ABSTRACT
Background
Burn injuries, particularly extensive severe burns, often have a fatal prognosis. However, many prognostic predictors are based on changes in the clinical course of treatment, and no prognostic predictors can be estimated in the early phases of injury. Using the Burn Index (BI) for evaluations requires familiarity with daily burn treatment, such as being able to evaluate the change from a second degree burn to a third degree burn appropriately. We sought to find a simpler and more quantitative prognostic prediction index.
Methods We hypothesized that, in addition to the current prognostic predictors, the number of neutrophils in severe burns may correlate with the prognosis, and analyzed its usefulness. The neutrophil and white blood cell counts were measured within 48 hours of injury in 35 burn patients who required inpatient treatment at our own institution. Mann–Whitney test was used to determine the significant of differences between the Survivor and Non-survivor groups.
Results
Compared to the Survivor group, neutrophil (P = 0.038) and white blood cell counts were increased significantly in the Non-survivor group (P = 0.004). Neutrophil counts and white blood cell counts correlated positively with the length of hospital stay, total body surface area, Prognostic Burn Index (PBI), and BI. The BI and PBI correlated with patient prognosis, as did neutrophil and white blood cell counts.
Conclusion
These results suggested that neutrophil and white blood cell counts in the early phases of burn injuries might be another factor in the prognosis of burn patients in addition to the current predictors.
Keywords: early injury, extensive burns, simplified prognostic indicators
According to a 2020 Ministry of Health, Labor, and Welfare patient survey, the number of burn patients in Japan is estimated to be about 5,100 per year. Of these, about 600 patients are hospitalized, and the hospitalization rate in terms of the number of injured patients amounted to about11.8%.1 Artz’s criterion2 is known as the standard for defining severe burns, but in the Japanese Burn Guidelines, prognostic factors are only listed based on expert opinions, and the level of evidence is not high.3 The American Burn Association’s Advanced Burn Life Support recommends referral to a burn center for second degree burns of 10% or more of the body surface area4 and early transfer to a burn center. In the event of a large-scale disaster, many burn patients will present at the same time. In such a scenario, it will be necessary to select a hospital and disperse transportation in anticipation of the prognosis.
Previous studies have shown that the extent of the burn injury as a percentage of the total body surface area of the patient (TBSA), Burn Index (BI), Prognostic Burn Index (PBI), white blood cell count, serum albumin level, lactate dehydrogenase level, and total cholesterol level are useful predictors of in-hospital death in burn patients.5 As other prognostic methods for burn patients, both the Revised Baux score (rBaux score) and the Abbreviated Burn Severity Index (ABSI) have been reported to be useful for predicting mortality, length of hospital stay, and length of intensive care unit (ICU) stay in patients with severe burns.6 Kaita et al. also reported that PBI was an excellent prognostic indicator, and that PBI > 105 was associated with deaths in severe burn patients in specialized burn care facilities.7 In order to evaluate the BI, familiarity with burn treatment on a regular basis is required. Therefore, we sought to find a simpler and more quantitative prognostic index that could be used in addition to the current prognostic predictors. We hypothesized that neutrophil counts in severe burn patients may correlate with prognosis, and here evaluated this hypothesis.
MATERIALS AND METHODS
This study was approved by the Clinical Ethics Committee of Tottori University Faculty of Medicine (SDS No. 22A026). Consent from patients was obtained by opt-out. Among the patients admitted to the Emergency and Critical Care Center of Tottori University Hospital between March 1, 2020, and August 31, 2022, 39 patients (23 men and 16 women) with a “burn” disease diagnosis were included.
