Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic. The interplay between innate and adaptive immune responses plays a crucial role in managing COVID-19. Cell therapy has recently emerged as a promising strategy to modulate the immune system, offering immense potential for the treatment of COVID-19 due to its customizability and regenerative capabilities. This review provides an overview of the various subsets of immune cell subsets implicated in the pathogenesis of COVID-19 and a comprehensive summary of the current status of immune cell therapy in COVID-19 treatment.
Keywords: SARS-CoV-2, COVID-19, cell therapy, immunotherapy
1. Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a devastating impact worldwide for over four years. This novel infectious disease has resulted in millions of fatalities, with the death tolls continuing to rise due to the emergence of new variants [1]. COVID-19 presents with respiratory symptoms associated with viral replication, along with systemic effects, making it a complex illness [2]. Common symptoms include fever, cough, and respiratory distress, with severe cases progressing to respiratory failure and even death [3].
The pathophysiology of COVID-19 is primarily characterized by dysregulated immune responses against SARS-CoV-2, involving both innate and adaptive immune components [4]. This dysregulation not only hinders viral clearance but also leads to inflammation, tissue damage in the lungs, and multi-organ damage systemically [5]. Various immune cells, including T cells, natural killer (NK) cells, dendritic cells (DCs), and macrophages, play crucial roles in SARS-CoV-2 infections [6]. Hence, immunotherapy targeting these immune cells presents a promising avenue for mitigating the pathology of SARS-CoV-2 infection, preventing long-term complications, and improving overall survival rates in infectious diseases.
Cellular therapy, an innovative approach to disease treatment, involves the utilization of specialized cells with specific functions obtained through bioengineering and ex vivo expansion, followed by reinfusion into the patient’s body [7]. Chimeric antigen receptor (CAR) T-cell therapy, which has gained approval from the US Food and Drug Administration, has demonstrated significant advancements in the treatment of B-cell malignancies [8]. Immune-cell-based therapies offer distinct advantages over conventional treatments, including high selectivity, localized concentration, and personalization [9]. Over the past decade, cancer immunotherapy has witnessed remarkable success, establishing immune cell therapy as a transformative treatment modality for various diseases, including COVID-19.
In the context of COVID-19, immune cell therapy is a promising approach for effective treatment. This review provides an overview of the different immune cell subsets involved in the pathogenesis of COVID-19 and summarizes the progress in immune cell therapy research and clinical trials targeting COVID-19 (Table 1).
Table 1.
Immune-cell-based therapy clinical trials for COVID-19.
| Strategy | Study Title | Phase | NCT Number | Status |
|---|---|---|---|---|
| Specific T cell | Safety Infusion of Natural Killer cells or Memory T Cells as Adoptive Therapy in COVID-19 pneumonia or Lymphopenia | 1 and 2 | NCT04578210 | Completed |
| Generation of SARS-CoV-2-specific T Lymphocytes from Recovered Donors and Administration to High-risk COVID-19 Patients | 1 and 2 | NCT05447013 | Recruiting | |
| Novel Adoptive Cellular Therapy With SARS-CoV-2 Specific T Cells in Patients with Severe COVID-19 | 1 | NCT04351659 | Recruiting | |
| Part Two of Novel Adoptive Cellular Therapy With SARS-CoV-2 Specific T Cells in Patients with Severe COVID-19 | 1 and 2 | NCT04457726 | Unknown status |
|
