Abstract
Introduction: Cardiotocography (CTG) constitutes a major and generally used tool for the assessment of fetal well-being. Subjectivity is the main difficulty in the interpretation of CTG. Inter- and intra-observer variability are substantival features of the interpretation of CTGs. An auspicious answer for reduction of inter- and intra-observer variability is the computerized analysis of fetal heart rate (FHR). Moreover, computerized analysis contributes to the reduction of adverse maternal and fetal outcomes.
Objective: The aim of the present review was to compare the visual and computerized analysis of CTG for establishing whether computerized CTG was related to better perinatal outcomes.
Materials and methods: Three electronic medical related databases (PubMed, Scopus and Cochrane) were searched from May to June 2023 in order to find randomized controlled trials (RCTs) in English. Studies were evaluated for their methodological quality with the CONSORT checklist. The target population comprised pregnant or intrapartum women into cardiotocographic monitoring. The intervention was represented by the visual analysis of CTG, and the comparison intervention by the computerized analysis of CTG. Primary outcomes included adverse perinatal outcomes.
Results: A total of 47 studies relevant with the topic were examined. However, only five articles met all inclusion and methodological criteria; four of those demonstrated that computerized analysis had no significant reduction in the rate of metabolic acidosis or obstetric interventions, and one study found a lower incidence of adverse perinatal outcome with conventional CTG (with fetal blood sampling). However, all reviews propose further development of decision-support software and more large-scale RCTs in the future.
Conclusion: The computerized analysis of FHR is a promising solution for the reduction of adverse perinatal outcomes and elimination of inter- and intra-observer variability.
Keywords:CTG, fetal monitoring, computer analysis, computerized analysis, perinatal outcomes.
INTRODUCTION
Over the years, the evaluation of fetal heart rate (FHR) during pregnancy and labor has been of primary interest for obstetricians and midwives. The first attempt to hear the FHR was made in 1895, when a French obstetrician, Adolphe Pinard, designed the Pinard horn-fetoscope. Since then, in the late 60s, the cardiotocogram (CTG) was first introduced into maternity care (1). Cardiotocogram is a non-operative and undemanding tool which provides us a coincident presentation of FHR and contractions of the uterus with the use of an ultrasound sensor located on a woman’s abdomen (2). In clinical practice, the well-being of the fetus is recognized via the visual analysis of CTG. In 1986, the International Federation of Gynecology and Obstetrics (FIGO) issued the primary general criteria for elucidation of CTG based on changes of FHR in relation to contraction. This interpretation includes parameters such as baseline, variability, accelerations, decelerations and the sinusoidal pattern. Thereon, criteria have been altered and improved (3). Recent systematic reviews were conducted to compare the existing CTG interpretation guidelines (4, 5). Even though there are clinical guidelines to provide a framework for the evaluation and management of intrapartum fetal monitoring patterns (6), the visual analysis of FHR is subject to inter- and intra-observer variability. This variability can increase frequency of unnecessary operative vaginal births and cesarean deliveries (2, 7, 8). Therefore, many algorithms have been developed for the analysis of CTG and deduce reliable and useful information to use as a guide to eliminate this variability through experts (2).
In the 1980s, researchers created the first computer-assisted programs which could automatically analyze the signals of CTG noticing signs of fetal hypoxia through labor. Those systems used in clinical practice nowadays are mainly based on FIGO classification. Characteristics of FIGO, including baseline, accelerations, decelerations and variability, were the groundwork for the construction of manageable rules to build proper signs of fetal hypoxia.
Dawes and Redman were the first to present a very promising computerized analysis system in the 1980s. The system is mostly based on features like the ones defined in FIGO classification. Thereafter, other computerized systems with relevant methodologic basis were developed – an example is the Omniview-SisPorto system (Speculum S.A., Portugal), developed by Ayres-de- Campos et al in 1998, that includes a numerical transformation of FIGO recommendations. The most recent version of this system developed over the years is named SisPorto 4.0 in 2017. Recently, in 2017, Georgieva et al reported the OxSys system, which was primarily based on a single parameter, decelerations. Moreover, this system had real-time alerts for health professionals (9).
