Abstract
Monocytes (Mo) and macrophages (Mφ) play important roles in the function of tissues, organs, and systems of all animals during homeostasis, infection, injury, and disease. For decades, conventional wisdom has dictated that Mo and Mφ are end-stage cells that do not proliferate and that Mφ accumulation in tissues is the result of infiltration of Mo from the blood and subsequent differentiation to Mφ. However, reports from the early 1900s to the present describe evidence of Mo and Mφ proliferation in different tissues and contexts. The purpose of this review is to summarize both historical and current evidence for the contribution of Mφ proliferation to their accumulation in different tissues during homeostasis, infection, injury, and disease. Mφ proliferate in different organs and tissues, including skin, peritoneum, lung, heart, aorta, kidney, liver, pancreas, brain, spinal cord, eye, adipose tissue, and uterus, and in different species including mouse, rat, rabbit, and human. Mφ can proliferate at different stages of differentiation with infiltrating Mo-like cells proliferating in certain inflammatory contexts (e.g. skin wounding, kidney injury, bladder and liver infection) and mature resident Mφ proliferating in other inflammatory contexts (e.g. nematode infection, acetaminophen liver injury) and during homeostasis. The pathways involved in stimulating Mφ proliferation also may be context dependent, with different cytokines and transcription factors implicated in different studies. Although Mφ are known to proliferate in health, injury, and disease, much remains to be learned about the regulation of Mφ proliferation in different contexts and its impact on the homeostasis, injury, and repair of different organs and tissues.
Keywords: cell proliferation, inflammation, macrophage, monocyte
In this review, we summarize historical and current evidence for, and provide perspective on, the proliferation of monocytes and macrophages in different tissues during homeostasis, infection, injury, and disease.
1. Introduction
Monocytes (Mo) and macrophages (Mφ) play important roles in the function of tissues, organs, and systems of all animals. Mo are generated in the bone marrow and are mobilized to play critical roles in the response to tissue injury and infection.1,2 Tissue-resident Mφ arise from embryonic precursors as well as from bone marrow Mo, and these cells play important roles in development, tissue homeostasis, and the resolution of inflammation after injury and infection.3,4 In contrast to these positive roles, dysregulated Mo and Mφ can cause pathology, including poor infection control, impaired wound healing, tissue fibrosis, atherosclerosis, and tumor growth.5–9 However, much remains to be learned about the regulation of the diverse Mo and Mφ functions in homeostasis, injury, infection, and disease. Improved understanding of the regulation of these functions would lead to more specific targeting in a vast array of pathologies.
In the late 1960s, van Furth et al.10,11 proposed the mononuclear phagocyte system (MPS) as a way to classify Mo and Mφ along with Mo precursors based on similar morphology, origin, function, and kinetics. The MPS considers Mφ as end-stage cells that are differentiated from Mo, which, in turn, are differentiated from bone marrow precursors. One inference from this model is that resident tissue Mφ are thought to be derived from blood Mo. This concept has been challenged and has recently received intense scrutiny. Over the past 15 yr, an overall consensus has developed that resident Mφ in some tissues (e.g. microglia in brain and Langerhans cells in epidermis)12,13 are primarily derived from embryonic precursors that self-renew without input from blood Mo whereas resident Mφ in barrier tissues like the gut and dermis turn over more rapidly and are populated in adult animals by blood Mo.14,15 In still other tissues, like lung and liver, resident Mφ are thought to be a mix of embryo-derived and Mo-derived cells.15–17
Another inference from the MPS model is that, whereas bone marrow Mo precursors can proliferate, Mo lack the ability to proliferate after mobilization into the blood, and consequently Mo-derived Mφ are postmitotic. Thus, the prevailing view based on the MPS model has dictated that Mφ accumulation in tissues is the result of infiltration of Mo from the blood and subsequent differentiation to Mφ, particularly during the response to injury or infection. This concept was challenged recently with the demonstration that accumulation of Mφ in response to nematode infection occurs primarily via proliferation of resident cells.18 In fact, a review of the literature revealed reports from the early 1900s to the present describing evidence of Mφ proliferation. The purpose of this review is to summarize both historical and current evidence for the contribution of Mφ proliferation to their accumulation in different tissues in homeostasis, infection, injury, and disease. In addition, we posit future directions that we think important for better understanding of the role and regulation of Mφ proliferation in different physiological and pathological contexts.
2. Historical perspectives
2.1. Evidence against proliferation in the periphery
The conventional wisdom that peripheral Mo and Mφ do not proliferate was derived from studies that formed the basis for the MPS concept. In a series of studies, van Furth et al.10,19 reported that in vivo injection of H3-thymidine into mice under homeostatic conditions resulted in labeling of only ∼3% of blood Mo and ∼1% of peritoneal Mφ 1 h after H3-thymidine injection, a time point that was likely too short for significant bone marrow Mo mobilization. At the same time, ∼20% of bone marrow mononuclear cells were labeled, indicating active proliferation. Labeling of blood Mo and peritoneal Mφ increased over time and peaked 48 to 60 h after injection of H3-thymidine, indicating that progeny of proliferating bone marrow progenitors that had been mobilized from the bone marrow likely were responsible for the increased labeling. These in vivo studies were corroborated by in vitro studies of homeostatic cells showing that no blood Mo and only ∼2% of peritoneal Mφ incorporate H3-thymidine when cultured with the nucleoside for 24 h,10,19 whereas ∼30% of bone marrow mononuclear cells were labeled in similar experiments. In follow-up studies using intraperitoneal injection of newborn calf serum in mice as a model of inflammation, although blood Mo and peritoneal Mφ increased during the inflammatory response, the percentage of labeled cells remained close to homeostatic levels.19 These findings indicated that bone marrow Mo are the source of the increased Mo and Mφ during inflammation as well as homeostasis.
Volkman20 performed similar experiments using intraperitoneal injection of glycogen as an inflammatory stimulus in rats and found that 1% to 2% of peritoneal Mφ incorporate H3-thymidine 1 h after in vivo injection or 24 h after in vitro incubation. Additionally, in studies using Salmonella infection in rats, 0.1% to 2% of blood Mo were labeled when assessed 30 min after H3-thymidine injection, with labeling increasing to the high end of that range 4 to 5 d after infection.21 Volkman, van Furth, and others also performed a series of irradiation, parabiosis, and adoptive transfer experiments that implicated bone marrow Mo as the precursors to tissue Mφ, providing further evidence that proliferating bone marrow precursors are the primary source of blood Mo and ultimately tissue Mφ, in both homeostasis and inflammatory responses.
2.1.1. Early Evidence for Proliferation
Despite the prevailing view that blood Mo and tissue Mφ are end-stage cells that do not proliferate, even the studies that are primary underpinnings of the MPS showed a low level of H3-thymidine incorporation, typically 1% to 3% of the population.10,20 Evidence for proliferation of tissue Mφ was published as early as 1914 by Evans et al.,22 when injection of Trypan blue dye into rabbits was found to be taken up by liver Kupffer cells associated with rare morphological evidence of mitosis, a process that was amplified by infection with Mycobacterium tuberculosis. Using different vital dyes and a rabbit ear wound chamber to visualize Mo and Mφ in vivo, Ebert and Florey23 reported evidence that blood Mo are the primary source of wound Mφ in vivo, and morphological evidence of proliferation in the wound.
Additionally, Mackaness24 reported indirect evidence that resistance to Listeria reinfection in mice may result from proliferation of a resistant Mφ population, and Forbes and Mackaness25 reported more direct evidence that immunization followed by reinjection of albumin in mice induced peritoneal Mφ proliferation assessed by in vitro incorporation of H3-thymidine; >50% of Mφ incorporated H3-thymidine after 1 h incubation when Mφ were obtained from immunized mice compared with ∼1% in Mφ from naive mice. Forbes26 also reported the endotoxin injection into mice induced proliferation of peritoneal Mφ, assessed by in vitro H3-thymidine incorporation, which increased from <1% for control mice to 5% to 10% when Mφ were harvested 2 to 3 d after endotoxin injection and further reported that various other stimulants could induce peritoneal Mφ proliferation, including repeated puncture and mouse or rabbit serum.27 Morphologic evidence for proliferation was also observed in Mφ from the experimental mice in all these studies. Furthermore, thioglycolate-elicited peritoneal Mφ from mice have been shown to proliferate and form colonies in liquid culture as assessed by cell counting and H3-thymidine incorporation.28
Early reports also provided evidence of proliferation of liver and alveolar Mφ. North29 reported that infection of mice with Listeria monocytogenes resulted in increased local proliferation of liver sinusoid Mφ peaking on day 2 after infection, with 20% of Mφ incorporating H3-thymidine injected 30 min prior to tissue harvest. This was paralleled morphological evidence of proliferation at the same time point observed by light microscopy. Soderland and Naum30 reported that mouse alveolar Mφ could proliferate in vitro when cultured with conditioned medium from a lung cell line, and Golde et al.31 found that human mouse alveolar Mφ demonstrated uptake of H3-thymidine over a 1 h period in culture, with cells from smokers demonstrating higher rates of proliferation, although these tended to be <1%.
2.2. Section summary
These older studies, although not definitive in many cases, provided evidence of Mφ proliferation in different tissues and in different contexts, contrasting with the view that these cells are nonproliferating end-stage cells. In addition, the low level of proliferation during homeostasis appeared to be increased in different models of infection, which provided early evidence that Mφ proliferation contributes to macrophage accumulation needed for host defense.