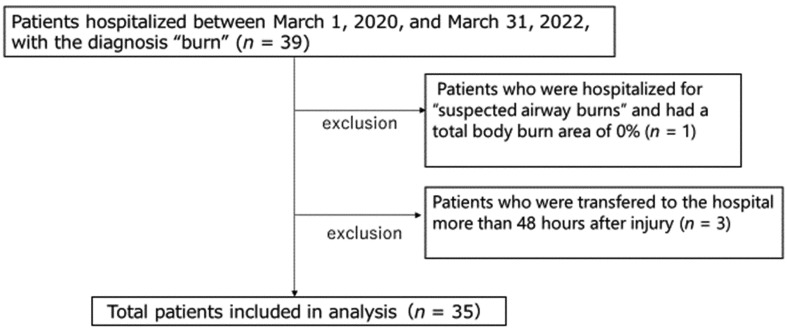
As shown in Fig. 1, one of the 39 patients was hospitalized with “suspected respiratory tract burns only” and TBSA was 0%; thus, this patient was excluded. In addition, three patients were excluded because they came to the hospital more than 48 hours after the injury. In addition, 12 patients developed sepsis and 15 patients required surgical intervention. Of the 35 entries, three patients died; all were male. The causes of death were fatal arrhythmia due to hyperkalemia, acute renal failure, and sepsis, respectively. BI and PBI were significantly higher in the death cases, as shown in Table 1, which was similar to previous reports.
Fig. 1.
Enrollment and screening of burn patients.
Table 1. Characteristics of patients (survivor and non-survivor).
| Outcome | Survivors | Non-survivors | P value |
| Number of patients males, females |
18, 14 | 3, 0 | |
| Mean Age (years) | 66 | 78 | 0.105 |
| Oldest Age (years) | 96 | 87 | |
| Youngest Age (years) | 0 | 65 | |
| Median Age (years) | 68 | 82 | |
| BI (mean) | 11.844 | 44.83 | |
| BI (median) | 5.25 | 41 | |
| PBI (mean) | 78 | 123 | |
| PBI (median) | 87.625 | 123 |
Burn patients hospitalized within 48 hours of injury (n = 35) from March 1, 2020, to March 31, 2022. BI, Burn Index; PBI, Prognostic Burn Index.
We investigated the significance of differences in the number of white blood cells and neutrophils (pcs) measured with a SYSMEX XN3000 (Kobe City, Japan), between the Survivor and Non-survivor groups, using the Mann–Whitney U test. P < 0.05 rejected the null hypothesis. In addition, we used Spearman’s rank correlation coefficient to examine the correlations of BI, PBI, TBSA, and hospital days with white blood cell counts and neutrophil counts.
In a previous study, Ueda et al. reported that the NSCORE (defined by sex and neutrophil count) was a useful prognostic predictor of sepsis.8 We therefore investigated whether the NSCORE and neutrophil score; which is a score used for patients with severe sepsis or septic shock. If the neutrophil count is 0~4999/mm3, the score is 3 points; if the neutrophil count is 5000~9999/mm3, the score is 1 point; and if the count is 10000/mm3 or more, the number of cells divided by 10000 is the score. This correlates with the APACHE II and SOFA scores. It is significantly higher in the mortality group than in the survival group of patients with severe sepsis and septic shock, with a cut-off value of 3.8, a sensitivity of 61.5%, and a specificity of 80.4%. It could be prognostic predictors of outcomes in burn patients.
In addition, the number of white blood cells and neutrophils at the time of hospital visit was also evaluated, using the Mann–Whitney test, to determine whether this could be a predictor of the onset of sepsis and the need for surgical intervention.
Statistical analyses were performed using EZR (Saitama, Japan).
