Abstract
The polyhydroxyalkanoate (PHA) synthase gene of Comamonas acidovorans DS-17 (phaCCa) was cloned by using the synthase gene of Alcaligenes eutrophus as a heterologous hybridization probe. Complete sequencing of a 4.0-kbp SmaI-HindIII (SH40) subfragment revealed the presence of a 1,893-bp PHA synthase coding region which was followed by a 1,182-bp β-ketothiolase gene (phaACa). Both the translated products of these genes showed significant identity, 51.1 and 74.2%, respectively, to the primary structures of the products of the corresponding genes in A. eutrophus. The arrangement of PHA biosynthesis genes in C. acidovorans was also similar to that in A. eutrophus except that the third gene, phaB, coding for acetoacetyl-coenzyme A reductase, was not found in the region downstream of phaACa. The cloned fragment complemented a PHA-negative mutant of A. eutrophus, PHB−4, resulting in poly-3-hydroxybutyrate accumulation of up to 73% of the dry cell weight when fructose was the carbon source. The heterologous expression enabled the incorporation of 4-hydroxybutyrate (4HB) and 3-hydroxyvalerate monomers. The PHA synthase of C. acidovorans does not appear to show any preference for 4-hydroxybutyryl-coenzyme A as a substrate. This leads to the suggestion that in C. acidovorans, it is the metabolic pathway, and not the specificity of the organism’s PHA synthase, that drives the incorporation of 4HB monomers, resulting in the efficient accumulation of PHA with a high 4HB content.
Poly(3-hydroxybutyrate) [P(3HB)] was identified as an intriguing bacterial inclusion body more than seven decades ago (21) and is now classified as one of the many different types of bacterial polyesters with the common name polyhydroxyalkanoate (PHA). Recently, due to increased awareness of global environmental issues, PHA has come under investigation because of its inherent property as a biodegradable thermoplastic (4, 37).
The biosynthesis of PHA has been studied in great detail in Alcaligenes eutrophus (28, 29, 36). In this bacterium, the biosynthesis process is initiated by the condensation of two acetyl coenzyme A (acetyl-CoA) molecules to acetoacetyl-CoA catalyzed by the enzyme β-ketothiolase (EC 2.3.1.9). The structural gene for this enzyme is designated phaA. Acetoacetyl-CoA is then reduced to the R enantiomer of 3-hydroxybutyryl-CoA by an NADPH-dependent acetoacetyl-CoA reductase (EC 1.1.1.36), the structural gene for which has been given the designation phaB. Both these enzymes have been thoroughly studied for several PHA-accumulating bacteria (8, 11, 12, 23, 25, 26, 38). Finally, the key enzyme, PHA synthase, which is encoded by a structural gene designated phaC, catalyzes the polymerization of (R)-3-hydroxybutyryl-CoA to P(3HB). All the three genes are constitutively expressed in A. eutrophus and form a single operon, with the order phaC-A-B.
PHA synthase genes from more than 20 different bacteria have been cloned and analyzed (20). The results show that PHA synthases are a class of highly versatile enzymes and are not specific to only one type of hydroxyalkanoic acid (HA) (42). Nevertheless, they can be broadly classified into two different types based on their primary amino acid sequences and substrate specificities. One type is active towards short-chain HA, consisting of three to five carbon atoms, and is represented by the PHA synthase of A. eutrophus. Some of the PHA synthases of this type were also found to incorporate into PHA 4- and 5-HA, such as 4-hydroxybutyric acid (4HB) (19), 4-hydroxyvaleric acid (4HV) (45), and 5HV (5). The other type, which is represented by the PHA synthase of Pseudomonas oleovorans, is active towards medium-chain-length HA, containing 6 to 14 carbon atoms. In addition, a few bacteria, such as Aeromonas caviae (7) and Rhodococcus ruber (10), have also been reported to have synthases that exhibit specificity for both short-chain and medium-chain-length HA.
PHAs containing monomer compositions that result in useful properties have constantly been sought. The physical char- acteristics of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] copolymers, having 4HB contents ranging from 0 to 100 mol%, show their great potential as biodegradable materials (32). Since 4HB is a linear monomer, it has the added advantage of being able to be degraded by a lipase as well as a depolymerase (31). In Comamonas acidovorans, it was shown that the 4HB monomer content could be easily controlled by supplying substrate mixtures of 4HB and other carbon sources. This results in the ability to biosynthesize PHAs with wide ranges of elasticity and tensile strength and, most importantly, controlled rates of biodegradation (31, 32).