3. Mϕ proliferation during homeostasis and repopulation
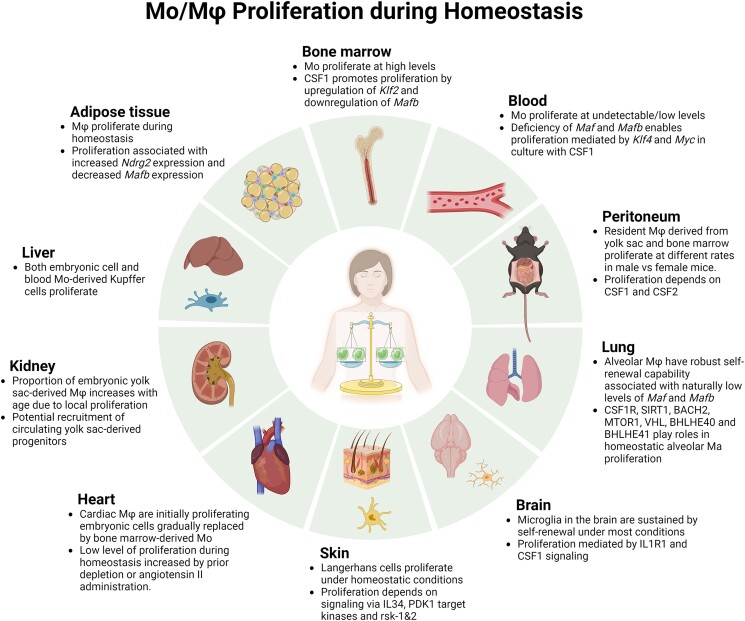
3.1. Bone marrow, blood, and spleen
A number of reports have corroborated earlier findings that, during homeostasis, Mo in the bone marrow proliferate at relatively high levels, whereas Mo in peripheral blood and spleen either do not proliferate or proliferate at very low levels in mice, rats, and humans.32–38 Studies over the past 30 yr have utilized flow cytometry and immunohistochemistry, along assessment of the cell cycle with DNA and Ki67 labeling, and DNA synthesis with BrdU or EdU labeling, to identify specific subpopulations of Mo and Mφ that proliferate in each tissue. These reports have also provided evidence that at least a subpopulation of blood Mo retain the ability to proliferate when stimulated with colony-stimulating factor 1 (CSF1),33,36 stored human serum containing oxidized low-density lipoprotein,36 advanced glycation end products39 or FLT3 ligand.37 A landmark study demonstrated that combined deficiency of the transcription factors Maf and Mafb in double knockout mice enabled Mo and Mφ derived from peripheral blood to proliferate long-term in culture with CSF1, an effect mediated by upregulation of transcription factors Klf4 and Myc.40 Similarly, a recent study demonstrated that a subpopulation of bone marrow–derived Mφ proliferate long term when cultured with CSF1, which appeared to be mediated by upregulation of the transcription factor Klf2 and downregulation of Mafb.41
3.2. Peritoneum and lung
Abundant studies demonstrate Mφ proliferation in the peritoneum and lung; early studies used either strontium-89 or X-irradiation to deplete blood Mo and demonstrated proliferation of local resident Mφ in the mouse via H3-thymidine incorporation, independent of blood Mo input.42,43 More recent studies have used flow cytometry assessment of phenotype markers to identify resident Mφ in the mouse peritoneum (e.g. F4/80hi cells) along with a variety of fate mapping approaches combined with cell cycle analysis to demonstrate proliferation of these cells particularly during postnatal development but persisting into adulthood.44,45 In an important study, a fate mapping approach was used to demonstrate that mouse peritoneal Mφ were long lived proliferating cells that were gradually replaced by bone marrow–derived Mo. The latter cells differentiated into Mφ that were phenotypically similar to embryonic Mφ but retained some differences.45 Interestingly, replacement of embryonic Mφ occurred faster in male vs. female mice, and newly arrived cells appeared to proliferate at a higher rate than the older cells.45 In other studies, female mice in the estrus phase or those treated with exogenous E2 (estradiol) exhibited increased proliferation of peritoneal Mφ, as shown by Ki67 labeling and BrdU incorporation,46 indicating that sex hormones influence Mφ proliferation. Another study took advantage of observations that many resident Mφ express CD169 and used CD169-DTR mice to deplete these cells and study repopulation of resident Mφ in the peritoneum and lung.47 Using CD169-DTR mice that were crossed with Ccr2 knockout mice to deplete blood Mo, repopulation was demonstrated to be independent of blood Mo. Further experiments using blocking antibodies and knockout mice indicated that, during repopulation, proliferation of resident Mφ in these tissues was found to be dependent on CSF1 and CSF2.
A number of studies have focused on the mechanisms underlying self-renewal of resident Mφ particularly in the peritoneum and lung. Soucie et al.48 used chromatin immunoprecipitation sequencing and Maf/Mafb double knockout bone marrow Mφ to identify enhancers associated with self-renewal and found that activated enhancers were associated with upregulation of a network of genes, directed by Klf2 and Myc, and repressed by Maf and Mafb. Importantly, alveolar Mφ were shown to naturally have low levels of Maf and Mafb and have robust self-renewal capability. Single-cell analysis also showed low levels of Maf and Mafb expression in resident Mφ of the peritoneum, liver, and spleen, which are also known to have self-renewal capacity. In addition, a recent study reported that inducible depletion of interstitial lung Mφ resulted in infiltration by circulating Ly6C+ Mo in a CCR2-dependent manner, which then proliferated, as shown by EdU incorporation, cell cycle analysis, and competitive bone marrow transfer experiments using Ccr2 knockout mice.49 In this report, CSF1 receptor (CSF1R) signaling was found to be required for proliferation in blocking antibody experiments and MAFB was implicated in the transition from proliferation to differentiation into the resident Mφ phenotype in experiments utilizing myeloid cell specific Mafb knockout mice. Other studies have reported important roles for SIRT1 in self-renewal of resident Mφ in the peritoneum and lung,50 and BACH2,51 MTOR1,52 VHL,53 BHLHE40 and BHLHE41,54 and mitochondrial metabolism55 in homeostatic alveolar Mφ proliferation in mice.
3.3. Brain and skin
Microglia in the brain and Langerhans cells in the skin are generally thought to be sustained by self-renewal under most conditions in adult animals.12,13 Using a multicolor fate mapping mouse model, combined with EdU/BrdU labeling, microglia self-renewal was found to be a random process during homeostasis with renewal rates showing regional differences in the brain.56 Interestingly, microglia proliferation correlated with proliferation of other cells in the same region, and proliferation was increased after facial nerve axotomy in a clonal manner. In another study, CX3CR1-DTR mice were used to deplete microglia, and repopulation of these cells occurred via proliferation of local resident cells assessed by BrdU labeling, mediated at least in part by interleukin-1 receptor 1 (IL1R1) signaling in experiments using an IL1R antagonist.57 These findings were supported by a subsequent report that utilized an CSF1 receptor antagonist to deplete microglia, along with different fate mapping approaches and EdU labeling to show that microglia were replenished via self-renewal. Another investigation demonstrated that administration of CSF1 locally to the brain in vivo or to cultured cells induced proliferation of microglia, as shown by Ki67 labeling and BrdU incorporation.58
In skin, using an inducible multicolor fate mapping approach and EdU labeling, Langerhans cells were found to be self-renewing cells that could be observed in proliferative clusters even in steady state, and such proliferation was increased following tape stripping.59 In other studies, self-renewal of Langerhans cells has been reported to rely on signaling via IL34 in experiments using Il34 knockout mice60 and the phosphoinositide-dependent kinase 1 target kinases, and ribosomal S6 kinases 1 and 2 also in knockout mouse experiments.61
3.4. Other tissues
Mφ proliferation has also been demonstrated in a variety of other tissues. In the heart, various fate mapping approaches in mice along with Ki67 and BrdU labeling have been used to demonstrate that cardiac Mφ are initially proliferating embryonic cells that are gradually replaced by bone marrow–derived Mo as the animals age.62,63 The low level of proliferation of cardiac Mφ during homeostasis was increased by prior depletion or angiotensin II administration.62 In contrast, the proportion of embryonic yolk sac–derived Mφ was reported to increase with age in the mouse kidney, using fate-mapping approaches along with parabiosis and Ki67 labeling.64 This increase was due to local proliferation and potentially recruitment of circulating yolk sac–derived progenitors, an intriguing possibility. In the liver, specific depletion of Kupffer cells using Clec4f-DTR mice resulted in repopulation by blood Mo. These Mo-derived Kupffer cells gained many of the characteristics of embryonic cell–derived Kupffer cells, including the ability to proliferate, as assessed using a protected bone marrow chimera approach and Ki67 labeling.65 Proliferation of adipose tissue Mφ has also been observed in mice using BrdU labeling, and in vitro experiments have implicated a neuropeptide FF-induced increase in Ndrg2 expression and decreased expression of proliferation inhibitors, including Ifi200 family members and Mafb in this process.66 Finally, local proliferation assessed by BrdU incorporation was found to contribute to the accumulation of both Mφ and dendritic cells in the mouse uterus during pregnancy.67
3.5. Section summary
Local proliferation of Mφ contributes to their maintenance during homeostasis, and to their repopulation after depletion, and there is evidence that both embryonically derived and adult bone marrow–derived cells possess this capacity. Initial work implicates the transcription factors MAF and MAFB, as well as the growth factor CSF1, as potential common mechanisms driving Mφ proliferation in different tissues. However, further study is needed to elucidate cell-intrinsic and cell-extrinsic mechanisms contributing to Mφ proliferation that are common to all tissues as well as mechanisms that may differ between tissues (Fig. 1).
Fig. 1.
Mo/Mφ proliferation during homeostasis. Local proliferation of Mo/Mφ contributes to their maintenance during homeostasis, and to their repopulation after depletion, including embryonically derived and adult bone marrow–derived cells.
