Abstract
Background and Objective
Crouch gait is the most common pathological gait pattern in cerebral palsy and is commonly seen in patients with spastic diplegia. It is characterized by excessive knee flexion throughout the stance phase of gait cycle. The aim of this review is to discuss the current literature about CG for a more comprehensive understanding.
Methods
A literature review about various aspects of crouch gait in cerebral palsy was undertaken. This included its etiology and pathophysiology, biomechanics in crouch gait, natural history of untreated crouch gait, clinical and radiological evaluation and different modalities of available treatment.
Results
The etiology is multifactorial and the pathophysiology is poorly understood. This makes its management challenging, thereby leading to a variety of available treatment modalities. Inadvertent lengthening of muscle–tendon units is an important cause and can be avoided. A meticulous clinical and radiological evaluation of patients, supplemented by observational and instrumented gait analysis is mandatory in choosing correct treatment modality and improving the treatment outcome. Younger children can be managed satisfactorily by various non-operative methods and spasticity reduction measures. However, crouch gait in cerebral palsy has a progressive natural history and surgical interventions are needed frequently. The current literature supports combination of various soft tissue and bony procedures as a part of single event multilevel surgery. Growth modulation in the form of anterior distal femur hemiepiphysiodesis for correction of fixed flexion deformity of knee has shown encouraging results and can be an alternative in younger children with sufficient growth remaining.
Conclusions
In spite of extensive research in this field, the current understanding about crouch gait has many knowledge gaps. Further studies about the etiopathogenesis and biomechanics of crouch using instrumented gait analysis are suggested. Similarly, future research should focus on the long term outcomes of different treatment modalities through comparative trials.
Keywords: Child, Cerebral palsy, Crouch gait, Knee, Spastic diplegia
Introduction
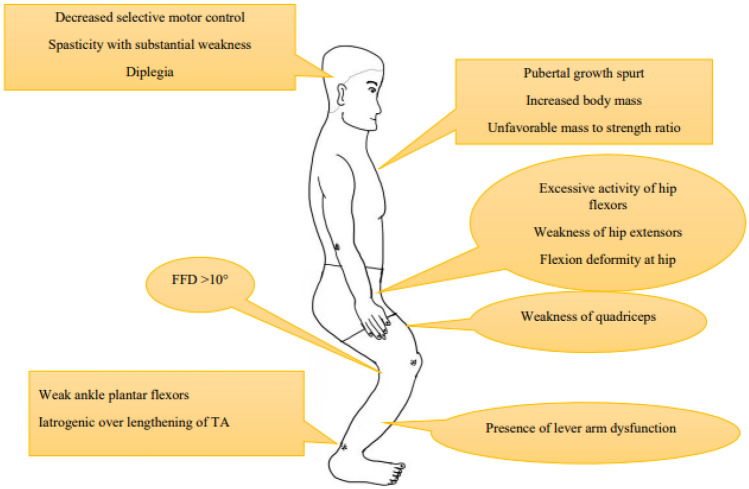
Crouch gait (CG) or flexed knee gait is the most common pathological gait pattern in cerebral palsy (CP) with prevalence as high as 76% [1]. It is characterized by excessive knee flexion throughout the stance phase of gait cycle. There is no consensus so far about the exact definition of CG. While majority see it as excessive hip and knee flexion and ankle dorsiflexion, others consider it as a flexed knee gait regardless of the ankle position [2, 3]. Severity of knee flexion and duration of flexed position during stance phase was considered as criteria in few studies [1]. Rotational deformities of femur, tibia and planovalgus foot deformity, referred to as lever arm disorders are also frequently seen [Fig. 1]. Five CG patterns have been identified in the order of increasing pathology [3]. These are mild crouch with mild equinus, moderate crouch, moderate crouch with anterior pelvic tilt, moderate crouch with equinus and severe crouch. The etiopathogenesis and biomechanics of CG in CP are still not very clearly understood. This makes its management challenging, thereby leading to a variety of available treatment modalities. The aim of this review is to discuss the current literature about CG for a more comprehensive understanding.
Fig. 1.
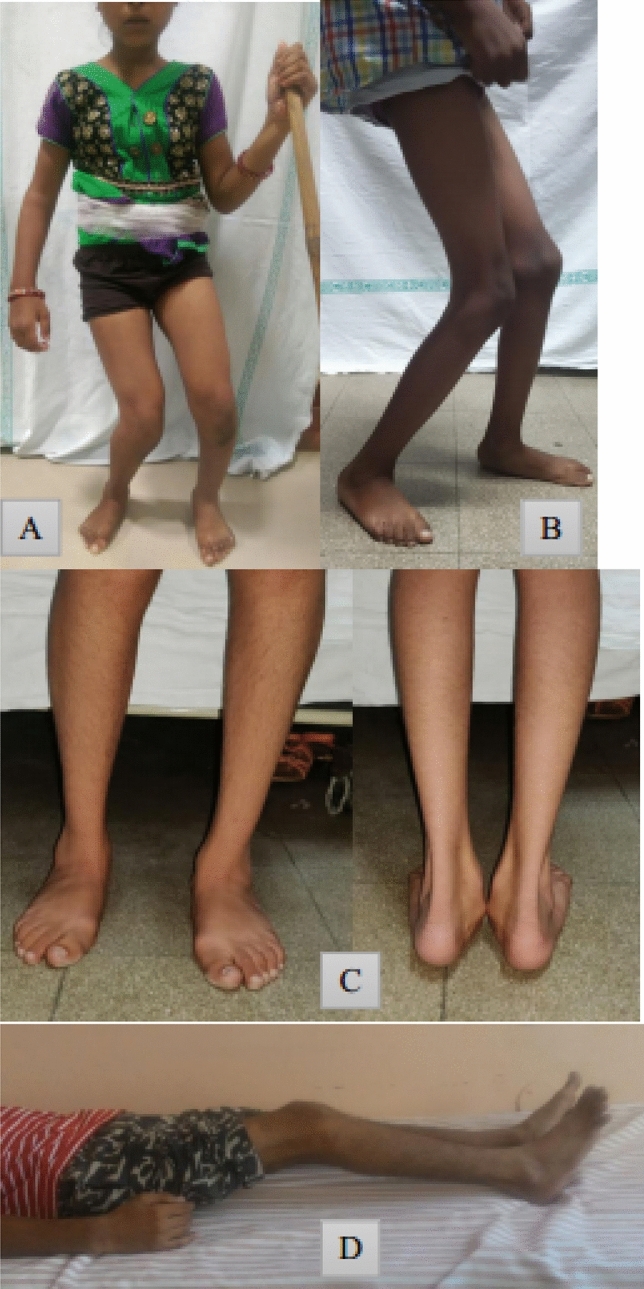
Characteristics of crouch gait. A & B—Flexion at hip and knee, dorsiflexion at ankle, rotational deformities in femur and tibia. C—Planovalgus foot deformity. D—Fixed flexion deformity at knee
Etiology
The etiology of CG is multifactorial [Fig. 2]. It is frequently seen in children with severe diplegia or quadriplegia and is less common in hemiplegic CP [2]. It is known that the sagittal gait pattern in diplegic CP is unstable and changes with the age of patient [4]. CG usually develops during the pubertal growth spurt and is related to increased body mass with an unfavorable mass to strength ratio [3]. In a retrospective observational study, the sagittal gait pattern in most of the children with diplegic CP changed from equinus or jump gait to a moderate or severe CG as they reached puberty [4]. There was no statistically significant increase in mean knee flexion in stance and passive knee flexion contracture but the ankle dorsiflexion in stance increased from − 0.3° to 9.0° (p < 0.001).
Fig. 2.
Etiopathology of crouch gait
Hamstring contracture was considered to be the primary culprit for CG. However, this concept was challenged by the studies reporting normal length of hamstrings in patients with CG [5]. Instead, fixed flexion deformity at the knee of more than 10° has been observed to be a more significant contributor. Weakness of quadriceps is another important factor as it prevents adequate knee extension during stance. Similarly, presence of overactive hip flexors and weak hip extensors results into a flexed position at hip. This is compensated by development of flexion at knee through mechanism of dynamic coupling [6].
