Abstract
Presently, obesity has emerged as a significant global public health concern due to its escalating prevalence and incidence rates. The gut microbiota, being a crucial environmental factor, has emerged as a key player in the etiology of obesity. Nevertheless, the intricate and specific interactions between obesity and gut microbiota, along with the underlying mechanisms, remain incompletely understood. This review comprehensively summarizes the gut microbiota characteristics in obesity, the mechanisms by which it induces obesity, and explores targeted therapies centered on gut microbiota restoration.
Keywords: gut microbiota, obesity, dysbiosis, metabolism
Introduction
The incidence of obesity, a chronic disease characterized by the abnormal or excessive accumulation of adipose tissue, is steadily rising and has indeed reached epidemic levels and has tripled since 1975 and is projected to continue increasing.1 Excessive body fat resulting from obesity is linked to cardio-metabolic complications such as hypertension, dyslipidemia, nonalcoholic fatty liver disease (NAFLD) and diabetes mellitus.2–5 The clustering of cardiovascular disease risk factors and abnormal metabolism-related factors defines the metabolic syndrome, including obesity, insulin resistance/glucose intolerance, hyperlipidemia, and hypertension.6 Currently, several effective anti-obesity treatments exist, including medications, lifestyle interventions (eg, diet and exercise), and bariatric surgery. However, these interventions can occasionally result in adverse events that lead to treatment discontinuation.7,8 Consequently, investigating the novel pathophysiological mechanisms related to obesity could facilitate the development of future prevention or treatment strategies.
The microbial communities are defined as multi-species assemblages, in which micro-organisms interact with each other in a contiguous environment. The gut serves as the primary reservoir for the colonization of various and abundant microbial communities, including bacteria, archaea, fungi, protists and algae, collectively referred to as the gut microbiota.9 The gut microbiota and their theatre of activity (microbial structural elements, gut environmental conditions and microbial metabolites) together form the term “gut microbiome”.10 The gut microbiota is regarded as a distinct organ that plays crucial physiological roles in regulating metabolic homeostasis. More specifically, the gut microbiota facilitates digestion, produces metabolites, maintains the integrity of gut barrier and shape the immune system.11–13
The gut microbiota and intestinal microecological environment are characterized by fragility and sensitivity, which are easily affected by genetic factors and environmental factors (eg, exposure, lifestyle, diet, exercise).14 Excessive adverse factors, such as smoking and excessive stress, frequently disrupt the homeostasis of the intestinal microecological environment, causing alterations in its composition and abundance that surpass the regulatory capacity of intestinal microecological homeostasis. This disruption further impairs the original physiological function of the gut microbiota, a condition referred to as gut microbiota dysbiosis. Notably, dysbiosis of gut microbiota could disrupt energy homeostasis through various mechanisms, resulting in obesity.15
In the realm of anti-obesity treatments, efforts have been directed towards rectifying gut microbiota dysbiosis and fostering a healthy intestinal microecological environment. For instance, research endeavors have delved into the potential of physical activity and exercise as non-pharmacological therapies. These studies have not only explored their capacity to diminish obesity-related inflammatory signaling pathways but have also examined their role in enhancing the variability of gut microbiota, reinstating the equilibrium of gut microbiota, and ameliorating gut microbiota dysbiosis associated with obesity.16,17 The prospect of leveraging physical activity to regulate gut microbiota, thereby enhancing overall health outcomes and mitigating obesity and its related comorbidities, holds immense appeal. Nevertheless, these mechanisms necessitate in-depth investigation to fully comprehend their intricacies and therapeutic potential.
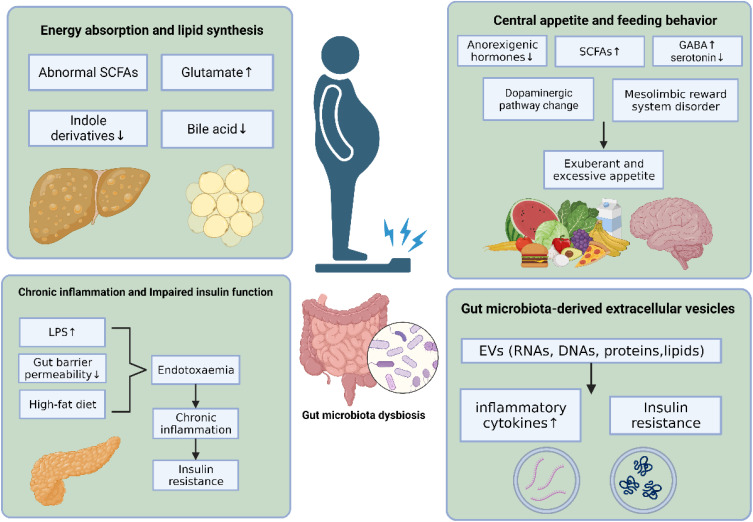
This review endeavors to systematically synthesize existing published evidence concerning the complex relationship between the gut microbiota and obesity. To achieve this, a meticulous search strategy was formulated, incorporating a combination of keywords and MeSH subject headings such as “obesity”, “adiposity”, “gut microbiota” and “dysbiosis”. Databases, including Medline, PubMed, and Web of Science, were systematically explored from the inception of the library to the publication cut-off in June 2023. Special attention was given to scrutinizing full-text articles published within the last five years. And this article presents an overview of the association between obesity and gut microbiota, elucidates the potential mechanisms through which gut microbiota influence obesity (Figure 1), and discusses pertinent therapeutic approaches for obesity treatment targeting gut microbiota.
Figure 1.
The mechanism of gut microbiota in the pathology of obesity.
Association of the Gut Dysbiosis with Obesity
The role of gut microbiota in obesity is initially illuminated through experimental studies in germ-free mice. These studies demonstrated that germ-free mice are leaner and exhibit greater resistance to high calorie intake compared to conventional mice, even when exposed to the same high-fat diet.18 When gut microbiota from obese mice (eg, Enterobacter cloacae), which has been strongly linked to obesity, are transplanted into germ-free mice, the latter display exacerbated metabolic characteristics, including reduced insulin sensitivity, impaired glucose tolerance, and obesity.19,20 Conversely, obese mice exhibiting the symptoms of metabolic syndrome experience a significant reversal of metabolic disadvantage upon receiving fecal microbiota from lean mice.21 The aforementioned evidences support the concept that gut microbiota may serve as a pivotal factor influencing the development and progression of obesity. Nevertheless, while the robust correlation between gut microbiota and obesity might imply a causal link, alternate explanation is also plausible——this association could be coincidental, necessitating a comprehensive exploration. Solely relying on animal models without pertinent human clinical trials hampers the comprehensive understanding of the gut microbiota’s role in human obesity.