RESULTS
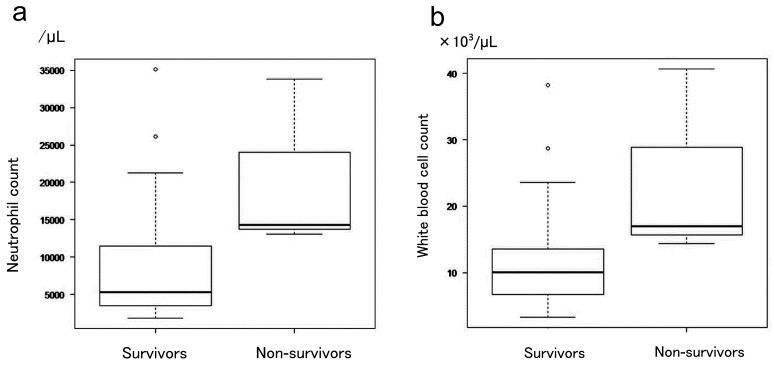
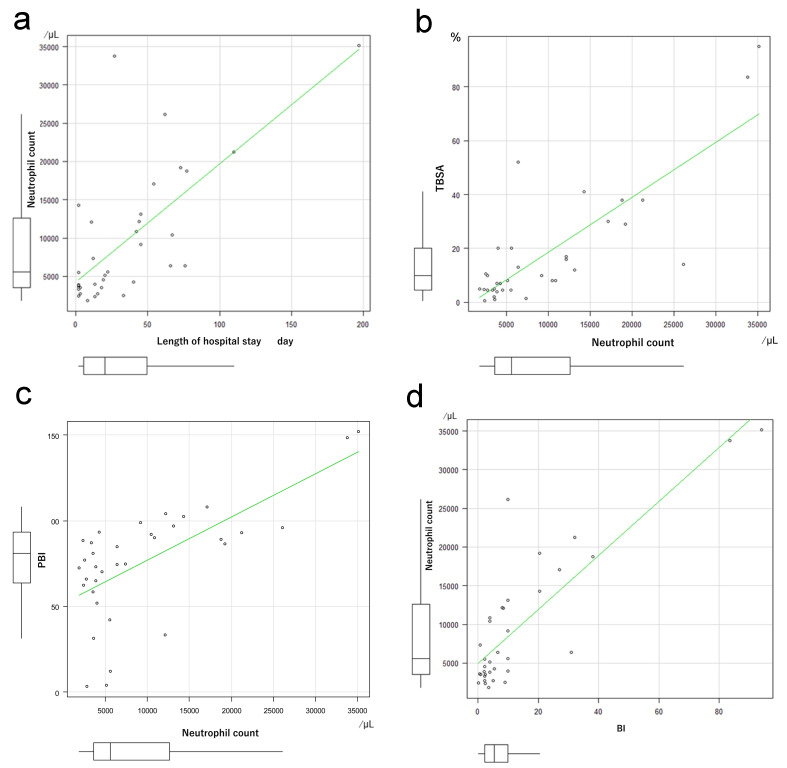
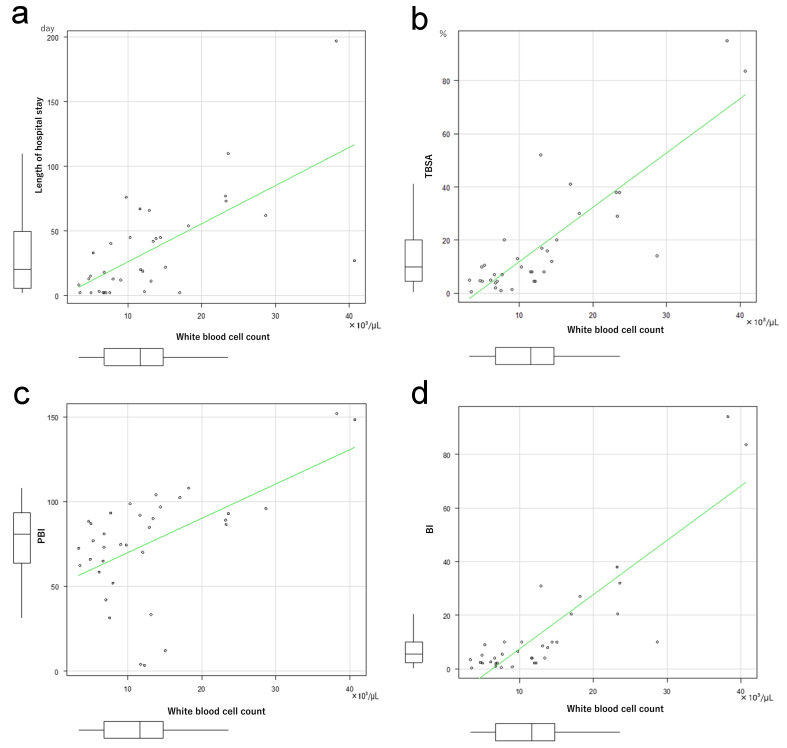
The number of neutrophils only among the total white blood cells, and the number of white blood cells per se on the day of admission were analyzed to assess the significance of differences between the Survivor and Non-survivor groups. The number of neutrophils was significantly higher in the Non-survivor than in the Survivor group (P = 0.038, Fig. 2a). The number of white blood cells was also increased significantly in the Non-survivor group (P = 0.004, Fig. 2b). Neutrophil count correlated with the length of hospital stay (Fig. 3a), TBSA (Fig. 3b), PBI (Fig. 3c), and BI (Fig. 3d). Similarly, the number of white blood cells correlated with the number of days spent in hospital (Fig. 4a), TBSA (Fig. 4b), PBI (Fig. 4c), and BI (Fig. 4d). In particular, the neutrophil count had a correlation coefficient of 0.6 or more for all items, indicating a strong correlation.
