Abstract
Myeloma is one of the most common types of haematological malignancies. We aimed to investigate the incidence rates of myeloma by sex, race, age, and histological subgroups in the United States (US) over 2000–2020. Data were retrieved from the the Surveillance, Epidemiology, and End Results (SEER) 22 database. The International Classification of Diseases for Oncology version 3 morphological codes 9731, 9732, and 9734 were assigned for solitary plasmacytoma of bone, plasma cell myeloma, and extraosseous plasmacytoma, respectively. Average annual percent change (AAPC) and the pairwise comparison with the parallelism and coincidence were reported. All estimates were reported as counts and age-adjusted incidence rates per 100,000 individuals. Over 2000–2019, most of myeloma cases were among those aged at least 55 years (85.51%), men (54.82%), and non-Hispanic Whites (66.67%). Among different subtypes, plasma cell myeloma with 193,530 cases had the highest frequency over the same period. Also, there was a significant decrease in the age-standardized incidence rate of myeloma across all races/ethnicities in both sexes within all age groups (AAPC: − 8.02; 95% confidence interval (CI): − 10.43 to − 5.61) and those aged < 55 (AAPC: − 8.64; 95% CI − 11.02 to − 6.25) from 2019 to November 2020. The overall trends of myeloma incidence rates were not parallel, nor identical. There was an increase in myeloma incidence in both sexes, with a highly increasing rate, particularly among younger Hispanic and non-Hispanic Black women over 2000–2019. However, a remarkable decline was observed in the incidence rates following the COVID-19 pandemic in 2020.
Subject terms: Cancer epidemiology, Myeloma
Introduction
Myeloma is a cancer affecting mature plasma cells which is typically preceded by a pre-cancerous condition called monoclonal gammopathy of undetermined significance1. It is the second most prevalent type of blood cancer, accounting for approximately 1% of all cancer cases, 2% of cancer-related deaths, and 12–15% of oncological and hematological diseases2–6. This condition can progress through stages of asymptomatic/smoldering myeloma before reaching the stage of overt myeloma1. Plasma cell myeloma is the most advanced and aggressive form of myeloma1,2. Two distinct types of localized plasma cell myelomas have been identified as solitary plasmacytoma of bone and extraosseous plasmacytoma, which constitutes approximately 4–5% and 1–3% of all plasma cell myelomas, respectively7.
The occurrence of myeloma has shown a rise in the United Kingdom, the United States (US), and Western Europe that can be due to improved access to healthcare services and enhanced diagnostic methods8. It is influenced by sex, race/ethnicity, and age, with a higher frequency observed among older individuals9. Men are more likely to develop the disease than women1. Furthermore, Hispanics are diagnosed with myeloma at a younger age and have survival rates comparable to those of non-Hispanic White (NHW) and non-Hispanic Black (NHB)10. A recent study showed ongoing advancements in the survival of patients with myeloma and the difficulty of enhancing long-term outcomes, particularly for older individuals and minority populations4.
The COVID-19 pandemic had substantial effects on the diagnostic process and treatment administration for myeloma. In this regard, a noticeable decline in the incidence of newly diagnosed myeloma cases in 2020 was demonstrated compared to previous years11,12. Also, it is estimated that between March 2020 and February 2021, there were 430,000 fewer patients referred for suspected cancers compared to the previous year13.
A previous study has reported the survival and mortality rate of plasma cell myeloma over the period of 1973–201414; however a more precise investigation of updated incidence trends of myeloma seems necessary. Therefore, we aimed to report the incidence trends of myeloma by age, sex, race/ethnicity and histological subgroups over 2000–2020 in the US using the The Surveillance, Epidemiology, and End Results (SEER) database. We also examined how the COVID-19 pandemic affected the overall incidence trends of myeloma.
Methods
Data sources
The SEER Programme of the National Cancer Institute is a comprehensive population-based source of cancer information in the US. SEER 22 covers about 48% of the US population and offers patient survival rates and cancer stage information at the time of diagnosis15. The SEER programme gathers information on patients' demographics, initial tumour site, tumour morphology, stage at diagnosis, first course of treatment, and vital status follow-up15. We used the SEER 22 database, which was released in April 2023 based on data submitted in November 2022, to estimate the incidence rates and annual percent changes (APCs) of myeloma cancer from 2000 to 202016,17. The SEER 22 database was accessed in accordance with the SEER Research Data Agreement for 1975–2020 Data (November 2022 Submission)18 and cancer statistics reported according to SEER 22 guideline19.
Definitions
Cancer cases are reported in terms of frequencies and percentages, with the incidence rate given in terms of cases per 100,000 people. The APCs of myeloma cancer for a specific time period indicate rate variation at a consistent fraction of the previous year's rate. The average annual percent changes (AAPCs) described the average of various APCs across the time. Hispanic, NHW, and NHB were classified. Due to the small sample size, the race and ethnicity groups of American Indian/Alaska Native, Native Hawaiian, and Asian/Pacific Islander cases were only used to calculate the parameters of all races and ethnicities. The patients with myeloma cancer were identified based on the International Classification of Diseases for Oncology version 3 (ICD-O-3). "According to the World Health Organization classification, "plasma cell neoplasm" serves as a broad category encompassing various conditions like monoclonal gammopathy of unknown significance, plasma cell myeloma, solitary plasmacytoma of bone, immunoglobulin deposition disease, extraosseous plasmacytoma, and osteosclerotic myeloma, while only plasma cell myeloma, solitary plasmacytoma of bone, and extraosseous plasmacytoma had reportable data"20. In this report, we used "myeloma" as the general term for different types of myeloma. The morphologies of myeloma cancer were classified as plasma cell myeloma (ICD-O-3 histologic code 9732), solitary plasmacytoma of bone (ICD-O-3 histologic code 9731), and extraosseous plasmacytoma (ICD-O-3 histologic code 9734), and they were also reported seperately.
Statistical analysis
To estimate the delay age-standardized incidence rate (ASIR) of myeloma, the SEER 22 Research Limited-Field Data with Delay-Adjustment database for the period from 2000 to 202016 was collected from SEER*Stat, version 8.4.1.221. The purpose of modelling reporting delay is to update the current case count to account for expected future data revisions (including additions and deletions)22. These adjusted counts, as well as the associated delay model, are useful in detecting current cancer trends more precisely22. The case selection was limited to malignant myeloma cancer diagnosed with a known age. The delay model was then run using delay adjustment factors such as cancer site, registry, age group, race and ethnicity, and the year of diagnosis23,24. In addition, to estimate the ASIRs of myeloma subtypes, the SEER 22 Research Limited-Field Data database for the period from 2000 to 202017 was retrieved from SEER*Stat, version 8.4.1.221. The SEER*Stat version 8.4.1.221 was used to estimate the ASIRs based on the 2000 US standard population and the accompanying 95% confidence intervals (CIs) with the Tiwari method25.
The Joinpoint Regression Program, version 5.0.226, was employed to estimate the APCs, AAPCs27, joinpoint regression modelling, parallelism test, and coincident test28 for ASIR29. Also, we used R software, version 4.3.2 (Vienna, Austria) for some data visualization like the age plots. The first year of the COVID-19 pandemic was 2020, which had a significant influence on the health system, leading to reductions in cancer screening and diagnosis, and resulted in a decrease in the 2020 incidence rates for the majority of cancer sites. As a result, the 2020 incidence data may induce bias in cancer incidence estimations, hence it was omitted from Joinpoint trends and solely displayed in graphics30. The ASIR of myeloma APCs were calculated by producing the best fit of least-squares regression lines on the natural logarithm of the ASIR, with the year of diagnosis as a regressor variable. The minimal number of observations between two joinpoints and from the joinpoint to either end of the data was set to two. Model selection was accomplished using the weighted Bayesian Information Criteria technique31. The empirical quantile method was used to obtain the 95% CIs of AAPCs32. The pairwise comparison using the parallelism test was used to see if the trends of the two groups were similar over time28. In addition, a pairwise comparison with the coincidence test was performed to see whether the rates of the two groups were identical over time.