| Viral Specific T Cell Therapy for COVID-19 Related Pneumonia | 1 | NCT04742595 | Recruiting | |
| Treg | REgulatory T Cell infuSion fOr Lung Injury Due to COVID-19 PnEumonia (RESOLVE) | 1 | NCT04468971 | Completed |
| RAPA-501-Allo Therapy of COVID-19-ARDS | 1 and 2 | NCT04482699 | Terminated | |
| NLow Dose of IL-2 In Acute Respiratory DistrEss Syndrome Related to COVID-19 (LILIADE-COVID) | 2 | NCT04357444 | Completed | |
| Tregs for the Treatment of Acute Respiratory Distress Syndrome (ARDS) Associated With COVID-19 (regARDS) | 1 | NCT05027815 | Terminated | |
| NK cells | A Phase I/II Study of Universal Off-the-shelf NKG2D-ACE2 CAR-NK Cells for Therapy of COVID-19 | 1 and 2 | NCT04324996 | Unknown status |
| Fase I Clinical Trial on NK Cells for COVID-19 | 1 | NCT04634370 | Unknown status |
|
| Natural Killer Cell (CYNK-001) Infusions in Adults With COVID-19 | 1 and 2 | NCT04365101 | Active, not recruiting | |
| Off-the-shelf NK Cells (KDS-1000) as Immunotherapy for COVID-19 | 1 and 2 | NCT04797975 | Withdrawn | |
| NK Cells Treatment for COVID-19 | 1 | NCT04280224 | Recruiting | |
| Dendritic cells | Injection and infusion of LV-SMENP DC vaccine and antigen-specific CTLs | 3 | NCT04276896 | Recruiting |
| Phase I-II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Adults | 1 and 2 | NCT04386252 | Withdrawn | |
| A Study to Evaluate the Efficacy, Immune Response, and Safety of a COVID-19 Vaccine in Adults ≥ 18 Years with a Pediatric Expansion in Adolescents (12 to <18 Years) at Risk for SARS-CoV-2 | 3 | NCT04611802 | Active, not recruiting | |
| Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals | 3 | NCT04368728 | Completed | |
| A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19 | 3 | NCT04470427 | Completed | |
| Dendritic Cell Vaccine to Prevent COVID-19 | 1 | NCT04685603 | Unknown status |
|
| Dendritic Cell Vaccine, AV-COVID-19, to Prevent COVID-19 Infection | 1 | NCT04690387 | Completed | |
| Phase I-II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Adults | 1 and 2 | NCT04386252 | Withdrawn | |
| Preventive Dendritic Cell Vaccine, AV-COVID-19, in Subjects Not Actively Infected with COVID-19 | 2 | NCT05007496 | Completed | |
| Training the Innate Immune System Against SARS-CoV-2 (COVID-19) Using the Shingrix Vaccine in Nursing Home Residents (NH-Shingrix) | 1 | NCT04523246 | Active, not recruiting | |
| Monocytes | The MONACO Cell Therapy Study: Monocytes as an Anti-fibrotic Treatment After COVID-19 (MONACO) | 1 and 2 | NCT04805086 | Unknown status |
| Mesenchymal stem cells | Mesenchymal Stem Cells Therapy in Patients With COVID-19 Pneumonia | Not applicable |
NCT04713878 | Completed |
| A Proof of Concept Study for the DNA Repair Driven by the Mesenchymal Stem Cells in Critical COVID-19 Patients (REPAIR) | Not applicable |
NCT04898088 | Completed | |
| NestaCell® Mesenchymal Stem Cell to Treat Patients with Severe COVID-19 Pneumonia (HOPE) | 2 | NCT04315987 | Completed | |
| An Exploratory Study of ADR-001 in Patients with Severe Pneumonia Caused by SARS-CoV-2 Infection (COVID-19) | 1 | NCT04522986 | Completed | |
| Therapeutic Study to Evaluate the Safety and Efficacy of DW-MSC in COVID-19 Patients (DW-MSC) | 1 | NCT04535856 | Completed | |
| Mesenchymal Stromal Cells for the Treatment of SARS-CoV-2 Induced Acute Respiratory Failure (COVID-19 Disease) | 1 and 2 | NCT04345601 | Completed | |
| Efficacy of Infusions of MSC from Wharton Jelly in the SARS-CoV-2 (COVID-19) Related Acute Respiratory Distress Syndrome (MSC-COVID19) | 2 | NCT04625738 | Completed | |
| Mesenchymal Stem Cells for the Treatment of COVID-19 | 1 | NCT04573270 | Completed | |
| A Randomized, Double-Blind, Single Center, Efficacy and Safety Study of Allogeneic HB-adMSCs Against COVID-19. | 2 | NCT04348435 | Completed | |
