Abstract
We describe herein two patients with epidermal growth factor receptor (EGFR) mutated non-small cell lung cancer (NSCLC) who developed cancer-associated ischemic stroke (CAIS), infarction caused by thromboembolism in the central nervous system. Case 1 was a 63-year-old man with Exon 19 deletion type EGFR mutated lung adenocarcinoma presenting with CAIS. Case 2 was a 71-year-old woman with Exon 21 L858R type EGFR mutated lung adenocarcinoma who developed CAIS during chemotherapy after EGFR-tyrosine kinase inhibitor (TKI) resistance. Although there was no recurrence of CAIS in these patients, anticancer therapy could be hampered by the comorbidity of CAIS. This can develop anytime from before clinical manifestations of NSCLC to the next treatment after EGFR-TKI resistance. The development of CAIS should be noted in patients with EGFR mutated NSCLC, who have a promising long-term prognosis. Anticancer and anticoagulant therapies as well as rehabilitation are important for patients who develop CAIS. Establishment of measurement tests to detect CAIS before onset is desired.
Keywords:cancer-associated ischemic stroke, epidermal growth factor receptor, non-small cell lung cancer, tyrosine kinase inhibitor, survival.
INTRODUCTION
Although rare, a few patients with malignant tumors develop ischemic stroke, arterial and venous thrombosis, pulmonary embolism, and non-bacterial thrombotic endocarditis (1-4). Thrombosis caused by cancer has been recognized for many years and many postmortem studies have confirmed an increased incidence of thromboembolic mortality in a variety of cancers, including lung cancer (1). This is commonly called cancer-as- sociated thrombosis or cancer-associated ischemic stroke (CAIS) (1-4). Causative malignant tumors are known to occur in gynecological tumors, gastrointestinal cancer and lung cancer (1). With regard to the treatment of lung cancer, the discovery of driver genes and the introduction of tyrosine kinase inhibitors (TKIs) against them have brought about a revolution (5). In particular, EGFR gene mutations and EGFR-TKIs, which were pioneering driver genes, became widespread in the 2000s. This dramatically improved the survival of patients with this mutation (6, 7). However, in patients with severe comorbidities, prognosis remains poor even with the presence of driver genes and corresponding TKIs (8). Most recently, a study of complications of EGFR-TKIs in propensity-matched non-small cell lung cancer (NSCLC) patients was reported (9). Interestingly, EGFR-TKI-treated patients seemed to have a higher incidence of cerebrovascular disorders. We herein report two EGFR-mutated patients with CAIS: concomitant diagnosis of lung cancer and CAIS in one patient, and metachronous diagnosis of CAIS during administration of cytotoxic anticancer drugs after development of EGFR-TKI resistance in another patient. CAIS in patients with EGFR-positive NSCLC has been reported to occur before, during, or after TKI therapy (10-15). It is important to pay attention to the development of CAIS even in EGFR gene-positive patients who could be expected to have a long-term survival. We do believe that this report will provide some suggestions for the future treatment of patients who have similar courses.
CASE REPORT
Case 1
A 63-year-old man presented with paresthesia and weakness in the left upper extremity and was referred to our hospital. He smoked for three years in his twenties but quitted. He had no history of hypertension, arrhythmia or diabetes. On physical examination, muscle weakness of the left upper extremity, left facial paresis and dysarthria were observed. Brain computed tomography (CT) revealed a low-density area with mass effect was confirmed in the right cerebral occipital lobe (Figure 1-A). A nodule was revealed in the lower lobe of the right lung in an examination upon admission (Figure 1-B). D-dimer was 29.7 mg/mL (<1.0 mg/mL) and CEA 702.7 (<5.0 mg/mL). He was diagnosed with CAIS, and heparin was started at a starting dose of 1000 unit/hour while monitoring activated partial thromboplastin time. He had cervical lymphadenopathy, and biopsy revealed lung adenocarcinoma with EGFR Ex19 deletion. His clinical stage was T4N3M1c stage IVB with multiple metastases in the vertebral bodies. Osimertinib was initiated two weeks after the start of heparin administration, and heparin administration was completed after two weeks. Irradiation were started for vertebral bone metastasis. Although the primary lesion decreased, the decline in activities of daily living due to the sequela of cerebral infarction progressed. Osimertinib was discontinued after 17 months because he became bedridden. Supportive care was then given, but he died five months after finishing Osimertinib. His total clinical course was 22 months.
