Abstract
Age and sex have effect on atherosclerosis. This study aimed to investigate their effect on non-stenotic intracranial atherosclerotic plaque (NIAP) in embolic stroke of undetermined source (ESUS) using high-resolution magnetic resonance imaging (HR-MRI). We retrospectively recruited consecutive ESUS patients who underwent intracranial HR-MRI to assess the plaque characteristics (remodeling index [RI], plaque burden [PB], fibrous cap [FC], discontinuity of plaque surface [DPS], intraplaque hemorrhage [IPH] and complicated plaque [CP]). We divided patients into three groups (< 60 years, 60–74 years, ≥ 75 years). 155 patients with ipsilateral NIAP were found from 243 ESUS patients, with 106 men (68.39%) and 49 women (31.61%). In total population or age group under 60 years, there were no significant differences in plaque characteristics between men and women (all p > 0.05). In age group of 60–74 years, men were associated with higher PB (66.27 ± 9.17% vs 60.91 ± 8.86%, p = 0.017) and RI (1.174 vs 1.156, p = 0.019), higher prevalence of DPS (82.50% vs 60.00%, p = 0.036) and complicated plaque (85.00% vs 63.33%, p = 0.036). For subjects ≥ 75 years old, PB were significantly higher in twomen vs men (68.85 ± 6.14% vs 62.62 ± 7.36%, p = 0.040). In addition, the probability for PBupper (≥ median PB), RIupper (≥ median RI) and vulnerable plaque increased as age increased, and its predictive power for index ESUS was higher in men than women. This study identified age-dependent sex differences in NIAP characteristics of ESUS patients, which will help us clarify their etiology.
Subject terms: Neuroscience, Medical research, Neurology
Introduction
In 2014, the Cryptogenic Stroke/ESUS International Working Group proposed the clinical concept of embolic strokes of undetermined source (ESUS)1. The criteria and the diagnostic algorithm for ESUS were recently updated2.Although there are substantial differences between men and women in terms of stroke occurrence3, how the effect of gender on intracranial plaque in patients with ESUS remains unexplored.
It is well known that age and sex have effect on atherosclerosis. Women tend to have higher prevalence of stable plaques compared with men4. Men also have more often a plaque with multiple vulnerable plaque components in symptomatic patients with mild-to-moderate carotid stenosis5. Plaque prevalence was higher in men than women in most age groups, until the age of 75, when carotid atherosclerosis was more common in women (81.2%) than men (76.5%)6. In addition, the effect of age and gender on ischemic stroke risk and pathophysiology is interactive and complex7. Since the clinical concept of ESUS was proposed1, the nature of these embolic sources is highly heterogeneous8–11. Growing evidence presented the importance of non-stenotic atherosclerotic plaques located extracranial12–14 or intracranial vessels15,16. Recently, a study based computed tomographic angiography (CTA) of the neck suggested that there were gender differences in carotid plaque composition in the ESUS cohort, but no significant difference in the atrial fibrillation (AF) cohort17.
In this context, we hypothesized that there may be sex differences in the characteristics of intracranial plaques, which could be affected by age. In the present study, we assess the characteristics of non-stenotic intracranial atherosclerotic plaque (NIAP) in women vs men with ESUS using 3.0 T high-resolution magnetic resonance imaging (MRI) to test the hypothesis.
Methods
Study enrollment and information collection
We used the same ESUS cohort, which has been reported in detail in our recent study15. We retrospectively recruited patients with acute ischemic stroke in the territory of a unilateral anterior circulation between January 2015 and December 2019. All patients included in the study completed HR-MRI within one week from onset and were eligible for proposed the diagnostic criteria for ESUS. In addition, we further excluded patients with nonstenosing carotid plaque ≥ 3 mm detected by computed tomography angiography (CTA) or carotid ultrasonography, aortic arch atherosclerotic plaque with ulceration or ≥ 4 mm by CTA or transesophageal echocardiogram (TEE), balloon dilatation and stent, previous radiation therapy to head or neck and malignant tumor. The more detailed inclusion/exclusion criteria were reported elsewhere15. The study was performed in accordance with the relevant guidelines and regulations, and approved by the Institutional Review Board of General Hospital of Northern Theater Command (IRB: k2019-57), which waived the requirement to obtain informed consent.
According to age, the patients were categorized into three groups: < 60 years group, 60–74 years group, and ≥ 75 years group. The categorization rationale was based on previous studies: (1) a study suggested that plaque prevalence was higher in men than women in most age groups, until the age of 75, when carotid atherosclerosis was more common in women (81.2%) than men (76.5%)6; (2) many studies were grouped by the age of 6018–20.
