Abstract
Background
Nectin-4 and Trop-2 are transmembrane targets of FDA-approved antibody-drug conjugates (ADC) Enfortumab-vedotin (EV) and Sacituzumab govitecan (SG), respectively, for the treatment of metastatic urothelial carcinoma (mUC). The expression and role of Nectin-4 and Trop-2 in mUC variant histology is poorly described.
Objective
We evaluate membranous and cytoplasmic protein expression, and mRNA levels of Nectin-4 and Trop-2 within matched primary and metastatic mUC samples to determine heterogeneity of ADC targets in mUC variants.
Results
Patients with mUC were consented for rapid autopsy immediately after death. Tissues from matched primary and metastatic lesions were collected. A total of 67 specimens from 20 patients were analyzed: 27 were UC, 17 plasmacytoid (PUC), 18 UC with squamous differentiation (UCSD), and 5 neuroendocrine (NE); 10 from primary and 57 from metastatic sites. All histology except NE expressed moderate-high levels of Nectin-4 and Trop-2 by both immunohistochemistry and RNAseq. Nectin-4 demonstrated prominent cytoplasmic staining in metastatic PUC and UCSD. Trop-2 demonstrated strong cytoplasmic and membrane staining in primary and metastatic tumors. Interestingly, Nectin-4 and Trop-2 expression are positively correlated at both mRNA and protein levels.
Conclusion
UC and non-NE variants express notable level of Nectin-4 and Trop-2 in both primary and metastatic lesions. Membrane staining of Nectin-4 and Trop-2 is present but cytoplasmic staining is a more common event in both mUC and mUC variant histology. These findings support evaluation of EV and SG in heavily treated variant histology BC and urge attention on the clinical relevance of cytoplasmic localization of ADC targets.
Keywords: bladder cancer, antibody-drug conjugates, variant histology, metastases
MicroAbstract
We sought to characterize the expression level and subcellular localization of Nectin-4 and Trop-2, targets of novel antibody-drug conjugates in metastatic urothelial cancer, within variant bladder cancer from matched primary and metastatic sites. We find significant levels of the targets in histologic variants. Additionally, high levels of cytoplasmic rather than membrane staining is seen, which may have important clinical implications.
Introduction
Patients with advanced bladder cancer (BC) have a grim prognosis despite advances in systemic therapy1. Recently, FDA approved two humanized antibody-drug conjugates (ADC) targeting membrane molecules in metastatic urothelial carcinoma (mUC). Enfortumab vedotin (EV) targeting Nectin-4, and Sacituzumab govitecan (SG) targeting Trop-2 have demonstrated improved survival in patients with mUC2,3. However, their potential utility to mUC histologic variants is unclear.
Variant histology BC is aggressive with low response rates to systemic therapy and poor outcomes. Moreover, their relative rarity adds significant challenges for conducting much-needed clinical trials. Standard of care treatments for variants has historically been extrapolated from non-variant UC studies, which partly contributed to the low response rate. Studies of Nectin-4 and Trop-2 expression to date in BC have focused on primary tumor4–6. However, genomic and phenotypic heterogeneity across metastatic sites are frequent and key to treatment resistance in advanced BC7. As such, characterizing the expression of Nectin-4 and Trop-2 in the aggressive but understudied UC histologic variants, as well as across metastatic sites, will allow understanding of potential activity of ADC in these challenging patient groups.
In this study, we evaluate expression level and report subcellular localization of Nectin-4 and Trop-2 in histologic variants of BC within matched primary and metastatic specimens acquired from 2015–2020 from the urothelial cancer rapid autopsy program7.
Materials and methods
Patients and tissue specimens
Specimens were obtained from patients who died of metastatic urothelial cancer in 2015–2020, and the rapid autopsy was typically performed within 6 hours of death7. All procedures involving human subjects were approved by the Institutional Review Board (IRB) of the University of Washington. All 20 patients have signed written informed consent for the rapid autopsy. Metastases and the primary tumor (if present) were identified and biopsied. Biopsies of all bone sites were obtained using a drill with an attached trephine. Rib metastasis was acquired by a Dyke cutter. All visceral metastases and matched primary tumor were embedded in Optimal Cutting Temperature compound (OCT; Tissue-Tek, Sakura Finetek, CA) or formalin-fixed and embedded in paraffin. Bone metastases were either snap frozen or decalcified in 10% formic acid before paraffin embedding.
Tissue microarray construction
Human tissue microarrays (TMA) were constructed for this bladder cancer rapid autopsy series. Formalin-fixed, paraffin-embedded specimens of paired primary (if available) and 2–5 metastatic specimens were used per patient, and TMA were constructed as previously described8. The TMA represented a total of 216 cores made from triplicate 1-mm cores taken from tumor regions of 72 specimens, as defined by two genitourinary pathologists (FVL and MPR).
Immunohistochemistry and analyses
Five-μm TMA sections were deparaffinized and rehydrated in sequential xylene and graded ethanol series. Antigen retrieval was performed in Tris/EDTA buffer (pH 9.0). Endogenous peroxidase and avidin/biotin were blocked (Vector Laboratories Inc., Burlingame, CA) and TMAs were incubated with 5% normal goat-horse-chicken serum for 1 hour at room temperature. Sections were incubated with rabbit monoclonal primary antibody to Nectin-45,9,10(EPR15613–68; Abcam, ab192033; 1:250) or to Trop-211 (EPR20043; Abcam, ab214488; 1:500) at 4°C overnight, followed by biotinylated secondary antibody (Vector Laboratories Inc.) and ABC reagent (Vector Laboratories Inc.). Of note, the anti-Nectin-4 antibody used differs from the Astellas and Seattle Genetics proprietary anti-Nectin-4 antibody (clone M22–321b41.112) which is not available. We utilized the publicly available alternative anti-Nectin-4 antibody employed by various other groups examining Nectin-4 in urothelial and other malignancies5,9,10. Staining was detected with stable DAB (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions. All sections were counterstained with hematoxylin and mounted with Cytoseal XYL (Richard Allan Scientific, San Diego, CA). Rabbit IgG were used as negative controls.