Recent studies have further established a close relationship between gut dysbiosis and obesity. Specifically, obese individuals show significant differences in the composition, abundance, and biodiversity of gut microbiota compared to their healthy counterparts.22 Literature reports a potential association between obesity and imbalanced proportions of two dominant bacterial phyla: Firmicutes and Bacteroidetes. More specifically, the gut microbiota of obese mice shows a significant increase in the proportion of Firmicutes and a proportional decrease in Bacteroidetes.23 Similar results are confirmed in humans as well. For instance, two human cohort studies demonstrate a significant elevation in the Firmicutes/Bacteroidetes ratio among obese individuals compared to those who were lean or of normal weight.24,25 However, several recent studies have shown contradictory findings, where some authors observed opposite variations in the Firmicutes/Bacteroidetes ratio, with no significant differences between obese and lean individuals.26,27 The lack of consensus on the relationship trend between obesity and the Firmicutes/Bacteroidetes ratio may be attributed to factors such as ethnicity, lifestyle variations, and dietary habits. Additionally, discrepancies in microbial DNA isolation, sequencing, and bioinformatic processing may also contribute to this variability. While endeavors to delineate a universally “healthy” gut microbiota face challenges arising from interindividual variations, the malleability of gut microbiota, and constraints in measurement methodologies, notable alterations in the gut microbiota of obese individuals, particularly at the genus level, have been documented in contrast to those with normal weight. Table 1 provides a comprehensive summary of the statistically significant changes discerned in the gut microbiota of obese patients.24–32 At the genus level, the abundance of Alistipes, Anaerococcus, Coprococcus, Fusobacterium, Parvimonas, Eubacterium sp., Roseburia sp., Faecalibacterium, Lachnospira, Veillonellaceae, Paraprevotellaceae and Clostridium are enriched in obese subjects compared to lean subjects. In contrast, the abundance of Bacteroides, Desulfovibrio, Lachnoanaerobaculum, Olsenella, Methanobrevibacter smithii, Akkermansia, Eggerthella, Dehalobacterium, Victivallis, and Oscillospira are retracted in such subjects.
Table 1.
Association Between Gut Microbiota and Obesity
| Study | Study Subjects | Comparison | Change of Gut Microbiota | |
|---|---|---|---|---|
| Increased | Decreased | |||
| Andoh 2016.24 | Japanese adult population | Obese vs Lean | Phylum: Firmicutes, Fusobacteria Genera: Alistipes, Anaerococcus, Coprococcus, Fusobacterium, Parvimonas |
Genera: Bacteroides, Desulfovibrio, Lachnoanaerobaculum, Olsenella |
| Kolida 2017.25 | Ukrainian adult cohort | Obese vs NW and UW | Phylum: Firmicutes |
Phylum: Bacteroidetes |
| Yun 2017.28 | Korean adult cohort | Obese and OW vs NW | Genera: Veillonellaceae, Paraprevotellaceae |
Genera: Akkermansia, Eggerthella, Adlercreutzia |
| Gao 2018.26 | Chinese adult population | Obese vs UW | Phylum: Bacteroidetes, Fusobacteria, Proteobacteria Genera: Fusobacterium |
|
| Peters 2018.29 | American adult population | Obese and OW vs NW | Genera: Blautia Streptococcus |
Genera: Dehalobacterium Oscillospira |
| Chavez-Carbajal 2019.30 | Mexican adult population | Obese vs NW | Phylum: Firmicutes, Verrucomicrobia, Spirochaetes, Fusobacteria Genera: Faecalibacterium, Roseburia, Lachnospira, Coprococcus |
Genera: Bacteroides |
| Oduaran 2020.31 | South African adult population | Obese vs Lean | Phylum: Bacteroidetes Genera: Acetanaerobacterium ClostridiumIntestinimonas Oscillibacter Phascolarctobacterium Ruminococcus |
Genera: Fusicatenibacter Parabacteroides Victivallis |
| Loftfield 2020.32 | Northern Finland birth cohort | Obese vs NW | Phylum: Bacteroidetes Genera: Blautia Dorea Roseburia |
|
| Murga-Garrido 2022.27 | Mexican children cohort | Obese vs NW | Genera: Eubacterium sp., Roseburia sp. |
Genera: Bacteroides rodentium, B. intestinalis, B. eggerthii, Methanobrevibacter smithii |
Notes: Comparisons of condition A vs condition B: “Increased” signifies an increase in condition A relative to condition B. “Decreased” signifies a decrease in condition A relative to condition B. There were statistically significant differences between different groups. OW, overweight; NW, normal weight; UW, underweight.
The Mechanism Underlying the Role of Gut Microbiota in Obesity
Energy Absorption and Lipid Synthesis
The human gut microbiota utilizes macronutrients from the diet to synthesize bioactive metabolites, including short-chain fatty acids, amino acids and their derivatives, and bile acids. Dysbiosis of gut microbiota in individuals with obesity results in alterations in the metabolite profile, potentially leading to increased energy absorption and lipid synthesis.
In contrast to conventional normal-weight counterparts, the gut microbiota of obese mice demonstrated heightened utilization of non-digestible carbohydrates (NDC) to produce additional monosaccharides and short-chain fatty acids (SCFAs) for energy acquisition and fat accumulation. These processes were mainly influenced by elevated levels of α-amylases and amylomaltases in gut microbiota associated with obesity.33 SCFAs play a pivotal role as substrates for energy generation and lipogenesis. They furnish 60 to 70% of the necessary caloric input for colonic epithelial cells and contribute to 5 to 15% of the overall caloric requirements for the human body.34,35
Although SCFAs are involved in the body’s energy metabolism, they show dual roles in regulating energy absorption and expenditure, and their specific functions are still controversial. SCFAs inhibit the release of fasting-induced adipocyte factors from intestinal epithelial cells, promoting the accumulation of Lipoprotein lipase-directed triglycerides into adipocytes and promote the development of obesity.36 However, SCFAs also have the potential to enhance thermogenesis and fatty acid oxidation by phosphorylating AMP-activated kinase and promoting the expression of mitochondrial uncoupled protein 1 and PPAR-γ coactivator 1α, thereby providing protection against obesity development.37 Blaak et al summarized a series of experimental animal studies, which demonstrated that dietary supplements containing single or combined SCFAs effectively attenuated weight gain from a high-fat diet.38 It is imperative to acknowledge that these findings are derived from experimental studies characterized by limited sample sizes, specific model animal groups, and a restricted number of human studies conducted thus far. In human studies, Schwiertz et al found that obese subjects have a different microbial and metabolite profile, resulting in higher SCFAs concentrations compared to their lean counterparts.39 Consistent with the above results, a related clinical study found higher SCFAs excretion to gut dysbiosis, gut permeability, excess adiposity, and cardiometabolic risk factors.40 Controlled and efficacious long-term clinical intervention studies investigating SCFAs in humans are crucially lacking. These studies should encompass precise measurements of SCFAs dosage and metabolic kinetics, considering metabolic variations arising from diverse human metabolic conditions. Additionally, research should focus on pinpointing advantageous dietary fiber types, fermentation locations, and the specific gut microbiota species involved.
