Abstract
Oligotrophic oceanic waters of the central ocean gyres typically have extremely low dissolved fixed inorganic nitrogen concentrations, but few nitrogen-fixing microorganisms from the oceanic environment have been cultivated. Nitrogenase gene (nifH) sequences amplified directly from oceanic waters showed that the open ocean contains more diverse diazotrophic microbial populations and more diverse habitats for nitrogen fixers than previously observed by classical microbiological techniques. Nitrogenase genes derived from unicellular and filamentous cyanobacteria, as well as from the α and γ subdivisions of the class Proteobacteria, were found in both the Atlantic and Pacific oceans. nifH sequences that cluster phylogenetically with sequences from sulfate reducers or clostridia were found associated with planktonic crustaceans. Nitrogenase sequence types obtained from invertebrates represented phylotypes distinct from the phylotypes detected in the picoplankton size fraction. The results indicate that there are in the oceanic environment several distinct potentially nitrogen-fixing microbial assemblages that include representatives of diverse phylotypes.
The productivity of the oceans controls the fluxes of many biogeochemically important compounds, including the rate of exchange of carbon dioxide between the open ocean and the atmosphere. In turn, oceanic carbon fixation is limited by the bioavailability of nutrients, including nitrogen, phosphorus, and iron (9, 10, 20). In contrast to the biogeochemical cycles of phosphorus and iron, nitrogen is present in relatively high concentrations in seawater as gaseous N2. Gaseous nitrogen is available only to microorganisms with the capability of biological nitrogen fixation, the reduction of atmospheric N2 to ammonium. Although large areas of the world’s oceans are virtually devoid of fixed dissolved inorganic nitrogen and primary production may be nitrogen limited, very few species of nitrogen-fixing organisms have been identified or isolated from the plankton. Trichodesmium, a filamentous aggregate-forming cyanobacterium, is an abundant diazotroph in tropical and subtropical waters (3, 5), but few other examples of diazotrophs from the open ocean are known (21, 35). The seeming low diversity of known nitrogen-fixing organisms in the open ocean stands in stark contrast to the presumptive nitrogen limitation in the world’s oceans and presents an evolutionary paradox.
Recently, biological nitrogen fixation has gained recognition as an important source of nitrogen for supporting oceanic primary production (3, 11, 18, 22). The nitrogen budget for the Atlantic Ocean does not balance because a source of nitrogen cannot be accounted for by current knowledge of fluxes and pools of nitrogen, even after including nitrogen fixation by Trichodesmium (22). It is speculated that rates of nitrogen fixation by known diazotrophic organisms have been underestimated (17), or as yet unidentified diazotrophic organisms are active in the ocean (18). Conventional nitrogenase, the enzyme that catalyzes biological dinitrogen reduction to ammonium, is composed of two highly conserved proteins: the iron (Fe) protein (encoded by the nifH gene) and the molybdenum iron (MoFe) protein (encoded by the nifDK genes). The nitrogenase enzyme is present in diverse lineages of prokaryotes and is generally believed to be ancient (38). Evolutionarily conserved amino acid sequences within the nifH (which encodes the Fe protein component of nitrogenase) gene have been exploited to design PCR primers to detect the genetic potential for nitrogen fixation in the marine environment (39). With this approach, the diversity of nitrogen-fixing microorganisms in oceanic water and marine plankton was determined. This report shows that there are far more diverse nitrogen-fixing populations and diverse habitats which can support nitrogen fixation in the open ocean than previously documented.
MATERIALS AND METHODS
Sample types, sampling locations, depths, and sampling dates are shown in Table 1. Samples were collected from the Bermuda Atlantic Time Series (BATS) and Hawaii Ocean Time Series (HOT) stations during routine sampling. Samples were also collected during cruises on ships of opportunity in the Atlantic and Pacific oceans and Caribbean Sea. Water was collected at depths ranging from 0 to 200 m, using Niskin water sampling bottles. Samples for picoplankton (0.22- to 20-μm size fraction) DNA extraction were prefiltered (through 20-μm nylon Nytex mesh) to remove Trichodesmium and zooplankton, and the microbial assemblages were filtered onto Gelman Supor 0.22-μm-pore-size filters. Water samples were typically 2 to 4 liters in volume except for the Atlantic equatorial samples and samples collected near the Bahama Islands, where 20 liters of water was concentrated in an Amicon DC10L tangential flow system fitted with a hollow-fiber (30,000-molecular-weight) cartridge. The concentrate from the tangential flow system (approximately 250 ml) was filtered onto Gelman filters as described above.
TABLE 1.
