ABSTRACT
Neuroscience has a long, rich history in embracing unusual animals for research. Over the past several decades, there has been a technology-driven bottleneck in the species used for neuroscience research. However, an oncoming wave of technologies applicable to many animals hold promise for enabling researchers to address challenging scientific questions that cannot be solved using traditional laboratory animals. Here, we discuss how leveraging the convergent evolution of physiological or behavioral phenotypes can empower research mapping genotype to phenotype interactions. We present two case studies using electric fish and poison frogs and discuss how comparative work can teach us about evolutionary constraint and flexibility at various levels of biological organization. We also offer advice on the potential and pitfalls of establishing novel model systems in neuroscience research. Finally, we end with a discussion on the use of charismatic animals in neuroscience research and their utility in public outreach. Overall, we argue that convergent evolution frameworks can help identify generalizable principles of neuroscience.
Keywords: Electric fish, Poison frogs, Convergent evolution, CRISPR, Neuroethology
Summary: Using functional genomics tools to study the convergent evolution of behavior allows a better understanding of genotype–phenotype relationships and how behavior evolves and diversifies.
Introduction
Karl von Frisch, Konrad Lorenz and Niko Tinbergen shared the Nobel Prize in 1973 for ‘their discoveries concerning organization and elicitation of individual social behavior patterns’. Their work is remarkable because they used clever experimental manipulations in the field to tease apart innate (or instinctual) versus acquired (or learned) behavior. These discoveries founded the field of neuroethology, a discipline of biologists who study the ‘neural basis of natural behavior’. This Nobel Prize winning research on bees, fishes and birds would now be considered as focusing on ‘non-model organisms’. Historically, neuroscience has a long and rich history of using unique organisms to tackle challenging questions in neurobiology, such as nerve conductance in the large axons of squid (Hodgkin and Huxley, 1952) or the circuit organization for sound localization in barn owls (Knudsen and Konishi, 1979). The focus on a few animals as primary organisms for neuroscience research is a rather recent phenomenon driven by technological advances. However, with the advent of new technologies applicable to any organism, the time is ripe for neuroscience to embrace other animals that enable us to address challenging questions in neuroscience from a different perspective. One rebuttal to such an argument is that researching unique animal attributes can lead to interesting basic science discoveries but such approaches lack translational applications. We advocate here that utilizing the convergent evolution of behavioral and physiological phenotypes can strengthen the search for generalizable principles in neuroscience versus species-specific mechanisms and both are valuable in understanding fundamental processes in the nervous system.
The spirit of inquiry within the field of neuroethology can be summarized by Krogh's principle, which states, ‘for such a large number of problems there will be some animal of choice, or a few such animals, on which it can be most conveniently studied’. For any given question in neuroscience, neuroethologists use ‘champion animals’ that are best suited to address particular questions given the animal's unique adaptations in physiology or behavior. Excellent examples of this approach can be found in Eve Marder's research on the stomatogastric ganglion in crabs to study fundamental properties of neural circuits or Eric Kandel's work with gill withdrawal reflexes in gastropod mollusks to understand the cellular basis of learning and memory (Castellucci et al., 1970). The idea of non-model and model organisms in laboratory research is a misguided concept, as all animals are studied by scientists to learn something fundamental about biological processes. Rather, the bottleneck in the diversity of research animals is due to species-specific technological advances like the creation of genetic resources (e.g. mouse genetic lines that differ in aspects of behavior or physiology). This bottleneck has been described as harmful to the field of modern neuroscience where findings in select species may be species specific rather than a generalizable property of biological systems (Brenowitz and Zakon, 2015).
With the advent of technologies to sequence and manipulate the genomes of a diverse array of organisms, there is a growing movement among biologists to connect genotype to phenotype. This is a difficult endeavor, even in organisms for which a vast array of tools is already available, such as fruit flies and mice. Moreover, mapping the genotype to phenotype landscape for behavior is even more difficult, as it is the cumulative output of a neuronal network weighing external environmental cues with internal physiological state and is therefore unlikely to be caused by a single mutation in a single protein (e.g. like hemoglobin and high-altitude adaptation; Storz, 2010). Another major theme in neuroethology is best stated by Theodosius Dobzhansky's famous statement that ‘Nothing in biology makes sense except in the light of evolution’. Studying the molecular mechanisms underlying behavior using an evolutionary framework lends power in establishing general genotype to behavioral phenotype connections rather than species-specific mechanisms. Indeed, studying the convergent evolution of traits (homoplasy) can be more useful in uncovering translational principles as they are more likely to be conserved across a wide variety of organisms (Katz, 2019). Here, we discuss how integration of a diverse array of functional genomics tools to study behavior and nervous systems within an evolutionary context represents a tractable approach towards understanding nervous system principles.
Choosing a champion
Studying the genotype to phenotype landscape of behavior is a difficult task. Behavior is the result of coordinated activity across many brain regions, neuronal cell types and proteins that integrate external signals with internal physiology. This becomes even more difficult when taking into account other individuals in social (conspecifics) or ecosystem (predator and prey) networks. Thus, parsing the components across various biological levels that influence behavioral output is often more challenging than studying other adaptive phenotypes, such as pigmentation changes traced to single gene mutations (Hubbard et al., 2010). After establishing novel model systems for our own neuroscience research programs, below we reflect on our own experiences and offer a few suggestions for choosing a champion model clade for investigating mechanisms underlying natural behavior.
Using convergent evolution as a framework
We argue that the most important consideration for choosing a champion model system for addressing scientific questions is trait variation within the clade. This usually relies on a rich literature in behavioral ecology or natural history on which one can build more mechanistic questions. A second and equally important consideration is whether particular phenotypes have evolved singly or multiply within the clade of interest (i.e. exhibit convergent evolution). This is particularly important as independent trait evolution events provide greater opportunity for biological inference and statistical power.
We chose animal clades for our own research that exhibit both trait variation and convergence at deep (wide evolutionary distances) and shallow (within the clade) levels of the phylogeny. For example, weakly electric fish vary in their electrical discharges within closely related species and have repeatedly evolved long-duration electric organ discharges (EODs) from short-duration EODs (Swapna et al., 2018) or simple EODs from complex ones (Gallant et al., 2011; Sullivan et al., 2002, 2004). Moreover, fish have evolved electrical organs many times, including in South America and Africa, and these specialized organs exhibit morphological, physiological and molecular convergence (Arnegard et al., 2010a; Gallant et al., 2014a; Zakon et al., 2006). Similarly, poison frogs vary in parental behavior and chemical defense between closely related species (Weygoldt, 2009). Similar to the evolutionary patterns seen in electric fish, parental behavior and chemical defenses have evolved many times in amphibians, including in poison frogs from South America and Africa (Heying, 2001). There are many other animals that have been established as organisms useful for laboratory research that also fulfill this charge of variability and convergence within the phylogeny. Teleost fish have been especially successful with research programs focusing on development and behavior in cavefish (Wilkens, 2010) (Kowalko, 2020, this issue), killifish (Passos et al., 2015; Poeschla and Valanzano, 2020, this issue) and sticklebacks (Bell, 2005) (Ishikawa and Kitano, 2020, this issue). Invertebrate examples include symbiosis and mimicry in rove beetles (Brueckner and Parker, 2020, this issue), eusociality in insects (Warner et al., 2019) and motor patterns in nudibranchs (Katz, 2016). Work from these systems with variation and evolutionary convergence at deep and shallow levels of the phylogeny allows neuroethologists to make conclusions about whether nervous system mechanisms are generalizable across many animals or whether there are many mechanistic solutions that give the same biological output.
Practical considerations
Organisms that may be excellent research organisms from an evolutionary or natural history perspective usually bring additional technical challenges that should be considered.
What methodological toolkits are available?
In order to establish a link between genotype and phenotype, technologies are needed to hone in on the gene(s) of interest and manipulate them. This can be done with transcriptomes via RNA sequencing, which is amenable to any organism, or with genome sequencing, which currently is more restricted to organisms with smaller genomes. Moreover, many promising genome manipulation technologies, like CRISPR, are only established for a handful of organisms, although this is changing. Embryos from animals with external fertilization are much easier to manipulate and thus genome manipulation technologies in insects, teleost fish and amphibians are more advanced than are those for amniote vertebrates with internal fertilization. There also seems to be variability in how easily some technologies can be transferred among organisms. For example, CRISPR technologies in teleost fish seem to be transferable across a wide range of teleost species while this may not be the case for amphibians, although this is mostly a factor of embryo rearing rather than CRISPR itself (discussed more below). Thus, one should consider the availability of methodological tools for a clade of interest or the ease of establishing such tools from scratch.