The gut microbiota exerts influence on the metabolic profile of amino acids and their derivatives in the host, thereby contributing to the pathogenesis of excessive lipid synthesis. Liu et al discovered previously unidentified associations between alterations in intestinal microbiota, circulating amino acids, and obesity. Specifically, they found that the abundance of Bacteroides thetaiotaomicron, a glutamate-fermenting bacteria, was significantly reduced in obese individuals and exhibited an inverse correlation with serum glutamate concentration. Elevated serum glutamate concentration contributes to substantial weight gain, heightened lipid synthesis, and alterations in body composition.41 Furthermore, in both obese mice and obese patients, the reduction in indole derivatives generated through tryptophan transformation by the gut microbiota results in an upregulation of miR-181 family expression downstream. This upregulation is associated with adipose tissue inflammation and excessive lipid synthesis, contributing to the development of obesity.42 While certain gut microbiota can produce metabolites, including amino acids and their derivatives, the specific contributors within the human gut remain largely unidentified. This entails confirming their precise functions in the host pathway and unraveling the mechanisms at work in different cells of the intestine and other tissues.
Additionally, the depletion of Bacteroides and Lactobacillus in obese patients results in a decline in bile acid levels, which serve as essential regulators of lipid synthesis and fat storage.43 Within the liver, the activation of Farnesoid X Receptor (FXR) by bile acids suppresses the expression of liver receptor homologue 1 through a mechanism mediated by a small molecule heterodimer partner. This, in turn, inhibits the transactivation of sterol regulatory binding protein 1c (SREBP1c), which is closely linked to genes associated with adipogenesis, ultimately repressing de novo lipogenesis in human hepatic cells and mitigating the development of obesity.44 In addition to the above pathways, FXR stimulates the fibroblast growth factor receptor 4 (FGFR4) on hepatocytes through the induction of fibroblast growth factor 19 (FGF19) release from the intestine. Furthermore, FXR directly inhibits SREBP1c by suppressing peroxisome proliferator-activated receptor-gamma coactivator 1b and its downstream targets.45 Therefore, these studies indicate that the reduced bile acid levels resulting from alterations in the gut microbiota promote hepatic de novo lipogenesis and facilitate fat storage.
The results above emphasize the significant relationship between the gut microbiota, microbial metabolite profile, and adipose tissue in energy storage. Elucidating these mechanisms in-depth will offer fresh perspectives and identify novel targets for obesity treatment.
Chronic Inflammation and Impaired Insulin Function
Chronic, systemic low-grade inflammation is commonly regarded as a key characteristic of metabolic diseases, primarily caused by an elevated concentration of lipopolysaccharide (LPS) in the bloodstream.46 Obese individuals exhibiting gut microbiota dysbiosis commonly display an overgrowth of LPS-producing gram-negative bacteria (eg, Veillonella) and a decrease in bacteria essential for preserving the integrity of the intestinal mucosal barrier (eg, Akkermansia muciniphila).47,48 These alterations in the microbiota composition result in elevated intestinal LPS levels, heightened intestinal mucosal barrier permeability, and enhanced transfer of toxic by-products from the intestine to the bloodstream. In this scenario, an excessive intake of fat from a high-fat diet leads to increased chylomicron production in the gut after a meal. This process further enhances the release of LPS into the circulation through the lymphatic system.49 Therefore, Disturbances in gut microbiota and alterations in intestinal permeability are potential triggers of inflammation in obesity. Unfavorable dietary environmental factors could synergistically contribute to inflammation in obesity.
LPS plays a crucial role in triggering the inflammatory signaling cascade. Elevated LPS levels can directly activate the TLR4/MyD88/IRAK4 signaling pathway in intestinal mucosal epithelial cells, thereby increasing intestinal tight junction permeability.50 LPS from the gut entering the peripheral circulation can further initiate related immune responses in liver and adipose tissue. LPS initially binds to the lipopolysaccharide binding protein (LBP) and subsequently forms a triplet complex with CD14. Subsequently, the triplet complex is transported to the Toll-Like receptor-4 (TLR-4)-myeloid differentiation protein-2 (MD-2) protein complex, where it binds to TLR-4 with the assistance of MD-2. This binding activates TLR-4, inducing dimerization and facilitating the transmission of extracellular molecular signals into the cell, thus activating intracellular signaling pathways. Intracellular signaling activates nuclear factor κB-induced kinase and transforming growth factor β-activated kinase, further leading to the activation of the corresponding nuclear factor kappa β and mitogen-activated protein kinase pathways. These two pathways eventually lead to the release of inflammatory mediators, including chemokines and cytokines (eg, IL-1, IL-6, and TNF-α), resulting in sepsis and inflammation.51–53 This process promotes chronic low-level inflammation, recruiting and activating numerous mature immune cells (including mast cells, macrophages, and dendritic cells) in metabolic tissues, particularly adipose tissue. Consequently, it alters the tissue environment and intensifies the inflammatory process.54 Fuchs et al reported that in healthy individuals, adipose tissue macrophages (ATMs) constitute only 10% to 15% of the stromovascular cells. However, in obese individuals, the presence of inflammatory mediators leads to an increase in the number of ATMs. Additionally, there is a shift from the predominance of anti-inflammatory M2-like macrophages towards pro-inflammatory M1-like macrophages.55 Investigating the subsequent inflammatory cascade and its connection with key inflammatory markers in relation to adverse outcomes of obesity and obesity-related diseases, as well as their potential utility in prognostic prediction models, requires further exploration.
In healthy individuals, an elevation in postprandial plasma glucose concentration effectively stimulates the secretion of insulin, initiating the utilization of extracellular glucose by peripheral organs. This, in turn, leads to glycolysis and oxidative respiration. Insulin resistance (IR), characterized by reduced or partial loss of insulin sensitivity in peripheral organs, results in impaired insulin action. This condition leads to fasting hyperglycemia and the pathological accumulation of fat in the liver and adipose tissue.56,57 The observation of elevated expression levels of inflammatory mediators in adipose tissue during obesity, along with the associated changes in insulin sensitivity and glucose intolerance, played a crucial role in establishing the link between chronic inflammation and metabolic dysfunction.58 Cytokines such as TNF-α or IL-1β present in adipose tissue of rodents and humans influence insulin sensitivity by modulating the gene expression of insulin receptor-1 (IRS-1), glucose transporter 4 (GLUT4), and PPAR-α. This modulation leads to the desensitization of the insulin signaling pathway.59 Additionally, TNF-α can activate critical components of the inflammatory signaling system, including intracellular signaling molecules such as c-Jun N-terminal kinases and inhibitor of kappa β kinase. This activation subsequently leads to a decrease in tyrosine phosphorylation of downstream insulin receptor substrate proteins, resulting in the attenuation of insulin signaling.60 Alterations in gut microbiota significantly impact the metabolism of toxic by-products within the intestines, leading to the promotion of low-grade inflammation. Various inflammatory mediators can influence distinct elements of the insulin signaling pathway, such as insulin receptors, downstream substrates, and transporters. This influence mediates the development of insulin resistance, consequently initiating subsequent events related to obesity.
Central Appetite and Feeding Behavior
Bioactive Substances Pathways
The gut microbiota plays a crucial role in regulating central appetite and feeding behavior through its modulation of intestinal hormones, gut metabolites, and neurotransmitters.