Case 2
A 71-year-old woman complained of persistent cough, expectoration and hemoptysis for one month. She referred to our hospital because her chest radiograph indicated a mass in her right lower lung field. She smoked for five years in her thirties. At the age of 58 she underwent breast cancer surgery, but she had no history of hypertension, arrhythmia or diabetes. Chest CT showed a 4-cm mass in the right lower lobe (Figure 2-A), and bronchoscopic biopsy confirmed lung adenocarcinoma and EGFR Exon 21 L858R mutation. She had metastases to the skull, was diagnosed as T2aN0M1c, stage IVB. Administration of Osimertinib was started. Right pleural effusion was detected five months after the start of Osimertinib. Recurrence of lung cancer was confirmed by cytology using specimens obtained by thoracentesis, and the patient was evaluated with progressive disease. Two courses of Carboplatin, Pemetrexed and Bevacizumab were performed as second-line therapy. Three months after starting this treatment, she developed a stroke. On brain CT, two low-density areas were confirmed in the right cerebral lateral lobe (Figure 2-B). D-dimer was 17.9 mg/mL (<1.0 mg/mL). She was diagnosed with CAIS, and heparin was started, which was gradually tapered over a month. After the onset of cerebral infarction, her activities of daily living decreased significantly, and she died three months after the onset of the infarction. Her total clinical course was 14 months.
DISCUSSION
Here, we report two patients with EGFR mutated NSCLC complicated by CAIS. One patient developed CAIS concomitantly to diagnosis of lung cancer, and the other patient developed CAIS during the course of cytotoxic chemotherapeutic drugs after EGFR-TKI-resistance. It should be noted that in EGFR mutated NSCLC, as observed in these patients, CAIS can develop at any time of treatment. In patients who unfortunately develop CAIS, it is important not only to perform anticancer therapy and anticoagulant therapy, but also to implement rehabilitation so as not to reduce ADL.
To our best knowledge, there have been six case reports of EGFR-mutant NSCLC patients with CAIS (10-15). Cancer-associated ischemic stroke preceded or coincided with the detection of lung cancer in three of these six patients (10, 11, 14). Two patients developed CAIS during TKI treatment: one patient developed symptoms two days after the start of the TKI (12), and the other one during interruption TKI due to adverse effects of the TKI (15). One patient developed CAIS during the course of cytotoxic chemotherapy after development of TKI resistance (13).
In the FLAURA trial, a trial comparing osimertinib with first-generation TKIs, median progression- free survival (PFS), overall survival (OS) for Osimertinib were 18.9 (95% CI: 15.2-21.4) months, 38.6 (95% CI: 34.5-41.8) months, PFS and OS for first-generation TKIs were 10.2 (95% CI: 9.6-11.1) months, 31.8 (95% CI: 26.6-36.0) months (6, 7). The PFS and OS of our two patients were 17 and 22 months for case 1 and 5 and 14 months for case 2, respectively. Among the above-mentioned six EGFR mutated NSCLC patients with CAIS, four cases of PFS were described in their reports, and median PFS of them was four months (2-10 months) (10, 14, 15). Overall survival was described in five patients, with their median OS being eight months (1-22 months) (10-12, 14, 15). Compared with FLAURA trial results, the prognosis of NSCLC patients with CAIS seemed to be poor.
Since the establishment of our institution in 2009, only two out of 75 (2.7%) EGFR mutated patients and two out of 477 (0.4%) EGFR wild-type patients were NSCLC patients with CAIS. However, these patients had different patient backgrounds such as age and sex, and comparison of prognosis was not appropriate. Most recently, Chang et al reported a study of complications of EGFR-TKIs in propensity-matched NSCLC patients (9). According to the authors, EGFR-TKI-treated patients had a higher incidence of cerebrovascular disorders (9). EGFR-TKIs are usually administered to EGFR-positive patients, but this report did not include information such as the presence or absence of EGFR mutations and the types of EGFR mutations in treated patients. As the author also states in the limitation, there was a lack of information on EGFR sequence variation–positive patients without TKI treatment. The possibility that the incidence of cerebrovascular accidents was higher in EGFR gene-positive patients, rather than in TKI-treated patients, was not ruled out. We suppose that the results of this study are subject to further verification.
There has been no established standard of therapy for CAIS; for many patients it is common to administer heparin at the time of onset, and after gradually tapering off heparin for periods ranging from several days to several months (10-12). Some patients first received thrombomodulin alfa or tissue plasminogen activator as initial treatment (13, 14), followed by clopidogrel, a direct oral anticoagulant, as subsequent treatment (15).
