Abstract
Purpose
3D bioprinting is capable of rapidly producing small-scale human-based tissue models, or organoids, for pathology modeling, diagnostics, and drug development. With the use of 3D bioprinting technology, 3D functional complex tissue can be created by combining biocompatible materials, cells, and growth factor. In today's world, 3D bioprinting may be the best solution for meeting the demand for organ transplantation. It is essential to examine the existing literature with the objective to identify the future trend in terms of application of 3D bioprinting, different bioprinting techniques, and selected tissues by the researchers, it is very important to examine the existing literature. To find trends in 3D bioprinting research, this work conducted an systematic literature review of 3D bioprinting.
Methodology
This literature provides a thorough study and analysis of research articles on bioprinting from 2000 to 2022 that were extracted from the Scopus database. The articles selected for analysis were classified according to the year of publication, articles and publishers, nation, authors who are working in bioprinting area, universities, biomaterial used, and targeted applications.
Findings
The top nations, universities, journals, publishers, and writers in this field were picked out after analyzing research publications on bioprinting. During this study, the research themes and research trends were also identified. Furthermore, it has been observed that there is a need for additional research in this domain for the development of bioink and their properties that can guide practitioners and researchers while selecting appropriate combinations of biomaterials to obtain bioink suitable for mimicking human tissue.
Significance of the Research
This research includes research findings, recommendations, and observations for bioprinting researchers and practitioners. This article lists significant research gaps, future research directions, and potential application areas for bioprinting.
Novelty
The review conducted here is mainly focused on the process of collecting, organizing, capturing, evaluating, and analyzing data to give a deeper understanding of bioprinting and to identify potential future research trends.
Keywords: 3D bioprinting, Biomaterials, Bioink, Meta-analysis, Systematic literature review
Introduction
To build 3D structures for bio-applications, layer-by-layer precise positioning of biological materials, biochemicals, and living cells is used in 3D bioprinting, along with spatial control of the positioning of functional components [1]. The technology has emerged as a key driver by precise deposition and assembly of biomaterials with patient's/donor cells. It is described as a collection of methods that use computer-aided printing technology to deposit living cells, biomaterials, extracellular matrix (ECM) elements, biochemical factors, proteins, or medications on a receptive solid or surface [2]. There are a few things that all three-dimensional printing methods have in common: (a) creating blueprints or computer-aided designs through pre-processing using surface laser scan, computed tomography, magnetic resonance, or other imaging modalities; (b) processing to create a real-world 3D duplicate of a created model; and (c) post-processing to accelerate organ development and transplantation [3]. Inkjet, micro extrusion, and laser-assisted bioprinting are the three basic types of 3D bioprinting technology [4]. Bioink or cells are delivered thermally or acoustically in inkjet bioprinting. In extrusion bioprinting process by applying a precise amount of bioink through a needle with the help of pneumatic pressure or mechanical pistons, bioprinting constructs are created, producing a three-dimensional structure. A high-energy laser is used to create a high-pressure system that ejects the biomaterial onto the collecting plate in laser-assisted bioprinters [5]. To create delicate structures, 3D bioprinting relies on the micrometer-scale deposition of biomaterials that either enclose cells or are afterward loaded with cells. Extruders that print bioink are often managed by a three-axis mechanical platform. The coordinates set by the designer and saved in a file format like G-code, which the printer can easily follow, regulate the algorithm and form that are necessary for this platform's movement.
Due to benefits including precise deposition, cost-effectiveness, simplicity, and controllable cell dispersion, 3D bioprinting has grown rapidly in popularity in recent years. As a result, there is a greater need for novel bioinks with the attributes necessary for appropriate printing, such as printability, printing fidelity, and mechanical qualities. This has resulted in a need for extensive research and development. The contributions of the researchers working in this field have created awareness about the 3D bioprinting and development of new bioink which can be suitable for human transplantation. The new bioink should be biocompatible when implants are to be used in a patient's body for a longer duration of time [6]. An investigation of the current bioprinting literatures is needed for this aim, as it has been done by many researchers in their field [7]. A literature review is also necessary to examine current biomaterials, identify new biomaterials, printing methods, and medicinal applications for bioprinting, all of which are crucial for successful bioprinting development. Important research topics pursued by this domain's researchers must also be visible at a glance. This type of research can suggest future research directions in this sector, which will be useful to a wide range of academics and practitioners.
As a result, to address the aforementioned issues, it is required to review the bioprinting literature. The first question for research is.
Que 1: What is the state of the bioprinting literature right now, where are the research gaps, and what are the expected trends?
It is simple to understand the extent to which the various bioprinting-related disciplines have been studied by earlier researchers, as well as the gaps and potential future developments. Because researchers had studied bioprinting using a variety of biomaterials and bioprinting methodologies, it was required to review the articles to acquire relevant information. As a result, the second research question was:
Que 2: What biomaterials and bioprinting processes have been studied or applied in previous bioprinting research?
This leads to the review of development of biomaterials and bioprinting processes for future usage.
Finding innovative biomaterials combinations for bioink development is a critical aspect in tissue growth and development. Although researchers have attempted to improve the bioprinting performance, more advancement in 3D printing technology are needed to boost the resolution without sacrificing scaffold design, strength, or flexibility. While emerging difficulties originating from an innovative approach to bioprinting were discussed, less emphasis was given on producing a new bioink for performance enhancement. The author’s attempts to raise awareness of bioprinting research by identifying key participants, interventions, and outcomes during the last two decades of research. The objective of present research is to identify the top universities, regions, journals, publishers, and authors of bioprinting research publications. The third important question is therefore,
Que 3: What are the trends in publications year over year, country over country, and publisher and university involvement in bioprinting research?
Six sections comprise this paper, the current one being one of them. Section 2 discusses the literature review on bioprinting, while Sect. 3 discusses the classification of the analysis and the study technique. Section 4 describes how articles were analyzed using several criteria that were included in the classification. Section 5 consists of a summary and discussion. The study's conclusion is detailed in Sect. 6, along with the study's key results, gaps, ramifications, and future trends.
Literature Review
The current section is divided into subsections and discusses existing literature review research publications. The background of bioprinting was studied and summarized based on existing research articles.
General Trends and Issues in the Bioprinting
The 3D bioprinting utilizes 3D printing techniques using biomaterials to manufacture biomedical parts. Table 1 compares extrusion, inkjet, stereo lithography, and laser-assisted bioprinting methods, with extrusion-based bioprinting being the mostly used.
Table 1.
Comparison of extrusion, inkjet, stereolithography, and laser-assisted bioprinting methods
| Method of bioprinting | Advantage | Disadvantage | Parameters | References |
|---|---|---|---|---|
| Extrusion | Printing diverse biomaterials is simple, capable of printing high cell densities | Works very well with sticky liquids only | Speed is slow | [8, 9] |
| Cell viability is 89.46 ± 2.51% | ||||
| Cell density is High | ||||
| Resolution is 100 µm | ||||
| Viscosity is 30–6 × 107 mPa s | ||||
| Inkjet | Simple biomaterial printing capability, low cost, high resolution and rapid production speed | Low cell densities, unable to give continuous flow, and not able to give vertical structural functionality | Speed is fast |
[10], [9] |
| Cell viability is 80–95% | ||||
| Cell density is Low | ||||
| Resolution is 50 µm | ||||
| Viscosity is < 10 mPa s | ||||
| Stereolithograpy | This method has the advantages of being nozzle-free, excellent accuracy, complex structure | The inability to print multi-cells, the toxicity of UV light to cells, and cell damage during photocuring | Speed is fast | [9, 11] |
| Cell viability is > 90% | ||||
| Cell density is Medium | ||||
| Resolution is 100 µm | ||||
| No limitation on Viscosity | ||||
| Laser-assisted | Resolution is very high for deposition of biomaterial in the solid or liquid phase | High price and thermal damage brought on by nanosecond/femtosecond lasers | Speed is medium | [4, 9] |
| Cell viability is < 85% | ||||
| Cell density is Medium | ||||
| Resolution is 10 µm | ||||
| Viscosity is 1–300 mPa s |
The bioink's rheological properties and surface tension, process-induced mechanical forces, printing flow rate control, and crosslinking mechanisms are all important qualities for bioprinting [12]. The important design elements in bioprinting include shape and resolution, cellular-material remodeling dynamism, and material heterogeneity [13]. 3D bioprinted components have been used for applications such as drug delivery and discovery, research models, and toxicology.