Neuroendocrine mechanisms are activated during the feeding process in response to various stimuli, including nutrients, changes in intestinal osmotic pressure, mechanical stimulation, and other factors. Enteroendocrine cells (EECs), widely distributed in the intestinal epithelium, promptly respond to these stimuli by secreting hormones that play crucial roles in metabolism. These hormones include peptide YY (PYY), pancreatic polypeptide (PP), and glucagon-like peptide 1 (GLP-1), each with distinct yet comparable functions. The primary biological effects of PYY involve inhibiting upper gastrointestinal motility, mucosal fluid and electrolyte secretion, and colonic transit. These combined actions contribute to the promotion of satiety.61 And PP can reduce food intake by slowing gastric emptying, increasing satiety, and increasing energy expenditure. These actions contribute to its role in anti-obesity.62 Similarly, GLP-1 lowers glucagon levels, slows gastric emptying, stimulates insulin synthesis, and reduces food intake.63 Recent evidence has shown that these hormones possess potent anorexigenic effects, influencing appetite and feeding behavior by entering the bloodstream or the enteric nervous system and binding to specific receptors located in enteric neurons, vagal afferents, the hypothalamus, and the brain stem.64 Interestingly, several studies have found that levels of peptide YY and GLP-1 are significantly lower in obese patients compared to normal individuals, and these hormone levels gradually increase after undergoing bariatric surgery.65,66 In a prior study conducted by Schéle et al, it was concluded that the gut microbiota reduces the expression of the anti-obesity neuropeptide GLP-1 precursor proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf). This reduction may contribute to increased food intake, thereby exacerbating the progression of obesity.67 Thus, the regulation of endogenous satiety gut hormones or the administration of exogenous hormone-like constructs to activate their respective receptors, thereby reducing food intake through satiety signaling mechanisms, appears to be a promising approach.
Previous studies have demonstrated that metabolites derived from the microbiota, such as bile acids, SCFAs, and indoles, are capable of regulating the release of these hormones.68–70 SCFAs interact with EECs through their binding to G-protein-coupled receptors GPR41 and GPR43, leading to the release of PYY and GLP-1. This interaction further modulates the expression profile of neuropeptides involved in the regulation of postprandial satiety.71 Butyrate, which is a component of short-chain fatty acids, exhibits the ability to cross the blood-brain barrier and bind directly to receptors in the hypothalamus. This activation of the vagus nerve subsequently impacts the host’s appetite and feeding behavior.72 Additionally, lactate, produced by Bifidobacterium and Lactobacillus, can serve as a substrate fuel for neuron cells, thereby extending postprandial satiety.73
The gut microbiota contributes to the production of neuroactive metabolites, including γ-aminobutyric acid (GABA) and serotonin, which influence the modulation of appetite and feeding behavior.74 GABA, a major inhibitory neurotransmitter in the human body, has been shown to play a stimulatory role in regulating hypothalamus-controlled feeding behavior in recent years.75 Existing literature strongly suggests that GABA-producing pathways are actively stimulated by various microbiota bacteria, including species like Bacteroides, Parabacteroides, Escherichia, Bifidobacterium, and Lactobacillus.76,77 Treatment of obese mice with GABA-producing Lactobacillus altered the expression of GABA mRNA in the brain and effectively improved the severe metabolic syndrome associated with obesity.78 Furthermore, recent findings by Kootte demonstrate that obese individuals treated with fecal microbiota transplantation (FMT) from lean donors experience an increase in plasma GABA concentrations.79 Serotonin primarily modulates satiety and stimulates peristalsis by regulating melanocortin neurons, which play a role in maintaining body weight homeostasis.80,81 Conversely, available evidence indicates that obesity is associated with reduced central serotonergic signaling.82 A recent study by Hartstra et al indicated that the gut microbiota can influence the central brain’s serotonin transporter binding potential in obese individuals through sympathetic tone.83
Hedonic and Reward Pathways
The regulation of hedonic and reward pathways also influences central appetite and food intake. The hedonic and reward system is an ancient mechanism essential for promoting survival and facilitating efficient energy storage. However, under certain conditions such as food availability, palatability, and social factors, the reward system can become dysregulated, leading to frequent binge eating and a significant positive energy balance. This reversal of its original survival effect highlights its involvement in the development of obesity.84,85
The involvement of the mesocorticolimbic dopamine pathway centered in the striatum in the hedonic system is now well established.86 The brain structures involved in this pathway include the ventral tegmental area (VTA), which contains dopamine-rich neurons, as well as the striatum regions where the nerve axons project, such as the nucleus accumbens (Nac) and the prefrontal cortex (PFC).87 Afferent signals, including those related to food intake and the subsequent modulation of gut hormone levels, can be transmitted through the vagus nerve, which is integrated in the nucleus tractus solitarius. These signals are then projected to the aforementioned regions that are crucial for energy balance regulation, stimulating the release of dopamine and completing the stimulus-action-reward mechanism cycle, thereby promoting feeding behavior.88,89 Recent studies have demonstrated an association between microbial dysbiosis in obesity and vagus innervation and signaling. For instance, variations in gut microbiota composition can result in changes in plasma lipopolysaccharide, which are breakdown products of Gram-negative bacteria. Elevated LPS disrupts signaling in the vagus nerve while simultaneously reducing the satiety effect of gastrointestinal hormones. This disruption and reduction result in the occurrence of excessive swallowing, hyperactive central appetite, and abnormal feeding behavior.90,91
From another perspective, alteration in the dopamine receptor and dopamine transporter involved in the hedonic and reward pathway have been associated with the changes in gut microbiota.92,93 More specifically, certain microbes belonging to genera such as Bacteroides, Lactobacillus, Clostridium have been demonstrated that modulate target receptors and transporters of the dopaminergic pathway in a direct or indirect manner.94 By transplanting the fecal microbiota from obese mice into lean subjects, de Wouters d’Oplinter et al observed that the dietary behavior of recipient mice was more similar as that of their obese donor. At the same time, they also detected that the recipient mice and the donor mice had parallel alterations in dopamine pathway, including the changes of dopamine D1 receptors and D2 receptors in the striatum, and the increased expression of dopaminergic transporter.95 What is more noteworthy is that the diet-induced gut microbiota turbulence and mesolimbic reward system disorder in obese maternal mice seem to be inherited to the next generation of mice, resulting in the change of gene expression in the mesolimbic reward system of offspring mice, thus promoting the offspring mice to consume more delicious food, leading to obesity.96
In summary, the gut microbiota modulates central appetite and feeding behavior via a variety of mechanisms, including but not limited to affecting the production of bioactive substances and participating in regulation hedonic and reward pathways. It seems reasonable to speculate that the gut microbiota is closely linked to the pathogenesis of obesity due to its significant roles in modulate central appetite and eating behavior.