| A Clinical Trial to Determine the Safety and Efficacy of HB-adMSCs to Provide Protection Against COVID-19 | 2 | NCT04349631 | Completed | |
| A First-In-Human Phase 1b Study of AmnioPul-02 in COVID-19/Other LRTI | 1 | NCT05348772 | Completed | |
| Menstrual Blood Stem Cells in Severe Covid-19 | 1 and 2 | NCT05019287 | Completed | |
| Treatment With Human Umbilical Cord-derived Mesenchymal Stem Cells for Severe Corona Virus Disease 2019 (COVID-19) | 2 | NCT04288102 | Completed | |
| Use of UC-MSCs for COVID-19 Patients | 1 and 2 | NCT04355728 | Completed | |
| Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Allogeneic Adult Mesenchymal Stem Cells of Expanded Adipose Tissue in Patients with Severe Pneumonia Due to COVID-19 | 1 and 2 | NCT04366323 | Completed | |
| Treatment of COVID-19 Associated Pneumonia with Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells | 1 and 2 | NCT04382547 | Completed | |
| The MEseNchymal coviD-19 Trial: MSCs in Adults with Respiratory Failure Due to COVID-19 or Another Underlying Cause (MEND) | 1 and 2 | NCT04537351 | Completed | |
| Clinical Use of Stem Cells for the Treatment of Covid-19 | 1 and 2 | NCT04392778 | Completed | |
| Efficacy and Safety Evaluation of Mesenchymal Stem Cells for the Treatment of Patients with Respiratory Distress Due to COVID-19 (COVIDMES) | 1 and 2 | NCT04390139 | Completed | |
| Evaluate the Safety and Efficacy of Allogeneic Umbilical Cord Mesenchymal Stem Cells in Patients With COVID-19 (UMSC01) | 1 and 2 | NCT05501418 | Active, not recruiting | |
| Regenerative Medicine for COVID-19 and Flu-Elicited ARDS Using Lomecel-B (RECOVER) (RECOVER) | 1 | NCT04629105 | Active, not recruiting | |
| Use of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Caused by COVID-19 | Early phase 1 |
NCT04456361 | Active, not recruiting | |
| Multiple Dosing of Mesenchymal Stromal Cells in Patients with ARDS (COVID-19) | 2 | NCT04466098 | Active, not recruiting | |
| Umbilical Cord Lining Stem Cells (ULSC) in Patients With COVID-19 ARDS (ULSC) | 1 and 2 | NCT04494386 | Active, not recruiting | |
| Study of the Safety of Therapeutic Tx with Immunomodulatory MSC in Adults With COVID-19 Infection Requiring Mechanical Ventilation | 1 | NCT04397796 | Active, not recruiting | |
| Efficacy and Safety Study of Allogeneic HB-adMSCs for the Treatment of COVID-19 | 2 | NCT04362189 | Terminated | |
| Study of Intravenous COVI-MSC for Treatment of COVID-19-Induced Acute Respiratory Distress | 2 | NCT04903327 | Terminated | |
| hCT-MSCs for COVID19 ARDS | 1 and 2 | NCT04399889 | Terminated | |
| MSCs in COVID-19 ARDS | 3 | NCT04371393 | Terminated | |
| Mesenchymal Stem Cell Infusion for COVID-19 Infection | 2 | NCT04444271 | Unknown status |
|
| Mesenchymal Stem Cell for Acute Respiratory Distress Syndrome Due for COVID-19 (COVID-19) | 2 | NCT04416139 | Unknown status |
|
| Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells | Early phase 1 |
NCT04302519 | Unknown status |
|
| Safety and Efficacy of Mesenchymal Stem Cells in the Management of Severe COVID-19 Pneumonia (CELMA) | 2 | NCT04429763 | Unknown status |
|
| Safety and Effectiveness of Mesenchymal Stem Cells in the Treatment of Pneumonia of Coronavirus Disease 2019 | Early phase 1 |
NCT04371601 | Unknown status |
|
| Mesenchymal Stem Cells in Patients Diagnosed With COVID-19 | 1 | NCT04611256 | Unknown status |
|
| Bone Marrow-Derived Mesenchymal Stem Cell Treatment for Severe Patients with Coronavirus Disease 2019 (COVID-19) | 1 and 2 | NCT04346368 | Unknown status |
|
| Administration of Allogenic UC-MSCs as Adjuvant Therapy for Critically-Ill COVID-19 Patients | 1 | NCT04457609 | Unknown status |
|
| Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With COVID-19 | 1 | NCT04252118 | Unknown status |
|
| Clinical Research of Human Mesenchymal Stem Cells in the Treatment of COVID-19 Pneumonia | 1 and 2 | NCT04339660 | Unknown status |