Fig. 2.
a: Significant difference in the neutrophil count between the surviving and deceased groups. b: Significant difference in the white blood cell count between the surviving and deceased groups. The deceased group demonstrated a significant increase in white blood cell counts.
Fig. 3.
a: Coefficient of correlation between neutrophil count and length of hospital stay. Spearman’s rank correlation coefficient: 0.651, P = 0.00002. b: Coefficient of correlation between neutrophil count and total body surface area (TBSA). Spearman’s rank correlation coefficient: 0.726, P = 0.0000008. c: Coefficient of correlation between neutrophil count and Prognotic Burn Index (PBI). Spearman’s rank correlation coefficient: 0.62, P = 0.0001 d: Coefficient of correlation between neutrophil count and Burn Index (BI). Spearman’s rank correlation coefficient: 0.731, P = 0.0000006.
Fig. 4.
a: Coefficient of correlation between white blood cell count and length of hospital stay. Spearman’s rank correlation coefficient: 0.637, P = 0.00004. b: Coefficient of correlation between white blood cell count and total body surface area % (TBSA). Spearman’s rank correlation coefficient: 0.745, P = 0.0000003. c: Coefficient of correlation between white blood cell count and Prognotic Burn Index (PBI). Spearman’s rank correlation coefficient: 0.494, P = 0.003. d: Coefficient of correlation between neutrophil count and Burn Index (BI). Spearman’s rank correlation coefficient: 0.741, P = 0.0000004.
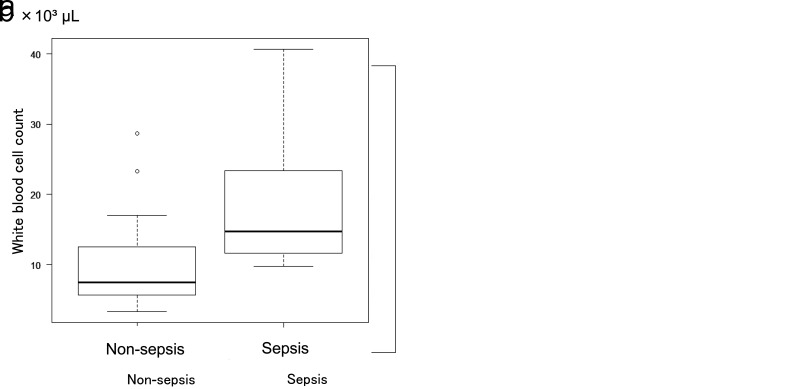
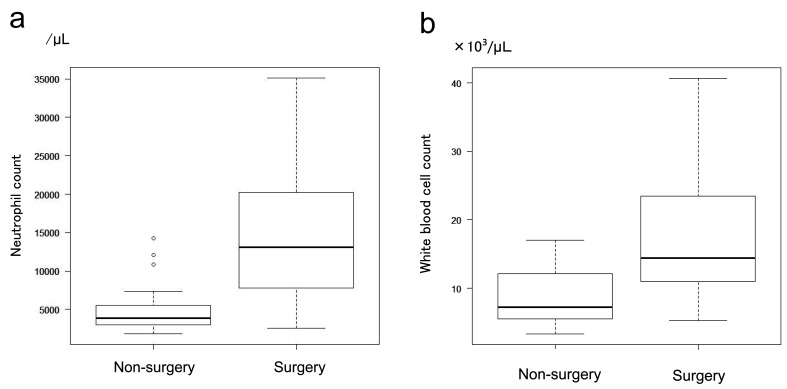
On the other hand, Spearman’s rank correlation coefficient between neutrophil count and the NSCORE was –0.29 (P = 0.091), and that between white blood cell count and the NSCORE was –0.226 (P = 0.191); thus, no significant correlations were observed. In addition, neutrophil counts were significantly higher in patients with than in those without sepsis (Fig. 5a). The number of white blood cells was also significantly higher in patients with than in those without sepsis (Fig. 5b). Neutrophil counts (Fig. 6a) and white blood cell counts (Fig. 6b) were significantly higher in patients who did not undergo surgery. Significant differences in age between the non-survival and survival groups were examined, and the white blood cell and neutrophil counts were also analyzed using Welch’s T-test.
