Abstract
Shigellosis, a leading cause of diarrhoeal mortality and morbidity globally, predominantly affects children under five years of age living in low- and middle-income countries. While whole genome sequence analysis (WGSA) has been effectively used to further our understanding of shigellosis epidemiology, antimicrobial resistance, and transmission, it has been under-utilised in sub-Saharan Africa. In this study, we applied WGSA to large sub-sample of surveillance isolates from South Africa, collected from 2011 to 2015, focussing on Shigella flexneri 2a and Shigella sonnei. We find each serotype is epidemiologically distinct. The four identified S. flexneri 2a clusters having distinct geographical distributions, and antimicrobial resistance (AMR) and virulence profiles, while the four sub-Clades of S. sonnei varied in virulence plasmid retention. Our results support serotype specific lifestyles as a driver for epidemiological differences, show AMR is not required for epidemiological success in S. flexneri, and that the HIV epidemic may have promoted Shigella population expansion.
Subject terms: Bacterial genetics, Bacterial infection, Infectious-disease epidemiology
As a leading cause of diarrhoeal mortality and morbidity, authors examine the epidemiology and genome dynamics of shigellosis in South Africa, utilising whole genome sequence analysis.
Introduction
Shigellosis is the second leading cause of diarrhoeal death, globally1,2, with the greatest disease burden on those living in low- and middle-income countries1,2. Of further concern is the high burden of disease among children under five years old (around 30% of cases and 38% of deaths), with repeat childhood infections bringing long-term health consequences, including stunted growth, impaired cognitive abilities, and chronic, functional bowel disorders3–10.
The causative agents, a group of Gram-negative bacteria called Shigella, are convergently evolved specialised pathovars of E. coli11. Made up of four serogroups (S. flexneri, S. sonnei, S. boydii and S. dysenteriae), they share an enteroinvasive pathogenesis mediated by acquisition of the large invasion, or virulence, plasmid (pINV)11. Each serogroup is epidemiologically distinct, though S. flexneri and S. sonnei are of primary importance due to their global distribution and high prevalence (accounting for approximately 90% of the global shigellosis burden)12–15. The two serogroups are also genetically distinct; S. sonnei has only one serotype and is highly clonal, while S. flexneri is far more diverse genetically and serotypically (having >20 different serotypes)16. Whole genome sequence analysis (WGSA) on large temporo-geographical collections of both serogroups has established new genomic nomenclatures, complementing traditional serological distinctions. These differentiate S. flexneri into eight phylogroups, and S. sonnei into five Lineages, and subsequently Clades and sub-Clades12,13,17.
The serogroups are also thought to have distinct ecologies, with a switch from S. flexneri dominance to S. sonnei being linked with increasing industrialisation14. This may be due to reduced Plesiomonas shigelloides exposure in industrialised settings leading to reduced cross-protection against S. sonnei from their shared O-antigen18,19. Though differences in pathogen lifestyle may also be involved, genetic differences in S. sonnei point to additional adaptations towards becoming an obligate pathogen (reduced pINV stability and O-antigen capsule expression)20,21. Understanding the epidemiological dynamics and pathogen ecologies of these two important pathogens is critical to their control.
Multidrug resistance (MDR) is widespread in both S. flexneri and S. sonnei and has been important in driving their epidemiology12,13,17,22. Increasing antimicrobial resistance (AMR) is such a risk to ongoing effective treatment that fluoroquinolone-resistant strains are classed as a WHO priority AMR pathogen and Shigella a recommended priority target organism for vaccines for AMR12,13,23–27. Genomic analyses reveal the genetic basis for AMR and enables tracking of their dissemination through the population. Many resistance determinants are carried on mobile genetic elements, such as the Shigella resistance locus (SRL), an important MDR element encoding resistance against ampicillin, chloramphenicol, streptomycin and tetracycline26,27, which is mobile alone or as part of the larger SRL pathogenicity island (PAI). The highest impact on number of deaths due to increased AMR is known to be in LMIC28.
Despite the greatest shigellosis disease burden falling on those living in sub-Saharan Africa (SSA) and Asia, the use of WGSA for studying shigellosis in SSA has been under-utilised. The largest study, to date, using WGSA on African isolates was The Global Enteric Multicentre Study (GEMS), which confirmed S. flexneri and S. sonnei to be the first and second most prevalent serogroups in three of four study countries (The Gambia, Mozambique, Mali)5,29,30. Further WGSA of the GEMS study isolates showed multiple Phylogroups of S. flexneri in each participating African country, highlighting the diversity of circulating strains16. While this provides a useful snapshot into the diversity of Shigella strains circulating regionally, detailed national studies are needed to further unpick the epidemiology of shigellosis in the region.
Non-genomic studies from across SSA highlight the importance of endemic disease in the region and confirm S. flexneri is typically the dominant serogroup of Shigella31–45. There is variation in shigellosis incidence, sub-continentally and nationally, with national attributable disease burden estimates of up to 63.3% of diarrhoeal cases, and intra-national variation as high as 34%46,47. The available data show AMR Shigella are present across the entire sub-continent, and MDR, where reported, is more common in S. flexneri31,46,48–50. South Africa has a high incidence (2.7 hospitalisations/100,000 persons per year) of shigellosis, caused predominantly by S. flexneri and S. sonnei, discovered through routine, national shigellosis surveillance, enabling detailed examination of the national epidemiology of serotypes from both globally important serogroups within SSA51–58.
In this study, we use WGSA to examine the epidemiology, ecology, and accessory genome dynamics of shigellosis in the sub-Saharan nation of South Africa using isolates collected as part of the national surveillance between 2011 and 2015. We focus on the two most prevalent serotypes (S. flexneri 2a and S. sonnei) that caused approximately 70% of shigellosis cases in the country during the study period51–58 and find evidence for distinct ecologies and AMR dynamics between the serotypes.
Results
The comparative epidemiology of S. sonnei and S. flexneri 2a in South Africa
We used reported case numbers from the annual surveillance reports by the Group for Enteric, Respiratory and Meningeal Diseases Surveillance in South Africa (GERMS-SA) to characterise the epidemiology of shigellosis in the country. Cases were unevenly distributed across the country, relative to provincial population size. More cases than expected were reported in Gauteng (+13.9%) and Western Cape (+11.8%) (χ2(26, n = 5078.01) = 2156, p = 0.0000), with the converse in Limpopo (−9.8%), Mpumalanga (−5.2%), North West (−5.7%) and KwaZulu–Natal (−5.6%)54–56. This may represent an imperfect surveillance system, differences in healthcare availability, differences in health-seeking behaviour, or genuine differences in shigellosis incidence across the country.
To conduct genomic epidemiological analysis, we created a representative surveillance subsample of the two most burdensome serotypes, S. flexneri 2a (n = 286) and S. sonnei (n = 275), collected between 2011 and 2015. Statistical analysis supported the representativeness of our sample relative to disease incidence (reported case numbers), with no significant difference between observed and expected isolate numbers by either province (χ2(26, n = 353) = 19.02, p = 0.890) or year (2011–2013, data unavailable 2014-2015; χ2(5, n = 353) = 2.75, p = 0.474) (Fig. 1A). When stratifying by serotype, however, there were slightly more S. sonnei than expected from Gauteng (+15%; χ2(8, n = 253) = 35.69, p = 0.0000) and S. flexneri 2a from the Western Cape (+11%; χ2(8, n = 260) = 31.091, p = 0.0001) (Fig. 1A). Similar over-representations were also observed in the reported cases; between 2011 and 2013, 44–66% of S. sonnei cases were from Gauteng and 22–38% of S. flexneri 2a were from the Western Cape54–56. Thus, this supports serotype-specific geographic distributions in the country, across the study period, rather than an over representation of these serotypes from these regions due to sub-sampling bias.
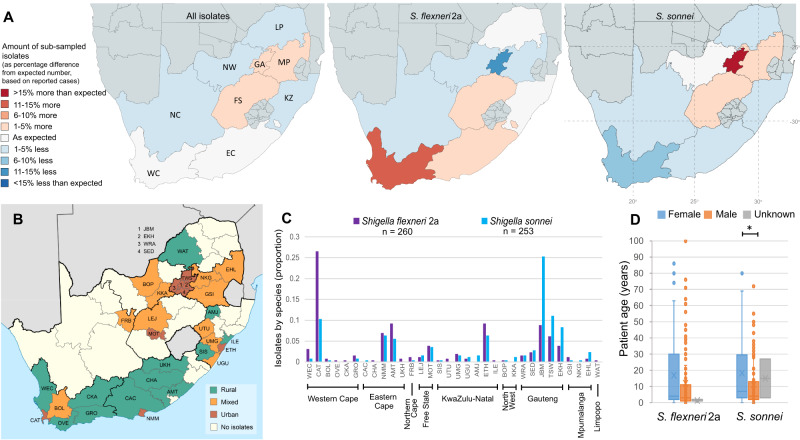
Fig. 1. Representativeness, and geographic and patient demographic features of the surveillance subsample.