Results
Myeloma
Overall incidence
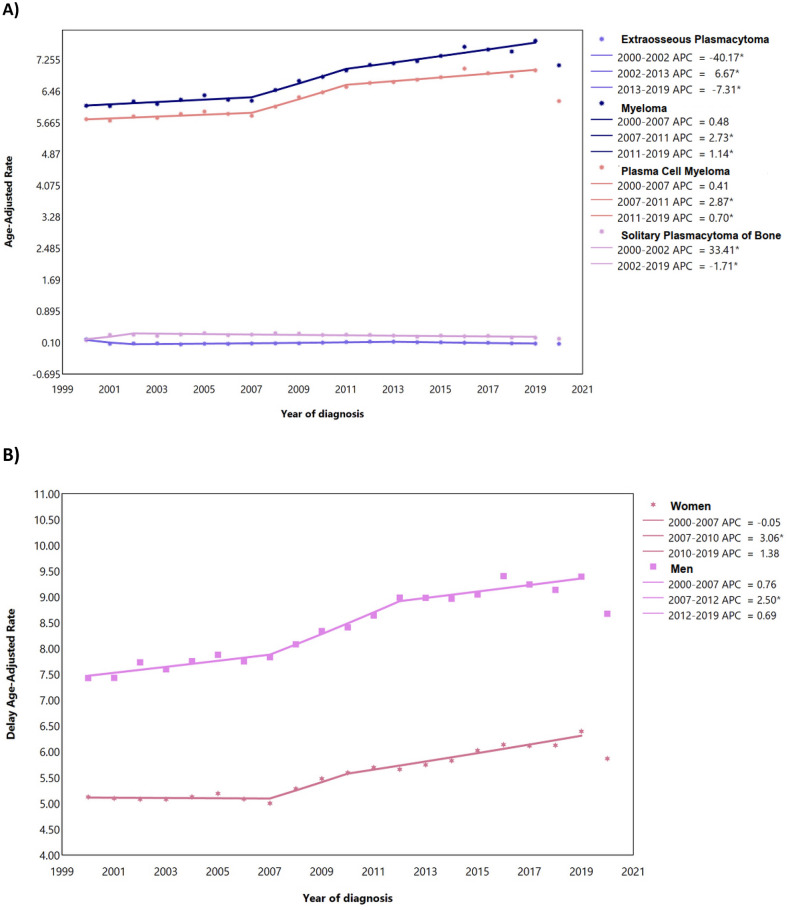
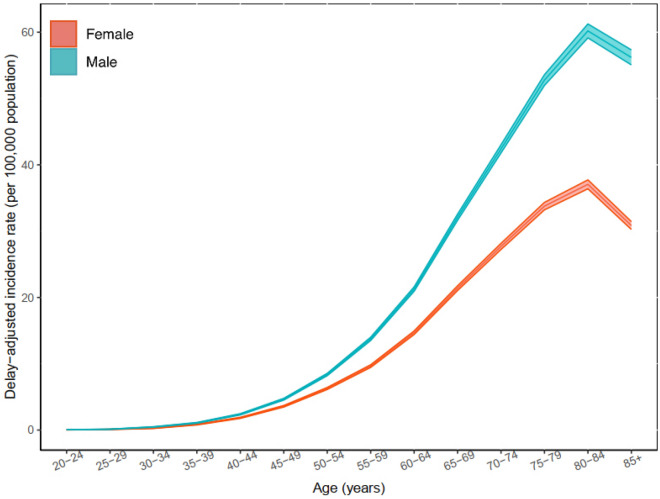
A total of 217,049 myeloma cases were reported in the US between 2000 and 2020. The majority were males (54.85%), aged ≥ 55 years (85.64%), living in urban area (88.45%), with median income between $50,000 and $65,000 per year (28.42%) (Table 1). From 2000 to 2019, a total of 204,872 cases of myeloma were recorded in the US among all ages. The most common reported subtype was plasma cell myeloma (94.46%) (Fig. 1A). The majority of cases were observed among those aged at least 55 years (85.51%), men (54.82%), and NHWs (66.67%) (Table 2; Fig. 1B–D). From 2000 to 2019, the delayed ASIR of myeloma per 100,000 population for men and women were 8.49 (95% CI 8.43 to 8.54) and 5.58 (95% CI 5.55 to 5.62), respectively, which showed 1.19% (95% CI 1.02 to 1.33) and 1.11% (95% CI 0.91 to 1.29) increase among men and women over 2000–2019, respectively (Table 2; Fig. 1B). The delayed-adjusted incidence rate of myeloma showed a gradual rise in both sexes and peaked in 80–84 age group (Fig. 2). By race/ethnicity, NHBs had higher delayed ASIRs among men (16.62; 95% CI 16.37 to 16.87) and women (12.00; 95% CI 11.83 to 12.17), and showed the highest AAPCs for men (1.55%; 95% CI 1.26 to 1.89) and women (1.64; 95% CI 1.22 to 2.13) (Table 2; Fig. 1C). The overall trends were not parallel and identical (Table S1 and Table S2).
Table 1.
Demographic characteristics of myeloma cases by the year of diagnosis.
| Characteristics | 2000–2004 N (%) | 2005–2009 N (%) | 2010–2014 N (%) | 2015–2019 N (%) | 2019–2020 N (%) | 2000–2019 N (%) | 2000–2020 N (%) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 21,086 (53.04) | 24,736 (54.54) | 30,808 (55.53) | 35,677 (55.49) | 6752 (55.45) | 112,307 (54.82) | 119,059 (54.85) |
| Female | 18,668 (46.96) | 20,615 (45.46) | 24,670 (44.47) | 28,612 (44.51) | 5425 (44.55) | 92,565 (45.18) | 97,990 (45.15) |
| Age | |||||||
| < 55 year | 6231 (15.67) | 7003 (15.44) | 7987 (14.40) | 8466 (13.17) | 1487 (12.21) | 29,687 (14.49) | 31,174 (14.36) |
| ≥ 55 year | 33,523 (84.33) | 38,348 (84.56) | 47,491 (85.60) | 55,823 (86.83) | 10,690 (87.79) | 175,185 (85.51) | 185,875 (85.64) |
| Median Income per yeara | |||||||
| < $35,000 | 162 (0.41) | 216 (0.48) | 378 (0.68) | 362 (0.56) | 63 (0.52) | 1118 (0.55) | 1181 (0.54) |
| $35,000–$49,999 | 2911 (7.32) | 4109 (9.06) | 6639 (11.97) | 5690 (8.85) | 820 (6.73) | 19,349 (9.44) | 20,169 (9.29) |
| $50,000–$64,999 | 9842 (24.76) | 12,700 (28.00) | 20,516 (36.98) | 16,117 (25.07) | 2508 (20.60) | 59,175 (28.88) | 61,683 (28.42) |
| ≥ $65,000 | 26,825 (67.48) | 28,316 (62.44) | 27,929 (50.34) | 42,116 (65.51) | 8785 (72.14) | 125,186 (61.10) | 133,971 (61.72) |
| Otherb | 14 (0.04) | 10 (0.02) | 16 (0.03) | 4 (0.01) | 1 (0.01) | 44 (0.02) | 45 (0.02) |
| Area of residence | |||||||
| Urban | 34,506 (86.80) | 39,879 (87.93) | 49,251 (88.78) | 57,414 (89.31) | 10,926 (89.73) | 181,050 (88.37) | 191,976 (88.45) |
| Rural | 5213 (13.11) | 5445 (12.01) | 6192 (11.16) | 6843 (10.64) | 1247 (10.24) | 23,693 (11.56) | 24,940 (11.49) |
| Otherc | 35 (0.09) | 27 (po0.06) | 35 (0.06) | 32 (0.05) | 4 (0.03) | 129 (0.06) | 133 (0.06) |
| Race/ethnicity | |||||||
| Hispanic | 4099 (10.31) | 5188 (11.44) | 7156 (12.90) | 9124 (14.19) | 1763 (2.74) | 25,567 (12.48) | 27,330 (12.59) |
| NHB | 7067 (17.78) | 8497 (18.74) | 10,834 (19.53) | 13,010 (20.24) | 2413 (3.75) | 39,408 (19.24) | 41,821 (19.27) |
| NHW | 27,140 (68.27) | 29,722 (65.54) | 34,671 (62.50) | 38,471 (59.84) | 7209 (11.21) | 130,004 (63.46) | 137,213 (63.22) |
| Reporting source | |||||||
| Autopsy only | 22 (0.06) | 12 (0.03) | 20 (0.04) | 7 (0.01) | 1 (0.01) | 61 (0.03) | 62 (0.03) |
| Death certificate only | 960 (2.41) | 890 (1.96) | 1015 (1.83) | 1187 (1.85) | 248 (2.04) | 4052 (1.98) | 4300 (1.98) |
| Hospital inpatient/outpatient or clinic | 35,575 (89.49) | 39,460 (87.01) | 47,377 (85.40) | 53,711 (83.55) | 10,104 (82.98) | 176,123 (85.97) | 186,227 (85.80) |
| Laboratory only (hospital or private) | 538 (1.35) | 559 (1.23) | 1091 (1.97) | 1098 (1.71) | 277 (2.27) | 3286 (1.60) | 3563 (1.64) |
| Nursing/convalescent home/hospice | 118 (0.30) | 120 (0.26) | 110 (0.20) | 114 (0.18) | 8 (0.07) | 462 (0.23) | 470 (0.22) |
| Other hospital outpatient unit or surgery center | 1087 (2.73) | 1829 (4.03) | 3087 (5.56) | 4392 (6.83) | 886 (7.28) | 10,395 (5.07) | 11,281 (5.20) |
| Physician’s office/private medical practitioner | 1296 (3.26) | 1443 (3.18) | 1195 (2.15) | 1278 (1.99) | 165 (1.36) | 5212 (2.54) | 5377 (2.48) |
| Radiation treatment or medical oncology center | 158 (0.40) | 1038 (2.29) | 1583 (2.85) | 2502 (3.89) | 488 (4.01) | 5281 (2.58) | 5769 (2.66) |
NHW Non-Hispanic White, NHB non-Hispanic Black.
aMedian household income adjusted to 2021 inflation.
bUnknown, missing, and no match.
cUnknown, missing, no match, and Alaska or Hawaii.