The role of weak plantar flexors at ankle is well established [1, 7]. It could be a result of primary brain insult or as a result of iatrogenic isolated heel cord over lengthening. Besides this, the increased activity of bi-articular gastrocnemius muscle may inhibit the knee extension during stance. However, Kanashvili et al. did not observe any statistically significant effect of gastrocsoleus complex lengthening at younger age on final knee position at maturity [4]. Similarly, Huh et al. reported development of calcaneus gait in 21% of children with diplegic CP without any history of earlier calf muscle–tendon lengthening [8].
Lever arm disorders may contribute in development of CG. External tibial torsion greater than 30° is known to inhibit the function of soleus and hip extensors [9]. Similarly, abnormal femoral anteversion may inhibit the extension of knee [10]. Planovalgus foot deformity with midfoot break leaves the midfoot in a loose unlocked position during stance and reduces the effect of lever arm. Additionally, the foot is externally rotated and is associated with external tibial torsion.
Pathophysiology and Biomechanics
In the second half of stance phase, knee extension is primarily maintained by plantar flexion–knee extension couple. Contraction of soleus inhibits progression of tibia and maintains the ground reaction force anterior to the knee joint, thereby keeping knee in extension without the activity of quadriceps. Due to weakness of soleus, this plantar flexion–knee extension couple is inefficient and the progression of tibia during stance phase is not inhibited. This brings the ground reaction force posterior to knee joint and produces knee flexion. The force is also anterior to hip, thereby producing hip flexion.
In majority of cases, excessive knee flexion is believed to be a result of short hamstring causing static knee contracture. When stretched, short hamstrings may generate excessive passive forces that limit the knee extension. In other words, hamstrings are working at abnormally short muscle tendon lengths and their surgical lengthening may improve knee extension by reducing the passive forces, thereby enabling them to work at longer muscle tendon lengths [5]. Contrary to this, hamstring lengthening procedure will be ineffective if muscle is not operating at shorter muscle tendon length. A short hamstring is not observed in every case of CG [11]. This could be due to the fact that shortening of hamstring produced by knee flexion contracture gets compensated by its stretching due to hip flexion contracture. Moreover, abnormally long hamstrings have been observed in some patients by Delp et al. [6]. Spastic hamstrings with normal muscle tendon length may be the reason for excessive knee flexion [11]. Besides this, increased and extended hamstring activation in stance phase has been observed [12]. In such cases, CG is due to exaggerated, velocity dependent resistance of hamstring to stretch and the muscle spasticity can be overcome at slower stretch velocities. Surgical lengthening of hamstrings in these cases may improve knee extension by reducing the response to stretch and enabling them to operate at greater stretch velocities [13]. Opposite to this, if hamstrings are not operating at short lengths or do not overcome spasticity at slow stretch velocities, patients may not benefit from a hamstring lengthening surgery [5]. Inadvertent lengthening of a normal length hamstring should be avoided as it may cause increased pelvic tilt, knee hyperextension and stiff knee gait [14].
Abbasi et al. further reported the influence of abnormal knee and ankle kinematics on characteristics of trunk movements. [15]. When compared to normal population, the trunk tilt range of motion in patients with CG did not show any significant difference. However, trunk bending and rotation were significantly reduced and this had a positive correlation with the severity of abnormal knee and ankle kinematics. Range of movements of the trunk decreased further with increase in knee flexion. Excessive dorsiflexion and plantar flexion at ankle were observed to produce a flexed and extended posture in trunk, respectively. Thus, correcting the abnormal knee and ankle kinematics in CG may improve the trunk kinematics and improve ambulation.
Natural history
The natural history of untreated CG is not clear. Two different patterns of worsening have been reported [16]. The one with slow progression seen in mild crouch (10–20° mid stance knee flexion) is well tolerated without any surgical intervention. Another pattern with rapid worsening observed in moderate (25–45° mid stance knee flexion) and severe (> 45° mid stance knee flexion) crouch needs timely surgical intervention. Irrespective of the etiology, an unlocked knee is pulled into flexion by the hamstrings, thereby causing compensatory increase in the activity of quadriceps. Increased activity of the quadriceps puts greater demand on hip extensors including the hamstrings. Thus a vicious cycle is initiated, causing increased flexion at hip and knee over time. The patellar tendon is stretched and elongated, causing further weakening of the quadriceps mechanism. The presence of patella alta increases force and pressure across the patello-femoral joint and causes anterior knee pain in untreated cases. Incidence of patellar or tibial tubercle stress fractures and apophysitis have also been reported [17]. Ambulation in severe cases has very high energy expenditure resulting in early fatigue of muscles across the knee, hip and back. The foot clearance during walking becomes difficult with time and orthosis are often not able to support the collapsing foot. Gradually, patients start losing motivation to walk and become wheelchair dependent [16].
Clinical evaluation
Clinical presentation of CG may vary from only mild disability to severe difficulty in ambulation. Evaluation of patients includes a comprehensive assessment of medical history, functional status, clinical examination and relevant investigations. A good evaluation is prerequisite for identifying the patients at the risk of further progression and helps in deciding the appropriate treatment, thereby giving best possible outcome.
Medical history should include the details of perinatal complications and the major milestones. History about existing medical comorbidities and ongoing medications should be taken. Information about the treatment taken so far for crouch, their outcomes and compliance of family towards the treatment measures helps in choosing further interventions and preparing the family in a better way. Similarly, an insight of current functional status in the terms of Gross Motor Function Classification System (GMFCS) and Functional Mobility Scale (FMS) helps in setting realistic goals and counselling the parents about best possible treatment outcome.
Clinical examination aims at identifying both static and dynamic components of deformity by examining the patient on a couch and by doing gait analysis, respectively. Any trunk imbalance and spine deformity should be noted and evaluated further. Static measures include assessment of muscle tone, muscle power, motor and sensory reflexes and deformities across the joints. Spasticity is usually evaluated by modified Ashworth scale and Tardieu scale which is based on passively stretching the muscle at different velocities and assessing the encountered resistance.
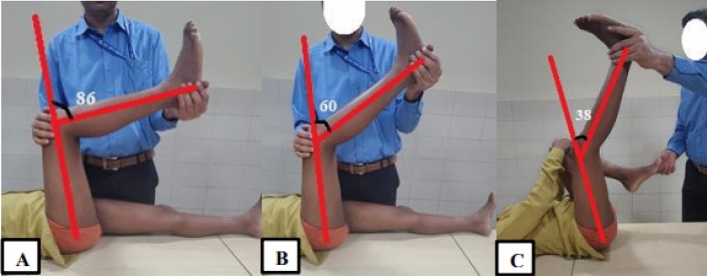
The fixed flexion deformity at knee is evaluated by popliteal angle test. Subsequently, hamstring shift test is used to exclude the contribution of concomitant hip flexion contracture and anterior pelvic tilt [Fig. 3]. The different muscle lengths and contractures across hip joint can be evaluated by specific clinical tests. Both Thomas’ hip flexion test and Staheli prone extension test can be used effectively but the later has been reported to be more accurate for CP [18]. The Duncan-Ely’s prone rectus test is used to assess rectus femoris contracture. Adductor contracture is tested with Phelp’s test to differentiate hip adductor contracture from medial hamstring contracture.
Fig. 3.
Popliteal angle test and hamstring shift test. The patient lies supine on a couch with both hip and knee extended. [A] – R1 position showing a value of 86°. [B] – R2 position showing improvement in popliteal angle with a value of 60°. [C] – Hamstring shift test showing further correction of the knee flexion deformity and popliteal angle of 38º
It is of utmost importance to look for a concomitant lever arm dysfunction in the form of planovalgus foot and torsional deformities in femur and tibia. Excessive femoral anteversion causes intoeing gait and is clinically assessed by Craig’s test or trochanteric prominence test. Similarly, tibial torsion can be identified and measured by thigh foot angle test, bimalleolar axis test and second toe test.
Muscle strength is commonly assessed by using Medical Research Council (MRC) grading for muscle strength assessment. However, for children with CP, the use of manual testing with Kendall scale or with a dynamometer is preferred [19]. The reduced selective motor control is documented and graded from 0 to 2 where grade 0 is no ability, while grade 2 is the complete ability for doing isolated movements.