Will animals breed and can you rear embryos in the laboratory?
In our experience, it is the difficulty in breeding animals and rearing embryos in the lab that dictates the pace of genome editing technology development. These difficulties include the ability to perform in vitro fertilization or obtain large quantities of single-cell embryos from natural matings. Large quantities are necessary for establishing egg injection parameters, efficiency rates and phenotype scoring. However, we note here that using CRISPR is not a savior for all systems studying genotype to phenotype interactions when taking into account species with long generation times (e.g. salamanders and turtles), groups studying multi-gene traits underlying behavior, or phenotypes driven by changes in cis-regulatory regions.
Can you conduct studies in the wild?
Studying ecologically relevant behaviors requires complementing laboratory studies of behavior with field studies in the wild. When choosing animals to study, one should also consider their ease of observation and capture in the field as well as the safety of field sites for trainees. In the Anthropocene epoch, many animals are threatened with extinction (Young et al., 2016) and scientists should carefully consider the population impact of studies requiring euthanasia. Moreover, if these organisms are found in a foreign country, partnering with local scientists or organizations with authentic collaborations is important to decolonize field-based science (Baker et al., 2019).
Electric fish and evolutionary novelties
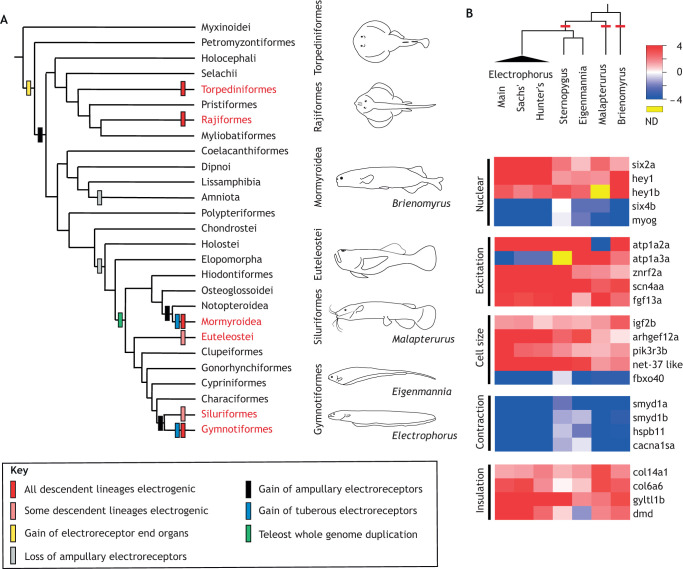
Electrogenesis, the ability to produce electric fields outside the body using an electric organ (EO), has convergently evolved six times in the history of vertebrates (Fig. 1A). In all cases, the highly derived EO derives from skeletal muscle precursors (Gallant et al., 2014a). A recent comparative analysis of electric fish genomes and transcriptomes demonstrated that EOs, despite their independent origins, show remarkably similar patterns of orthologous gene expression (Fig. 1), particularly in transcription factors that regulate the development of skeletal muscle cell size, and electrical excitability (Fig. 1B). This has motivated the hypothesis that electric fishes have evolved EOs using a ‘shared genetic toolkit’ (Gallant et al., 2014a).
Fig. 1.
‘Deep convergence’ illustrated by electric fish. (A) Electric organs (EOs) have evolved six times in the history of vertebrates (red font). The evolutionary history of electroreceptors is also shown, highlighting the independent origin of tuberous electroreceptors, which detect high-frequency (short-duration) electric organ discharge (EOD) signals in weakly electric fish. (B) A recent analysis illustrates remarkably similar patterns of gene expression in orthologous genes between independently derived EOs. The heatmap indicates relative gene expression for functionally important categories: red represents genes that are highly expressed in EOs, blue represents genes that are highly expressed in skeletal muscle (SM). ND, no data available. Note the similarity between independently derived taxa (phylogeny, above). Figure adapted from Gallant et al. (2014a).
Among these many lineages of electric fish, two lineages of teleosts, the mormyrid fishes of Africa and the gymnotiform fishes of South America, are remarkable in that they convergently evolved the ability to produce and detect brief, weak electric fields. Both lineages of weakly electric fish generate electric fields (electrogenesis) by discharging a specialized organ: the EO. These EODs result from the simultaneous action potentials of a thousand or more electrocytes which determine EOD waveform characteristics. EODs can be distinct between species, sexes and individuals, and EOD waveforms are modulated on time scales ranging from milliseconds to months (Markham, 2013; Stoddard et al., 2006). EODs are then detected by an array of electroreceptors located on the skin (electroreception). In contrast to the highly derived trait of electrogenesis, electroreception is an ancestral vertebrate trait (Fig. 1A). Electroreceptors are modified hair cells and are found in all major aquatic vertebrate groups, although the ancestors of teleosts (modern bony fish) lost this ancient sensory modality. Subsequently, electroreceptors were independently ‘re-invented’ at least twice in the ancestors of electric fish (Fig. 1A). Weakly electric fish analyze distortions of the electric field caused by nearby objects in the water to create high-resolution electrical images of their surroundings. Sensing changes in the EOD waveform and rate of nearby conspecifics lets them also use electricity as a social communication signal to secure/defend territory and to attract mates (Curtis and Stoddard, 2003; Hagedorn and Zelick, 1989). Thus, features of both electrosensory and electromotor systems in weakly electric fish evolved independently, but are highly convergent and thus lend themselves to replicable studies on the evolution of nervous systems and behavior (Kawasaki, 1996).
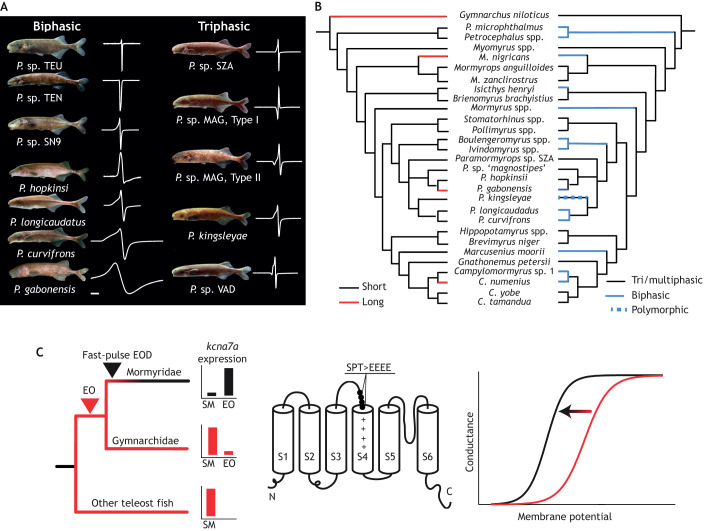
Both independently evolved clades of weakly electric fish are species rich, and African weakly electric fish (Mormyroidea) are among the most rapidly speciating groups of ray-finned fish (Rabosky et al., 2013). Field and laboratory playback experiments have revealed strong preferences for species-specific EOD waveforms (Fig. 2A) among weakly electric fishes (Arnegard et al., 2006; Hopkins and Bass, 1981; Nagel et al., 2018a,b). The most variable traits among these are EOD duration and complexity (Fig. 2A,B). Recent work has demonstrated that EOD diversification can greatly outpace other forms of ecological and morphological diversification in mormyrids (Arnegard et al., 2010b). The combination of these two lines of evidence has motivated the hypothesis that mate preferences may be responsible for EOD diversity (Arnegard et al., 2005, 2010b). A growing body of work has identified a number of additional selective forces that are likely to contribute to EOD evolution as well. Among these are the high energetic demands of electrogenesis (e.g. Dunlap et al., 2016; Lewis et al., 2014; Salazar et al., 2013). Other factors may include background noise (Hopkins, 1973), predation by electroreceptive predators (Dunlap et al., 2016; Stoddard, 1999, 2002) and constraints imposed by electrolocation (Gottwald, 2018), which are not yet well understood in an ecological context.
Fig. 2.
‘Shallow convergence’ illustrated by weakly electric fish. (A) A sympatric assemblage of weakly electric mormyrid fish from the Ivindo River (genus Paramormyrops). This genus exhibits high morphological similarity, but substantial diversity in EOD signals, particularly in the number of phases present in the EOD (all EODs on the left are biphasic, all EODs on the right are triphasic) as well as the duration. Scale bar: 1 ms. (B) The evolution of EOD duration (left, red and black) and number of phases (right, blue and black) is superimposed upon the most recent phylogenetic tree proposed by Sullivan et al. (2000). It is evident that there have been numerous transitions between long and short EOD waveforms as well as simple and complex EOD waveforms. (C) The duplicate genes kcna7a and kcna7b encode the Kv1.7 potassium channel, and both are ancestrally expressed in SM. kcna7a expression shifts to the EO at the transition between Gymnarchus and other mormyrid fish, along with a burst of molecular evolution. Specifically, the substitution of negatively charged amino acids at the S3–S4 linker (SPT>EEEE) causes a shift in Kv1.7 voltage sensitivity at more hyperpolarized potentials, leading to brief action potentials. A was adapted from Picq et al. (2019 preprint) and C from Swapna et al. (2018).