Classification of major types of nifH sequences obtained from marine picoplankton and zooplankton samples, showing sample source and location
| Phylogenetic affiliation | GenBank accession no. | Sample type | Location/date | Depth (m) | Sequence(s)a |
|---|---|---|---|---|---|
| Heterocystous cyanobacteria | AF059624 | Plankton | BATS station/August 8, 1996 | 1 | BT1101 |
| AF059625 | Plankton | BATS station/February 15, 1996 | 200 | BT1118 | |
| Unicellular cyanobacteria | AF016616 | Picoplankton | Atlantic Ocean (19.1°N 58.2°W)/May 29, 1994 | 160 | AO11 |
| AF059626 | Picoplankton | HOT station (22°45′N 158°W)/May 22, 1996 | 175 | HT1103 | |
| AF059627 | Picoplankton | HOT station (22°45′N 158°W)/May 6, 1997 | 25 | HT1150 | |
| Filamentous nonheterocystous cyanobacteria | AF059628 | Plankton | HOT station (22°45′N 158°W)/May 6, 1997 | 25 | HT1169 |
| α proteobacteria | AF016612 | Picoplankton | BATS station/February 15, 1996 | 10 | BT13 |
| AF016610, AF016611 | Picoplankton | BATS station/February 15, 1996 | 75 | BT11, BT12 | |
| AF016615 | Picoplankton | Atlantic Ocean (23.1°N 69.4°W)/May 25, 1994 | 125 | AO12 | |
| AF059644 | Diatom collections | Pacific Ocean/September 1, 1992 | Surface | PO3120 | |
| AF059645 | Diatom (Hemiaulus) collections | Pacific Ocean/September 22, 1995 | PO3133 | ||
| β proteobacteria | AF016618 | Picoplankton | Atlantic Ocean (17.3°N 53.2°W)/May 31, 1994 | 40 | AO14 |
| AF059643 | Picoplankton | Near Bahama Islands/September 4, 1991 | Surface | BH1132 | |
| AF016602 | Acartia tonsa | Gulf of Mexico (29°N 84°W) | 2 | GM26 | |
| AF059647 | Diatom collections | Pacific Ocean/September 4, 1992 | PO3137 | ||
| AF059646 | Diatom collections | Pacific Ocean/September 4, 1992 | PO3135 | ||
| Distantly related to γ proteobacteria | AF016592 | Labidocera aestiva | Gulf of Mexico (29°N 84°W) | 2 | GM23 |
| AF016601, AF016600 | A. tonsa | Gulf of Mexico (29°N 84°W) | 2 | GM24, GM21 | |
| γ proteobacteria | AF016603, AF016609 | A. tonsa | Gulf of Mexico (29°N 84°W) | 2 | GM25, GM22 |
| AF016614 | Picoplankton | Atlantic Ocean (23°N 69.2°W)/May 25, 1994 | 160 | AO13 | |
| AF016617 | Picoplankton | Atlantic Ocean (19.1°N 58.23°W)/May 29, 1994 | 70 | AO16 | |
| AF059622 | Picoplankton | Atlantic Ocean (18–23°N 43–62°W)/October 21, 1996 | 20 | AO1104 | |
| AF059623 | Picoplankton | Atlantic Ocean (18–23°N 43–62°W)/October 21, 1996 | 40 | AO1113 | |
| AF016613 | Picoplankton | Atlantic Ocean (23°N 69.2°W)/May 25, 1994 | 125 | AO15 | |
| AF059629 | Picoplankton | HOT station (22°45′N 158°W)/May 6, 1997 | 50 | HT1177 | |
| AF059621 | Picoplankton | Atlantic Ocean (18–23°N 43–62°W)/October 21, 1996 | 0 | AO1102 | |
| Unidentified relatives of clostridia and sulfate reducers | AF016595, AF016598, AF016597, AF016599, AF016596 | A. tonsa | Gulf of Mexico (29°N 84°W) | 2 | GM215, GM216, GM27, GM29, GM210 |
| AF016594, AF016593 | L. aestiva | Gulf of Mexico (29°N 84°W) | 2 | GM212, GM214 |
Sequence prefixes: AO, Atlantic Ocean; GM, Gulf of Mexico; BT, BATS station; PO, Pacific Ocean; HT, HOT station; BH, near Bahama Islands.
Zooplankton were collected by 150-μm net tow at a depth of 2 m in the Gulf of Mexico near Florida (29°N, 84°W) in the fall of 1994. Live adult individuals of the calanoid copepods Labidocera aestiva and Acartia tonsa were sorted from the mixed assemblage by using wide-bore pipettes. Sorted individuals were washed in prefiltered (through a 0.2-μm-pore-size filter) seawater prior to preservation.
All samples were frozen in 10 mM Tris (pH 8.0)–100 mM EDTA (pH 8.0) until analyzed. DNA was extracted from the filter and zooplankton samples by using a slight modification of the method of Giovannoni et al. (13).
Nitrogenase Fe protein genes (nifH) were amplified from picoplankton- and zooplankton-derived genomic DNA, using the PCR primers of Zehr and McReynolds (41). The samples were amplified by PCR in a mixture containing 4 mM MgCl2 (Promega, Madison, Wis.), the enzyme manufacturer’s buffer (Promega), 200 μM deoxynucleoside triphosphates, 100 pmol of each primer, and 2.5 U of Taq polymerase (Promega) in 50-μl volumes for 35 cycles (1 min at 94°C, 1 min at 54°C, and 1 min at 72°C).