A Provincial representation the surveillance cases within isolate subsample. The under or over representation of cases by sub-sampled isolates (, where O = actual number of sub-sampled isolates and E = expected number of isolates based on reported case numbers) is shown for all isolates (left), S. flexneri 2a (middle), and S. sonnei (right), coloured by percentage divergence from expected number of isolates based on reported cases, according to the inlaid key. NB: data are shown for 2011 to 2013 due to a lack of reported data from 2014 and 2015. Latitude and longitude are overlaid on S. sonnei map. B Urbanisation of sampled districts, level of urbanisation indicated by colour as shown in inlaid key (bottom right). C Proportion of subsample by district (x axis) and province (overlying horizontal bars) of origin and serotype (S. flexneri 2a = purple, S. sonnei = blue). D Distribution of patient ages among the surveillance subsample by Shigella serotype (x axis) and sex (female = blue and male = orange, unknown sex = grey). Data are presented as Box plots: S. flexneri 2a (male and female, respectively) minima = 0 and 0, maxima = 86 and 100, median = 3 and 4, IQR (bounds of box) = 1–11 and 2–30, whiskers = 23 and 63; S. sonnei (male and female, respectively) minima = 0, maxima = 80 and 72, median = 4 and 7, IQR (bounds of box) = 2–12.8 and 3–28.5, whiskers = 29 and 69.* = Mann–Whitney U (two-sided) p value = 0.007187, S. flexneri 2a male n = 128, female n = 129, and unknown sex = 3, S. sonnei male n = 129, female n = 122 and unknown sex = 2. WEC West Coast, CAT City of Cape Town, BOL Cape Winelands, OVE Overberg, CKA Central Karoo, GRO Garden State, CAC Sarah Baartman, CHA Chris Hani, NMM Nelson Mandela Bay, AMT Amathole, UKH Joe Gqabi, FRB Frances Baard, LEJ Lejweleputswa, MOT Manguang, SIS Harry Gwala, UTU uThukela, UMG uMhlanga, UGU =Ugu, AMJ Amajuba, ETH City of eThekwini, ILE iLembe, BOP Bojanala Platinum, KKA Dr. Kenneth Kaunda, WRA West Rand, SED Sedibeng, JBM City of Johannesburg, TSW City of Tshwane, EKH Ekurhuleni, GSI Gert Sibande, NKG Nkangala, EHL Ehlanzeni, WAT Waterberg. Maps in A were created using editable MapChart maps (https://www.mapchart.net/), edited and used according to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-sa/4.0/). Map in B was adapted from image made available at Wikimedia Commons (https://commons.wikimedia.org/wiki/File:Map_of_South_Africa_with_district_borders_(2011).svg), and used in accordance to the Creative Commons Attribution 3.0 International License (https://creativecommons.org/licenses/by-sa/3.0/deed.en). Breakdown of isolate numbers are found in (A and C). Source data are provided as a Source Data file.
To look at the potential influence of urbanisation on serotype distribution, we categorised districts by level of urbanisation according to methods laid out by the European Commission (March 2020). Most study isolates were from urban districts (72% total; 69% S. flexneri 2a and 76% S. sonnei) and the fewest from mixed districts (9%; 8% S. flexneri 2a and 10% S. sonnei). Greater representation of rural districts than mixed could be due to socioeconomic factors influencing disease incidence or differential surveillance efforts (Fig. 2B, C). Consistent with reports of S. sonnei being a pathogen of urban and urbanising areas14, statistical analysis showed S. sonnei was more likely to be from mixed and urban districts than S. flexneri 2a (χ2(2, n = 513) = 7.489, p = 0.0024), extending the granularity of this international observation to be relevant at a national level.
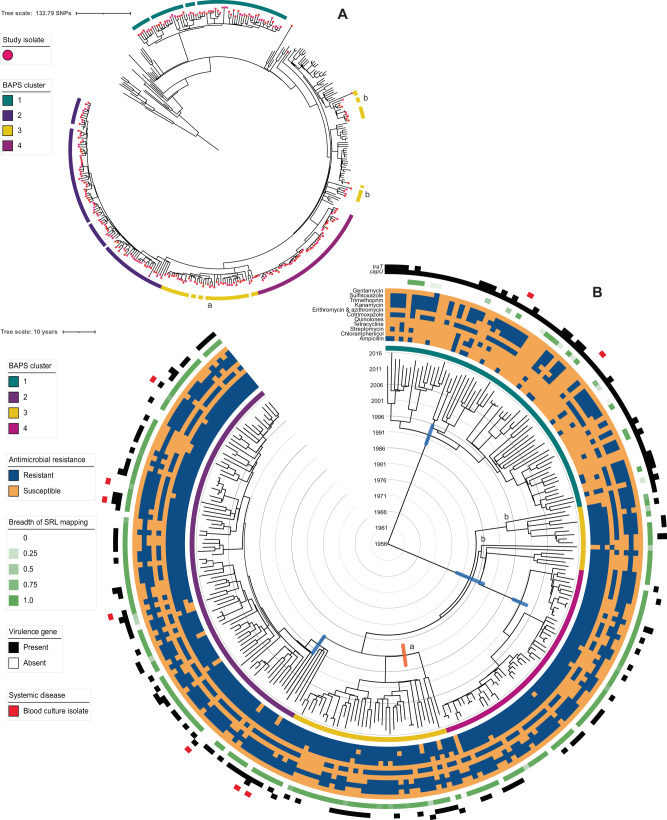
Fig. 2. The contextualised population structure, antimicrobial resistance, and genetic features of South African Shigella flexneri 2a.
The maximum likelihood phylogeny A shows the S. flexneri 2a isolates South African sub-sampled isolates (indicated by pink dot overlying the terminal node) among Phylogroup 3 representatives. The BAPS clusters for the South African samples are shown in the outer ring according, coloured to the inlaid key. Sub-clusters of polyphyletic Cluster 3 are indicated with ‘a’ (locally circulating) and ‘b’ (possibly imported). B A Maximum Clade Credibility tree from Bayesian analysis shows the population structure of the S. flexneri 2a with branch lengths in time, scaled in years by the concentric rings radiating from the tree MRCA in the centre. BAPS clusters are indicated in the ring immediately adjacent to tree tips, coloured according to top inlaid key) and their MRCA 95% HPDs are indicated by blue bars for BAPS Clusters 1, 2, and 4. The circulating sub-clusters of polyphyletic BAPS Cluster 3 are labelled ‘a’ and ‘b’ and the MRCA 95% HPD of locally circulating ‘a’ indicated at the defining node in orange. Other metadata rings (from inner to outer, coloured according to the inlaid keys) show the: antimicrobial resistance profiles by antimicrobial class (combination of predicted and phenotypic resistance—see methods; blue = resistant and orange = susceptible), breadth of mapping coverage across the SRL (green), the presence of virulence genes capU and traT (black), and where an isolate was derived from a blood sample (rather than stool; red). MRCA most recent common ancestor, HPDs highest posterior density interval, BAPS Bayesian analysis of population structure, SRL Shigella resistance locus. Source data are provided as a Source Data file.
We also explored possible associations between available patient demographic characteristics and shigellosis caused by either serotype. The median age of infection (all isolates) was higher in females (5 years old, IQR = 2–30) than males (3.5 years old, IQR = 1.25–11.75; Mann–Whitney U W = 18685, p = 0.0027) (raw data shown by serotype Fig. 1). This age difference was more pronounced in S. sonnei (3-year difference in median patient age) than S. flexneri 2a (1-year difference); reflected as a statistically significant difference being observed only in S. sonnei when stratifying by serotype (Mann–Whitney U test S. sonnei: W = 5739.5, p = 0.0072; S. flexneri: W = 7487, p = 0.0789) (Fig. 1). Secondary infection within the home has been shown to a driver of S. sonnei transmission59,60, and it may be that the higher age in females results from onward infection during caring responsibilities for ill children in this setting. We found no association between sex and serotype.
Shigella flexneri 2a population dynamics
We used phylogenetics to examine and contextualise the South African S. flexneri 2a population structure. All S. flexneri 2a study isolates belonged to Phylogroup 3 (PG3) (Supplementary fig. 1), according to a single nucleotide polymorphism (SNP)-based maximum likelihood (ML) phylogeny of our isolates (n = 260) and global reference isolates (n = 116). For a more granular visualisation of the population structure, we generated a PG3 only ML phylogeny of our isolates and PG3 reference isolates (n = 116) (Fig. 2A). We also used Bayesian phylogenetics to characterise the population dynamics of S. flexneri 2a in South Africa through time. We identified four co-existing strains, two of which were likely recent introductions, and found evidence of population diversification beginning in the 1990s (Fig. 2B, Supplementary fig. 3). The geographical clustering of the South African isolates in the global context shows the epidemiology of S. flexneri 2a in South Africa is mainly driven by local transmission.
Bayesian analysis of population structure (BAPS) clustering, used to facilitate statistical analysis, grouped the S. flexneri subsample into four clusters (Sf clusters). Each Sf cluster was typically monophyletic among the study isolates and mostly in the global context, indicating sustained local transmission (referred to as endemic throughout), with the exception of Sf cluster 3 which grouped an apparently endemic sub-lineage (Sf cluster 3a, Fig. 2) with possibly imported strains (Sf cluster 3b, Fig. 2). The fifteen isolates in Sf cluster 3b were inferred as possibly imported by their genetic distance from the endemic lineages and close relatedness to global references (Fig. 2A).
Owing to their potential role in driving population dynamics, genes relating to AMR and virulence were characterised among the isolates. Partial-phenotyping and in silico genotyping confirmed MDR, resistance to three or more antimicrobial classes, is widespread in South African S. flexneri 2a (91.5% of isolates). All but one cluster (Sf cluster 1) were predominantly (≥98%) MDR (Fig. 2). Only 67% of Sf cluster 1 were MDR; all (n = 19) predicted pan-susceptible isolates belonged to the cluster and resistance to ampicillin, chloramphenicol, tetracycline, and streptomycin was significantly lower relative to other clusters (χ2(1, n = 260) = 126.51, 191.70, 201.85 and 138.38, respectively; p = 0.0000 for all four antimicrobials) (Fig. 2B, Supplementary Data 1). Greater drug susceptibility due to the absence of the SRL, a MDR element encoding resistance to these four antimicrobials, was confirmed by poor mapping to the region (Fig. 2B, Supplementary Data 1).