In the past decade there has been a proliferation of genomics resources available for weakly electric fish (Gallant et al., 2014a, 2017; Guth et al., 2013; Kim et al., 2009; Lamanna et al., 2014; Pinch et al., 2016; Swapna et al., 2018; Traeger et al., 2015, 2017). A major goal of this work has been to understand how changes at the molecular level result in phenotypic changes at the organismal level.
Key to understanding the link between genotype and EOD phenotype has been the investigation of voltage-gated ion channels, particularly sodium and potassium channels (Arnegard et al., 2010a; Nagel et al., 2017; Swapna et al., 2018; Zakon et al., 2006). As a result, weakly electric fish are a textbook example of how gene duplication events may lead to divergence of gene function (Futuyma, 2013). Molecular evolution of neofunctionalized duplicate sodium channels (scn4aa, scn4ab) correlates with the origin of the two major electrogenic clades, and positive selection on sites affecting channel kinetics has been demonstrated to occur in parallel. Fish muscle expresses two paralogous voltage-gated Na+ channel genes (scn4aa, scn4ab), homologs of a gene expressed in muscle in all vertebrates (scn4a) (Thompson et al., 2014). However, in both lineages of weakly electric fish, one gene (scn4aa) is compartmentalized in the EO where it evolves rapidly, presumably under selection pressures associated with electrical signaling and sensing, whereas the other (scn4ab) retains its expression in muscle where it is functionally constrained and evolves slowly (Arnegard et al., 2010a; Zakon et al., 2006). Evolution of potassium channels seems to follow a parallel trajectory in mormyrids (Swapna et al., 2018). In teleost fish, kcna7a and kcna7b are ancestrally expressed in skeletal muscle. Among mormyrid electric fish, selection for brief-duration EODs has likely led to the neofunctionalization of kcna7a. Expression of this gene shifts to the electric organ and is accompanied by a burst of molecular evolution: among these changes are substitutions at a key site affecting channel kinetics. Specifically, the substitution of negatively charged amino acids at the S3–S4 linker causes a shift in Kv1.7 conductance at lower potentials, leading to brief action potentials (Fig. 2C).
Another contributor to EOD diversity is electrocyte cell shape, which can vary greatly in mormyrids, and can influence the pathway that current travels through the EO. This variation has considerable consequences for the number of phases present in the EOD (Alves-Gomes et al., 1997; Bennett, 1970; Gallant et al., 2011), leading to the repeated convergent evolution of biphasic EODs (Fig. 2A,B). To understand the genetic underpinnings of EOD waveform shape differences, a recent study by Losilla and Gallant (2019) examined patterns of gene expression between multiple sets of closely related Paramormyrops species, identifying a set of candidate cytoskeletal proteins, extracellular matrix proteins and membrane-associated proteins that exhibit patterns of differentially expression associated with biphasic and triphasic EODs. The precise role of these genes in determining electrocyte cell shape will require both developmental and functional genomics studies in the coming years.
Thus far, selective factors that shape EOD signals have been identified and some genetic substrates that contribute to this diversity have been identified, though this work is ongoing. The missing link between these two datasets is functional tools to interrogate how specific genetic variants result in ion channels and speciation. The promise of these technologies used in conjunction with genetic manipulation techniques have been described more broadly for electric fish elsewhere (see Pitchers et al., 2016). We highlight here that CRISPR/Cas9 gene editing has recently been applied to the weakly electric fish system. Constantinou et al. (2019) demonstrated effective knock out of scn4aa function in both gymnotiform and mormyrid electric fish, obtaining F0 mutants that are effectively ‘electrically silent’. We note that this protocol provides video and written instructions that detail husbandry, molecular biology and injection techniques, and is linked to supplemental genomic resources and target design tools (http://efishgenomics.integrativebiology.msu.edu), which should facilitate widespread use of this technology within the electric fish community.
While CRISPR/Cas9 gene editing is still in a nascent stage for the electric fish community, the availability of functional tools in electric fish increases the tractability of several key experiments necessary for understanding the link between genotype and behavior. First and most obviously, in vivo manipulation studies could provide direct evidence linking genetic changes to behavioral outputs. Second, the prospect of stable transgenic lines that have ‘dissected’ different components of EOD diversity within single species could offer a new generation of highly controlled behavioral assays designed to elucidate the selective advantages of particular signal features. As mutagenesis efficiencies increase through more widespread community use, we see a third and exciting possibility of introducing transgenic materials into weakly electric fish, which may allow the use of optogenetic and reporter genes for dissecting neural circuits and assessing electrocyte physiology.
Poison frog innovations in physiology and behavior
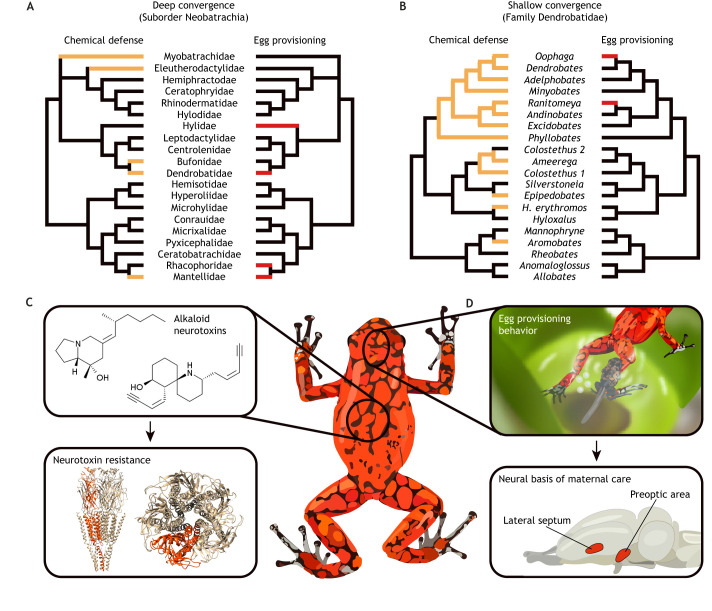
Several lineages of frogs have repeatedly evolved suites of traits that earn them the name ‘poison frogs’. These groups include Central and South American dendrobatids and bufonids, Malagasy mantellids, Australian myobatrachids and Cuban eleutherodactylids (Saporito et al., 2012) (Fig. 3A). Each have independently evolved aposematic warning coloration that advertises their toxicity to potential predators (Santos et al., 2003). There is also variation within some of these groups, where chemical defenses have evolved four times independently within Central and South American dendrobatids (Santos et al., 2003) (Fig. 3B). The alkaloid small molecules they sequester from their diet to their skin are used for chemical defense against predators and pathogens (Santos et al., 2016). In addition to chemical defenses, some poison frogs have also evolved elaborate social behavior, including monogamy and parental behavior. Here, we discuss the open questions and current progress on using the convergent evolution of correlated traits in poison frogs to map the genotype to phenotype landscape.
Fig. 3.
Convergent evolution in chemical defenses and social behavior in poison frogs. (A) Frogs have evolved chemical defenses (orange families in left phylogeny) and maternal egg provisioning to tadpoles (red families in right phylogeny) several times independently; phylogeny adapted from Pyron (2014). (B) The family Dendrobatidae shows convergence in a number of traits, including the independent evolution of chemical defenses (orange genera in left phylogeny) and maternal egg provisioning to tadpoles (red genera in right phylogeny); phylogeny adapted from Santos et al. (2016). (C) Poison frogs sequester lipophilic alkaloids to their skin and many of these compounds target ion channels in the nervous system. Poison frogs have ion channel mutations that confer resistance to some neurotoxins. For example, dendrobatids have mutations in the beta subunit (shown in orange) of the nicotinic acetylcholine receptor (nAchR) that reduces sensitivity to epibatidine (Tarvin et al., 2017); shown is the structure of the human nAchR (Unwin, 2005). (D) Poison frogs also vary in parental behavior and have convergently evolved egg provisioning behavior, where mothers feed developing tadpoles with nutritive unfertilized eggs. Neural activity in the lateral septum and preoptic area is correlated with this nursing behavior, although the activation of oxytocin neurons differs between independent evolutionary origins (Fischer et al., 2019).