4. Mϕ proliferation during infection
4.1. Peritoneum and lung
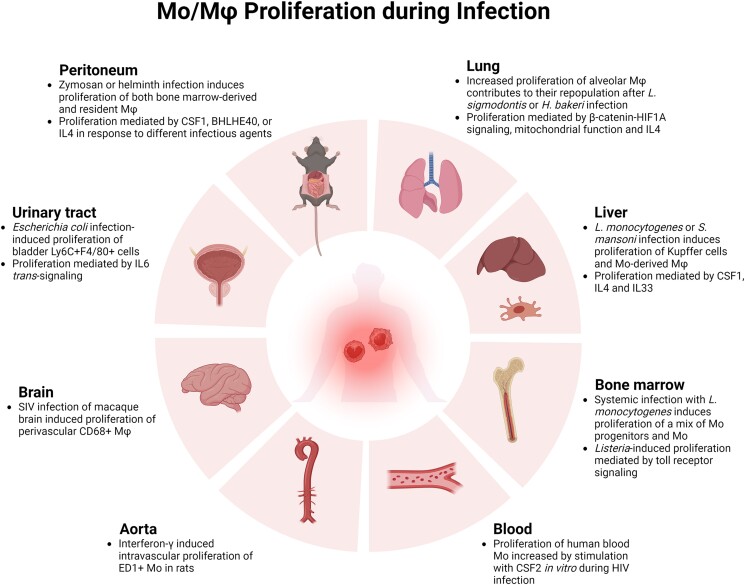
Both infiltrating Mo-derived and resident Mφ have been shown to proliferate at a higher rate after injection in different tissues, and such proliferative responses have been most extensively studied in peritoneum and lung. In addition to their findings on peritoneal Mφ proliferation during postnatal development, Davies et al.44,68 reported that zymosan, which is found in the yeast Saccharomyces cerevisiae, increased proliferation of adult mouse peritoneal Mφ as assessed by Ki67 labeling and cell cycle analysis. Injection of zymosan increased proliferation of both bone marrow–derived and tissue-resident Mφ, differentiated by levels of F4/80 and Ly6B expression, and proliferation appeared to depend on CSF1 but not on IL4 in blocking antibody experiments.68 A later study demonstrated that infiltrating bone marrow–derived Mφ can persist long term in the peritoneal cavity but do not completely phenocopy tissue-resident Mφ, including a higher capacity for proliferation of the newly arrived cells as indicated by Ki67 labeling.69 Furthermore, infection with the helminth Heligmosomoides polygyrus results in proliferation of large peritoneal macrophages, assessed by cell cycle analysis and BrdU incorporation, which appears to be dependent on the transcription factor BHLHE40, as demonstrated in experiments with Bhlhe40 knockout mice.70 BHLHE40 also appeared to mediate the proliferative response to an IL4 agonist, potentially via negative regulation of Maf and Mafb and positive regulation of cell cycle genes.
Infection with the nematode Litomosoides sigmodontis was shown to increase proliferation of resident Mφ in the lung of C57Bl/6 mice, and infection with H. polygyrus bakeri was shown to increase proliferation of resident Mφ in the peritoneum of BALB/c mice, and these responses were blocked in Il4 knockout mice and myeloid-specific IL4ra knockout mice.18,71 In addition, administration of an IL4 agonist could induce proliferation of resident peritoneal and lung Mφ, and of inflammatory peritoneal Mφ induced by thioglycolate, further implicating IL4 as a mediator of Mφ proliferation. Other experiments showed that proliferation of resident alveolar Mφ was associated with resistance to infection with L. sigmodontis in C57Bl/6 mice, whereas BALB/c mice did not exhibit robust resident Mφ expansion but instead demonstrated accumulation of infiltrating Mφ associated with susceptibility to infection.72 In these latter studies, tissue-resident and infiltrating Mφ were differentiated by surface levels of F4/80 and Ly6C, along with GATA6 and CD102.
Following influenza virus infection, alveolar Mφ were initially reduced and their repopulation appeared to be due, at least in part, to local proliferation as assessed by Ki67 labeling.73 In these studies, a β-catenin-HIF1A signaling pathway appeared to mediate an inflammatory Mφ phenotype and inhibited their proliferation and repopulation capacity. Another study by the same group showed that influenza infection decreased expression of Tfam and causes mitochondrial damage, which may lead to impaired self-renewal and increased susceptibility to severe infection.74
4.2. Liver
An early clue that liver Kupffer cells may proliferate in response to infection came from a study showing that glucan administration induced proliferation of liver Mφ as assessed by H3-thymidine incorporation, despite induction of monocytopenia via strontium-89.75 In addition, consistent with early studies by North et al,29 a more recent study showed that L. monocytogenes infection induced proliferation of liver Mφ as assessed by Ki67 labeling.76 In this latter study, lineage tracing studies using CX3CR1 and MaFIA reporter mice along with Ccr2 knockout mice demonstrated that the proliferating Mφ were Mo-derived cells and further experiments demonstrated that CSF1, IL4, and IL33 are involved in this proliferative response. Another study demonstrated that infection with Schistosoma mansoni resulted in depletion of resident liver Mφ and replacement with Mo-derived cells using congenic bone marrow lineage tracing experiments.77 During this process, proliferation of Ly6Chi Mo-like cells appeared to contribute to the repopulation of liver Mφ, as assessed by EdU labeling, whereas proliferation of more mature Mφ was negligible.
4.3. Bone marrow and blood
Systemic infection with L. monocytogenes induced proliferation of bone marrow cells that were Ly6Chi, and either CD11b+ or CD11b–, as assessed by BrdU incorporation.78 These cells likely represent a mix of Mo progenitors and Mo, and Listeria-induced proliferation appeared to be dependent on toll receptor signaling. In studies on the fungus Cryptococcus neoformans, Fc-mediated phagocytosis of live or heat-killed fungus, or even polystyrene beads, by the J774 Mφ-like cell line, bone marrow–derived Mφ, or peritoneal Mφ, resulted in proliferation of these cells as assessed by cell cycle analysis and BrdU incorporation.79 This process did not appear to require ingestion, because it was also induced by incubating cells on IgG1-coated plates. In a study on the mechanisms of HIV infection, in vitro proliferation of human blood Mo was increased by stimulation with CSF2, as assessed by H3-thymidine incorporation.80 Interestingly, such proliferation was required for productive HIV infection.
4.4. Other tissues
Consistent with the idea that proliferation of Mo-like cells may contribute to Mφ accumulation during the response to infection, intravenous administration of interferon-γ to rats induced intravascular proliferation of ED1+ Mo as assessed by pulsed BrdU incorporation.81 In addition, infection of the brain of macaques with simian immunodeficiency virus results proliferation of infected perivascular CD68+ Mφ assessed by Ki67 and BrdU labeling.82 Finally, urinary tract infection in mice with Escherichia coli induced proliferation of Ly6C+ F4/80+ cells in the bladder, and these cells were shown to be recruited from blood via congenic bone marrow transfer experiments.83 Proliferation of these cells was found to depend on IL6 trans-signaling via administration of soluble gp130.
4.5. Section summary
Bacterial, viral, fungal, and helminth infection all result in proliferation of Mφ in peripheral tissues. Accumulated evidence indicates that both Mo-derived and/or resident Mφ can proliferate, potentially depending on the specific pathogen and tissue involved. Proliferation of these cells can be induced by various factors, including CSF1, IL4, IL6, and the hypoxia inducible factor 1 subunit alpha pathway, but further study is needed to determine mechanisms that are common to different types of infection, and which are pathogen and/or tissue dependent (Fig. 2).
Fig. 2.
Mo/Mφ proliferation during infection. Infection with various pathogens results in proliferation of Mo-derived and/or resident Mφ potentially depending on the specific pathogen and tissue involved. H. bakeri = Heligmosomoides polygyrus bakeri; HIF1A = hypoxia inducible factor 1 subunit alpha; L. monocytogenes = Listeria monocytogenes; L. sigmodontis = Litomosoides sigmodontis; SIV = simian immunodeficiency virus.
5. Mϕ proliferation during tissue injury and repair
5.1. Skin
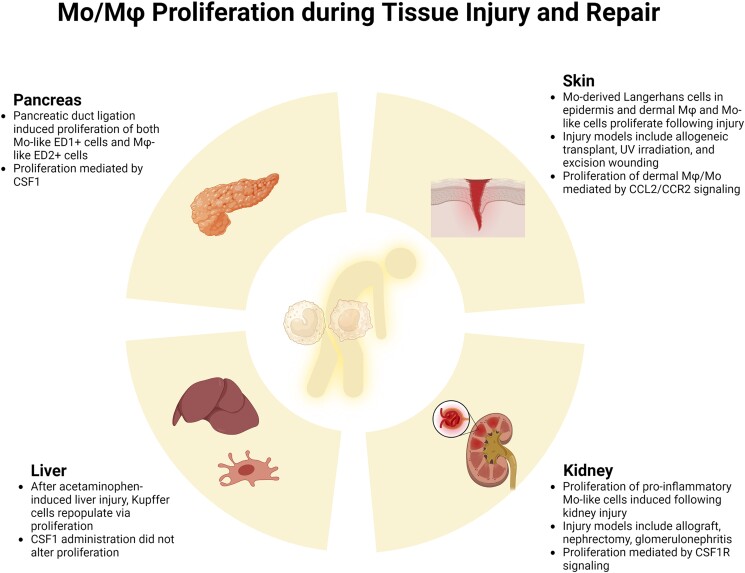
Different types of tissue injury also result in increased proliferation of both infiltrating Mo-derived and resident Mφ. In skin, epidermal and dermal Mφ both proliferate following injury in different species. Langerhans cells in the epidermis are thought to be embryo-derived self-renewing cells during homeostasis, but after immune injury induced by allogeneic hematopoietic stem cell transplantation, donor Mo replace damaged Langerhans cells in the epidermis.84 These Mo-derived Langerhans cells undergo sequential differentiation and proliferation that matches that of embryonic-derived cells as assessed by Ki67 labeling. In addition, ultraviolet irradiation of human skin resulted in expansion of a dermal Mφ population that expressed CD11b, CD36, and HLA-DR but not CD1 and cell cycle analysis indicated a high proportion of these cells in the proliferative S/G2/M phases of the cell cycle.85 Similarly, in vitro stimulation of human skin explants with substance P increased the population of dermal CD68+ cells but this increase was not associated with increased proliferation of CD68+ cells, assessed by Ki67 labeling.86 Instead, substance P stimulation increased a population of Ki67+ CD34+ in proximity to CD68+ cells and the authors suggested the former cells may be the source of increased dermal CD68+ cells.