Sf cluster 1 also had a distinct virulence profile, with a higher proportion of isolates containing capU and traT relative to other clusters, possibly indicating greater relative virulence (Fig. 2B, Supplementary Data 1). This is partially supported by the concentration of isolates derived from blood-culture (indicative of invasive infection) in this cluster (Fig. 2, Supplementary Data 1). The influence of pathogen factors on disease presentation is not well understood, but traT may have a role in innate immunity evasion61, while capU was identified on a virulence plasmid associated with enteroaggregative Escherichia coli62.
To further understand how the identified cluster characteristics have shaped shigellosis epidemiology in South Africa over time, we conducted temporal population dynamic modelling (implemented in BEAST, see methods). The previous emergence estimate for S. flexneri PG3 was 184813, while our S. flexneri 2a subsample likely emerged around 100 years later (1956; 95% HDP = late 1945 to late 1965). Our study emergence date instead coincides with the emergence (1948) of a rapidly expanding, intercontinental, PG3 subcluster13. The South Africa S. flexneri 2a population is split into two main Clades: 1) the reduced AMR Sf cluster 1 and 2) an MDR Clade comprising the remaining Sf clusters. Emergence dating suggests Sf cluster 1 established in South Africa in 1992 (95% HPD = mid-1989 to late 1995) after the MDR Clade (1983; 95% HPD = early 1979 to mid-1988) (Fig. 2B). Thus, there was a successful introduction of a less AMR lineage into the country when MDR strains were already circulating, possibly aided by the increased presence of virulence genes in Sf cluster 1.
The other endemic clusters (Sf clusters 2, 3a and 4) all emerged during the 1990s; evidence of population diversification which is supported by the estimated increasing S. flexneri 2a population size between 2001 and 2009, (Supplementary fig. 3). Clusters 2 and 3a likely diverged from the same ancestral population in 1994 and 1991 (95% HPDs: mid-1991 to early 1997, and late 1987 to mid-1994), respectively (Fig. 2). A long branch preceding the Sf cluster 4 MRCA suggests an evolutionary bottleneck, perhaps formed by migration of the strain out of South Africa then reintroduction in 2001 (95% HPD = early 1999 to late 2003) (Fig. 2B).
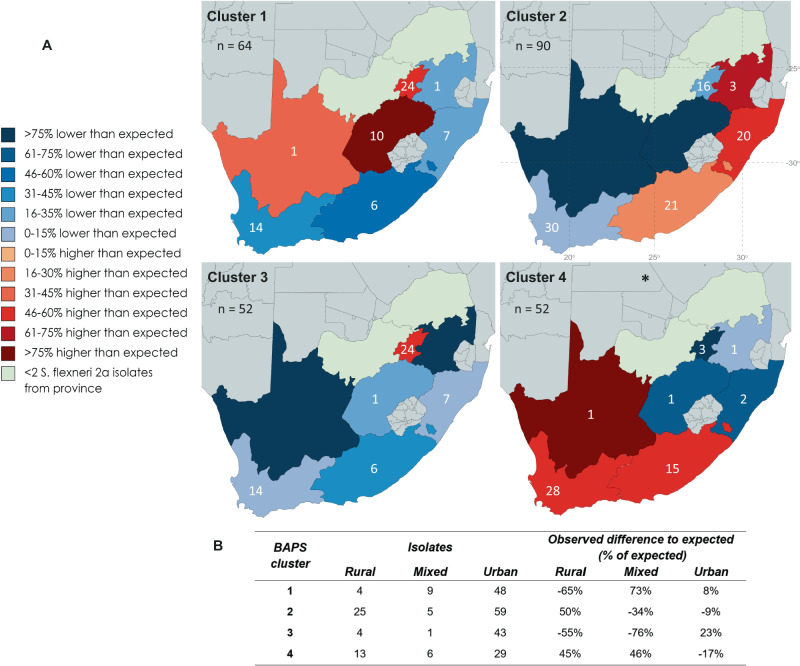
To determine the sub-national distribution of the identified genomic subtypes, we assessed the Sf cluster distribution across the country. Together, the Sf clusters were unevenly distributed between provinces (Fisher’s exact p = 0.0005, Fig. 3). This suggests that co-existence of locally circulating strains in a country may feature sub-regional dominance by strains. We also found an association between Sf cluster and level of district urbanisation (Fisher’s exact p = 0.0001) Owing to their potential role in driving population dynamics, genes relating to AMR and virulence were characterised among the isolates. Partial-phenotyping and in silico genotyping confirmed MDR, resistance to three or more antimicrobial classes, is widespread in South African S. flexneri 2a (91.5% of isolates). All but one cluster (Sf cluster 1) were predominantly (≥98%) MDR (Fig. 2). Only 67% of Sf cluster 1 were MDR; all (n = 19) predicted pan-susceptible isolates belonged to the cluster and resistance to ampicillin, chloramphenicol, tetracycline, and streptomycin was significantly lower relative to other clusters (χ2(1, n = 260) = 126.51, 191.70, 201.85 and 138.38, respectively; p = 0.0000 for all four antimicrobials) (Fig. 2B, Supplementary Data 1). Greater drug susceptibility due to the absence of the SRL, a MDR element encoding resistance to these four antimicrobials, was confirmed by poor mapping to the region (Fig. 2B, Supplementary Data 1), perhaps reflecting genuine associations with level of urbanisation, or maybe a consequence of province associations.
Fig. 3. Geographic distribution of S. flexneri 2a BAPS clusters within South Africa.
Each cluster has a distinct distribution by province across South Africa. A The under or over representation of each BAPS cluster by province based on the total number of isolates from each province; blue = fewer isolates than expected, red = more isolates than expected) (percentage difference determined using: , where O = actual number of cluster isolates and E = expected number of cluster isolates based on the total number of sample set isolates). Individually, Sf cluster 4 is statistically associated with Northern Cape, Western Cape, and Eastern Cape. Latitude and longitude are overlaid on BAPS cluster 2 map. B The presence of each cluster also varies by level of urbanisation, defined at the district level. * Fisher’s exact p value p = 0.0005. Source data are provided as a Source Data file. Maps in A were created using editable MapChart maps (https://www.mapchart.net/), edited and used according to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-sa/4.0/). BAPS Bayesian analysis of population structure.
The distribution of AMR reflected the distribution of the underlying Sf clusters. Free State province, predominantly Sf cluster 1, was negatively associated with MDR (9/13 isolates, −24%; Fisher’s exact p = 0.008) (Owing to their potential role in driving population dynamics, genes relating to AMR and virulence were characterised among the isolates. Partial-phenotyping and in silico genotyping confirmed MDR, resistance to three or more antimicrobial classes, is widespread in South African S. flexneri 2a (91.5% of isolates). All but one cluster (Sf cluster 1) were predominantly (≥98%) MDR (Fig. 2). Only 67% of Sf cluster 1 were MDR; all (n = 19) predicted pan-susceptible isolates belonged to the cluster and resistance to ampicillin, chloramphenicol, tetracycline, and streptomycin was significantly lower relative to other clusters (χ2(1, n = 260) = 126.51, 191.70, 201.85 and 138.38, respectively; p = 0.0000 for all four antimicrobials) (Fig. 2B, Supplementary Data 1). Greater drug susceptibility due to the absence of the SRL, a MDR element encoding resistance to these four antimicrobials, was confirmed by poor mapping to the region (Fig. 2B, Supplementary Data 1).
Shigella sonnei population dynamics
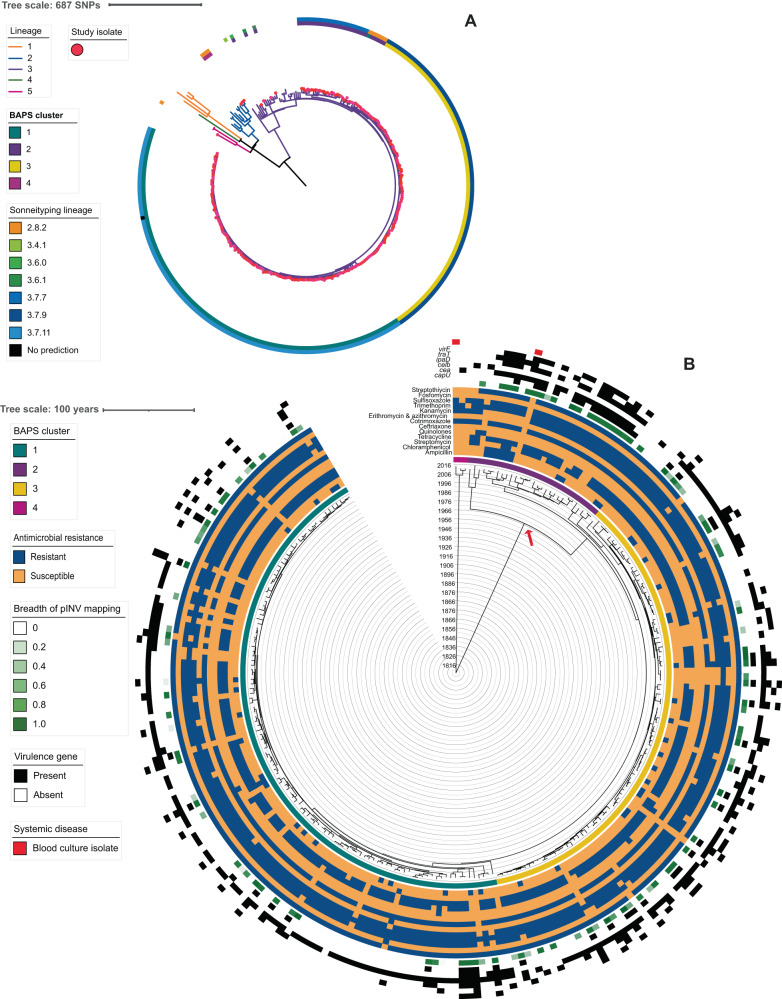
We performed similar phylogenetic analyses on the South African S. sonnei subsample. The SNP-based maximum likelihood phylogeny contextualised the S. sonnei subsample (n = 253) within the global population (40 references), identifying the majority (98.4%) as Global Lineage III (Clade 3.7) isolates (Fig. 4A). We similarly found that the epidemiology of S. sonnei-attributable shigellosis was mainly driven by local transmission. We also found evidence population diversification, again starting in the 1990s (Fig. 4B, Supplementary Figs. 2 and 3). Most isolates (246/253) comprised three sub-clades of Lineage 3, Clade 3.7; sub-Clades 3.7.7, 3.7.9, 3.7.11 (Fig. 4A). Five strains were dispersed throughout the remainder of Clade 3.7 and seven were even more distantly related isolates (one Lineage 5, three sub-Clade 2.8.2, two Clade 3.6, and one Clade 3.4).