In addition to C. acidovorans, A. eutrophus (19) and Alcaligenes latus (15) have been reported to produce PHA containing 4HB as a monomer. Among these wild-type bacteria, only C. acidovorans is capable of producing PHA copolymers with a very high (>90 mol%) 4HB monomer content, more than 20% of the dry cell weight (31). Invariably, however, 4HB is incorporated into PHA only when related carbon sources, such as 4HB, γ-butyrolactone, and 1,4-butanediol, are provided in the culture media. An attempt was made to produce P(3HB-co-4HB) in recombinant Escherichia coli using an unrelated carbon source, such as glucose. For this purpose, succinate degradation genes from Clostridium kluyveri were introduced into E. coli together with the PHA biosynthesis genes from A. eutrophus. It is known from a previous study (41) that 4-hydroxybutyryl-CoA, which is an immediate precursor for the PHA synthase of A. eutrophus, occurs as an intermediate in the cofermentation of succinic acid and ethanol in Clostridium kluyveri. Therefore, it was anticipated that 4-hydroxybutyryl-CoA could be generated from succinic acid in E. coli if the genes coding for necessary enzymes were introduced. However, despite the theoretically attractive approach, a maximum of 2.8 mol% 4HB was incorporated into PHA by the recombinant E. coli (44).
To this end, C. acidovorans seems to be a very promising wild-type candidate with a metabolic pathway suitable for the production of P(3HB-co-4HB) with a 0 to 100 mol% 4HB monomer content. In this paper we report the cloning and characterization of the PHA biosynthesis genes of C. acidovorans DS-17 (JCM10181). In addition, the substrate specificity of C. acidovorans PHA synthase was evaluated by heterologous expression of the cloned PHA synthase gene. The discussion emphasizes the capacity of the metabolic pathways in C. acidovorans to produce PHA with a high 4HB monomer content.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
All strains and plasmids used in this study are listed in Table 1. C. acidovorans and A. eutrophus were grown in a nutrient-rich (NR) medium containing meat extract (10 g/liter), Bacto Peptone (10 g/liter), and yeast extract (2 g/liter) at 30°C. E. coli strains were grown at 37°C in Luria-Bertani (LB) medium. For maintenance of plasmids, 25 mg of tetracycline per liter or 50 mg of ampicillin or kanamycin per liter was added.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant phenotype | Source or reference |
|---|---|---|
| Strains | ||
| C. acidovorans DS-17 (JCM10181) | Wild type | 31 |
| A. eutrophus PHB−4 (DSM541) | PHA-negative mutant of wild-type H16 | 35 |
| E. coli | ||
| S17-1 | recA and tra genes of plasmid RP4 integrated into the chromosome; auxotrophic for proline and thiamine | 40 |
| DH5α | deoR endA1 gyrA96 hsdR17(rK− mK+) recA1 relA1 supE44 thi-1 Δ(lacZYA-argFV169) φ80ΔlacZΔM15 F− λ− | Clontech |
| Plasmids | ||
| pLA2917 | Cosmid; Kmr Tcr | 1 |
| pLACa | pLA2917 derivative; 20-kbp gDNA fragment | This study |
| pJRD215 | Cosmid; Kmr Smr RSF1010 replicon; Mob+ | 3 |
| pJRDEV80 | pJRD215 derivative; phaCCa phaACa | This study |
| pBluescript II KS(+) | AprlacPOZ; T7 and T3 promoter | Stratagene |
| pEV80 | pBluescript derivative; phaCCa phaACa | This study |
Analysis of PHA accumulation.
PHA accumulation was analyzed in both one-stage and two-stage batch cultivation. For one-stage cultivation, filter-sterilized carbon source was added to 100 ml of mineral salts (MS) medium consisting of 0.9 g of Na2HPO4 · 12H2O, 0.15 g of KH2PO4, 0.05 g of NH4Cl, and 0.02 g of MgSO4 · 7H2O and supplemented with 0.1 ml of trace element solution (18). For two-stage cultivation, cells were initially grown in 100 ml of NR medium for 12 h before being harvested and transferred to the same volume of nitrogen-free MS medium. Different carbon sources were then tested for their ability to promote PHA synthesis. To determine the polyester content and composition, 10 to 25 mg of lyophilized cell material was subjected to methanolysis in the presence of 15% (vol/vol) sulfuric acid. The resulting hydroxyacyl methyl esters were then analyzed by gas chromatography (GC) (2). For PHA copolymer containing high levels of 4HB, solvent extraction was employed and the content (weight percent) was determined gravimetrically. Lyophilized cells were stirred for 2 days in chloroform at room temperature (39). The insoluble cellular material was then collected by filtration and methanolyzed for GC analysis to check for incomplete extraction, while the chloroform extract was concentrated prior to precipitation of the polymer in methanol. The precipitate was then washed in methanol and ether followed by drying in vacuo, and it was then subjected to gravimetric measurement and compositional analysis by GC.