Reviews of the Available Literature on Bioprinting
Before starting the literature review, it is necessary to research prior literature review papers from the relevant subject. A thorough awareness of current advances and research fields can be acquired by carefully examining the available literature review publications. Table 2 presents the findings of a comprehensive examination of recent papers in the bioprinting domain.
Table 2.
Analysis of available literature review articles on bioprinting
| Title | Year | Biomaterial studied | Study focus | Study finding | Total citations |
|---|---|---|---|---|---|
| Synthesis and application of fish gelatin for hydrogels/ composite hydrogels: A review | 2022 | Gelatin | Fabrication of fish gelatin | Gelatin's tripeptide pattern promotes cell adhesion | 3 |
| Conductive and injectable hyaluronic acid/gelatin/gold Nano rod hydrogels for enhanced surgical translation and bioprinting | 2022 | Hyaluronic acid, gelatin hydrogel | High aspect ratio citrate-gold nanorods (GNRs) were added to a hyaluronic acid and gelatin hydrogel to generate a conductive hydrogel | There are three main obstacles to be addressed in the development of conductive biomaterials: (3) Many conductive biomaterials are pre-formed scaffolds that cannot be injected. (1) Many conductive components are cytotoxic. (2) Many conductive biomaterials are pre-formed scaffolds that cannot be injected | 0 |
| Development of Silk Fibroin Scaffolds by Using Indirect 3D-Bioprinting Technology | 2022 | Silk fibroin | Using indirect 3D bioprinting technology, natural polymer silk fibroin can be used to create new scaffolds | The flexibility of the scaffolds could be regulated by changing the solvent for the silk fibroin solution used to make them | 4 |
| Printable gelatin, alginate and boron nitride nanotubes hydrogel-based ink for 3D bioprinting and tissue engineering applications | 2022 | Alginate, Gelatin, Boron nitride nanotubes (BNNTs) | A new printable hydrogel-based ink solution was created using gelatin-alginate (GA) and boron nitride nanotubes (BNNTs) | Increasing the concentration of BNNTs in GA resulted in a larger compressive stress load | 3 |
| Hybrid bio fabrication of 3D osteo conductive constructs comprising Mg-based nanocomposites and cell-laden bioinks for bone repair | 2022 | Magnesium hydroxide nanoparticles (Mg), polycaprolactone (PCL) | The creation of a strong and bioactive bone regeneration scaffold for 3D printing using magnesium hydroxide nanoparticles (Mg) and polycaprolactone (PCL) thermoplastic nanocomposite biomaterial ink (Mg-PCL) | In an accelerated-degradation assay, Mg-PCL degrades faster than standard PCL, which has implications for in vivo implant degradation and bone regeneration | 4 |
| Challenges and recent trends with the development of hydrogel fibre for biomedical applications | 2022 | Nano-cellulose-based hydrogel | Current hydrogel development trends and challenges for biological applications | Surface energy, intermolecular interactions, and hydrogel adhesion force interactions are all key issues in the production of hydrogels | 2 |
| Chitosan as an underrated polymer in modern tissue engineering | 2021 | Chitosan | Chitosan and its alterations in a unique application | The molecular structure of chitosan, as well as the presence of active chemical groups, allows for material customization to fit specific needs | 2 |
| Clay minerals as bioink ingredients for 3d printing and 3d bioprinting: Application in tissue engineering and regenerative medicine | 2021 | Clays, hydroxyapatite, graphene, carbon nanotubes, silicate nanoparticles | Use of clays (both natural and synthetic) for tissue engineering and regenerative medicine | Clay is a naturally occurring substance with well-known biocompatibility and bioactivity, making it ideal for this cutting-edge technology | 1 |
| The effect of silk–gelatin bioink and TGF-β3 on mesenchymal stromal cells in 3D bioprinted chondrogenic constructs: A proteomic study | 2021 | Silk fibroin–gelatin (SF–G) bioink | An SF-G-based 3D bioprinted construct can be used for articular cartilage | SF-G bioink enhanced various chondrogenic pathways, including Wnt, HIF-1, and Notch, when combined with hMSCs | 1 |
| Nano clay Reinforced Biomaterials for Mending Musculoskeletal Tissue Disorders | 2021 | Graphene, carbon nanotubes, MXenes, nanoclays | Application of nanomaterials for in vivo study | Properties of Nanomaterials including graphene, carbon nanotubes, MXenes, and nanoclays can be transferred to biomaterials by a simple inclusion technique | 2 |
| Pectin as rheology modifier of a gelatin-based biomaterial ink | 2021 | Gelatin, pectin, GPTMS(3-glycidyloxypropyltrimethoxysilane) | Using pectin as a rheology modification of gelatin to improve gelatin bioprinting performance | Pectin improves the viscosity and yield stress of gelatin solutions with a low viscosity | 6 |
| Determination of the geometrical and viscoelastic properties of scaffolds made by additive manufacturing using bio plotter | 2017 | Polylactic acid (PLA) and, polyhydroxybutyrate (PHB), | On the qualitative, mechanical, and geometrical features of printed items, printing parameters and technological pre-processing of the material have an impact | The material is more stable once it has been dried before it is printed | 0 |
| A dual crosslinking strategy to tailor rheological properties of gelatin methacryloyl | 2017 | Gelatin methacryloyl (GelMA) | An enzymatic crosslinking method powered by Ca2 + -independent microbial transglutaminase (MTGase) was introduced to catalyze the development of isopeptide linkages between chains of GelMA, which may improve its rheological characteristics, mainly its viscosity | It is feasible to adjust the fluid viscosity and rapidly stabilize the gelatin macromolecules by combining enzymatic crosslinking and light crosslinking | 35 |
| Bioprinting and bio fabrication with peptide and protein biomaterials | 2017 | Protein, peptide-derived biomaterials | Application specific, peptide-based bioprinting approaches | Numerous regenerative applications, including both organ bioprinting and non-organ bioprinting, have made use of these materials' capacity to produce highly printable hydrogels that are similar to the natural ECM | 15 |
| Development of scaffolds for vascular tissue engineering: Biomaterial mediated neovascularization | 2017 | Synthetic polymers with polysaccharides | The biocompatibility and mechanisms underlying stem cells' proliferation, migration, adhesion, differentiation, and organization in vascular networks are highlighted in recent research on the polymers and scaffolds used to improve neovascularization | Conjugation of synthetic polymers with polysaccharides or proteins attempt to improve the biocompatibility of scaffolds | 3 |
| Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications | 2017 | Mitogenic hydrogel, alginate, sulfate | To convert alginate sulfate to a printable bioink by combining with nanocellulose | The non-printed bioink substance encouraged cell spreading, proliferation, and collagen II synthesis by the encapsulated cells while the alginate sulfate/nano-cellulose ink demonstrated good printing capabilities | 261 |
| Bio-printing cell-laden Matrigel-agarose constructs | 2016 | Matrigel, agarose | Development of a hybrid Matrigel-agarose hydrogel system | Agarose helps in maintenance of 3D-printed structures, Matrigels provides essential microenvironment for cell growth | 83 |