The upper extremities should be evaluated in a similar way for tone, muscle strength, deformities and function. This is especially important for children ambulating with support who need good hand function for daily activities. The routinely used functional scales for upper extremity are the manual ability classification system (MACS) and quality of upper extremity skill test (QUEST).
Observational gait analysis and video recordings
Analysis of gait pattern provides information about the dynamic component of deformity and reveals relative functional deviations. Observational gait analysis refers to watching the children while they are walking. Child is exposed from foot to umbilicus and is made to walk several times with examiner seated with his eyes at the level of patient’s knee. Child is observed meticulously while walking from front, side and back and the joint angles at hip, knee and ankle are noted at different stages of gait cycle. Simultaneous video recording of gait (videographic gait analysis) enables the examiner to analyze the gait pattern several times at his own convenience and in slow motion. Observational gait analysis though subjective and not very precise, provides sufficient information in experienced hands. It is an important tool especially in developing nations where the majority of health centers lack proper infrastructure and do not have access to instrumented 3D gait analysis.
Instrumented 3D Gait analysis (I3DGA)
In conjunction with physical examination and observational gait analysis, I3DGA offers quantifiable data to direct treatment for gait problems and evaluate its effectiveness in children with CG. It provides a more detailed and precise assessment of the gait pattern, which can aid in the development of targeted interventions to improve gait function. Furthermore, the assessment of dynamic muscle tendon length using I3DGA can provide additional insight into the underlying causes of the crouch gait pattern, which can inform treatment decisions. Yet, decision making is challenging due to lack of defined standards to interpret the data and select the surgery [20].
According to a recent review, I3DGA adds objective and evidence-based value to clinical examination by providing correct number and selection of treatment procedures. It allowed greater insight to the causes of gait problems, particularly for internal hip rotation and flexed knee gait. Earlier, these issues were attributed to muscular spasticity; however, with I3DGA, they were attributed to structural abnormalities like patellar tendon laxity, femoral anteversion, and knee flexion contractures [21]. I3DGA altered surgical recommendations in 52% of cases of spastic CP [7]. Incidence of severe CG decreased from 25 to 4% as a result of practice adjustments after inclusion of I3DGA [22]. Surgical decision post I3DGA demonstrated agreement with clinical assessment in 86% patients, whereas there was agreement with clinical assessment in 97% cases for non-operative treatment [22]. Overall, the results led to a reduction in the number of patients recommended for surgery. Also, recommendations for the degree or type of surgery to be performed changed in 40% of cases.
Most studies reported a good agreement between I3DGA and clinical assessment for bone surgery, suggesting that clinical evaluation of torsional problems was fairly reliable. However, there was poor agreement for soft tissue procedures, probably reflecting the added difficulties in clinical assessment of tone-related problems. Agreement between the two measures was greater for hamstrings and gastrocnemius muscle than for other muscle groups, probably as their length facilitates clinical assessment better than shorter muscle groups [23]. Few studies have reported better effects of using I3DGA on surgical outcomes. Previous studies have reported insufficiency of physical evaluation in decision-making for femoral derotation osteotomy (FDRO) [24]. I3DGA helped in deciding the need as well as degree of derotation in such patients and they showed considerable improvement in their CG when the recommended FDRO was performed [20, 24]. Similarly, passive and dynamic hip rotation demonstrated minimal improvement, when treatment was given without I3DGA data [23]. Improvement in knee flexion angle was positively associated with three biomechanical variables that were drawn from findings of I3DGA: (i) adequate hamstrings lengths and velocities, (ii) normal tibial torsion, possibly achieved via tibial derotation osteotomy, and (iii) sufficient muscle strength [20].
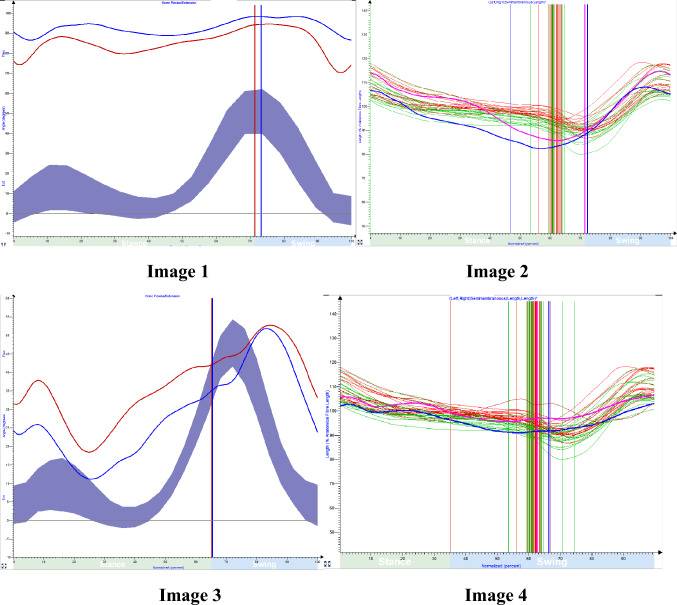
Example [Fig. 4]:
Fig. 4.
Image 1 & 2 represent the knee kinematic and dynamic muscle tendon length–semimembranosus muscle of 14-year-old with spastic cerebral palsy. Image 3 & 4 represent knee kinematics and dynamic muscle tendon length (DMTL) of 17 year old with spastic CP
The comparison of 3-dimensional gait analysis and bedside clinical evaluation of a 17-year-old child and a 14-year-old child with spastic diplegia revealed a similar crouch gait pattern. This was demonstrated by both children exhibiting a similar shortening of hamstrings length using modified popliteal angle test during bedside clinical evaluation. However, the 3D gait analysis of the 14-year-old child revealed a crouch angle of 90°, whereas the 17-year-old child demonstrated a crouch angle of 40°. Additionally, instrumented 3D gait analysis demonstrated that both the children with crouch gait had dynamic hamstring muscle tendon length well within the reference range.
Thus, the combination of bedside clinical evaluation and I3DGA can provide a more comprehensive assessment of CG in children with spastic diplegia.
Radiological evaluation
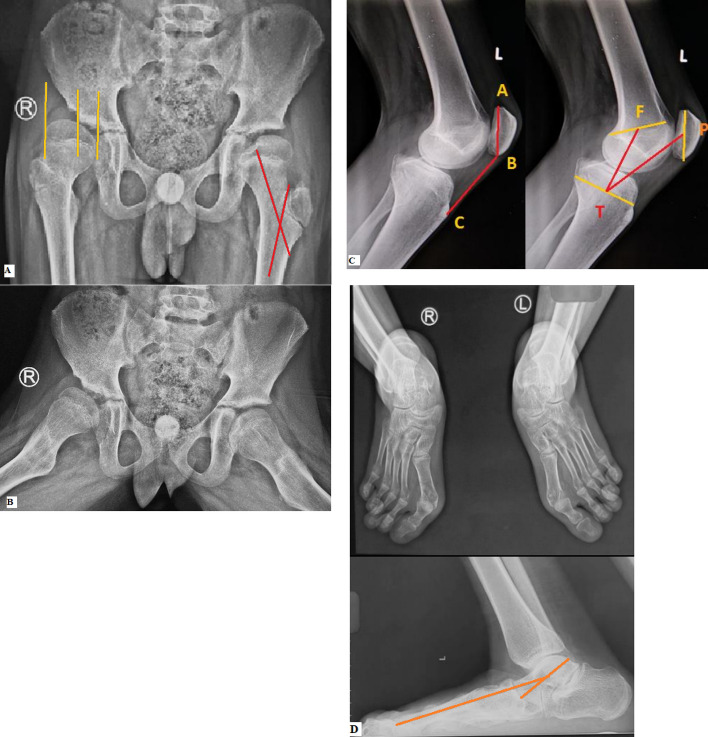
Radiological evaluation consists of anteroposterior (AP) and lateral view radiographs of pelvis with both hips and of both knees. Hip radiographs are looked for any dysplastic changes, deformity and uncovering of femoral head. Radiographs of the knees are taken with the knee in maximum possible extension to document and quantify any coronal plane deformity and fixed flexion deformity around knee. Another lateral radiograph with knee flexed in 30–90° evaluates the position of patella by calculating Insall Salvati Index and Koshino index [Fig. 5]. Weight bearing radiographs of the feet are useful for evaluating and planning treatment of foot deformity. An anteroposterior and lateral X-ray scanogram of both lower limbs helps in better analysis of deformities and to rule out any limb length discrepancy.