Figure 1.
Age-adjusted incidence rate of myeloma over 2000–2019 and in 2020 in the United States, by histologic type (A), sex (B), race/ethnicity (C), and age (D). APC annual percent change. *Represent p-value less than 0.05.
Table 2.
Counts and age-standardized rate of myeloma incidence per 100,000 and average annual percent change from 2000 to 2019 in the United States, by age, sex, and race.
| All age | Age ≥ 55 | Age < 55 | |
|---|---|---|---|
| All races/ethnicities | |||
| Women | |||
| Cases (%) | 92,565 (45.18) | 79,645 (38.88) | 12,920 (6.31) |
| Delayed ASIR (95% CI) | 5.58 (5.55 to 5.62) | 21.97 (21.81 to 22.12) | 1.13 (1.11 to 1.15) |
| AAPC (95% CI) | 1.11 (0.91 to 1.29) | 0.91 (0.75 to 1.05) | 2.15 (0.75 to 1.05) |
| Men | |||
| Cases (%) | 112,307 (54.82) | 95,540 (46.63) | 16,767 (8.18) |
| Delayed ASIR (95% CI) | 8.49 (8.43 to 8.54) | 34.18 (33.95 to 34.40) | 1.51 (1.48 to 1.53) |
| AAPC (95% CI) | 1.19 (1.02 to 1.33) | 1.22 (1.00 to 1.54) | 1.68 (1.21 to 2.18) |
| Hispanic | |||
| Women | |||
| Cases (%) | 11,878 (46.46) | 9454 (36.98) | 2424 (9.48) |
| Delayed ASIR (95% CI) | 5.75 (5.65 to 5.86) | 22.80 (22.33 to 23.27) | 1.12 (1.08 to 1.17) |
| AAPC (95% CI) | 0.89 (0.36 to 1.86) | 0.69 (0.16 to 1.49) | 2.27 (1.27 to 3.49) |
| Men | |||
| Cases (%) | 13,689 (53.54) | 10,533 (41.20) | 3156 (12.34) |
| Delayed ASIR (95% CI) | 8.19 (8.04 to 8.34) | 33.04 (32.37 to 33.72) | 1.44 (1.39 to 1.49) |
| AAPC (95% CI) | 0.79 (0.42 to 1.23) | 0.68 (0.27 to 1.18) | 1.48 (0.58 to 2.56) |
| NHB | |||
| Women | |||
| Cases (%) | 20,079 (50.95) | 16,181 (41.06) | 3898 (9.90) |
| Delayed ASIR (95% CI) | 12.00 (11.83 to 12.17) | 47.06 (46.33 to 47.80) | 2.84 (2.75 to 2.93) |
| AAPC (95% CI) | 1.64 (1.22 to 2.13) | 1.5 (1.05 to 2.03) | 2.22 (1.29 to 3.24) |
| Men | |||
| Cases (%) | 19,329 (49.05) | 15,435 (39.16) | 3894 (9.88) |
| Delayed ASIR (95% CI) | 16.62 (16.37 to 16.87) | 66.02 (64.91 to 67.14) | 3.20 (3.10 to 3.30) |
| AAPC (95% CI) | 1.55 (1.26 to 1.89) | 1.99 (1.24 to 2.66) | 1.55 (0.67 to 5.55) |
| NHW | |||
| Women | |||
| Cases (%) | 56,072 (43.13) | 50,232 (38.64) | 5840 (4.50) |
| Delayed ASIR (95% CI) | 4.78 (4.74 to 4.82) | 19.25 (19.08 to 19.42) | 0.85 (0.83 to 0.87) |
| AAPC (95% CI) | 1.00 (0.78 to 1.27) | 0.76 (0.54 to 1.09) | 2.18 (1.60 to 2.79) |
| Men | |||
| Cases (%) | 73,932 (56.87) | 65,149 (50.11) | 8783 (6.75) |
| Delayed ASIR (95% CI) | 7.86 (7.80 to 7.92) | 32.04 (31.79 to 32.29) | 1.29 (1.26 to 1.32) |
| AAPC (95% CI) | 1.34 (1.12 to 1.58) | 1.21 (0.96 To 1.58) | 1.42 (0.99 to 1.85) |
NHW Non-Hispanic White, NHB Non-Hispanic Black, ASIR Age-standardized incidence rate, CI Confidence interval, AAPC Average annual percent change.
Figure 2.
Delay-adjusted incidence rate of myeloma in the United States among males and females in each age group. Shaded areas are the confidence interval range for the point estimates. Note: Estimates were only provided for those with more than 16 cases.
Older adults (ages ≥ 55)
Over 2000–2019, a total of 175,185 cases of myeloma were diagnosed in those aged ≥ 55 years. Among older adults, plasma cell myeloma was the most common subtype (95.17%). Moreover, the majority of cases were males (54.53%) and NHWs (69.10%). Delayed ASIR per 100,000 population was 34.18 (95% CI 33.95 to 34.40) in men and 21.97 (95% CI 21.81 to 22.12) in women (Table 2). The incidence rate of myeloma in older adults increased over 2000–2019 in males and females with AAPC of 1.22 (95% CI 1.00 to 1.54) and 0.91 (95% CI 0.75 to 1.05), respectively (Table 2). Also, there was a steady increase in the incidence rate in all races, with NHB men having the highest AAPC (1.99%, 95% CI 1.24 to 2.66) (Table 2). The parallel and identical trends of myeloma among older adults are provided in Table S1 and Table S2.
Younger adults (ages < 55)
A total of 29,687 cases of myeloma cancer were reported over 2000–2019 among adults aged less than 55 years in the US. Most of the cases were plasma cell myeloma (90.30%). The majority of cases occurred in men (56.48%) and NHW (52.23%). The delayed ASIR per 100,000 population was 1.51 (95% CI 1.48 to 1.53) in men and 1.13 (95% CI 1.11 to 1.15) in women. Over 2000–2019, there was a higher increase in the ASIR of myeloma in women than men (AAPC: 2.15 vs. 1.68). There was an increase in incidence rates within all groups, with Hispanic women having the highest AAPC (2.27%; 95% CI 1.27 to 3.49) (Table 2). The trends of myeloma incidence over 2000–2019 among young adults in the US were not identical nor parallel (Table S1 and Table S2).
COVID-19 impact on myeloma
There was a significant decrease in the ASIR of myeloma across all races/ethnicities in both sexes within all age groups (AAPC: − 8.02; 95% CI − 10.43 to − 5.61) and those aged ≥ 55 (AAPC: − 8.64; 95% CI − 11.02 to − 6.25) from 2019 to November 2020. Moreover, there was a significant decrease in AAPC for females (− 8.28; 95% CI − 11.61 to − 4.96) and males (− 7.66; 95% CI − 10.78 to − 4.54) of all age groups during the COVID-19 pandemic (Table 3).
Table 3.