Effects of Gut Microbiota-Derived Extracellular Vesicles on Obesity
Extracellular vesicles (EVs) derived from the gut microbiota can encapsulate a wide range of bioactive cargo, such as RNAs, DNAs, proteins, and lipids. These cargos have the potential to modulate gut barrier integrity and regulate tissue immune responses.97 Growing evidence has confirmed that EVs derived from the gut microbiota may serve as relevant mediators of microbial-host interactions, influencing intestinal homeostasis and potentially contributing to the pathogenesis of metabolic diseases. Chelakkot et al reported a direct relationship between Akkermansia muciniphila EVs and the improvement of gut barrier integrity and metabolic characteristics in obese mice induced by a high-fat diet. Specifically, the EVs up-regulated the expression of the intestinal barrier protein occludin, reduced intestinal barrier permeability, and consequently led to decreased weight gain and improved glucose tolerance.98 Hiippala et al confirmed the protective effect of commensal bacterial EVs-mediated inflammatory response in a study. They found that EVs derived from commensal Odoribacter splanchnicus 57 exhibited immunomodulatory characteristics and reduced the production of the inflammatory cytokine IL-8 in intestinal epithelial cells treated with E. coli LPS.99
On the other hand, additional studies have revealed the detrimental roles of specific gut microbiota EVs in triggering pro-inflammatory responses and promoting insulin resistance. Previous studies have shown that EVs derived from commensal gut microbiota can increase the secretion of pro-inflammatory cytokines, including IL-6, IL-8, and TNF-α. This suggests that EVs from these strains play a role in regulating intestinal inflammatory responses.100 Importantly, the profile alteration of gut microbiota EVs is more susceptible than changes in gut microbe composition. Due to the specific structure and low molecular weight of EVs compared to the gut microbiota, EVs can more easily penetrate the circulation and be transported to insulin-acting peripheral organs. This impacts metabolism-related pathways and worsens metabolic disorders in the context of gut microbiota dysbiosis. Choi verified this hypothesis and reported that EVs can impair glucose metabolism by promoting insulin resistance in adipose tissue, thereby exacerbating the metabolic disorders induced by a high-fat diet.101 Additionally, Gao demonstrated that intestinal EVs can move into the host circulation and transfer microbial DNA into pancreatic β-cells of obese individuals. This process activates the cGAS/STING pathway, initiating an inflammatory response and impairing insulin secretion from β-cells, thus worsening the condition of obesity.102
It is noteworthy that gut microbiota is intricately linked to the expression profile of EVs. These EVs, crucial in intercellular communication, can transport products from harmful bacteria across the intestinal mucosal barrier and into the systemic circulation, thereby exacerbating the onset and progression of obesity. However, a critical step forward involves a comprehensive screening of the specific pathogenic molecules within the cargo transported by EVs.
The Emerging Role of Gut Microbiota in Obesity Potential Therapies
FMT
FMT is defined as the introduction of a fecal supernatant suspension, obtained from a screened healthy donor, into the gastrointestinal tract of a recipient with the goal of restoring gut microbiota and treating associated diseases.103 In 2013, Els et al conducted the pioneering application of FMT in a randomized clinical trial to assess its efficacy in treating clostridioides difficile infection (CDI).104 Subsequently, the scope of FMT’s applications broadened significantly. Its clinical use transitioned from infectious diseases to encompass non-infectious conditions such as ulcerative colitis, Crohn’s disease, and obesity.105 Recent landmark studies have shown metabolic improvements in patients with obesity who received FMT from lean healthy donors, including increased insulin sensitivity and restoration of metabolite composition.106 Additionally, Mocanu made further enhancements to the FMT technique by combining it with adjuvant fiber supplementation. Clinical human trials demonstrated that this approach effectively delayed the progressive loss of donor microbe engraftment and significantly improved insulin sensitivity in severely obese patients over a six-week period.107 Remarkably, FMT has the ability to transfer the entire gut microbiota, as well as by-products, from healthy lean donors to recipients. However, improper donor screening and inadequate analysis of fecal donor material can lead to infection with opportunistic pathogenic bacteria. This can further worsen dysbiosis in obese recipients, leading to severe adverse effects such as vomiting and sepsis. These safety concerns raise apprehensions regarding the use of FMT.108,109 Despite the promising potential of FMT, recent randomized clinical trials have shown that it does not have significant effects on metabolic profiles and weight loss.110,111 These conflicting findings necessitate further investigation.
Probiotics
Probiotics are defined as living microorganisms that confer beneficial effects on the host when administered in sufficient quantities.112 In contrast to FMT, probiotic therapy has been shown to exert anti-obesity effects through the antagonism of pathogenic microorganism growth, reduction of intestinal barrier permeability, and modulation of the gastrointestinal immune system. Several probiotics, whether used alone or in symbiotic mixtures, have demonstrated these effects.113 More specifically, Lactobacillus and Bifidobacterium have been successfully utilized in established animal models of obesity and clinical trials, resulting in varying degrees of improvement in insulin resistance and reduction of fat accumulation compared to a placebo.114 Abenavoli et al summarized the specific strains that have been validated for their anti-obesity effects in animal or clinical trials, including Lactobacillus (eg, L. Casei strain Shirota (LAB13), L. Gasseri, L. Rhamnosus and L. Plantarum) and Bifidobacterium (eg, B. Infantis, B. Longum, and B. Breve B3) species.115 However, a recent meta-analysis of randomized controlled human studies reported that the relationship between probiotic treatment and weight loss was not significant.116 Furthermore, it is worthwhile to conduct further investigations on isolating specific and effective bacterial species, as well as increasing the survival and longevity of beneficial bacteria.
Bariatric Surgery
Bariatric surgery is widely regarded as an effective treatment for obesity, offering substantial and long-lasting metabolic improvements to severely obese patients. Common bariatric surgery procedures include adjustable gastric banding (AGB), sleeve gastrectomy (SG), and Roux-en-Y gastric bypass (RYGB).117 In a clinical study with a small sample size, the authors observed a significant improvement in the composition of gut microbiota in patients who underwent bariatric surgery. This finding suggests that the surgery may alter the intestinal microenvironment, thereby impacting food digestion and absorption.118 Li et al conducted animal experiments and discovered that RYGB caused a significant alteration in the abundance of twenty-two microbial species. Moreover, these changes exhibited a significant correlation with weight control as well as improvements in metabolic and inflammatory parameters.119 Subsequently, Liou AP et al have transferred microbiota from mice that underwent RYGB to non-operated obese mice and observed that these recipient mice experienced weight loss and a reduction in fat mass.120 Collectively, these findings provide substantial support for the hypothesis that a connection exists between alterations in gut microbiota and weight loss in hosts, leading to improved metabolism after weight loss surgery.
Physical Activity and Exercise
Physical activity is described as any bodily movement that engages skeletal muscles and results in energy expenditure in daily life.121 Exercise is a subset of physical activity, specifically denoting planned, structured, and repetitive movements aimed at enhancing the health of specific body parts.122 Physical activity and exercise persist as the predominant and highly advocated methods for weight loss. Research has demonstrated that physical activity can enhance microbial diversity and rebalance the Firmicutes/Bacteroidetes ratio.123 These effects play a pivotal role in halting the advancement of obesity and facilitating weight reduction. Research conducted by Bressa et al and Mahdieh et al revealed that short doses of physical activity resulted in a significant increase in the health-promoting bacteria, notably Bifidobacterium spp., Akkermansia muciniphila, P. Roseburia hominis, and Faecalibacterium prausnitzii.124,125 In adherence to the World Health Organization recommendations for physical activity guidelines, physical activity appears to counteract detrimental changes in the gut microbiota, consequently impeding the exacerbation of metabolic disorders in obesity, even in the absence of dietary intervention. The effectiveness of anti-obesity strategies centered around physical exercise is notably impacted by socioeconomic and lifestyle factors. Low compliance among obese patients further hampers the efficacy of these approaches. Currently, integrating physical activity with other treatments presents a promising avenue. Such combined interventions have the potential to yield synergistic anti-obesity effects.