Poison frogs do not synthesize toxins themselves, but acquire them through their specialist diet of ants and mites (Daly et al., 1994). The repeated evolution of chemical defenses in amphibians raises several questions on the evolution of novel physiological traits. The most obvious is whether the repeated evolution of alkaloid bioaccumulation involves similar or unique mechanisms, a question that remains open. Another mystery is how poison frogs manage to resist poisoning by their own toxins. Many poison frog alkaloids target ion channels in the nervous system, including sodium channels and nicotinic acetylcholine receptors. Several studies have utilized the independent evolution of toxicity as a framework to identify mutations in ion channels that could confer resistance to alkaloid neurotoxins (Fig. 3C). For example, by comparing the gene encoding the sodium channel Nav1.4 across both Central and South American dendrobatids and Malagasy mantellids, several mutations were predicted to confer resistance to the potent neurotoxin batrachotoxin (Tarvin et al., 2016; Yuan and Wang, 2018). A similar comparative approach was used to demonstrate that mutations in nicotinic acetylcholine receptors can confer resistance to the neurotoxin epibatidine (Tarvin et al., 2017). Similar relationships have been identified in other chemical defense systems (Ujvari et al., 2015), demonstrating that autoresistance of neurotoxins in many animals can be a simple genetic trait, where clear genotype to phenotype relationships can be established. Although gene editing has not been used to test these relationships in vivo, the path forward to do so is clear and will clarify whether these observations are a general principle in animal chemical defenses.
Some poison frogs have evolved elaborate parental behaviors, including tadpole transport and egg provisioning (Heying, 2001; Weygoldt, 2009). While these behaviors occur in other non-poison frog amphibians, the repeated evolutionary origins and behavioral diversity within the group allows investigation of the neural basis of behavior and determining whether these behaviors have similar or different underlying mechanisms. For example, some Central and South American dendrobatid frogs and a Malagasy mantellid have evolved egg provisioning behavior that promotes healthy tadpole development (Heying, 2001) (Fig. 2D). This ‘nursing’ behavior has evolved at least twice in dendrobatids, including the Oophaga clade and in the Mimetic poison frog (Ranitomeya imitator), as well as once in the climbing mantella (Mantella laevigata). In these species, mothers provision developing tadpoles with nutritive unfertilized eggs. We investigated the mechanisms of egg provisioning in the diablito frog (Oophaga sylvatica) and M. laevigata, which diverged roughly 140 million years ago (Fischer et al., 2019). We found that, in addition to providing nutrition, egg provisioning also provides alkaloid chemical defenses to tadpoles. Poison frogs acquire their chemical defenses from leaf litter arthropods, which tadpoles do not have access to in their aquatic environments. Thus, egg provisioning could evolve as a way to provide tadpoles with chemical defenses earlier. When looking at overall brain activation, we found more neuronal activity in the lateral septum and preoptic area in nursing mothers compared with controls. The preoptic area is well known for mediating care for offspring across vertebrates (Fischer et al., 2019), suggesting evolving parental behavior relies on hypothalamic neural networks. Oxytocin is a well-known mediator of maternal behavior in mammals (Leng et al., 2008), and so we next tested the hypothesis that oxytocin promotes egg provisioning behavior in amphibians. To our surprise, we found increased activity of oxytocin neurons during nursing behavior in M. laevigata, but not O. sylvatica, suggesting that the underlying mechanisms of maternal behavior may be different in these repeated evolutionary origins.
Our work utilizing convergent evolution of maternal care in amphibians has taught us several important lessons. First, although parental behavior may use similar mechanisms at the level of brain regions (e.g. the preoptic area) across independent evolutionary origins, the neuronal cell types facilitating a behavior may be different. In other words, there may be many mechanistic ‘solutions’ for promoting a similar behavior. Maternal care is ubiquitous in mammals and only comparative work across independent origins allowed us to highlight alternative mechanisms for behavioral regulation. Second, this also serves as a cautionary tale to behavioral neuroscientists, where the underlying neural mechanisms across species that promote a very similar behavior may be different and comparative work is necessary to understand patterns that are species specific versus generalizable.
Patterns and principles of convergence in physiology and behavior
A major goal of contemporary biology is to understand how changes at the molecular level result in phenotypic changes at the organismal level. The proliferation of genetic and genomic resources for a wide variety of organisms has already been intellectually profitable in this regard. Unsurprisingly, many of the known genetic variants underlying variation in many animal phenotypes have been loci of major effects for relatively ‘simple’ phenotypes, such as pigmentation (Hubbard et al., 2010). Additionally, we have discussed here how duplications and mutations in ion channels have potentially large effects on physiology with the function of electric organs in fish and toxin autoresistance in poison frogs. This proliferation of data has motivated attempts to extract general principles from the relationship between genotype and phenotype (Gallant et al., 2014b; Martin and Orgogozo, 2013; Stern and Orgogozo, 2009). Martin and Orgogozo (2013) cataloged 1008 alleles linked to phenotype across the literature spanning eukaryotes, and found a surprisingly high incidence of genomic ‘hotspots of evolution’ that accumulate mutations and lead to similar convergent phenotypic effects. This phenomenon is exemplified by work in Limeninitis and Heliconius butterflies: both lineages, which last shared an ancestor 65 million years ago, have evolved mutations in the cis-regulatory region of the signal ligand WntA that produce variation in melanization phenotype on butterfly wings (Gallant et al., 2014a,b).
In this article, we have advocated for approaching the genetic underpinnings of behavior within a comparative framework, drawing upon ‘champion species’ which exhibit repeated evolution of behavioral traits over different phylogenetic scales. We feel that this is a formidable strategy for two reasons. First, conclusions about evolutionary processes are greatly enhanced by assessing statistically independent points (Stone et al., 2011). Second, exploring convergent phenotypes at different taxonomic scales provides a natural opportunity to probe the nature of constraint and predictability of evolution. Convergent phenotypic evolution is thought to occur at the genetic level through three mechanisms: (1) the evolution of independent mutations in parallel lineages, (2) the exchange of alleles by hybridization (introgression) and (3) the repeated fixation of polymorphic alleles from ancestral populations (Stern, 2013). Two of these processes (introgression and fixation of ancient polymorphism) are considerably more likely to occur within closely related taxa than distantly related ones. The occurrence of genetic convergence through independent mutations has been well documented on both shallow (e.g. Sugawara et al., 2005) and deep (e.g. Gallant et al., 2014a; Gallant et al., 2014b) time scales. The tantalizing insight that there are ‘genetic paths of least resistance’ (Martin and Orgogozo, 2013) motivates the question of how extensible this concept is to complex phenotypes such as behavior. Evidence of convergent behavioral evolution by independently occurring mutations would be the most compelling evidence of ‘genetic paths of least resistance’ in behavior. To summarize: leveraging convergent evolution over multiple phylogenetic scales provides greater statistical power, enabling researchers to better ask ‘how’ behavioral phenotype and genotype relate to each other, but also ‘how likely’ it is that particular genotypic changes will lead to particular behavioral effects.
Although the technological revolutions in molecular biology and genomics have propelled many fields forward, it is important to remember that from an organismal perspective, behavior and physiology are the substrates of selection. In other words, natural and sexual selection shape species from the ‘top down’, starting with physiology and behavior. In this regard, many wild model systems provide an advantage in terms of understanding genotype–phenotype connections, given often a rich literature on behavioral ecology and natural history in contrast with traditional laboratory models. For example, we found that poison frogs that evolved maternal egg provisioning behavior on different continents had very similar behaviors, but different underlying mechanisms, highlighting multiple mechanistic paths towards the same behavioral output (Fischer et al., 2019). Another example of deep convergence is the loss of flight in birds, where examining the genomes across multiple independent losses of wing function highlighted that convergent changes in regulatory regions, rather than mutations of protein-coding genes, was associated with trait loss (Sackton et al., 2019). Overall, we argue that examining the deep convergence of trait gains or losses provides more power in mapping the genotype–phenotype landscape compared with single species approaches.
Perils and pitfalls in linking genotype to behavioral phenotypes in unusual organisms
While there is growing progress in understanding the genomic underpinnings of phenotypic differences between organisms, this progress has largely been made for relatively ‘simple’ phenotypes. This point is particularly vexing for those interested in the mechanistic underpinnings of behavior and the sheer complexity of the nervous system. We predict that this will be an ‘Achilles heel’ for neuroethologists for some time, as most behaviors are widely perceived to have a complex genetic basis (York, 2018). Just as early progress in understanding the molecular basis of genetic disease in humans largely focused on the ‘one gene–one disorder’ approach (Plomin et al., 1994), we strongly suspect that those attempting to understand the genetic underpinnings of behavior will focus on genes of major effect and relatively simple behaviors. Put another way: demonstration that a behavioral trait has a genetic basis may become increasingly trivial over time. By placing these genotype to phenotype findings in a comparative context, these insights have the potential to inform deeper insights than a taxon-limited approach.