| Four-Dimensional Printing Hierarchy Scaffolds with Highly Biocompatible Smart Polymers for Tissue Engineering Applications | 2016 | Poly-caprolactone | 4D printing of novel biomimetic gradient tissue | Glass transition temperature range is in 8 °C to 35 °C | 98 |
| Tissue engineering with gellan gum | 2016 | Gellan gum | Purification and modification of gellan gum | Gellan gum is an anionic polysaccharide | 90 |
| Differences in time-dependent mechanical properties between extruded and molded hydrogels | 2016 | Gelatin | Mechanical and swelling properties of conventional molded gelatin-based hydrogel | Young's modulus and the ideal extruding pressure increased with the amount of polymer used while printing resolution increased with both printing speed and nozzle gauge | 23 |
| Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers | 2016 | Poly-caprolactone (PCL) | Use of synthetic polymer in in fabrication of cell-printed constructs | PCL layer prevent the thermal damage | 50 |
| Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting | 2016 | Gelatin-methacryloyl (gelMA), gellan gum | Investigating whether gelMA/gellan is appropriate for cartilage bioprinting | A promising bioink is gelatin-methacryloyl (gelMA) combined with gellan gum | 2 |
| Bio plotting of a bioactive alginate dialdehyde-gelatin composite hydrogel containing bioactive glass nanoparticles | 2016 | Alginate dialdehyde-gelatin (ADA-GEL), BGNPs | Bio-plotting of bioactive alginate | EDS analysis suggested that the BGNPs loading promoted the growth of bone-like apatite laye | 69 |
| Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells | 2016 | Alginate, carboxymethyl-chitosan, agarose | Human neural stem cells have been used to print neural tissue | Bicuculline-induced enhanced calcium response in differentiated neurons | 256 |
|
Polyvinyl alcohol-graft-polyethylene glycol hydrogels improve utility and bio functionality of injectable collagen biomaterials |
2016 | Collagen | Interactions of polyvinyl alcohol blend variants, as non-polymer surfactants | Stabilization of collagen solution by addition of Polyvinyl alcohol hydrogel | 8 |
| Bio-Orthogonally Crosslinked, Engineered Protein Hydrogels with Tunable Mechanics and Biochemistry for Cell Encapsulation | 2016 | Arginine–glycine–aspartic acid (RGD) | Development of engineered elastin-like proteins (ELPs) | ELP hydrogels with SPAAC crosslinks are appealing materials for therapeutic cell injection and bioprinting | 114 |
| Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair | 2016 | Gelatin-methacryloyl (gelMA) | Development of GelMA hydrogel by changing combination of gelatin's inherent bioactivity and photo-cross linkable hydrogels' physicochemical tailor ability | GelMA will enhance the development of bio fabricated constructions containing cells | 484 |
|
Investigation of thermal degradation with extrusion-based dispensing modules for 3D bioprinting technology |
2016 | Poly-lactic-co-glycolic acid (PLGA) | Polylactic-co-glycolic acid(PLGA) prepared by syringe type and filament type | The Filament dispensing module retained the characteristics of the PLGA scaffold, but the syringe dispensing module caused thermal deterioration | 21 |
| Recent progress in stem cell differentiation directed by material and mechanical cues | 2016 | Polyacrylamide (PAM),polydimethylsiloxane (PDMS) | The use of biophysical signals to drive stem cell differentiation could be beneficial | The main materials are polyacrylamide (PAM) and polydimethylsiloxane (PDMS) hydrogels | 68 |
| Silk fibroin as biomaterial for bone tissue engineering | 2016 | Silk fibroin (SF) | different fabrication and functionalization methods of silk fibroin | Silk fibroin and HA were combined to generate a composite scaffold that closely resembles the natural bone environment | 542 |
| Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering | 2016 | Chitosan | chitosan-based biomaterials modified for functional 3D bioprinting | Chitosan-based hydrogels have outstanding room-temperature printability, high shape accuracy in printed 3D constructions, and strong biocompatibility with fibroblast skin cells | 206 |
| Methacrylate gelatin and mature adipocytes are promising components for adipose tissue engineering | 2016 | Methacrylate gelatin (GM) | For the composition of fatty tissue equivalents in vitro, mature adipocytes are a hugely relevant cell source | Methacrylate gelatin that can be photo crosslinked is an effective tissue scaffold | 90 |
Researchers Lapomarda et al. [14] identified that pectin can be used for enhancing the rheological properties of gelatin. Pectin improves viscosity as well as yield stress of solution made from gelatin. According to Boyd-Moss et al. [15] protein- and peptide-based biomaterials can easily imitate the native ECM by forming highly printable hydrogels. Further, Müller et al. [16] investigated that the combination of alginate sulfate and nano-cellulose had good printability; whereas, Fan et al. [17] developed a hybrid matrigel-agarose hydrogel system, which had provided essential microenvironment for cell growth. Miao et al. [18] discussed about 4D printing of novel biometric gradient tissues. Stevens et al. [19] discussed about purification and modification methods of gellan gum; however, Ersumo et al. [20] highlighted the mechanical and swelling properties of gelatin-based hydrogel and also discussed the ways of enhancing the mechanical properties. Additionally, Kim et al. [21] discussed about the use of synthetic polymer for cell-printed constructs and Mouser et al. [22] investigated the suitability of GelMA supplemented with gellan gum, and found that this combination can be suitable to use as a bioink. Leite et al. [23] investigated the bio-plotting characteristics of bioactive alginate and by EDS (energy-dispersive X-ray spectroscopy) analysis, it was observed that growth of bone-like apatite layer happened. However, Gu et al. [24] discussed printing neural tissue from human neural stem cells that have in situ grown into active neurons and supporting neuroglia. Further, Hartwell et al. [25] discussed about the stabilization of collagen solution by the addition of polyvinyl alcohol hydrogel and Madl et al. [26] developed the engineered elastin-like protein, which can be a appealing material for therapeutic cell injection by SPAAC(strain-promoted azide-alkyne cycloaddition) crosslink; whereas, Lee et al. [27] investigated the PLGA (poly D,L-lactic-co-glycolic acid) scaffold preparation method by syringe type and filament type based on extrusion-based dispensing. Researchers observed that filament-based module was more suitable. Melke et al. [28] discussed regarding different fabrication, functionalization method of silk fibroin and observed that silk fibroin combined with HA(hydroxyapatite) can provide scaffold that closely resembles the natural bone. The two tissue research investigations below indicate how the use of scaffolds composed of various biomaterials might improve bone growth naturally. The presence of mesenchymal stem cells, chondroprogenitor cells, and pluripotent cells can be enhanced in an in vitro examination applying thermos-reversible gelatin, which may be useful for treating osteoarthritic chondrocytes in elderly patients [29]. SCID mice with severe combined immunodeficiency (SCID) were used to study bone formation using β-tricalciumphosphate (β-TCP) scaffolds coated with hBMSC and hRIA-MSC [30].
The Research Strategy Implemented in the Study
Every research project should include literature analysis because it enables you to examine current trends and active areas in a certain field. The selection and categorization of the articles will be covered in this part.