Fig. 5.
Radiological evaluation of crouch gait. [A] AP radiograph of pelvis and both hips showing dysplastic changes and increased uncovering of femoral head (migration index = 60%) on right side. Coxa valga (neck shaft angle = 152.4°) is present on left side. [B] Frog leg lateral radiograph of same patient showing dysplastic changes on right side. [C] Lateral radiograph of knee showing patella alta (Insall Salvati Index = 1.57, Koshino Index = 1.35) [D] Plano valgus deformity in foot
Computerized tomogram (CT) scan of femur and tibia are used to analyze rotational deformities. It also identifies early stress fracture of patella and avulsion injury of tibial tuberosity in severe crouch which may not be apparent on radiographs. Finally, magnetic resonance imaging (MRI) of knee can be helpful in evaluating a painful knee and in revealing subtle findings like bone marrow oedema, meniscal tears and issues in ligaments around the knee.
Treatment
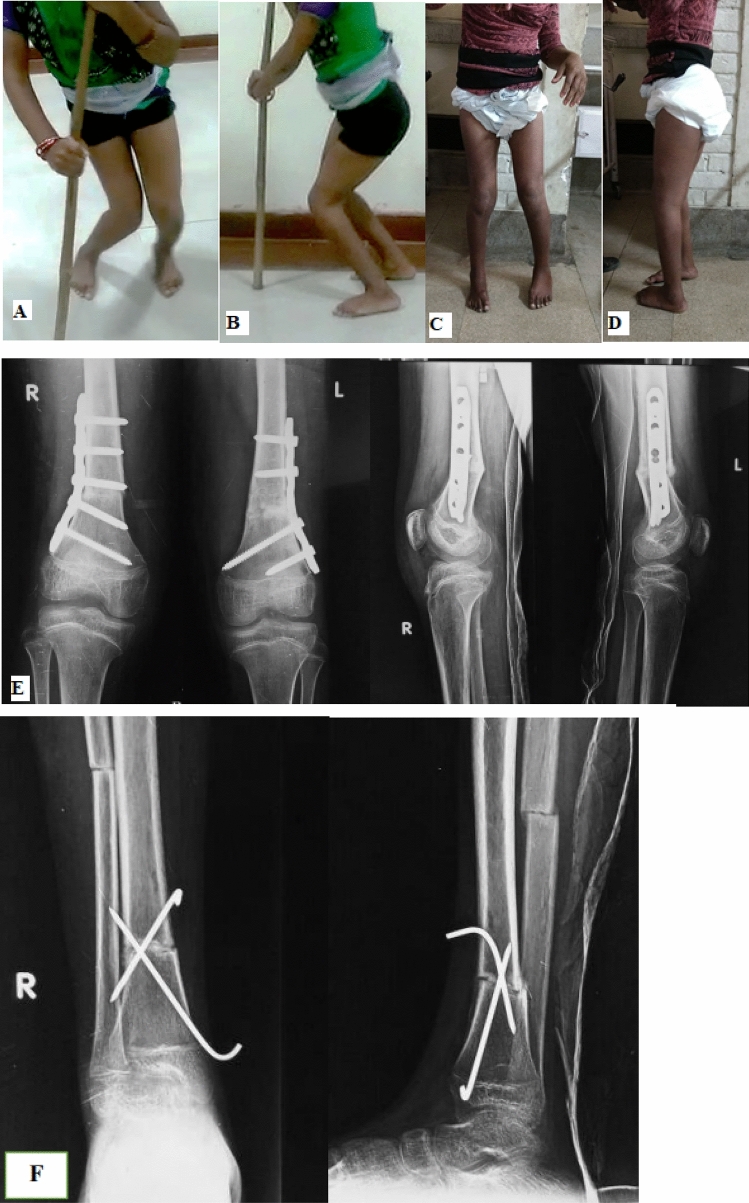
The treatment of CG should be directed towards its cause and should take care of patient’s specific musculoskeletal issues. A variety of nonsurgical and surgical treatment modalities have been suggested. Some factors deciding the type of intervention include the age of patient, severity of spasticity, presence of dystonia, severity of deformity and patient’s GMFCS level. Nonsurgical methods alone like physiotherapy, orthosis and spasticity management can be effective in treatment of younger children and those with mild crouch. Besides, they have a role in further rehabilitation of surgically treated patients. Surgical intervention is indicated when conservative treatment fails. It aims to correct the flexion deformity at knee, reduce hamstring spasticity and restore length, thereby facilitating hip and knee extension [Fig. 6].
Fig. 6.
Case example of a patient with crouch gait treated surgically. [A & B] Preoperative clinical images [C & D] Post-operative clinical images [E & F] Post-operative radiographs showing correction of knee deformities and lever arm dysfunction
Non-operative
Exercises
Even though, there is lack of convincing evidence about effectiveness of physiotherapy measures in treatment of CG, they are frequently advised. Some functional improvement has been reported with strength training [25]. However, it was refuted by Scianni et al. after a meta-analysis of randomized trials [26]. Steele et al. reviewed the effect of progressive resistance strengthening program and identified the presence of hamstring spasticity and slower walkers as poor prognostic factors for improvement in knee extension [27]. Early encouraging results of robot assistance to soleus and functional electrical stimulation of quadriceps has been published [28, 29]. But effectiveness of these methods needs further validation by doing longer follow up studies.
Bracing
The role of bracing and type of orthosis in management of CG remains controversial. Due to excessive ankle dorsiflexion the standard ankle foot orthosis is poorly tolerated. The Ground Reaction Ankle Foot Orthosis (GRAFO) avoids excessive dorsiflexion at ankle and maintains plantar flexion–knee extension couple by imparting extension force over anterior aspect of leg, thereby maintaining the knee extension during stance phase [30]. However, Ries et al. did not observe any difference in outcome between solid ankle foot orthosis (SAFO) and GRAFO [31]. Furthermore, the role of GRAFO has been questioned in presence of fixed deformities, excessive tibial torsion and weak quadriceps [30].
Spasticity reduction
The methods of spasticity reduction could be global or local and may have either temporary or permanent effect. A variety of treatment modalities ranging from the use of oral muscle relaxants to invasive procedures like selective dorsal rhizotomy have been described. Currently, the method of injecting botulinum toxin in the target muscles is preferred [32]. It is also used as a diagnostic test before muscle–tendon complex lengthening to anticipate the outcome after surgery. However, the overall functional improvement after botulinum toxin injection is reportedly small and short lasting [33].
When initiated at an early age, non-operative measures can delay the need for surgical intervention later. However, due to typical natural history of CG being progressive over time, these measures are poorly tolerated. Surgical interventions are commonly needed and the rate of bony procedures remains unchanged.
Operative
SEMLS
Single event multilevel surgery (SEMLS) refers to dealing with all deformities simultaneously and has become the standard practice for ambulatory patients with CP [34]. It prepares the child instantly for rehabilitation and is more convenient to the family by reducing the cost of treatment and incidences of missing school.
Hamstring lengthening (HL) and Hamstring transfer (HT)
Selective medial HL has been reported to improve the knee flexion contracture and overall functional outcome [35]. Traditional open approach through a posterior midline incision is commonly used. Recently, percutaneous technique has been reported to be equally effective [36]. Combined medial and lateral HL offers greater improvement and is reserved for severe flexion contracture. However, it increases the risk of knee hyperextension, neurological stretch injury and postoperative calf spasticity. The HL procedure should be used judiciously as it reduce the power of muscle and can have a contrary effect on the relevant joint kinematics. Moreover, a large proportion of patients with CG have normal length of hamstrings [5]. As hamstrings also act as hip extensor, its inadvertent lengthening specially in GMFCS III, may increase the hip flexion deformity, anterior pelvic tilt and lumbar lordosis, therewith worsening the knee deformity further [37]. Increased tone in rectus and a weak hip extensor before surgery could predict for increased pelvic tilt after HL [38]. Recurrence of crouch after HL is usually due to factors like quadriceps insufficiency and weak soleus muscle [39]. Repeating HL in such cases is less effective and hence interventions other than repeat hamstring lengthening should be considered [39, 40].