Conclusions
Despite the existing heterogeneity in the research data and the absence of a unanimous definition for a “healthy” gut microbiota, consistent observations demonstrate the presence of gut microbiota dysbiosis in obese patients. Furthermore, discernible distinctions in gut microbiota characteristics exist between obese and non-obese individuals. Gut microbiota dysbiosis is likely implicated in the pathogenesis of obesity due to its influence on host energy absorption, lipid synthesis, chronic inflammation, central appetite regulation, and involvement in exosome-driven pathways. Currently, certain therapeutic interventions aimed at restoring gut microbiota show potential as treatments for obesity, including probiotics, bariatric surgery, FMT, physical activity and exercise. Despite the significant potential of these therapies in obesity treatment, their safety and efficacy require rigorous evaluation, and further exploration is necessary concerning the key strains and their related pathogenic mechanisms.
Acknowledgments
Zequn Zhuang and Peng Zhou are co-first authors for this study. We thank all the participants who contributed to this article. We express our gratitude to the Biorender website for providing us with significant convenience in creating figure for this review.
Funding Statement
This work was supported by the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (BJ2020032), Project of Wuxi Translational Medicine Center (2020ZHYB10), Project of Wuxi Science and Technology Development Fund (Y20212004), Wuxi Taihu Lake Talent Plan, Team in Medical and Health Profession (2021), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX23_0690).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. doi: 10.1016/j.metabol.2022.155217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and Cardiovascular Disease: a Scientific Statement From the American Heart Association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15(4):230–240. doi: 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- 4.Makri E, Goulas A, Polyzos SA. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch Med Res. 2021;52(1):25–37. doi: 10.1016/j.arcmed.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 5.Piche ME, Tchernof A, Despres JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res. 2020;126(11):1477–1500. doi: 10.1161/CIRCRESAHA.120.316101 [DOI] [PubMed] [Google Scholar]
- 6.Lemieux I, Després JP. Metabolic Syndrome: past, Present and Future. Nutrients. 2020;12(11):3501. doi: 10.3390/nu12113501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe BM, Kvach E, Eckel RH. Treatment of Obesity: weight Loss and Bariatric Surgery. Circ Res. 2016;118(11):1844–1855. doi: 10.1161/CIRCRESAHA.116.307591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera R, Murad MH, Chandar AK, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: a Systematic Review and Meta-analysis. JAMA. 2016;315(22):2424–2434. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–1262. doi: 10.1126/science.1224203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg G, Rybakova D, Fischer D, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8(1):103. doi: 10.1186/s40168-020-00875-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul Rahim MBH, Chilloux J, Martinez-Gili L, et al. Diet-induced metabolic changes of the human gut microbiome: importance of short-chain fatty acids, methylamines and indoles. Acta Diabetol. 2019;56(5):493–500. doi: 10.1007/s00592-019-01312-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leibovitzh H, Lee SH, Xue M, et al. Altered Gut Microbiome Composition and Function Are Associated With Gut Barrier Dysfunction in Healthy Relatives of Patients With Crohn’s Disease. Gastroenterology. 2022;163(5):1364–1376.e1310. doi: 10.1053/j.gastro.2022.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Li W, Deng Y, Chu Q, Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41–47. doi: 10.1016/j.canlet.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 14.Gacesa R, Kurilshikov A, Vich Vila A, et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature. 2022;604(7907):732–739. [DOI] [PubMed] [Google Scholar]
- 15.Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022;147:112678. doi: 10.1016/j.biopha.2022.112678 [DOI] [PubMed] [Google Scholar]
- 16.Silva JSC, Seguro CS, Naves MMV. Gut microbiota and physical exercise in obesity and diabetes - A systematic review. Nutr Metab Cardiovasc Dis. 2022;32(4):863–877. doi: 10.1016/j.numecd.2022.01.023 [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro FM, Silva MA, Lyssa V, et al. The molecular signaling of exercise and obesity in the microbiota-gut-brain axis. Front Endocrinol (Lausanne). 2022;13:927170. doi: 10.3389/fendo.2022.927170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB j. 2010;24(12):4948–4959. doi: 10.1096/fj.10-164921 [DOI] [PubMed] [Google Scholar]
- 19.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. Isme j. 2013;7(4):880–884. doi: 10.1038/ismej.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John GK, Mullin GE. The Gut Microbiome and Obesity. Curr Oncol Rep. 2016;18(7):45. doi: 10.1007/s11912-016-0528-7 [DOI] [PubMed] [Google Scholar]
- 21.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916.e917. doi: 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 22.Pinart M, Dotsch A, Schlicht K, et al. Gut Microbiome Composition in Obese and Non-Obese Persons: a Systematic Review and Meta-Analysis. Nutrients. 2021;14(1):12. doi: 10.3390/nu14010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andoh A, Nishida A, Takahashi K, et al. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr. 2016;59(1):65–70. doi: 10.3164/jcbn.15-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. doi: 10.1186/s12866-017-1027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao X, Zhang M, Xue J, et al. Body Mass Index Differences in the Gut Microbiota Are Gender Specific. Front Microbiol. 2018;9:1250. doi: 10.3389/fmicb.2018.01250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murga-Garrido SM, Orbe-Orihuela YC, Díaz-Benítez CE, et al. Alterations of the Gut Microbiome Associated to Methane Metabolism in Mexican Children with Obesity. Children. 2022;9(2). doi: 10.3390/children9020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun Y, Kim HN, Kim SE, et al. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017;17(1):151. doi: 10.1186/s12866-017-1052-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters BA, Shapiro JA, Church TR, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8(1):9749. doi: 10.1038/s41598-018-28126-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chávez-Carbajal A, Nirmalkar K, Pérez-Lizaur A, et al. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int J Mol Sci. 2019;20(2):438. doi: 10.3390/ijms20020438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oduaran OH, Tamburini FB, Sahibdeen V, et al. Gut microbiome profiling of a rural and urban South African cohort reveals biomarkers of a population in lifestyle transition. BMC Microbiol. 2020;20(1):330. doi: 10.1186/s12866-020-02017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loftfield E, Herzig KH, Caporaso JG, et al. Association of Body Mass Index with Fecal Microbial Diversity and Metabolites in the Northern Finland Birth Cohort. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2289–2299. doi: 10.1158/1055-9965.EPI-20-0824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27(2):201–214. doi: 10.1177/0884533611436116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mithieux G. New data and concepts on glutamine and glucose metabolism in the gut. Curr Opin Clin Nutr Metab Care. 2001;4(4):267–271. doi: 10.1097/00075197-200107000-00004 [DOI] [PubMed] [Google Scholar]
- 35.Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev. 2013;14(12):950–959. doi: 10.1111/obr.12068 [DOI] [PubMed] [Google Scholar]