|
| Mesenchymal Stem Cell Therapy for SARS-CoV-2-related Acute Respiratory Distress Syndrome | 2 and 3 | NCT04366063 | Unknown status |
|
| Safety and Efficacy Study of Allogeneic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe COVID-19 Patients | 1 and 2 | NCT04336254 | Unknown status |
|
| Treatment of COVID-19 Patients Using Wharton’s Jelly-Mesenchymal Stem Cells | 1 | NCT04313322 | Unknown status |
|
| Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Severe COVID-19 | Not applicable |
NCT04273646 | Unknown status |
|
| Treatment of Coronavirus COVID-19 Pneumonia (Pathogen SARS-CoV-2) With Cryopreserved Allogeneic P_MMSCs and UC-MMSCs | 1 and 2 | NCT04461925 | Unknown status |
|
| Treatment of Severe COVID-19 Patients Using Secretome of Hypoxia-Mesenchymal Stem Cells in Indonesia | 2 | NCT04753476 | Unknown status |
|
| A Study of Cell Therapy in COVID-19 Subjects with Acute Kidney Injury Who Are Receiving Renal Replacement Therapy | 1 and 2 | NCT04445220 | Unknown status |
|
| A Study to Collect Bone Marrow for Process Development and Production of BM-MSC to Treat Severe COVID19 Pneumonitis (COMET20d) | Observational | NCT04397471 | Unknown status |
|
| Safety and Efficacy of Intravenous Wharton’s Jelly Derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Due to COVID 19 | 1 and 2 | NCT04390152 | Unknown status |
|
| Umbilical Cord (UC)-Derived Mesenchymal Stem Cells (MSCs) Treatment for the 2019-novel Coronavirus(nCOV) Pneumonia | 2 | NCT04269525 | Unknown status |
|
| Mesenchymal Stromal Cells for the Treatment of Patients With COVID-19. | 1 and 2 | NCT05433298 | Withdrawn | |
| ASC Therapy for Patients with Severe Respiratory COVID-19 (ASC COVID-19) | 1 and 2 | NCT04341610 | Withdrawn | |
| Study of Allogeneic Adipose-Derived Mesenchymal Stem Cells to Treat Post COVID-19 “Long Haul” Pulmonary Compromise | 2 | NCT04909892 | Withdrawn | |
| Study of Intravenous Administration of Allogeneic Adipose-Derived Mesenchymal Stem Cells for COVID-19-Induced Acute Respiratory Distress | 2 | NCT04728698 | Withdrawn | |
| Study of Allogeneic Adipose-Derived Mesenchymal Stem Cells for Non-COVID-19 Acute Respiratory Distress Syndrome | 2 | NCT04909879 | Withdrawn | |
| Umbilical Cord Tissue (UC) Derived Mesenchymal Stem Cells (MSCs) Versus Placebo to Treat Acute Pulmonary Inflammation Due to COVID-19 (COVID-19) | 1 | NCT04490486 | Withdrawn | |
| BAttLe Against COVID-19 Using MesenchYmal Stromal Cells | 2 | NCT04348461 | Suspended | |
| A Study of ADR-001 in Patients with Severe Pneumonia Caused by SARS-CoV-2 Infection (COVID-19) | 2 | NCT04888949 | Recruiting | |
| A Clinical Study on Safety and Effectiveness of Mesenchymal Stem Cell Exosomes for the Treatment of COVID-19. | Early phase 1 |
NCT05787288 | Recruiting | |
| Cord Blood-Derived Mesenchymal Stem Cells for the Treatment of COVID-19 Related Acute Respiratory Distress Syndrome | 1 and 2 | NCT04565665 | Recruiting | |
| Application and Research of Mesenchymal Stem Cells in Alleviating Severe Development of COVID-19 Infection | 1 and 2 | NCT05741099 | Recruiting | |
| UC-MSCs in the Treatment of Severe and Critical COVID-19 Patients | 3 | NCT05682586 | Recruiting | |
| Allogenic UCMSCs as Adjuvant Therapy for Severe COVID-19 Patients (UCMSC) | 2 and 3 | NCT05132972 | Recruiting | |
| A Phase II Study in Patients with Moderate to Severe ARDS Due to COVID-19 | 2 | NCT04780685 | Recruiting | |
| Study of Allogeneic Adipose-Derived Mesenchymal Stem Cells for Treatment of COVID-19 Acute Respiratory Distress | 2 | NCT04905836 | Recruiting | |
| Randomized Double-Blind Phase 2 Study of Allogeneic HB-adMSCs for the Treatment of Chronic Post-COVID-19 Syndrome (HBPCOVID02) | 2 | NCT05126563 | Recruiting | |