Transfer of semitendinosus to the adductor magnus or adductor tubercle of femur inhibits its activity as knee flexor, while allowing it to act as a hip extensor [41]. Both HL and HT have been reported to produce identical improvement in knee range of movement and the long term outcome [35, 42]. Combining semitendinosus transfer with lengthening of other hamstrings may have added benefits [41]. It may improve knee kinematics and is indicated for knee flexion contractures with concomitant anterior pelvic tilt [43].
Posterior knee capsulotomy
Posterior capsulotomy of knee can effectively correct mild to moderate knee flexion deformity. When combined with medial hamstring lengthening it improves both static and dynamic knee flexion and walking velocity and avoids bony procedure in younger children [44]. However, forceful extension of knee after the procedure is discouraged to avoid sciatic nerve stretch injury. Instead, serial casting on weekly basis is recommended to correct the residual deformity.
Concomitant soft tissue surgeries around hip and ankle
Concomitant soft tissue surgeries around hip and ankle should be performed if indicated, as a part of SEMLS. The most common interventions around hip are psoas and rectus femoris lengthening. As Iliacus muscle is usually spared, aponeurotic lengthening of psoas at pelvic brim is encouraged instead of iliopsoas tendon lengthening [45]. Similarly, resection of the direct head of rectus femoris is preferred, keeping the reflected head intact to avoid unwanted weakness in knee extension. Around the ankle, decision for lengthening of gastrocnemius or tendo-Achilles is made (based on the findings of Silfverskiold test) after correction of knee and foot deformity. Achilles tendon shortening may be needed in presence of plantar flexion lag [46].
Anterior distal femoral hemiepiphysiodesis (ADFH)
ADFH has been reported to correct knee flexion deformity effectively without causing physeal arrest [47–49]. It offers gradual correction of deformity is less invasive and allows early weight bearing. The use of two 4.5 mm cannulated screws across the anterior 1/3rd of physis is preferred over 8 plate due to less incidence of post-operative anterior knee pain [50]. Correction rate of 0.5–1° per month has been reported. ADFH, when combined with hamstring lengthening, produces greater improvement in popliteal angle, knee flexion contracture and peak knee extension during stance [47]. A similar benefit is not observed in combination with patellar tendon shortening (PTS) and a simultaneous PTS has been suggested only for severe contractures more than 30° [48]. There is consensus for ADFH in ambulating children with less than 30° of flexion contracture and more than two years of growth remaining [51].
Distal Femur Extension Osteotomy (DFEO) and Patellar Tendon Advancement (PTA)
Stout et al. advocated the combined use of DFEO and PTA to correct flexion deformity of knee in CP and reported better clinical, radiological and functional outcome when compared to isolated DFEO or PTA [52]. Subsequently, this method has evolved as the standard practice for correction of CG in CP with many studies reporting satisfactory results [Table 1] [46, 52–72].
Table 1.
Literature review of studies on DFEO and PTA
| Study | Sample size (n) | Mean age (years) | Mean follow up (months) | Level of evidence | Important conclusions |
|---|---|---|---|---|---|
| Stout et al. 2008 [52] | 73 | 13.8 | 15 | III | PTA combined with DFEO has better results when compared to both procedures in isolation |
| Filho et al. 2008 [54] | 12 | 13.1 | 28 | IV | DFEO + medial hamstring lengthening without PTA is effective in improving knee extension but recurrence and anterior pelvic tilt is common |
| Novacheck et al. 2009 [55] | 73 | NA | NA | III | Inclusion of PTA with DFEO is necessary to achieve optimal results |
| Joseph et al. 2010 [56] | 17 | 12.4 | 24 | IV |
Two staged surgery is recommended 6 weeks apart Stage 1—DFEO + PTA) Stage 2—Hamstring lengthening |
| Ganjwala 2011 [46] | 18 | 14.6 | 24 | IV | Multilevel surgery including DFEO + PTA improves mobility and function and the results are maintained till 2 years after surgery |
| Healy et al. 2011 [57] | 32 | NA | NA | IV | Concomitant hamstring lengthening is rarely needed with DFEO + PTA |
| Das et al. 2012 [58] | 14 | 13.6 | 36 | IV | DFEO + PTA improve function and knee extension and reduce knee pain |
| Inan et al. 2015 [59] | 28 | 13 | NA | IV | Reported 10% incidence of neurological complications. However it was not correlated with amount of deformity and correction |
| Lenhart et al. 2017 [53] | Experimental study | DFEO alone stretches the hamstrings and shortens the femur and quadriceps. A cuneiform wedge resection reduces the stretch on hamstring while PTA takes care of quadriceps shortening | |||
| Klotz et al. 2017 [60] | 22 | 12.1 | 15.6 | IV | PTA increases the anterior pelvic tilt |
| Boyer et al. 2017 [61] | 51 | 20 | 96 | III | DFEO + PTA causes knee extensor dysfunction during sit to stand activity |
| Boyer et al. 2018 [62] | 51 | 26.1 | 156 | III | DFEO + PTA improves knee extension in short term but does not impart any significant benefit in long term |
| Filho et al. 2018 [63] | 95 | 14.3 | 32.1 | III | Addition of PTA and hamstring lengthening increases the anterior pelvic tilt |
| Salami et al. 2018 [64] | 19 | 13 | 13–60 | III | DFEO + PTA improve knee kinematics at mid-term but does not increase the length or velocity of hamstring muscle |
| Pelrine et al. 2020 [65] | 51 | 12.8 | 12 | III | Surgery does not decrease the prevalence of knee pain |
| Aroojis et al. 2019 [66] | 26 | 14.3 | 22 | IV | DFEO + PTA are effective in treatment of crouch gait. Pediatric condylar locking compression plate provides stable fixation and allows for early mobilization |
| Park et al. 2019 [67] | 33 | 12.2 | 26.9 | IV | DFEO + PTA improve knee kinematics but increases anterior pelvic tilt and incidence of stiff knee gait |
| Hefny et al. 2020 [68] | 20 | 12.5 | 24 | IV | DFEO + PTA improve knee kinematics. Simultaneous hamstring lengthening increases anterior pelvic tilt |
| Hyer et al. 2021 [69] | 28 | 13.2 | 12 | IV | DFEO + PTA improves clinical, radiological and gait analysis parameters around knee |
| Emara et al. 2021 [70] | 20 | 11.1 | 16.2 | IV | DFEO + PTA improve the range and strength of knee extension and reduces knee pain |
| Liou et al. 2022 [71] | 25 | 11 | 12 | III | DFEO at a lower level with a distally placed plate near physis can produce genu valgum deformity. Fixation of osteotomy in slight varus is recommended |
| Erdal et al. 2022 [72] | 12 | NA | 37 | IV | The use of intraoperative neuromonitoring decreases the incidence of neurological complications |
The technique consists of removing an anterior-based wedge from distal femur and closing the osteotomy by extending and posteriorly translating the distal fragment. A trapezoid shape wedge is advised for flexion contractures greater than 30° to add shortening and avoiding stretch injury to the posterior neurovascular structures. A higher location of osteotomy should be avoided as it necessitates greater translation of distal fragment. Any rotational deformity in the femur is corrected simultaneously. PTA is accomplished by shortening the patellar tendon in skeletally immature children and by advancing the tibial tuberosity to a more distal position when physis is closed. Concomitant hamstring lengthening has been suggested for deformities greater than 30°, however, Healy et al. did not find it necessary [57].
The incidence of neurological complications after surgery varies between 3 and 12% and is an important concern [52, 60]. However, it does not correlate with the severity of flexion deformity before surgery, the amount of correction done or the scarring from previous surgeries [59, 73]. Leaving the posterior cortex intact as suggested by Stout et al. may lead to the stretching of posterior neurovascular structures [52]. Adequate femoral shortening, avoiding forceful correction and a stable internal fixation are the key factors in reducing the incidence of stretch injuries. [59, 73].