Study aim
The aim of the present systematic review was to explore the available literature for comparing conventional analysis of CTG and computerized analysis of CTG in terms of better perinatal outcomes.
METHODS
Search strategy
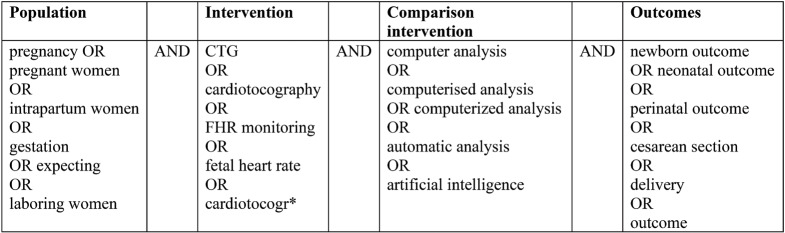
A systematic review of electronic databases concerning medical care (PubMed-Medline, Scopus and the Cochrane Library) was conducted from May to June 2023. The PubMed-Medline search terms were used according to PICO acronym, as shown in Table 1. Search terms were customized to be suitable for each database.
In order to identify researches which had not raised from the primary search, we further investigated the reference lists of studies from the initial search. Two authors carried out data collection together with data analysis.
Study selection
Inclusion criteria for eligible studies were the following:
• Target population: pregnant or intrapartum women into cardiotocographic monitoring
• Intervention: visual analysis of CTG (conventional CTG)
• Comparison intervention: computerized analysis of CTG
• Outcomes: perinatal outcomes (maternal and neonatal) such as cesarian section, operative vaginal delivery, fetal hypoxia, admissions to neonatal unit and low Apgar score
• Study design: randomized control trials (RCTs), English language
Quality assessment of included studies
Selected trials that met the inclusion criteria were evaluated for their reporting clarity by using the CONSORT statement – a validated checklist consisting of 25 items (10). A presence of each item was recorded using Yes/No after reading the full text. We considered the CONSORT compliance rates as high compliance when they were higher than 85%, moderate compliance when they ranged between 70%-85% and low compliance when they were lower than 70%.
RESULTS
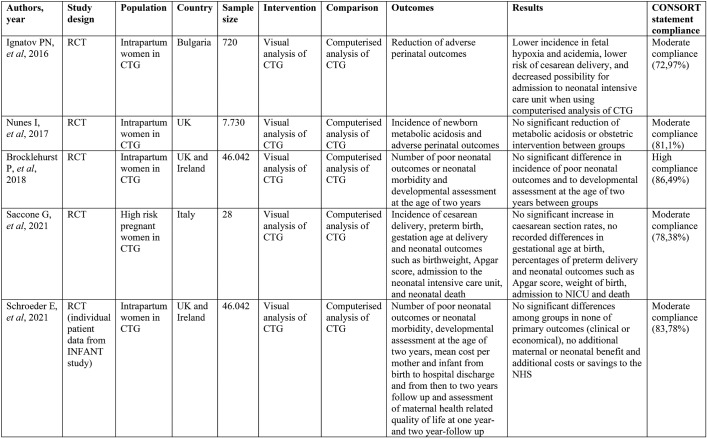
The initial search generated 47 studies. Titles and abstracts were examined for relevance to the aim of the present study. After screening, two studies were rejected as duplicates and 40 studies did not meet the inclusion criteria. Finally, only five studies were eligible for inclusion in our systematic review. Thus, data of the five remaining studies were analyzed and assessed for their methodological quality. A flow diagram that illustrates the article filtering process is shown in Figure 1. The five eligible studies were also methodologically suitable, and their characteristics and results were summarized in Table 2.