Fig. 5.
a: Coefficient of correlation neutrophil count and sepsis complications. The number of neutrophils was significantly higher in the group with sepsis at P = 0.0006. b: Correlation between white blood cell count and sepsis complications. The white blood cell count was significantly higher in the group with sepsis at P = 0.001.
Fig. 6.
a: Correlation between neutrophil count and surgical intervention. Neutrophil counts were significantly higher in the operative intervention group (P = 0.0001). b: Correlation between white blood cell count and operative intervention. The white blood cell count was high in the operative intervention group with P = 0.001.
Both comparisons showed a P-value > 0.05, and no significant difference was observed regarding age. The underlying health conditions in the non-survival group were hypertension and abnormal glucose tolerance that did not require insulin, but there were no major underlying health conditions such as chronic renal failure or dialysis induction.
DISCUSSION
In our study, the BI and PBI correlated with patient prognosis, as previously reported.7
Neutrophil count and white blood cell count correlated with patient outcomes, similar to the BI and PBI. This suggests that neutrophil count and white blood cell count might be another factor in the prognosis of burn patients in addition to the current predictors. Death in burn patients has various causes, such as hypovolemic shock, respiratory failure, infection, and multiple organ failure. There are reports that syndecan-1 correlates with the development of abdominal compartment syndrome,9 and the onset of disseminated intravascular coagulation, antithrombin, protein S, plasminogen activator inhibitor-1, SOFA scores on the 3rd day after injury, and thrombin on the 7th day after injury, and reported that the antithrombin complex was positively correlated with ICU mortality.10
We must investigate the prognostic factors because these reports showed that changes in blood parameters and severity scores over time do not facilitate initial care or assessment early after injury, as the prognosis can only be evaluated from the degree of change in test item values. Therefore, since poor prognoses can only be predicted after and during intensive care intervention, time may be lost in confirming a likely poor prognosis, which may impact survival.
We focused on the number of neutrophils in the early stages of injury because this can be easily tested even in university hospitals and community hospitals that are not high-level medical institutions. It has been reported that burn injury typically occurs at night, between 6 p.m. and 10 p.m.,11 and since the generalized neutrophil count can be tested even during night hours, we considered that a prognostic estimation based on the number of neutrophils could be used by any medical institution. Although the APACHE II score, which currently applied all over the world, is useful for prognostic measures in critically ill patients, it requires parameters observed for 24 hours after admission.12 Osuka et al. report that the severity can be predicted by a decrease in white blood cell and platelet counts two to three days after injury. In this case, as with the APACHE II score, it is not possible to predict within 24 hours of injury, and there is a possibility that it will be affected by complications such as infection and sepsis.9 The white blood cell and neutrophil counts proposed by us are less susceptible to these complications because they are evaluated immediately after injury, and we think that it is useful for predicting the severity before treatment.