Filho et al. reported increase in anterior pelvic tilt after DFEO with PTA but it was lesser when compared to DFEO or PTA alone [63]. The tilt was higher when hamstring lengthening was added to the procedure. Opposite to this, Klotz et al. reported comparatively greater increase in anterior pelvic tilt in patients undergoing DFEO with PTA when compared to DFEO alone [60]. Boyer et al. reported greater knee extension dysfunction during sit to stand after DFEO with PTA when compared to those receiving other conventional methods of treatment [61]. Subsequently, they compared the long term outcomes between the two groups and reported better improvement of knee flexion contracture and stance phase knee extension in earlier group [62]. However, slight subsequent decline of the short term improvement was noted. Moreover, these benefits did not improve the overall functional outcome, mobility and participation of patients in various activities. The knee pain and osteoarthritis scores in early adulthood were also similar in both groups. Thus, even though DFEO with PTA improves the knee kinematics, these benefits are not converted into a better quality of life in long term.
Correction of lever arm dysfunction
Derotation osteotomies should be added to correct the rotational deformities in femur and tibia. For femur, it can be performed simultaneously with DFEO. Tibial derotation is commonly performed through a supramalleolar osteotomy. For planovalgus foot deformity, MOSCA procedure, consisting of lateral column lengthening and medial soft tissue plication is recommended if deformity it flexible and not very severe. More severe and stiff foot deformities will need complex mid foot osteotomies or a triple arthrodesis.
Conclusion
CG is fairly common in patients of diplegic CP. The etiology is multifactorial and in majority, it is beyond the control of treating physician. However, one should be aware of the effect of unnecessary over lengthening of muscle–tendon units, particularly, the soleus and hamstrings in causing CG. Meticulous clinical and radiological evaluation in combination with I3DGA helps in planning appropriate treatment. Younger children are managed effectively with methods of temporary spasticity management and physiotherapy measures, while soft tissue lengthening is reserved for joint contractures. ADFH is a viable option for fixed flexion deformity of knee before maturity. For those around maturity, bony procedures with concomitant soft tissue surgeries are known to give satisfactory results. In spite of extensive research in this field, the current understanding about crouch gait remains deficient. Further studies focusing on etiopathogenesis, biomechanics and long term outcome of different treatment modalities are suggested.
Author Contributions
ANJ: conceptualized the study, made the study design, reviewed, edited and did proof reading. RAP: drafted the manuscript, reviewed, edited and did proof reading. Triveni: drafted the manuscript, reviewed, edited and did proof reading. All authors read and approved the final draft of manuscript.
Funding
None.
Data availability
A data availability statement is not applicable for this manuscript as being a narrative review, no actual data was used in writing of this manuscript.
Declarations
Conflict of interest
The authors state that they have no conflict of interest, financial or otherwise, concerning the material or methods used in this study or the findings specified in this paper.
Ethics approval and consent to participate
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wren TA, Rethlefsen S, Kay RM. Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery. Journal Of Pediatric Orthopaedics. 2005;25(1):79–83. doi: 10.1097/00004694-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland DH, Davids JR. Common gait abnormalities of the knee in cerebral palsy. Clinical Orthopaedics and Related Research. 1993;288:139–147. [PubMed] [Google Scholar]
- 3.Rozumalski A, Schwartz MH. Crouch gait patterns defined using k-means cluster analysis are related to underlying clinical pathology. Gait & Posture. 2009;30(2):155–160. doi: 10.1016/j.gaitpost.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Kanashvili B, Miller F, Church C, Lennon N, Howard JJ, Henley JD, Niiler T, Sees JP, Rogers KJ, Shrader MW. The change in sagittal plane gait patterns from childhood to maturity in bilateral cerebral palsy. Gait & Posture. 2021;90:154–160. doi: 10.1016/j.gaitpost.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AS, Liu MQ, Schwartz MH, Ounpuu S, Delp SL. The role of estimating muscle-tendon lengths and velocities of the hamstrings in the evaluation and treatment of crouch gait. Gait & Posture. 2006;23(3):273–281. doi: 10.1016/j.gaitpost.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Delp SL, Arnold AS, Speers RA, Moore CA. Hamstrings and psoas lengths during normal and crouch gait: implications for muscle-tendon surgery. Journal of Orthopaedic Research. 1996;14:144–151. doi: 10.1002/jor.1100140123. [DOI] [PubMed] [Google Scholar]
- 7.Vuillermin C, Rodda J, Rutz E, Shore BJ, Smith K, Graham HK. Severe crouch gait in spastic diplegia can be prevented. J Bone Joint Surg Br. 2011;93-B(12):1670–75. [DOI] [PubMed]
- 8.Huh K, Rethlefsen S, Wren T, Kay R. Development of calcaneal gait without prior triceps surae lengthening: an examination of predictive factors. Journal of Pediatric Orthopedics. 2010;30(3):240–243. doi: 10.1097/BPO.0b013e3181d4117d. [DOI] [PubMed] [Google Scholar]
- 9.Hicks J, Arnold A, Anderson F, Schwartz M, Delp S. The effect of excessive tibial torsion on the capacity of muscles to extend the hip and knee during single-limb stance. Gait & Posture. 2007;26(4):546–552. doi: 10.1016/j.gaitpost.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ounpuu S, DeLuca P, Davis R, Romness M. Long-term effects of femoral derotation osteotomies: an evaluation using three-dimensional gait analysis. Journal of Pediatric Orthopedics. 2002;22:139–145. doi: 10.1097/01241398-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kwak YH, Kim HW, Park KB. Muscle–tendon lengths according to sagittal knee kinematics in patients with cerebral palsy: differences between recurvatum and crouch knee. Journal of Pediatric Orthopedics. Part B. 2014;23:76–85. doi: 10.1097/BPB.0b013e3283654d30. [DOI] [PubMed] [Google Scholar]
- 12.Hoffinger SA, Rab GT, Abou-Ghaida H. Hamstrings in cerebral palsy crouch gait. Journal of Pediatric Orthopedics. 1993;13:722–726. doi: 10.1097/01241398-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Arnold AS, Liu MQ, Schwartz MH, Ounpuu S, Dias LS, Delp SL. Do the hamstrings operate at increased muscle-tendon lengths and velocities after surgical lengthening? Journal of Biomechanics. 2006;39:1498–1506. doi: 10.1016/j.jbiomech.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Gordon AB, Baird GO, McMulkin ML, Caskey PM, Ferguson RL. Gait analysis outcomes of percutaneous medial hamstring tenotomies in children with cerebral palsy. Journal of Pediatric Orthopedics. 2008;28:324–329. doi: 10.1097/BPO.0b013e318168d1c0. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi L, Rojhani-Shirazi Z, Razeghi M, Raeisi SH. Trunk Kinematic Analysis during Gait in Cerebral Palsy Children with Crouch Gait Pattern. J Biomed Phys Eng. 2018;8(3):281–288. [PMC free article] [PubMed] [Google Scholar]
- 16.O'Sullivan R, Horgan F, O'Brien T, French H. The natural history of crouch gait in bilateral cerebral palsy: a systematic review. Research in Developmental Disabilities. 2018;80:84–92. doi: 10.1016/j.ridd.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Elhassan Y, Mahon J, Kiernan D, O Brien T. A greenstick fracture of the patella: a unique fracture in CP crouch gait. BMJ Case Rep. 2013;22;2013:bcr2013009717. [DOI] [PMC free article] [PubMed]
- 18.Lee KM, Chung CY, Kwon DG, Han HS, Choi IH, Park MS, et al. Reliability of physical examination in the measurement of hip flexion contracture and correlation with gait parameters in cerebral palsy. Journal of Bone and Joint Surgery. American Volume. 2011;93:150–158. doi: 10.2106/JBJS.J.00252. [DOI] [PubMed] [Google Scholar]