The study by Ignatov and colleagues (11), a RCT conducted in Bulgaria in 2016, aimed to evaluate the effectiveness of a computerized decision-support system to reduce adverse perinatal outcomes compared to a conventional CTG. The study sample included 720 women in active labor, of which 360 were randomized to the intervention group and 360 to the control group. The intervention was represented by a computerized decision-support system that could calculate predicted pH values. In the control group, CTG traces were evaluated by clinicians according to FIGO guidelines and when an abnormal CTG trace was present, a fetal blood sampling was performed. After birth, blood gases were measured in all newborns in both study groups. Primary outcomes were hypoxia, acidemia, cesarean section and use of forceps. Low Apgar score, newborn seizures and admission to the neonatal intensive care unit (NICU) composed the secondary outcomes. The incidence of adverse perinatal outcomes was lower among women who were allocated to the intervention group compared to those who were monitored using the conventional fetal blood sampling. More specifically, the above study supported that there was a noteworthy reduction in fetal hypoxia, acidemia, cesarean section rate and admission to the NICU. The study by Ignatov et al achieved moderate compliance to CONSORT checklist with almost 73% agreement.
The study by Nunes et al (12) was a RCT conducted in the UK in 2017. Its objective was to identify whether the computerized analysis of intrapartum CTG and real-time alerts can contribute to the reduction of obstetric intervention and/or neonatal metabolic acidosis when compared to conventional CTG. The above-mentioned authors studied 7.730 women in active labor (but not in active second stage), of which 3.961 were allocated to computer CTG analysis and real-time alerts, and the remaining 3.769 to visual analysis. Those who were randomized to the intervention arm had continuous CTG with computer analysis and real-time alerts in a central monitoring station, while women randomized to the control arm had continuous CTG, displayed in the same central monitoring station but without computer analysis or alerts. The primary outcome was the incidence of newborn metabolic acidosis and secondary outcomes included operative delivery, low Apgar score, admission to neonatal intensive care unit, hypoxic– ischemic encephalopathy and perinatal death. No significantly reduction of the rate of metabolic acidosis or obstetric intervention was found in the study arm. The authors proposed that continued refinement of interpretation algorithms may be required in the future. Nunes et al’s study reported a moderate compliance to CONSORT checklist with 81% agreement.
The study carried out by Brocklehurst et al (13) was a large RCT conducted in the UK and Ireland in 2017. Its aim was to determine whether the help of a decision-support software in the analysis of CTG reflected the number of poor neonatal outcomes. Brocklehurst et al studied 46.042 women (22.987 in the decision-support group and 23.055 in the no-decision-support group). Primary and secondary outcomes were separated to short-term and long-term. Adverse neonatal outcomes, including significant morbidity, death and admission to neonatal care unit within two days after birth, were the primary short-term outcomes. Developmental progress at two years of age was related with long-term outcomes. Secondary short-term outcomes related with neonatal (intrapartum stillbirth, low Apgar, seizures) and maternal (operative delivery, admission to higher level of care) and long-term outcomes related with infant such as vocabulary subscale, late deaths up to two years, major disability and breastfeeding. There was no difference in the incidence of poor neonatal outcomes and to developmental assessment at the age of two years between the two groups. The authors proposed that further development of decision-support software could improve assistance of the system provides to clinicians to change the outcomes. The study by Brocklehurst et al showed a high compliance to CONSORT checklist with almost 86% agreement.
The study by Saccone et al (14) was a RCT conducted in Italy in 2021 that studied whether antenatal computerized analysis of CTG increased the rate of cesarean section in women with high-risk gestations. Saccone et al explored 28 high-risk pregnancies. Women were divided into two groups: 14 to the control group and 14 to the intervention one. Those who were randomized to the computerized CTG arm had antenatal CTG with computerized analysis and real- time alerts in a central monitoring station; CTG was connected to a system that analyzed several parameters, including baseline of FHR and short-term variability (STV). Women randomized to the control arm had antenatal CTG with standard non-stress test (NST) with visual analysis and real-time alerts. The primary outcome was the incidence of cesarean delivery and secondary outcomes included preterm birth, gestational age at delivery and neonatal outcomes such as birthweight, Apgar score, admission to the neonatal intensive care unit and neonatal death. The result of the study was that the use of computerized CTG did not show a significant rise in cesarean delivery compared with standard CTG. As for the secondary outcomes, there were no significant differences in preterm birth, gestational age at delivery, Apgar score and birth weight between groups. No neonatal deaths were reported in the study population. The study by Saccone et al achieved a moderate compliance according to CONSORT checklist with almost 78% agreement.