Isolation and manipulation of DNA.
Isolation of total genomic DNA (gDNA) from C. acidovorans, plasmid DNA isolation, agarose gel electrophoresis, and transformation of E. coli were carried out according to standard procedures (33). Restriction endonucleases and all other DNA-manipulating enzymes and kits were used according to the manufacturers’ protocols. Transconjugation of A. eutrophus with E. coli S17-1 harboring broad-host-range plasmids was performed as described by Friedrich et al. (6).
Construction of gDNA library.
gDNA isolated from C. acidovorans was partially digested with HindIII, and the genomic fragments were ligated to the HindIII site of a broad-host-range cosmid vector, pLA2917 (1). The resulting concatemers were then packaged in vitro with a Gigapack II packaging kit (Stratagene). Mature phage particles containing the genomic fragments were then used to infect E. coli S17-1. Transformants harboring the hybrid cosmids were selected by plating on LB agar plates containing tetracycline (12.5 mg/liter).
Southern blot analysis and colony hybridization.
A 1.8-kbp Csp45I-AatI fragment harboring the PHA synthase gene of A. eutrophus was used as a probe. Preparation of labeled probe and visualization of successful hybridization were carried out with the digoxigenin nucleic acid labeling and detection kit (Boehringer Mannheim Biochemicals).
Nucleotide sequence analysis.
DNA fragments to be sequenced were subcloned into pBluescript II KS(+), and nested sets of deletion clones were generated by using exonuclease III (14). DNA sequencing was carried out by the dideoxy chain termination method with the Prism 310 DNA sequencer (Applied Biosystems, Inc.) employing the dye terminator labeling procedure (Perkin Elmer Corp.). Nucleic acid sequence data and the deduced amino acid sequences were analyzed with GENETYX-MAC software (Software Development Co., Tokyo, Japan). Similarity searches were performed with the BLAST (Basic Local Alignment Search Tool) program and the NCBI (National Center for Biotechnology Information) databases.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases with the accession no. AB009273.
RESULTS
Identification, cloning, and nucleotide sequence of the C. acidovorans PHA biosynthesis genes.
The digoxigenin-labeled PHA synthase gene of A. eutrophus (digoxigenin-labeled phaCAe) was used as a heterologous hybridization probe to detect the homologous gene of C. acidovorans. A relatively strong single hybridization signal appeared in the 20-kbp HindIII-digested fragments of C. acidovorans gDNA. Based on this observation, a genomic library was constructed with HindIII-digested gDNA. Screening of the gDNA library by colony hybridization with the digoxigenin-labeled phaCAe probe resulted in the isolation of one positive monocolony harboring a recombinant cosmid, designated pLACa. To confirm the functional activity of the cloned gDNA fragment, pLACa was mobilized from the E. coli S17-1 host cells into a PHA-negative mutant strain of A. eutrophus, PHB−4. The ability to accumulate PHA was restored to the mutant upon transconjugation. Cells of E. coli S17-1 harboring pLACa, however, did not accumulate PHA when they were cultivated in LB medium supplemented with 0.5% (wt/vol) glucose and/or 0.5% (wt/vol) 4HB.
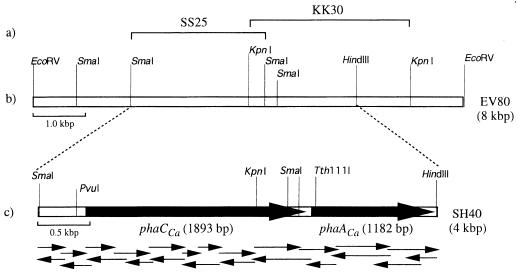
A positive 8-kbp EcoRV subfragment (EV80) from the isolated cosmid pLACa was cloned into pBluescript II KS(+), and to facilitate sequencing two smaller subclones which comprise the center region of EV80 were constructed (Fig. 1a); pSS25 harbors a 2.5-kbp SmaI fragment, while pKK30 harbors a 3-kbp KpnI fragment. The nucleotide sequence of a 4-kbp SmaI-HindIII region (Fig. 1c) was obtained from overlapping partial sequences determined for both strands. Two open reading frames (ORF), ORF1 (1,893 bp) and ORF2 (1,182 bp), which were separated by 123 nucleotides, were identified.