Until recently, the accumulation of Mφ following skin injury was thought to result solely from infiltration of blood Mo that differentiate into Mφ. Using an excisional wound model, our laboratory demonstrated that wounding increased Mo-like Ly6C+ F4/80lo cells in the proliferative S/G2/M phases of the cell cycle, peaking at ∼25% of these cells on day 6 postinjury.35 Blood Mo did not show evidence of proliferation, nor did more mature Ly6C-F4/80+ cells in wounds, indicating that environmental factors may induce proliferation in a maturation stage–dependent manner. Importantly, impaired wound healing in diabetic mice was associated with increase proliferation and accumulation of Ly6C+ F4/80lo cells in wounds.34 Although proliferation of Ly6C+ Mφ was reminiscent of the response to urinary tract infection,83 proliferation was not altered in Il6 knockout mice in our studies. Instead, studies utilizing administration of recombinant CCL2 and adoptive transfer with CCR2 knockout Mo indicated that CCL2/CCR2 signaling induces proliferation of Ly6C+ Mφ. Thus CCL2 may contribute to persistent accumulation of Ly6C+ Mφ in wounds of diabetic mice by inducing both infiltration and proliferation.34 Interestingly, CCL2 also stimulated proliferation of cultured microglia, indicating that this phenomenon may not be restricted to skin wound Mφ.87
5.2. Kidney
Mφ proliferation has also been demonstrated in a number of different models of kidney injury in mouse, rat and humans. Robust local proliferation of ED1+ Mφ, identified by double labeling with proliferating cell nuclear antigen (PCNA), were observed within a kidney allograft undergoing acute rejection.88 ED1+ cells are typically considered to be proinflammatory Mo-like Mφ, similar to Ly6C+ Mφ in mice. In this study, the immunosuppressant drug deoxyspergualin inhibited Mφ proliferation in the graft. Local proliferation of ED1+ Mφ was also observed in a rat model of glomerulonephritis induced by anti-glomerular basement membrane antibody.89 In this study, proliferation was assessed by PCNA labeling and confirmed by BrdU labeling, was restricted to ED1+ ED2– ED3– Mo-like cells, and was confined to areas of severe damage. Local proliferation of Mφ was also observed in regions of damage after partial nephrectomy in rats (ED1+ PCNA+ cells;90), and in human glomerulonephritis (CD68+ PCNA+ cells;91).
Local Mφ proliferation assessed by PCNA labeling has been correlated with CSF1 expression in both rat models of kidney damage and in human glomerulonephritis.92,93 Furthermore, in a mouse model of unilateral ureteric obstruction, an anti-CSF1R blocking antibody largely prevented the proliferation of Mac-1+ cells, assessed by PCNA and BrdU labeling, and blocked the accumulation of these cells.94 These latter studies implicate CSF1R and CSF2R signaling in the local proliferation of Mφ following kidney injury. Recent studies have also reported local proliferation of Mφ in models of chronic ischemia assessed by pulsed BrdU labeling95 and ischemia/reperfusion assessed by PCNA labeling,96 with periostin implicated as an inducer of proliferation following ischemia/reperfusion.
5.3. Other tissues
In the liver, Kupffer cells identified as CD11bhi MHCIIhiCD64hi F4/80hi CX3CR1neg/lo Mφ were reduced following acetaminophen-induced injury in mice and repopulated by self-renewal as demonstrated by BrdU and Ki67 labeling.97 Monocyte adoptive transfer and CCR2 knockout mice were used to demonstrate lack of monocyte input into repopulating Kupffer cells and CSF1 administration did not affect Kupffer cells proliferation. In the pancreas, duct ligation in the rat resulted in proliferation of both Mo-like ED1+ cells and Mφ-like ED2+ cells as assessed by BrdU pulse labeling.98 Proliferation was robust, peaking on day 2 postligation at 20% to 30% of the parent population. Pancreatic duct ligation in mice also induced local Mφ proliferation peaking on day 3 postligation at 10% to 30% of the parent population as assessed by BrdU pulse labeling and Ki67 labeling.99 Proliferation was enhanced in CCR2 knockout mice, indicating a compensatory effect for lack of Mo input to damaged pancreas and inhibited by a CSF1R blocking antibody, indicating that CSF1 promotes Mφ proliferation in this model.
5.4. Section summary
Contrary to conventional wisdom, Mφ proliferation contributes to accumulation of these cells following immune, chemical, and physical injury to skin, liver, kidney, and pancreas. In some cases, infiltrating Mo-like cells show robust proliferative capacity that may exceed the proliferative capacity of resident cells. The factors inducing Mφ proliferation following tissue injury include CSF1, CCL2, and substance P, but further study is needed to better understand mechanisms that are generalizable over different types of injury in different tissues and mechanisms that may be context dependent (Fig. 3).
Fig. 3.
Mo/Mφ proliferation during tissue injury and repair. Mφ proliferation contributes to their accumulation following injury to skin, liver, kidney, and pancreas. In many cases, infiltrating Mo-like cells show robust proliferative capacity. UV = ultraviolet.
6. Mϕ proliferation during disease
6.1. Adipose tissue
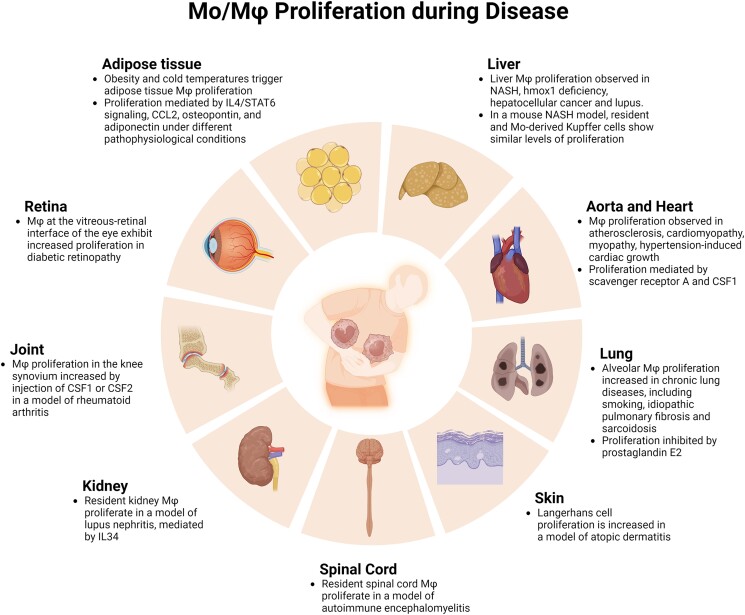
Mφ proliferation has been observed in a variety of disease states, including metabolic disease in both humans and rodents. Adipose tissue exhibits increased Mφ accumulation in obese humans, in genetically obese mice and in mice fed a high-fat diet (HFD), and this accumulation is due at least in part to local proliferation. Proliferating Mφ were localized to crown-like structures assessed by Ki67 labeling in both obese mouse and human adipose tissue and such proliferation increased over time in mice fed a HFD.100 Using bone marrow transfer experiments in which adipose tissue was shielded from irradiation, bone marrow–derived Mo were found to contribute little to adipose tissue Mφ accumulation early (8 wk) in the accumulation of fat mass in HFD mice, but their contribution increased at later time points (12 wk).101 Proliferation of both the resident and newly arrived cells, assessed by Ki67 and EdU labeling, contributed to adipose tissue Mφ accumulation throughout the time course of obesity, and IL4/STAT6 signaling appeared to contribute to this phenomenon. Another study demonstrated that adipose tissue Mφ proliferation in both genetically obese ob/ob and HFD mice, assessed by Ki67 and EdU labeling, was associated with an increase in CCL2, that CCL2 treatment increased Mφ proliferation in adipose tissue explants, and that local Mφ proliferation was reduced in CCL2 knockout mice.102 Osteopontin is also increased in adipose tissue of HFD mice and enhances survival and proliferation of bone marrow–derived Mφ in culture; importantly, adipose tissue Mφ proliferation induced by HFD obesity was blocked in osteopontin knockout mice.103 Interestingly, chronic cold exposure also resulted in proliferation of adipose tissue Mφ assessed by EdU labeling, a process associated with adaptive thermogenesis.104 Chronic cold also increased adiponectin expression, which appeared to be required for adipose tissue F4/80+ CD206+ Mφ proliferation as such proliferation was eliminated in adiponectin knockout mice. Thus, a number of pathways appear to trigger adipose tissue Mφ proliferation under different pathophysiological conditions.
6.2. Liver
Feeding mice a diet deficient in methionine and choline is a model of nonalcoholic steatohepatitis (NASH), and is associated with infiltration of Mo, whereas numbers of resident Kupffer cells do not appear to be altered.105,106 Parabiosis and bone marrow transfer experiments with CCR2 knockout mice were used to demonstrate that Mo-derived cells partially replace resident Kupffer cells during development of NASH, and are more proinflammatory, although both resident and Mo-derived cells show similar levels of proliferation as assessed by Ki67 labeling.106 Another study demonstrated similar levels of proliferation of resident and Mo-derived Kupffer cells in mice recovering from NASH, when they were switched back to a normal diet.105 Furthermore, heme oxygenase 1 (Hmox1) deficiency in humans is a lethal disease characterized by severe anemia, and Hmox1 knockout mice recapitulate this disease phenotype. Wild-type bone marrow–derived Mφ infused into Hmox1 knockout mice engrafted into the liver and proliferated as assessed by BrdU labeling, rescuing these mice from disease.107 In human hepatocellular cancer, tumor Mφ proliferation assessed by Ki67 labeling was positively correlated with Mφ accumulation and poor prognosis.108 In this study, tumor Mφ proliferation appeared to be stimulated by adenosine signaling. Finally, in the lupus prone mouse strain MRL lpr/lpr, liver Mφ are increased compared with control mice.109 The increased Mφ population was associated with increased proliferative potential of non-parenchymal cells, which included Mo, Mφ and potentially precursor cells, assessed by CSF2-induced H3-thymidine incorporation in vitro. In short, liver Mφ proliferation appears to be involved in a variety of diseases.
6.3. Aorta and heart
Early studies provided evidence for local Mφ proliferation in atherosclerotic plaques of mice, rabbits, and humans using H3-thymidine or BrdU incorporation, or PCNA labeling along with immunohistochemical detection of Mφ markers in histological sections.110–112 Using a BrdU labeling strategy in Apoe knockout mice fed a high-cholesterol diet, Mφ turnover was found to be surprisingly rapid, and local proliferation contributed to this turnover as assessed by cell cycle analysis, Ki67, and phospho-histone H3 labeling along with adoptive transfer and parabiosis experiments.113 Mφ proliferation appeared to depend on the lesion microenvironment, and scavenger receptor A was implicated in the process in competitive bone marrow transfer experiments. CSF1 has also been shown to promote Mφ proliferation in atherosclerotic lesions in mice treated with Ldlr antisense oligonucleotides and fed a high-cholesterol diet.114 Proliferation of Mac-3+ and CD68+ Mφ was reduced in lesions of Csf1+/− mice as well in smooth muscle cell– and endothelial cell–specific Csf1 knockout mice as assessed by labeling with Ki67 or BrdU, indicating that these cells were important sources of CSF1 in lesions.