Additionally, we encourage behavioral biologists to move beyond accumulating genome and transcriptome resources in their research programs and towards measuring genetic variation. Efforts to connect genotypic variation to complex phenotypic variation in humans and other canonical laboratory models have been largely facilitated by quantitative approaches which leverage datasets that encompass genetic variation among individuals, strains, populations and hybrids. Presently, as many of the ‘wild’ models are at the stage of assembling and finishing genomes, datasets that encompass genetic variation are still relatively rare. Researchers should consider collecting genotypic data from populations of organisms, leveraging field-based collections, laboratory crosses and the specimens available in natural history museums (Bi et al., 2013), which will facilitate these approaches in the future.
Finally, we emphasize the importance of strong functional tools as a necessary component of any research strategy. As discussed above, highly efficient mutagenesis techniques such as CRISPR/Cas9 gene editing are beginning to democratize functional genomics tools in a wide variety of systems. Applying these techniques successfully in wild models is challenging enough and researchers need to carefully consider how mutagenesis results may be interpreted in the context of behavior. As behaviors are typically the result of coordinated output of neural circuits across many brain regions, simple knock-out mutants may not yield major behavior changes. Importantly, lack of a phenotype does not imply the target gene plays no role in the phenotype of interest as there could be compensatory influences in tissues as robust and plastic as the brain. In this regard, there will be a considerable impetus to develop techniques that restrict mutagenesis in time and space, avoiding potentially pleiotropic or compensatory effects on the organism as a whole.
Fantastic beasts and how to use them for public engagement
Using unusual model systems for research occasionally comes with skepticism about usefulness for humanity, especially from a biomedical or translational viewpoint. Research on this theme is sometimes targeted by politicians to incite public opinion about spending taxpayer dollars on research that may not be directly involved in understanding and treating human disease. As recipients of taxpayer funds, scientists are obligated to convey their research findings to the public in a way that communicates the value of basic research and within a framework where the public are stakeholders in this new knowledge. This is even more important in current times, where public opinion can be contrary to scientific evidence, such as climate change (Leiserowitz et al., 2013), childhood vaccinations (Baumgaertner et al., 2018) and genetically modified foods (Malyska et al., 2016). Programs that broaden the impact of research are required of some funding agencies, like the National Science Foundation in the USA. However, it is often difficult for scientists to create dynamic, meaningful and engaging learning experiences that reach beyond the university and formal training in education is frequently lacking. Yet, most people are inherently drawn to unusual and charismatic animals through natural curiosity, which can be used as a pathway for engaging in scientific topics that otherwise may not receive attention. For example, children are more engaged in learning when exotic species are used to convey scientific concepts compared with local species (Ballouard et al., 2011; Trainin et al., 2005). Below, we summarize strategies for outreach development and describe our own outreach programs that incorporate the unique biology of our research organisms.
When establishing outreach programs, start by connecting how your unique path into science can appeal to stakeholders or how your unique model system can be used to communicate specific scientific concepts. Every scientist has a unique background and every model system is used for some special reason to address a critical gap in knowledge. In education, programs are designed using Logic Models (Kaplan and Garrett, 2005) that articulate the goals and resources of an outreach program prior to its implementation. A critical aspect of meaningful outreach programs is to ensure they are achieving the desired goals by using strong assessment strategies. Much like scientific experiments, outreach programs need allocated funds and a logical plan that includes quality control assessments to measure impact and refine the approach in subsequent iterations.
The Gallant laboratory leverages the unusual biology of electric fish to motivate elementary students to consider forms of energy. While electricity is pervasive in the lives of young students, understanding how chemical energy is transformed into electricity is a difficult concept illustrated well by biological systems, particularly electric fish behavior. This curriculum, initially developed as a module for Grade 5 students in rural New York State (USA), is now shared yearly on an elementary school tour in the area of East Lansing, Michigan (USA) and reaches approximately 300 students per year. One of the more innovative aspects of this curriculum has been the focus on developing hands-on, interactive displays that relate the foreign concept of electrosensation and electrogenesis into more relatable sensory experiences (audio, visual and tactile). For instance, using low-cost, open-source hardware, the Gallant laboratory developed a method of transducing electric fish signals into flashing lights that power holiday decorations (http://bit.ly/efishxmas). A key component of developing these experiences has been assessment: in the case of the Grade 5 student program, the curriculum was evaluated by a combination of simple pre- and post-experience quizzes (administered weekly) as well as narrative assessments in the form of artwork and composition writing, that were developed collaboratively with instructors.
To communicate the contribution of poison frogs to scientific research in biology and chemistry, the O'Connell lab has developed several programs to increase public engagement. First, to connect with the public in a meaningful way, Lauren O'Connell specifically focuses on community college students with the goal of increasing genuine research experiences in that population. Lauren attended a rural community college and can uniquely communicate and mentor that group, an example of using past experience to connect with an underserved population. Moreover, many students from underrepresented and/or low socioeconomic groups start their education at community colleges (Ma and Baum, 2016) and increasing research exposure with these students is an excellent way to diversify the scientific workforce. This program is assessed by student publications, graduation from a university, and career choice compared with a control population that did not have research experiences. Second, the O'Connell lab created the Frogger School Program, which places (non-toxic) poison frogs into classrooms and equips teachers with lesson plans on integrating the frog ‘anchor phenomenon’ into biology and chemistry education. Each summer, some teachers participate in genuine research and then develop an ‘Education Transfer Plan’ to integrate their research experience into their classrooms. The participation of science teachers in genuine research experiences improves retention in the profession, increases inquiry-based instruction in their classrooms and enhances their students' scientific achievements on placement exams (Melear et al., 2000; Silverstein et al., 2009). In our case, assessment of teacher participation in genuine research is based on teacher pre- and post-program surveys and scientific publications with the teachers and their classrooms as co-authors (Moskowitz et al., 2019 preprint).
When designing outreach programs with the public, there are several characteristics that should be considered (Krajcik, 2015). (1) Is it feasible? Consider the target age group and available time constraints. (2) Is it ethical? Are the appropriate animal ethics (for vertebrate animals) or human ethics (involvement of minors in research, use of surveys, etc.) approvals in place from local regulatory committees? (3) Is it meaningful? Learning happens best when people are engaged with the material because they find it interesting and/or important to their lives. Using unusual and charismatic animals in research is particularly useful in gaining interest, especially with children (Ballouard et al., 2011; Trainin et al., 2005). (4) Does it meet scientific learning goals? For effective outreach, the target audience needs to walk away with the concepts you want them to know (Varner, 2014). Informal science outreach can often be assessed with surveys or discussions with participants (Keeley, 2008), while larger programs can accommodate formal evaluations with appropriate control groups (Margoluis et al., 2009). (5) Is the education effort sustainable? Is this a one-time engagement or can the teacher, classroom or community continue the research effort and what resources are necessary to do that? Finally, we encourage scientists to reach out to local education experts in their communities for collaborations on broadening the impact of their research. Just as one would reach out to experts from different scientific fields to collaborate on new directions or techniques in a research program, education requires the same expertise and collaboration to make a meaningful impact.
The adoption of unusual ‘champion’ animals to address fundamental biological questions in research programs comes with the need to perform additional justification to funding agencies and the general public on why such research is valuable. While we may not feel the need to do this within our own scientific community, it is imperative that the value of neuroethological approaches be communicated through outreach if we wish to move the field forward with continued funding from the public.
Summary
Here, we describe how studying behavior and neural function within a convergent evolution framework allows unique insights into whether patterns of genetic or neuronal function are generalizable versus species specific, using our work in electric fish and poison frogs as case studies in convergent evolution of unusual traits. We advocate that gene editing technologies open the door to using a diverse set of organisms in neuroscience research, although this enormous potential comes with many challenges that should be carefully assessed. Finally, using the charismatic animals in outreach is useful in gaining public support for basic science research. We hope this framework will inspire others to leverage the convergent evolution of physiological or behavioral phenotypes to strengthen research programs mapping genotype to phenotype interactions.
Acknowledgements
We would like to thank Michael Dickinson, Julian Dow and Leslie Vosshall for organizing the Journal of Experimental Biology 2019 symposium on ‘Genome editing for comparative physiology’.