The amplified fragments were cloned into Promega pGEM-T vector (Promega). Clones were screened by restriction digestion to identify those with the correct insert, and DNA from the selected clones was used for DNA sequencing by the Sanger dideoxynucleotide chain termination method (28). DNA sequences were obtained on both strands; the consensus sequence was used to determine the deduced amino acid sequence, using the Genetic Data Environment package (30). Deduced amino acid sequences were aligned manually, and the aligned sequences were used for phylogenetic analysis using PHYLIP 3.5c (12) or TREECON for Windows (34).
Nucleotide sequence accession numbers.
The sequences obtained in this study were submitted to GenBank under accession no. AF016592 to AF016618 and AF059621 to AF059649 (which includes two new nifH sequences obtained from cultivated isolates of Chromatium purpuratum).
RESULTS AND DISCUSSION
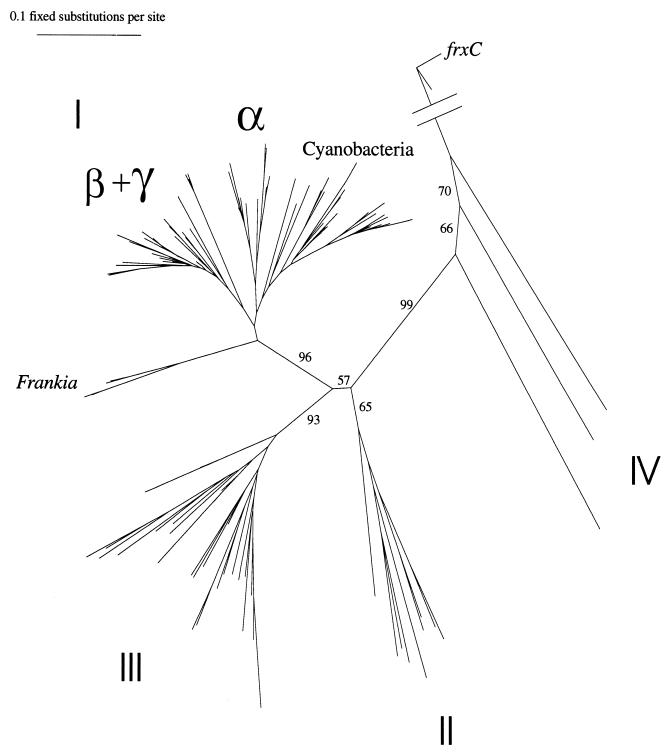
Nitrogenase (nifH) genes were amplified from oligotrophic surface water picoplankton from the tropical Atlantic and Pacific oceans and from the Sargasso and Caribbean seas, using PCR and universal primers for the nitrogenase Fe protein gene (41). nifH or nifH-related gene sequences cluster in four major groups (Fig. 1, I to IV [7]). nifH genes not only were found in the picoplankton size fraction from these diverse water samples but also were amplified from marine crustacean zooplankton. Sequences from the marine invertebrate and oceanic picoplankton samples were found only in clusters I (conventional nifH) and group III (divergent nifH from sulfate reducers and clostridia) (Fig. 2 and 3). The nif sequences obtained from free-living picoplankton and invertebrate samples represented diverse heterotrophic and photoautotrophic lineages (Fig. 2 and 3), but more importantly, the sequence types were distinctly different from the two sample types, highlighting the differences in diversity between the two microbial habitats.
FIG. 1.
Radial phylogenetic tree indicating major clusters of nifH genes (groups I to IV as defined in reference 7). A nifH-like gene related to chlorophyllide reductase (frxC; GenBank sequence X60490) was used to root the tree. The radial tree was constructed by using sequences presented in Fig. 2 plus GenBank sequences from groups II (X56072, M23528, P09553, X70033, P25767, X87971, U75887, U23648, and P51602) and IV (P08624, P06119, and P08625). Group I includes conventional nifH sequences from proteobacterial clades (α, β, and γ) and cyanobacteria. Group II represents second alternative nifH genes (from non-molybdenum-, non-vanadium-containing nitrogenases). Group III includes sequences from sulfate reducers (δ proteobacteria) and clostridia (gram-positive bacteria). Group IV sequences are from divergent genes that may not be derived from active nitrogenases.