Imaging analysis
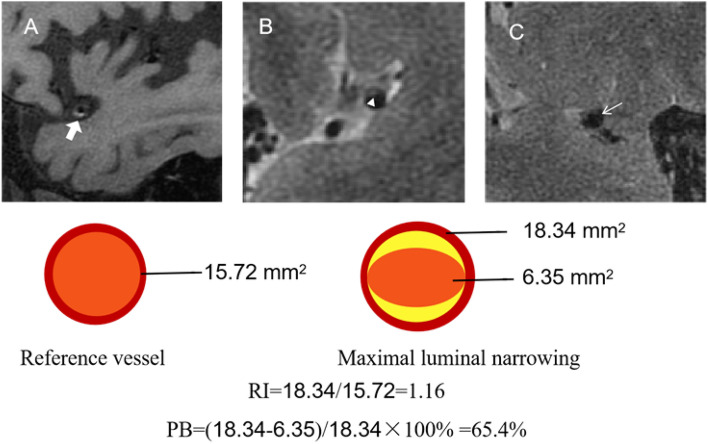
In agreement with our previous study21, the culprit plaque was defined as a lesion of ipsilateral proximal vascular territory of infarction with clinical symptoms. The detailed image protocol was reported in our recent study15,21. As shown in Fig. 1, multidimensional parameters were evaluated using 3.0 T high-resolution magnetic resonance imaging, including plaque remodeling index (RI), plaque burden (PB), fibrous cap (FC), discontinuity of plaque surface (DPS), intraplaque hemorrhage (IPH) and complicated plaque (CP).
Figure 1.
Schematic diagram of multidimensional parameters using 3.0 T high-resolution magnetic resonance imaging. Top: A, the plaque with IPH; B, the plaque with a thick FC; C, the plaque with DPS. Bottom: Schematic diagram of PB and remodeling index RI. IPH, intraplaque hemorrhage; FC, fibrous cap; DPS, discontinuity of plaque surface; PB, plaque burden; RI, remodeling index.
The plaque at the maximal luminal narrowing (MLN) site was selected in the cross-sectional images of vessels, while the reference sites were chosen as the neighboring plaque-free or was calculated as the average of the minimal lesion segment proximal and distal to the MLN site due to vessel tapering. The RI was defined as vessel area at the MLN site divided by reference vessel area, and RIupper was defined as the value ≥ median of remodeling index. The PB was calculated as (vessel area luminal area/vessel area at MLN site) × 100%, and PBupper was calculated as the value ≥ median of plaque burden. The thick FC was defined as a continuous band of T2 high signal adjacent to the lumen. DPS was defined as irregularity of plaque luminal surface, such as FC rupture, ulcer plaque, or formation of overlying mural thrombus. IPH was defined as a bright T1 signal ≥ 150% of T1 signal of adjacent muscle or pons. Complicated plaque (CP) was defined as any or both of DPS and IPH based on the definition of a complicated American Heart Association type VI plaque22.
Statistical analysis
We used Student’s t-test (normally distributed) or the Wilcoxon rank sum test (not normally distributed), as appropriate, for continuous variables, and chi-square test or Fisher’s exact for categorical variables, to compare the difference of baseline clinical, plaque characteristics between men and women among different age groups. To explore the age distribution of plaque characteristics across genders, we performed violin plots and percentage bar charts. To assess probability of vulnerable plaque and facilitate statistical analysis, we transformed PB and RI into binary variables by the median, and then we performed probability curves for plaque characteristics. Univariable and multivariable logistic regression analyses were performed to identify whether vulnerable features of plaques were related to mechanism of the ESUS between men and women. All analyses were performed using SPSS version 22, GraphPad software Inc., Prism Version 8 and a p-value (2-tailed) < 0.05 were considered to indicate statistical significance.
Ethics approval
This retrospective study was approved by the Institutional Review Board of General Hospital of Northern Theater Command (IRB: k2019-57).
Results
From the initial cohort of 587 ESUS patients, we excluded patients with bilateral or posterior circulation ESUS (n = 193), carotid plaque of ≥ 3 mm in thickness (n = 91), aortic arch atherosclerosis with ulceration or ≥ 4 mm in thickness (n = 15), paradoxical embolism (n = 27) and poor image quality or incomplete information (n = 18). The final cohort included 243 ESUS patients, of whom 155 (31.6% women) had ipsilateral NIAP and 88 patients had not ipsilateral NIAP.