Footnotes
Funding
J.R.G. is supported by the National Science Foundation [IOS-1856243, IOS-1655965, IOS-1455405, IOS-1557657]. L.A.O. is supported by the National Institutes of Health [DP2HD102042], the National Science Foundation [IOS-1845651, IOS-1827333 and IOS-1822025], the Rita Allen Foundation and L'Oreal USA For Women in Science. Deposited in PMC for release after 12 months.
References
- Alves-Gomes, J. A., Hopkins, C. D. and Rose, C. D. (1997). Molecular insights into the phylogeny of mormyriform fishes and the evolution of their electric organs. Brain Behav. Evol. 49, 324-350. 10.1159/000113001 [DOI] [PubMed] [Google Scholar]
- Arnegard, M. E., Bogdanowicz, S. M. and Hopkins, C. D. (2005). Multiple cases of striking genetic similarity between alternate electric fish signal morphs in sympatry. Evolution 59, 324-343. 10.1111/j.0014-3820.2005.tb00993.x [DOI] [PubMed] [Google Scholar]
- Arnegard, M. E., Jackson, B. S. and Hopkins, C. D. (2006). Time-domain signal divergence and discrimination without receptor modification in sympatric morphs of electric fishes. J. Exp. Biol. 209, 2182-2198. 10.1242/jeb.02239 [DOI] [PubMed] [Google Scholar]
- Arnegard, M. E., Zwickl, D. J., Lu, Y. and Zakon, H. H. (2010a). Old gene duplication facilitates origin and diversification of an innovative communication system–twice. Proc. Natl. Acad. Sci. USA 107, 22172-22177. 10.1073/pnas.1011803107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnegard, M. E., McIntyre, P. B., Harmon, L. J., Zelditch, M. L., Crampton, W. G. R., Davis, J. K., Sullivan, J. P., Lavoué, S. and Hopkins, C. D. (2010b). Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Am. Nat. 176, 335-356. 10.1086/655221 [DOI] [PubMed] [Google Scholar]
- Baker, K., Eichhorn, M. P. and Griffiths, M. (2019). Decolonizing field ecology. Biotropica 51, 288-292. 10.1111/btp.12663 [DOI] [Google Scholar]
- Ballouard, J.-M., Brischoux, F. and Bonnet, X. (2011). Children prioritize virtual exotic biodiversity over local biodiversity. PLoS ONE 6, e23152. 10.1371/journal.pone.0023152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgaertner, B., Carlisle, J. E. and Justwan, F. (2018). The influence of political ideology and trust on willingness to vaccinate. PLoS ONE 13, e0191728. 10.1371/journal.pone.0191728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. M. (2005). Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464-473. 10.1111/j.1420-9101.2004.00817.x [DOI] [PubMed] [Google Scholar]
- Bennett, M. V. L. (1970). Comparative physiology: electric organs. Annu. Rev. Physiol. 32, 471-528. 10.1146/annurev.ph.32.030170.002351 [DOI] [PubMed] [Google Scholar]
- Bi, K., Linderoth, T., Vanderpool, D., Good, J. M., Nielsen, R. and Moritz, C. (2013). Unlocking the vault: next-generation museum population genomics. Mol. Ecol. 22, 6018-6032. 10.1111/mec.12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz, E. A. and Zakon, H. H. (2015). Emerging from the bottleneck: benefits of the comparative approach to modern neuroscience. Trends Neurosci. 38, 273-278. 10.1016/j.tins.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueckner, A. and Parker, J. (2020). Molecular evolution of gland cell types and chemical interactions in animals. J. Exp. Biol. 223, jeb211938. 10.1242/jeb.211938. [DOI] [PubMed] [Google Scholar]
- Castellucci, V., Pinsker, H., Kupfermann, I. and Kandel, E. R. (1970). Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167, 1745-1748. 10.1126/science.167.3926.1745 [DOI] [PubMed] [Google Scholar]
- Constantinou, S. J., Nguyen, L., Kirschbaum, F., Salazar, V. L. and Gallant, J. R. (2019). Silencing the spark: CRISPR/Cas9 genome editing in weakly electric fish. J. Vis. Exp. 152, e60253. 10.3791/60253 [DOI] [PubMed] [Google Scholar]
- Curtis, C. C. and Stoddard, P. K. (2003). Mate preference in female electric fish, Brachyhypopomus pinnicaudatus. Anim. Behav. 66, 329-336. 10.1006/anbe.2003.2216 [DOI] [Google Scholar]
- Daly, J. W., Martin Garraffo, H., Spande, T. F., Jaramillo, C. and Stanley Rand, A. (1994). Dietary source for skin alkaloids of poison frogs (Dendrobatidae)? J. Chem. Ecol. 20, 943-955. 10.1007/BF02059589 [DOI] [PubMed] [Google Scholar]
- Dunlap, K. D., Tran, A., Ragazzi, M. A., Krahe, R. and Salazar, V. L. (2016). Predators inhibit brain cell proliferation in natural populations of electric fish, Brachyhypopomus occidentalis. Proc. Biol. Sci. 283. 10.1098/rspb.2015.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, E. K., Nowicki, J. P. and O'Connell, L. A. (2019). Evolution of affiliation: patterns of convergence from genomes to behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180242. 10.1098/rstb.2018.0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, E. K., Roland, A. B., Moskowitz, N. A., Vidoudez, C., Ranaivorazo, N., Tapia, E. E., Trauger, S. A., Vences, M., Coloma, L. A. and O'Connell, L. A. (2019). Mechanisms of convergent egg-provisioning in poison frogs. Curr. Biol. 29, 4145-4151. 10.1016/j.cub.2019.10.032 [DOI] [PubMed] [Google Scholar]
- Futuyma (2013). Evolution, 3 edn. Sinauer Associates Inc. [Google Scholar]
- Gallant, J. R., Arnegard, M. E., Sullivan, J. P., Carlson, B. A. and Hopkins, C. D. (2011). Signal variation and its morphological correlates in Paramormyrops kingsleyae provide insight into the evolution of electrogenic signal diversity in mormyrid electric fish. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 197, 799-817. 10.1007/s00359-011-0643-8 [DOI] [PubMed] [Google Scholar]
- Gallant, J. R., Traeger, L. L., Volkening, J. D., Moffett, H., Chen, P.-H., Novina, C. D., Phillips, G. N., Jr, Anand, R., Wells, G. B., Pinch, M., et al. (2014a). Genomic basis for the convergent evolution of electric organs. Science 344, 1522-1525. 10.1126/science.1254432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant, J. R., Evans, B., Reed, R. D., Chamberlain, N. L., Pote, B. L., Peterson, C., Smith, G. E., Savage, W. K., Mullen, S. P., Martin, A., et al. (2014b). Ancient homology underlies adaptive mimetic diversity across butterflies. Nat. Commun. 5, 4817. 10.1038/ncomms5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant, J. R., Losilla, M., Tomlinson, C. and Warren, W. C. (2017). The genome and adult somatic transcriptome of the mormyrid electric fish paramormyrops kingsleyae. Genome Biol. Evol. 9, 3525-3530. 10.1093/gbe/evx265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald, H. (2018). Der lange Schatten der Hidschra. Duncker and Humblot. [Google Scholar]
- Guth, R., Pinch, M. and Unguez, G. A. (2013). Mechanisms of muscle gene regulation in the electric organ of Sternopygus macrurus. J. Exp. Biol. 216, 2469-2477. 10.1242/jeb.082404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn, M. and Zelick, R. (1989). Relative dominance among males is expressed in the electric organ discharge characteristics of a weakly electric fish. Anim. Behav. 38, 520-525. 10.1016/S0003-3472(89)80045-4 [DOI] [Google Scholar]
- Heying, H. E. (2001). Social and reproductive behaviour in the Madagascan poison frog, Mantella laevigata, with comparisons to the dendrobatids. Anim. Behav. 61, 567-577. 10.1006/anbe.2000.1642 [DOI] [Google Scholar]
- Hodgkin, A. L. and Huxley, A. F. (1952). Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 116, 449-472. 10.1113/jphysiol.1952.sp004717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, C. D. (1973). Lightning as background noise for communication among electric fish. Nature 242, 268. 10.1038/242268a0 [DOI] [Google Scholar]
- Hopkins, C. D. and Bass, A. H. (1981). Temporal coding of species recognition signals in an electric fish. Science 212, 85-87. 10.1126/science.7209524 [DOI] [PubMed] [Google Scholar]
- Hubbard, J. K., Uy, J. A. C., Hauber, M. E., Hoekstra, H. E. and Safran, R. J. (2010). Vertebrate pigmentation: from underlying genes to adaptive function. Trends Genet. 26, 231-239. 10.1016/j.tig.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Ishikawa, A. and Kitano, J. (2020). Diversity in reproductive seasonality in the three-spined stickleback, Gasterosteus aculeatus. J. Exp. Biol. 223, jeb208975. 10.1242/jeb.208975 [DOI] [PubMed] [Google Scholar]