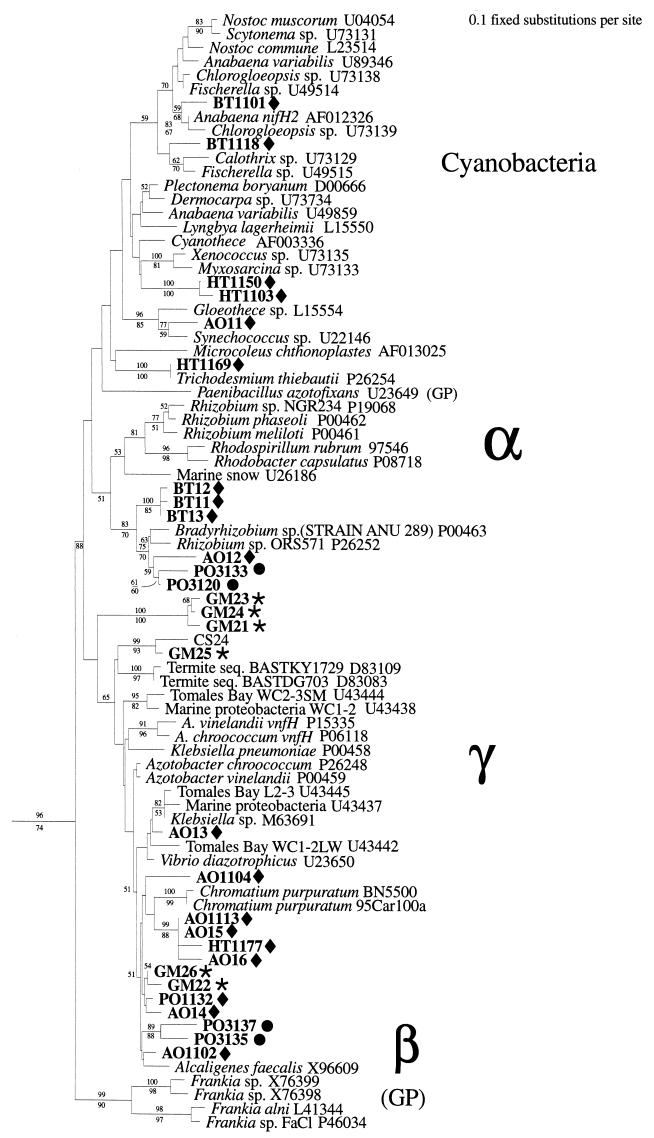
FIG. 2.
Phylogenetic analysis of nifH genes obtained from oceanic picoplankton and zooplankton included in α, β, and γ proteobacteria and cyanobacterial clades. The tree includes nifH sequences representing cultivated organisms (genus and species) and uncultivated organisms (e.g., from termites and from marine [e.g., Tomales Bay] samples) (GenBank sequence numbers are indicated). The data set was bootstrapped 100 times, and bootstrap values greater than 50% are indicated at the relevant nodes (distance and parsimony methods are represented by values above and below the nodes, respectively). Sample information and identifications are given in Table 1. GP, gram positive; •, diatom associated; ⧫, picoplankton; 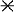 , zooplankton associated.
, zooplankton associated.
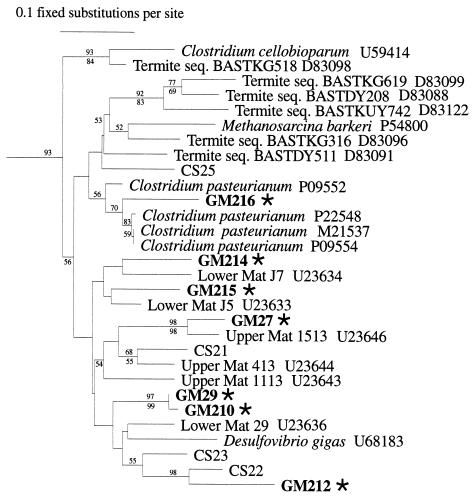
FIG. 3.
Phylogenetic analysis of nifH genes obtained from oceanic picoplankton and zooplankton included in a large set of deeply branching clusters containing divergent sequences derived from clades including sulfate-reducing bacteria and clostridia. nifH sequences representing cultivated organisms and uncultivated organisms are included on the tree (GenBank sequence numbers are indicated). The data sets were bootstrapped 100 times, and bootstrap values greater than 50% are indicated at the relevant nodes (distance and parsimony methods represented above and below the nodes, respectively). Sequences and analysis are as for Fig. 2. •, diatom associated; ⧫, picoplankton associated; 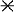 , zooplankton associated.
, zooplankton associated.
Picoplankton nif genes included sequences clearly derived from representatives of the α and γ proteobacteria, β proteobacteria, and unicellular cyanobacteria clades (Table 1; Fig. 2). The phylogeny of nifH clearly distinguishes between filamentous nonheterocystous, heterocystous, and unicellular strains (40). nifH sequences from this study clustered with sequences from group V heterocystous strains and group I unicellular strains. Unicellular cyanobacterial nifH sequences were found in low-latitude oligotrophic waters of the Atlantic and at the BATS and HOT sites (Table 1). The phylotypes obtained from the Pacific and the Atlantic water samples were distinctly different, however. The nifH sequences obtained from the Pacific Ocean (sequence types HT1150 and HT1103) cluster with the group II genera Myxosarcina and Xenococcus, whereas the sequence type amplified from the Atlantic Ocean (AO11) clusters more closely with the nifH sequences from Gloeothece and Cyanothece (previously called Synechococcus), which are representatives of group I cyanobacteria (unicellular, dividing in one plane). However, the resolution of these groups in the nifH tree may currently be limited by the availability of representative nifH sequences. The unicellular cyanobacterial nifH genes from the HOT site were recovered in two different years and from multiple depths spanning the mixed layer. nifH genes derived from filamentous species were also detected in the picoplankton size fraction. Although filamentous cyanobacteria would have been expected to be largely removed by the prefiltration procedure, the presence of cells that presumably dissociated from filaments indicates that filamentous cyanobacteria (nonheterocystous species from the HOT station and heterocystous species from the BATS site) may be present in the net plankton.