Fig. 4. The contextualised population structure, antimicrobial resistance, and genetic features of South African Shigella sonnei.
The maximum likelihood phylogeny A shows the S. sonnei sub-sampled isolates (indicated by pink dot overlying the terminal node) among some global representatives. The previously defined Lineages are indicated by branch colour while the outer ring shows the predicted genotype for the study isolates, predicted by SonneiTyping software, coloured according to inlaid key. B A Maximum Clade Credibility tree from Bayesian analysis shows the population structure of the S. sonnei with branch lengths in time, scaled in years by the concentric rings radiating from the tree MRCA in the centre. BAPS clusters are indicated in the ring immediately adjacent to tree tips, coloured according to the top inlaid key. Other metadata rings (from inner to outer, coloured according to the inlaid keys) show: antimicrobial resistance profiles by antimicrobial class (combination of predicted and phenotypic—see methods; blue = resistant and orange = susceptible), breadth of mapping coverage across the pINV (green), presence of virulence genes capU, cea, celb, ipaD, traT, virF (black), and derived from a blood sample (rather than stool; red). MRCA for endemic clusters indicated by red arrow. BAPS Bayesian analysis of population structure, pINV large virulence plasmid. MRCA most recent common ancestor, HPDs highest posterior density interval, BAPS Bayesian analysis of population structure. Source data are provided as a Source Data file.
In common with S. flexneri 2a, partial phenotyping and in silico genotyping showed MDR was widespread in S. sonnei (93.7% of isolates). We also identified low-level AMR against two of the remaining widely effective antimicrobials, fluoroquinolones and third generation cephalosporins. Specifically, resistance against cephalosporins was phenotypically detected in two isolates, conferred by blaCMY-2 and blaCMY-4. The only predicted fluoroquinolone-resistant (FQR) S. sonnei isolate, that contained triple mutations in the quinolone resistance determining regions (QRDR), (specifically gyrA S83L, gyrA D87G and parC S80I), was reported as phenotypically susceptible to ciprofloxacin. Single point mutations in gyrA (n = 6) were also detected in all five of the phenotypically quinolone-resistant isolates (Supplementary Data 1). One quinolone-resistant isolate and the QRDR triple mutant were from the Asia-associated sub-Clade 3.6.1 (Fig. 4)63. The remaining quinolone-resistant isolates were locally circulating strains, showing possible potential for de novo emergence of FQR strains in the country.
In contrast to S. flexneri 2a, we found no evidence of AMR profile or geographical distribution differences between circulating sub-Clades of S. sonnei, however, variation was seen in their virulence profiles. Most cluster-associated virulence genes were positively associated with Ss cluster 2 (Fig. 4B, Supplementary Data 1). Though this may be linked to the relatively higher presence of the pINV in Ss cluster 2, relative to other clusters (Pearson χ2(1, n = 253) = 6.919, p = 0.009) (Fig. 4B). Both virF and ipaD are known to be carried on the pINV64,65, while capU was associated with pINV retention in the S. sonnei subsample (n = 53; χ2 = 120.4, p = 0.0000).
Bayesian population clustering, used again to aid statistical analysis, grouped the Lineage III isolates into three clusters (Ss clusters 1–3), the rest forming another cluster (Ss cluster 4), roughly corresponding with the S. sonnei subclade designations, with the exception of a 3.7 sub-Clade mis-identified as 2.8.2 (Fig. 4). Bayesian phylogenetics highlighted that the most recent common ancestor of all circulating S. sonnei in South Africa emerged in 1967 (95% HPD = late 1957 to early 1978) (red arrow, Fig. 4B). The circulating strain likely diversified into the identified sub-Clades in the 1990s. All emerging around the same time, sub-Clades 3.7.11 and 3.7.9 (Ss clusters 1 and 3, respectively) and the two sub-Clades of Ss cluster 2 (3.7.7 and the mis-identified 2.8.2 sub-Clade within Clade 3.7) likely emerged in 1996 (95% HPD = mid-1993 to late 1999), 2001 (95% HPD = late 1998 to late 2003), 1998 (95% HPD: 1996–2001), and 1996 (95% HPD: 1993-1998), respectively (Fig. 4). Population diversification in S. sonnei was also supported by an estimated increase in population size, though the increase was likely later than in S. flexneri 2a from around 2007–2010 to 2013 (Supplementary Fig. 3). Our S. sonnei Bayesian modelling dating estimates are consistent with prior estimates, with our estimated MRCA date for all S. sonnei subtypes (1786; 95% HPD = mid-1714 to early 1849) (Fig. 4B), overlapping with the prior MRCA estimate for all S. sonnei (median = 1669, 95% HPD = 1554–1763)12.
Discussion
This study has highlighted several distinctions between the epidemiology of S. flexneri 2a and S. sonnei in South Africa. The four circulating clusters of S. flexneri 2a varied in their AMR and virulence profiles while the phylogenetically identifiable, circulating sub-Clades of S. sonnei had little variation. The lack of evidence for MSM-associated Lineages is believed to be due to the high prevalence of shigellosis in the general population in South Africa, relative to nations where MSM-associated Lineages are observed. This high endemic transmission is likely to have obscured the signal of MSM-associated transmission rather than a lack of MSM-associated transmission.
The diversity of S. flexneri 2a clusters, and presence of all clusters across the whole study period (Supplementary fig. 4), supports strain co-existence, previously observed on a global level13. Contrastingly, clonal replacement in S. flexneri was found on a national level before in countries in South East Asia17 though this occurred over a 5–20 year period, so our study may not have covered a sufficient time period to observe clonal replacement17. We found no evidence that the introduced, reduced AMR S. flexneri 2a Lineage was replacing the already present MDR Clade. However, on a regional (Southeast Asia) and global level, clonal replacement in S. flexneri is less obvious; with re-emergence of strains after their apparent replacement13,17. The epidemiological dynamics of Shigella in South Africa may be more like the regional dynamics identified in the Southeast Asian study17 as South Africa is a comparable land mass to the total Southeast Asian region studied. This is supported by the distinct provincial distributions of the identified circulating S. flexneri 2a clusters observed in this study.
The AMR profile similarity and geographic homogeneity of S. sonnei clusters are consistent with clonal replacement observed in previous global and national (Vietnam, China) studies of S. sonnei12,66–68. Greater pINV retention was seen in the smaller circulating S. sonnei sub-Clade (3.7.7) despite having emerged around the same time as the other sub-Clades (3.7.9 and 3.7.11) (1996–2001), possibly indicating a link between epidemiological success and pINV stability. Reduced stability of the pINV in S. sonnei, compared to other Shigella, is likely a lifestyle adaptation towards becoming reliant on human to human transmission21. The presence of all clusters across the study period (Supplementary fig. 4), however, does not support clonal replacement driven by differences in pINV retention. While it is clear further study is needed to confirm a link between pINV stability and epidemiological success, our study supports pathogen ecology, in particular lifestyle, drives Shigella epidemiology.
Our observed epidemiological differences between S. sonnei and S. flexneri 2a add to the existing evidence of serogroup-specific lifestyles. Prior evidence includes variation in AMR-linked epidemiological success; clonal replacement and intercontinental dissemination are driven by acquisition of AMR in S. sonnei but not S. flexneri, except amongst men who have sex with men (MSM)13,67,69,70. Epidemiological success may in MSM be linked to AMR regardless of serotype, promoted by, almost exclusively, direct contact transmission and high rates of antimicrobial use among the sub-population of affected MSM71–73. Here, we have found a reduced AMR lineage (Sf cluster 1) was successfully introduced to South Africa in the early 1990s when MDR strains were already present highlighting that AMR is not always necessary for S. flexneri strain success. Loss of the SRL has been reported previously27, as has the establishment of a drug-susceptible lineage of Salmonella enterica subspecies enterica, serovar Typhimurium, in the presence of MDR strains circulating in Malawi74.