- 36.Mandard S, Zandbergen F, van Straten E, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281(2):934–944. doi: 10.1074/jbc.M506519200 [DOI] [PubMed] [Google Scholar]
- 37.Lin HV, Frassetto A, Kowalik Jr EJ Jr, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaak EE, Canfora EE, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11(5):411–455. doi: 10.3920/BM2020.0057 [DOI] [PubMed] [Google Scholar]
- 39.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190–195. doi: 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- 40.de la Cuesta-Zuluaga J, Mueller NT, Alvarez-Quintero R, et al. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients. 2018;11(1):51. doi: 10.3390/nu11010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–868. doi: 10.1038/nm.4358 [DOI] [PubMed] [Google Scholar]
- 42.Virtue AT, McCright SJ, Wright JM, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019;11(496). doi: 10.1126/scitranslmed.aav1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200 [DOI] [PubMed] [Google Scholar]
- 44.Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28(1):220–224. doi: 10.1159/000282091 [DOI] [PubMed] [Google Scholar]
- 45.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284(15):10023–10033. doi: 10.1074/jbc.M808818200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55. doi: 10.1016/j.immuni.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology. 2016;31(4):283–293. doi: 10.1152/physiol.00041.2015 [DOI] [PubMed] [Google Scholar]
- 48.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747–756. doi: 10.1016/S2468-1253(17)30147-4 [DOI] [PubMed] [Google Scholar]
- 50.Guo S, Nighot M, Al-Sadi R, Alhmoud T, Nighot P, Ma TY. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J Immunol. 2015;195(10):4999–5010. doi: 10.4049/jimmunol.1402598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne). 2012;3:181. doi: 10.3389/fendo.2012.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neal MD, Leaphart C, Levy R, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176(5):3070–3079. doi: 10.4049/jimmunol.176.5.3070 [DOI] [PubMed] [Google Scholar]
- 53.Behzadi P, García-Perdomo HA, Karpiński TM. Toll-Like Receptors: general Molecular and Structural Biology. J Immunol Res. 2021;2021:9914854. doi: 10.1155/2021/9914854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chng MH, Alonso MN, Barnes SE, Nguyen KD, Engleman EG. Adaptive Immunity and Antigen-Specific Activation in Obesity-Associated Insulin Resistance. Mediators Inflamm. 2015;2015:593075. doi: 10.1155/2015/593075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuchs A, Samovski D, Smith GI, et al. Associations Among Adipose Tissue Immunology, Inflammation, Exosomes and Insulin Sensitivity in People With Obesity and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161(3):968–981.e912. doi: 10.1053/j.gastro.2021.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gasmi A, Noor S, Menzel A, Doşa A, Pivina L, Bjørklund G. Obesity and Insulin Resistance: associations with Chronic Inflammation, Genetic and Epigenetic Factors. Curr Med Chem. 2021;28(4):800–826. doi: 10.2174/0929867327666200824112056 [DOI] [PubMed] [Google Scholar]
- 58.Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375–C391. doi: 10.1152/ajpcell.00379.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. doi: 10.1186/s13073-016-0303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–198. doi: 10.1038/nm1185 [DOI] [PubMed] [Google Scholar]
- 61.Lafferty RA, Flatt PR, Irwin N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides. 2018;100:269–274. doi: 10.1016/j.peptides.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 62.Khandekar N, Berning BA, Sainsbury A, Lin S. The role of pancreatic polypeptide in the regulation of energy homeostasis. Mol Cell Endocrinol. 2015;418(Pt 1):33–41. doi: 10.1016/j.mce.2015.06.028 [DOI] [PubMed] [Google Scholar]
- 63.Salehi M, Purnell JQ. The Role of Glucagon-Like Peptide-1 in Energy Homeostasis. Metab Syndr Relat Disord. 2019;17(4):183–191. doi: 10.1089/met.2018.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng Z, Zhang L, Yang L, Chu H. The critical role of gut microbiota in obesity. Front Endocrinol (Lausanne). 2022;13:1025706. doi: 10.3389/fendo.2022.1025706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol. 2009;587(1):19–25. doi: 10.1113/jphysiol.2008.164269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Federico A, Dallio M, Tolone S, et al. Gastrointestinal Hormones, Intestinal Microbiota and Metabolic Homeostasis in Obese Patients: effect of Bariatric Surgery. Vivo. 2016;30(3):321–330. [PubMed] [Google Scholar]
- 67.Schéle E, Grahnemo L, Anesten F, Hallén A, Bäckhed F, Jansson JO. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology. 2013;154(10):3643–3651. doi: 10.1210/en.2012-2151 [DOI] [PubMed] [Google Scholar]
- 68.Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9(4):1202–1208. doi: 10.1016/j.celrep.2014.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Modasia A, Parker A, Jones E, et al. Regulation of Enteroendocrine Cell Networks by the Major Human Gut Symbiont Bacteroides thetaiotaomicron. Front Microbiol. 2020;11:575595. doi: 10.3389/fmicb.2020.575595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larraufie P, Martin-Gallausiaux C, Lapaque N, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8(1):74. doi: 10.1038/s41598-017-18259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong DH, Beiko RG. Transfer of energy pathway genes in microbial enhanced biological phosphorus removal communities. BMC Genomics. 2015;16(1):526. doi: 10.1186/s12864-015-1752-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silberbauer CJ, Surina-Baumgartner DM, Arnold M, Langhans W. Prandial lactate infusion inhibits spontaneous feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278(3):R646–R653. doi: 10.1152/ajpregu.2000.278.3.R646 [DOI] [PubMed] [Google Scholar]
- 74.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–239. [DOI] [PubMed] [Google Scholar]
- 75.Delgado TC. Glutamate and GABA in Appetite Regulation. Front Endocrinol (Lausanne). 2013;4:103. doi: 10.3389/fendo.2013.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi: 10.1038/s41564-018-0307-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693(Pt B):128–133. doi: 10.1016/j.brainres.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patterson E, Ryan PM, Wiley N, et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci Rep. 2019;9(1):16323. doi: 10.1038/s41598-019-51781-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kootte RS, Levin E, Salojärvi J, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26(4):611–619.e616. doi: 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 80.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. 2016;8(1). doi: 10.3390/nu8010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51(2):239–249. doi: 10.1016/j.neuron.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 82.van Galen KA, Ter Horst KW, Booij J, la Fleur SE, Serlie MJ. The role of central dopamine and serotonin in human obesity: lessons learned from molecular neuroimaging studies. Metabolism. 2018;85:325–339. doi: 10.1016/j.metabol.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 83.Hartstra AV, Schüppel V, Imangaliyev S, et al. Infusion of donor feces affects the gut-brain axis in humans with metabolic syndrome. Mol Metab. 2020;42:101076. doi: 10.1016/j.molmet.2020.101076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Araujo IE, Schatzker M, Small DM. Rethinking Food Reward. Annu Rev Psychol. 2020;71(1):139–164. doi: 10.1146/annurev-psych-122216-011643 [DOI] [PubMed] [Google Scholar]
- 85.Riediger T. The receptive function of hypothalamic and brainstem centres to hormonal and nutrient signals affecting energy balance. Proc Nutr Soc. 2012;71(4):463–477. doi: 10.1017/S0029665112000778 [DOI] [PubMed] [Google Scholar]