FIG. 1.
Organization of the PHA biosynthetic genes of C. acidovorans in EV80 subfragment. (a) Subfragments relevant for nucleotide sequence analysis; (b) restriction map; (c) strategy for sequencing of the SH40 subfragment.
Structure of the putative gene product of ORF1.
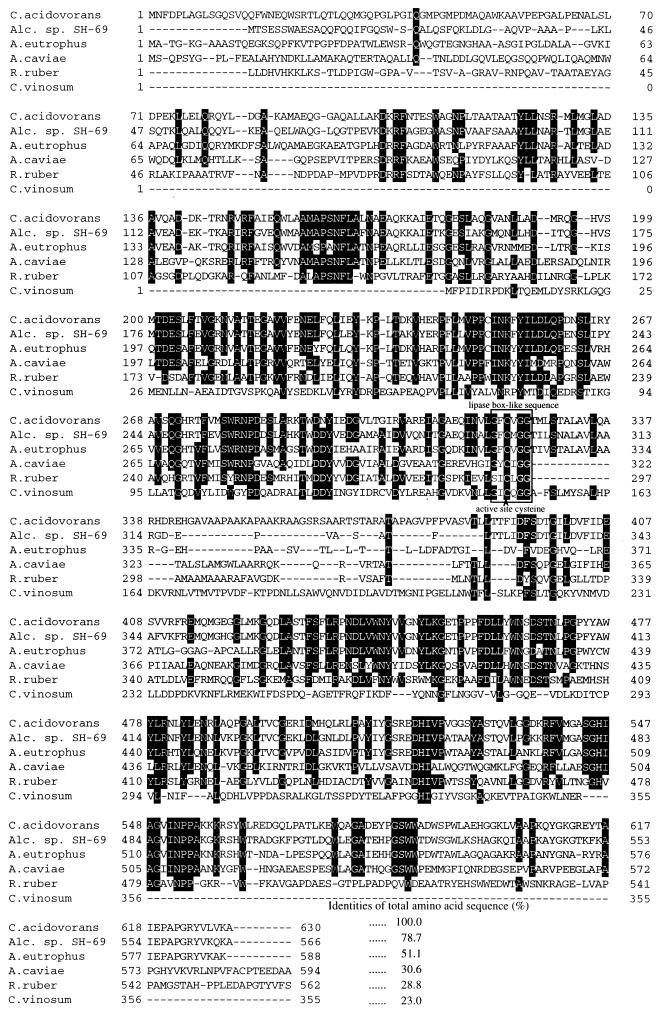
ORF1 (1,893 bp) starts 748 bp downstream from the SmaI site (Fig. 2). Several potential translation initiation codons were present in the region between 700 and 900 bp (at bp 748, 841, 874, 883, and 892), but the ATG beginning at position 748 was considered the most probable translation initiation codon based on the presence of a reliable Shine-Dalgarno sequence 6 bp upstream. ORF1 encodes a protein of 630 amino acids with a calculated relative molecular weight (Mr) of 69,068, and this gene was referred to as the PHA synthase gene of C. acidovorans (phaCCa) because the translated product showed strikingly high identities to the synthases of Alcaligenes sp. strain SH-69 (78.7%) and A. eutrophus (51.1%) (Fig. 3). In order to maximize homology, several gaps had to be introduced into the amino acid sequences of these synthases, mainly in the regions that correspond to the phaCCa-encoded primary structure between amino acids 338 and 389 (Fig. 3). This extra region encoded by phaCCa apparently contributes to the main difference between the primary structures of these synthases. The primary structure of the product of phaCCa, however, exhibited relatively lower identities to other synthases capable of incorporating short-chain HA, such as those of Rhodobacter sphaeroides (35.4% identity) (17) and Methylobacterium extorquens (33.1% identity) (46). The phaCCa product showed about 40% identity to the medium-chain-length PHA synthases encoded by phaC1 and phaC2 of both P. oleovorans (16) and Pseudomonas aeruginosa (43). Moreover, a highly conserved cysteine residue that has been well documented for the catalytic cycle (9) was also well preserved in the deduced amino acid sequence for phaCCa (Fig. 2). Computer identity search and promoter analysis did not reveal any other PHA-related genes or promoter region in the 736-bp DNA sequence immediately upstream of the tentative ribosome binding site (RBS).
FIG. 2.
Nucleotide sequence of a 3,973-bp region containing the phaCCa and phaACa genes with the amino acid sequences. Stop codons are indicated by asterisks. SD, Shine-Dalgarno site (RBS).