Criteria for Selection of Articles
The main objective of this analysis is to use the Scopus database to discuss developments and research areas in the field of bioprinting. All academic papers having the phrase "Bioprinting and Biomaterial" in their titles or keywords that had been published in peer-reviewed journals were indexed for this study. Book chapters, brief notes, conference papers, and editorial remarks were among the items that were initially filtered out. This study includes articles authored between 2004 and 2022. It would be difficult to include bioprinting in a single discipline due to its wide range of applications; as a result, peer-reviewed publications from respected publishers including Taylor & Francis, Elsevier, MDPI AG, Emerald, and SAGE Publications Ltd. have been chosen for research purposes. These publications are recommended since they provide high-quality papers on bioprinting and have numerous applications in several sectors. The exploring method used to choose this analysis produced 1002 articles encompassing a variety of contexts, including biomaterial, bioink, bioprinting methods, etc. These articles were then reviewed utilizing a system of groupings between different points of view. The selection and classification of articles are shown in Fig. 1.
Fig. 1.
Flow chart for the research methodology
In summary, 1002 research articles on the topic of bioprinting and biomaterials were chosen for the study. It i probable that some crucial publications were missed, though, because the authors worked through a procedure before selecting the papers for the study. In an effort to identify biomaterials, bioprinting techniques, and applications, the papers now are further categorized based on the many parameters as shown. The study's conclusions also analyzed its findings, research gaps, implications, and future research direction. The classification strategy developed themes, and research findings are expected to be useful to both researchers and clinicians.
Classification Scheme for Analysis
For the examination of research publications on bioprinting, a classification technique was suggested. The following nine essential criteria are used to evaluate and categorize papers:
Classification based on year of publication.
Classification based on articles and publishers.
Classification based on nation.
Classification based on affiliation of author.
Authors who are currently working in bioprinting.
Top bioprinting publications in terms of citations.
Natural and synthetic polymer-based biomaterial classification. Classification on the basis of Polymer based biomaterials
Bioprinting method-based classification.
Application area of bioprinting.
The classification described above will provide a roadmap for additional research by highlighting the domain's progressive development, research types, applications, and obstacles.
Evaluation of Articles Based on Bioprinting
The short-listed research article of bioprinting, along with their detailed analysis, is discussed in the current section to provide some insight into the research topic.
Classification Based on Year of Publication
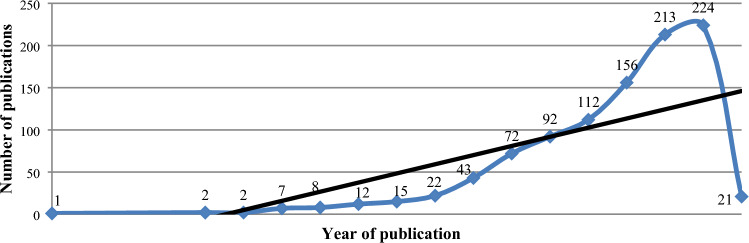
In this study, articles published between 2004 and 2022 were selected, for the year 2022 articles were selected up to January. Figure 2 represents the regression line and trends for the number of articles published per year. Linear trend represents the most appropriate points for the selection and that is why it was selected.
Fig. 2.
No. of articles published per year
Because of the trend line's positive linear shape, there has been an increase in publications since 2011. Since the number of publications has also significantly increased since 2014 and will continue to do so until 2021. The regression equation for the obtained curve shows that there has been and will continue to be an increase in the number of research publications. Additionally, the number of articles published over the past ten years has increased by more than 60%, demonstrating the growing significance of bioprinting in view of the growing demands for organ transplants, drug testing, and biological research models purpose. Various traditional methods available like autograft, allograft, and xenograft are having their own advantages and disadvantages and these methods cannot fulfill the demand needs of patients waiting for transplantation. On the other hand, there is scarcity of biological models for research and drug testing. So, for overcoming the above-mentioned challenges, bioprinting is the best way in which cells, proteins, and biomaterials are used as building blocks for 3D-printed biological models, biological systems, and pharmaceutical products [31]. This analysis helps in answering research question number one and three.
Classification Based on Articles and Publishers
The electronic database in which the chosen research publications were published was used to further categorize them. The Institute of Physics Publishing, John Wiley and Sons Inc., Elsevier Ltd., Elsevier B.V., and MDPI (Multidisciplinary Digital Publishing Institute) are the primary electronic databases mentioned in this research (Multidisciplinary Digital Publishing Institute). Table 3 provides a summary of the distribution of articles based on journals and publishers for the top 10 journals. The answers to research questions one and three may be found with the help of this analysis.
Table 3.
Journal-based classification
| Sr. no | Source title | Publisher | Total articles | Citation* | H-index# | Main theme of research |
|---|---|---|---|---|---|---|
| 1 | Bio fabrication | Institute of Physics Publishing | 63 | 5264 | 80 | Crosslinking, hydrogel scaffold, bioink development |
| 2 | Advanced Healthcare Materials | John Wiley and Sons Inc | 43 | 2013 | 90 | Bioink, silk fibroin, bone tissue engineering |
| 3 | Acta Biomaterialia | Elsevier Ltd | 42 | 3383 | 190 | Bone regeneration, cartilage tissue engineering, hyaluronic acid |
| 4 | Bioprinting | Elsevier B.V | 36 | 132 | 16 | Extrusion-based bioprinting, gelatin, biocompatibility |
| 5 | International Journal Of Molecular Sciences | MDPI Multidisciplinary Digital Publishing Institute | 28 | 449 | 162 | Scaffold, antibacterial polymer, bioink development |
| 6 | Biomaterials | Elsevier BV | 27 | 4280 | 381 | Bioink, GelMa, hydrogel |
| 7 | Biomedical Materials Bristol | IOP Publishing Ltd | 16 | 367 | 72 | Hydrogel, biomaterial, microfabrication |
| 8 | Tissue Engineering Part B Reviews | Mary Ann Liebert Inc | 16 | 673 | 91 | Bioink, skin bioprinting, bone substitutes |
| 9 | ACS Biomaterials Science And Engineering | American Chemical Society | 15 | 513 | 50 | Hydrogel, GelMA, bone tissue engineering |
| 10 | ACS Applied Materials And Interfaces | American Chemical Society | 14 | 348 | 50 | Mechanical properties, GelMA, biomaterials |
*Citations are for specific publications that have been evaluated in that particular journal and can be found at https://www.scimagojr.com
Classification Based on Nation
A close examination of the chosen papers demonstrates that bioprinting is a global phenomenon, with publications in 67 nations. Because of the growing demand for organ transplantation, 3D bioprinting is gaining popularity due to its capacity to print a variety of cell types in specific spatial areas, making it suited to regenerative medicine to address the demand for transplantable organs and tissue[32]. Several governments are encouraging scientists to conduct bioprinting research and develop cutting-edge technology. Table 4 summarizes the top ten countries that contribute to the bioprinting domain. This analysis can assist to find answer for research questions one and three.
Table 4.