- 19.Berry ET, Giuliani CA, Damiano DL. Intrasession and intersession reliability of handheld dynamometry in children with cerebral palsy. Pediatric Physical Therapy. 2004;16:191–198. doi: 10.1097/01.PEP.0000145932.21460.61. [DOI] [PubMed] [Google Scholar]
- 20.Hicks JL, Delp SL, Schwartz MH. Can biomechanical variables predict improvement in crouch gait? Gait & posture. 2011;34(2):197–201. doi: 10.1016/j.gaitpost.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wren TAL, Tucker CA, Rethlefsen SA, Gorton GE, 3rd, Õunpuu S. Clinical efficacy of instrumented gait analysis: systematic review 2020 update. Gait & Posture. 2020;80:274–279. doi: 10.1016/j.gaitpost.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Cook RE, Schneider I, Hazlewood ME, Hillman SJ, Robb JE. Gait analysis alters decision-making in cerebral palsy. Journal of Pediatric Orthopedics. 2003;23(3):292–295. doi: 10.1097/01241398-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Wren TA, Lening C, Rethlefsen SA, Kay RM. Impact of gait analysis on correction of excessive hip internal rotation in ambulatory children with cerebral palsy: a randomized controlled trial. Developmental Medicine and Child Neurology. 2013;55:919–925. doi: 10.1111/dmcn.12184. [DOI] [PubMed] [Google Scholar]
- 24.Dreher T, Wolf S, Braatz F, Patikas D, Doderlein L. Internal rotation gait in spastic diplegia–critical considerations for the femoral derotation osteotomy. Gait & Posture. 2007;26:25–31. doi: 10.1016/j.gaitpost.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Damiano DL, Arnold AS, Steele KM, Delp SL. Can strength training predictably improve gait kinematics? A pilot study on the effects of hip and knee extensor strengthening on lower-extremity alignment in cerebral palsy. Physical Therapy. 2010;90:269–279. doi: 10.2522/ptj.20090062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scianni A, Butler JM, Ada L, Teixeira-Salmela LF. Muscle strengthening is not effective in children and adolescents with cerebral palsy: a systematic review. The Australian Journal of Physiotherapy. 2009;55:81–87. doi: 10.1016/S0004-9514(09)70037-6. [DOI] [PubMed] [Google Scholar]
- 27.Steele KM, Damiano DL, Eek MN, Unger M, Delp SL. Characteristics associated with improved knee extension after strength training for individuals with cerebral palsy and crouch gait. J Pediatr Rehabil Med. 2012;5(2):99–106. doi: 10.3233/PRM-2012-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerner ZF, Damiano DL, Park HS, Gravunder AJ, Bulea TC. A robotic exoskeleton for treatment of crouch gait in children with cerebral palsy: design and initial application. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2017;25(6):650–659. doi: 10.1109/TNSRE.2016.2595501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khamis S, Martikaro R, Wientroub S, Hemo Y, Hayek S. A functional electrical stimulation system improves knee control in crouch gait. Journal of Children's Orthopaedics. 2015;9(2):137–143. doi: 10.1007/s11832-015-0651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Böhm H, Matthias H, Braatz F, Döderlein L. Effect of floor reaction ankle-foot orthosis on crouch gait in patients with cerebral palsy: what can be expected? Prosthetics and Orthotics International. 2018;42(3):245–253. doi: 10.1177/0309364617716240. [DOI] [PubMed] [Google Scholar]
- 31.Ries AJ, Schwartz MH. Ground reaction and solid ankle-foot orthoses are equivalent for the correction of crouch gait in children with cerebral palsy. Developmental Medicine and Child Neurology. 2019;61(2):219–225. doi: 10.1111/dmcn.13999. [DOI] [PubMed] [Google Scholar]
- 32.Kim SK, Rha DW, Park ES. Botulinum toxin type A injections impact hamstring muscles and gait parameters in children with flexed knee gait. Toxins. 2020;12(3):145. doi: 10.3390/toxins12030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camargo CH, Teive HA, Zonta M, et al. Botulinum toxin type A in the treatment of lower-limb spasticity in children with cerebral palsy. Arquivos de Neuro-Psiquiatria. 2009;67:62–68. doi: 10.1590/S0004-282X2009000100016. [DOI] [PubMed] [Google Scholar]
- 34.Sung HK, Chung CY, Lee KM. Long term outcome of single multilevel surgery in spastic diplegia with flexed knee gait. Gait & Posture. 2013;37:536–541. doi: 10.1016/j.gaitpost.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Do P, Feng J, Sussman MD. Long-term outcome of hamstring lengthening versus transfer and the role of biceps femoris lengthening in patients with spastic diplegia and dynamic knee flexion in gait. Journal of Children's Orthopaedics. 2022;16(6):429–441. doi: 10.1177/18632521221128593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nazareth A, Rethlefsen S, Sousa TC, Mueske NM, Wren TAL, Kay RM. Percutaneous hamstring lengthening surgery is as effective as open lengthening in children with cerebral palsy. Journal of Pediatric Orthopedics. 2019;39(7):366–371. doi: 10.1097/BPO.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 37.Pierz K, Brimacombe M, Õunpuu S. Percutaneous hamstring lengthening in cerebral palsy: technique and gait outcomes based on GMFCS level. Gait & Posture. 2022;91:318–325. doi: 10.1016/j.gaitpost.2021.10.035. [DOI] [PubMed] [Google Scholar]
- 38.Böhm H, Hösl M, Döderlein L. Predictors for anterior pelvic tilt following surgical correction of flexed knee gait including patellar tendon shortening in children with cerebral palsy. Gait & Posture. 2017;54:8–14. doi: 10.1016/j.gaitpost.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Rethlefsen SA, Yasmeh S, Wren TA, Kay RM. Repeat hamstring lengthening for crouch gait in children with cerebral palsy. Journal of Pediatric Orthopedics. 2013;33(5):501–504. doi: 10.1097/BPO.0b013e318288b3e7. [DOI] [PubMed] [Google Scholar]
- 40.Morais Filho MC, Blumetti FC, Kawamura CM, Fujino MH, Matias MS, Lopes JAF. Comparison of the results of primary versus repeat hamstring surgical lengthening in cerebral palsy. Journal of Pediatric Orthopedics. 2020;40(5):e380–e384. doi: 10.1097/BPO.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 41.Feng L, Patrick Do K, Aiona M, Feng J, Pierce R, Sussman M. Comparison of hamstring lengthening with hamstring lengthening plus transfer for the treatment of flexed knee gait in ambulatory patients with cerebral palsy. Journal of Children's Orthopaedics. 2012;6(3):229–235. doi: 10.1007/s11832-012-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Morais Filho MC, Fujino MH, Blumetti FC, Dos Santos CA, Kawamura CM, Ramos BCA, Lopes JAF. Comparison between semitendinosus transfer to distal femur and medial hamstrings surgical lengthening for treatment of flexed knee gait in cerebral palsy. Journal of Orthopaedic Surgery (Hong Kong) 2020;28(1):2309499020910978. doi: 10.1177/2309499020910978. [DOI] [PubMed] [Google Scholar]
- 43.Sung KH, Lee J, Chung CY, Lee KM, Cho BC, Moon SJ, Kim J, Park MS. Factors influencing outcomes after medial hamstring lengthening with semitendinosus transfer in patients with cerebral palsy. Journal of NeuroEngineering and Rehabilitation. 2017;14:83. doi: 10.1186/s12984-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moen TC, Dias L, Swaroop VT, Gryfakis N, Kelp-Lenane C. Radical posterior capsulectomy improves sagittal knee motion in crouch gait. Clinical Orthopaedics and Related Research. 2011;469:1286–1290. doi: 10.1007/s11999-010-1719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novacheck TF, Trost JP, Schwartz MH. Intramuscular psoas lengthening improves dynamic hip function in children with cerebral palsy. Journal of Pediatric Orthopedics. 2002;22:158–164. doi: 10.1097/01241398-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Ganjwala D. Multilevel orthopedic surgery for crouch gait in cerebral palsy: an evaluation using functional mobility and energy cost. Indian J Orthop. 2011;45(4):314–319. doi: 10.4103/0019-5413.82334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long JT, Laron D, Garcia MC, McCarthy JJ. Screw anterior distal femoral hemiepiphysiodesis in children with cerebral palsy and knee flexion contractures: a retrospective case-control study. Journal of Pediatric Orthopedics. 2020;40:e873–e879. doi: 10.1097/BPO.0000000000001634. [DOI] [PubMed] [Google Scholar]
