a variety of antibodies targeting tumor antigens [e.g., EGFR, human epidermal growth factor receptor 2(HER2), CD44, and prostate-specific membrane antigen (PSMA), etc.] [1, 19–21]. NIR-PIT can also be used to eliminate subpopulations of cells in the TME to enhance anti-tumor immunity [22]. Previously, CD25-targeted NIR-PIT and CTLA4-targeted NIR-PIT [4, 6], which mainly target Tregs [23], showed impressive tumor suppressive effects. NIR-PIT can dramatically alter the local immune environment and is a promising new tool in experimental immunobiology. MDSCs are widely known as suppressor cells [24, 25], but so far, there is no report of NIR-PIT targeting MDSCs. A previous study has shown that repeated administration of anti-mouse VISTA mAb (clone 13F3) decreased Tregs and MDSCs and showed anti-tumor effects [8]. However, in this study, a single dose of anti-VISTA-IR700 followed by just one dose of NIR light irradiation showed therapeutic effects, suggesting that dramatic changes in local tumor immunity can lead to robust anti-tumor effects.
There are several limitations to this study. First, we used only one clone of an Armenian hamster derived anti-VISTA mAb. The results may differ if other clones of anti-VISTA mAb are used. Second, in this study, we only performed experiments using subcutaneous tumor models because it is technically easier to measure tumor size, create stable tumors, and take images. However, the orthotopic tumor models would be preferable because they can represent more organ-specific TME depending on the type of cancer, thus having more clinical significance than the subcutaneous tumor models [26, 27]. Therefore, further studies are needed to show if VISTA-targeted NIR-PIT is effective in the orthotopic tumor mouse models. Third, we performed VISTA-targeted NIR-PIT targeting immune cells rather than cancer cells. VISTA has been reported to be expressed in some cancers [9–12], and VISTA-targeted NIR-PIT may be useful in such tumors. VISTA is expressed in mouse and human microglia, which are the major myeloid cells of the central nervous system (CNS). Therefore, it would be important to monitor for potential adverse events in the CNS when performing NIR-PIT targeting VISTA near the CNS [28]. Since we treated subcutaneous tumors distant from the CNS, we were not able to evaluate adverse events in the CNS, and further studies are needed.
In conclusion, this is the first study to demonstrate the efficacy of VISTA-targeted NIR-PIT to deplete immunosuppressive cells in the TME resulting in the activation of T cell host immunity and an anti-tumor response culminating in prolonged survival.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (ZIA BC 011513). FI was also supported with a Grant from National Center for Global Health and Medicine Research Institute, Tokyo, Japan.
Declarations
Conflicts of interest
No potential conflicts of interest were disclosed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi H, Choyke PL. Near-infrared photoimmunotherapy of cancer. Acc Chem Res. 2019;52:2332–2339. doi: 10.1021/acs.accounts.9b00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato K, Ando K, Okuyama S, et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: a mechanism of cancer cell cytotoxicity after near-infrared photoimmunotherapy. ACS Cent Sci. 2018;4:1559–1569. doi: 10.1021/acscentsci.8b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL, Hasegawa Y, Kobayashi H. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci Transl Med. 2016;8:352ra110. doi: 10.1126/scitranslmed.aaf6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada R, Maruoka Y, Furusawa A, Inagaki F, Nagaya T, Fujimura D, Choyke PL, Kobayashi H. The effect of antibody fragments on CD25 targeted regulatory T cell near-infrared photoimmunotherapy. Bioconjug Chem. 2019;30:2624–2633. doi: 10.1021/acs.bioconjchem.9b00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada R, Kato T, Furusawa A, Inagaki F, Wakiyama H, Choyke PL, Kobayashi H. Local depletion of immune checkpoint ligand CTLA4 expressing cells in tumor beds enhances antitumor host immunity. Adv Ther (Weinh) 2021;4:2000269. doi: 10.1002/adtp.202000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L. VISTA regulates the development of protective antitumor immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villarroel-Espindola F, Yu X, Datar I, et al. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24:1562–1573. doi: 10.1158/1078-0432.CCR-17-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong S, Yuan Q, Xia H, et al. Analysis of VISTA expression and function in renal cell carcinoma highlights VISTA as a potential target for immunotherapy. Protein Cell. 2019;10:840–845. doi: 10.1007/s13238-019-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie S, Huang J, Qiao Q, Zang W, Hong S, Tan H, Dong C, Yang Z, Ni L. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother. 2018;67:1685–1694. doi: 10.1007/s00262-018-2227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulati K, Hamanishi J, Matsumura N, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer. 2019;120:115–127. doi: 10.1038/s41416-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan L, Tatineni J, Mahoney KM, Freeman GJ. VISTA: a mediator of quiescence and a promising target in cancer immunotherapy. Trends Immunol. 2021;42:209–227. doi: 10.1016/j.it.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov Phase 1 Study of CI-8993 Anti-VISTA Antibody in Patients With Advanced Solid Tumor Malignancies. https://clinicaltrials.gov/ct2/show/study/NCT04475523. Accessed 11 Feb 2021
- 15.Maruoka Y, Furusawa A, Okada R, et al. Combined CD44- and CD25-targeted near-infrared photoimmunotherapy selectively kills cancer and regulatory T cells in syngeneic mouse cancer models. Cancer Immunol Res. 2020;8:345–355. doi: 10.1158/2326-6066.CIR-19-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Nat Acad Sci. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baras AS, Drake C, Liu JJ, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology. 2016;5:e1134412. doi: 10.1080/2162402X.2015.1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Allen C, Kobayashi H. Syngeneic mouse models of oral cancer are effectively targeted by anti-CD44-based NIR-PIT. Mol Cancer Res. 2017;15:1667–1677. doi: 10.1158/1541-7786.MCR-17-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Kobayashi H. Near-infrared photoimmunotherapy targeting prostate cancer with prostate-specific membrane antigen (PSMA) antibody. Mol Cancer Res. 2017;15:1153–1162. doi: 10.1158/1541-7786.MCR-17-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakiyama H, Kato T, Furusawa A, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy of cancer; possible clinical applications. Nanophotonics. 2021;10:3135–3151. doi: 10.1515/nanoph-2021-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato T, Wakiyama H, Furusawa A, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy: a review of targets for cancer therapy. Cancers. 2021 doi: 10.3390/cancers13112535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression: implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–359. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 28.Borggrewe M, Grit C, Den Dunnen WFA, Burm SM, Bajramovic JJ, Noelle RJ, Eggen BJL, Laman JD. VISTA expression by microglia decreases during inflammation and is differentially regulated in CNS diseases. Glia. 2018;66:2645–2658. doi: 10.1002/glia.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.