Nation-based bioprinting articles
| Sr. no | Nation | No. of articles | No. of citation | Topics of research |
|---|---|---|---|---|
| 1 | United States | 374 | 22,765 | Bioink development, hydrogel |
| 2 | China | 176 | 3802 | Biomaterial selection, cartilage regeneration |
| 3 | Germany | 84 | 2175 | Alginate-based bioink, bioink development |
| 4 | South Korea | 84 | 2153 | Crosslinking mechanism |
| 5 | United Kingdom | 70 | 1380 | Skin regeneration, medical device development |
| 6 | India | 67 | 1653 | Biomaterials development, printing process parameters |
| 7 | Australia | 62 | 1750 | Printability, bioethics |
| 8 | Canada | 54 | 1848 | Hybrid Scaffold, bone tissues engineering |
| 9 | Netherlands | 52 | 3837 | Drug delivery, scaffold |
| 10 | Italy | 42 | 505 | Standardization, scaffold, tissue regeneration |
According to Table 4, the US has contributed a total of 374 articles. Researchers from nations like China, Germany, South Korea, the United Kingdom, and India have contributed to the study's foundation and strength.
Classification Based on Affiliation of Author
Researchers who have written about bioprinting are associated with a university. Researchers claim that the increased demand for organ transplants and the scarcity of donors are compelling scientists and medical professionals to adopt the bioprinting idea, which makes complex tissue architectures simple to print. Researchers working with various universities are motivated by the current situation to conduct bioprinting research. Examining the contributions made by various nations in this sector was necessary to comprehend the future trend in this domain. 160 universities from around the world have submitted 1002 articles on bioprinting. Research questions one and three of this study are addressed in Table 5, which provides information about the top 10 universities.
Table 5.
University-based classification of bioprinting articles
| Sr. no | Affiliation | No. of documents | No. of citation* | Main theme |
|---|---|---|---|---|
| 1 | Harvard Medical School | 44 | 5269 | Bone defect, GelMa, bioink development |
| 2 | Brigham and Women's Hospital | 37 | 4724 | Bone regeneration, hydrogels, cell-laden fibers |
| 3 | Massachusetts Institute of Technology | 31 | 5338 | Regenerative engineering, hydrogels, bone regeneration |
| 4 | Wake Forest School of Medicine | 26 | 3096 | Bioink, collagen, biocompatibility |
| 5 | Harvard-MIT Health Sciences and Technology | 26 | 3835 | Vascularization, hydrogels, Tissue Engineering |
| 6 | University of California, Los Angeles | 24 | 1179 | Multi-material 3D bioprinting, personalized medicine, tissue engineering |
| 7 | Chinese Academy of Sciences | 21 | 1125 | Skin tissue engineering, scaffold, bone tissue engineering |
| 8 | Pohang University of Science and Technology | 20 | 1296 | Natural polymer, stem cell engineering, silk-based bioink |
| 9 | Harvard University | 18 | 3025 | Bioink development, bioprinting, hydrogels |
| 10 | Tsinghua University | 18 | 1429 | Biomaterials, hydrogels, tissue engineering |
*Determines the references for each document from a certain university that has been reviewed
44 scientific articles from Harvard Medical School addressed important issues such as bone deformity and bioink creation. Silk-based bioink is being developed by scientists at Pohang University of Science and Technology.
Authors Who are Currently Working in Bioprinting
Researchers and practitioners had contributed to bioprinting research and tried to develop bioink and select suitable combination of biomaterial and cells as per the requirement of tissue application. The contributions of numerous researchers are listed in this article, and Table 6 lists the top ten authors.
Table 6.
Active author-based classification of bioprinting articles
| Sr. No | Author name | Total article | Total citations* | Overall h-index # | Theme |
|---|---|---|---|---|---|
| 1 | Khademhosseini, A | 25 | 93,369 | 153 | Bioengineering, drug delivery, biomaterials, tissue engineering, regenerative medicine |
| 2 | Malda, J | 16 | 19,007 | 69 | Bio fabrication in translation |
| 3 | Cho, D.W | 15 | 19,860 | 76 | 3D printing, cell printing, tissue engineering |
| 4 | Zhang, Y.S | 14 | 21,113 | 73 | 3D bioprinting, biomaterials, regenerative engineering, organ-on-a-chip, bioanalysis |
| 5 | Moroni, L | 13 | 11,490 | 55 | Biofabrication 3D cell culture, scaffolds, regenerative medicine, biomaterials |
| 6 | Ashammakhi, N | 9 | 9312 | 49 | Tissue engineering, biomaterials, 3D printing, organ-on-a-chip |
| 7 | Gelinsky, M | 9 | 11,017 | 57 | Biomaterials, tissue engineering, 3D printing, bioprinting, bio fabrication |
| 8 | Burdick, J.A | 8 | 41,355 | 113 | Biomaterials, tissue engineering, bioengineering, hydrogels |
| 9 | De Maria, C | 8 | 1656 | 19 | Biofabrication, additive manufacturing, open source medical devices |
| 10 | Dokmeci, M.R | 8 | 22,047 | 77 | Bioengineering, biosensors, organs on a chip, biomaterials |
*Citations are for specific documents that the author has studied; data were obtained from Google Scholar
Khademhosseini A. addressed many applications of bioprinting with his maximum contribution of twenty-five articles, including bioengineering, drug delivery, biomaterials, tissue engineering, and regenerative medicine. During his work, he explained about cell responsive behavior of gelatin methacrylate (GelMA). Furthermore, the researcher Malda, J. contributed sixteen articles on bio fabrication in translation. Researchers worked on designing of porous scaffold in which by varying PEGT (poly-ethylene glycol-terephthalate)/PBT (poly-butylene terephthalate) composition, porosity and pore geometry 3D-deposited scaffolds were produced with a range of mechanical properties [33]. This analysis can assist to find answer for research questions one and three.
Top Bioprinting Publications in Terms of Citations
Numerous experts in the field of bioprinting contributed to the investigations and wrote papers that were included in prestigious journals. It was important to find major publications in this topic to learn more about bioprinting. As indicated in Table 7, the authors of this research attempted to identify the top ten most cited articles in the field of bioprinting.
Table 7.
Top citation-based classification of bioprinting articles
| Sr. no | Authors | Title | Year | Source title | Citation | Theme |
|---|---|---|---|---|---|---|
| 1 | Kang H.-W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A | A 3D bioprinting system to produce human-scale tissue constructs with structural integrity | 2016 | Nature biotechnology | 1342 | Tissue construct |
| 2 | Malda J., Visser J., Melchels F.P., Jüngst T., Hennink W.E., Dhert W.J.A., Groll J., Hutmacher D.W | 25th anniversary article: Engineering hydrogels for bio fabrication | 2013 | Advanced materials | 1028 | Design and tailoring of hydrogels |
| 3 | Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A | Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels | 2015 | Biomaterials | 962 | GelMA-based hydrogels |
| 4 | Chia H.N., Wu B.M | Recent advances in 3D printing of biomaterials | 2015 | Journal of biological engineering | 897 | Biomaterials review |
| 5 | Mandrycky C., Wang Z., Kim K., Kim D.-H | 3D bioprinting for engineering complex tissues | 2016 | Biotechnology Advances | 680 | Review on complex bioprinting |
| 6 | Kim Y., Yuk H., Zhao R., Chester S.A., Zhao X | Printing ferromagnetic domains for untethered fast-transforming soft materials | 2018 | Nature | 654 | ferromagnetic domains program for soft material |
| 7 | Guillotin B., Souquet A., Catros S., Duocastella M., Pippenger B., Bellance S., Bareille R., Rémy M., Bordenave L., Amédée j J., Guillemot F | Laser assisted bioprinting of engineered tissue with high cell density and microscale organization | 2010 | Biomaterials | 471 | Precision for printing miniaturized tissue |
| 8 | Jia W., Gungor-Ozkerim P.S., Zhang Y.S., Yue K., Zhu K., Liu W., Pi Q., Byambaa B., Dokmeci M.R., Shin S.R., Khademhosseini A | Direct 3D bioprinting of perfusable vascular constructs using a blend bioink | 2016 | Biomaterials | 457 | Bioink formulation |
| 9 | Hölzl K., Lin S., Tytgat L., Van Vlierberghe S., Gu L., Ovsianikov A | Bioink properties before, during and after 3D bioprinting | 2016 | Bio fabrication | 453 | Review on effect of cells on hydrogel processing |
| 10 | Schuurman W., Levett P.A., Pot M.W., van Weeren P.R., Dhert W.J.A., Hutmacher D.W., Melchels F.P.W., Klein T.J., Malda J | Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs | 2013 | Macromolecular bioscience | 441 | Study of GelMA hydrogel |
With 1342 citations, the research publications authored by Kang et al. [34] was the most cited of the 1002 articles. The integrated tissue–organ printer (ITOP) described in this paper can build any shape of stable, human-scale tissue constructs. Madl et al. [26] aimed to develop new ideas and improvements in hydrogel design and tailoring. Yue et al. [35] aimed to identify GelMa-based hydrogels application to engineer cardiac, vascular, bone, and cartilage tissues. These articles may assist researchers, particularly beginners, in better understanding of the bioprinting concept. As previously stated, this knowledge solves research question number one and three.