Staining for both membrane and cytoplasm was graded in a blinded fashion by two pathologists (FKL and MPR). For the variant histology specimens in this study, all the variants are either pure (PUC) or predominant (neuroendocrine [NE] and UC with squamous differentiation [UCSD], >50–95% variant), and only the variant component was scored. Primary and metastatic specimen had concordant histology except for in one patient, 19–022, which was pure UC primary but one of 3 metastases (H2-Adrenal) was UCSD. Signal intensity (0–3: 0 for negative stain, 1 for faint/equivocal stain, 2 for moderate stain, and 3 for intense stain) and percentage of signal coverage (0–100) of each core were scored, and the product of the intensity and coverage was represented as an H-score (0–300) as previously described8,13. Nectin-4 and Trop-2 was considered positive when the H-score is ≥ 30 and ≥ 100, respectively. Owing to tissue loss during the immunohistochemistry (IHC) process, a total of 201 cores from 67 bladder cancer specimens were evaluated. The score for each specimen was an average of triplicated cores.
RNA sequencing and analyses
Primary or metastatic specimens selected for sequencing were macro-dissected to represent greater than 80% tumor cellularity by genitourinary pathology review (FVR and MPR). RNA was extracted from 75 OCT-embedded specimens representing 31 UCC, 16 PUC, 21 UCSD, and 7 NE specimens. Briefly, RNA was extracted using RNA STAT 60 (Tel-Test, Friendswood, TX) and RNeasy Mini kit, followed by DNase digestion in solution prior to purification (Qiagen, Germantown, MD) as described before7. RNA integrity number was determined using the Agilent Bioanalyzer (Agilent, Santa Clara, CA). Total RNA (300 ng) was used to construct RNA-seq libraries using the Illumina TruSeq RNA Exome Sample Prep Kit according to the manufacturer’s protocol (Illumina, San Diego, CA). Barcoded libraries were pooled and sequenced using Illumina HiSeq 2500 system to obtain 50-bp paired-end reads (Illumina, San Diego, CA). The reads were aligned to the human hg38 using STAR v2.1.014. Gene level abundance was quantitated from the filtered human alignments in python using HTSeq-count15.
Statistical Analyses
Statistical comparisons were conducted by Mann-Whitney U test for continuous variables, and Chi-square test for categorical variables based on normality of data assessment using Shapiro-Wilk test (p<0.05 across all groups). Central tendency data are presented as medians with interquartile range (IQR). Kaplan-Meier survival curves were estimated, and log-rank tests were used to compare survival between high and low expression groups. Pearson correlation analysis was performed for all correlations between RNA and protein levels. All statistical analyses were performed using Statistical Package for the Social Sciences, Version 22.0 (IBM Corp., 2020, Armonk, NY) and figures were generated using GraphPad Prism version 9.4.0 (GraphPad Software, San Diego, CA).
Results
Patient population and specimen histology
A total of 67 specimens from 20 patients were analyzed, consisted of 27 UC, 17 plasmacytoid (PUC), 18 UC with squamous differentiation (UCSD), and 5 neuroendocrine (NE); 10 specimens were collected from primary tumor and 57 from metastases. Histology in metastases was consistent with that of the primary tumor. Thirteen of 20 patients (65%) received systemic chemotherapy and 14 (70%) received immunotherapy. Specific chemotherapy regimens can be seen in Table 1. None of the patients received EV or SG. Clinicopathologic data and biospecimen information are shown in Fig. 1A and 1B, and Supplementary Table 1.
Table 1.
Treatment information for individual bladder cancer patient in the rapid autopsy cohort.
| Patient | Gender | Race (C:Caucasian, A: Asian) | Surgery | BCG | Chemotherapy | PD-1/PD-L1-based Immunotherapy | Radiation (site) | Other treatment(s) |
|---|---|---|---|---|---|---|---|---|
| 15–108 | M | C | Y | Y | None | None | Lung | |
| 15–109 | M | C | Y | N | Gemcitabine and cisplatin (GC), Docetaxel | None | No | Rapamycin |
| 16–070 | M | C | N | N | Gemcitabine and cisplatin (GC), Docetaxel, Gemcitabine and Paclitaxel | Atezolizumab | No | OGX427–02, IMC-18F1, Pemetrexed, AGS-15E, Eribulin |
| 16–079 | F | C | N | N | Gemcitabine and carboplatin, Docetaxel | Atezolizumab | No | |
| 16–097 | F | C | Y | N | Gemcitabine and cisplatin (GC) | Atezolizumab | No | AGS-15E |
| 17–020 | M | A | N | N | Gemcitabine and carboplatin, Docetaxel | None | No | |
| 17–026 | M | C | N | N | Gemcitabine and cisplatin (GC) | None | Bladder and pubic ramus | |
| 17–030 | M | C | Y | N | Gemcitabine and cisplatin (GC) | Atezolizumab | Rapamycin, Pemetrexed | |
| 17–047 | F | C | Y | N | None | Atezolizumab | Pelvis | |
| 17–071 | M | C | Y | N | Gemcitabine and cisplatin (GC), Cisplatin | Pembrolizumab | Pelvis | |
| 18–016 | M | C | N | N | None | None | ||
| 18–065 | F | C | N | N | None | Pembrolizumab | ||
| 18–101 | M | C | N | N | Gemcitabine and cisplatin (GC) | Pembrolizumab | ||
| 18–120 | M | C | Y | N | accelerated MVAC (aMVAC) | Pembrolizumab | ||
| 19–001 | M | C | Y | N | None | Atezolizumab | ||
| 19–004 | M | C | Y | Y | None | None | Bladder | |
| 19–007 | M | C | N | N | Gemcitabine and cisplatin (GC) | Atezolizumab | Bladder, brain | |
| 19–022 | M | C | Y | Y | None | Pembrolizumab | Lung | |
| 19–037 | M | C | N | N | Etoposide and Cisplatin (EP), Paclitaxel | Pembrolizumab | Brain, spine | |
| 19–044 | F | C | Y | N | Gemcitabine and cisplatin (GC) | Atezolizumab |
M: Male, F: Female
C:Caucasian, A: Asian
Y: Yes, N: No
Fig. 1. Clinical and specimen information for the bladder cancer rapid autopsy series.