FIG. 3.
Alignment and identities of the deduced sequence of PHA synthase from C. acidovorans with those from Alcaligenes (Alc.) sp. strain SH-69 (GenBank accession no. U78047), A. eutrophus (29, 36), Aeromonas caviae (7), Rhodococcus ruber (30), and Chromatium vinosum (22), which have the ability to incorporate short-chain HA into PHA. Amino acids identical in at least four sequences are boxed in black.
Structure of the putative gene product of ORF2.
ORF2 (1,182 bp), which was located immediately downstream of phaCCa, has a spacer 123 bp from the transcriptional termination codon of ORF1 (Fig. 2). A potential RBS preceded the start codon at a distance of 9 bp. ORF2 was calculated to encode a 393-amino-acid protein with an Mr of 40,238. This deduced amino acid sequence, when subjected to an identity search, was found to have significant identity (74.2%) to the primary structure of β-ketothiolase from A. eutrophus (28). Therefore, ORF2 was concluded to represent a structural gene for C. acidovorans β-ketothiolase, phaACa. It also showed significantly high identities, i.e., >60%, to other thiolases known to be involved in the biosynthesis of PHA (27, 34). This prompted us to determine the nucleotide sequence of the downstream region of phaACa with the anticipation of finding a third gene involved in PHA biosynthesis, i.e., the gene coding for acetoacetyl-CoA reductase. However, despite the similarity of the arrangement of phaCCa and phaACa to the arrangement of genes in the PHA biosynthetic operon of A. eutrophus, a gene corresponding to the third gene, phaB, was not found in the region downstream of phaACa.
Heterologous expression studies.
Expression of the cloned 20-kbp C. acidovorans gDNA in A. eutrophus PHB−4 complemented the mutant strain, resulting in the accumulation of significant amounts of PHA, as shown in Table 2. P(3HB) homopolymer, as well as the copolymers P(3HB-co-3HV) and P(3HB-co-4HB), could be synthesized by the recombinant PHB−4 strain from suitable carbon sources. Use of fructose as a carbon source resulted in the production of P(3HB) homopolymer in both one-stage (results not shown) and two-stage cultivations. Comparison with the monomer composition of PHA produced by wild-type A. eutrophus showed that the ability to incorporate 3HB monomers was significantly improved in the recombinant PHB−4 strain. When pentanoate was supplied as the carbon source, the 3HB monomer content was increased to about 30 mol% more than that in the wild-type A. eutrophus. A similar increase was also observed when 4HB was the carbon source. The final PHA content in the recombinant PHB−4 strain was not very different from that in the wild type when either fructose or pentanoate was the carbon source. However, the PHA contents accumulated in the wild-type C. acidovorans incubated with pentanoate or 4HB were 43 and 31% of the dry cell weight, respectively, and the latter was greater than that produced by the wild-type A. eutrophus or the recombinant PHB−4 strain.
TABLE 2.
PHA accumulation by A. eutrophus PHB−4 complemented with pLACa and by wild types of A. eutrophus and C. acidovoransa
| Bacterium and carbon source | Dry cell wt (g/liter) | PHA content (wt%)b | PHA composition (mol%)
|
||
|---|---|---|---|---|---|
| 3HB | 3HV | 4HB | |||
| A. eutrophus PHB−4(pLACa) | |||||
| Fructose | 4.2 | 73 | 100 | 0 | 0 |
| Pentanoate | 1.8 | 27 | 56 | 44 | 0 |
| 4HB | 1.5 | 5 | 86 | 0 | 14 |
| 1,4-Butanediol | 1.3 | 2 | 100 | 0 | 0 |
| A. eutrophus H16 | |||||
| Fructose | 4.3 | 72 | 100 | 0 | 0 |
| Pentanoate | 2.0 | 65 | 25 | 75 | 0 |
| 4HB | 1.3 | 16 | 66 | 0 | 34 |
| 1,4-Butanediol | 1.2 | 22 | 89 | 0 | 11 |
| C. acidovorans DS-17 | |||||
| Fructose | 2.4 | 7 | 100 | 0 | 0 |
| Pentanoate | 2.4 | 43 | 39 | 61 | 0 |
| 4HB | 2.2 | 31 | 0 | 0 | 100 |
| 1,4-Butanediol | 2.1 | 17 | 3 | 0 | 97 |
Cells were cultivated in 100 ml of NR medium for 12 h and then transferred into nitrogen-free MS medium containing 1% (wt/vol) carbon source for 48 h at 30°C.