Unicellular cyanobacteria and prochlorophytes are numerous in the open ocean (8, 16, 36), but it is likely that most of these species are not able to fix atmospheric nitrogen. Only a single open-ocean unicellular nitrogen-fixing strain of cyanobacteria has been found in low-latitude Atlantic waters (37). The few known nitrogen-fixing unicellular cyanobacterial strains (e.g., of the genera Gloeothece and Cyanothece) are distinctly different in morphology from the typically abundant oceanic unicellular cyanobacterial strains and are quite large (5 μm in diameter) (6). Since an oceanic cyanobacterial nitrogen-fixing isolate has been obtained (36) and the results presented here suggest that this type of organism may be more widely distributed in the Pacific and Atlantic Ocean basins than previously documented, these organisms should receive closer attention.
The presence of numerous α and γ proteobacterial (referred to for simplicity as α and γ) nifH genes in the picoplankton is consistent with the documentation of numerous α and γ 16S rRNA phylotypes in oceanic waters (23, 29). Noncyanobacterial nitrogenase genes found in the bulk water include γ genes closely related to nifH genes from the genera Azotobacter and Vibrio (Fig. 2; Table 1). The γ nifH phylotypes were found numerous times in samples (35 sequences obtained that were >98% identical at the nucleotide level [data not shown]) from the equatorial Atlantic waters. Interestingly, this γ nifH sequence type was not obtained from the zooplankton samples. Very closely related Vibrio nifH sequences (31) and strains (32) have been found in coastal studies, suggesting that these nitrogen-fixing γ proteobacteria are major nitrogenase gene-containing phylotypes in the marine environment. A second cluster of γ nifH sequences were relatively closely related to sequences obtained from the purple sulfur bacterium C. purpuratum (Fig. 2). Sequences in this cluster, as well as the Atlantic Ocean picoplankton samples, were obtained from the HOT station. Relatively closely related to this cluster are a group of sequences that group between the Alcaligenes faecalis nifH sequence and γ nifH. These nifH sequences, which may be derived from phylotypes of β proteobacteria, were obtained in the Atlantic Ocean picoplankton samples as well as from diatom mats collected in the Pacific Ocean (Fig. 2).
α 16S genes have been previously discovered in the open ocean and include the important SAR (Sargasso Sea) clusters (14). The α nifH sequences recovered in this study (Fig. 2) could even be derived from the SAR-11 α phylotypes. Interestingly, the α nifH phylotypes have not yet been recovered from the HOT station, although two sequences (PO3133 and PO3120) were obtained from planktonic Pacific Ocean diatom mats. The Atlantic Ocean α nifH phylotype clustered with the Pacific diatom-associated nifH, but a distinct cluster of α nifH sequences was obtained from BATS (Fig. 2). Although the most closely related nifH sequences from cultivated organisms are those from Rhizobium spp., the branches are long relative to the diversity of α nifH sequences on the tree, and thus it is likely that the nitrogen-fixing α proteobacteria represented by these phylotypes are only distantly related to known organisms.
The nifH sequences obtained from amplification of DNA extracted from planktonic crustacean zooplankton (calanoid copepods L. aestiva and A. tonsa) clustered with different nifH clusters than did sequences obtained from the picoplankton. In comparison to the picoplankton samples, the zooplankton nifH gene sequences represented distinct lineages, with abundant sequences clustering with sequences from sulfate reducers and clostridia (group III nitrogenase [Fig. 3]). Interestingly, sequences in this cluster obtained from direct DNA extracts of marine zooplankton were also obtained from microbial enrichments initiated with zooplankton biomass (2). The diverse set of nifH genes amplified from individual copepods (A. tonsa and L. aestiva) collected from Gulf of Mexico waters (Fig. 1 and 2; Table 1) suggests that nitrogen-fixing microorganisms may be associated with the exoskeleton and/or the gut tract of planktonic crustacea. With one exception, all of the nifH genes obtained by amplification from zooplankton were distinctly different from the nifH sequences obtained from the picoplankton. Although the two zooplankton sequences, GM22 and GM26, form a distinct cluster, there is some similarity of sequences GM22 and GM26 obtained from the calanoid copepod A. tonsa and the sequences AO14 and BH1132 obtained from the Atlantic Ocean and Bahama Island picoplankton samples.