An important finding of this study was the evidence of population diversification, in both serotypes, beginning in the 1990s. The emergence and introduction of new Shigella strains in the country occurred during the 1990s coinciding with the end of Apartheid (1992–1994) and the beginning of the HIV epidemic in the country75, both of which may have driven changes in shigellosis epidemiology. Regarding post-Apartheid influences, changes in cross-border travel during this time may be linked to the introduction of Sf cluster 1 into South Africa in 1992 and promoted S. flexneri 2a population expansion through an influx of immunologically naïve hosts. However, population expansion and strain diversification could also be linked with increasing HIV prevalence. Strain divergence in S. sonnei (clusters 1 and 3) and S. flexneri 2a (clusters 2 and 3a) occurred from 1991 to 2001, coinciding with the peak of new HIV infections (2000 and 2001); the number of people living with HIV in South Africa continues to increase75 (Supplementary fig. 3). The influence of HIV is supported by previous research, as HIV is a risk factor for both shigellosis infection and systemic disease23,24,76–81. Furthermore, similar dynamics have been observed in Salmonella and tuberculosis (TB). Specifically, invasive Salmonella Typhimurium in SSA, was predominantly caused by two Lineages82 whose clonal expansions were linked to peaks in HIV prevalence and the expansion of HIV-182, and HIV is a known driver of the TB epidemic83,84.
While this study is unable to determine the relative importance of the end of Apartheid and the HIV epidemic on shigellosis in South Africa, understanding the link between HIV and shigellosis is particularly important for effective public health policy. Particularly as the disparity in the average age of infection between males and females, identified in this study, may additionally be partially due to the greater numbers of women over the age of fifteen years living with HIV in South Africa75 (rather than child care responsibilities as explored above). Another limitation of this study relates to the paucity of surveillance data for gastrointestinal illnesses in endemic regions. Furthermore, the sample comes only from people attending public hospitals and there is low sampling coverage in rural areas. However, we have tried to mitigate this by carefully selecting our subsample relative to case reporting data, but the potential for sampling bias to have impacted our overall findings cannot be completely ruled out.
Our study is the first detailed examination of shigellosis genomic epidemiology in an African nation and shows that shigellosis epidemiology in South Africa is serotype specific. Our findings offer insights into likely drivers of Shigella evolution and epidemiology, supporting pathogen lifestyle as a driver of shigellosis epidemiology, explaining the observed serogroup differences in AMR trends and pINV stability in this study, and globally. We also highlight the potential importance of the HIV epidemic on shigellosis and underline the importance of further research into the relationship between HIV and shigellosis. Based on the findings of this study, we suggest that serogroup-specific public health approaches, which take HIV into account, may be needed for effective shigellosis control nationally, across SSA, and globally.
Methods
Ethics statement
No individual consent was required or sought as all isolates were collected as part of routine surveillance and ethical approval for the use of patient data for public health activities was granted by the Human Research Ethics Committee of the University of the Witwatersrand (Protocol Numbers: M060449 and M110499).
Sample collection, sub-sampling, and sequencing
We created a sub-sample (n = 561) from all the biochemically identified Shigella flexneri 2a (n = 286) or Shigella sonnei (n = 275) collected during routine surveillance (of all age groups) in South Africa (2011–2015) carried out by The Group for Enteric, Respiratory and Meningeal Diseases Surveillance in South Africa (GERMS-SA)54–58. Total Shigella isolated during the study period was 8251, of which 2287 were S. flexneri 2a and 1841 were S. sonnei. Isolates were randomly sub-sampled across those collected throughout the study period. Random sub-sampling was achieved by selecting every 8th (S. flexneri 2a) or 5th (S. sonnei) isolate, based on SA lab number, from the database of collected, surveillance isolates (collected from any body site, 1 January 2011–31 December 2015, for which demographic data was available), up to a minimum of 250 isolates. SA lab number was assigned upon arrival at the reference laboratory, from a surveillance site. If selected isolate could not be located, then the previous isolate in the database was selected instead. All surveillance sites were public hospitals.
All study isolates were whole genome sequenced using Illumina HiSeq 4000 sequencing equipment and the DNA library was prepared using the Illumina Nextera XT DNA Library Prep Kit (Illumina, FC-131-1096)85. The quality of some of the isolate sequences (S. flexneri 2a n = 119 and S. sonnei n = 65) was poor (due to GC library bias caused by the transposon used for tagmentation) and were subsequently re-sequenced at the Centre for Genomic Research (CGR, University of Liverpool) using the Illumina NovaSeq 6000 platform; the DNA library was constructed using the NEBNext Ultra II FS DNA Library Prep Kit for Illumina16. Re-sequenced reads were pooled with the original isolate reads prior to quality trimming.
Two separate public data sets were used as references for the maximum likelihood phylogenetics; one for S. flexneri 2a (n = 286) and one for S. sonnei (n = 40) (accession numbers available in Supplementary Data 1). For, both sample sets, isolates were selected from across the known global Phylogeny, with care taken to include isolates from all genomic subtypes (e.g., Phylogroups/Lineages). A subset of the S. flexneri 2a reference sample set (n = 116) was used to generate a Phylogroup 3 phylogeny, and exclusively contained all the Phylogroup 3 isolates from the larger reference sample set.
Data collection
National surveillance involved a network of public hospitals and laboratories from across all nine provinces, further details can be found in GERMS-SA annual reports54–58. Shigella isolates were identified as part of active laboratory-based surveillance for bacterial causes of diarrhoea. Patients presenting at health-care facilities with a history of diarrhoea defined as three or more loose stools per day, either with or without blood, were eligible to have a stool specimen submitted for microscopy and culture for bacterial pathogens; final decision on stool specimen submission rested with the treating healthcare practitioner. All positive bacterial cultures were forwarded to CED for further characterisation, according to standard characterisation procedures. Some patient data was collected during surveillance, including age and sex (based on medical records); no records were collected on sexuality. A single isolate only was collected from each patient sample. Isolates came from 257 males (128 S. flexneri 2a and 129 S. sonnei) and 151 females (129 S. flexneri 2a and 122 S. sonnei).
Recorded case numbers, taken from the GERMS-SA annual reports, were used to examine the background epidemiology shigellosis in the country, and the representation of the larger surveillance dataset by our study isolates54–58. Due to missing data from the GERMS-SA annual reports, comparisons were made using data collected during 2011 to 2013.
Each province in South Africa is sub-divided into district municipalities and metropolitan municipalities, both referred to as districts throughout and categorised by degree of urbanisation according to the methods laid out by the European Commission in March 2020 (https://ec.europa.eu/16urostat/cros/system/files/bg-item3j-recommendation-e.pdf). Densely populated districts are referred to, here, as ‘urban’, intermediate density as ‘mixed’, and thinly populated as ‘rural’.
Quality control
All raw sequence reads were quality trimmed with Trimmomatic (v0.38) and SeqTK (v1.3) (https://github.com/lh3/seqtk)86. In addition to quality trimming, a further eight bases were removed from the ends of all South African study isolate reads with SeqTK due to poor quality.
Quality was assessed before and after trimming with FastQC (v0.11.8) and MultiQC (v1.5)87,88. Read mapping quality was assessed using Qualimap (v2.2.2-dev)89. Genome assembly quality was assessed using Quast (v8.13) against the read mapping reference genome90,91. All included isolates had sequences with: (1) per base mean Phred score ≥28, (2) per sequence Phred score ≥28, (3) even per base sequence content, (4) even per sequence GC content curve and peak GC content between 48–54%, 5) per base N content <5, (6) < 5% overrepresented sequences, and (7) < 5% sequences with adaptor sequences.
No sequencing data was received for thirteen S. flexneri 2a and five S. sonnei. Twenty S. flexneri 2a isolates were excluded from further analysis: seven for poor sequence quality, two for uneven per base sequence content, five for uneven GC content, two for poor mapping against the complete S. flexneri 2a reference genome (<20 mean read depth), and four for being mis-identified as S. flexneri 2a.
Seventeen S. sonnei were excluded from further analysis: four for uneven per base sequence content, seven poor mapping to the S. sonnei reference genome (<20 mean read depth), and six for being misidentified as S. sonnei. All unpaired reads for both serotypes were also excluded from further analysis due to poor per base sequence content. Of the included 260 S. flexneri 2a and 253 S. sonnei isolates, 111 and 57 were re-sequenced, respectively. All unpaired reads were excluded due to poor per base sequence content.
In silico strain typing
In silico species confirmation and prediction of serotype was done using ShigaTyper (v1.0.6) (https://github.com/CFSAN-Biostatistics/shigatyper) and SonneiTyping sonnei_genotype.py (v1) (https://github.com/katholt/sonneityping) was used to predict genotype of S. sonnei isolates63. Both software tools were run using isolate sequence reads; raw reads for ShigaTyper but trimmed reads for SonneiTyping.
Maximum likelihood phylogenetics
All maximum likelihood phylogenetic trees were generated from core-SNP alignments using RaxML-NG (v0.6.6; GTR + G substitution model, 1000 bootstrap validation and mid-point rooted)92. Core-SNP alignments (S. flexneri 2a: all Phylogroups phylogeny = 34,768 SNPs and Phylogroup 3 phylogeny = 13,279 SNPs, S. sonnei: 6870 SNPS) were generated from quality-trimmed study isolate sequence reads and phylogenetic reference isolate sequence reads. Sequence read mapping, performed with bwa mem (v0.7.17), was to either the S. flexneri 2a 301 strain complete genome (accessions: NC_004337.2, NC_004851.1) or the S. sonnei 53 G strain complete genome (accessions: HE616528.1, HE616529.1, HE616530.1, HE616531.1, HE616532.1). Samtools (v1.9), samclip (v2.27.1) and Picard (v2.23.8) (https://broadinstitute.github.io/picard/) were used to sort, filter and index mapped reads. Variant calling was performed with samtools mpileup (v1.9) and bcftools (v1.9)93–95. Consensus sequences were defined with samtools vcfutils.pl (min. depth = 4), with plasmids, mobile genetic elements, and phaster (https://phaster.ca/) identified phage sequences masked with bedtools (v2.27.1)93–98. Masked consensus sequences were run through gubbins (v2.4.1)99.