Percent change in age-standardized, delay-adjusted incidence rates from 2019 to 2020, by cancer site and sex, using the November 2022 data submission.
| Races/ethnicities | Sex | Age | 2019 delayed ASIR (95% CI) | 2020 delayed ASIR (95% CI) | AAPC (95% CI) |
|---|---|---|---|---|---|
| All | Both | All | 7.73 (7.6 to 7.87) | 7.11 (6.99 to 7.24) | − 8.02 (− 10.43 to − 5.61) |
| All | Both | < 55 | 1.53 (1.46 to 1.61) | 1.46 (1.39 to 1.54) | − 4.58 (− 11.66 to 2.51) |
| All | Both | ≥ 55 | 30.56 (30.01 to 31.12) | 27.92 (27.38 to 28.46) | − 8.64 (− 11.02 to − 6.25) |
| All | Female | All | 6.4 (6.24 to 6.57) | 5.87 (5.71 to 6.03) | − 8.28 (− 11.61 to − 4.96) |
| All | Male | All | 9.4 (9.18 to 9.62) | 8.68 (8.47 to 8.89) | − 7.66 (− 10.78 to − 4.54) |
| All | Female | < 55 | 1.39 (1.29 to 1.49) | 1.34 (1.24 to 1.44) | − 3.6 (− 13.39 to 6.2) |
| All | Female | ≥ 55 | 24.85 (24.18 to 25.53) | 22.55 (21.9 to 23.2) | − 9.26 (− 12.82 to − 5.69) |
| All | Male | < 55 | 1.68 (1.57 to 1.8) | 1.59 (1.48 to 1.7) | − 5.36 (− 15 to 4.28) |
| All | Male | ≥ 55 | 37.8 (36.87 to 38.74) | 34.78 (33.88 to 35.7) | − 7.99 (− 11.3 to − 4.68) |
| Hispanic | Both | All | 7.21 (6.88 to 7.56) | 6.55 (6.24 to 6.88) | − 9.15 (− 15.2 to − 3.11) |
| Hispanic | Both | < 55 | 1.29 (1.16 to 1.44) | 1.38 (1.24 to 1.53) | 6.98 (− 8.6 to 22.55) |
| Hispanic | Both | ≥ 55 | 29 (27.53 to 30.53) | 25.61 (24.24 to 27.03) | − 11.69 (− 18.29 to − 5.09) |
| Hispanic | Female | all | 6.16 (5.75 to 6.59) | 5.37 (5 to 5.77) | − 12.82 (− 21.45 to − 4.2) |
| Hispanic | Female | < 55 | 1.24 (1.05 to 1.44) | 1.29 (1.1 to 1.5) | 4.03 (− 18.78 to 26.84) |
| Hispanic | Female | ≥ 55 | 24.28 (22.5 to 26.16) | 20.41 (18.8 to 22.11) | − 15.94 (− 25.2 to − 6.68) |
| Hispanic | Male | All | 8.57 (8.02 to 9.15) | 8.07 (7.54 to 8.63) | − 5.83 (− 14.78 to 3.11) |
| Hispanic | Male | < 55 | 1.35 (1.16 to 1.56) | 1.47 (1.27 to 1.69) | 8.89 (− 13.58 to 31.36) |
| Hispanic | Male | ≥ 55 | 35.15 (32.67 to 37.76) | 32.39 (30.03 to 34.88) | − 7.85 (− 17.39 to 1.69) |
| NHB | Both | All | 16.05 (15.44 to 16.68) | 14.29 (13.71 to 14.89) | − 10.97 (− 15.94 to − 5.99) |
| NHB | Both | < 55 | 3.58 (3.25 to 3.93) | 3.42 (3.09 to 3.76) | − 4.47 (− 17.34 to 8.4) |
| NHB | Both | ≥ 55 | 61.97 (59.39 to 64.64) | 54.3 (51.86 to 56.81) | − 12.38 (− 17.81 to − 6.95) |
| NHB | Female | All | 14.14 (13.39 to 14.93) | 12.69 (11.97 to 13.43) | − 10.25 (− 17.31 to − 3.19) |
| NHB | Female | < 55 | 3.37 (2.94 to 3.84) | 3.29 (2.86 to 3.77) | − 2.37 (− 21.07 to 16.32) |
| NHB | Female | ≥ 55 | 53.8 (50.69 to 57.05) | 47.29 (44.36 to 50.35) | − 12.1 (− 19.67 to − 4.53) |
| NHB | Male | All | 18.78 (17.74 to 19.85) | 16.73 (15.74 to 17.77) | − 10.92 (− 18.23 to − 3.6) |
| NHB | Male | < 55 | 3.82 (3.33 to 4.35) | 3.56 (3.08 to 4.08) | − 6.81 (− 24.67 to 11.06) |
| NHB | Male | ≥ 55 | 73.84 (69.36 to 78.53) | 65.23 (60.95 to 69.71) | − 11.66 (− 19.68 to − 3.64) |
| NHW | Both | All | 6.95 (6.8 to 7.11) | 6.45 (6.3 to 6.6) | − 7.19 (− 10.27 to − 4.12) |
| NHW | Both | < 55 | 1.28 (1.18 to 1.37) | 1.18 (1.09 to 1.28) | − 7.81 (− 18.23 to 2.6) |
| NHW | Both | ≥ 55 | 27.85 (27.21 to 28.5) | 25.83 (25.2 to 26.47) | − 7.25 (− 10.37 to − 4.14) |
| NHW | Female | All | 5.5 (5.31 to 5.7) | 5.08 (4.9 to 5.27) | − 7.64 (− 12.49 to − 2.79) |
| NHW | Female | < 55 | 1.07 (0.95 to 1.21) | 0.99 (0.87 to 1.12) | − 7.48 (− 22.45 to 7.5) |
| NHW | Female | ≥ 55 | 21.8 (21.03 to 22.59) | 20.14 (19.39 to 20.91) | − 7.61 (− 12.32 to − 2.91) |
| NHW | Male | All | 8.71 (8.45 to 8.97) | 8.11 (7.85 to 8.36) | − 6.89 (− 10.89 to − 2.89) |
| NHW | Male | < 55 | 1.48 (1.34 to 1.63) | 1.37 (1.24 to 1.52) | − 7.43 (− 20.06 to 5.2) |
| NHW | Male | ≥ 55 | 35.33 (34.25 to 36.44) | 32.89 (31.83 to 33.96) | − 6.91 (− 11.07 to − 2.74) |
NHW Non-Hispanic White, NHB Non-Hispanic Black, ASIR Age-standardized incidence rate, CI Confidence interval, AAPC Average annual percent change.
Plasma cell myeloma
Overall incidence
From 2000 to 2019, there were 193,530 cases of plasma cell myeloma in all age groups in the US. The majority of cases were men (54.40%), NHWs (66.52%), and aged ≥ 55 years (86.15%) (Table 3; Figs. S1, S2, S3). The ASIR per 100,000 population was 7.84 (95% CI 7.80 to 7.89) for men and 7.86 (95% CI 7.82 to 7.91) for women. The AAPCs for men and women were 1.04 (95% CI 0.90 to 1.15) and 0.89 (95% CI 0.74 to 1.02), respectively (Table 4; Fig. S1). NHB men had the highest ASIR (15.64, 95% CI 15.40 to 15.88) and NHB women had the highest AAPC (1.35; 95% CI 0.92 to 1.85) (Table 4; Fig. S2). The trends were not parallel, nor identical over 2000–2019.
Table 4.
Counts and age-standardized rate of plasma cell myeloma incidence per 100,000 and average annual percent change from 2000 to 2019 in the United States, by age, sex, and race.
| All age | Age ≥ 55 | Age < 55 | |
|---|---|---|---|
| All races/ethnicities | |||
| Women | |||
| Cases (%) | 88,231 (45.60) | 76,289 (39.42) | 11,942 (6.17) |
| ASIR (95% CI) | 7.86 (7.82 to 7.91) | 31.97 (31.76 to 32.18 ) | 1.32 (1.30 to 1.34) |
| AAPC (95% CI) | 0.89 (0.74 to 1.02) | 0.68 (0.52 to 0.82) | 2.25 (1.84 to 2.71) |
| Men | |||
| Cases (%) | 105,299 (54.40) | 90,432 (46.73) | 14,867 (7.68) |
| ASIR (95% CI) | 7.84 (7.8 to 7.89) | 31.87 (31.67 to 32.08) | 1.32 (1.30 to 1.34) |
| AAPC | 1.04 (0.90 to 1.15) | 0.97 (0.77 to 1.14) | 1.74 (1.29 to 2.23) |
| Hispanic | |||
| Women | |||
| Cases (%) | 11,182 (47.00) | 9017 (37.91) | 2165 (9.10) |
| ASIR (95% CI) | 5.34 (5.24 to 5.45) | 21.38 (20.94 to 21.84) | 0.99 (0.95 to 1.03) |
| AAPC (95% CI) | 0.85 (0.32 to 1.50) | 0.65 (0.77 to 1.25) | 1.99 (0.87 to 3.38) |
| Men | |||
| Cases (%) | 12,607 (53.00) | 9892 (41.58) | 2715 (11.41) |
| ASIR (95% CI) | 7.51 (7.37 to 7.65) | 30.61 (29.97 to 31.26) | 1.23 (1.18 to 1.28) |
| AAPC (95% CI) | 0.23 (− 0. 27 to 0.75) | 0.54 (0.10 to 1.09) | 1.42 (055 to 2.46) |
| NHB | |||
| Women | |||
| Cases (%) | 19,371 (51.18) | 15,692 (41.46) | 3679 (9.72) |
| ASIR (95% CI) | 11.65 (11.48 to 11.82) | 44.83 (44.12 to 45.55) | 2.63 (2.55 to 2.72) |
| AAPC (95% CI) | 1.35 (0.92 to 1.85) | 1.17 (0.69 to 1.73) | 2.11 (1.24 to 3.08) |
| Men | |||
| Cases (%) | 18,478 (48.82) | 14,870 (39.29) | 3608 (9.53) |
| ASIR (95% CI) | 15.64 (15.40 to 15.88) | 62.49 (61.42 to 63.56) | 2.92 (2.82 to 3.01) |
| AAPC (95% CI) | 1.27 (0.90 to 1.79) | 1.21 (0.76 to 1.87) | 1.52 (0.66 to 2.46) |
| NHW | |||
| Women | |||
| Cases (%) | 53,343 (43.54) | 47,945 (39.13) | 5398 (4.41) |
| ASIR (95% CI) | 4.49 (4.45 to 4.53) | 18.14 (17.98 to 18.31) | 0.78 (0.76 to 0.80) |
| AAPC (95% CI) | 0.75 (0.53 to 1.09) | 0.51 (0.26 to 0.70) | 2.10 (1.48 to 2.76) |
| Men | |||
| Cases (%) | 69,167 (56.46) | 61,463 (50.17) | 7704 (6.29) |
| ASIR (95% CI) | 7.26 (7.21 to 7.32) | 29.88 (29.64 to 30.12) | 1.12 (1.09 to 1.14) |
| AAPC (95% CI) | 1.05 (0.80 to 1.34) | 0.98 (0.68 to 1.31) | 1. 53 (1.07 to 1.99) |
NHW Non-Hispanic White, NHB Non-Hispanic Black, ASIR Age-standardized incidence rate, CI Confidence interval, AAPC Average annual percent change.