Supplemental Table 1 summarizes the baseline characteristics in male and female groups. Among ESUS patients with ipsilateral NIAP, compared with men, women were associated with lower prevalence of smoking (14.2% vs 60.3%, p < 0.001) and alcohol drinking (4.0% vs 52.8%, p < 0.001), higher NT-proBNP (126.00 [IQR 63.15–251.2] pg/mL vs 87.17 [IQR 36.04–159.38] pg/mL, p < 0.021), total cholesterol (5.09 ± 1.11 mmol/L vs 4.47 ± 1.18 mmol/L, p = 0.002), HDL (1.06 [0.92–1.29] mmol/L vs 0.94 [0.79–1.08] mmol/L, p < 0.001) and LDL (3.05 ± 0.83 mmol/L vs 2.65 ± 0.81 mmol/L, p < 0.001), but lower creatinine (54.2 [48.87–63.16] umol/L vs 70.55 [62.93–80.90] umol/L, p < 0.001) and homocysteine (10.56 [8.55–13.62] umol/L vs 12.16 [9.96–16.11] umol/L, p < 0.001). We also found similar results in the age group of 60–74 years. In the age group under 60 years, there were differences in smoking, drinking and the level of creatinine between men and women (p < 0.05). For subjects ≥ 75 years old, there was difference in initial NIHSS between men and women (p < 0.05).
Table 1 and Fig. 2 summarizes sex differences in ipsilateral NIAP characteristics of ESUS among different age groups. Among all ESUS patients, there was a trend of higher prevalence of complicated plaque in men, which did not reach statistical significance (men vs women: 81.1% vs 67.3%, p = 0.059). In the age group under 60 years, there were no significant differences in plaque characteristics between men and women (all p > 0.05). In the age group of 60–74 years, we found that men were associated with higher PB (66.27 ± 9.17% vs 60.91 ± 8.86%, p = 0.017; Fig. 2A) and RI (1.17 [IQR1.14–1.21] vs 1.16 [1.11–1.18], p = 0.019; Fig. 2A), higher prevalence of DPS (82.50% vs 60.00%, p = 0.036; Fig. 2B) and complicated plaque (85.00% vs 63.33%, p = 0.036; Fig. 2B). For subjects ≥ 75 years old, PB were significantly higher in women vs men (68.85 ± 6.14% vs 62.62 ± 7.36%, p = 0.040; Fig. 2A). There were not sex differences in contralateral NIAP among different age groups (Supplemental Table 2). In addition, we also divided patients into two groups based on the median of age (62 years), and a similar trend was found as the results of three groups (< 60, 60–74, and ≥ 75 years) (Supplemental Table 3).
Table 1.
Sex differences in ipsilateral NIAP of ESUS among different age groups.
| Total | Age < 60 years | Age 60–74 years | Age ≥ 75 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 106) | Female (n = 49) | p | Male (n = 54) | Female (n = 8) | p | Male (n = 40) | Female (n = 30) | p | Male (n = 12) | Female (n = 11) | p | |
| PB, % | 64.30 ± 8.98 | 62.72 ± 8.70 | 0.304 | 63.22 ± 9.06 | 61.08 ± 7.97 | 0.529 | 66.27 ± 9.17 | 60.91 ± 8.86 | 0.017 | 62.62 ± 7.36 | 68.85 ± 6.14 | 0.040 |
| RI | 1.17 (1.13–1.19) | 1.16 (1.11–1.19) | 0.565 | 1.16 (1.12–1.18) | 1.170 (1.11–1.19) | 0.578 | 1.17 (1.14–1.21) | 1.16 (1.11–1.18) | 0.019 | 1.15 (1.09–1.22) | 1.19 (1.16–1.23) | 0.356 |
| DPS | 82 (77.3) | 32 (65.3) | 0.114 | 40 (74.07) | 1 (12.50) | 0.672 | 33 (82.50) | 18 (60.00) | 0.036 | 9 (75.00) | 9 (81.82) | 1.000 |
| IPH | 29 (27.3) | 12 (24.4) | 0.707 | 14 (25.93) | 3 (37.50) | 0.672 | 13 (32.50) | 5 (16.67) | 0.172 | 2 (16.67) | 4 (36.36) | 0.371 |
| Thick FC | 36 (35.0) | 17 (35.4) | 0.956 | 23 (42.59) | 2 (25.00) | 0.456 | 8 (20.00) | 12 (40.00) | 0.089 | 5 (45.45) | 3 (30.00) | 0.659 |
| CP | 86 (81.1) | 33 (67.3) | 0.059 | 42 (77.77) | 5 (62.50) | 0.388 | 34 (85.00) | 19 (63.33) | 0.036 | 10 (83.33) | 9 (81.82) | 1.000 |
NIAP, non-stenotic intracranial atherosclerotic plaque; ESUS, embolic stroke of undetermined source; PB, plaque burden; RI, remodeling index; DPS, discontinuity of plaque surface; FC, thick fibrous cap; IPH, intraplaque hemorrhage; CP, complicated plaque; values are mean ± SD, median (interquartile range), or n (%).
Figure 2.
Age-dependent sex differences in ipsilateral NIAP characteristics. Figure A presented age-dependent sex differences in PB and RI; Figure B presented age-dependent probability of DPS, IPH, thick FC or complicated plaque in male vs female ESUS. NIAP, non-stenotic intracranial atherosclerotic plaque; PB, plaque burden; RI, remodeling index; DPS, discontinuity of plaque surface; FC, thick fibrous cap; IPH, intraplaque hemorrhage; CP, complicated plaque.