Bayesian phylogenetics
Serotype-specific time trees were generated with Bayesian Evolutionary Analysis by Sampling Trees software (BEAST2) (v2.6.3). Generated from study isolate only core-SNP alignments (S. flexneri 2a: 7936 SNPs, S. sonnei: 4161 SNPs), created using same methods as for maximum likelihood phylogenetics. The fasta alignment was converted to nex with seqmagick (v0.8.0) (https://github.com/fhcrc/seqmagick/).
Two isolates were excluded from each serotype-specific BEAST phylogeny due to being outliers for the molecular clock signal, visually identified in TempEST (v.1.5.3) using RaxML-ng (bootstraps, GTR + G model) generated, study isolate-only, serotype-specific, maximum likelihood phylogenies92,100. Once outlier isolates were excluded, estimated (in TempEST) molecular clock rates were 3.1921e-4 and 6.0361e−4 substitutions per site per year (correlation co-efficient = 0.27 and 0.39) for S. flexneri 2a and S. sonnei, respectively.
We used an extended coalescent Bayesian skyline tree model, with a relaxed clock and a log normal prior distribution (prior rate = 1e-6), and site model averaging with BmodelTest, transition-transversion split (prior mutation rate = 1.0), all other priors were left as default101.
For S. flexneri 2a we ran a single MCMC chain with a length of 5,043,000,000 (sampling every 1,000,000), 39% burn-in was removed. While for S. sonnei we ran three MCMC chains with a length of 3,300,000,000 (sampling every 100000), removing around 67% burn-in from all three while combining with logcombiner. The tree topology was generated with treeannotator (v2.6.3) and visualised in FigTree (v1.4.4) (https://github.com/rambaut/figtree) and the interactive tree of life (ITOL) online platform102. A minimum effective sample size (ESS) of 200 was achieved for all model parameters, checked using Tracer (v2.6.3)103.
Study isolates population clusters were defined using RhierBAPS (v1.1.3) and serotype-specific, study isolate only core-SNP alignment in Rstudio (v1.4.1717; R v4.1.0)104.
Genome assembly and annotation
Draft genomes were assembled using unicycler (v0.4.7) and quality-trimmed sequence reads105. Genome annotation was achieved with Prokka (v1.14.5) against the Escherichia database.
Antimicrobial resistance profiling
AMR profiles were defined based on phenotypic data, where available, or in silico prediction. Gene determinants were identified by starAMR (v0.5.1), or AMRfinderPlus (v3.2.3) for chloramphenicol resistance in S. flexneri 2a (due to better accuracy against phenotypic data). (Fluoro)quinolone resistance conferring point mutations in the QRDR were detected in silico with SonneiTyping (S. sonnei) or manual extraction of amino acids in gyrA at positions 83 and 87 and parC position 80 (S flexneri 2a). Partial phenotypic data was available for ampicillin, chloramphenicol, streptomycin, tetracycline, nalidixic acid, ciprofloxacin, trimethoprim, and ceftriaxone, and are detailed in the Supplementary Data 1 alongside the detection of genotypic markers.
Accuracy of the in silico AMR phenotype predictions were assessed against recorded phenotype. Accuracy by antimicrobial, for both AMRfinder and starAMR, was defined as number of correct resistant phenotype predictions and number of correct susceptible phenotype predictions. Association with resistance was also assessed for each antimicrobial resistance determinant, using genotype. A minimum association of 50% was required for a resistance determinant to be accepted as associated with resistance and, if present, used to predict a resistance phenotype. In S. sonnei, aadA1 or aadA2 alone were poor predictors of streptomycin resistance, though if present together the association was strong enough that a resistant phenotype was predicted. In S. flexneri 2a, aadA2 was not used to predict resistance to streptomycin.
Virulence profiling
A virulence profile was generated for all study isolates by comparing the draft genome assemblies against the VirulenceFinder database, using VirulenceFinder (v2.0.4–1), and against a local, curated virulence genome database, based off a previous study (Supplementary Data 1), with BLASTn (v2.10.0+)33. Only genes with ≥99% sequence identity and database gene coverage were accepted.
The presence of the SRL-PAI, SRL and pINV was assessed by mapping isolate reads to these known virulence loci, within a reference genome. Quality-trimmed reads were mapped to various reference genomes, known to contain the relevant loci, with bwa mem (v0.7.17) (Supplementary Data 1). Read mapping of in silico generated positive and negative controls were also assessed, using random reads (paired, 100-70 bases, insert size 150-300) generated from positive and negative control genomes with the bbmap randomreads.sh script (v38.00) to 60x coverage (www.sourceforge.net/projects/bbmap/) (Supplementary Data 1). Reads converted into a set of forward and a set of reverse reads with seqkit (v0.10.1)171.
Statistics
Most associations were compared with a chi-squared test of association, carried out using raw numbers and reported, in most cases, as a percentage difference. For background epidemiology (using data extracted from GERMS-SA annual reports54–58) and sample set representativeness, percentage differences are defined as the observed cases or samples (O) as a fraction of the national total (T) minus the expected (E) fraction of the national total (), where To is the total observations (cases or samples) and Tc is the total reported cases. For associations between isolate or patient metadata, percentage difference is defined as the difference between observed and expected isolate numbers as a fraction of the expected number of isolates ().
Associations between AMR determinant, or profile, and geographic region was tested with a Fisher’s exact test. Chi-squared tests were used to examine the associations between AMR determinants, or profile, and Shigella sub-population. Meanwhile, associations between virulence gene and Shigella sub-population, were assessed using odds ratios and two-tailed Z tests (Supplementary Data 1). The Bonferroni correction was used to adjust the statistical significance threshold for multiple comparisonsTable 1.
Table 1.
Number of sequenced samples from each province and district by serotype
| Shigella flexneri 2a | Shigella sonnei | Total | |
|---|---|---|---|
| Eastern Cape | 48 | 32 | 80 |
| Amathole DM | 24 | 14 | 38 |
| Chris Hani DM | 1 | 1 | |
| Nelson Mandela Bay MM | 18 | 16 | 34 |
| Joe Gqabi DM | 2 | 2 | |
| Sarah Baartman DM | 1 | 1 | |
| District unknown | 3 | 1 | 4 |
| Free State | 13 | 13 | 26 |
| Lejweleputswa DM | 4 | 7 | |
| Mangaung MM | 10 | 9 | 19 |
| Gauteng | 64 | 134 | 198 |
| City of Johannesburg MM | 23 | 64 | 87 |
| City of Tshwane MM | 16 | 27 | 43 |
| Ekurhuleni MM | 10 | 21 | 31 |
| Sedibeng DM | 6 | 7 | 13 |
| West Rand DM | 4 | 4 | 8 |
| City of Tshwane MM | 1 | 1 | |
| District unknown | 5 | 10 | 15 |
| KwaZulu-Natal | 36 | 29 | 65 |
| Amajuba DM | 4 | 4 | |
| iLembe DM | 1 | 1 | |
| Ugu DM | 2 | 3 | 5 |
| uMgungundlovu DM | 5 | 4 | 9 |
| City of eThekwini MM | 24 | 16 | 40 |
| Harry Gwala DM | 1 | 1 | 2 |
| Uthukela DM | 2 | 2 | |
| District unknown | 1 | 1 | 2 |
| Limpopo | 1 | 1 | |
| Waterberg DM | 1 | 1 | |
| Mpumalanga | 5 | 8 | 13 |
| Ehlanzeni DM | 2 | 6 | 8 |
| Gert Sibande DM | 3 | 1 | 4 |
| Nkangala DM | 1 | 1 | |
| Northern Cape | 3 | 1 | 4 |
| Frances Baard DM | 3 | 1 | 4 |
| North West | 1 | 4 | 5 |
| Bojanala Platinum DM | 1 | 1 | 2 |
| Dr Kenneth Kaunda MM | 3 | 3 | |
| Western Cape | 89 | 32 | 121 |
| Cape Winelands DM | 2 | 1 | 3 |
| Central Karoo DM | 1 | 1 | |
| City of Cape Town MM | 69 | 26 | 95 |
| Overberg DM | 1 | 1 | |
| West Coast DM | 8 | 2 | 10 |
| Garden Route DM | 4 | 2 | 6 |
| Unknown district | 4 | 1 | 5 |
MM metropolitan municipality, DM district municipality.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank all participants of the NICD GERMS-SA Laboratory Surveillance Network for submission of clinical isolates of Shigella species to the NICD. The surveillance network includes laboratories belonging to the Department of Health (NHLS laboratories) and laboratories that form part of the private sector. Next-generation sequencing and library construction were delivered via the BBSRC National Capability in Genomics and Single Cell Analysis (BB/CCG1720/1, N.H.) at Earlham Institute, by members of the Genomics Pipelines Group. The project was supported by both a Global Challenges Research Fund (GCRF) data & resources grant BBS/OS/GC/000009D (N.H.) and the BBSRC Core Capability Grant to the Earlham Institute BB/CCG1720/1 (N.H.) and Core Strategic Programme Grant BBS/E/T/000PR9817 (N.H.). This work was also supported by Medical Research Council grant MR/R020787/1 (K.B.). For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
Author contributions
Conceptualisation: K.S.B., K.K. Data curation: K.S.B., N.H., G.E.S. Formal analysis: G.E.S. Funding acquisition: K.S.B., M.I.G. Investigation: G.E.S. Methodology: G.E.S., R.J.B. Project administration: G.E.S. Resources: N.H., K.K., A.S., J.T. Supervision: K.S.B., M.I.G., A.S., J.T., K.K. Visualisation: G.E.S. Writing—original draft: G.E.S. Writing—reviewing and editing: all authors.