Lastly, the study conducted by Schroeder E. et al in 2021 (15) was a cost-consequence analysis of individual patient data from the INFANT study, a large RCT-previously referred as the study by Brocklehurst et al in 2017 (13), which took place in maternity units in the UK and Ireland. The study objective was an economic evaluation of computerized analysis of CTG intrapartum. Similarly to the INFANT trial, 46.042 women and 46.614 infants were included, and the intervention consisted of the use of a computerized analysis support system. Statistics about unit costs were selected from national sources (Personal Social Services Research Unit, National Health Service-NHS Reference costs). The overall costs two years following labor evaluated by combining charges from postpartum discharge to one year and 1-2 years after. Primary outcomes comprised the number of poor neonatal outcomes or neonatal morbidity, developmental assessment at the age of two years, mean cost per mother and newborn from birth to hospital discharge and from then to two-year follow up and assessment of maternal health related quality of life at one year- and two-year-follow up. There were no remarkable differences in any of the clinical or economic primary outcomes among intervention and control groups. The reviewers concluded that the use of computerized analysis intrapartum did not lead to additional maternal or neonatal benefit and to additional costs or savings to the NHS. The study by Schroeder E. et al achieved a moderate compliance according to CONSORT checklist with almost 84% agreement.
DISCUSSION
The purpose of the present review is to compare visual and computerized analysis of CTG in terms of better perinatal outcomes, including caesarian section, operative vaginal delivery, fetal hypoxia, admissions to neonatal unit and low Apgar. Furthermore, variability through experts’ and clinicians’ interpretations was an additional reason to examine.
In recent decades, CTG has remained the main method for screening the well-being of the fetus. Since 1895 until today, a rapid progress has been made to analyze the CTG signals for fetal hypoxia. Many studies are related with the analysis of the CTG and the way to analyze it and confirm the well-being of the fetus. The main difficulty in the interpretation of CTG is the subjectivity. Intra- and inter-observer variability are substantival features of the interpretation of CTGs. Variation in the interpretation of visual analysis of CTG was reviewed by several reet al (16) examined intra- and inter-observer agreement in midwives’ visual interpretations of intrapartum CTGs. Additionally, in 1997, Bernardes and colleagues (17) evaluated the interobserver agreement in visual analysis by three expert obstetricians. Later, in 2022, Amadori and colleagues (18) evaluated the intra- and inter- operator agreement in CTG by both midwives and obstetricians and whether their educational background influenced the final result. All above-mentioned studies demonstrated the need to develop non-invasive methods of CTG assessment in childbirth. In particular, Bernardes et al and Amadori et al (15) proposed an electronic analysis of CTG as a suitable solution to eliminate intra- and inter-observer variability.
Towards this direction, several studies reviewed the contribution of computerized analysis to an optimal interpretation of CTG. The findings of this systematic review of relevant studies showed that computerized CTG had not significantly reduced the rate of metabolic acidosis or obstetric intervention. Only one study, which was carried out by Ignatov et al in 2016 (11), found a lower incidence of adverse perinatal outcomes with the computerized decision-support system that could calculate predicted pH values.
However, it is worthwhile mentioning that a prospective review conducted by Costa and colleagues in 2010 (19) examined how the use of computerized analysis of CTG altered experts’ prediction of umbilical artery blood (UAB) pH and Apgar score in first five minutes after birth. The total number of CTG tracings were randomized to the intervention arm, which had a computer analysis system support, and to the control arm, without that support. All tracings were independently evaluated by three experts, who had to predict the UAB pH of newborns and five-minute Apgar. It was found that, when clinicians had access to computerized analysis of CTG tracings, they had significantly higher agreement and accuracy in prediction of UAB pH. Also, an increased inter-observer agreement in prediction of five-minute Apgar was seen in the intervention arm, but it was not too high to reach statistical significance. The authors found that maybe the experts’ previous experience with the system had an impact on the results.