In the mouse heart, doxorubicin-induced cardiomyopathy was associated with accumulation of Mφ that were derived from blood Mo, as shown by parabiosis and lineage tracing experiments, whereas resident Mφ were depleted.115 Both the newly arrived and resident Mφ proliferated in the heart, with resident cells proliferating at a higher rate, induced in part by a scavenger receptor A1-c-Myc axis, contributing to the recovery of resident Mφ during recovery from myopathy. In addition, in a mouse model of hypertension-induced cardiac growth, Mo-derived cells transiently accumulated early followed by later accumulation of resident Mφ as shown by Cx3cr1 fate mapping.116 Accumulation of TimD4hi subsets of resident Mφ was associated with proliferation as assessed by BrdU incorporation. In short, Mφ proliferation contributes to both adaptive and pathophysiological processes in the cardiovascular system.
6.4. Lung
Alveolar Mφ from patients with chronic lung inflammatory disease, including smokers, idiopathic pulmonary fibrosis, and sarcoidosis, showed increased proliferation in vitro compared with Mφ from healthy control subjects, as assessed by H3-thymidine incorporation.117 These findings were confirmed by flow cytometric cell cycle analysis and morphological evidence of mitosis. In addition, chronic exposure of mice to particulate matter resulted in a time-dependent accumulation of bone marrow–derived Mo into the alveolar Mφ population, as shown by shielded bone marrow transfer experiments.118 The accumulation of bone marrow–derived Mφ was associated with reduced proliferation of resident alveolar Mφ as assessed by BrdU incorporation and a chronic inflammatory phenotype. Furthermore, in mice exposed to cigarette smoke, increased alveolar Mφ proliferation assessed by EdU incorporation was associated with reduced prostaglandin E2 levels.119 This study also demonstrated that reduced alveolar Mφ numbers in aged mice was associated with increased prostaglandin E2 levels. In vitro, prostaglandin E2 inhibited CSF2-induced expansion of alveolar Mφ, suggesting that this eicosanoid limits proliferation.
6.5. Other tissues
In the skin, Langerhans cells undergo robust proliferation during mouse development, as assessed by Ki67 labeling, with much lower levels of proliferation during homeostasis in the adult.120 Langerhans cell proliferation in the adult mouse is dramatically increased by local treatment with a vitamin D3 analog, which induces inflammation resembling atopic dermatitis; increased Langerhans cell proliferation was also seen in skin human atopic dermatitis patients.120 In a mouse model of autoimmune encephalomyelitis, proliferation of resident Mφ subsets, assessed by lineage tracing and Ki67 labeling, was observed in the spinal cord, which contributed to their accumulation alongside infiltration of Mo and their differentiation into Mφ .121 In the MRL-Faslpr lupus mouse model, IL34 appears to contribute to lupus nephritis by increasing Mφ accumulation in the kidney, via increased production of bone marrow Mo and by local Mφ proliferation, assessed by Ki67 labeling.122 In a mouse model of rheumatoid arthritis, injection of CSF1 or CSF2 increased local proliferation of Mφ assessed by BrdU labeling and exacerbated pathology.123 Local proliferation of Mφ has also been reported at the vitreous-retinal interface of the eye in diabetic retinopathy in mice and humans, assessed by BrdU and Ki67 labeling.124
6.6. Section summary
Local Mφ proliferation of both Mo-derived and resident Mφ contribute to the inflammatory response and pathogenesis in a variety of diseases, including metabolic disease, chronic inflammatory/immune disease, cancer, and cardiovascular disease. A number of factors have been reported to induce Mφ proliferation in these diseases, including CSF1, CSF2, IL4, IL34, CCL2, adiponectin, and prostaglandin E2. However, additional research is needed to elucidate the mechanisms that contribute to Mφ proliferation in each disease state and whether targeting these mechanisms can ameliorate disease pathology (Fig. 4).
Fig. 4.
Mo/Mφ proliferation during disease. Local Mφ proliferation contributes to the inflammatory response in a variety of tissues and diseases.
7. Summary and future directions
In summary, numerous studies have shown that Mφ can proliferate during homeostasis as well as during the response to infection, injury, and disease. Mφ proliferate in different organs and tissues, including skin, peritoneum, lung, heart, aorta, kidney, liver, pancreas, brain, spinal cord, eye, adipose tissue, and uterus, and in different species including mouse, rat, rabbit, and human. Mφ can proliferate at different stages of differentiation with infiltrating Mo-like cells proliferating in certain inflammatory contexts (e.g. skin wounding, kidney injury, bladder infection) and mature resident Mφ proliferating in other inflammatory contexts (e.g. helminth infection, fungal infection, and metabolic disease) and during homeostasis. The pathways involved in stimulating Mφ proliferation also appear to be context dependent, with IL-1, IL4, IL6, IL34, CSF1, CSF2, CCL2, SIRT1, mTOR, VHL, osteopontin, PGE2, Flt3 ligand, oxidized low-density lipoprotein, and transcription factors MAF, MAFB, KLF2, KLF4, MYC, BACH2, BHLHE 40, and BHLHE41 implicated in different studies. Although much has been learned about the role and regulation of Mφ proliferation in health, injury, and disease, further research is needed on both generalizable and context-dependent mechanisms involved and the impact of Mφ proliferation on the homeostasis, injury, and repair of different organs and tissues.
7.1. Regulation of Mϕ proliferation
An intriguing observation is that Mo proliferate in bone marrow, but do not proliferate after their mobilization to peripheral blood, and then can proliferate again after recruitment to sites of inflammation during the response to infection, injury, and disease. Evidence suggests that the microenvironment plays a role in stimulating Mo and Mφ proliferation, and different factors have been implicated in triggering proliferation, including IL1, IL4, IL6, IL34, CSF1, CSF2, and CCL2, but a comprehensive understanding of the cell-intrinsic and cell-extrinsic pathways that regulate Mo and Mφ proliferation in the bone marrow, in the blood, and at sites of inflammation remains to be elucidated. In addition, whether Mo and Mφ preferentially proliferate in specific locations or niches of different tissues remains to be determined. The transcription factors MAF and MAFB are likely to be involved as a number of studies have demonstrated that these transcription factors are part of a pathway that blocks Mo and Mφ proliferation, and that downregulation of these factors permits proliferation.40,48,49,70
In addition, the self-renewal capacity of Mφ has been reported to be influenced by sex, with higher levels of peritoneal Mφ proliferation in male vs female mice that appears to be driven by the local environment.45 These findings appear to contrast with findings that both administration of exogenous estradiol and the endogenous hormone surge in female mice increase proliferation of peritoneal Mφ .46 Thus, the pathways that influence sexual dimorphism in Mφ proliferation remain to be elucidated, and the impact of sex differences in Mφ proliferation on the inflammatory response during infection, injury, and disease should be a fruitful area of future study.
7.2. Impact on function
The studies reviewed have provided evidence for proliferation of infiltrating Mo-derived Mφ as well as tissue-resident Mφ that appear to be context dependent. Mo-derived Mφ and tissue-resident Mφ appear to retain somewhat different phenotypes even when exposed to the same environment, with Mo-derived cells tending to have proinflammatory roles and resident cells tending to contribute to resolution and repair at least in some contexts.1,3,12,125,126 Thus, differential proliferation may be a mechanism by which the function of the total Mφ population is regulated. This idea could be extended to subsets within the Mo-derived Mφ and tissue-resident Mφ populations if subsets of these cells have different capacities to proliferate. Recent studies have identified heterogeneity of blood Mo that could affect their function after infiltration into tissues and subsequent differentiation, supporting the idea that both cell-intrinsic and cell-extrinsic factors play a role in regulating the function of Mφ .127,128 Further study is needed to determine whether subsets of Mo-derived Mφ and tissue-resident Mφ proliferate differently in different contexts, and if so, to determine the impact on the function of Mφ in those contexts.
7.3. Need for human studies
Most studies on Mφ proliferation have been performed in rodents, particularly mice, whereas fewer studies have been performed in humans or on human cells. Most human studies have used peripheral blood Mo stimulated in culture, or other cells that are relatively easy to obtain, including alveolar or adipose tissue Mφ. A few studies have capitalized on the ability to obtain cells from diseased organs, including heart, kidney, liver, and intestine. Other interesting studies have taken advantage of the accessibility of skin and the utility of skin allografts to study the ability of resident Langerhans cells and dermal Mφ to proliferate in the graft, the ability of infiltrating host Mo to replace these resident cells, and the respective roles of host and donor cells in the function of the graft.129–131 Barriers to human studies include logistical and technical difficulties in obtaining cells in a form suitable for identifying cells and cell subsets and assessing proliferation, as well as difficulties in performing mechanistic studies especially in vivo. However, the need for such studies is emphasized by differences observed in mouse vs human immune systems132; there is need both for studies that translate findings from mouse studies and for those that make context-specific observations in humans that can be mechanistically tested in mice.
Further work in these areas will improve our understanding of the role of Mo and Mφ proliferation in physiological and pathological conditions in various organs and tissues, how proliferation is regulated, and how proliferation can be targeted to improve outcomes of a number of different disorders and diseases.
Acknowledgments
The authors thank Dr. Giamila Fantuzzi for input on a previous draft of this review. The figures were created with BioRender.com
Contributor Information
Jingbo Pang, Center for Wound Healing and Tissue Regeneration, Department of Kinesiology and Nutrition, University of Illinois at Chicago, 1919 West Taylor Street, Chicago, IL 60612-7246, United States.
Timothy J Koh, Center for Wound Healing and Tissue Regeneration, Department of Kinesiology and Nutrition, University of Illinois at Chicago, 1919 West Taylor Street, Chicago, IL 60612-7246, United States.
Author contributions
J.P. helped write the manuscript and made the figures. T.J.K. helped write the manuscript.
Funding
This study was supported by the National Institute of General Medical Sciences through grant R35GM136228 to T.J.K.