Peer review
Peer review information
Nature Communications thanks Martyn Kirk and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The study isolates’ raw sequences have been deposited in the European Nucleotide Archive under project accession PRJEB55173 and individual isolate accession numbers can be found in the Supplementary Data 1. Accession numbers for reference isolates used in the study are also provided in the supplementary table. All patient metadata, and all data generated from the analysis of this metadata, used in this study are provided in the Supplementary Data 1 and Source Data files, respectively. Source data are provided with this paper.
Competing interests
The authors declare the following competing interests: M.I.G. is currently an employee of GSK, but this work was completed prior to her joining. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
George E. Stenhouse, georgiesten@googlemail.com
Kate S. Baker, Email: kb827@cam.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43345-5.
References
- 1.Institue for Health Metrics and Evaluation. Global Burden of Disease. https://vizhub.healthdata.org/gbd-results/ 2019.
- 2.Troeger CE, et al. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: an analysis of the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2020;20:37–59. doi: 10.1016/S1473-3099(19)30401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granfors K, et al. Bacterial lipopolysaccharide in synovial fluid cells in Shigella triggered reactive arthritis [4] J. Rheumatol. 1992;19:500. [PubMed] [Google Scholar]
- 4.Khalil IA, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the global burden of disease study 1990–2016. Lancet Infect. Dis. 2018;18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 6.Lee G, et al. Effects of shigella-, campylobacter- and ETEC-aSsociated diarrhea on childhood growth. Pediatric Infectious Disease Journal. 2014;33:1004–1009. doi: 10.1097/INF.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 7.Pinkerton R, et al. Early childhood diarrhea predicts cognitive delays in later childhood independently of malnutrition. Am. J. Trop. Med. Hyg. 2016;95:1004–1010. doi: 10.4269/ajtmh.16-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter CK, et al. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology. 2008;135:781–786. doi: 10.1053/j.gastro.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 9.Schiellerup P, Krogfelt KA, Locht H. A comparison of self-reported joint symptoms following infection with different enteric pathogens: effect of HLA-B27. J. Rheumatol. 2008;35:480–487. [PubMed] [Google Scholar]
- 10.Schuster HJ, et al. An adult case with shigellosis-aSsociated encephalopathy. BMJ Case Rep. 2018;2018:bcr2017222372. doi: 10.1136/bcr-2017-222372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seferbekova Z, et al. High rates of genome rearrangements and pathogenicity of Shigella spp. Front. Microbiol. 2021;12:628622. doi: 10.3389/fmicb.2021.628622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt KE, et al. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global diSsemination from Europe. Nat. Genet. 2012;44:1056–1059. doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor TR, et al. Species-wide whole genome sequencing reveals historical global spread and recent local persistence in Shigella flexneri. eLife. 2015;4:e07335. doi: 10.7554/eLife.07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson CN, Duy PT, Baker S. The rising dominance of shigella sonnei: an intercontinental shift in the etiology of bacillary dysentery. PLoS Negl. Trop. Dis. 2015;9:e0003708. doi: 10.1371/journal.pntd.0003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bengtsson RJ, et al. Pathogenomic analyses of Shigella isolates inform factors limiting shigellosis prevention and control acroSs LMICs. Nat. Microbiol. 2022;7:251–261. doi: 10.1038/s41564-021-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung The H, et al. Evolutionary histories and antimicrobial resistance in Shigella flexneri and Shigella sonnei in Southeast Asia. Commun. Biol. 2021;4:353. doi: 10.1038/s42003-021-01905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayeed S, Sack DA, Qadri F. Protection from Shigella sonnei infection by immunisation of rabbits with Plesiomonas shigelloides (SVC 01) J. Med. Microbiol. 1992;37:382–4. doi: 10.1099/00222615-37-6-382. [DOI] [PubMed] [Google Scholar]
- 19.Kotloff KL, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 1999;77:651–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Caboni M, et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLOS Pathog. 2015;11:e1004749. doi: 10.1371/journal.ppat.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McVicker G, Tang CM. Deletion of toxin–antitoxin systems in the evolution of Shigella sonnei as a host-adapted pathogen. Nat. Microbiol. 2016;2:16204. doi: 10.1038/nmicrobiol.2016.204. [DOI] [PubMed] [Google Scholar]
- 22.Chung The H, Baker S. Out of Asia: the independent rise and global spread of fluoroquinolone-resistant Shigella. Microb. Genom. 2018;4:e000171. doi: 10.1099/mgen.0.000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO press (2017).
- 24.WHO, DRAFT WHO preferred product characteristics for vaccines against Shigella. WHO press (2020).
- 25.Toro CS, et al. Antimicrobial resistance dynamics in chilean shigella sonnei strains within two decades: role of shigella resistance locus pathogenicity island and ClaSs 1 and ClaSs 2 integrons. Front. Microbiol. 2022;12:794470. doi: 10.3389/fmicb.2021.794470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luck SN, et al. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 2001;69:6012–21. doi: 10.1128/IAI.69.10.6012-6021.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner SA, et al. Nested deletions of the SRL pathogenicity island of Shigella flexneri 2a. J. Bacteriol. 2001;183:5535–43. doi: 10.1128/JB.183.19.5535-5543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray CJL, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livio S, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014;59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotloff KL, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 2012;55:S232–45. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenhouse GE, et al. Whole genome sequence analysis of Shigella from Malawi identifies fluoroquinolone resistance. Microb. Genom. 2021;7:000532. doi: 10.1099/mgen.0.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali Nor BS, Menza NC, Musyoki AM. Multidrug-resistant shigellosis among children aged below five years with diarrhea at Banadir Hospital in Mogadishu, Somalia. Can. J. Infect. Dis. Med. Microbiol. 2021;2021:6630272. doi: 10.1155/2021/6630272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver WM, et al. Etiology and pathogenicity of bacterial isolates: a croSs sectional study among diarrheal children below five years in central regions of Kenya. PAMJ. 2018;31:88. doi: 10.11604/pamj.2018.31.88.15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilongosi Webale M, et al. Epidemiological patterns and antimicrobial resistance of bacterial diarrhea among children in Nairobi City, Kenya. Gastroenterol. Hepatol. Bed Bench. 2020;12:8. [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlinac PB, et al. Failure of syndrome-based diarrhea management guidelines to detect shigella infections in kenyan children. J. Pediatric Infect. Dis. Soc. 2015;5:366–374. doi: 10.1093/jpids/piv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyanga PL, et al. Escherichia coli pathotypes and Shigella sero-groups in diarrheic children in Nairobi city, Kenya. Gastroenterol. Hepatol. Bed. Bench. 2017;10:220–228. [PMC free article] [PubMed] [Google Scholar]
- 37.Phiri AFND, et al. Burden, antibiotic resistance, and clonality of shigella spp. implicated in community-acquired acute diarrhoea in Lilongwe, Malawi. Trop. Med. Infect. Dis. 2021;6:63. doi: 10.3390/tropicalmed6020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langendorf C, et al. Enteric bacterial pathogens in children with diarrhea in Niger: diversity and antimicrobial resistance. PLoS One. 2015;10:e0120275. doi: 10.1371/journal.pone.0120275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, et al. A prospective study of etiological agents among febrile patients in Sierra Leone. Infect. Dis. Ther. 2021;10:1645–1664. doi: 10.1007/s40121-021-00474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tosisa W, et al. Prevalence and antimicrobial susceptibility of Salmonella and Shigella species isolated from diarrheic children in Ambo town. BMC Pediatrics. 2020;20:91. doi: 10.1186/s12887-020-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Njuguna C, et al. Enteric pathogens and factors aSsociated with acute bloody diarrhoea, Kenya. BMC Infect. Dis. 2016;16:477. doi: 10.1186/s12879-016-1814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chissaque A, et al. The epidemiology of diarrhea in children under 5 years of age in Mozambique. Curr. Trop. Med. Rep. 2018;5:115–124. doi: 10.1007/s40475-018-0146-6. [DOI] [Google Scholar]
- 43.Breurec S, et al. Serotype distribution and antimicrobial resistance of shigella species in Bangui, Central African Republic, from 2002 to 2013. Am. J. Trop. Med. Hyg. 2018;99:283–286. doi: 10.4269/ajtmh.17-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaumburg F, et al. Molecular characterization of Shigella spp. from patients in Gabon 2011–2013. Trans. R. Soc. Trop. Med. Hyg. 2015;109:275–279. doi: 10.1093/trstmh/tru175. [DOI] [PubMed] [Google Scholar]
- 45.Kalule JB, et al. Prevalence and antibiotic susceptibility patterns of enteric bacterial pathogens in human and non-human sources in an urban informal settlement in Cape Town, South Africa. BMC Microbiol. 2019;19:244. doi: 10.1186/s12866-019-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussen S, Mulatu G, Yohannes Kassa Z. Prevalence of Shigella species and its drug resistance pattern in Ethiopia: a systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2019;18:22. doi: 10.1186/s12941-019-0321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mero S, et al. Prevalence of diarrhoeal pathogens among children under five years of age with and without diarrhoea in Guinea-BiSsau. PLoS Negl. Trop. Dis. 2021;15:e0009709. doi: 10.1371/journal.pntd.0009709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiyangi H, et al. Identification and antimicrobial resistance patterns of bacterial enteropathogens from children aged 0–59 months at the University Teaching Hospital, Lusaka, Zambia: a prospective croSs sectional study. BMC Infect. Dis. 2017;17:117. doi: 10.1186/s12879-017-2232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vubil D, et al. Antibiotic resistance and molecular characterization of shigella isolates recovered from children aged less than 5 years in Manhiça, Southern Mozambique. Int. J. Antimicrob. Agents. 2018;51:881–887. doi: 10.1016/j.ijantimicag.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Irenge LM, et al. Antimicrobial resistance of bacteria isolated from patients with bloodstream infections at a tertiary care hospital in the Democratic Republic of the Congo. S. Afr. Med. J. 2015;105:752–755. doi: 10.7196/SAMJnew.7937. [DOI] [PubMed] [Google Scholar]
- 51.Group for Enteric, R.a.M.d.S.i.S.A., GERMS-SA Annual Report 2007. Available at: https://www.nicd.ac.za/assets/files/2007_GERMS-SA_Annual_Report.pdf, 2007.