As shown in Fig. 3, the probability for PBupper and RIupper and vulnerable plaque increased as age increased. Interestingly, the probability of PBupper and RIupper was similar between men and women around age 75. For subjects < 75 years old, the probability of PBupper and RIupperb were higher in men, while the probability of PBupper and RIupperb were higher in women over 75 years old. Furthermore, the probability of DPS, IPH, CP was higher in men regardless of age. In addition, the probability of thick FC was decreased in men as age increased, but increased in women.
Figure 3.
Probability curves for plaque characteristics in male vs female ESUS. PBupper, the value ≥ median of plaque burden; RIupper, the value ≥ median of remodeling index; DPS, discontinuity of plaque surface; FC, thick fibrous cap; IPH, intraplaque hemorrhage; CP, complicated plaque.
Table 2 and Fig. 4 summarizes the sex difference of NIAP ipsilateral vs contralateral to ESUS. The univariable logistic regression analyses adjusting for age or excluding more than 75 years old patients showed that PB, RI, DPS and complicated plaque were related with an index ESUS in total patients or in men (all p < 0.05). In women, only RI was related with an index ESUS (p < 0.05), but the association disappeared after excluding more than 75 years old patients (p = 0.120).
Table 2.
Univariable logistic regression analyses of ipsilateral NIAP for index ESUS, after adjusted for age or age ≤ 75 years.
| Univariable OR (95% CI) |
p | Adjust age OR (95% CI) |
p | Age ≤ 75 years OR (95% CI) |
p | |
|---|---|---|---|---|---|---|
| Total | ||||||
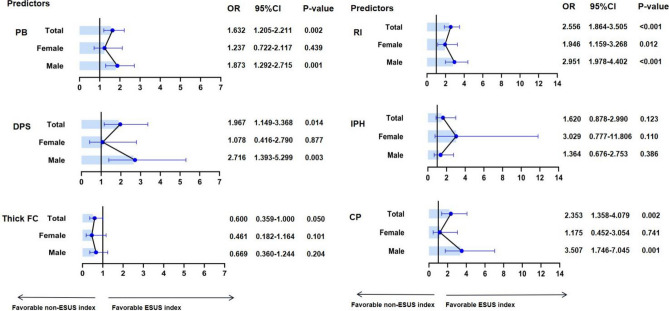
| PB *10 | 1.593 (1.182–2.149) | 0.002 | 1.632 (1.205–2.211) | 0.002 | 1.449 (1.054–1.992) | 0.022 |
| RI*10 | 2.553 (1.861–3.503) | < 0.001 | 2.556 (1.864–3.505) | < 0.001 | 2.387 (1.703–3.346) | < 0.001 |
| DPS | 1.884 (1.109–3.200) | 0.019 | 1.967 (1.149–3.368) | 0.014 | 1.920 (1.081–3.411) | 0.026 |
| IPH | 1.609 (0.872–2.968) | 0.128 | 1.620 (0.878–2.990) | 0.123 | 1.465 (0.760–2.842) | 0.255 |
| Thick FC | 0.607 (0.365–1.011) | 0.055 | 0.600 (0.359–1.000) | 0.050 | 0.638 (0.365–1.114) | 0.114 |
| CP | 2.239 (1.304–3.845) | 0.003 | 2.353 (1.358–4.079) | 0.002 | 2.250 (1.254–4.038) | 0.007 |
| Male | ||||||
| PB *10 | 1.826 (1.266–2.632) | 0.001 | 1.873 (1.292–2.715) | 0.001 | 1.803 (1.232–2.638) | 0.002 |
| RI*10 | 2.962 (1.985–4.421) | < 0.001 | 2.951 (1.978–4.402) | < 0.001 | 2.913 (1.905–4.454) | < 0.001 |
| DPS | 2.522 (1.317–4.830) | 0.005 | 2.716 (1.393–5.299) | 0.003 | 2.800 (1.406–5.577) | 0.003 |
| IPH | 1.342 (0.666–2.702) | 0.410 | 1.364 (0.676–2.753) | 0.386 | 1.468 (0.700–3.080) | 0.310 |
| Thick FC | 0.688 (0.372–1.273) | 0.234 | 0.669 (0.360–1.244) | 0.204 | 0.593 (0.309–1.138) | 0.116 |
| CP | 3.174 (1.620–6.217) | 0.001 | 3.507 (1.746–7.045) | < 0.001 | 3.401 (1.673–6.913) | 0.001 |
| Female | ||||||
| PB *10 | 1.184 (0.702–1.997) | 0.527 | 1.237 (0.722–2.117) | 0.439 | 0.807 (0.436–1.493) | 0.495 |
| RI*10 | 1.897 (1.131–3.179) | 0.015 | 1.946 (1.159–3.268) | 0.012 | 1.541 (0.894–2.657) | 0.120 |
| DPS | 1.035 (0.404–2.656) | 0.942 | 1.078 (0.416–2.790) | 0.877 | 0.767 (0.251–2.341) | 0.641 |
| IPH | 3.027 (0.779–11.759) | 0.110 | 3.029 (0.777–11.806) | 0.110 | 1.600 (0.375–6.820) | 0.525 |
| Thick FC | 0.452 (0.180–1.136) | 0.091 | 0.461 (0.182–1.164) | 0.101 | 0.778 (0.262–2.306) | 0.650 |
| CP | 1.134 (0.440–2.926) | 0.794 | 1.175 (0.452–3.054) | 0.741 | 0.857 (0.279–2.631) | 0.788 |
NIAP, non-stenotic intracranial atherosclerotic plaque; ESUS, embolic stroke of undetermined source; PB, plaque burden; RI, remodeling index; DPS, discontinuity of plaque surface; FC, thick fibrous cap; IPH, intraplaque hemorrhage; CP, complicated plaque.