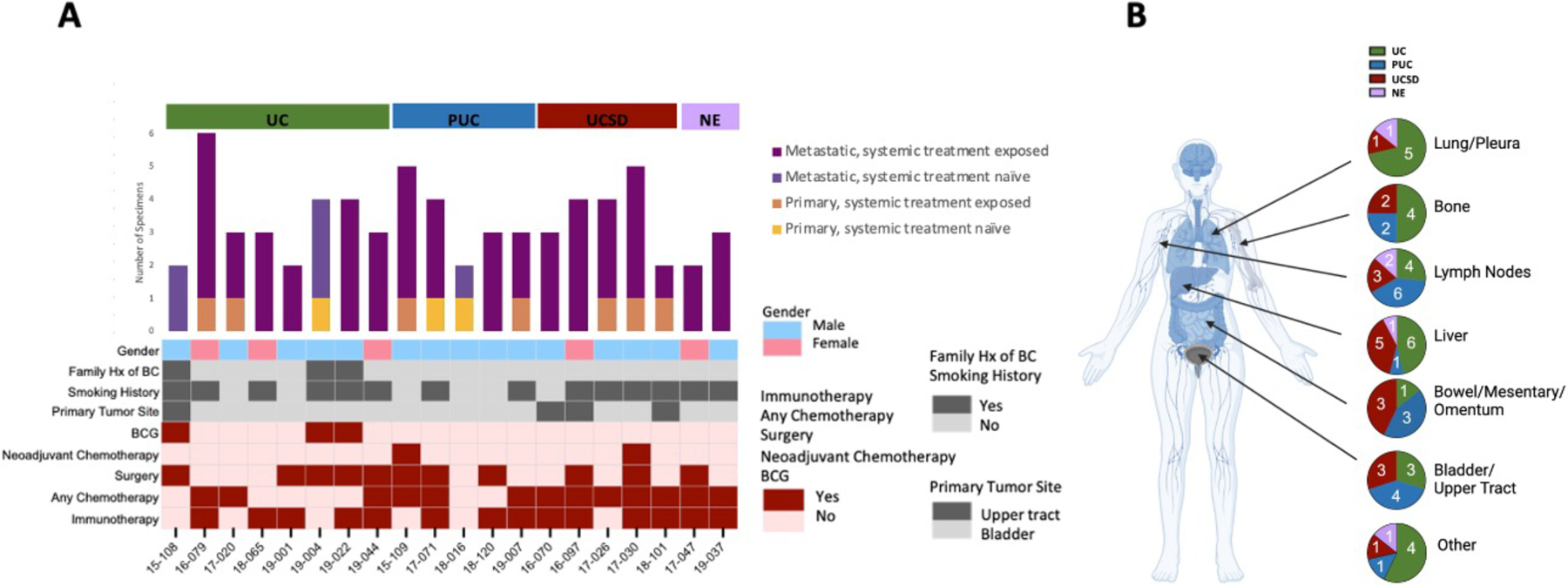
A) Demographics, treatment history, and number of specimens (n=67) analyzed for each patient. B) Tissue source and histology of specimens analyzed.
PVRL4 and TACSTD2 mRNA expression is correlated in patient specimens
We first evaluated the mRNA expression of PVRL4 (Nectin-4) and TACSTD2 (Trop-2) in UC and its variants and determine the expression heterogeneity across metastases within an individual. RNA sequencing results showed a high expression of PVRL4 and TACSTD2 in UC and histological variants PUC and UCSD, but not in NE (Fig. 2A). The high expression of both PVRL4 and TACSTD2 were largely conserved across multiple metastases within an individual showing conventional UC histology or variant histology (Fig. 2A). Interestingly, a significant positive correlation was noted for PVRL4 and TACSTD2 in each specimen (Fig. 2B and C; r = 0.81, p < 0.001).
Fig. 2. RNA expression by specimens in the bladder cancer rapid autopsy series.