In addition, some reports have described prognostic measures using the APACHE II score in combination with urokinase plasminogen activator receptors,13 but both of these are over time and specialized tests, and it is difficult to test them in many hospitals. To resolve these problems, Ueda et al. proposed a new simplified scoring system (the NSCORE) using white blood cell and neutrophil counts. Although the NSCORE is calculated based only on sex and neutrophil count, it correlated with the APACHE II score and the SOFA score; thus, it was proposed to be a scoring system that could easily estimate mortality related to sepsis and septic shock. In the APACHE II score, SOFA score, etc., the higher the score, the more severe is the condition, but it is considered difficult to use the NSCORE for severity assessment because the condition may be severe whether the number of neutrophils is small or large. However, Ueda et al. succeeded in developing a simple scoring system by scoring neutrophil counts and adding sex factors. The survival rate of sepsis patients is higher in women than in men,14,15,16 and this difference was scored by adding +2 points for men and +1 points for women.8 In our study, all non-survivors were male, indicating that it is possible that being male is a poor prognostic factor. However, Sakuma et al. reported that sex is not a poor prognostic factor.17 The assessment of severity taking into account sex differences may require further research.
Neutrophils phagocytose bacteria, and digest them in the phagosome. The cytoplasmic granules of neutrophils contains enzymes, such as neutrophil elastase, cathepsin G, and myeloperoxidase. Excessive release of these enzymes and reactive oxygen groups outside of the cell by activation of cytokines and adhesion molecules (TNFα, interleukin-1β, interluekin-8, etc.) causes tissue damage.17 Mediators produced by neutrophils include neutrophil elastase, neutral protease, reactive oxygen species, leukotrienes, platelet activators, and nitric oxide, which interact with each other to produce more potent tissue-damaging substances (hypochlorous acid, peroxynitrite, etc.).18 Neutrophils play a very important role as part of the innate immunity. Pathogen-associated molecular pattern molecules (PAMPs) are released by bacteria and viruses that invade the body, which bind to pattern recognition receptors, such as Toll-like receptors, activate intracellular signaling, and induce an inflammatory response to infections.19 On the other hand, the inflammatory response in the uninfected state is mediated by damage-associated molecular pattern molecules (DAMPs) released from damaged tissue or cells undergoing necrosis. DAMPs bind to pattern recognition receptors similar to PAMPs and induce an inflammatory response.20 In sepsis, a decrease in total lymphocytes and decreased HLA-DR expression in macrophages have been reported.21 This is considered to be the mechanism underlying the low white blood cell count in severe sepsis. In animal experiments, it has been reported that the white blood cell count peaks 24-48 hours after injury,22 but there was no specific description of when, in hours, it peaks. There are reports that the number of white blood cells decreases after a few days in burn patients, but it can also increase, so this study did not focus on the increase or decrease in the number of white blood cells but on the number of white blood cells in the early stages of injury.
We have not been able to analyze the peak of the white blood cell count in this study, and we would like to address it as a future issue.
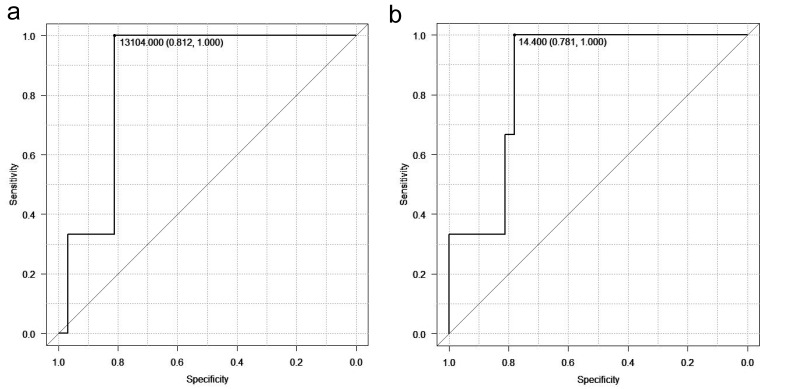
In this study, blood test results obtained immediately after the burn injury occurred were used, and an increase in neutrophil and white blood cell counts was observed in all cases. This suggests that severe sepsis does not develop immediately after the burn injury, and that the number of leukocytes and neutrophils at this time reflected cell damage. The NSCORE reflects neutrophil count reduction because it focuses on sepsis. In this study, it was found that a neutrophil count of 13104/μL or more and a white blood cell count of 14400/μL or more on the ROC curve is an indicator of severe burns. These initial treatment numbers are useful as a prediction of the severity of the burns (Figs. 7a and b). White blood cell and neutrophil counts were more prognostic than NSCOREs in early burn patients. In the future, determination of cutoff values may be useful in determining treatment strategies. Neutrophil and white blood cell counts correlated with length of hospital stay, and these two numbers were also significantly higher in patients who underwent surgery or who developed sepsis. This can be explained by the positive correlation of neutrophil counts and white blood cell counts with the BI and TBSA. Patients with a high BI have a large burn area and become infected due to the breakdown of the skin barrier; thus, early debridement surgery is necessary to prevent infection and sepsis.