There is one other point worth noting. Case 2 developed ischemic stroke during chemotherapy including Bevacizumab. Bevacizumab is an angiogenesis inhibitor that suppresses vascular endothelial cell growth factor, and there have been many reports pointing out the relationship between this drug and hemorrhagic brain injury, and ischemic injuries (16, 17). When cancer patients receiving this drug develop ischemic stroke, it is important to differentiate it from CAIS.
Our patients were treated with heparin for a month and had no recurrence of infarction. Establishment of standard therapy is desired. Regarding the onset of CAIS, there have been reports recommending monitoring of FDP and D-dimer (10, 11). However, considering the frequency of complication of CAIS, there is room for discussion from the perspective of clinical practice and health economics about what kind of monitoring tests and when them should be carried out, and it will be a future issue.
CONCLUSIONS
Close monitoring of CAIS in clinical, hematology, and diagnostic imaging is important in EGFR mutated NSCLC patients as CAIS could develop at any time from before to after TKI therapy. Poor general condition adversely affects the performance of TKI and subsequent anticancer treatment, so it is important for patients who unfortunately develop CAIS not only to undergo anticoagulation and anticancer treatment but also to undergo rehabilitation to maintain ADL.
Statement of ethics: This study was approved by the institutional ethics committee of our institute (NO 1639). Written comprehensive informed consent at the time of admission for obtaining pathological specimens was obtained from the patients.
Conflict of interests: none declared.
Financial support: none declared.
FIGURE 1.
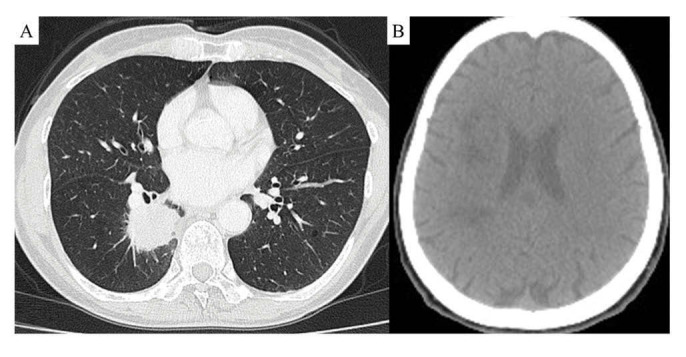
Chest CT scan in an examination upon admission revealed a nodule with pleural indentation in the lower lobe of the right lung (A); brain CT scan taken at admission showed a low-density area with mass effect was confirmed in the right cerebral occipital lobe (B)
FIGURE 2.
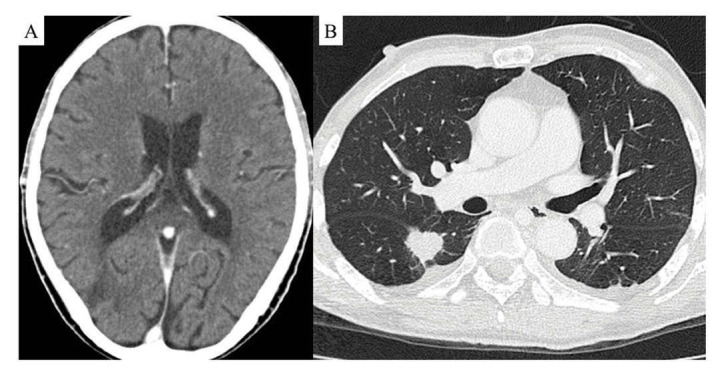
Brain CT taken at the onset of stroke revealed two low-density areas were confirmed in the right cerebral lateral lobe (A); chest CT scan taken at admission showed a 4 cm mass in the right lower lobe (B)
Contributor Information
Manato TAGUCHI, Division of Respiratory Medicine, University of Tsukuba Hospital, University of Tsukuba, Amakubo, Tsukuba, Ibaraki, Japan.
TAKESHI KAWAKAMI, Division of Respiratory Medicine, University of Tsukuba Hospital, University of Tsukuba, Amakubo, Tsukuba, Ibaraki, Japan.
Sachie HASEGAWA, Division of Respiratory Medicine, University of Tsukuba Hospital, University of Tsukuba, Amakubo, Tsukuba, Ibaraki, Japan.