Percent of the dry cell weight.
A smaller, 8-kbp EcoRV subfragment (EV80) (Fig. 1b), which comprises the phaCCa and phaACa sequences and about 2 kbp of unknown sequences on both ends, was subcloned into the unique StuI restriction site of a broad-host-range vector, pJRD215. The resulting plasmid, pJRDEV80, was then mobilized from E. coli S17-1 to A. eutrophus PHB−4 by means of transconjugation. The heterologous expression of the EV80 subfragment under the conditions used for expression of the 20-kbp gDNA fragment, however, failed to induce PHA accumulation in the A. eutrophus PHB−4 transconjugant.
DISCUSSION
The genes for the key enzymes involved in PHA biosynthesis, PHA synthases, from more than 20 different bacteria have been cloned and analyzed with the objectives of elucidating the mechanism of PHA biosynthesis and reproducing the steps leading to the synthesis of desired bacterial polyesters. In this study, we report the cloning and characterization of the PHA synthase gene (phaCCa) and the β-ketothiolase gene (phaACa) of C. acidovorans DS-17 (JCM10181). Based on the types of PHAs produced, both the PHA synthases of C. acidovorans and A. eutrophus show specificity for short-chain 3- and 4-hydroxyacyl-CoA thioesters. The main difference is that C. acidovorans is able to produce a homopolymer of 4HB in substantial quantities (>20% of the cell dry mass) from 4HB and 1,4-butanediol (32). This prompted us to clone and characterize the PHA biosynthesis genes of C. acidovorans, to study the substrate specificity of the phaCCa-encoded product, and to determine if the product shows any preference for 4-hydroxybutyryl-CoA as a monomer.
The nucleotide sequence analysis of a 4-kbp SmaI-HindIII (Fig. 1) subfragment revealed that phaCCa and phaACa were arranged as they are in the PHA biosynthesis operon of A. eutrophus (phaC-A-B), but there was a difference for the third gene, the gene encoding acetoacetyl-CoA reductase (phaB), which was not located in the region immediately downstream of phaACa. This distinguishes the PHA biosynthesis operon of C. acidovorans from all those that have been reported to date. Comparison of the deduced amino acid sequence of the phaCCa product by alignment with other known synthases showed the highest identities to the synthases that are specific for short-chain HAs, especially to the synthases of Alcaligenes sp. strain SH-69 and A. eutrophus (Fig. 3). In contrast, the primary structure of phaCCa showed much lower identities to the synthases of Rhodobacter sphaeroides (17), M. extorquens (46), and Rhodococcus ruber (30), which were 35.4, 33.1, and 28.8%, respectively.
The heterologous expression in A. eutrophus PHB−4 of a 20-kbp gDNA fragment from C. acidovorans containing phaCACa suggests that the substrate specificity of C. acidovorans PHA synthase is indeed similar to that of A. eutrophus. Conditions that favored the production of P(4HB) homopolymer in C. acidovorans did not enhance the incorporation of 4HB monomers by the PHB−4 transconjugant. This indicates that, more than the specificity of the PHA synthase, the monomer-supplying pathway plays an important role in determining the type of PHA that can be produced. It is known that A. eutrophus is unable to produce P(4HB) homopolymer in quantities of more than 1 or 2% of the dry cell weight (24). But the coexpression of phaCAe and orfZ of Clostridium kluyveri, which putatively encodes a 4HB-CoA transferase, had enabled the construction of an E. coli strain that produces P(4HB) in larger quantities (13). These results support the idea that a metabolic environment which can supply the necessary precursors for PHA biosynthesis is the most crucial aspect of improving 4HB incorporation into PHA. In A. eutrophus, the channeling of 4HB into the 3-hydroxybutyryl-CoA production pathway may be more efficient than that into the 4-hydroxybutyryl-CoA production pathway, whereas the opposite may be true for C. acidovorans. No preference for 4-hydroxybutyryl-CoA was shown by the phaCCa-encoded product when expressed in A. eutrophus PHB−4; instead, the 3HB content was clearly increased (Table 2). The PHA content in wild-type C. acidovorans when supplied with 4HB was higher than in wild-type A. eutrophus and the recombinant PHB−4 strain. This further suggests that the ability of C. acidovorans to produce PHA with a high 4HB monomer content is due to its metabolic capacity to efficiently supply 4HB monomers rather than to the specificity of its PHA synthase.
ACKNOWLEDGMENTS
We are indebted to H. G. Schlegel (Georg-August-Universität) for the kind gifts of A. eutrophus PHB−4 and E. coli S17-1 and to B. Witholt (ETH) for plasmid pJRD215 used in this study.