References
- 1. Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018:49(4):595–613. 10.1016/j.immuni.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 2. Hume DA, Irvine KM, Pridans C. The mononuclear phagocyte system: the relationship between monocytes and macrophages. Trends Immunol. 2019:40(2):98–112. 10.1016/j.it.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 3. Bleriot C, Chakarov S, Ginhoux F. Determinants of resident tissue macrophage identity and function. Immunity. 2020:52(6):957–970. 10.1016/j.immuni.2020.05.014 [DOI] [PubMed] [Google Scholar]
- 4. Jenkins SJ, Allen JE. The expanding world of tissue-resident macrophages. Eur J Immunol. 2021:51(8):1882–1896. 10.1002/eji.202048881 [DOI] [PubMed] [Google Scholar]
- 5. Kuznetsova T, Prange KHM, Glass CK, de Winther MPJ. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat Rev Cardiol. 2020:17(4):216–228. 10.1038/s41569-019-0265-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barman PK, Koh TJ. Macrophage dysregulation and impaired skin wound healing in diabetes. Front Cell Dev Biol. 2020:8:528. 10.3389/fcell.2020.00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016:44(3):450–462. 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davuluri GVN, Chan CH. Regulation of intrinsic and extrinsic metabolic pathways in tumour-associated macrophages. FEBS J. 2022:290(12):3040–3058. 10.1111/febs.16465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reece MD, Taylor RR, Song C, Gavegnano C. Targeting macrophage dysregulation for viral infections: novel targets for immunomodulators. Front Immunol. 2021:12:768695. 10.3389/fimmu.2021.768695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968:128(3):415–435. 10.1084/jem.128.3.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972:46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- 12. Ginhoux F, Guilliams M. Tissue-Resident macrophage ontogeny and homeostasis. Immunity. 2016:44(3):439–449. 10.1016/j.immuni.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 13. Doebel T, Voisin B, Nagao K. Langerhans cells—the macrophage in dendritic cell clothing. Trends Immunol. 2017:38(11):817–828. 10.1016/j.it.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 14. Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014:260(1):102–117. 10.1111/imr.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott CL, Henri S, Guilliams M. Mononuclear phagocytes of the intestine, the skin, and the lung. Immunol Rev. 2014:262(1):9–24. 10.1111/imr.12220 [DOI] [PubMed] [Google Scholar]
- 16. Li W, Chang N, Li L. Heterogeneity and function of Kupffer cells in liver injury. Front Immunol. 2022:13:940867. 10.3389/fimmu.2022.940867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bain CC, MacDonald AS. The impact of the lung environment on macrophage development, activation and function: diversity in the face of adversity. Mucosal Immunol. 2022:15(2):223–234. 10.1038/s41385-021-00480-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011:332(6035):1284–1288. 10.1126/science.1204351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Furth R, Diesselhoff-den Dulk MC, Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973:138(6):1314–1330. 10.1084/jem.138.6.1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volkman A. The origin and turnover of mononuclear cells in peritoneal exudates in rats. J Exp Med. 1966:124(2):241–254. 10.1084/jem.124.2.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volkman A, Collins FM. The cytokinetics of monocytosis in acute salmonella infection in the rat. J Exp Med. 1974:139(2):264–277. 10.1084/jem.139.2.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans HM, Bowman FB, Winternitz MC. An experimental study of the histogenesis of the miliary tubercle in vitally stained rabbits. J Exp Med. 1914:19(3):283–302. 10.1084/jem.19.3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ebert RH, Florey HW. The extravascular development of the monocyte observed in vivo. Br J Exp Pathol. 1939:20(4):342–356. [Google Scholar]
- 24. Mackaness GB. Cellular resistance to infection. J Exp Med. 1962:116(3):381–406. 10.1084/jem.116.3.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forbes IJ, Mackaness GB. Mitosis in macrophages. Lancet. 1963:2(7319):1203–1204. 10.1016/S0140-6736(63)92927-1 [DOI] [PubMed] [Google Scholar]
- 26. Forbes IJ. Induction of mitosis in macrophages by endotoxin. J Immunol. 1965:94(1):37–39. 10.4049/jimmunol.94.1.37 [DOI] [PubMed] [Google Scholar]
- 27. Forbes IJ. Mitosis in mouse peritoneal macrophages. J Immunol. 1966:96(4):734–743. 10.4049/jimmunol.96.4.734 [DOI] [PubMed] [Google Scholar]
- 28. Stewart CC, Lin HS, Adles C. Proliferation and colony-forming ability of peritoneal exudate cells in liquid culture. J Exp Med. 1975:141(5):1114–1132. 10.1084/jem.141.5.1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. North RJ. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969:130(2):315–326. 10.1084/jem.130.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soderland SC, Naum Y. Letter: growth of pulmonary alveolar macrophages in vitro. Nature. 1973:245(5421):150–152. 10.1038/245150a0 [DOI] [PubMed] [Google Scholar]
- 31. Golde DW, Byers LA, Finley TN. Proliferative capacity of human alveolar macrophage. Nature. 1974:247(5440):373–375. 10.1038/247373a0 [DOI] [PubMed] [Google Scholar]
- 32. Westermann J, Ronneberg S, Fritz FJ, Pabst R. Proliferation of macrophage subpopulations in the adult rat: comparison of various lymphoid organs. J Leukoc Biol. 1989:46(3):263–269. 10.1002/jlb.46.3.263 [DOI] [PubMed] [Google Scholar]
- 33. Finnin M, Hamilton JA, Moss ST. Characterization of a CSF-induced proliferating subpopulation of human peripheral blood monocytes by surface marker expression and cytokine production. J Leukoc Biol. 1999:66(6):953–960. 10.1002/jlb.66.6.953 [DOI] [PubMed] [Google Scholar]
- 34. Pang J, Maienschein-Cline M, Koh TJ. Enhanced proliferation of Ly6C(+) monocytes/macrophages contributes to chronic inflammation in skin wounds of diabetic mice. J Immunol. 2021:206(3):621–630. 10.4049/jimmunol.2000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pang J, Urao N, Koh TJ. Proliferation of Ly6C+ monocytes/macrophages contributes to their accumulation in mouse skin wounds. J Leukoc Biol. 2020:107(4):551–560. 10.1002/JLB.3HI1119-389RRRR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asakura E, Tojo N, Tanabe T. Monocyte proliferation induced by modified serum is associated with endogenous M-CSF production: evidence for involvement of a signalling pathway via scavenger receptors. Cell Prolif. 1999:32(4):185–194. 10.1046/j.1365-2184.1999.3240185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SW, Choi SM, Choo YS, Kim IK, Song BW, Kim HS. Flt3 ligand induces monocyte proliferation and enhances the function of monocyte-derived dendritic cells in vitro. J Cell Physiol. 2015:230(8):1740–1749. 10.1002/jcp.24824 [DOI] [PubMed] [Google Scholar]
- 38. Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009:324(5925):392–397. 10.1126/science.1170540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin X, Liu L, Zhang Y, Xiang Y, Yin G, Lu Y, Shi L, Dong J, Shen C. Advanced glycation End products enhance murine monocyte proliferation in bone marrow and prime them into an inflammatory phenotype through MAPK signaling. J Diabetes Res. 2018:2018:2527406. 10.1155/2018/2527406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aziz A, Soucie E, Sarrazin S, Sieweke MH. Mafb/c-maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009:326(5954):867–871. 10.1126/science.1176056 [DOI] [PubMed] [Google Scholar]
- 41. Nasser H, Adhikary P, Abdel-Daim A, Noyori O, Panaampon J, Kariya R, Okada S, Ma W, Baba M, Takizawa H, et al. Establishment of bone marrow–derived M-CSF receptor-dependent self-renewing macrophages. Cell Death Discov. 2020:6(1):63. 10.1038/s41420-020-00300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sawyer RT, Strausbauch PH, Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982:46(2):165–170. [PubMed] [Google Scholar]
- 43. Tarling JD, Coggle JE. Evidence for the pulmonary origin of alveolar macrophages. Cell Tissue Kinet. 1982:15(6):577–584. 10.1111/j.1365-2184.1982.tb01064.x [DOI] [PubMed] [Google Scholar]
- 44. Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 2011:41(8):2155–2164. 10.1002/eji.201141817 [DOI] [PubMed] [Google Scholar]
- 45. Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, Mack M, Joshi A, Guilliams M, Mowat AM, et al. Long-lived self-renewing bone marrow–derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat Commun. 2016:7(1):ncomms11852. 10.1038/ncomms11852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pepe G, Braga D, Renzi TA, Villa A, Bolego C, D'Avila F, Barlassina C, Maggi A, Locati M, Vegeto E. Self-renewal and phenotypic conversion are the main physiological responses of macrophages to the endogenous estrogen surge. Sci Rep. 2017:7(1):44270. 10.1038/srep44270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013:38(4):792–804. 10.1016/j.immuni.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soucie EL, Weng Z, Geirsdottir L, Molawi K, Maurizio J, Fenouil R, Mossadegh-Keller N, Gimenez G, VanHille L, Beniazza M, et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science. 2016:351(6274):aad5510. 10.1126/science.aad5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vanneste D, Bai Q, Hasan S, Peng W, Pirottin D, Schyns J, Marechal P, Ruscitti C, Meunier M, Liu Z, et al. MafB-restricted local monocyte proliferation precedes lung interstitial macrophage differentiation. Nat Immunol. 2023:24(5):827–840. 10.1038/s41590-023-01468-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Imperatore F, Maurizio J, Vargas Aguilar S, Busch CJ, Favret J, Kowenz-Leutz E, Cathou W, Gentek R, Perrin P, Leutz A, et al. SIRT1 Regulates macrophage self-renewal. EMBO J. 2017:36(16):2353–2372. 10.15252/embj.201695737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ebina-Shibuya R, Matsumoto M, Kuwahara M, Jang KJ, Sugai M, Ito Y, Funayama R, Nakayama K, Sato Y, Ishii N, et al. Inflammatory responses induce an identity crisis of alveolar macrophages, leading to pulmonary alveolar proteinosis. J Biol Chem. 2017:292(44):18098–18112. 10.1074/jbc.M117.808535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deng W, Yang J, Lin X, Shin J, Gao J, Zhong XP. Essential role of mTORC1 in self-renewal of murine alveolar macrophages. J Immunol. 2017:198(1):492–504. 10.4049/jimmunol.1501845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Izquierdo HM, Brandi P, Gomez MJ, Conde-Garrosa R, Priego E, Enamorado M, Martinez-Cano S, Sanchez I, Conejero L, Jimenez-Carretero D, et al. Von Hippel-Lindau protein is required for optimal alveolar macrophage terminal differentiation, self-renewal, and function. Cell Rep. 2018:24(7):1738–1746. 10.1016/j.celrep.2018.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rauschmeier R, Gustafsson C, Reinhardt A, A-Gonzalez N, Tortola L, Cansever D, Subramanian S, Taneja R, Rossner MJ, Sieweke MH, et al. Bhlhe40 and Bhlhe41 transcription factors regulate alveolar macrophage self-renewal and identity. EMBO J. 2019:38(19):e101233. 10.15252/embj.2018101233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wessendarp M, Watanabe-Chailland M, Liu S, Stankiewicz T, Ma Y, Kasam RK, Shima K, Chalk C, Carey B, Rosendale LR, et al. Role of GM-CSF in regulating metabolism and mitochondrial functions critical to macrophage proliferation. Mitochondrion. 2022:62:85–101. 10.1016/j.mito.2021.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017:20(6):793–803. 10.1038/nn.4547 [DOI] [PubMed] [Google Scholar]