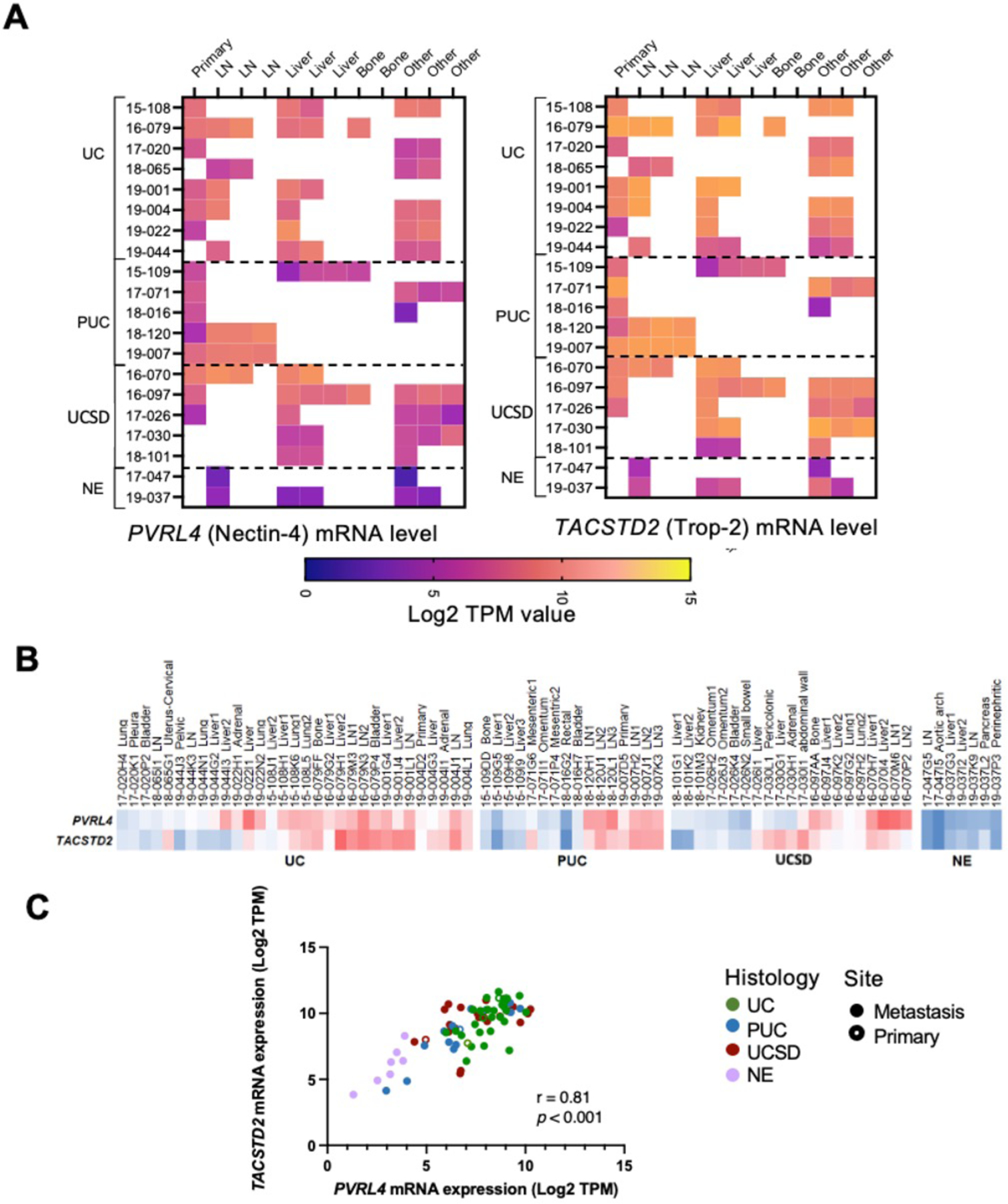
A) Absolute RNA expression represented by log2TPM (Transcripts Per Million) value for PVRL4 (Nectin-4) and TACSTD2 (Trop-2). LN denotes lymph node, ‘other’ includes lung, pleura, omentum, mesentery, bowel, vasculature, adrenal gland, pancreas, and abdominal wall. B) Mean-centered RNA expression comparison among the 76 specimens from A). Red denotes higher gene expression; blue denotes lower gene expression. C) Correlation analysis of PVRL4 and TACSTD2 with Pearson correlation coefficient, r = 0.81, p < 0.001.
Cytoplasmic Nectin-4 and Trop-2 is prominent in UC and its variants
Next, we assessed the membrane protein expression for Nectin-4 and Trop-2 based on their approved utility as targets for ADCs. Pathological evaluation noted a remarkable cytoplasmic presence of both Nectin-4 and Trop-2, in addition to their anticipated membranous localization. As such, all specimens were independent evaluated by our GU pathologists for membrane and cytoplasmic staining separately. IHC staining for Nectin-4 and Trop-2 proteins revealed both prominent membranous and cytoplasmic staining in UC and its variants, except NE (Fig. 3A–B, Supplementary Fig. 1A). Nectin-4 was expressed heterogeneously and at moderate levels in conventional UC samples (Fig. 3B), with modest enrichment in the cytoplasm (56%) compared to the membrane (30%, p = 0.03; Fig. 3B and