- 48.Rethlefsen SA, Hanson AM, Wren TAL, Abousamra O, Kay RM. Anterior distal femoral hemiepiphysiodesis with and without patellar tendon shortening for fixed knee flexion contractures in children with cerebral palsy. Journal of Children's Orthopaedics. 2020;14:415–420. doi: 10.1302/1863-2548.14.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al Badi H, Lorange JP, Alzeedi M, Marwan Y, Bernstein M, Hamdy RC. Distal Femur Anterior Hemiepiphysiodesis for Fixed Knee Flexion Deformity in Neuromuscular Patients: A Systematic Review. JBJS Rev. 2023 Jun 5;11(6). [DOI] [PubMed]
- 50.Nazareth A, Gyorf i MJ, Rethlefsen SA, Wiseley B, Noonan K, Kay RM. Comparison of plate and screw constructs versus screws only for anterior distal femoral hemiepiphysiodesis in children. J Pediatr Orthop B. 2020;29:53–61. [DOI] [PubMed]
- 51.Shore JB, McCarthy J, Shrader MW, et al. Anterior distal femoral hemiepiphysiodesis in children with cerebral palsy: establishing surgical indications and techniques using the modified Delphi method and literature review. Journal of Children's Orthopaedics. 2022;16(1):65–74. doi: 10.1177/18632521221087529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stout JL, Gage JR, Schwartz MH, Novacheck TF. Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. Journal of Bone and Joint Surgery. American Volume. 2008;90:2470–2484. doi: 10.2106/JBJS.G.00327. [DOI] [PubMed] [Google Scholar]
- 53.Lenhart RL, Smith CR, Schwartz MH, Novacheck TF, Thelen DG. The effect of distal femoral extension osteotomy on muscle lengths after surgery. Journal of Children's Orthopaedics. 2017;11(6):472–478. doi: 10.1302/1863-2548.11.170087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morais Filho MC, Neves DL, Abreu FP, Juliano Y, Guimara˜es L. Treatment of fixed knee flexion deformity and crouch gait using distal femur extension osteotomy in cerebral palsy. J Child Orthop 2008;2:37–43. [DOI] [PMC free article] [PubMed]
- 55.Novacheck TF, Stout JL, Gage JR, Schwartz MH. Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. Surgical technique. J Bone Joint Surg Am. 2009 Oct 1;91 Suppl 2:271–86. [DOI] [PubMed]
- 56.Joseph B, Reddy K, Varghese RA, Shah H, Doddabasappa SN. Management of severe crouch gait in children and adolescents with cerebral palsy. Journal of Pediatric Orthopedics. 2010;30:832–839. doi: 10.1097/BPO.0b013e3181fbfd0e. [DOI] [PubMed] [Google Scholar]
- 57.Healy MT, Schwartz MH, Stout JL, Gage JR, Novacheck TF. Is simultaneous hamstring lengthening necessary when performing distal femoral extension osteotomy and patellar tendon advancement? Gait & Posture. 2011;33:1–5. doi: 10.1016/j.gaitpost.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 58.Das SP, Pradhan S, Ganesh S, Sahu PK, Mohanty RN, Das SK. Supracondylar femoral extension osteotomy and patellar tendon advancement in the management of persistent crouch gait in cerebral palsy. Indian J Orthop. 2012;46:221–228. doi: 10.4103/0019-5413.93677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.İnan M, Sarikaya İA, Yildirim E, Güven MF. Neurological complications after supracondylar femoral osteotomy in cerebral palsy. Journal of Pediatric Orthopedics. 2015;35(3):290–295. doi: 10.1097/BPO.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 60.Klotz MCM, Hirsch K, Heitzmann D, Maier MW, Hagmann S, Dreher T. Distal femoral extension and shortening osteotomy as a part of multilevel surgery in children with cerebral palsy. World J Pediatr. 2017;13(4):353–359. doi: 10.1007/s12519-016-0086-y. [DOI] [PubMed] [Google Scholar]
- 61.Boyer ER, Stout JL, Laine JC, Gutknecht SM, Oliveira LH, Munger ME, Schwartz MH, Novacheck TF. Evidence of knee extensor dysfunction during sit-to-stand following distal femoral extension osteotomy and patellar tendon advancement in young adults with cerebral palsy: a pilot study. Gait & Posture. 2017;58:527–532. doi: 10.1016/j.gaitpost.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Boyer ER, Stout JL, Laine JC, Gutknecht SM, Araujo de Oliveira LH, Munger ME, Schwartz MH, Novacheck TF. Long-term outcomes of distal femoral extension osteotomy and patellar tendon advancement in individuals with cerebral palsy. J Bone Joint Surg Am. 2018;100:31–41. [DOI] [PubMed]
- 63.de Morais Filho MC, Blumetti FC, Kawamura CM, Leite JBR, Lopes JAF, Fujino MH, Neves DL. The increase of anterior pelvic tilt after crouch gait treatment in patients with cerebral palsy. Gait & Posture. 2018;63:165–170. doi: 10.1016/j.gaitpost.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Salami F, Wagner J, van Drongelen S, Klotz MCM, Dreher T, Wolf SI, Niklasch M. Mid-term development of hamstring tendon length and velocity after distal femoral extension osteotomy in children with bilateral cerebral palsy: a retrospective cohort study. Developmental Medicine and Child Neurology. 2018;60(8):833–838. doi: 10.1111/dmcn.13739. [DOI] [PubMed] [Google Scholar]
- 65.Pelrine ER, Novacheck TF, Boyer ER. Knee pain and crouch gait in individuals with cerebral palsy: what impact does crouch-related surgery have? Developmental Medicine and Child Neurology. 2020;62(6):709–713. doi: 10.1111/dmcn.14438. [DOI] [PubMed] [Google Scholar]
- 66.Aroojis A, Patel M, Shah A, Sarathy K, Vaidya S, Mehta R. Distal femoral extension osteotomy with 90° pediatric condylar locking compression plate and patellar tendon advancement for the correction of crouch gait in cerebral palsy. Indian J Orthop. 2019;53:45–52. doi: 10.4103/ortho.IJOrtho_410_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park H, Park BK, Park KB, Abdel-Baki SW, Rhee I, Kim CW, Kim HW. Distal femoral shortening osteotomy for severe knee flexion contracture and crouch gait in cerebral palsy. Journal of Clinical Medicine. 2019;8(9):1354. doi: 10.3390/jcm8091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hefny H, Mahran M, Fayyad T, Ahmed M. Supra condylar femoral extension osteotomy with patellar tendon advancement to treat fixed knee flexion deformity in crouching ambulatory adolescents with cerebral palsy. Ain Shams Medical Journal. 2020;71(1):101–118. doi: 10.21608/asmj.2020.106405. [DOI] [Google Scholar]
- 69.Hyer LC, Carpenter AM, Saraswat P, Davids JR, Westberry DE. Outcomes of patellar tendon imbrication with distal femoral extension osteotomy for treatment of crouch gait. Journal of Pediatric Orthopedics. 2021;41(5):e356–e366. doi: 10.1097/BPO.0000000000001793. [DOI] [PubMed] [Google Scholar]
- 70.Emara KM, El-Ghazaly SA, Mahran MA, Alsehemy MA. Distal Femoral Extension Osteotomy and Patellar Tendon Advancement in Management of Fixed Knee Flexion Deformity and Crouch Gait in Cerebral Palsy, QJM: An International Journal of Medicine. 2021;114(1):hcab104.012.
- 71.Liou YL, Lee WC, Kao HK, Yang WE, Chang CH. Genu valgum after distal femur extension osteotomy in children with cerebral palsy. Journal of Pediatric Orthopedics. 2022;42(4):e384–e389. doi: 10.1097/BPO.0000000000002076. [DOI] [PubMed] [Google Scholar]
- 72.Erdal OA, Gorgun B, Sarikaya IA, Inan M. Intraoperative neuromonitoring during distal femoral extension osteotomy in children with cerebral palsy. Journal of Pediatric Orthopedics. Part B. 2022;31(2):194–201. doi: 10.1097/BPB.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 73.Asirvatham R, Mukherjee A, Agarwal S, et al. Supracondylar femoral extension osteotomy: its complications. Journal of Pediatric Orthopedics. 1993;13:642–645. doi: 10.1097/01241398-199313050-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data availability statement is not applicable for this manuscript as being a narrative review, no actual data was used in writing of this manuscript.