- 57. Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015:43(1):92–106. 10.1016/j.immuni.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 58. Pepe G, De Maglie M, Minoli L, Villa A, Maggi A, Vegeto E. Selective proliferative response of microglia to alternative polarization signals. J Neuroinflammation. 2017:14(1):236. 10.1186/s12974-017-1011-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ghigo C, Mondor I, Jorquera A, Nowak J, Wienert S, Zahner SP, Clausen BE, Luche H, Malissen B, Klauschen F, et al. Multicolor fate mapping of Langerhans cell homeostasis. J Exp Med. 2013:210(9):1657–1664. 10.1084/jem.20130403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL34 Is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012:13(8):753–760. 10.1038/ni.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zaru R, Matthews SP, Edgar AJ, Prescott AR, Gomez-Nicola D, Hanauer A, Watts C. The PDK1-rsk signaling pathway controls Langerhans cell proliferation and patterning. J Immunol. 2015:195(9):4264–4272. 10.4049/jimmunol.1501520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014:40(1):91–104. 10.1016/j.immuni.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014:211(11):2151–2158. 10.1084/jem.20140639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ide S, Yahara Y, Kobayashi Y, Strausser SA, Ide K, Watwe A, Xu-Vanpala S, Privratsky JR, Crowley SD, Shinohara ML, et al. Yolk-sac-derived macrophages progressively expand in the mouse kidney with age. Elife. 2020:9:e51756. 10.7554/eLife.51756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016:7(1):10321. 10.1038/ncomms10321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waqas SFH, Hoang AC, Lin YT, Ampem G, Azegrouz H, Balogh L, Thuroczy J, Chen JC, Gerling IC, Nam S, et al. Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages. J Clin Invest. 2017:127(9):3559. 10.1172/JCI95841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF1. J Exp Med. 2011:208(9):1901–1916. 10.1084/jem.20110866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow–derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013:4(1):1886. 10.1038/ncomms2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Louwe PA, Badiola Gomez L, Webster H, Perona-Wright G, Bain CC, Forbes SJ, Jenkins SJ. Recruited macrophages that colonize the post-inflammatory peritoneal niche convert into functionally divergent resident cells. Nat Commun. 2021:12(1):1770. 10.1038/s41467-021-21778-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jarjour NN, Schwarzkopf EA, Bradstreet TR, Shchukina I, Lin CC, Huang SC, Lai CW, Cook ME, Taneja R, Stappenbeck TS, et al. Bhlhe40 mediates tissue-specific control of macrophage proliferation in homeostasis and type 2 immunity. Nat Immunol. 2019:20(6):687–700. 10.1038/s41590-019-0382-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL4 Directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF1. J Exp Med. 2013:210(11):2477–2491. 10.1084/jem.20121999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Campbell SM, Knipper JA, Ruckerl D, Finlay CM, Logan N, Minutti CM, Mack M, Jenkins SJ, Taylor MD, Allen JE. Myeloid cell recruitment versus local proliferation differentiates susceptibility from resistance to filarial infection. Elife. 2018:7:e30947. 10.7554/eLife.30947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhu B, Wu Y, Huang S, Zhang R, Son YM, Li C, Cheon IS, Gao X, Wang M, Chen Y, et al. Uncoupling of macrophage inflammation from self-renewal modulates host recovery from respiratory viral infection. Immunity. 2021:54(6):1200–1218.e9. 10.1016/j.immuni.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gao X, Zhu B, Wu Y, Li C, Zhou X, Tang J, Sun J. TFAM-Dependent Mitochondrial metabolism is required for alveolar macrophage maintenance and homeostasis. J Immunol. 2022:208(6):1456–1466. 10.4049/jimmunol.2100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yamada M, Naito M, Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukoc Biol. 1990:47(3):195–205. 10.1002/jlb.47.3.195 [DOI] [PubMed] [Google Scholar]
- 76. Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015:42(1):145–158. 10.1016/j.immuni.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 77. Rolot M, M A, Javaux D, Lallemand J, Machiels F, Martinive B, Gillet P, Dewals L, G B. Recruitment of hepatic macrophages from monocytes is independent of IL4Ralpha but is associated with ablation of resident macrophages in schistosomiasis. Eur J Immunol. 2019:49(7):1067–1081. 10.1002/eji.201847796 [DOI] [PubMed] [Google Scholar]
- 78. Serbina NV, Hohl TM, Cherny M, Pamer EG. Selective expansion of the monocytic lineage directed by bacterial infection. J Immunol. 2009:183(3):1900–1910. 10.4049/jimmunol.0900612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Luo Y, Tucker SC, Casadevall A. Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S. J Immunol. 2005:174(11):7226–7233. 10.4049/jimmunol.174.11.7226 [DOI] [PubMed] [Google Scholar]
- 80. Schuitemaker H, Kootstra NA, Koppelman MH, Bruisten SM, Huisman HG, Tersmette M, Miedema F. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. J Clin Invest. 1992:89(4):1154–1160. 10.1172/JCI115697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Steiniger B, Schroder D, Luck R, Luciano L, van der Meide PH. Gamma interferon treatment in vivo provokes accumulation of activated monocytes in the venous circulation of rats. Am J Pathol. 1990:136(4):967–978. [PMC free article] [PubMed] [Google Scholar]
- 82. Filipowicz AR, McGary CM, Holder GE, Lindgren AA, Johnson EM, Sugimoto C, Kuroda MJ, Kim WK. Proliferation of perivascular macrophages contributes to the development of encephalitic lesions in HIV-infected humans and in SIV-infected macaques. Sci Rep. 2016:6(1):32900. 10.1038/srep32900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dixit A, Bottek J, Beerlage AL, Schuettpelz J, Thiebes S, Brenzel A, Garbers C, Rose-John S, Mittrucker HW, Squire A, et al. Frontline science: proliferation of Ly6C(+) monocytes during urinary tract infections is regulated by IL6 trans-signaling. J Leukoc Biol. 2018:103(1):13–22. 10.1189/jlb.3HI0517-198R [DOI] [PubMed] [Google Scholar]
- 84. Ferrer IR, West HC, Henderson S, Ushakov DS, Santos ESP, Strid J, Chakraverty R, Yates AJ, Bennett CL. A wave of monocytes is recruited to replenish the long-term Langerhans cell network after immune injury. Sci Immunol. 2019:4(38):eaax8704. 10.1126/sciimmunol.aax8704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Meunier L, Bata-Csorgo Z, Cooper KD. In human dermis, ultraviolet radiation induces expansion of a CD36+ CD11b+ CD1- macrophage subset by infiltration and proliferation; CD1+ Langerhans-like dendritic antigen-presenting cells are concomitantly depleted. J Invest Dermatol. 1995:105(6):782–788. 10.1111/1523-1747.ep12326032 [DOI] [PubMed] [Google Scholar]
- 86. Gherardini J, Uchida Y, Hardman JA, Cheret J, Mace K, Bertolini M, Paus R. Tissue-resident macrophages can be generated de novo in adult human skin from resident progenitor cells during substance P-mediated neurogenic inflammation ex vivo. PLoS One. 2020:15(1):e0227817. 10.1371/journal.pone.0227817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 Modulation of microglial activation and proliferation. J Neuroinflammation. 2011:8(1):77. 10.1186/1742-2094-8-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kerr PG, Nikolic-Paterson DJ, Lan HY, Tesch G, Rainone S, Atkins RC. Deoxyspergualin suppresses local macrophage proliferation in rat renal allograft rejection. Transplantation. 1994:58(5):596–601. 10.1097/00007890-199409150-00012 [DOI] [PubMed] [Google Scholar]
- 89. Lan HY, Nikolic-Paterson DJ, Mu W, Atkins RC. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int. 1995:48(3):753–760. 10.1038/ki.1995.347 [DOI] [PubMed] [Google Scholar]
- 90. Yang N, Wu LL, Nikolic-Paterson DJ, Ng YY, Yang WC, Mu W, Gilbert RE, Cooper ME, Atkins RC, Lan HY. Local macrophage and myofibroblast proliferation in progressive renal injury in the rat remnant kidney. Nephrol Dial Transplant. 1998:13(8):1967–1974. 10.1093/ndt/13.8.1967 [DOI] [PubMed] [Google Scholar]
- 91. Yang N, Isbel NM, Nikolic-Paterson DJ, Li Y, Ye R, Atkins RC, Lan HY. Local macrophage proliferation in human glomerulonephritis. Kidney Int. 1998:54(1):143–151. 10.1046/j.1523-1755.1998.00978.x [DOI] [PubMed] [Google Scholar]
- 92. Isbel NM, Hill PA, Foti R, Mu W, Hurst LA, Stambe C, Lan HY, Atkins RC, Nikolic-Paterson DJ. Tubules are the major site of M-CSF production in experimental kidney disease: correlation with local macrophage proliferation. Kidney Int. 2001:60(2):614–625. 10.1046/j.1523-1755.2001.060002614.x [DOI] [PubMed] [Google Scholar]