Fig. 4A). However, UC variants PUC and UCSD demonstrated significantly enriched cytoplasmic staining (cNectin-4) when compared to membranous (mNectin-4; PUC: 94% cytoplasmic vs. 6% membranous, p < 0.001; UCSD: 83% cytoplasmic vs. 44% membranous, p = 0.01; Fig. 3A–B and Fig. 4A). NE had no detectable Nectin-4 (Fig. 3A–B and Fig 4A).
Fig. 3. Nectin-4 and Trop-2 expression in primary and metastatic BC variants.
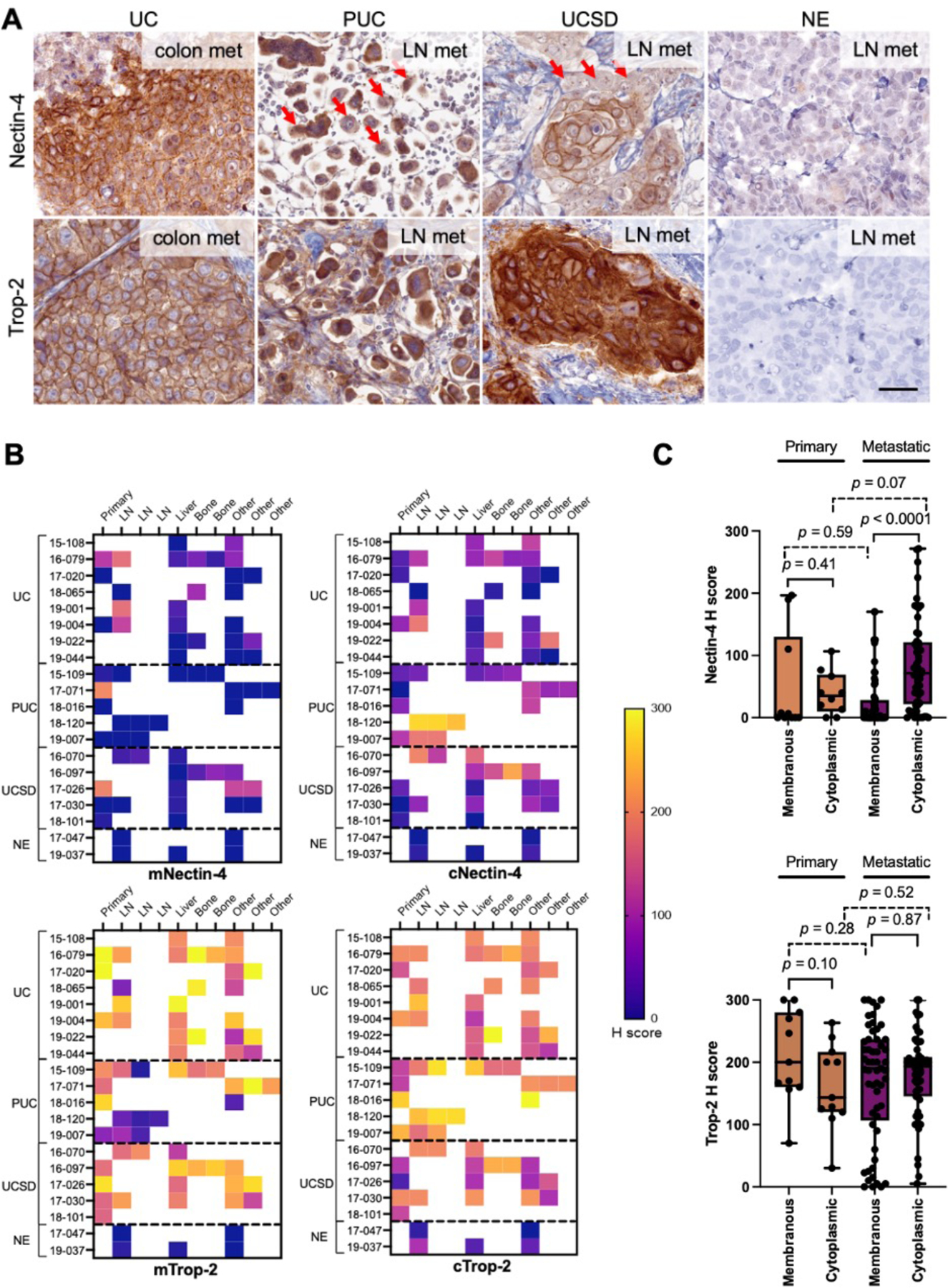
A) Representative Nectin-4 and Trop-2 IHC images by histology. Both Nectin-4 and Trop-2 showed prominent membranous and cytoplasmic staining in non-NE variants. In PUC and UCSD, cells with predominantly cytoplasmic staining were noted for Nectin-4 (red arrows). Magnification:400×, scale bar = 50μm. B) H score by specimen (n=67), separated for membranous and cytoplasmic staining. LN denotes lymph node, ‘other’ includes lung, pleura, omentum, mesentery, bowel, vasculature, adrenal gland, pancreas, and abdominal wall. C) Box-and-whisker plot of primary and metastatic H scores, separated by membranous and cytoplasmic subcellular localization (n=67 each). The P value from Mann-Whitney U test was shown.
Fig. 4. Protein expression level and box-and-whisker plot for each histology and specimen site for A) Nectin-4 and B) Trop-2.
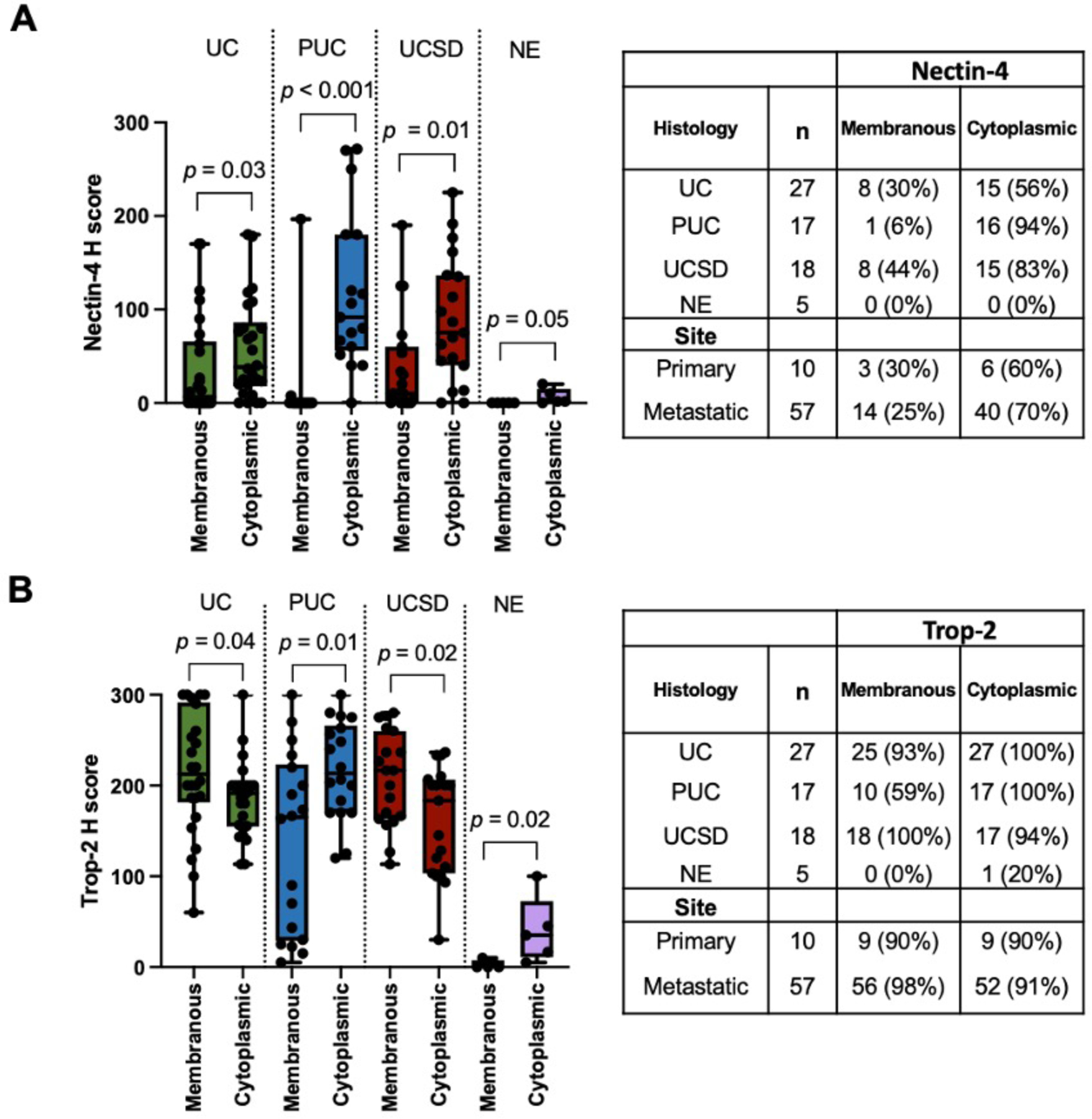
The P value from Mann-Whitney U test is shown in the box-and-whisker plot.