Although the summation of this review proposes further development of decision-support software and more large-scale RCTs in the future. Randomized controlled trials with larger sample sizes should be conducted to have more statistically significant results.
The strength of the present research is that it was a contemporary review conducted between April–June 2023, which included recent studies published from 2016 to 2021. However, the current systematic review has also some limitations, as the inclusion criteria were met by only five studies, which had several restrictions such as small sample size, long recruitment period and limited reproducibility across countries.
CONCLUSION
In this systematic review we established a recent and thorough search of the existing data that compared visual and computerized analysis of CTG for assessing which method was related to better perinatal outcomes. According to our results, there was no significant difference between interventions, in contrast to our initial hypothesis.
The computerized analysis of FHR is a promising solution for reduction of adverse perinatal outcomes and elimination of variability using experts’ opinions. The technology will help reduce disputes between experts and provide a more reliable solution. The combination of experts and computer systems seems to be the best option. Finally, further large-scale RCTs comparing cardiotocography with or without computerized analysis could be useful for the development of optimal strategies in daily clinical practice.
Conflict of interests: none declared.
Financial support: none declared.
TABLE 1.
Search terms used in the study according to the PICO acronym
FIGURE 1.
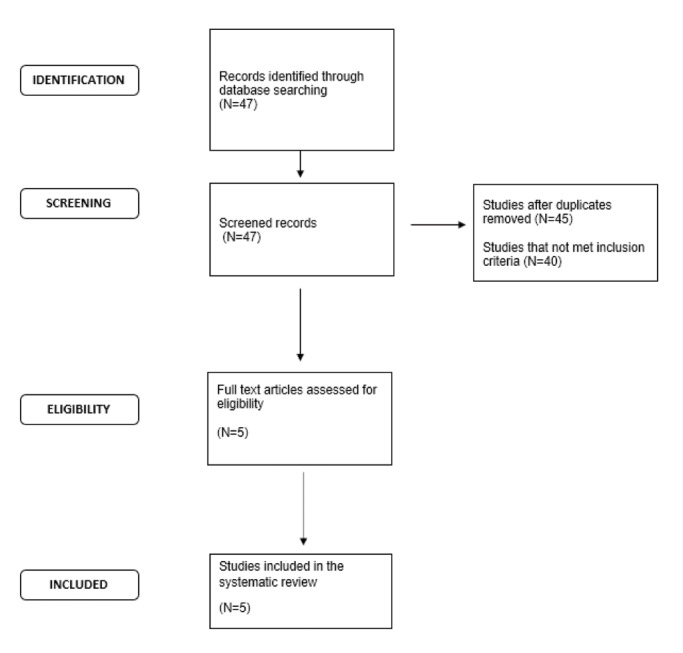
Flow diagram illustrating the article filtering process
TABLE 2.
Characteristics and results of included studies
Contributor Information
Aikaterini TSIPOURA, Department of Midwifery, University of West Attica, Athens, Greece.
Paraskevi GIAXI, Department of Midwifery, University of West Attica, Athens, Greece.
Antigoni SARANTAKI, Department of Midwifery, University of West Attica, Athens, Greece.
Kleanthi GOUROUNTI, Department of Midwifery, University of West Attica, Athens, Greece.