Older adults (ages ≥ 55)
A total of 166,721 cases of plasma cell myeloma were reported between 2000 and 2019 in the US among those aged ≥ 55 years. The majority of the older adults were men (54.24%) and NHWs (68.86%). The ASIR per 100,000 population was 31.87 (95% CI 31.67 to 32.08) in men and 31.97 (95% CI 31.76 to 32.18) in women. Men had higher AAPC for plasma cell myeloma incidence rate among older adults than women (0.97 vs. 0.68) (Table 4). Over 2000–2019, all races/ethnicities had significant increases in the incidence rates and NHB men had the highest increase (AAPC: 62.49; 95% CI 61.42 to 63.56) (Table 4).
Younger adults (ages < 55)
Over 2000–2019, a total of 26,809 cases of plasma cell myeloma were reported in the US among those aged < 55 years. The majority of them were men (55.45%) and NHWs (51.85%). The ASIR per 100,000 population was 1.32 (95% CI 1.30 to 1.34) for men and women. The AAPCs were higher in women than men (2.25 vs. 2.71) (Table 4). All groups experienced an increase between 2000 and 2019, with NHB women having the highest rate of increase (AAPC: 2.11; 95% CI 1.24 to 3.08) (Table 4).
Extraosseous plasmacytoma
Overall incidence
Over 2000–2019, a total of 2864 cases of extraosseous plasmacytoma in all age groups in the US were reported. The majority of them were men (62.88%) and NHWs (71.18%), and aged ≥ 55 years (72.87%) (Table 5; Figs. S4; S5, S6). The ASIR per 100,000 population was 0.13 (95% CI 0.12 to 0.14) and 0.06 (95% CI 0.06 to 0.07) for men and women, respectively. There was a higher rate of decline in ASIR of extraosseous plasmacytoma among men than women (AAPC: − 3.41; 95% CI − 6.02 to − 0.94 for women and AAPC: − 4.10; 95% CI − 5.87 to − 2.22 for men) (Table 5; Fig. S4). NHB women had the greatest decline in ASIR of extraosseous plasmacytoma with an AAPC of − 7.67 (95% CI − 12.05 to − 3.66) (Table 5).
Table 5.
Counts and age-standardized rate of extraosseous plasmacytoma incidence per 100,000 and average annual percent change from 2000 to 2019 in the United States, by age, sex, and race.
| All age | Age ≥ 55 | Age < 55 | |
|---|---|---|---|
| All races/ethnicities | |||
| Women | |||
| Cases (%) | 1063 (37.12) | 798 (27.86) | 265 (9.25) |
| ASIR (95% CI) | 0.06 (0.06 to 0.07) | 0.22 (0.20 to 0.23) | 0.02 (0.02 to 0.03) |
| AAPC (95% CI) | − 3.41 (− 6.02 to − 0.94) | − 3.91 (− 6.19 to − 1.47) | 0.44 (− 2.39 to 3.61) |
| Men | |||
| Cases (%) | 1801 (62.88) | 1289 (45.01) | 512 (17.88) |
| ASIR (95% CI) | 0.13 (0.12 to 0.14) | 0.44 (0.42 to 0.47) | 0.05 (0.04 to 0.05) |
| AAPC (95% CI) | − 4.10 (− 5.87 to − 2.22) | − 3.74 (− 5.33 to − 1.84) | − 3.39 (− 7.03 to 1.41) |
| Hispanic | |||
| Women | |||
| Cases (%) | 164 (37.18) | 95 (21.54) | 69 (15.65) |
| ASIR (95% CI) | 0.07 (0.06 to 0.08) | 0.22 (0.18 to 0.27) | 0.03 (0.02 to 0.04) |
| AAPC (95% CI) | 1.51 (− 1.23 to 5.53) | 2.59 (− 1.03 to 8.47) | |
| Men | |||
| Cases (%) | 277 (62.82) | 161 (36.51) | 116 (26.30) |
| ASIR (95% CI) | 0.14 (0.12 to 0.16) | 0.48 (0.4 to 0.56) | 0.05 (0.04 to 0.06) |
| AAPC (95% CI) | − 1.15 (− 5.61 to 4.82) | − 1.59 (− 6.13 to 3.43) | |
| NHB | |||
| Women | |||
| Cases (%) | 157 (46.45) | 110 (32.54) | 47 (13.91) |
| ASIR (95% CI) | 0.09 (0.08 to 0.11) | 0.3 (0.25 to 0.37) | 0.03 (0.02 to 0.04) |
| AAPC (95% CI) | − 7.67 (− 12.05 to − 3.66) | ||
| Men | |||
| Cases (%) | 181 (53.55) | 118 (34.91) | 63 (18.64) |
| ASIR (95% CI) | 0.14 (0.12 to 0.17) | 0.49 (0.40 to 0.59) | 0.05 (0.04 to 0.07) |
| AAPC (95% CI) | − 4.97 (− 9.72 to − 1.58) | − 1.01 (− 14.11 to 15.24) | − 7.4 (− 11.3 to − 3.6) |
| NHW | |||
| Women | |||
| Cases (%) | 677 (35.19) | 548 (28.49 | 129 (6.70) |
| ASIR (95% CI) | 0.06 (0.06 to 0.06) | 0.21 (0.19 to 0.23) | 0.02 (0.02 to 0.02) |
| AAPC (95% CI) | − 3.05 (− 6.15 to − 0.36) | − 3.75 (− 6.36 to − 1.19) | 1.53 (− 3.94 to 7.93) |
| Men | |||
| Cases (%) | 1247 (64.81) | 948 (49.27) | 299 (15.54) |
| ASIR (95% CI) | 0.13 (0.12 to 0.14) | 0.45 (0.42 to 0.48) | 0.05 (0.04 to 0.05) |
| AAPC (95% CI) | − 3.65 (− 5.21 to − 2.21) | − 3.72 (− 6.22 to − 1.29) | − 0.19 (− 3.52 to 3.14) |
NHW Non-Hispanic White, NHB Non-Hispanic Black, ASIR Age-standardized incidence rate, CI Confidence interval, AAPC Average annual percent change.
Older adults (ages ≥ 55)
Over 2000–2019, a total of 2087 cases of extraosseous plasmacytoma among older adults in the US were reported. The majority of them were men (61.76%) and NHWs (75.55%). The ASIR per 100,000 population was 0.44 (95% CI 0.42 to 0.47) for men and 0.22 (95% CI 0.20 to 0.23) for women. Women experienced a slightly higher decline than men over 2000–2019 (AAPC: − 3.91; 95% CI − 6.19 to − 1.47 vs. AAPC: − 3.74; 95% CI − 5.33 to − 1.84). NHW women had the highest rate of decline (AAPC: − 3.75; 95% CI − 6.36 to − 1.19) (Table 5).