- 52.Group for Enteric, R.a.M.d.S.i.S.A., GERMS-SA Annual Report 2008. Available at: https://www.nicd.ac.za/assets/files/2008_GERMS-SA_Annual_Report(1).pdf 2008.
- 53.Group for Enteric, R.a.M.d.S.i.S.A., GERMS-SA Annual Report 2009. Available at: https://www.nicd.ac.za/assets/files/2009GERMS-SA_Annual_Report.pdf 2009.
- 54.Group for Enteric, R.a.M.d.S.i.S.A., GERMS-SA Annual Report 2011. Available at: http://www.nicd.ac.za/assets/files/2011%20GERMS-SA%20Annual%20report%20pub%20final(1).pdf 2011.
- 55.Group for Enteric, R.a.M.d.S.i.S.A., GERMS-SA Annual Report 2012. Available at: http://www.nicd.ac.za/assets/files/GERMS-SA%202012%20Annual%20Report.pdf 2012.
- 56.Group for Enteric, R.a.M.d.S.i.S.A., GERMS-SA Annual Report 2013. Available at: http://www.nicd.ac.za/assets/files/GERMS-SA%20AR%202013(1).pdf 2013.
- 57.Group for Enteric, R.a.M.d.S.i.S.A., GERMS-SA Annual Report 2014. Available at: http://www.nicd.ac.za/assets/files/GERMS-SA%20AR%202014(1).pdf 2014.
- 58.Group for Enteric, R.a.M.d.S.i.S.A.. GERMS-SA Annual Report 2015. Available at: http://www.nicd.ac.za/assets/files/GERMS-SA%20AR%202015-1.pdf 2015.
- 59.Garrett V, et al. A recurring outbreak of Shigella sonnei among traditionally observant Jewish children in New York City: the risks of daycare and household transmiSsion. Epidemiol. Infect. 2006;134:1231–6. doi: 10.1017/S0950268806006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDonnell J, et al. Retrospective analysis of whole genome sequencing compared to prospective typing data in further informing the epidemiological investigation of an outbreak of Shigella sonnei in the UK. Epidemiol. Infect. 2013;141:2568–2575. doi: 10.1017/S0950268813000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agüero ME, et al. A plasmid-encoded outer membrane protein, TraT, enhances resistance of Escherichia coli to phagocytosis. Infect. Immun. 1984;46:740–6. doi: 10.1128/iai.46.3.740-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujiyama R, et al. The shf gene of a shigella flexneri homologue on the virulent plasmid pAA2 of enteroaggregative escherichia coli 042 is required for firm biofilm formation. Curr. Microbiol. 2008;56:474–480. doi: 10.1007/s00284-008-9115-y. [DOI] [PubMed] [Google Scholar]
- 63.Hawkey J, et al. Global population structure and genotyping framework for genomic surveillance of the major dysentery pathogen, Shigella sonnei. Nat. Commun. 2021;12:2684. doi: 10.1038/s41467-021-22700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Martino ML, et al. The multifaceted activity of the VirF regulatory protein in the shigella lifestyle. Front. Mol. Biosci. 2016;3:61. doi: 10.3389/fmolb.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roehrich AD, et al. Shigella IpaD has a dual role: signal transduction from the type III secretion system needle tip and intracellular secretion regulation. Mol. Microbiol. 2013;87:690–706. doi: 10.1111/mmi.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holt KE, et al. Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc. Natl. Acad. Sci. USA. 2013;110:17522–7. doi: 10.1073/pnas.1308632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thanh Duy P, et al. Commensal Escherichia coli are a reservoir for the tranSfer of XDR plasmids into epidemic fluoroquinolone-resistant Shigella sonnei. Nat. Microbiol. 2020;5:256–264. doi: 10.1038/s41564-019-0645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu S, et al. A Shigella sonnei clone with extensive drug resistance aSsociated with waterborne outbreaks in China. Nat. Commun. 2022;13:7365. doi: 10.1038/s41467-022-35136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung The H, et al. South Asia as a reservoir for the global spread of ciprofloxacin-resistant shigella sonnei: a cross-sectional study. PLoS Med. 2016;13:e1002055. doi: 10.1371/journal.pmed.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung The H, et al. DiSsecting the molecular evolution of fluoroquinolone-resistant Shigella sonnei. Nat. Commun. 2019;10:4828. doi: 10.1038/s41467-019-12823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker KS, et al. Intercontinental diSsemination of azithromycin-resistant shigellosis through sexual transmiSsion: a croSs-sectional study. Lancet Infect. Dis. 2015;15:913–921. doi: 10.1016/S1473-3099(15)00002-X. [DOI] [PubMed] [Google Scholar]
- 72.Baker KS, et al. Horizontal antimicrobial resistance tranSfer drives epidemics of multiple Shigella species. Nat. Commun. 2018;9:1462. doi: 10.1038/s41467-018-03949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aragón TJ, et al. Case-control study of shigellosis in San Francisco: the role of sexual transmiSsion and HIV infection. Clin. Infect. Dis. 2007;44:327–334. doi: 10.1086/510593. [DOI] [PubMed] [Google Scholar]
- 74.Pulford CV, et al. Stepwise evolution of Salmonella Typhimurium ST313 causing bloodstream infection in Africa. Nat. Microbiol. 2021;6:327–338. doi: 10.1038/s41564-020-00836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.UNAIDS. AIDS info: epidemic and response. https://aidsinfo.unaids.org/ 2021.
- 76.Baer JT, et al. HIV infection as a risk factor for shigellosis. Emerg. Infect. Dis. 1999;5:820–823. doi: 10.3201/eid0506.990614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keddy KH, et al. Systemic shigellosis in South Africa. Clin. Infect. Dis. 2012;54:1448–1454. doi: 10.1093/cid/cis224. [DOI] [PubMed] [Google Scholar]
- 78.Tsai C-S, et al. Changing epidemiology of shigellosis in Taiwan, 2010-2019: an emerging threat to HIV-infected patients and men who have sex with men. Emerg. Microbes Infect. 2022;11:498–506. doi: 10.1080/22221751.2022.2031309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiou CS, et al. The worldwide spread of ciprofloxacin-resistant Shigella sonnei among HIV-infected men who have sex with men, Taiwan. Clin. Microbiol. Infect. 2016;22:383.e11–383.e16. doi: 10.1016/j.cmi.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 80.Gaufin T, et al. Antimicrobial-resistant Shigella spp. in San Diego, California, USA, 2017-2020. Emerg. Infect. Dis. 2022;28:1110–1116. doi: 10.3201/eid2806.220131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaudreau C, et al. Clinical and genomic investigation of an international ceftriaxone- and azithromycin-resistant shigella sonnei cluster among men who have sex with men, Montréal, Canada 2017-2019. Microbiol. Spectr. 2022;10:e0233721. doi: 10.1128/spectrum.02337-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okoro CK, et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat. Genet. 2012;44:1215–1221. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawn SD, Wood R. Incidence of tuberculosis during highly active antiretroviral therapy in high-income and low-income countries. Clin. Infect. Dis. 2005;41:1783–1786. doi: 10.1086/498308. [DOI] [PubMed] [Google Scholar]
- 84.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perez-Sepulveda BM, et al. An acceSsible, efficient and global approach for the large-scale sequencing of bacterial genomes. Genome Biol. 2021;22:349. doi: 10.1186/s13059-021-02536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andrews, S. FastQC: a quality control tool for high throughput sequence data. 2014.
- 88.Ewels P, et al. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–8. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okonechnikov K, Conesa A, Garcia-Alcalde F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32:292–4. doi: 10.1093/bioinformatics/btv566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gurevich A, et al. QUAST: quality aSseSsment tool for genome aSsemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wick RR, et al. Bandage: interactive visualization of de novo genome aSsemblies. Bioinformatics. 2015;31:3350–3352. doi: 10.1093/bioinformatics/btv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H. A statistical framework for SNP calling, mutation discovery, aSsociation mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–93. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Danecek P, et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics (Oxford, England) 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arndt D, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Y, et al. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39:W347–52. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Croucher NJ, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kozlov AM, et al. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35:4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bouckaert RR, Drummond AJ. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017;17:42. doi: 10.1186/s12862-017-0890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rambaut A, et al. Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tonkin-Hill G, et al. RhierBAPS: an R implementation of the population clustering algorithm hierBAPS [version 1; peer review: 2 approved] Wellcome Open Res. 2018;3:93. doi: 10.12688/wellcomeopenres.14694.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wick RR, et al. Unicycler: resolving bacterial genome aSsemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The study isolates’ raw sequences have been deposited in the European Nucleotide Archive under project accession PRJEB55173 and individual isolate accession numbers can be found in the Supplementary Data 1. Accession numbers for reference isolates used in the study are also provided in the supplementary table. All patient metadata, and all data generated from the analysis of this metadata, used in this study are provided in the Supplementary Data 1 and Source Data files, respectively. Source data are provided with this paper.