Fig.7.
a: Neutrophil counts as predictors of severity in burns. The receiver operating characteristic (ROC) curve for neutrophil counts are shown. b: White blood cell counts as predictors of severity in burns. The ROC curve for white blood cell counts are shown.
At our department, debridement surgery is performed on the day of the admission if the TBSA is large, with a clear third degree burn, and surgical intervention is performed after the burn site is clarified when the area of a second degree burn is large (TBSA > 30%). These results suggest that neutrophil counts and white blood cell counts in the early stages of injury can be used as indicators of the need for early surgery.
As limitations, this study was limited in that it was a retrospective study from a single institution. Moreover, the Non-survivor group may have been biased because the number of deaths (3 of 35) was low and were all male patients. Since the NSCORE is a prognostic scoring method for sepsis and is not intended to be applied to burns, it may be possible to use it for prognostic prediction if the NSCORE scoring method is modified. This study is a single-center study and is characterized by a better prognosis compared to previous reports. The average length of hospitalization cannot be compared in burn patients because TBSAs and injury causes differ and vary, but the mortality rate is extremely low.23 We believe that one of the reasons for this is that our facility has more experience and better-educated staff as a specialized burn facility. Other factors with favorable life prognoses will be examined in the future.
We here reported that the prognosis of burn patients is correlated with the number of white blood cells and neutrophils. White blood cell and neutrophil counts were also correlated with the length of hospitalization, TBSA, BI, surgery, and the onset of sepsis. This suggests that, even if TBSA at the first admission does not indicate severe burns, if the numbers of neutrophils and white blood cells are high in blood samples obtained at the time of admission, the prognosis is likely to be poor. These high values can be an indicator for surgical intervention and treatment for sepsis, as well as for deciding whether to transfer the patient to a higher medical institution. In addition, since this index is an objective and simple evaluation, it can be used without the need for specialized facilities or departments. Since no specific cut-off value was established in our study, more cases should be accumulated and examined using multicenter research and burn registries in future. Further studies are also needed on the application of the NSCORE.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Director-general for statistics, information policy and industrial relations. Patient Survey 2020(Classification of diseases) [Internet]. Tokyo: Ministry of Health, Labor, and Welfare; 2020. [cited 2023 Jul 1]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/10syoubyo/dl/r02syobyo.pdf. Japanese.
- 2.Artz CP,Moncrief JA. The Treatment of Burns. WB Saunders Philadelphia.1969:94-98. [Google Scholar]
- 3.Japanese Dermatological Association. [Guidelines for wounds, pressure ulcers, and burns-6]. Nihon Hihuka Gakkai Zasshi. 2017;127:2261-92. Japanese. 10.14924/dermatol.127.2261 [DOI] [Google Scholar]
- 4.American Burn Association. Advanced Burn Life Support Course Provider’s Manual [Internet]. Chicago: American Burn Association; [cited2023 Jul 1]. Available from: https://www.vascomedical.gr/training/ABLS%20Provider%20Manual%202011.pdf.