Figure 4.
Subgroup analyses by sex stratification for predicting ESUS. All models adjusted for age. PB, plaque burden; RI, remodeling index; DPS, discontinuity of plaque surface; FC, thick fibrous cap; IPH, intraplaque hemorrhage; CP, complicated Plaque.
Univariable logistic regression analyses were performed to identify the sex difference of ipsilateral vs contralateral NIAP in different age groups (Supplemental Table 4 and Fig. 5). In the age group under 60 years, PB, RI, DPS, and complicated plaque were related with an index ESUS in the overall cohort or in men (p < 0.05), but none of these were related with an index ESUS in women (p > 0.05). In the age group of 60–74 years, RI was related with an index ESUS in total patients (p < 0.05), while PB, RI with an index ESUS in men (p < 0.05), but none of these were related with an index ESUS in women. For subjects ≥ 75 years old, RI was related with an index ESUS in the overall cohort, men or women, and PB were related with an index ESUS in total patients or women. On multivariable logistic regression analysis (Table 3), RI was related with an index ESUS in the overall cohort, men or women, and CP was related with an index ESUS in the overall cohort in the age group under 60 years (p < 0.05). In the age group of 60–74 years, RI was related with an index ESUS in the overall cohort or in men (p < 0.05). For subjects ≥ 75 years old, only RI was related with an index ESUS in the overall cohort (p < 0.05) (Table 3). When combined with PB, RI and complicated plaque for predicting index ESUS (Fig. 6), no significant difference in AUC values was found between men and women (AUC: 0.777 vs o.768) in the age group under 60 years. In the age group of 60–74 years, AUC values were higher in men than women (AUC: 0.710 vs o.617). For subjects ≥ 75 years old, AUC values were higher in women than men, with good predictive power.
Figure 5.
Forest plot of association between plaque characteristics and ESUS index among different age groups. PB, plaque burden; RI, remodeling index; DPS, discontinuity of plaque surface; FC, thick fibrous cap; IPH, intraplaque hemorrhage; CP, complicated Plaque.
Table 3.
Multivariable logistic regression analyses of ipsilateral NIAP for index ESUS in different age groups.
| Age < 60 years OR (95% CI) |
p | Age 60–74 years OR (95% CI) |
p | Age ≥ 75 years OR(95% CI) |
p | |
|---|---|---|---|---|---|---|
| Total | ||||||
| PB *10 | 1.262 (0.726–2.193) | 0.409 | 1.036 (0.627–1.710) | 0.891 | 2.756 (0.931–8.158) | 0.067 |
| RI*10 | 1.980 (1.163–3.372) | 0.012 | 2.415 (1.484–3.930) | < 0.001 | 3.212 (1.301–7.926) | 0.011 |
| Complicated Plaque | 3.136 (1.217–8.087) | 0.018 | 0.781 (0.304–2.008) | 0.608 | 2.170 (0.353–13.345) | 0.403 |
| Male | ||||||
| PB *10 | 1.210 (1.789–1.854) | 0.382 | 1.172 (0.577–2.380) | 0.661 | 1.135 (0.239–5.382) | 0.873 |
| RI*10 | 2.554 (1.678–3.888) | < 0.001 | 3.321 (1.574–7.007) | 0.002 | 4.053 (0.756–21.722) | 0.102 |
| Complicated Plaque | 1.924 (0.898–4.121) | 0.092 | 1.655 (0.441–6.213) | 0.456 | 0.422 (0.017–10.446) | 0.598 |
| Female | ||||||
| PB *10 | 1.176 (0.669–2.067) | 0.574 | 0.759 (0.345–1.668) | 0.492 | 159.382 (0.185–137,535.146) | 0.141 |
| RI*10 | 2.300 (1.125–3.148) | 0.016 | 1.765 (0.843–3.696) | 0.132 | 35.876 (0.558–2307.143) | 0.092 |
| Complicated Plaque | 6.667 (0.324–2.465) | 0.155 | 0.319 (0.064–1.589) | 0.163 | 173.660 (0.105–285,876.507) | 0.172 |
“PB *10”, “RI*10” and “Complicated Plaque” were included in the multivariable analysis. NIAP, non-stenotic intracranial atherosclerotic plaque; PB, plaque burden; RI, remodeling index; DPS, discontinuity of plaque surface; FC, thick fibrous cap; IPH, intraplaque hemorrhage. Values are presented as odds ratio and 95% CIs.