Shinichiro OKAUCHI, Division of Respiratory Medicine, Mito Medical Center, University of Tsukuba-Mito Kyodo General Hospital, Mito, Ibaraki, Japan.
Gen OHARA, Division of Respiratory Medicine, Mito Medical Center, University of Tsukuba-Mito Kyodo General Hospital, Mito, Ibaraki, Japan.
Hiroaki SATOH, Division of Respiratory Medicine, Mito Medical Center, University of Tsukuba-Mito Kyodo General Hospital, Mito, Ibaraki, Japan.
Nubuyuki HIZAWA, Division of Respiratory Medicine, University of Tsukuba Hospital, University of Tsukuba, Amakubo, Tsukuba, Ibaraki, Japan.
References
- 1.Akinbo DB, Ajayi OI. Thrombotic pathogenesis and laboratory diagnosis in cancer patients, an update. Int J Gen Med. 2023;16:259–272. doi: 10.2147/IJGM.S385772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kono T, Ohtsuki T, Hosomi N, et al. Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int. 2012;12:468–474. doi: 10.1111/j.1447-0594.2011.00796.x. [DOI] [PubMed] [Google Scholar]
- 3.Grazioli S, Paciaroni M, Agnelli G, et al. Cancer-associated ischemic stroke: A retrospective multicentre cohort study. Thromb Res. 2018;165:33–37. doi: 10.1016/j.thromres.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZM, Lin ZH, Zhu GL. Tirofiban in the treatment of cancer-associated ischemic stroke. Eur Rev Med Pharmacol Sci. 2023;27:3590–3596. doi: 10.26355/eurrev_202304_32142. [DOI] [PubMed] [Google Scholar]
- 5.Dowell JE. Epidermal growth factor receptor mutations in non-small cell lung cancer: a basic science discovery with immediate clinical impact. Am J Med Sci. 2006;331:139–149. doi: 10.1097/00000441-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 7.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 8.Enewold L, Thomas A. Real-world patterns of EGFR testing and treatment with erlotinib for non-small cell lung cancer in the United States. PLoS One. 2016;11:e0156728. doi: 10.1371/journal.pone.0156728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang WT, Lin HW, Chang TC, et al. Assessment of tyrosine kinase inhibitors and survival and cardiovascular outcomes of patients with non-small cell lung cancer in Taiwan. JAMA Netw Open. 2023;6:e2313824. doi: 10.1001/jamanetworkopen.2023.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masubuchi H, Maeno T, Uchida M, et al. A case of Trousseau syndrome caused by pulmonary adenocarcinoma that was controlled for one year and 10 months with thrombosis treatment using an EGFR tyrosine kinase inhibitor and chemotherapy. Respir Med Case Rep. 2015;15:101–105. doi: 10.1016/j.rmcr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Miao J, Wang L, et al. EGFR-mutant NSCLC presenting with stroke and massive systemic embolization as the first manifestation: case report. BMC Neurol. 2021;21:221. doi: 10.1186/s12883-021-02236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naito H, Nezu T, Hosomi N, et al. Antithrombotic therapy strategy for cancer-associated ischemic stroke: a case series of 26 patients. J Stroke Cerebrovasc Dis. 2018;27:e206–e211. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Nonagase Y, Takeda M, Tanaka K, et al. Treatment of EGFR mutation-positive non-small cell lung cancer complicated by Trousseau syndrome with gefitinib followed by osimertinib: a case report. Oncotarget. 2018;9:29532–29535. doi: 10.18632/oncotarget.25687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kai Y, Ohara H, Matsuda M, et al. Endovascular therapy for cerebral infarction due to Trousseau syndrome in a patient with non-small cell lung cancer. Respir Med Case Rep. 2021;34:101531. doi: 10.1016/j.rmcr.2021.101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai ML, Tan EC, Ang CC, Liam CK. Recurrent cerebral infarcts secondary to marantic endocarditis in a patient with adenocarcinoma of the lung. Singapore Med J. 2016;57:524–525. doi: 10.11622/smedj.2016157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo PY, Chen XL, Liu YW, et al. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PLoS One. 2014;9:e102484. doi: 10.1371/journal.pone.0102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon J, Jee D, Lim SH. Would intravitreal bevacizumab injection increase risk of cerebral infarction? Eur J Neurol. 2018;25:1177–1181. doi: 10.1111/ene.13683. [DOI] [PubMed] [Google Scholar]