| Study to Evaluate the Efficacy and Safety of AstroStem-V in Treatment of COVID-19 Pneumonia | 1 and 2 | NCT04527224 | Recruiting | |
| Safety and Efficacy of Umbilical Cord Mesenchymal Stem Cell Exosomes in Treating Chronic Cough After COVID-19 | Early phase 1 |
NCT05808400 | Recruiting | |
| UC-MSCs in the Treatment of Severe and Critical COVID-19 Patients with Refractory Hypoxia | 3 | NCT05689008 | Recruiting | |
| Study of Descartes-30 in Acute Respiratory Distress Syndrome | 1 and 2 | NCT04524962 | Recruiting | |
| Mesenchymal Stem Cells for the Treatment of Various Chronic and Acute Conditions | 1 and 2 | NCT04684602 | Recruiting | |
| Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST) (REALIST | 1 and 2 | NCT03042143 | Recruiting | |
| Autologous Adipose-derived Stem Cells (AdMSCs) for COVID-19 | 2 | NCT04428801 | Not yet recruiting |
|
| Mesenchymal Stromal Cells for COVID-19 and Viral Pneumonias (SAMPSON-1) | 1 | NCT05286255 | Not yet recruiting |
|
| Clinical Study for Subjects With COVID-19 Using Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells (AdMSCs) | 2 | NCT05017298 | Not yet recruiting |
|
| Treatment of Long COVID Symptoms Utilizing Autologous Stem Cells Following COVID-19 Infection | 1 | NCT05669261 | Not yet recruiting |
|
| Study of Allogeneic Adipose-Derived Mesenchymal Stem Cells to Treat Post COVID-19 “Long Haul” Pulmonary Compromise (BR) | 2 | NCT04992247 | Not yet recruiting |
|
| Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cells in the Treatment of Long COVID-19 | 2 | NCT05719012 | Not yet recruiting |
|
| Mesenchymal Stem Cells (MSCs) in Inflammation-Resolution Programs of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS) | 2 | NCT04377334 | Not yet recruiting |
|
| Use of hUC-MSC Product (BX-U001) for the Treatment of COVID-19 With ARDS | 1 and 2 | NCT04452097 | Not yet recruiting |
|
| AllogeneiC Expanded Human MSC Therapy in Patients Recovering From COVID-19 Acute Respiratory Distress Trial (ACE_CARD) | 1 | NCT05491681 | Not yet recruiting |
2. T Cell Therapy
T lymphocytes, derived from lymphoid stem cells in the bone marrow, undergo differentiation and maturation in the thymus. They then circulate through the lymph and blood, playing a critical role in cellular immunity throughout various immune organs and tissues in the body. Pathological excessive inflammation, associated with lymphocyte reduction and dysregulated T cell responses, is one of the immunological characteristics of COVID-19 [10]. The success of T cell therapy in treating life-threatening viral infections supports its potential application in COVID-19 treatment [11].
2.1. Specific T Cell Therapy in COVID-19
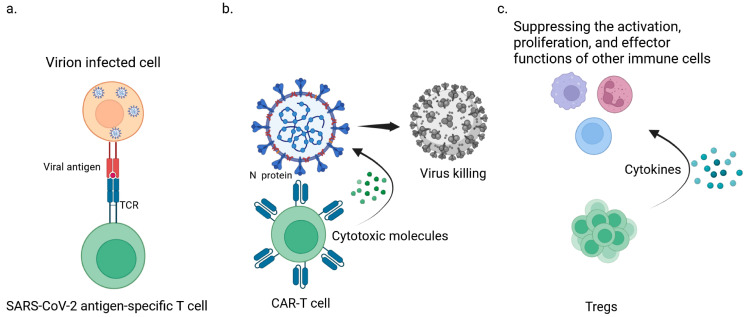
Specific T cell therapy involves isolating and expanding virus-specific T cells that can recognize the structural proteins of SARS-CoV-2, particularly the membrane protein (Figure 1a). Notably, blood from donors exposed to SARS-CoV-2 contains memory CD4 and CD8+ T cells capable of recognizing various viral antigens, including spike proteins, nucleocapsid proteins, and membrane antigens [12]. Cultivating SARS-CoV-2-specific T cells in the presence of interleukin (IL)-2/4/7 can increase their proliferation by over 1000 times while preserving their phenotype, function, and antigen recognition hierarchy compared to baseline samples [13]. This approach allows for the generation of expanded cytotoxic T lymphocytes (CTLs) targeting the structural proteins of SARS-CoV-2, including the receptor-binding domain of the spike protein [13]. Incorporating HLA-E-restricted CD8 T cells into T cell immunotherapy for COVID-19 offers several advantages, including the ability to eliminate infected cells, inhibit intracellular infection, reduce inflammatory reactions, and limit tissue damage, which are important mechanisms in the pathogenesis of COVID-19 [14].