Classification on the Basis of Polymer-Based Biomaterial
Biomaterials serve a variety of uses in prosthetics and medical devices. Polymers are the broadest category of biomaterials [36]. Natural and synthetic polymer biomaterial are mostly used for bioprinting. It is important for future researchers to understand what the various polymer-based biomaterial used in bioprinting are. As shown in Table 8, natural biomaterial, Alginate with 101 articles represents its application in tissue engineering for bone, cartilage, and regenerative medicine. Collagen with 68 articles shows application in skin tissue engineering, vascularized bone model.
Table 8.
Classification on the basis of polymer-based biomaterial used
| Sr. no | Biomaterial | Total articles | Application of research |
|---|---|---|---|
| Natural biomaterial | |||
| 1 | Collagen | 68 | Skin tissue engineering, vascularized bone model, skeletal muscle cell |
| 2 | Fibrin | 17 | Vessel substitute, skin tissue engineering, regenerative medicine |
| 3 | Silk | 8 | Bone tissue engineering, biomedicine, therapeutic application |
| 4 | Agarose | 10 | Skeletal muscle, bio-artificial bone, cartilage tissue engineering |
| 5 | Alginate | 101 | Bone tissue engineering, cartilage tissue engineering, regenerative medicine |
| 6 | Hyaluronic | 2 | Wound healing, hydrogel formation |
| 7 | Chitosan | 29 | Bone tissue engineering, regenerative medicine, skin tissue engineering |
| Synthetic biomaterial | |||
| 1 | Poly-lactic acid (PLA) | 212 | Bone tissue engineering, skin regeneration, cartilage tissue engineering |
| 2 | Poly-caprolactone (PCL) | 25 | Bone tissue engineering, skin tissue engineering, regenerative medicine |
| 3 | Polyethylene glycol (PEG) | 4 | Bio-artificial vascular graft, cartilage tissue engineering, shape morphing hydrogel |
| 4 | Polyacrylamide (PAAm) | 3 | Skin tissue engineering, vascularization |
| 5 | Poly-vinyl alcohol (PVA) | 2 | Skin tissue engineering, regenerative medicine, bone tissue engineering |
Synthetic polymer biomaterial, poly-lactic acid (PLA) with 212 articles represents its application in bone tissue engineering, skin regeneration, and cartilage tissue engineering. Poly-caprolactone (PCL) with 25 articles has various applications for regenerative medicine, skin tissue engineering, and bone tissue engineering. This analysis can assist to find answer for research questions one, two, and three.
Bioprinting Method-Based Classification
The medical sector and the manufacturing of medical materials have been radically impacted as a result of the rapid rise of 3D bioprinting in the medical profession. Complex tissues and organ constructs have been created using this technology. 3D bioprinting is divided into several categories based on how it works. Extrusion-based bioprinting, the most popular method, uses bioinks that are continually dispensed or extruded as filaments to create three-dimensional objects[1]. Extrusion-based bioprinting has 31 articles which show its importance in bioprinting techniques. It is mainly used for scaffold development of smart bioink and skin tissue engineering. Thermal inkjet printing with 20 articles was mainly used for its fast fabrication speed and resolution. It is mostly applied in the field of bone tissue engineering, cartilage tissue engineering, and skin tissue engineering as shown in Table 9. This analysis can assist to find answer for research questions one, two, and three.
Table 9.
Classification on the basis of bioprinting method
| Sr. no | Bioprinting method | No. of article | Main theme |
|---|---|---|---|
| 1 | Thermal inkjet printing | 20 | Skin tissue engineering, cartilage tissue engineering, bone tissue engineering |
| 2 | Mechanical/Pneumatic extrusion | 31 | Scaffold development of smart bioink, skin tissue engineering |
| 3 | Laser-guided direct writing | 14 | Skin tissue engineering, vascularized tissue bio fabrication |
| 4 | Stereolithography (SLA) | 2 | Cartilage tissue engineering, scaffold |
| 5 | Digital light processing (DLP) | 4 | Vascularized tissue engineering, cardiac tissue engineering |
Application Area of Bioprinting
Bioprinting is gaining more attention because of its unique feature of recreating the complicated human tissue with accuracy. As there is a lack of donor available for fulfilling the requirement of transplantation, bioprinting is a playing a major role for fulfilling that gap. Table 10 represents various application of bioprinting addressed by several researchers of the selected articles. Cartilage tissue with 46 articles represents highest application of bioprinting. It is used in a variety of applications besides cartilage tissue, including bone tissue engineering, skin tissue, cardiac tissue, heart valve, neural tissue, etc. In various tissue applications, scaffolding is main aim. This analysis can assist to find answer for research questions one, two and three.
Table 10.
Classification on the basis of application area
| Sr. no | Application area | No. of articles | Main research theme |
|---|---|---|---|
| 1 | Bone tissue | 24 | Development of bioink, cell-laden scaffolding |
| 2 | Cardiac tissue | 21 | Cardiac construct, reconstruction of the heart |
| 3 | Cartilage tissue | 46 | In vivo human cartilage formation, scaffolding for nasal cartilage defect |
| 4 | Heart valve | 4 | Cardiac valve, tissue engineering of human heart valve |
| 5 | Neural tissue | 4 | Scaffold to repair the damaged spinal cord, functional 3D neural mini-tissue |
| 6 | Skin tissue | 26 | Scaffold for skin wound healing, skin disease modeling |
Summary and Analysis
The presented literature research examined 1002 bioprinting articles to identify significant problems and potential developments. Researchers in this domain have conducted study and presented their findings on the several key aspects of bioprinting. Despite numerous studies in the field of bioprinting, issues with the bioprinted component's mechanical strength must be resolved. However, over the past 18 years, 1002 articles in peer-reviewed journals have been found that contain the phrase "Bioprinting and Biomaterial" either in their title or in the keywords. Research in this area is still in its infancy, and many lines of inquiry remain open, according to a study of the bioprinting studies that made the shortlist. Although many studies have contributed to the backdrop for bioprinting, bioink development is still necessary to increase the adoption and performance of bioprinted tissues and models. Many academics have pinpointed beneficial findings and future prospects, but very few authors have attempted to identify gaps in this field's domain of research.
The Study's Utmost Important Findings
The following is a summary of study conclusions based on a comprehensive review of the documents:
It was noticed while reading the numerous literature reviews available on bioprinting that literature review papers focus on specific tissue application. The purpose of the research described in this article is to provide a multi-dimensional view of bioprinting.