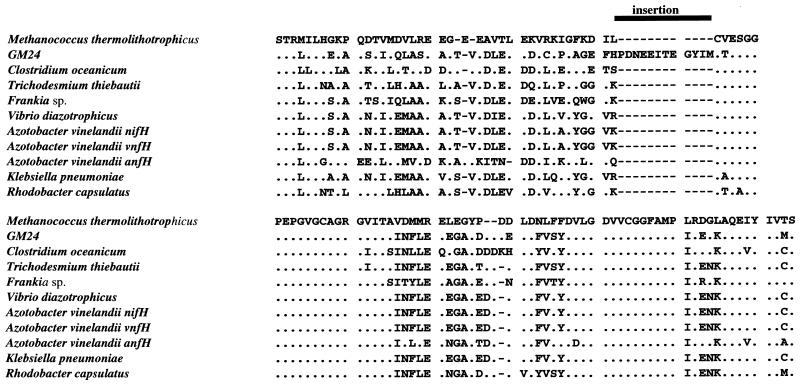
The γ nifH sequences obtained from zooplankton were distantly related to the γ nifH sequences derived from the picoplankton (Fig. 2). It is noteworthy that genes in one of the clusters of γ nifH genes recovered from the zooplankton are unique with respect to nifH genes described thus far. The nifH sequences in this cluster contain a 12-amino-acid residue insertion (Fig. 4). It is unlikely that these nifH genes are pseudogenes. The reading frame is maintained throughout the insertion, and the physical location of the insertion in the three-dimensional structure of the Fe protein is on the surface of the protein (data not shown). This loop would not likely interfere with nucleotide binding or the FeS cluster, and it is not near the interface where the MoFe protein docks with the Fe protein. The chance that such an extensive insertion would have occurred as an evolutionary event in a pseudogene without associated extensive accumulation of mutations in the rest of the amino acid sequence is extremely low. Phylogenetically, the unique sequences are relatively closely related to other proteobacterial sequences (Fig. 2). Thus, the evidence suggests that the nifH sequences were derived from potentially active, novel nitrogenases.
FIG. 4.
Unique nifH deduced amino acid sequences (GM24) obtained from amplification of nifH genes from zooplankton samples. Partial sequences (corresponding to amino acid residues 45 to 153 of the nifH gene from Azotobacter vinelandii [GenBank sequence M20568]) are aligned to diverse representative sequences of archaea (Methanotrophicus thermolithotrophicus), gram-positive, low-GC content bacteria (Clostridium oceanicum), cyanobacteria (Trichodesmium thiebautii), gram-positive, high-GC content bacteria (Frankia sp.), γ proteobacteria (Vibrio diazotrophicus and Klebsiella pneumoniae), α proteobacteria (Rhodobacter capsulatus), and the conventional and alternative nifH genes of a γ proteobacterium (Azotobacter vinelandii). GM21, GM23, and GM24 nifH sequences contain a unique large (12-amino-acid) insertion (represented by the solid bar above the sequences and dashes within the aligned sequences).
Most of the other nitrogenase gene sequences obtained from zooplankton clustered with a diverse clade that includes sequences from sulfate-reducing bacteria and from clostridia (Fig. 3). All of the cultivated species whose nitrogenase genes are included in this cluster, such as sulfate reducers and clostridia, are strict anaerobes.
Recently, nitrogenase gene sequences included in this cluster have been recovered from anoxic environments (including marine sediments and soils [33, 42]) and, most interestingly, from termite guts (25). The nitrogen-fixing species associated with zooplankton appear to be largely anaerobic microorganisms, which is consistent with the postulated existence of such organisms in the zooplankton gut (27). Therefore, since the nifH sequences obtained from zooplankton were largely representative of anaerobic microbes, the nitrogen-fixing microorganisms associated with zooplankton were probably derived from the gut rather than associated with the exoskeleton.
The intriguing finding that nitrogen fixation in the open ocean may occur in marine invertebrate guts is analogous to nitrogen fixation in guts of terrestrial insects (25) and marine shipworms (4). Sequences of this cluster are not found in the bulk water samples, indicating that the organisms from which these sequences were derived may be permanent residents of the zooplankton gut and may even be symbiotic with zooplankton. Planktonic invertebrates, including copepods, are known to have gut microflora (24), but this is the first report that demonstrates that copepod gut microflora includes diverse populations of microorganisms with the genetic potential for nitrogen fixation. Since nitrogen fixation is energetically expensive, zooplankton gut tracts may be an important environment for nitrogen fixation in oligotrophic oceans. Copepods are the most abundant zooplankton in the ocean (19), and since copepods are responsible for consuming much of the phytoplankton productivity in the world’s oceans, they would provide the microflora with a continuous supply of energy-rich substrates for microbial metabolism. Anoxic conditions within the gut could protect nitrogenase from oxygen inactivation. Furthermore, it has recently been argued that nitrogen fixation in the ocean is limited by iron availability (11), but copepod gut tracts undergo pH (26) and redox changes during feeding and digestion that could be important in increasing the bioavailability of trace elements, such as iron, for nitrogen fixers in the gut tract. Copepod grazing clearly solubilizes phytoplankton cellular iron (15), which provides a potential mechanism for making iron available to copepod-associated nitrogen-fixing gut microflora.