- 93. Isbel NM, Nikolic-Paterson DJ, Hill PA, Dowling J, Atkins RC. Local macrophage proliferation correlates with increased renal M-CSF expression in human glomerulonephritis. Nephrol Dial Transplant. 2001:16(8):1638–1647. 10.1093/ndt/16.8.1638 [DOI] [PubMed] [Google Scholar]
- 94. Le Meur Y, Tesch GH, Hill PA, Mu W, Foti R, Nikolic-Paterson DJ, Atkins RC. Macrophage accumulation at a site of renal inflammation is dependent on the M-CSF/c-fms pathway. J Leukoc Biol. 2002:72(3):530–537. 10.1189/jlb.72.3.530 [DOI] [PubMed] [Google Scholar]
- 95. Puranik AS, Leaf IA, Jensen MA, Hedayat AF, Saad A, Kim KW, Saadalla AM, Woollard JR, Kashyap S, Textor SC, et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci Rep. 2018:8(1):13948. 10.1038/s41598-018-31887-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kormann R, Kavvadas P, Placier S, Vandermeersch S, Dorison A, Dussaule JC, Chadjichristos CE, Prakoura N, Chatziantoniou C. Periostin promotes cell proliferation and macrophage polarization to drive repair after AKI. J Am Soc Nephrol. 2020:31(1):85–100. 10.1681/ASN.2019020113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, Varol C. Infiltrating monocyte-derived macrophages and resident Kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014:193(1):344–353. 10.4049/jimmunol.1400574 [DOI] [PubMed] [Google Scholar]
- 98. Goto M, Matsuno K, Yamaguchi Y, Ezaki T, Ogawa M. Proliferation kinetics of macrophage subpopulations in a rat experimental pancreatitis model. Arch Histol Cytol. 1993:56(1):75–82. 10.1679/aohc.56.75 [DOI] [PubMed] [Google Scholar]
- 99. Van Gassen N, Van Overmeire E, Leuckx G, Heremans Y, De Groef S, Cai Y, Elkrim Y, Gysemans C, Stijlemans B, Van de Casteele M, et al. Macrophage dynamics are regulated by local macrophage proliferation and monocyte recruitment in injured pancreas. Eur J Immunol. 2015:45(5):1482–1493. 10.1002/eji.201445013 [DOI] [PubMed] [Google Scholar]
- 100. Haase J, Weyer U, Immig K, Kloting N, Bluher M, Eilers J, Bechmann I, Gericke M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014:57(3):562–571. 10.1007/s00125-013-3139-y [DOI] [PubMed] [Google Scholar]
- 101. Zheng C, Yang Q, Cao J, Xie N, Liu K, Shou P, Qian F, Wang Y, Shi Y. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 2016:7(3):e2167. 10.1038/cddis.2016.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014:19(1):162–171. 10.1016/j.cmet.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tardelli M, Zeyda K, Moreno-Viedma V, Wanko B, Grun NG, Staffler G, Zeyda M, Stulnig TM. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Mol Metab. 2016:5(11):1131–1137. 10.1016/j.molmet.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KS, et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015:22(2):279–290. 10.1016/j.cmet.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 105. Devisscher L, Scott CL, Lefere S, Raevens S, Bogaerts E, Paridaens A, Verhelst X, Geerts A, Guilliams M, Van Vlierberghe H. Non-alcoholic steatohepatitis induces transient changes within the liver macrophage pool. Cell Immunol. 2017:322:74–83. 10.1016/j.cellimm.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 106. Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gelineau A, Marcelin G, Magreau-Davy E, Ouhachi M, Lesnik P, et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity. 2020:53(3):627–640 e5. 10.1016/j.immuni.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 107. Kim KS, Zhang DL, Kovtunovych G, Ghosh MC, Ollivierre H, Eckhaus MA, Rouault TA. Infused wild-type macrophages reside and self-renew in the liver to rescue the hemolysis and anemia of Hmox1-deficient mice. Blood Adv. 2018:2(20):2732–2743. 10.1182/bloodadvances.2018019737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang J, Wang Y, Chu Y, Li Z, Yu X, Huang Z, Xu J, Zheng L. Tumor-derived adenosine promotes macrophage proliferation in human hepatocellular carcinoma. J Hepatol. 2021:74(3):627–637. 10.1016/j.jhep.2020.10.021 [DOI] [PubMed] [Google Scholar]
- 109. Muller M, Emmendorffer A, Lohmann-Matthes ML. Expansion and high proliferative potential of the macrophage system throughout life time of lupus-prone NZB/W and MRL lpr/lpr mice. Lack of down-regulation of extramedullar macrophage proliferation in the postnatal period. Eur J Immunol. 1991:21(9):2211–2217. 10.1002/eji.1830210932 [DOI] [PubMed] [Google Scholar]
- 110. Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990:10(5):680–687. 10.1161/01.ATV.10.5.680 [DOI] [PubMed] [Google Scholar]
- 111. Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990:87(12):4600–4604. 10.1073/pnas.87.12.4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lutgens E, Daemen M, Kockx M, Doevendans P, Hofker M, Havekes L, Wellens H, de Muinck ED. Atherosclerosis in APOE*3-Leiden transgenic mice: from proliferative to atheromatous stage. Circulation. 1999:99(2):276–283. 10.1161/01.CIR.99.2.276 [DOI] [PubMed] [Google Scholar]
- 113. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013:19(9):1166–1172. 10.1038/nm.3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sinha SK, Miikeda A, Fouladian Z, Mehrabian M, Edillor C, Shih D, Zhou Z, Paul MK, Charugundla S, Davis RC, et al. Local M-CSF (macrophage colony-stimulating factor) expression regulates macrophage proliferation and apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2021:41(1):220–233. 10.1161/ATVBAHA.120.315255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang H, Xu A, Sun X, Yang Y, Zhang L, Bai H, Ben J, Zhu X, Li X, Yang Q, et al. Self-Maintenance of cardiac resident reparative macrophages attenuates doxorubicin-induced cardiomyopathy through the SR-A1-c-Myc axis. Circ Res. 2020:127(5):610–627. 10.1161/CIRCRESAHA.119.316428 [DOI] [PubMed] [Google Scholar]
- 116. Zaman R, Hamidzada H, Kantores C, Wong A, Dick SA, Wang Y, Momen A, Aronoff L, Lin J, Razani B, et al. Selective loss of resident macrophage-derived insulin-like growth factor-1 abolishes adaptive cardiac growth to stress. Immunity. 2021:54(9):2057–2071 e6. 10.1016/j.immuni.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 117. Bitterman PB, Saltzman LE, Adelberg S, Ferrans VJ, Crystal RG. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984:74(2):460–469. 10.1172/JCI111443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gangwar RS, Vinayachandran V, Rengasamy P, Chan R, Park B, Diamond-Zaluski R, Cara EA, Cha A, Das L, Asase C, et al. Differential contribution of bone marrow–derived infiltrating monocytes and resident macrophages to persistent lung inflammation in chronic air pollution exposure. Sci Rep. 2020:10(1):14348. 10.1038/s41598-020-71144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Penke LR, Speth JM, Draijer C, Zaslona Z, Chen J, Mancuso P, Freeman CM, Curtis JL, Goldstein DR, Peters-Golden M. PGE(2) accounts for bidirectional changes in alveolar macrophage self-renewal with aging and smoking. Life Sci Alliance. 2020:3(11):e202000800. 10.26508/lsa.202000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009:206(13):3089–3100. 10.1084/jem.20091586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jordao MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019:363(6425):eaat7554. 10.1126/science.aat7554 [DOI] [PubMed] [Google Scholar]
- 122. Wada Y, Gonzalez-Sanchez HM, Weinmann-Menke J, Iwata Y, Ajay AK, Meineck M, Kelley VR. IL34-Dependent Intrarenal and Systemic Mechanisms Promote Lupus Nephritis in MRL-Fas(lpr) mice. J Am Soc Nephrol. 2019:30(2):244–259. 10.1681/ASN.2018090901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bischof RJ, Zafiropoulos D, Hamilton JA, Campbell IK. Exacerbation of acute inflammatory arthritis by the colony-stimulating factors CSF1 and granulocyte macrophage (GM)-CSF: evidence of macrophage infiltration and local proliferation. Clin Exp Immunol. 2000:119(2):361–367. 10.1046/j.1365-2249.2000.01125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yamaguchi M, Nakao S, Wada I, Matoba T, Arima M, Kaizu Y, Shirane M, Ishikawa K, Nakama T, Murakami Y, et al. Identifying hyperreflective foci in diabetic retinopathy via VEGF-induced local self-renewal of CX3CR1+ vitreous resident macrophages. Diabetes. 2022:71(12):2685–2701. 10.2337/db21-0247 [DOI] [PubMed] [Google Scholar]
- 125. Mu X, Li Y, Fan GC. Tissue-Resident macrophages in the control of infection and resolution of inflammation. Shock. 2021:55(1):14–23. 10.1097/SHK.0000000000001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Milich LM, Ryan CB, Lee JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 2019:137(5):785–797. 10.1007/s00401-019-01992-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Menezes S, Melandri D, Anselmi G, Perchet T, Loschko J, Dubrot J, Patel R, Gautier EL, Hugues S, Longhi MP, et al. The heterogeneity of Ly6C(hi) monocytes controls their differentiation into iNOS(+) macrophages or monocyte-derived dendritic cells. Immunity. 2016:45(6):1205–1218. 10.1016/j.immuni.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yanez A, Coetzee SG, Olsson A, Muench DE, Berman BP, Hazelett DJ, Salomonis N, Grimes HL, Goodridge HS. Granulocyte-Monocyte progenitors and monocyte-dendritic cell progenitors independently produce functionally distinct monocytes. Immunity. 2017:47(5):890–902 e4. 10.1016/j.immuni.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Czernielewski JM, Demarchez M. Further evidence for the self-reproducing capacity of Langerhans cells in human skin. J Invest Dermatol. 1987:88(1):17–20. 10.1111/1523-1747.ep12464659 [DOI] [PubMed] [Google Scholar]
- 130. Kanitakis J, Morelon E, Petruzzo P, Badet L, Dubernard JM. Self-renewal capacity of human epidermal Langerhans cells: observations made on a composite tissue allograft. Exp Dermatol. 2011:20(2):145–146. 10.1111/j.1600-0625.2010.01146.x [DOI] [PubMed] [Google Scholar]
- 131. Mielcarek M, Kirkorian AY, Hackman RC, Price J, Storer BE, Wood BL, Leboeuf M, Bogunovic M, Storb R, Inamoto Y, et al. Langerhans cell homeostasis and turnover after nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation. Transplantation. 2014:98(5):563–568. 10.1097/TP.0000000000000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Masopust D, Sivula CP, Jameson SC. Of mice, dirty mice, and men: using mice to understand human immunology. J Immunol. 2017:199(2):383–388. 10.4049/jimmunol.1700453 [DOI] [PMC free article] [PubMed] [Google Scholar]