Younger adults (ages < 55)
Over 2000–2019, a total of 777 cases of extraosseous plasmacytoma among young adults in the US were reported. The majority of the patients were men (65.89%) and NHWs (59.19%). The ASIR per 100,000 population was 0.05 (95% CI 0.04 to 0.05) for men and 0.02 (95% CI 0.02 to 0.03) for women. NHB men were the only group that showed a significant decrease in AAPC (− 7.40; 95% CI − 11.30 to − 3.60) (Table 5).
Solitary plasmacytoma of bone
Overall incidence
Over 2000–2019, 8478 cases of solitary plasmacytoma of bone in all age groups in the US were reported. The majority of the cases were men (61.41%), NHWs (68.52%), and aged ≥ 55 years (75.22%) (Table 6; Figs. S7, S8, and S9). The ASIR per 100,000 population was 0.38 (95% CI 0.37 to 0.39) for men and 0.20 (95% CI 0.19 to 0.20) for women. There were not significant changes in ASIR over 2000–2019 for men and women (Table 6). Only NHW women had a significant increase in AAPC (2.41; 95% CI 0.10 to 5.08) (Table 6).
Table 6.
Counts and age-standardized rate of solitary plasmacytoma of bone incidence per 100,000 and average annual percent change from 2000 to 2019 in the United States, by age, sex, and race.
| All age | Age ≥ 55 | Age < 55 | |
|---|---|---|---|
| All races/ethnicities | |||
| Women | |||
| Cases (%) | 3271 (38.58) | 2558 (30.17) | 713 (8.41) |
| ASIR (95% CI) | 0.20 (0.19 to 0.2) | 0.69 (0.67 to 0.72) | 0.06 (0.06 to 0.07) |
| AAPC (95% CI) | 1.98 (− 0.62 to 3.72) | − 0.05 (− 1.18 to 2.34) | − 0.17 (− 2.29 to 1.96) |
| Men | |||
| Cases (%) | 5207 (61.42) | 3819 (45.05) | 1388 (16.37) |
| ASIR (95% CI) | 0.38 (0.37 to 0.39) | 1.31 (1.26 to 1.35) | 0.12 (0.12 to 0.13) |
| AAPC (95% CI) | 0.01 (− 3.22 to 3.11) | − 0.22 (− 2.15 to 1.94) | 0.56 (− 0.92 to 2.01) |
| Hispanic | |||
| Women | |||
| Cases (%) | 532 (39.80) | 342 (25.58) | 190 (14.21) |
| ASIR (95% CI) | 0.24 (0.22 to 0.26) | 0.79 (0.71 to 0.88) | 0.08 (0.07 to 0.1) |
| AAPC (95% CI) | − 2.23 (− 4.55 to 1.16) | − 1.74 (− 4.60 to 1.70) | NA |
| Men | |||
| Cases (%) | 805 (60.20) | 480 (35.90) | 325 (24.31) |
| ASIR (95% CI) | 0.40 (0.37 to 0.43) | 1.38 (1.25 to 1.52) | 0.14 (0.12 to 0.15) |
| AAPC (95% CI) | − 1.12 (− 4.41 to 2.9) | − 2.12 (− 4.77 to 0.80) | − 0.27 (− 3.14 to 3.12) |
| NHB | |||
| Women | |||
| Cases (%) | 551 (45.13) | 379 (31.04) | 172 (14.08) |
| ASIR (95% CI) | 0.32 (0.29 to 0.35) | 1.04 (0.94 to 1.15) | 0.12 (0.11 to 0.14) |
| AAPC (95% CI) | − 0.77 (− 3.76 to 2.6) | − 0.53 (− 3.76 to 3.47) | − 1.99 (− 4.34 to 0.29) |
| Men | |||
| Cases (%) | 670 (54.87) | 447 (36.62) | 223 (18.26) |
| ASIR (95% CI) | 0.52 (0.48 to 0.56) | 1.77 (1.60 to 1.95) | 0.18 (0.16 to 0.21) |
| AAPC (95% CI) | 0.40 (− 6.18 to 6.63) | 0.93 (− 3.06 to 5.8) | − 2.08 (− 5.00 to 0.77) |
| NHW | |||
| Women | |||
| Cases (%) | 2052 (36.84) | 1739 (31.22) | 313 (5.62) |
| ASIR (95% CI) | 0.18 (0.17 to 0.19) | 0.66 (0.63 to 0.69) | 0.05 (0.04 to 0.05) |
| AAPC (95% CI) | 2.41 (0.10 to 5.08) | 1.73 (0.09 to 3.6) | 2.15 (− 4.13 to 6.68) |
| Men | |||
| Cases (%) | 3518 (63.16) | 2738 (49.16) | 780 (14.00) |
| ASIR (95% CI) | 0.37 (0.36 to 0.38) | 1.30 (1.25 to 1.35) | 0.12 (0.11 to 0.13) |
| AAPC (95% CI) | 0.09 (− 1.29 to 1.55) | − 0.11 (− 2.11 to 2.03) | − 0.02 (− 2.89 to 1.77) |
NHW Non-Hispanic White, NHB Non-Hispanic Black, ASIR Age-standardized incidence rate, CI Confidence interval, AAPC Average annual percent change, NA Not available.
Older adults (ages ≥ 55)
A total of 6377 cases were reported among older adults in the US over 2000–2019, with the majority of them were men (59.88%) and NHWs (73.09%). The ASIR per 100,000 population was 1.31 (95% CI 1.26 to 1.35) for men and 0.69 (95% CI 0.67 to 0.72) for women. There were not significant changes in AAPC from 2000 to 2019 for men and women. Only NHW women had a remarkable increase in AAPC (2.41; 95% CI 0.10 to 5.08) (Table 6).
Younger adults (ages < 55)
Over 2000–2019 a total of 2101 patients with solitary plasmacytoma of bone among young adults in the US were reported. The majority of them were men (66.06%) and NHWs (54.56%). The ASIR per 100,000 population were 0.12 (95% CI 0.12 to 0.13) for men and 0.06 (95% CI 0.06 to 0.07) for women. The AAPC did not have any significant changes by sex and race/ethnicity over the study period (Table 6).
Discussion
This study presented the trends in incidence rates of myeloma in the US across a two-decade interval. To the best of our knowledge, this is the first US population-based study that utilized a large data integrated in the SEER program, focusing on different subtypes of myeloma to perform a comprehensive analysis of the epidemiological trends of myeloma within 2000–2020. Overall, our findings demonstrated an increasing trend of myeloma incidence in both sexes within 2000–2019. Men constituted the majority of myeloma cases (54.82%) and a male predominance was observed in all three morphological subtypes. The incidence rates of myeloma were increasing at a relatively higher rate in older aged men compared to older aged women. However, in individuals younger than 55, the incidence rates increased at a higher pace in women. While myeloma had most commonly occurred in NHWs throughout the study period, NHBs exhibited the highest increase in incidence rate both in men and women among all races/ethnicities. However, racial and ethnic disparities were evident in the trends of myeloma incidence in old and young adult participants. Accordingly, NHBs had the highest AAPC in both older men and women. However, in younger individuals, Hispanic women and NHB men exhibited the highest AAPC. Collectively, the concerning rise in the incidence rate of myeloma among younger women, specifically among young women of Hispanic ethnicity, should be carefully acknowledged when formulating public health strategies to address these worrisome patterns.
Our analysis of plasma cell myeloma incidence trends, which is the most prevalent and extensively studied subtype of myeloma, revealed a consistent pattern similar to the overall trend observed for myeloma. However, NHBs exhibited the highest rate of increase in both younger and older adults across both sexes. In a recent study by Huang et al., an increasing trend of myeloma incidence was reported globally from 2001 to 201933. According to their report and consistent with our observed trends, the incidence of myeloma in the US increased both in men and women, with a higher AAPC observed for men33. Previous reports on the incidence rate of myeloma between 1993 and 2012 in the US, have also pointed out the increased incidence and a tendency for younger age at diagnosis; however, the only significant increases were reported to occur in NHW women and men, as well as NHB men34. Our findings on the other hand, suggest an increase among all races/ethnicities with higher rates of increase in NHBs. Moreover, as observed in the general trend of myeloma, the highest AAPC for myeloma in our study also belongs to the younger women, suggesting that these individuals should be considered at risk with increasing rates of myeloma.