Figure 6.
Comparison of ROC analysis of plaque vulnerability among different age groups for predicting ESUS. The model included PB, RI and CP. PB, plaque burden; RI, remodeling index; CP, complicated plaque.
Discussion
The current study assesses age-dependent sex differences of intracranial plaque characteristics in ESUS, and identifies significant sex difference of vulnerable plaques characteristics in men vs women, as well as an effect of age on the sex differences in plaque characteristics.
We found that there were significant differences in PB, RI, DPS and complicated plaque in women vs men. Men had higher PB and RI, higher prevalence of DPS and complicated plaque. The logistic regression analyses showed that PB, RI, DPS and complicated plaque were related with an index ESUS in the overall cohort or men, but only RI was related with an index ESUS in women. The results were well in agreement with previous studies in which men tend to have a higher prevalence of plaques with vulnerable features (large intraplaque haemorrhage, thin fibrous cap, large lipid core, more inflammatory cells) than women6,23–27. The gender difference could be related with secretion of estrogen. It is widely accepted that oestradiol has sex-specific protective effects in women against atherosclerosis, a finding which was also confirmed in experimental animal models28–31. Unfortunately, no estrogen-related information was collected in our study. However thick FC and IPH was not found to be a significant predictor for index ESUS, they were related to atherosclerotic plaque as PB, RI, DPS and complicated plaque. We speculate that the difference may be related to the moderate sample size, and the imbalance of sample size among groups.
Furthermore, the sex difference was significantly affected by age in the current study. In the 60–74 year group, the vulnerable characteristics of ipsilateral NIAP were found more frequently in men, however, for patients ≥ 75 years old, the vulnerable characteristics occurred more frequently in women. The results were consistent with previous studies: the Tromsø Study found that carotid plaque prevalence was higher in men than women in most of age groups, but more common in women (81.2%) than men (76.5%) until the age of 756. Also, carotid IPH, an unstable plaque component, was shown to have delayed onset in women32. Similarly, coronary histopathology study showed that women over 50 were much more likely to have vulnerable plaque than younger, premenopausal women33. The protection of oestradiol diminishes with age after menopause, and there was a marked reduction in circulating oestradiol in postmenopausal women4.In addition, no significant differences were found between men and women with regard to plaque characteristics in the age group under 60 years, which may be due to the smaller sample size of women vs men (8 vs 54).
In the present study, we found that the prevalence of DPS, IPH, CP was higher in men regardless of age, which further supported the high prevalence of plaque vulnerability in men. However, the probability of PBupper and RIupper was similar between men and women around age 75. Considered together with the high prevalence of vulnerable characteristics in ≥ 75 years old women, these results could suggest the hypothesis that the high prevalence of plaque vulnerability changed from men to women at this time window. Higher plaque burden, positive remodeling and complicated plaques are more likely linked to an embolic stroke, as the respond to the increased plaque burden, and outward expansion of vessel wall will lead to high risk of vulnerability to rupture causing embolic stroke34,35. This could provide a possible explanation for the different proportions of gender at different ages of ESUS. Besides, we also found that plaques characteristics exhibited different predictive powers for ESUS index in men vs women, or among different age groups. Collectively, these results suggest that the differential effect of age and gender on the probability of specific mechanisms underlying ESUS could be considered in future studies.
The strength of this study is that it is the first to assess age-dependent sex difference in intracranial plaque characteristics of ESUS, which could facilitate tailored secondary prevention and clinical trial design for ESUS patients. The main limitations are the retrospective nature, the moderate sample size, and the imbalance of sample size of women vs men, which render this finding from the study less powerful. In addition, the gender difference might be related with secretion of estrogen, but no estrogen-related information was collected in our study. Given the high prevalence of intracranial atherosclerotic disease in Chinese populations, the findings may have limited generalizability to other populations.
Conclusion
This study reported age-related sex differences in the characteristics of intracranial plaques in ESUS patients. The findings could inform secondary prevention strategies and clinical trial design in ESUS.
Supplementary Information
Author contributions
N.L. and Z.Y.S. retrospectively enrolled patients, acquired the data and did the literature search. N.L. wrote the paper. N.L. and Z.Y.S. did statistical analysis. B.Q.Y acquired the imaging data. H.S.C. designed the study and critically revised the manuscript. G.N. critically revised the manuscript.