The results of this study show that there are diverse nitrogen-fixing phylotypes in the oceanic environment in the plankton, as well as associated with diatom aggregates and planktonic crustacea. It has yet to be demonstrated whether these phylotypes fix nitrogen or how abundant these phylotypes are in the ocean. Recently, an approach for quantifying nifH genes was applied to the estuarine environment and showed that nif gene abundance decreased from freshwater sources to the mesohaline reaches (1). In comparison to results from the estuarine study, the amplification products from marine samples indicate that the concentration of nitrogen-fixing organisms is much lower in oceanic environments than in coastal environments. However, even at low densities, active populations of nitrogen-fixing microorganisms over vast areas of the open ocean could contribute substantially to nitrogen inputs in the world’s oceans. This study provides the first evidence of the presence and diversity of open-ocean diazotrophs, which may be key to balancing the nitrogen budget of the oceans (22). The results indicate that there are far more diverse nitrogen-fixing microorganisms in the oceanic plankton than previously believed.
ACKNOWLEDGMENTS
We thank Bermuda Biological Station personnel for sample collection and logistic support at the BATS station collections, and we thank D. Karl and L. Tupas for facilitating sample collection at the HOT station. We also thank D. Capone and T. Villareal for providing equatorial Atlantic Ocean picoplankton and Pacific Ocean diatom samples, respectively. L. Proctor provided copepod samples from the Gulf of Mexico and reviewed the manuscript.
This research was supported by NSF grants OCE-950353 and IBN 9629314 to J.P.Z.
REFERENCES
- 1.Affourtit J. Characterization and quantitation of nitrogenase genes along a salinity gradient in the Neuse River. M.S. thesis. Troy, N.Y: Rensselaer Polytechnic Institute; 1997. [Google Scholar]
- 2.Braun, S., L. Proctor, S. Zani, M. T. Mellon, and J. P. Zehr. Molecular evidence for zooplankton-associated nitrogen-fixing anaerobes based on amplification of the nifH gene. Submitted for publication.
- 3.Capone D G, Zehr J P, Paerl H W, Bergman B, Carpenter E J. Trichodesmium: a globally significant marine cyanobacterium. Science. 1997;276:1221–1229. [Google Scholar]
- 4.Carpenter E J, Culliney J L. Nitrogen fixation in marine shipworms. Science. 1975;187:551–552. doi: 10.1126/science.187.4176.551. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter E J, Romans K. Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science. 1991;254:1356–1358. doi: 10.1126/science.254.5036.1356. [DOI] [PubMed] [Google Scholar]
- 6.Castenholz R W, Waterbury J B. Group I. Cyanobacteria. In: Staley J T, editor. Bergey’s manual of determinative bacteriology. Baltimore, Md: Williams & Wilkins; 1989. pp. 1710–1727. [Google Scholar]
- 7.Chien Y-T, Zinder S H. Cloning, DNA sequencing, and characterization of a nifD-homologous gene from the archaeon Methanosarcina barkeri 227 which resembles nifD1 from the eubacterium Clostridium pasteurianum. J Bacteriol. 1994;176:6590–6598. doi: 10.1128/jb.176.21.6590-6598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisholm S W, Frankel S L, Goericke R, Olson R J, Palenick B, Waterbury J B, West-Johnsrud L, Zettler E R. Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b. Arch Microbiol. 1992;157:297–300. [Google Scholar]
- 9.Dugdale R C, Goering J J. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol Oceanogr. 1967;12:196–206. [Google Scholar]
- 10.Dugdale R C, Menzel D W, Ryther J H. Nitrogen fixation in the Sargasso Sea. Deep-Sea Res. 1961;7:298–300. [Google Scholar]
- 11.Falkowski P G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. [Google Scholar]
- 12.Felsenstein J. PHYLIP—phylogeny inference package (version 3.5c). Seattle, Wash: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 13.Giovannoni S J, DeLong E J, Schmidt T M, Pace N R. Tangential flow filtration and preliminary phylogenetic analysis of marine picoplankton. Appl Environ Microbiol. 1990;56:2572–2575. doi: 10.1128/aem.56.8.2572-2575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannoni S J, Britschgi T B, Moyer C L, Field K G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990;345:60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- 15.Hutchins D A, Wang W-X, Fisher N S. Copepod grazing and the biogeochemical fate of diatom iron. Limnol Oceanogr. 1995;40:989–994. [Google Scholar]
- 16.Johnson P W, Sieburth J M. Chroococcoid cyanobacteria in the sea: a ubiquitous and diverse phototrophic biomass. Limnol Oceanogr. 1979;24:928–935. [Google Scholar]
- 17.Karl D, Letelier R, Tupas L, Dore J, Christian J, Hebel D. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature. 1997;388:533–538. [Google Scholar]