- Kaplan, S. A. and Garrett, K. E. (2005). The use of logic models by community-based initiatives. Eval Program Plann. 28, 167-172. 10.1016/j.evalprogplan.2004.09.002 [DOI] [Google Scholar]
- Katz, P. S. (2016). Evolution of central pattern generators and rhythmic behaviours. Phil. Trans. R. Soc. B 371, 20150057. 10.1098/rstb.2015.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, P. S. (2019). The conservative bias of life scientists. Curr. Biol. 29, R666-R667. 10.1016/j.cub.2019.05.066 [DOI] [PubMed] [Google Scholar]
- Kawasaki, M. (1996). Comparative analysis of the jamming avoidance response in african and south american wave-type electric fishes. Biol. Bull. 191, 103. 10.2307/1543070 [DOI] [PubMed] [Google Scholar]
- Keeley, P. (2008). Science Formative Assessment: 75 Practical Strategies for Linking Assessment, Instruction, and Learning. Corwin. [Google Scholar]
- Kim, H.-J., Güth, R., Jonsson, C. B. and Unguez, G. A. (2009). S. macrurus myogenic regulatory factors (MRFs) induce mammalian skeletal muscle differentiation; evidence for functional conservation of MRFs. Int. J. Dev. Biol. 53, 993-1002. 10.1387/ijdb.082672hk [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, E. I. and Konishi, M. (1979). Mechanisms of sound localization in the barn owl (Tyto alba). J. Comp. Physiol. 133, 13-21. 10.1007/BF00663106 [DOI] [Google Scholar]
- Kowalko, J. (2020). Utilizing the blind cavefish Astyanax mexicanus to understand the genetic basis of behavioral evolution. J. Exp. Biol. 223, jeb208835. 10.1242/jeb.208835 [DOI] [PubMed] [Google Scholar]
- Krajcik, J. (2015). Three-dimensional instruction: using a new type of teaching in the science classroom. Science and Children 053. 10.2505/4/sc15_053_03_6 [DOI] [Google Scholar]
- Lamanna, F., Kirschbaum, F. and Tiedemann, R. (2014). De novo assembly and characterization of the skeletal muscle and electric organ transcriptomes of the African weakly electric fish Campylomormyrus compressirostris (Mormyridae, Teleostei). Mol. Ecol. Resour. 14, 1222-1230. 10.1111/1755-0998.12260 [DOI] [PubMed] [Google Scholar]
- Leiserowitz, A., Maibach, E. W., Roser-Renouf, C., Feinberg, G. and Howe, P. (2013). Climate change in the American mind: Americans’ global warming beliefs and attitudes in April 2013. Yale University and George Mason University. New Haven, CT: Yale Project on Climate Change Communication. [Google Scholar]
- Leng, G., Meddle, S. L. and Douglas, A. J. (2008). Oxytocin and the maternal brain. Curr. Opin. Pharmacol. 8, 731-734. 10.1016/j.coph.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Lewis, J. E., Gilmour, K. M., Moorhead, M. J., Perry, S. F. and Markham, M. R. (2014). Action potential energetics at the organismal level reveal a trade-off in efficiency at high firing rates. J. Neurosci. 34, 197-201. 10.1523/JNEUROSCI.3180-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losilla, M. and Gallant, J. R. (2019). The transcriptional correlates of divergent electric organ discharges in Paramormyrops electric fish. Evol. Biol. 223. 10.1101/625475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. and Baum, S. (2016). Trends in community colleges: enrollment, prices, student debt, and completion. College Board Research Brief 4, 1-23. [Google Scholar]
- Malyska, A., Bolla, R. and Twardowski, T. (2016). The role of public opinion in shaping trajectories of agricultural biotechnology. Trends Biotechnol. 34, 530-534. 10.1016/j.tibtech.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Margoluis, R., Stem, C., Salafsky, N. and Brown, M. (2009). Design alternatives for evaluating the impact of conservation projects. New Directions for Evaluation 2009, 85-96. 10.1002/ev.298 [DOI] [Google Scholar]
- Markham, M. R. (2013). Electrocyte physiology: 50 years later. J. Exp. Biol. 216, 2451-2458. 10.1242/jeb.082628 [DOI] [PubMed] [Google Scholar]
- Martin, A. and Orgogozo, V. (2013). The Loci of repeated evolution: a catalog of genetic hotspots of phenotypic variation. Evolution 67, 1235-1250. 10.1111/evo.12081 [DOI] [PubMed] [Google Scholar]
- Melear, C. T., Goodlaxson, J. D., Warne, T. R. and Hickok, L. G. (2000). Teaching preservice science teachers how to do science: responses to the research experience. JSTE 11, 77-90. 10.1023/A:1009479915967 [DOI] [Google Scholar]
- Moskowitz, N. A., Dorritie, B., Fay, T., Nieves, O. C., Vidoudez, C., Cambridge Lindge and Latin 2017 Biology Class, Class, M. 2017 B., Fischer, E. K., Trauger, S. A., Coloma, L. A., et al. (2019). Land use impacts poison frog chemical defenses through changes in leaf litter ant communities. bioRxiv. 10.1101/745976 [DOI] [Google Scholar]
- Nagel, R., Kirschbaum, F. and Tiedemann, R. (2017). Electric organ discharge diversification in mormyrid weakly electric fish is associated with differential expression of voltage-gated ion channel genes. J Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 203, 183-195. 10.1007/s00359-017-1151-2 [DOI] [PubMed] [Google Scholar]
- Nagel, R., Kirschbaum, F., Engelmann, J., Hofmann, V., Pawelzik, F. and Tiedemann, R. (2018a). Male-mediated species recognition among African weakly electric fishes. R. Soc. Open Sci. 5, 170443. 10.1098/rsos.170443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel, R., Kirschbaum, F., Hofmann, V., Engelmann, J. and Tiedemann, R. (2018b). Electric pulse characteristics can enable species recognition in African weakly electric fish species. Sci. Rep. 8, 10799. 10.1038/s41598-018-29132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos, C., Tassino, B., Rosenthal, G. and Reichard, M. (2015). Reproductive behavior and sexual selection in annual fishes. Annual Fishes 207-230. 10.1201/b19016-16 [DOI] [Google Scholar]
- Picq, S., Sperling, J., Cheng, C. J., Carlson, B. A. and Gallant, J. R. (2019). Genetic drift does not sufficiently explain patterns of electric signal variation among populations of the mormyrid electric fish Paramormyrops kingsleyae. bioRxiv 154047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinch, M., Guth, R., Samanta, M. P., Chaidez, A. and Unguez, G. A. (2016). The myogenic electric organ of Sternopygus macrurus: a non-contractile tissue with a skeletal muscle transcriptome. PeerJ 4, e1828. 10.7717/peerj.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers, W. R., Constantinou, S. J., Losilla, M. and Gallant, J. R. (2016). Electric fish genomics: progress, prospects, and new tools for neuroethology. J. Physiol. Paris 110, 259-272. 10.1016/j.jphysparis.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Plomin, R., Owen, M. J. and McGuffin, P. (1994). The genetic basis of complex human behaviors. Science 264, 1733-1739. 10.1126/science.8209254 [DOI] [PubMed] [Google Scholar]
- Poeschla, M. and Valenzano, D. R. (2020). The turquoise killifish: a genetically tractable model for the study of aging. J. Exp. Biol. 223, jeb209296. 10.1242/jeb.209296 [DOI] [PubMed] [Google Scholar]
- Pyron, R. A. (2014). Biogeographic analysis reveals ancient continental vicariance and recent oceanic dispersal in amphibians. Syst. Biol. 63, 779-797. 10.1093/sysbio/syu042 [DOI] [PubMed] [Google Scholar]
- Rabosky, D. L., Santini, F., Eastman, J., Smith, S. A., Sidlauskas, B., Chang, J. and Alfaro, M. E. (2013). Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat. Commun. 4, 1958. 10.1038/ncomms2958 [DOI] [PubMed] [Google Scholar]
- Sackton, T. B., Grayson, P., Cloutier, A., Hu, Z., Liu, J. S., Wheeler, N. E., Gardner, P. P., Clarke, J. A., Baker, A. J., Clamp, M., et al. (2019). Convergent regulatory evolution and loss of flight in paleognathous birds. Science 364, 74-78. 10.1126/science.aat7244 [DOI] [PubMed] [Google Scholar]