Trop-2 was detected at high levels both on the membrane and within the cytoplasm in UC and its histologic variants, except for NE (Fig. 3A–B and Fig. 4B). Despite a few of the histological variant PUC showed very weak membrane Trop-2 (mTrop-2) staining at metastases, most specimens (UC and its variants) showed moderate – high level of mTrop-2 staining (Fig. 3B–C), suggesting Trop-2 is a relatively conserved membrane target for UC from primary to metastasis.
Correlation of Nectin-4 and Trop-2 mRNA and protein levels
In view of the notable membrane and cytoplasmic protein expression of both NEctin-4 and Trop-2, we questioned whether mRNA was useful in predicting localization of the proteins. For Nectin-4, we observed the PVRL4 mRNA level was significantly correlated with cNectin-4 (Supplementary Fig. 2A; r = 0.55, p < 0.001) but not with mNectin-4 protein (r = 0.10, p = 0.45). For Trop-2, TACSTD2 mRNA level was significantly correlated with both membranous Trop-2 (mTrop-2; Supplementary Fig. 2A; r = 0.36, p = 0.01) and cytoplasmic Trop-2 protein (cTrop-2; r = 0.42, p = 0.002). These correlations, despite statistically significant, are of moderate strength. Interestingly, a significant positive correlation between mNectin-4 and mTrop-2 (Supplementary Fig 2B; r = 0.42, p = 0.002) and cNectin-4 and cTrop-2 (r = 0.59, p < 0.001) was observed.
Nectin-4 and Trop-2 protein expression in UC metastases
In query of Nectin-4 and Trop-2 protein expression in metastatic tumors, we observed similar levels in metastatic compared to primary sites, though Nectin-4 was enriched in the cytoplasm in metastases (Fig. 3C and Fig. 4A, p<0.0001). Trop-2 was highly expressed in primary and metastatic sites (Fig. 3B–C and Fig. 4B), except for NE histology. Despite the small sample size, we found no significant difference in Nectin-4 or Trop-2 protein expression in BC vs upper tract UC (data not shown), and in specimens post-chemotherapy or immunotherapy (vs naïve; Supplementary Fig. 3).
Discussion
Our study reports for the first time prominent cytoplasmic staining of both Nectin-4 and Trop-2, in addition to their anticipated membrane staining in UC. Specifically, Nectin-4 is enriched in the cytoplasm within non-NE histologic variants in BC, especially within metastatic specimens. Trop-2 proteins (membrane and cytoplasmic) are expressed at moderate-high levels in both primary and metastatic specimens within UC and non-NE histologic variants. These data support prospective trials of ADC targeting Nectin-4 and Trop-2 in mUC variant histology.
Nectin-4 and Trop-2 protein expression have been reported in primary UC and its variants, as well as some metastatic UC. Nectin-4 expression is high/moderate in 60% of primary UC16 and its expression is heterogeneous in primary UC histologic variants5,6; whereas Trop-2 is expressed at high level in most primary UC17. However, these important observations on clinical specimens were exclusively focused on total protein staining. Although Nectin-4 protein expression was detected for all UC specimens available in the EV-201 trial, the objective response rate (ORR) is 44–52%12,18. Likewise, Trop-2 is thought to be commonly and highly expressed in UC19 and therefore staining was not included as an inclusion criteria for TROPHY-U-01 trial, where the ORR is only 27%3. These results demonstrating total target protein expression (without differentiating membrane or cytoplasm) argue many of the patients expressing Nectin-4 or Trop-2 did not respond to ADCs. This discordance between abundant target expression and limited therapeutic response of ADCs raised questions regarding the subcellular localization of these otherwise membrane protein targets.
In our study, cytoplasmic staining of Nectin-4 and Trop-2 is a common event in addition to the anticipated membrane staining. A recent report found that low mNectin-4 was associated with reduced progression-free survival on EV20, supporting the relevance of confirming membrane presence of the protein. The same study reported a decrease in mNectin-4 within metastatic specimens compared to primary20. However, we observed the expression of both Nectin-4 and Trop-2 are largely conserved from primary to multiple metastatic sites within an individual in patients within conventional UC and non-NE variants. Additionally, despite the heavily treated nature in this cohort, Nectin-4 and Trop-2 protein expression did not appear to be impacted by prior chemotherapy or immune checkpoint inhibitor (ICI) exposure.
The clinical implications of subcellular localization of ADC targets have been scantly described in other malignancies. One study identified primarily cytoplasmic localization of Nectin-4 in hepatocellular carcinoma, which is associated with worse progression-free and overall survival21. In contrast, mNectin-4 expression predicted a shorter disease-free and metastasis-free survival compared to cNectin-4 expression in patients with breast cancer22. A study in breast cancer identified that mTrop-2 was associated with a significantly higher risk of relapse and death23; and loss of mTrop-2 was associated with SG resistance in triple-negative breast cancer24. In our limited cohort, we did not observe differences in overall survival based on localization of either protein (data not shown) and none of our patients received either ADCs.