This work was supported by CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation (JST).
REFERENCES
- 1.Allen L N, Hanson R S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985;161:955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunegg G, Sonnleitner B, Lafferty R M. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol. 1978;6:29–37. [Google Scholar]
- 3.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 4.Doi Y. Microbial polyesters. New York, N.Y: VCH; 1990. [Google Scholar]
- 5.Doi Y, Tamaki A, Kunioka M, Soga K. Biosynthesis of terpolyesters of 3-hydroxybutyrate, 3-hydroxyvalerate, and 5-hydroxyvalerate in Alcaligenes eutrophus from 5-chloropentanoic and pentanoic acids. Makromol Chem Rapid Commun. 1987;8:631–635. [Google Scholar]
- 6.Friedrich B, Hogrefe C, Schlegel H G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981;147:198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukui T, Doi Y. Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J Bacteriol. 1997;179:4821–4830. doi: 10.1128/jb.179.15.4821-4830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukui T, Ito M, Saito T, Tomita K. Purification and characterization of NADP-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Biochim Biophys Acta. 1987;917:365–371. [PubMed] [Google Scholar]
- 9.Gerngross T U, Snell K D, Peoples O P, Sinskey A J, Csuhai E, Masamune M, Stubbe J. Overexpression and purification of the soluble polyhydroxyalkanoate synthase from Alcaligenes eutrophus. Evidence for a required posttranslational modification for catalytic activity. Biochemistry. 1994;33:9311–9320. doi: 10.1021/bi00197a035. [DOI] [PubMed] [Google Scholar]
- 10.Haywood G W, Anderson A J, Williams G A, Dawes E A, Ewing D F. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int J Biol Macromol. 1991;13:83–87. doi: 10.1016/0141-8130(91)90053-w. [DOI] [PubMed] [Google Scholar]
- 11.Haywood G W, Anderson A J, Chu L, Dawes E A. Characterization of two 3-ketothiolases in the polyhydroxyalkanoate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol Lett. 1988;52:91–96. [Google Scholar]
- 12.Haywood G W, Anderson A J, Chu L, Dawes E A. The role of NADH- and NADPH-linked acetoacetyl-CoA reductases in the poly-3-hydroxyalkanoate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol Lett. 1988;52:259–264. [Google Scholar]
- 13.Hein S, Söhling B, Gottschalk G, Steinbüchel A. Biosynthesis of poly(4-hydroxybutyric acid) by recombinant strains of Escherichia coli. FEMS Microbiol Lett. 1997;153:411–418. doi: 10.1111/j.1574-6968.1997.tb12604.x. [DOI] [PubMed] [Google Scholar]
- 14.Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 15.Hiramitsu M, Doi Y. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Alcaligenes latus. Biotechnol Lett. 1993;15:461–464. [Google Scholar]
- 16.Huisman G W, Wonink E W, Meima R, Kazemier B, Tersptra P, Witholt B. Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. J Biol Chem. 1991;266:2191–2198. [PubMed] [Google Scholar]
- 17.Hustede E, Steinbüchel A. Characterization of the polyhydroxyalkanoate synthase gene locus of Rhodobacter sphaeroides. Biotechnol Lett. 1993;15:709–714. [Google Scholar]
- 18.Kato M, Bao H J, Kang C K, Fukui T, Doi Y. Production of a novel copolyester of 3-hydroxybutyric acids and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl Microbiol Biotechnol. 1996;45:363–370. [Google Scholar]
- 19.Kunioka M, Nakamura Y, Doi Y. New bacterial copolyesters produced in Alcaligenes eutrophus from organic acids. Polymer Commun. 1988;29:174–176. [Google Scholar]
- 20.Lee S Y. Bacterial polyhydroxyalkanoates. Biotechnol Bioeng. 1996;49:1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Lemoigne M. Products of dehydration and of polymerization of β-hydroxybutyric acid. Bull Soc Chem Biol. 1926;8:770–782. [Google Scholar]
- 22.Liebergesell M, Steinbüchel A. Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem. 1992;209:135–150. doi: 10.1111/j.1432-1033.1992.tb17270.x. [DOI] [PubMed] [Google Scholar]
- 23.Mothes G, Rivera I S, Babel W. Competition between β-ketothiolase and citrate synthase during poly(β-hydroxybutyrate) synthesis in Methylobacterium rhodesianum. Arch Microbiol. 1997;166:405–410. doi: 10.1007/BF01682987. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Doi Y, Scandola M. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Macromolecules. 1992;25:4237–4241. [Google Scholar]