- 86.Luo SX, Huang EJ. Dopaminergic Neurons and Brain Reward Pathways: from Neurogenesis to Circuit Assembly. Am J Pathol. 2016;186(3):478–488. doi: 10.1016/j.ajpath.2015.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, Pöppel E. Dopaminergic reward system: a short integrative review. Int Arch Med. 2010;3:24. doi: 10.1186/1755-7682-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ueno H, Nakazato M. Mechanistic relationship between the vagal afferent pathway, central nervous system and peripheral organs in appetite regulation. J Diabetes Investig. 2016;7(6):812–818. doi: 10.1111/jdi.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Son J, Koekkoek LL, La Fleur SE, Serlie MJ, Nieuwdorp M. The Role of the Gut Microbiota in the Gut-Brain Axis in Obesity: mechanisms and Future Implications. Int J Mol Sci. 2021;22(6):2993. doi: 10.3390/ijms22062993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sen T, Cawthon CR, Ihde BT, et al. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol Behav. 2017;173:305–317. doi: 10.1016/j.physbeh.2017.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de La Serre CB, de Lartigue G, Raybould HE. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav. 2015;139:188–194. doi: 10.1016/j.physbeh.2014.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jadhav KS, Peterson VL, Halfon O, et al. Gut microbiome correlates with altered striatal dopamine receptor expression in a model of compulsive alcohol seeking. Neuropharmacology. 2018;141:249–259. doi: 10.1016/j.neuropharm.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 93.Nettleton JE, Klancic T, Schick A, et al. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients. 2019;11(6):1248. doi: 10.3390/nu11061248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamamah S, Aghazarian A, Nazaryan A, Hajnal A, Covasa M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines. 2022;10(2):436. doi: 10.3390/biomedicines10020436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Wouters d’Oplinter A, Huwart SJP, Cani PD, Everard A. Gut microbes and food reward: from the gut to the brain. Front Neurosci. 2022;16:947240. doi: 10.3389/fnins.2022.947240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nettleton JE, Cho NA, Klancic T, et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut. 2020;69(10):1807–1817. doi: 10.1136/gutjnl-2018-317505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schaack B, Hindré T, Quansah N, Hannani D, Mercier C, Laurin D. Microbiota-Derived Extracellular Vesicles Detected in Human Blood from Healthy Donors. Int J Mol Sci. 2022;23(22):13787. doi: 10.3390/ijms232213787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chelakkot C, Choi Y, Kim DK, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50(2):e450. doi: 10.1038/emm.2017.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hiippala K, Barreto G, Burrello C, et al. Novel Odoribacter splanchnicus Strain and Its Outer Membrane Vesicles Exert Immunoregulatory Effects in vitro. Front Microbiol. 2020;11:575455. doi: 10.3389/fmicb.2020.575455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cañas MA, Fábrega MJ, Giménez R, Badia J, Baldomà L. Outer Membrane Vesicles From Probiotic and Commensal Escherichia coli Activate NOD1-Mediated Immune Responses in Intestinal Epithelial Cells. Front Microbiol. 2018;9:498. doi: 10.3389/fmicb.2018.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi Y, Kwon Y, Kim DK, et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep. 2015;5:15878. doi: 10.1038/srep15878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao H, Luo Z, Ji Y, et al. Accumulation of microbial DNAs promotes to islet inflammation and beta cell abnormalities in obesity in mice. Nat Commun. 2022;13(1):565. doi: 10.1038/s41467-022-28239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antushevich H. Fecal microbiota transplantation in disease therapy. Clin Chim Acta. 2020;503:90–98. doi: 10.1016/j.cca.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 104.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 105.Wang JW, Kuo CH, Kuo FC, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc. 2019;118(Suppl 1):S23–s31. doi: 10.1016/j.jfma.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 106.de Groot P, Scheithauer T, Bakker GJ, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69(3):502–512. doi: 10.1136/gutjnl-2019-318320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mocanu V, Zhang Z, Deehan EC, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled Phase 2 trial. Nat Med. 2021;27(7):1272–1279. doi: 10.1038/s41591-021-01399-2 [DOI] [PubMed] [Google Scholar]
- 108.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437 [DOI] [PubMed] [Google Scholar]
- 109.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117–127. doi: 10.1016/j.jhin.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 110.Leong KSW, Jayasinghe TN, Wilson BC, et al. Effects of Fecal Microbiome Transfer in Adolescents With Obesity: the Gut Bugs Randomized Controlled Trial. JAMA Netw Open. 2020;3(12):e2030415. doi: 10.1001/jamanetworkopen.2020.30415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu EW, Gao L, Stastka P, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17(3):e1003051. doi: 10.1371/journal.pmed.1003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beyaz Coşkun A, Sağdiçoğlu Celep AG. Therapeutic modulation methods of gut microbiota and gut-liver axis. Crit Rev Food Sci Nutr. 2022;62(23):6505–6515. doi: 10.1080/10408398.2021.1902263 [DOI] [PubMed] [Google Scholar]
- 113.Marttinen M, Ala-Jaakkola R, Laitila A, Lehtinen MJ. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients. 2020;12(10):2936. doi: 10.3390/nu12102936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cerdó T, García-Santos JA, Campoy C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients. 2019;11(3):635. doi: 10.3390/nu11030635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abenavoli L, Scarpellini E, Colica C, et al. Gut Microbiota and Obesity: a Role for Probiotics. Nutrients. 2019;11(11):2690. doi: 10.3390/nu11112690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dror T, Dickstein Y, Dubourg G, Paul M. Microbiota manipulation for weight change. Microb Pathog. 2017;106:146–161. doi: 10.1016/j.micpath.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 117.Phillips BT, Shikora SA. The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metabolism. 2018;79:97–107. doi: 10.1016/j.metabol.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 118.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60(9):1214–1223. doi: 10.1136/gut.2010.234708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra141. doi: 10.1126/scitranslmed.3005687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peluso MA, Guerra de Andrade LH. Physical activity and mental health: the association between exercise and mood. Clinics. 2005;60(1):61–70. doi: 10.1590/S1807-59322005000100012 [DOI] [PubMed] [Google Scholar]
- 122.Carbone S, Del Buono MG, Ozemek C, Lavie CJ. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 2019;62(4):327–333. doi: 10.1016/j.pcad.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 123.Mitchell CM, Davy BM, Hulver MW, Neilson AP, Bennett BJ, Davy KP. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med Sci Sports Exerc. 2019;51(1):160–167. doi: 10.1249/MSS.0000000000001760 [DOI] [PubMed] [Google Scholar]
- 124.Bressa C, Bailén-Andrino M, Pérez-Santiago J, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. 2017;12(2):e0171352. doi: 10.1371/journal.pone.0171352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mahdieh MS, Maryam J, Bita B, et al. A pilot study on the relationship between Lactobacillus, Bifidibactrium counts and inflammatory factors following exercise training. Arch Physiol Biochem. 2023;129(3):778–787. doi: 10.1080/13813455.2021.1871763 [DOI] [PubMed] [Google Scholar]