According to our findings, extraosseous plasmacytoma was the only subtype with decreasing AAPC in both men and women. Racial disparities were also observed in the incidence rate of extraosseous plasmacytoma, as NHBs demonstrated the highest rate of decrease in both sexes across all ages. However, in older ages, NHWs witnessed the highest rate of decline in incidence rate. On the other hand, no significant changes in the incidence rate of solitary plasmacytoma of bone were observed throughout the study period, except for NHW women who exhibited significant AAPC increase, which was pronounced in the older age women. While plasmacytoma and myeloma are plasma cell disorders that share certain cytological and immunophenotypic characteristics, their tendency to affect different sites suggest that variations exist between these entities35,36. A previous study on the incidence of myeloma subtypes in the US has highlighted that the incidence of myeloma was 16 times higher than plasmacytoma overall, and the incidence of plasmacytoma of the bone was 40% higher than extraosseous plasmacytoma37. Similar to our findings, rates for plasmacytomas were higher among males, but with increasing incidence over 1992–200437. A more recent study on the US population reported overall ASIRs among adults to be 0.45 for solitary plasmacytoma and 0.12 for extraosseous plasmacytoma and the incidence rates were higher in men in both diseases38.
In order to address the impact of the COVID-19 pandemic on the reported incidence of myeloma in the US, we analyzed the corresponding data for 2020 and the change in AAPC compared to the preceding year. Findings revealed a remarkable decrease in the ASIR of myeloma within all races and both sexes following the outbreak of the pandemic. However, the differential impact of the pandemic was observed on different age groups and races. More precisely, the decline in ASIR was highly pronounced in older age groups, as no significant changes in the ASIR of myeloma were observed in the < 55 age group across any race or sex groups. Notably, male Hispanics were the only group who were not affected by the pandemic in any of the age groups. Overall, findings suggest that the incidence of myeloma among older individuals of all races except for male Hispanics was significantly affected by the COVID-19 pandemic. Supporting these observations, a recent umbrella review has identified a substantial decrease in screening and diagnosis of several cancers during the pandemic, which was more remarkable in regions that implemented a lockdown strategy39. Among these cancers, myeloma is no exception as previous global reports have indicated that the number of newly diagnosed myeloma cases was reduced in 2020 compared to 201911. The reduced incidence of myeloma during the COVID-19 pandemic, as observed in our study and previous reports on other cancer entities40,41, could be attributed to impaired screening and utilization of diagnostic measures during the pandemic42. The overload of medical centers with COVID-19, as well as the reluctance of patients to seek diagnostic procedures might have resulted in underreporting of cancer patients. Supporting this, studies indicate that cancer biopsies, including bone marrow biopsies which are crucial in diagnosing hematological malignancies, have remarkably decreased with the outbreak of the pandemic43.
The underreporting and delayed diagnosis of myeloma has resulted in increased disease burden post-pandemic. Accordingly, a report by Carmichael et al. indicated an increasing presentation of myeloma cases to emergency services with heightened rates of osseous and extra-medullary manifestations44. Findings of another study have also confirmed decreased survival of myeloma cases after the pandemic11. Moreover, reports from the early COVID-19 pandemic have also highlighted that compared with the pre-pandemic era, myeloma patients during the COVID-19 pandemic were less likely to initiate treatment or had initiated treatment later45. Therefore, both delayed diagnosis and postponed treatment initiation might contribute to decreased survival. The delay in treatment and changes in treatment pattern, may also cause the pandemic to leave a mark on the survival of myeloma patients in the subsequent years44. Although the long-term effects of these changes may not be observable for a considerable period, it is evident that the COVID-19 pandemic, and its broader influence on healthcare delivery, have led to alterations in the epidemiology and burden of individuals with myeloma and these changes could potentially have significant implications for the patients’ future prospects.
There are several strengths and limitations to this study. This is the first study to utilize the updated SEER database with robust statistical measures to provide a comprehensive US population-based study on incidence trends of myeloma. Besides analyzing the general trends of myeloma incidence, our study provided subgroup analyses on distinct pathological subtypes, including plasma cell myeloma and two less prevalent, yet critical subtypes, to better elucidate the incidence rate across various pathologic classes. Due to the unneglectable impact of the COVID-19 pandemic on cancer diagnosis, we also provided a detailed overview of changes in incidence rates of myeloma in the first year of the pandemic outbreak compared to the preceding year across all races, age groups and sexes. However, our findings might be influenced by the limitations of the SEER database. For instance, misclassification bias due to the collection of demographic data, including race and ethnicity, from various sources such as administrative databases, patient intake or provider notes is probable. Furthermore, there is uncertainty regarding whether self-identification of race and ethnicity accurately reflects ancestry or if it is primarily based on cultural factors, particularly in individuals who have mixed racial backgrounds. This ambiguity adds complexity to the interpretation of data related to race and ethnicity and should be taken into consideration when generalizing our findings. Additionally, our analysis was not sub-grouped according to race and ethnicity groups of American Indian/Alaskan Native, Asian/Pacific Islander, and Native Hawaiian due to the small sample size. Therefore, our findings may not fully represent the characteristics of individuals from these specific racial and ethnic backgrounds. The diagnostic methods of cancers can influence the incidence trends of myeloma and lead to under- or over-estimation of cancers. So, providing valid and reliable information for diagnostic methods and development of additional biomarkers data in SEER can improve the estimation of incidence of myeloma in future SEER data46. Also, there might be some other potential contributing factors to myeloma such as socioeconomic status that were not the aim of the study and can be considered in future studies. Moreover, data of some potential factors like occupation was not available in the current SEER iteration and can be taken into account for next SEER versions. Regarding the effects of COVID-19, there was a decreasing trend in the myeloma diagnosis by 14% in 2020 compared with 2019 at the global level11. The diagnosis of hematological malignancies was also decreased in the US during the COVID-19 pandemic compared with pre-pandemic period, especially due to the lockdown strategies47. As a result, the incidence data in 2020 was excluded from joinpoint trends. Nevertheless, it should be acknowledged as a study limitation and be considered in the interpretation of results.
Conclusions
There was an increase in myeloma incidence in both sexes, with a highly increasing rate, particularly among younger Hispanic and NHB women between 2000 and 2019. The findings of our study also underscore the significant impact of the COVID-19 pandemic on the reported incidence rate of myeloma in 2020 across most races/ethnicities, particularly in older ages.
Supplementary Information
Acknowledgements
We would like to thank the National Institute of Cancer staff and its collaborators of the Surveillance, Epidemiology, and End Results Program (SEER) who prepared these data.
Author contributions
S.E.M. and S.A.N. designed the study. S.E.M. analyzed the data and performed the statistical analyses. S.E.M., M.I., A.A., Z.Y., and S.A.N. drafted the initial manuscript. S.E.M., Z.Y., and S.A.N. critically edited and revised the initial draft of the manuscript. S.E.M. and S.A.N. supervised the project. All authors reviewed the drafted manuscript for critical content. All authors approved the final version of the manuscript.
Data availability
The data presented in this study are available at https://seer.cancer.gov/data-software/
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seyed Ehsan Mousavi, Email: mousavi.e@tbzmed.ac.ir, Email: drehsanmousavii@gmail.com.
Seyed Aria Nejadghaderi, Email: ariang20@gmail.com, Email: ariaghaderi@sbmu.ac.ir.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-47906-y.