Previous studies have focused on primary UC and its variants, and our study brings in perspectives on Nectin-4 and Trop-2 expression in more aggressive metastatic variants which treatments are essentially non-existing. While UC and UCSD has reasonable levels of mNectin-4 staining, we find the mNectin-4 staining within PUC is rare. Conversely, Trop-2 is a more commonly (over 90%) expressed targets in both membrane and cytoplasm for both UC and UCSD, and was robustly observed in PUC (membrane 59% and cytoplasmic 100%). In view of the prominent cytoplasmic staining for both Nectin-4 and Trop-2, it may be reasonable to ascertain the presence of membrane staining before commencing ADC therapy and evaluate the biological implication for cytoplasmic retention of the protein.
Our study reports strong correlation between Nectin-4 and Trop-2 expression at both mRNA and protein levels. Clinically, responses with SG were noted after progression on EV in advanced UC3. As such, studies evaluating the combination (NCT04724018) or the optimal sequence of ADCs are of clinical importance. Our study showed mRNA levels were associated with cNectin-4, but weak surrogates for mNectin-4. In light of the potential importance of mNectin-4 on durability of EV response20, it is logical to evaluate membrane presence of Nectin-4, rather than mRNA levels, within ongoing trials.
Our study is limited by the small sample size which prevents robust clinical correlations and comparison across different metastatic sites. We did not observe significant differences based on tumor site (BC vs UTUC), chemotherapy, and immunotherapy, yet larger sample sizes are needed to confirm this. While our research identifies prominent cytoplasmic staining of both Nectin-4 and Trop-2, the biological significance of such enrichment or retention remains to be evaluated. It would be clinically important to observe if the loss of membrane staining (or the retention of cytoplasmic staining), not the mRNA level in general, is associated with treatment resistance.
To our knowledge, this unique rapid autopsy cohort represents a comprehensive mRNA and protein expression, and subcellular localization of novel ADC targets in matched primary and metastatic UC and its variants. Nectin-4 and Trop-2 are expressed within UC and non-NE variants and correlate strongly with each other at both mRNA and protein levels. PUC and UCSD were enriched with cytoplasmic Nectin-4, especially in metastatic sites. Trop-2 was expressed highly in both the membrane and cytoplasm in UC and non-NE variants. The clinical impact of Nectin-4 and Trop-2 localization and expression warrants further investigation. Taken together, the moderate to high expression of Nectin-4 and Trop-2 in variant histology BC (except in NE type), at both primary and metastatic sites, provides evidence supporting clinical trials evaluating ADCs in BC variants.
Supplementary Material
Supplementary Fig. 1. Representative Nectin-4 and Trop-2 IHC images by histology showing homogeneous high expression of Nectin-4 and Trop-2 proteins in the cancer cells in non-NE variants. Magnification: 200×.
Supplementary Fig. 2. A) Correlation plots between mRNA and protein expression of Nectin-4 and Trop-2 showing a significant positive correlation between mRNA and protein expression for both proteins. B) A correlation matrix heatmap showing a significant positive correlation between mNectin-4 and mTrop-2, and cNectin-4 and cTrop-2.
Supplementary Fig. 3. Box-and-whisker plot showing the impact of systemic chemo on the protein level of A) Nectin-4 and B) Trop-2. The impact of immunotherapy on C) Nectin-4 and D) Trop-2 is also illustrated. The P value from Mann-Whitney U test is shown.
Supplementary Table 1. Survival information for the rapid autopsy cohort.
Acknowledgements
We are grateful to the patients and their families who participated in this study. We thank the support of the University of Washington Genitourinary Cancer Research Laboratory and the rapid autopsy team, and the technical support by Ms. Yan Wang for the immunohistochemistry study. The work was supported by Seattle Translational Tumor Research, Howard Cohen Foundation, and Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation. HML is a recipient of an Idea Development Award from the Department of Defense (W81XWH-19-1-0624) and Imagination Award from the Kuni Foundation. MPR is a recipient of a research scholarship from the Institute of Prostate Cancer Research.
Financial support:
The work was supported by Seattle Translational Tumor Research, Howard Cohen Foundation, and Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation. HML is a recipient of an Idea Development Award from the Department of Defense (W81XWH-19-1-0624) and Imagination Award from the Kuni Foundation. MPR is a recipient of a research scholarship from the Institute of Prostate Cancer Research.
Conflict of interest:
Evan Yu:
Institutional grants: Daiichi-Sankyo, Taiho, Dendreon, Merck, Seattle Genetics, Blue Earth,,Bayer - DAROL and citDNA, Lantheus
Consulting with honorarium (in the last 3 years): Jansen, Merck, Advanced Accelerator Applications, Bayer, Exelixis, Clovis, Abbvie, Sanofi-Genzyme
Funda Vakar-Lopez:
AstraZeneca - Virtual Advisory Board Member on metastatic urothelial cancer
Petros Grivas:
Institutional grants: Bavarian Nordic; Bristol-Myers Squibb; Clovis Oncology; Debiopharm; Merck KGaA; Gilead; Pfizer; MSD; QED Therapeutics; GlaxoSmithKline; G1 Therapeutics; Mirati TherapeuticsConsulting: Aadi Bioscience; AstraZeneca; Asieris Pharmaceuticals, Astellas Pharma, BMS; Boston Gene, CG Oncology, Dyania Health; Exelixis; Lucence Health; Fresenius Kabi, G1 Therapeutics; Gilead; Guardant Health; ImmunityBio; Infinity Pharmaceuticals; Janssen; Merck KGaA; Mirati Therapeutics; MSD; Genentech/Roche; Pfizer; PureTech; Regeneron Pharmaceuticals; QED Therapeutics; Seattle Genetics, Strata Oncology, 4D Pharma PLC, UroGen, Silverback Therapeutics
Bruce Montgomery:
Institutional grants: AstraZeneca, Janssen Oncology, Clovis Oncology, Astellas Pharma, BeiGene
John K. Lee:
Institutional grants: Immunomedics
Michael Schweizer:
Institutional grants: Zenith Epigenetics, Bristol Myers Squibb, Merck, Immunomedics, Janssen, AstraZeneca, Pfizer, Madison Vaccines, Hoffman-La Roche, Tmunity, SignalOne Bio and Ambrx, Inc.