- 18.Lipschultz F, Owens N J P. An assessment of nitrogen fixation as a source of nitrogen to the North Atlantic Ocean. Biogeochemistry. 1996;35:261–274. [Google Scholar]
- 19.Longhurst A R. Relationship between diversity and the vertical structure of the upper ocean. Deep-Sea Res. 1985;32:1535–1570. [Google Scholar]
- 20.Martin J H, Gordon M, Fitzwater S E. The case for iron. Limnol Oceanogr. 1991;36:1793–1802. [Google Scholar]
- 21.Martinez L, Silver M W. Nitrogen fixation by floating diatom mats: a source of new nitrogen to oligotrophic ocean waters. Science. 1983;221:152–154. doi: 10.1126/science.221.4606.152. [DOI] [PubMed] [Google Scholar]
- 22.Michaels A F, Olson D, Sarmiento J L, Ammerman J W, Fanning K, Jahnke R, Knap A H, Lipschultz F, Prospero J M. Inputs, losses and transformations of nitrogen and phophorus in the pelagic North Atlantic Ocean. Biogeochemistry. 1996;35:181–226. [Google Scholar]
- 23.Mullins T D, Britschgi T B, Krest R L, Giovannoni S J. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol Oceanogr. 1995;40:148–158. [Google Scholar]
- 24.Nagasawa S, Nemoto T. Presence of bacteria in guts of marine crustaceans and on their fecal pellets. J Plankton Res. 1988;10:559–564. [Google Scholar]
- 25.Ohkuma M, Noda S, Usami R, Horikoshi K, Kudo T. Diversity of nitrogen fixation genes in the symbiotic intestinal microflora of the termite Reticulitermes speratus. Appl Environ Microbiol. 1996;62:2747–2752. doi: 10.1128/aem.62.8.2747-2752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pond D W, Harris R P, Brownlee C. A microinjection technique using a pH sensitive dye to determine the gut pH of Calanus helgolandicus. Mar Biol. 1995;123:75–79. [Google Scholar]
- 27.Proctor L M. Nitrogen-fixing, photosynthetic, anaerobic bacteria associated with pelagic copepods. Aquat Microb Ecol. 1997;12:105–113. [Google Scholar]
- 28.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt T M, DeLong E F, Pace N R. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J Bacteriol. 1991;173:4371–4378. doi: 10.1128/jb.173.14.4371-4378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith S W, Overbeek R, Woese C R, Gilbert W, Gillevet P M. The Genetic Data Environment: an expandable GUI interface for multiple sequence analysis. Comput Appl Biosci. 1994;10:671–675. doi: 10.1093/bioinformatics/10.6.671. [DOI] [PubMed] [Google Scholar]
- 31.Steppe T F, Olson J B, Paerl H W, Litaker R W, Belnap J. Consortial N-2 fixation—a strategy for meeting nitrogen requirements of marine and terrestrial cyanobacterial mats. FEMS Microbiol Ecol. 1996;21:149–156. [Google Scholar]
- 32.Tibbles B J, Rawlings D E. Characterization of nitrogen-fixing bacteria from a temperate saltmarsh lagoon, including isolates that produce ethane from acetylene. Microb Ecol. 1994;27:65–80. doi: 10.1007/BF00170115. [DOI] [PubMed] [Google Scholar]
- 33.Ueda T, Suga Y, Yahiro N, Matsuguchi T. Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J Bacteriol. 1995;177:1414–1417. doi: 10.1128/jb.177.5.1414-1417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 35.Villareal T A, Carpenter E J. Nitrogen fixation, suspension characteristics and chemical composition of Rhizosolenia mats in the central North Pacific gyre. Biol Oceanogr. 1989;6:387–405. [Google Scholar]
- 36.Waterbury J B, Watson S W, Guillard R R L, Brand L E. Widespread occurrence of a unicellular, marine, planktonic, cyanobacterium. Nature. 1979;277:293–294. [Google Scholar]
- 37.Waterbury J B, Watson S W, Valois F W. Temporal separation of photosynthesis and dinitrogen fixation in the marine unicellular cyanobacterium Erythrosphaera marin. Eos. 1988;69:1089. [Google Scholar]
- 38.Young J P W. Phylogenetic classification of nitrogen-fixing organisms. In: Stacey G, Evans H J, Burris R H, editors. Biological nitrogen fixation. New York, N.Y: Chapman and Hall; 1992. pp. 43–86. [Google Scholar]
- 39.Zehr J P, Capone D G. Problems and promises of assaying the genetic potential for nitrogen fixation in the marine environment. Microb Ecol. 1996;32:263–281. doi: 10.1007/BF00183062. [DOI] [PubMed] [Google Scholar]
- 40.Zehr J P, Mellon M T, Hiorns W D. Phylogeny of cyanobacterial nifH genes: evolutionary implications and potential applications to natural assemblages. Microbiology. 1997;143:1443–1450. doi: 10.1099/00221287-143-4-1443. [DOI] [PubMed] [Google Scholar]
- 41.Zehr J P, McReynolds L A. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium spp. Appl Environ Microbiol. 1989;55:2522–2526. doi: 10.1128/aem.55.10.2522-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zehr J P, Mellon M, Braun S, Litaker W, Steppe T, Paerl H W. Diversity of heterotrophic nitrogen fixation genes in a marine cyanobacterial mat. Appl Environ Microbiol. 1995;61:2527–2532. doi: 10.1128/aem.61.7.2527-2532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]