References
- 1.Padala SA, Barsouk A, Barsouk A, Rawla P, Vakiti A, Kolhe R, et al. Epidemiology, staging, and management of multiple myeloma. Med. Sci. 2021;9(1):66. doi: 10.3390/medsci9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghazaryan N, Danelyan S, Bardakhchyan S, Saharyan A, Sahakyan L. Multiple myeloma in Armenia during the period 2006–2018: Facts and discussion. BMC Cancer. 2021;21(1):941. doi: 10.1186/s12885-021-08676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bora K. Distribution of multiple myeloma in India: Heterogeneity in incidence across age, sex and geography. Cancer Epidemiol. 2019;59:215–220. doi: 10.1016/j.canep.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017;1(4):282–287. doi: 10.1182/bloodadvances.2016002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres M, Feller A, Arndt V, Group NW. Trends of incidence, mortality, and survival of multiple myeloma in Switzerland between 1994 and 2013. Cancer Epidemiol. 2018;53:105–10. doi: 10.1016/j.canep.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Tsang M, Le M, Ghazawi FM, Cyr J, Alakel A, Rahme E, et al. Multiple myeloma epidemiology and patient geographic distribution in Canada: A population study. Cancer. 2019;125(14):2435–2444. doi: 10.1002/cncr.32128. [DOI] [PubMed] [Google Scholar]
- 7.Fend F, Dogan A, Cook JR. Plasma cell neoplasms and related entities-evolution in diagnosis and classification. Virchows Arch. 2023;482(1):163–177. doi: 10.1007/s00428-022-03431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curado MP, Oliveira MM, Silva DRM, Souza DLB. Epidemiology of multiple myeloma in 17 Latin American countries: An update. Cancer Med. 2018;7(5):2101–2108. doi: 10.1002/cam4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Liu W, Mi L, Zeng X, Cai C, Ma J, et al. Incidence and mortality of multiple myeloma in China, 2006–2016: An analysis of the Global Burden of Disease Study 2016. J. Hematol. Oncol. 2019;12(1):136. doi: 10.1186/s13045-019-0807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur G, Mejia Saldarriaga M, Shah N, Catamero DD, Yue L, Ashai N, et al. Multiple myeloma in hispanics: Incidence, characteristics, survival, results of discovery, and validation using real-world and connect MM registry data. Clin. Lymphoma Myeloma Leuk. 2021;21(4):e384–e397. doi: 10.1016/j.clml.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Lopez J, Hernandez-Ibarburu G, Alonso R, Sanchez-Pina J, Zamanillo I, Lopez-Muñoz N, et al. Impact of COVID-19 in patients with multiple myeloma based on a global data network. Blood Cancer J. 2021;11(12):198. doi: 10.1038/s41408-021-00588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Lopez J, Hernandez-Ibarburu G, Alonso R, Sanchez-Pina JM, Zamanillo I, Lopez-Munoz N, et al. Impact of COVID-19 in patients with multiple myeloma based on a global data network. Blood Cancer J. 2021;11(12):198. doi: 10.1038/s41408-021-00588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmichael J, Seymour F, McIlroy G, Tayabali S, Amerikanou R, Feyler S, et al. Delayed diagnosis resulting in increased disease burden in multiple myeloma: the legacy of the COVID-19 pandemic. Blood Cancer J. 2023;13(1):38. doi: 10.1038/s41408-023-00795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milano AF. Plasma cell myeloma-20-year comparative survival and mortality of three plasma cell myeloma ICD-O-3 oncologic phenotypes by age, sex, race, stage, cohort entry time-period and disease duration: A systematic review of 111,041 cases for diagnosis years 1973–2014: (SEER*Stat 8.3.4) J. Insur. Med. 2018;47(4):203–11. doi: 10.17849/insm-47-04-1-9.1. [DOI] [PubMed] [Google Scholar]
- 15.SEER. About the SEER Program—SEER. Retrieved Jun 11, 2023, from https://seer.cancer.gov/about/overview.html
- 16.databse TNCIsS. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER Research Limited-Field Data with Delay-Adjustment, 22 Registries, Malignant Only, Nov 2022 Sub (2000–2020)—Linked To County Attributes—Time Dependent (1990–2021) Income/Rurality, 1969–2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
- 17.databse TNCIsS. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER Research Limited-Field Data, 22 Registries, Nov 2022 Sub (2000–2020)—Linked To County Attributes—Time Dependent (1990–2021) Income/Rurality, 1969–2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
- 18.SEER. SEER Research Data Agreement. (n.d.). SEER. Retrieved Jun 11, 2023, from https://seer.cancer.gov/data-software/documentation/seerstat/nov2022/seer-dua-nov2022.html
- 19.Institute TNC. Impact of COVID on 2020 SEER Cancer Incidence Data. 2023.
- 20.NCI's Surveillance E, and End Results (SEER). Plasma cell neoplasm 2023. Available from: https://seer.cancer.gov/seertools/hemelymph/5331a216e4b0626b192764b4/
- 21.SEER. Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.4.1.2.
- 22.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J. Natl. Cancer Inst. 2002;94(20):1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 23.@@@Technical Notes—Reporting delay. In (eds Altekruse, S. F., Kosary, C. L., Krapcho, M., Neyman, N., Aminou, R., Waldron, W., Ruhl, J., Howlader, N., Tatalovich, Z., Cho, H., Mariotto, A., Eisner, M. P., Lewis, D. R., Cronin, K., Chen, H. S., Feuer, E. J., Stinchcomb, D. G., & Edwards, B. K.) SEER Cancer Statistics Review, 1975–2007, National Cancer Institute. Bethesda, MD, based on November 2009 SEER data submission, posted to the SEER website 12–16 (2010).
- 24.Institute TNC. Development of the Delay Model (2023).
- 25.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 26.Institute NC. Joinpoint Regression Program, Version 5.0.2—May 2023; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- 27.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat. Med. 2009;28(29):3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60(4):1005–1014. doi: 10.1111/j.0006-341X.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Chen HS, Byrne J, Wheeler B, Feuer EJ. Twenty years since Joinpoint 1.0: Two major enhancements, their justification, and impact. Stat. Med. 2022;41(16):3102–30. doi: 10.1002/sim.9407. [DOI] [PubMed] [Google Scholar]
- 30.NCI's Surveillance E, and End Results (SEER). Impact of COVID on 2020 SEER Cancer Incidence Data 2023. Available from: https://seer.cancer.gov/data/covid-impact.html.
- 31.Institute TNC. Weighted BIC (WBIC).
- 32.Kim HJ, Luo J, Chen HS, Green D, Buckman D, Byrne J, et al. Improved confidence interval for average annual percent change in trend analysis. Stat. Med. 2017;36(19):3059–3074. doi: 10.1002/sim.7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Chan SC, Lok V, Zhang L, Lucero-Prisno DE, Xu W, et al. The epidemiological landscape of multiple myeloma: A global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022;9(9):e670–e677. doi: 10.1016/S2352-3026(22)00165-X. [DOI] [PubMed] [Google Scholar]
- 34.Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017;1(4):282–287. doi: 10.1182/bloodadvances.2016002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremer M, Ott G, Nathrath M, Specht K, Stecker K, Alexiou C, et al. Primary extramedullary plasmacytoma and multiple myeloma: Phenotypic differences revealed by immunohistochemical analysis. J. Pathol. 2005;205(1):92–101. doi: 10.1002/path.1680. [DOI] [PubMed] [Google Scholar]
- 36.Bladé J, Beksac M, Caers J, Jurczyszyn A, von Lilienfeld-Toal M, Moreau P, et al. Extramedullary disease in multiple myeloma: A systematic literature review. Blood Cancer J. 2022;12(3):45. doi: 10.1038/s41408-022-00643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: Incidence and survival in the United States, 1992–2004. Brit. J. Haematol. 2009;144(1):86–94. doi: 10.1111/j.1365-2141.2008.07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellington TD, Henley SJ, Wilson RJ, Wu M, Richardson LC. Trends in solitary plasmacytoma, extramedullary plasmacytoma, and plasma cell myeloma incidence and myeloma mortality by racial-ethnic group, United States 2003–2016. Cancer Med. 2021;10(1):386–395. doi: 10.1002/cam4.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muka T, Li JJ, Farahani SJ, Ioannidis JP. An umbrella review of systematic reviews on the impact of the COVID-19 pandemic on cancer prevention and management, and patient needs. Elife. 2023;12:66. doi: 10.7554/eLife.85679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Medina S, Gil S, Jimenez B, Rodriguez-Brazzarola P, Diaz-Redondo T, Cazorla M, et al. Significant decrease in annual cancer diagnoses in Spain during the COVID-19 pandemic: A real-data study. Cancers. 2021;13(13):3215. doi: 10.3390/cancers13133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrew T, Alrawi M, Lovat P. Reduction in skin cancer diagnoses in the UK during the COVID-19 pandemic. Clin. Exp. Dermatol. 2021;46(1):145–146. doi: 10.1111/ced.14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on cancer care: How the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin. Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amador M, Matias-Guiu X, Sancho-Pardo G, Martinez JC, de la Torre-Montero JC, Saiz AP, et al. Impact of the COVID-19 pandemic on the care of cancer patients in Spain. ESMO Open. 2021;6(3):100157. doi: 10.1016/j.esmoop.2021.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmichael J, Seymour F, McIlroy G, Tayabali S, Amerikanou R, Feyler S, et al. Delayed diagnosis resulting in increased disease burden in multiple myeloma: The legacy of the COVID-19 pandemic. Blood Cancer J. 2023;13(1):38. doi: 10.1038/s41408-023-00795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neparidze N, Wang R, Zeidan AM, Podoltsev NA, Shallis RM, Ma X, et al. Changes in multiple myeloma treatment patterns during the early COVID-19 pandemic period. Leukemia. 2022;36(8):2136–2139. doi: 10.1038/s41375-022-01633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) program and pathology: Toward strengthening the critical relationship. Am. J. Surg. Pathol. 2016;40(12):e94–e102. doi: 10.1097/PAS.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhandari S, Chawla J, Jain P, Kaushiva A, Lagor C, Lal LS, et al. Racial disparities in newly diagnosed hematological malignancies in the United States during the COVID-19 pandemic, January 2020 to March 2021. Blood. 2022;140(Supplement 1):13269–13270. doi: 10.1182/blood-2022-170098. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available at https://seer.cancer.gov/data-software/