- 5.Yamamoto Y,Masuda K,Okamoto J,Nagao Y. [Examination of prognostic factors in patients with severe burns in our hospital]. Soushou. 2016;7:9-14. Japanese. 10.11310/jsswc.7.9 [DOI] [Google Scholar]
- 6.Wu G,Zhuang M,Jiang Y,Fan J,Sun Y,Zhou Z,et al. Can systemic inflammatory response syndrome score at admission predict clinical outcome in patients with severe burns? Burns. 2019;45:860-8. 10.1016/j.burns.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 7.Kaita Y,Tarui T,Tanaka Y,Suzuki J,Yoshikawa K,Yamaguchi Y. Reevaluation for prognostic value of prognostic burn index in severe burn patients. Acute Med Surg. 2020;7:e499. 10.1002/ams2.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda T,Aoyama-Ishikawa M,Nakao A,Yamada T,Usami M,Kotani J. A simple scoring system based on neutrophil count in sepsis patients. Med Hypotheses. 2014;82:382-6. 10.1016/j.mehy.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 9.Osuka A,Kusuki H,Yoneda K,Matsuura H,Matsumoto H,Ogura H,et al. Glycocalyx shedding is enhanced by age and correlates with increased fluid requirement in patients with major burns. Shock. 2018;50:60-5. 10.1097/SHK.0000000000001028 [DOI] [PubMed] [Google Scholar]
- 10.Lavrentieva A,Kontakiotis T,Bitzani M,Papaioannou-Gaki G,Parlapani A,Thomareis O,et al. Early coagulation disorders after severe burn injury: impact on mortality. Intensive Care Med. 2008;34:700-6. 10.1007/s00134-007-0976-5 [DOI] [PubMed] [Google Scholar]
- 11.Baba K,Tokuda R. [Statistics of Burn Patients in Bedded Clinics]. Nesshou. 1999;25:222-9. Japanese. [Google Scholar]
- 12.Standage SW,Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther. 2011;9:71-9. 10.1586/eri.10.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ,Norrby-Teglund A,Mylona V,Savva A,Tsangaris I,Dimopoulou I,et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care. 2012;16:R149. 10.1186/cc11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitrolaki DM,Dimitriou H,Kalmanti M,Briassoulis G. CD64-Neutrophil expression and stress metabolic patterns in early sepsis and severe traumatic brain injury in children. BMC Pediatr. 2013;13:31. 10.1186/1471-2431-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adrie C,Azoulay E,Francais A,Clec’h C,Darques L,Schwebel C,et al. ; OutcomeRea Study Group. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786-93. 10.1378/chest.07-0420 [DOI] [PubMed] [Google Scholar]
- 16.Russell JA,Singer J,Bernard GR,Wheeler A,Fulkerson W,Hudson L,et al. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000;28:3405-11. 10.1097/00003246-200010000-00005 [DOI] [PubMed] [Google Scholar]
- 17.Sakuma T,Kaneda T,Saitou K,Suzuki T. [Examination of prognostic factors in burn patients]. Sosei. 2013;32:7-10. Japanese. 10.11414/jjreanimatology.32.7 [DOI] [Google Scholar]
- 18.Yasui K. [Pathophysiology and clinical practice of neutrophilic inflammation]. Enshou/Saisei. 2005;25:173-6. Japanese. 10.2492/jsir.25.173 [DOI]
- 19.Fleer A,Krediet TG. Innate immunity: toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn. Neonatology. 2007;92:145-57. 10.1159/000102054 [DOI] [PubMed] [Google Scholar]
- 20.Rubartelli A,Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429-36. 10.1016/j.it.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Drewry AM,Samra N,Skrupky LP,Fuller BM,Compton SM,Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42:383-91. 10.1097/SHK.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao T,Gomez BI,Heard TC,Dubick MA,Burmeister DM. Increased oxidative phosphorylation in lymphocytes does not atone for decreased cell numbers after burn injury. Innate Immun. 2020;26:403-12. 10.1177/1753425918805544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yakupu A,Zhang J,Dong W,Song F,Dong J,Lu S. The epidemiological characteristic and trends of burns globally. BMC Public Health. 2022;22:1596. 10.1186/s12889-022-13887-2 [DOI] [PMC free article] [PubMed] [Google Scholar]