- Salazar, V. L., Krahe, R. and Lewis, J. E. (2013). The energetics of electric organ discharge generation in gymnotiform weakly electric fish. J. Exp. Biol. 216, 2459-2468. 10.1242/jeb.082735 [DOI] [PubMed] [Google Scholar]
- Santos, J. C., Coloma, L. A. and Cannatella, D. C. (2003). Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc. Natl. Acad. Sci. USA 100, 12792-12797. 10.1073/pnas.2133521100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, J. C., Tarvin, R. D. and O'Connell, L. A. (2016). A review of chemical defense in poison frogs (Dendrobatidae): ecology, pharmacokinetics, and autoresistance. CSIV 13, 305-337. 10.1007/978-3-319-22026-0_21 [DOI] [Google Scholar]
- Saporito, R. A., Donnelly, M. A., Spande, T. F. and Martin Garraffo, H. (2012). A review of chemical ecology in poison frogs. Chemoecology 22, 159-168. 10.1007/s00049-011-0088-0 [DOI] [Google Scholar]
- Silverstein, S. C., Dubner, J., Miller, J., Glied, S. and Loike, J. D. (2009). Teachers’ participation in research programs improves their students’ achievement in science. Science 326, 440-442. 10.1126/science.1177344 [DOI] [PubMed] [Google Scholar]
- Stern, D. L. (2013). The genetic causes of convergent evolution. Nat. Rev. Genet. 14, 751-764. 10.1038/nrg3483 [DOI] [PubMed] [Google Scholar]
- Stern, D. L. and Orgogozo, V. (2009). Is genetic evolution predictable? Science 323, 746-751. 10.1126/science.1158997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard, P. K. (1999). Predation enhances complexity in the evolution of electric fish signals. Nature 400, 254-256. 10.1038/22301 [DOI] [PubMed] [Google Scholar]
- Stoddard, P. K. (2002). The evolutionary origins of electric signal complexity. J. Physiol. Paris 96, 485-491. 10.1016/S0928-4257(03)00004-4 [DOI] [PubMed] [Google Scholar]
- Stoddard, P. K., Zakon, H. H., Markham, M. R. and McAnelly, L. (2006). Regulation and modulation of electric waveforms in gymnotiform electric fish. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 192, 613-624. 10.1007/s00359-006-0101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, G. N., Nee, S. and Felsenstein, J. (2011). Controlling for non-independence in comparative analysis of patterns across populations within species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1410-1424. 10.1098/rstb.2010.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz, J. F. (2010). Genes for high altitudes. Science 329, 40-41. 10.1126/science.1192481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara, T., Terai, Y., Imai, H., Turner, G. F., Koblmüller, S., Sturmbauer, C., Shichida, Y. and Okada, N. (2005). Parallelism of amino acid changes at the RH1 affecting spectral sensitivity among deep-water cichlids from Lakes Tanganyika and Malawi. Proc. Natl. Acad. Sci. USA 102, 5448-5453. 10.1073/pnas.0405302102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, J. P., Lavoue, S. and Hopkins, C. D. (2000). Molecular systematics of the African electric fishes (Mormyroidea: teleostei) and a model for the evolution of their electric organs. J. Exp. Biol. 203, 665-683. [DOI] [PubMed] [Google Scholar]
- Sullivan, J. P., Lavoué, S. and Hopkins, C. D. (2002). Discovery and phylogenetic analysis of a riverine species flock of African electric fishes (Mormyridae: Teleostei). Evolution 56, 597-616. 10.1111/j.0014-3820.2002.tb01370.x [DOI] [PubMed] [Google Scholar]
- Sullivan, J. P., Lavoué, S., Arnegard, M. E. and Hopkins, C. D. (2004). AFLPs resolve phylogeny and reveal mitochondrial introgression within a species flock of African electric fish (Mormyroidea: Teleostei). Evolution 58, 825-841. 10.1111/j.0014-3820.2004.tb00415.x [DOI] [PubMed] [Google Scholar]
- Swapna, I., Ghezzi, A., York, J. M. J. M., Markham, M. R. M. R., Halling, D. B. B., Lu, Y., Gallant, J. R. and Zakon, H. H. (2018). Electrostatic tuning of a potassium channel in electric fish. Curr. Biol. 28, 2094-2102.e5. 10.1016/j.cub.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvin, R. D., Santos, J. C., O'Connell, L. A., Zakon, H. H. and Cannatella, D. C. (2016). Convergent substitutions in a sodium channel suggest multiple origins of toxin resistance in poison frogs. Mol. Biol. Evol. 33, 1068-1081. 10.1093/molbev/msv350 [DOI] [PubMed] [Google Scholar]
- Tarvin, R. D., Borghese, C. M., Sachs, W., Santos, J. C., Lu, Y., O'Connell, L. A., Cannatella, D. C., Harris, R. A. and Zakon, H. H. (2017). Interacting amino acid replacements allow poison frogs to evolve epibatidine resistance. Science 357, 1261-1266. 10.1126/science.aan5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, A., Vo, D., Comfort, C. and Zakon, H. H. (2014). Expression evolution facilitated the convergent neofunctionalization of a sodium channel gene. Mol. Biol. Evol. 31, 1941-1955. 10.1093/molbev/msu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traeger, L. L., Volkening, J. D., Moffett, H., Gallant, J. R., Chen, P. H., Novina, C. D., Phillips, G. N., Anand, R., Wells, G. B., Pinch, M., et al. (2015). Unique patterns of transcript and miRNA expression in the South American strong voltage electric eel (Electrophorus electricus). BMC Genomics 16, 243. 10.1186/s12864-015-1288-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traeger, L. L., Sabat, G., Barrett-Wilt, G. A., Wells, G. B. and Sussman, M. R. (2017). A tail of two voltages: proteomic comparison of the three electric organs of the electric eel. Sci. Adv. 3, e1700523. 10.1126/sciadv.1700523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainin, G., Wilson, K., Wickless, M. and Brooks, D. (2005). Extraordinary animals and expository writing: zoo in the classroom. J. Sci. Educ. Technol. 14, 299-304. 10.1007/s10956-005-7195-z [DOI] [Google Scholar]
- Ujvari, B., Casewell, N. R., Sunagar, K., Arbuckle, K., Wüster, W., Lo, N., O'Meally, D., Beckmann, C., King, G. F., Deplazes, E., et al. (2015). Widespread convergence in toxin resistance by predictable molecular evolution. Proc. Natl. Acad. Sci. USA 112, 11911-11916. 10.1073/pnas.1511706112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin, N. (2005). Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 346, 967-989. 10.1016/j.jmb.2004.12.031 [DOI] [PubMed] [Google Scholar]
- Varner, J. (2014). Scientific outreach: toward effective public engagement with biological science. Bioscience 64, 333-340. 10.1093/biosci/biu021 [DOI] [Google Scholar]
- Warner, M. R., Qiu, L., Holmes, M. J., Mikheyev, A. S. and Linksvayer, T. A. (2019). Convergent eusocial evolution is based on a shared reproductive groundplan plus lineage-specific plastic genes. Nat. Commun. 10, 2651. 10.1038/s41467-019-10546-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygoldt, P. (2009). Evolution of parental care in dart poison frogs (Amphibia: Anura: Dendrobatidae). J. Zool. Syst. Evol. Res. 25, 51-67. 10.1111/j.1439-0469.1987.tb00913.x [DOI] [Google Scholar]
- Wilkens, H. (2010). Genes, modules and the evolution of cave fish. Heredity 105, 413-422. 10.1038/hdy.2009.184 [DOI] [PubMed] [Google Scholar]
- York, R. A. (2018). Assessing the genetic landscape of animal behavior. Genetics 209, 223-232. 10.1534/genetics.118.300712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, H. S., McCauley, D. J., Galetti, M. and Dirzo, R. (2016). Patterns, causes, and consequences of anthropocene defaunation. Ann. Rev. Ecol. Evol. Syst. 47, 333-358. 10.1146/annurev-ecolsys-112414-054142 [DOI] [Google Scholar]
- Yuan, M. L. and Wang, I. J. (2018). Sodium ion channel alkaloid resistance does not vary with toxicity in aposematic Dendrobates poison frogs: an examination of correlated trait evolution. PLoS ONE 13, e0194265. 10.1371/journal.pone.0194265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon, H. H., Lu, Y., Zwickl, D. and Hillis, D. M. (2006). Sodium channel genes and the evolution of diversity in communication signals of electric fishes: convergent molecular evolution. Proc. Natl. Acad. Sci. USA 103, 3675-3680. 10.1073/pnas.0600160103 [DOI] [PMC free article] [PubMed] [Google Scholar]