- 25.Nishimura T, Saito T, Tomita K. Purification and properties of β-ketothiolase from Zoogloea ramigera. Arch Microbiol. 1978;116:21–27. doi: 10.1007/BF00408729. [DOI] [PubMed] [Google Scholar]
- 26.Oeding V, Schlegel H G. β-Ketothiolase from Hydrogenomonas eutropha H16 and its significance in the regulation of poly-β-hydroxybutyrate metabolism. Biochem J. 1973;134:239–248. doi: 10.1042/bj1340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peoples O P, Masamune S, Walsh C T, Sinskey A J. Biosynthetic thiolase from Zoogloea ramigera. III. Isolation and characterization of the structural gene. J Biol Chem. 1987;262:97–102. [PubMed] [Google Scholar]
- 28.Peoples O P, Sinskey A J. Poly-β-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding β-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem. 1989;264:15293–15297. [PubMed] [Google Scholar]
- 29.Peoples O P, Sinskey A J. Poly-β-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC) J Biol Chem. 1989;264:15298–15303. [PubMed] [Google Scholar]
- 30.Pieper U, Steinbüchel A. Identification, cloning and sequence analysis of the poly(3-hydroxyalkanoic acid) synthase gene of the gram-positive bacterium Rhodococcus ruber. FEMS Microbiol Lett. 1992;96:73–80. doi: 10.1016/0378-1097(92)90459-2. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Doi Y. Microbial synthesis and properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int J Biol Macromol. 1994;16:99–104. doi: 10.1016/0141-8130(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Nakamura S, Hiramitsu M, Doi Y. Microbial synthesis and properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Polymer Int. 1996;39:169–174. [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schembri M A, Bayly R C, Davies J K. Phosphate concentration regulates transcription of the Acinetobacter polyhydroxyalkanoic acid biosynthetic genes. J Bacteriol. 1995;177:4501–4507. doi: 10.1128/jb.177.15.4501-4507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlegel H G, Lafferty R, Krauss I. The isolation of mutants not accumulating poly-β-hydroxybutyric acid. Arch Mikrobiol. 1970;71:283–294. doi: 10.1007/BF00410161. [DOI] [PubMed] [Google Scholar]
- 36.Schubert P, Steinbüchel A, Schlegel H G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-β-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988;170:5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seebach D, Brunner A, Bachmann B M, Hoffmann T, Kühnle F N M, Lengweiler U D. Biopolymers and -oligomers of (R)-3-hydroxyalkanoic acids. Contributions of synthetic organic chemists. Berlin, Germany: Ernst Schering Research Foundation; 1995. [Google Scholar]
- 38.Senior P J, Dawes E A. The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J. 1973;134:225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi F, Gross R A, Rutherford D R. Microbial polyester synthesis: effects of poly(ethylene glycol) on product composition, repeat unit sequence, and end group structure. Macromolecules. 1996;29:10–17. [Google Scholar]
- 40.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering. Transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 41.Söhling B, Gottschalk G. Molecular analysis of the anaerobic succinate degradation pathway in Clostridium kluyveri. J Bacteriol. 1996;178:871–880. doi: 10.1128/jb.178.3.871-880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentine H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev. 1992;103:217–230. doi: 10.1111/j.1574-6968.1992.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 43.Timm A, Steinbüchel A. Cloning and molecular analysis of the poly(3-hydroxyalkanoic acid) gene locus of Pseudomonas aeruginosa PAO1. Eur J Biochem. 1992;209:15–30. doi: 10.1111/j.1432-1033.1992.tb17256.x. [DOI] [PubMed] [Google Scholar]
- 44.Valentin H E, Dennis D. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate in recombinant Escherichia coli grown on glucose. J Biotechnol. 1997;58:33–38. doi: 10.1016/s0168-1656(97)00127-2. [DOI] [PubMed] [Google Scholar]
- 45.Valentin H E, Schönebaum A, Steinbüchel A. Identification of 4-hydroxyvaleric acid as a constituent in biosynthetic polyhydroxyalkanoic acids from bacteria. Appl Microbiol Biotechnol. 1992;36:507–514. [Google Scholar]
- 46.Valentin H E, Steinbüchel A. Cloning and characterization of the Methylobacterium extorquens polyhydroxyalkanoic-acid-synthase structural gene. Appl Microbiol Biotechnol. 1993;40:699–709. doi: 10.1007/BF00192084. [DOI] [PubMed] [Google Scholar]