Funding
The work was supported by grants from the Science and Technology Project Plan of Liao Ning Province (2022JH2/101500020).
Data availability
Data are available upon reasonable request. The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-48091-8.
References
- 1.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 2.Diener HC, Easton JD, Hart RG, Kasner S, Kamel H, Ntaios G. Review and update of the concept of embolic stroke of undetermined source. Nat. Rev. Neurol. 2022 doi: 10.1038/s41582-022-00663-4. [DOI] [PubMed] [Google Scholar]
- 3.Ntaios G, Lip GYH, Vemmos K, Koroboki E, Manios E, Vemmou A, et al. Age- and sex-specific analysis of patients with embolic stroke of undetermined source. Neurology. 2017;89:532–539. doi: 10.1212/WNL.0000000000004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasbarrino K, Di Iorio D, Daskalopoulou SS. Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur. Heart J. 2022;43:460–473. doi: 10.1093/eurheartj/ehab756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dam-Nolen DHK, van Egmond NCM, Dilba K, Nies K, van der Kolk AG, Liem MI, et al. Sex differences in plaque composition and morphology among symptomatic patients with mild-to-moderate carotid artery stenosis. Stroke. 2022;53:370–378. doi: 10.1161/strokeaha.121.036564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joakimsen O, Bonaa KH, Stensland-Bugge E, Jacobsen BK. Age and sex differences in the distribution and ultrasound morphology of carotid atherosclerosis: The tromsø study. Arterioscler. Thromb. Vasc. Biol. 1999;19:3007–3013. doi: 10.1161/01.atv.19.12.3007. [DOI] [PubMed] [Google Scholar]
- 7.Roy-O'Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. 2018;159:3120–3131. doi: 10.1210/en.2018-00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ntaios G, Pearce LA, Veltkamp R, Sharma M, Kasner SE, Korompoki E, et al. Potential embolic sources and outcomes in embolic stroke of undetermined source in the navigate-esus trial. Stroke. 2020;51:1797–1804. doi: 10.1161/STROKEAHA.119.028669. [DOI] [PubMed] [Google Scholar]
- 9.Ntaios G, Perlepe K, Lambrou D, Sirimarco G, Strambo D, Eskandari A, et al. Prevalence and overlap of potential embolic sources in patients with embolic stroke of undetermined source. J. Am. Heart Assoc. 2019;8:e012858. doi: 10.1161/JAHA.119.012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: A systematic review and clinical update. Stroke. 2017;48:867–872. doi: 10.1161/STROKEAHA.116.016414. [DOI] [PubMed] [Google Scholar]
- 11.Ntaios G. Embolic stroke of undetermined source: Jacc review topic of the week. J. Am. Coll. Cardiol. 2020;75:333–340. doi: 10.1016/j.jacc.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Ntaios G, Wintermark M, Michel P. Supracardiac atherosclerosis in embolic stroke of undetermined source: The underestimated source. Eur. Heart J. 2021;42:1789–1796. doi: 10.1093/eurheartj/ehaa218. [DOI] [PubMed] [Google Scholar]
- 13.Ntaios G, Swaminathan B, Berkowitz SD, Gagliardi RJ, Lang W, Siegler JE, et al. Efficacy and safety of rivaroxaban versus aspirin in embolic stroke of undetermined source and carotid atherosclerosis. Stroke. 2019;50:2477–2485. doi: 10.1161/STROKEAHA.119.025168. [DOI] [PubMed] [Google Scholar]
- 14.Ntaios G, Pearce LA, Meseguer E, Endres M, Amarenco P, Ozturk S, et al. Aortic arch atherosclerosis in patients with embolic stroke of undetermined source: An exploratory analysis of the navigate esus trial. Stroke. 2019;50:3184–3190. doi: 10.1161/STROKEAHA.119.025813. [DOI] [PubMed] [Google Scholar]
- 15.Tao L, Li XQ, Hou XW, Yang BQ, Xia C, Ntaios G, et al. Intracranial atherosclerotic plaque as a potential cause of embolic stroke of undetermined source. J. Am. Coll. Cardiol. 2021;77:680–691. doi: 10.1016/j.jacc.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Ameriso SF, Amarenco P, Pearce LA, Perera KS, Ntaios G, Lang W, et al. Intracranial and systemic atherosclerosis in the navigate esus trial: Recurrent stroke risk and response to antithrombotic therapy. J. Stroke Cerebrovasc. Dis. 2020;29:104936. doi: 10.1016/j.jstrokecerebrovasdis.2020.104936. [DOI] [PubMed] [Google Scholar]