References
- 1.Jauniaux E, Prefumo F. Fetal heart monitoring in labour: from pinard to artificial intelligence. BJOG. 2016;123:870. doi: 10.1111/1471-0528.13844. [DOI] [PubMed] [Google Scholar]
- 2.Ponsiglione AM, Cosentino C, Cesarelli G, et al. A Comprehensive Review of Techniques for Processing and Analyzing Fetal Heart Rate Signals. Sensors (Basel) 2021;21:6136. doi: 10.3390/s21186136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayres-de-Campos D, Spong CY, Chandraharan E. FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet. 2015;131:13–24. doi: 10.1016/j.ijgo.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Brown J, Kanagaretnam D, Zen M. Clinical practice guidelines for intrapartum cardiotocography interpretation: A systematic review. Aust N Z J Obstet Gynaecol. 2023;63:278–289. doi: 10.1111/ajo.13667. [DOI] [PubMed] [Google Scholar]
- 5.Mohan M, Ramawat J, La Monica G, et al. Electronic intrapartum fetal monitoring: a systematic review of international clinical practice guidelines. AJOG Glob Rep. 2021;1:100008. doi: 10.1016/j.xagr.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists. Practice bulletin no. 116: Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116:1232–1240. doi: 10.1097/AOG.0b013e3182004fa9. [DOI] [PubMed] [Google Scholar]
- 7.Gatellier MA, De Jonckheere J, Storme L, et al. Fetal heart rate variability analysis for neonatal acidosis prediction. J Clin Monit Comput. 2021;35:771–777. doi: 10.1007/s10877-020-00535-6. [DOI] [PubMed] [Google Scholar]
- 8.Houzé de l'Aulnoit A, Génin M, Boudet S, et al. Use of automated fetal heart rate analysis to identify risk factors for umbilical cord acidosis at birth. Comput Biol Med. 2019;115:103525. doi: 10.1016/j.compbiomed.2019.103525. [DOI] [PubMed] [Google Scholar]
- 9.Ben M'Barek I, Jauvion G, Ceccaldi PF. Computerized cardiotocography analysis during labor – A state-of-the-art review. Acta Obstet Gynecol Scand. 2023;102:130–137. doi: 10.1111/aogs.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. [Google Scholar]
- 11.Ignatov PN, Lutomski JE. Quantitative cardiotocography to improve fetal assessment during labor: a preliminary randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2016;205:91–97. doi: 10.1016/j.ejogrb.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Nunes I, Ayres-de-Campos D, Ugwumadu A, et al. Central Fetal Monitoring With and Without Computer Analysis: A Randomized Controlled Trial. Obstet Gynecol. 2017;129:83–90. doi: 10.1097/AOG.0000000000001799. [DOI] [PubMed] [Google Scholar]
- 13.Brocklehurst P, Field D, Greene K, et al. Computerized interpretation of the fetal heart rate during labour: a randomised controlled trial (INFANT). Health Technol Assess. 2018;22:1–186. doi: 10.3310/hta22090. [DOI] [PubMed] [Google Scholar]
- 14.Saccone G, Tagliaferri S, Grasso A, et al. Antenatal cardiotocography with and without computer analysis in high-risk pregnancy: a randomized clinical trial. Am J Obstet Gynecol MFM. 2021;3:100284. doi: 10.1016/j.ajogmf.2020.100284. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder E, Yang M, Brocklehurst P, et al. Economic evaluation of computerized interpretation of fetal heart rate during labour: a cost-consequence analysis alongside the INFANT study. Arch Dis Child Fetal Neonatal Ed. 2021;106:143–148. doi: 10.1136/archdischild-2020-318806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devane D, Lalor J. Midwives' visual interpretation of intrapartum cardiotocographs: intra- and inter-observer agreement. J Adv Nur. 2005;52:133–141. doi: 10.1111/j.1365-2648.2005.03575.x. [DOI] [PubMed] [Google Scholar]
- 17.Bernardes J, Costa-Pereira A, Ayres-de-Campos D, et al. Evaluation of interobserver agreement of cardiotocograms. Int J Gynaecol Obstet. 1997;57:33–37. doi: 10.1016/s0020-7292(97)02846-4. [DOI] [PubMed] [Google Scholar]
- 18.Amadori R, Vaianella E, Tosi M, et al. Intrapartum cardiotocography: an exploratory analysis of interpretational variation. J Obstet Gynaecol. 2022;42:2753–2757. doi: 10.1080/01443615.2022.2109131. [DOI] [PubMed] [Google Scholar]
- 19.Costa A, Santos C, Ayres-de-Campos D, et al. Access to computerized analysis of intrapartum cardiotocographs improves clinicians' prediction of newborn umbilical artery blood pH. BJOG. 2010;117:1288–1293. doi: 10.1111/j.1471-0528.2010.02645.x. [DOI] [PubMed] [Google Scholar]