Paid consultant and/or received Honoria: Sanofi, AstraZeneca, PharmaIn and Resverlogix.
Jonathan Wright
Institutional grants: Merck, Veracyte, Pacific Edge, Janssen, Nucleix.
Consultant: Immunity Bio
Royalties: UpToDate
Other authors declare no potential conflicts of interest.
References
- 1.Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review. JAMA. 2020;324(19):1980–1991. doi: 10.1001/jama.2020.17598 [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2019;37(29):2592–2600. doi: 10.1200/JCO.19.01140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol. 2021;39(22):2474–2485. doi: 10.1200/JCO.20.03489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi W, Lombardo K, Patel S, et al. A Molecular Inquiry into the Role of Antibody-Drug Conjugates in Bacillus Calmette-Guérin-exposed Non-muscle-invasive Bladder Cancer. Eur Urol. Published online November 1, 2021:S0302–2838(21)02077–7. doi: 10.1016/j.eururo.2021.10.009 [DOI] [PubMed] [Google Scholar]
- 5.Hoffman-Censits JH, Lombardo KA, Parimi V, et al. Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes. Appl Immunohistochem Mol Morphol. 2021;29(8):619–625. doi: 10.1097/PAI.0000000000000938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wucherpfennig S, Rose M, Maurer A, et al. Evaluation of Therapeutic Targets in Histological Subtypes of Bladder Cancer. Int J Mol Sci. 2021;22(21):11547. doi: 10.3390/ijms222111547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winters BR, De Sarkar N, Arora S, et al. Genomic distinctions between metastatic lower and upper tract urothelial carcinoma revealed through rapid autopsy. JCI Insight. 4(13):e128728. doi: 10.1172/jci.insight.128728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winters BR, Vakar-Lopez F, Brown L, et al. Mechanistic target of rapamycin (MTOR) protein expression in the tumor and its microenvironment correlates with more aggressive pathology at cystectomy. Urol Oncol. 2018;36(7):342.e7–342.e14. doi: 10.1016/j.urolonc.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 9.Choi W, Lombardo K, Patel S, et al. A Molecular Inquiry into the Role of Antibody-Drug Conjugates in Bacillus Calmette-Guérin-exposed Non–muscle-invasive Bladder Cancer. European Urology. 2022;81(2):138–142. doi: 10.1016/j.eururo.2021.10.009 [DOI] [PubMed] [Google Scholar]
- 10.Teo MY, Rosenberg JE. NECTIN4 Heterogeneity and Molecular Diversity in Bladder Cancers: Deconstructing the Activity of An Antibody-Drug Conjugate. Clin Cancer Res. 2021;27(18):4950–4952. doi: 10.1158/1078-0432.CCR-21-1807 [DOI] [PubMed] [Google Scholar]
- 11.Chou J, Trepka K, Sjöström M, et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur Urol Oncol. Published online February 22, 2022:S2588–9311(21)00215–7. doi: 10.1016/j.euo.2021.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J Clin Oncol. 2020;38(10):1041–1049. doi: 10.1200/JCO.19.02044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam HM, Nguyen HM, Labrecque MP, et al. Durable Response of Enzalutamide-resistant Prostate Cancer to Supraphysiological Testosterone Is Associated with a Multifaceted Growth Suppression and Impaired DNA Damage Response Transcriptomic Program in Patient-derived Xenografts. Eur Urol. 2020;77(2):144–155. doi: 10.1016/j.eururo.2019.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016;76(10):3003–3013. doi: 10.1158/0008-5472.CAN-15-1313 [DOI] [PubMed] [Google Scholar]
- 17.Stepan LP, Trueblood ES, Hale K, Babcook J, Borges L, Sutherland CL. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem. 2011;59(7):701–710. doi: 10.1369/0022155411410430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu EY, Petrylak DP, O’Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):872–882. doi: 10.1016/S1470-2045(21)00094-2 [DOI] [PubMed] [Google Scholar]
- 19.Avellini C, Licini C, Lazzarini R, et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget. 2017;8(35):58642–58653. doi: 10.18632/oncotarget.17407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klümper N, Ralser DJ, Ellinger J, et al. Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clinical Cancer Research. Published online December 19, 2022:CCR-22–1764. doi: 10.1158/1078-0432.CCR-22-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Sheng Z, Lv Y, et al. Expression and clinical significance of Nectin-4 in hepatocellular carcinoma. Onco Targets Ther. 2016;9:183–190. doi: 10.2147/OTT.S96999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lattanzio R, Ghasemi R, Brancati F, et al. Membranous Nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis. 2014;3(9):e118. doi: 10.1038/oncsis.2014.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrogi F, Fornili M, Boracchi P, et al. Trop-2 Is a Determinant of Breast Cancer Survival. PLoS One. 2014;9(5):e96993. doi: 10.1371/journal.pone.0096993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coates JT, Sun S, Leshchiner I, et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov. 2021;11(10):2436–2445. doi: 10.1158/2159-8290.CD-21-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Representative Nectin-4 and Trop-2 IHC images by histology showing homogeneous high expression of Nectin-4 and Trop-2 proteins in the cancer cells in non-NE variants. Magnification: 200×.
Supplementary Fig. 2. A) Correlation plots between mRNA and protein expression of Nectin-4 and Trop-2 showing a significant positive correlation between mRNA and protein expression for both proteins. B) A correlation matrix heatmap showing a significant positive correlation between mNectin-4 and mTrop-2, and cNectin-4 and cTrop-2.
Supplementary Fig. 3. Box-and-whisker plot showing the impact of systemic chemo on the protein level of A) Nectin-4 and B) Trop-2. The impact of immunotherapy on C) Nectin-4 and D) Trop-2 is also illustrated. The P value from Mann-Whitney U test is shown.
Supplementary Table 1. Survival information for the rapid autopsy cohort.