- 17.Song JW, Cao Q, Siegler JE, Thon JM, Woo JH, Cucchiara BL. Sex differences in carotid plaque composition in patients with embolic stroke of undetermined source. J. Am. Heart Assoc. 2021;10:e020143. doi: 10.1161/JAHA.120.020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B, Lau KK, Li L, Lovelock C, Liu M, Kuker W, et al. Age-specific associations of renal impairment with magnetic resonance imaging markers of cerebral small vessel disease in transient ischemic attack and stroke. Stroke. 2018;49:899–904. doi: 10.1161/strokeaha.117.019650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazcano U, Cuadrado-Godia E, Grau M, Subirana I, Martínez-Carbonell E, Boher-Massaguer M, et al. Increased covid-19 mortality in people with previous cerebrovascular disease: A population-based cohort study. Stroke. 2022;53:1276–1284. doi: 10.1161/strokeaha.121.036257. [DOI] [PubMed] [Google Scholar]
- 20.Jiménez MC, Manson JE, Cook NR, Kawachi I, Wassertheil-Smoller S, Haring B, et al. Racial variation in stroke risk among women by stroke risk factors. Stroke. 2019;50:797–804. doi: 10.1161/strokeaha.117.017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo N, Shang ZY, Tao L, Yang BQ, Chen HS. Atherosclerosis as a potential cause of deep embolic stroke of undetermined source: A 3T high-resolution magnetic resonance imaging study. J. Am. Heart Assoc. 2022;11(21):e026737. doi: 10.1161/JAHA.122.026737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–1373. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 23.Hellings WE, Pasterkamp G, Verhoeven BA, De-Kleijn DP, De-Vries JP, Seldenrijk KA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J. Vasc. Surg. 2007;45:289–296. doi: 10.1016/j.jvs.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 24.Sangiorgi G, Roversi S, Biondi Zoccai G, Modena MG, Servadei F, Ippoliti A, et al. Sex-related differences in carotid plaque features and inflammation. J. Vasc. Surg. 2013;57:338–344. doi: 10.1016/j.jvs.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 25.Ota H, Reeves MJ, Zhu DC, Majid A, Collar A, Yuan C, et al. Sex differences in patients with asymptomatic carotid atherosclerotic plaque: In vivo 3.0-t magnetic resonance study. Stroke. 2010;41:1630–1635. doi: 10.1161/STROKEAHA.110.581306. [DOI] [PubMed] [Google Scholar]
- 26.Ota H, Reeves MJ, Zhu DC, Majid A, Collar A, Yuan C, et al. Sex differences of high-risk carotid atherosclerotic plaque with less than 50% stenosis in asymptomatic patients: An in vivo 3t mri study. AJNR Am. J. Neuroradiol. 2013;34(1049–1055):s1041. doi: 10.3174/ajnr.A3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendorff C, Wendorff H, Pelisek J, Tsantilas P, Zimmermann A, Zernecke A, et al. Carotid plaque morphology is significantly associated with sex, age, and history of neurological symptoms. Stroke. 2015;46:3213–3219. doi: 10.1161/STROKEAHA.115.010558. [DOI] [PubMed] [Google Scholar]
- 28.Marsh MM, Walker VR, Curtiss LK, Banka CL. Protection against atherosclerosis by estrogen is independent of plasma cholesterol levels in ldl receptor-deficient mice. J. Lipid Res. 1999;40:893–900. doi: 10.1016/S0022-2275(20)32124-6. [DOI] [PubMed] [Google Scholar]
- 29.Elhage R, Arnal JF, Pieraggi MT, Duverger N, Fievet C, Faye JC, et al. 17 beta-estradiol prevents fatty streak formation in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1997;17:2679–2684. doi: 10.1161/01.atv.17.11.2679. [DOI] [PubMed] [Google Scholar]
- 30.Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein e-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler. Thromb. Vasc. Biol. 2017;37:746–756. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh N, Moody AR, Zhang B, Kaminski I, Kapur K, Chiu S, et al. Age-specific sex differences in magnetic resonance imaging-depicted carotid intraplaque hemorrhage. Stroke. 2017;48:2129–2135. doi: 10.1161/STROKEAHA.117.017877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J. Am. Coll. Cardiol. 2021;77:1426–1435. doi: 10.1016/j.jacc.2021.01.038. [DOI] [PubMed] [Google Scholar]
- 34.Shi MC, Wang SC, Zhou HW, Xing YQ, Cheng YH, Feng JC, et al. Compensatory remodeling in symptomatic middle cerebral artery atherosclerotic stenosis: A high-resolution mri and microemboli monitoring study. Neurol. Res. 2012;34:153–158. doi: 10.1179/1743132811Y.0000000065. [DOI] [PubMed] [Google Scholar]
- 35.Qiao Y, Anwar Z, Intrapiromkul J, Liu L, Zeiler SR, Leigh R, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke. 2016;47:434–440. doi: 10.1161/STROKEAHA.115.009955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request.