The United States, China, Germany, South Korea, the United Kingdom, and India were among the source nations where 1002 research articles on bioprinting were examined. To meet their need for organ supply for the patients waiting for their transplants, countries have been seen conducting substantial study in this area. Prominent researchers, Khademhosseini, A., Malda, J., Cho, D.W., Zhang, Y.S., Moroni, L., Ashammakhi, N., etc. have made a considerable impact on bioprinting-related research.
The biomaterials, bioprinting procedures, and bioink development techniques that have been discovered by numerous bioprinters researchers have been compiled in this publication and can be used as a ready-to-use resource by present and future researchers and practitioners. Bioprinting researchers work on a variety of topics, but the most frequent ones are bone tissue engineering, skin bioprinting, and cartilage regeneration. The themes addressed throughout this study can serve as a guide for future scholars in this field.
Many studies have concentrated on the development of bioink, but only a few have focused on improving the qualities of bioprinted components such as biocompatibility, biodegradability, mechanical strength, and structural stability.
Research Gaps
The literature review given identifies the following research gaps:
In the medical field, there has been a considerable paradigm shift in bioprinting compared to standard graft implantation, as documented by literature. There is, however, a paucity of material for the development of a new bioink and improving the properties like mechanical/electrical, biocompatibility, and biodegradability of bioprinted components for direct use in transplantation.
While collecting the polymer-based biomaterials for bioprinting, it was discovered that just a few biomaterial combinations had been investigated for the development of bioink.
Only a few studies have shown the use of bioprinting in a variety of medical sectors.
Several bioprinting studies have found that the bioprinting aspect of bioink requires a lot of parameter control, such as composition and structure, biocompatibility, surface functionalization and characterization, etc., as well as the proper composition of biomaterial that can match the exact composition of the human body matrix. Bioprinted components are still in the testing phase before being implanted due to the lack of appropriateness of the above features.
Several articles discussed the benefits of bioprinting; however, there is still a lot of work to be done in terms of bioink development and improving the stability of bioprinted components for replacing missing tissues and replacing damaged tissues with a bioprinted component.
Implications of Research
A thorough literature assessment and analysis of a few key articles on bioprinting and biomaterials was conducted to review the current status of research in the chosen topic and to determine potential future research directions. The anticipated research implications of the study for researchers, clinicians, and doctors are listed below.
The review's findings may be helpful to practitioners and researchers in assessing the state of research in the fields of biomaterials and bioprinting. Additionally, this will help learners understand how bioprinting research has developed since 2004 and where it stands today globally.
Researchers and practitioners may find it useful to use the current study's analysis of contributions by publisher and source to find information sources for upcoming research and application. They can use this as a database for future research and to increase their understanding.
The discovered bioprinting technologies and polymer-based biomaterials may help practitioners and doctors choose the appropriate bioprinting technology and biomaterial for the application. Furthermore, the study's findings may assist doctors and researchers in determining the benefits and drawbacks of adopting a specific type of biomaterial. This will help in the implementation of bioprinted component preventative measures before a problem emerges, making bioprinted component adoption for tissue engineering applications easier. Furthermore, researchers may get motivated to examine and discover new biomaterials combinations for the development of bioink for use in bioprinting.
This could also serve as a platform for future bioprinted component enhancements. This could help surgeons choose biomaterials and bioprinting procedures for ready-to-use tissue transplantation in damaged or missing areas.
By helping new researchers learn significant information and develop their skills, the top authors and resources featured here could boost their contributions to bioprinting research. Researchers and practitioners may also be able to find important documents and authors who have contributed significantly by doing this..
Finally, after careful review of the selected research articles, the framework was proposed for future research directions as shown in Fig. 3.
Fig. 3.
Proposed framework for the future research directions
Conclusion and Future Scope
The goal of the study is to conduct a thorough assessment of the literature pertaining to the chosen bioprinting articles and to highlight recent advancements in the field. A total of 1002 research articles with the phrase "Bioprinting and Biomaterial" in the title or keywords were gathered for this study from the Scopus database between 2004 and 2022. The selected research articles were further analyzed for the assessment of neoteric developments in bioprinting research. According to this study, early trends focused primarily on bioprinting techniques, while later trends turned to the creation of bioink. Recently, several articles on the development of bioink employing biomaterial based on natural polymers were published. Among these, most of the literature highlights the use of natural biomaterial like Collagen, Silk, Alginate, Chitosan etc. for removing the issues of toxicity and non-biodegradability. Leading journals, publishers, academic institutions, writers, and other participants who have actively contributed to the field of bioprinting research have been highlighted. This study also includes bioprinting techniques, polymer-based biomaterials, and bioprinting applications that have been reported by different authors. Implementing bioprinting in actual practice will require knowledge of the biomaterials utilized in the process, the bioprinting techniques, and the application area indicated in this research. Researchers and practitioners can both benefit from a deeper understanding of the topic thanks to the classification of bioprinting documents that is presented here based on many criteria. Even though the Scopus database contains a large number of research publications, the subject is still evolving, and the proposed framework lists a number of further prospects. It was also mentioned that numerous studies based on the development of bioink have successfully documented their research's findings. A limited amount of literature has been found to address the optimization of various parameters and to enhance the functionality of bioprinting. Research on bioprinting has been heavily influenced by nations including the United States, China, Germany, South Korea, United Kingdom, and India. Despite these efforts, bioprinted models are still in the research stage and are unable to be successfully used as transplants for various tissues because they lack the desired mechanical and morphological qualities. Future research on bioprinted components is also anticipated, and gaps must be filled. Future study is predicted to follow existing Graft implantation methods such as autograft, allograft, and xenograft by various researchers. Patients who are waiting for a donor for their damaged or missing tissues can easily meet their demand using bioprinted components. Future researchers on bioprinting will need to think about how to increase acceptability of the technology. To provide practitioners with guidance, it is important to identify and enhance the numerous factors that affect the adoption of bioprinting. To highlight the recent advancements in the field of bioprinting, this study adopts a classification of earlier work based on a number of different criteria. This will help in understanding bioprinting and be a useful resource for practitioners. However, the generalized technologies and applications of bioprinting in the area of bone tissue engineering, for bioprinting customized bone tissue can be included in the future studies for the benefits of academicians as well as for the orthopedic doctors. However, the authors of this research believe that the study has a few drawbacks, which are noted below:
Our research is limited to looking at specific publications that include the term "Bioprinting and Biomaterials" in the title or keywords of the article, even though we acknowledge that there are several research articles that could not have used the term "Bioprinting and Biomaterials" in the keyword, the description could have focused on bioprinting.
Journals published by the Institute of Physics Publishing, John Wiley and Sons Inc., and MDPI (Multidisciplinary Digital Publishing Institute) were excluded from this research. There are, however, research publications published by other publishers that can be utilized in the bioprinting and biomaterials-based studies. The collection is not exhaustive, but it is considered comprehensive because it includes a wide range of scholarly publications from a number of well-regarded journals.
The authors of this study attempted to incorporate a wide range of parameters as well as all of the necessary bases for comparing the articles. However, the authors believe that a more complete study is needed to have a better knowledge of bioprinting and its application in the medical field.
Declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nature Biotechnology. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 2.Xu F, Celli J, Rizvi I, Moon S, Hasan T, Demirci U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnology Journal. 2011;6(2):204–212. doi: 10.1002/biot.201000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends in Biotechnology. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Zhang Y. Tissue engineering applications of three-dimensional bioprinting. Cell Biochemistry and Biophysics. 2015;72(3):777–782. doi: 10.1007/s12013-015-0531-x. [DOI] [PubMed] [Google Scholar]
- 5.Hong N, Yang GH, Lee JH, Kim GH. 3D bioprinting and its in vivo applications. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2018;106(1):444–459. doi: 10.1002/jbm.b.33826. [DOI] [PubMed] [Google Scholar]
- 6.Rouf S, Malik A, Raina A, Irfan Ul Haq M, Naveed N, Zolfagharian A, et al. Functionally graded additive manufacturing for orthopedic applications. Journal of Orthopaedics. 2022;33:70–80. doi: 10.1016/J.JOR.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pansare R, Yadav G, Nagare M. Reconfigurable manufacturing system: A systematic review, meta-analysis and future research directions. Journal of Engineering, Design and Technology. 2021 doi: 10.1108/JEDT-05-2021-0231. [DOI] [Google Scholar]
- 8.Lin W, Shen H, Fu J, Wu S. Online quality monitoring in material extrusion additive manufacturing processes based on laser scanning technology. Precision Engineering. 2019;60:76–84. doi: 10.1016/j.precisioneng.2019.06.004. [DOI] [Google Scholar]
- 9.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioactive Materials. 2018;3(2):144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Wen X, Vyavahare NR, Boland T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials. 2008;29(28):3781–3791. doi: 10.1016/j.biomaterials.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Negro A, Cherbuin T, Lutolf MP. 3D inkjet printing of complex, cell-laden hydrogel structures. Scientific Reports. 2018;8(1):1–9. doi: 10.1038/s41598-018-35504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning L, Gil CJ, Hwang B, Theus AS, Perez L, Tomov ML, et al. Biomechanical factors in three-dimensional tissue bioprinting. Applied Physics Reviews. 2020 doi: 10.1063/5.0023206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JM, Yeong WY. Design and printing strategies in 3D bioprinting of cell-hydrogels: A review. Advanced Healthcare Materials. 2016;5(22):2856–2865. doi: 10.1002/adhm.201600435. [DOI] [PubMed] [Google Scholar]
- 14.Lapomarda A, Pulidori E, Cerqueni G, Chiesa I, De Blasi M, Geven MA, et al. Pectin as rheology modifier of a gelatin-based biomaterial ink. Materials. 2021;14(11):1–16. doi: 10.3390/ma14113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd-Moss M, Fox K, Brandt M, Nisbet D, Williams R. Bioprinting and biofabrication with peptide and protein biomaterials. Peptides and Peptide-based Biomaterials and their Biomedical Applications. 2017 doi: 10.1007/978-3-319-66095-0_5. [DOI] [PubMed] [Google Scholar]
- 16.Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M. Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Annals of Biomedical Engineering. 2017;45(1):210–223. doi: 10.1007/s10439-016-1704-5. [DOI] [PubMed] [Google Scholar]
- 17.Fan R, Piou M, Darling E, Cormier D, Sun J, Wan J. Bio-printing cell-laden Matrigel-agarose constructs. Journal of Biomaterials Applications. 2016;31(5):684–692. doi: 10.1177/0885328216669238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao S, Zhu W, Castro NJ, Leng J, Zhang LG. Four-dimensional printing hierarchy scaffolds with highly biocompatible smart polymers for tissue engineering applications. Tissue Engineering Part C: Methods. 2016;22(10):952–963. doi: 10.1089/ten.tec.2015.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens LR, Gilmore KJ, Wallace GG, In het Panhuis M Tissue engineering with gellan gum. Biomaterials Science. 2016;4(9):1276–1290. doi: 10.1039/c6bm00322b. [DOI] [PubMed] [Google Scholar]
- 20.Ersumo N, Witherel CE, Spiller KL. Differences in time-dependent mechanical properties between extruded and molded hydrogels. Biofabrication. 2016;8(3):1–12. doi: 10.1088/1758-5090/8/3/035012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BS, Jang J, Chae S, Gao G, Kong JS, Ahn M, et al. Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers. Biofabrication. 2016;8(3):1–14. doi: 10.1088/1758-5090/8/3/035013. [DOI] [PubMed] [Google Scholar]
- 22.Mouser VHM, Melchels FPW, Visser J, Dhert WJA, Gawlitta D, Malda J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication. 2016;8(3):1–13. doi: 10.1088/1758-5090/8/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leite ÁJ, Sarker B, Zehnder T, Silva R, Mano JF, Boccaccini AR. Bioplotting of a bioactive alginate dialdehyde-gelatin composite hydrogel containing bioactive glass nanoparticles. Biofabrication. 2016;8(3):1–8. doi: 10.1088/1758-5090/8/3/035005. [DOI] [PubMed] [Google Scholar]
- 24.Gu Q, Tomaskovic-Crook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, et al. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Advanced Healthcare Materials. 2016;5(12):1429–1438. doi: 10.1002/adhm.201600095. [DOI] [PubMed] [Google Scholar]
- 25.Hartwell R, Chan B, Elliott K, Alnojeidi H, Ghahary A. Polyvinyl alcohol-graft-polyethylene glycol hydrogels improve utility and biofunctionality of injectable collagen biomaterials. Biomedical Materials (Bristol) 2016;11(3):35013. doi: 10.1088/1748-6041/11/3/035013. [DOI] [PubMed] [Google Scholar]
- 26.Madl CM, Katz LM, Heilshorn SC. Bio-orthogonally crosslinked, engineered protein hydrogels with tunable mechanics and biochemistry for cell encapsulation. Advanced Functional Materials. 2016;26(21):3612–3620. doi: 10.1002/adfm.201505329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H, Yoo JJ, Kang HW, Cho DW. Investigation of thermal degradation with extrusion-based dispensing modules for 3D bioprinting technology. Biofabrication. 2016;8(1):15011. doi: 10.1088/1758-5090/8/1/015011. [DOI] [PubMed] [Google Scholar]
- 28.Melke J, Midha S, Ghosh S, Ito K, Hofmann S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomaterialia. 2016;31:1–16. doi: 10.1016/j.actbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Katoh S, Yoshioka H, Iwasaki M, Senthilkumar R, Rajmohan M, Karthick R, et al. A three-dimensional in vitro culture environment of a novel polymer scaffold, yielding chondroprogenitors and mesenchymal stem cells in human chondrocytes derived from osteoarthritis-affected cartilage tissue. Journal of Orthopaedics. 2021;23:138–141. doi: 10.1016/j.jor.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westhauser F, Höllig M, Reible B, Xiao K, Schmidmaier G, Moghaddam A. Bone formation of human mesenchymal stem cells harvested from reaming debris is stimulated by low-dose bone morphogenetic protein-7 application in vivo. Journal of Orthopaedics. 2016;13(4):404–408. doi: 10.1016/J.JOR.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun W, Starly B, Daly AC, Burdick JA, Groll J, Skeldon G, et al. The bioprinting roadmap. Biofabrication. 2020 doi: 10.1088/1758-5090/ab5158. [DOI] [PubMed] [Google Scholar]
- 32.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 33.Woodfield TBF, Malda J, De Wijn J, Péters F, Riesle J, Van Blitterswijk CA. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004;25(18):4149–4161. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnology. 2016;34(3):312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 35.Yue K, Trujillo-de SG, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piskin, E. (1994). Biodegradable polymers as biomaterials. Journal of Biomaterials Science (March 2013), 37–41. [DOI] [PubMed]