Figure 1.
Three types of T cell therapies available for treating life-threatening viral infections. (a) SARS-CoV-2-specific T cells that specifically recognize the structural antigen membrane protein of SARS-CoV-2. (b). CAR-T cells specially designed to identify the viral antigen N protein of SARS-CoV-2. (c). Tregs play a key role in suppressing the activation, proliferation, and effector functions of other immune cells, thereby playing a protective role in inhibiting autoimmune inflammatory reactions in COVID-19 patients with excessive inflammation and cytokine storms.
In vitro experiments have demonstrated that clinically relevant levels of SARS-CoV-2-specific T cells maintain their functionality and proliferative capacity while retaining their specific cytotoxic potential. These T cells exhibit functional and phenotypic stability within days after enrichment [15]. In vitro expansion and isolation of SARS-CoV-2-specific T cells producing IFNγ have been successfully achieved, showing peptide-specific cytolytic and proliferative responses upon re-exposure to the antigen [16]. Furthermore, in vitro expanded SARS-CoV-2 antigen-specific T cells using partially HLA-matched third-party products have shown potential in the treatment of severe cases of COVID-19 unresponsive to previous interventions [17]. Currently, a phase I clinical trial is underway to assess the safety and feasibility of immunotherapy with infused CD45RA memory T cells, including SARS-CoV-2-specific T cells, for the treatment of moderate to severe COVID-19 cases [18]. Additionally, a randomized (2:1), open-label phase I/II trial is evaluating the safety and efficacy of pre-made, partially human leukocyte antigen (HLA)-matched SARS-CoV-2-specific T cells from recovered patients (CoV-2-STs) in combination with standard-of-care therapy (SoC) for the treatment of severe COVID-19 patients [19]. Numerous clinical trials investigating specific T cell therapies for COVID-19 are currently underway (Table 1).
2.2. CAR-T Cell Therapy in COVID-19
CAR-T cells, which are engineered T cells with the ability to specifically recognize and attack tumor cells, have emerged as a promising approach for combating COVID-19. These CAR-T cells are designed to target SARS-CoV-2 viral antigens, particularly the N protein(Figure 1b). Mathematical models have been developed to describe the dynamics of infection involving the virus, CAR-T cells, and memory cells. Theoretical analysis suggests that this approach can generate positive therapeutic effects by delaying viral production, which is particularly beneficial in the early stages of infection. This study highlights the potential use of CAR-T cells as an antiviral therapy for COVID-19 [20].
3. Tregs Therapy
Regulatory T cells (Tregs) are a group of immune-suppressive cells that play a crucial role in maintaining immune homeostasis and preventing autoimmune reactions [21,22]. Some studies have indicated a decrease in the number of Tregs in the peripheral blood of COVID-19 patients [23,24,25,26]. Mahmoud et al. evaluated the frequencies of Tregs and Th17 cells in healthy individuals and COVID-19 patients using flow cytometry. The results showed a significant decrease in the frequency of Tregs and an increase in the frequency of Th17 cells and the Th17/Treg ratio, particularly in severe COVID-19 patients. Moreover, the expression levels of ROR-γt and FoxP3 were found to increase and decrease, respectively, as the disease progressed [24]. Another study demonstrated that non-survivor patients had lower levels of Tregs and lower FOXP3 expression compared to survivors. However, conflicting results regarding changes in Treg levels in COVID-19 patients have also been reported. Some studies have shown a significant increase in the frequency of CD25+CD127-Foxp3+ Tregs in critically ill COVID-19 patients [27,28].
However, Tregs play a crucial role in suppressing the activation, proliferation, and effector functions of other immune cells, and they are involved in inhibiting self-immune inflammatory reactions. Tregs may exert a protective effect on COVID-19 patients with excessive inflammation and cytokine storms [29,30]. Based on the anti-inflammatory characteristics of Tregs, Treg-based immunotherapy may provide a viable option for treating COVID-19 (Figure 1c). This viewpoint is supported by a recent case study that examined the effects of Treg transplantation from umbilical cord blood. Two patients with acute respiratory distress syndrome received two to three different allogeneic umbilical-cord-blood-derived Tregs that were expanded in vitro, resulting in a significant reduction in the production of inflammatory mediators and achieving good efficacy. Further clinical trials are being conducted to explore the use of umbilical-cord-blood-derived Tregs for the treatment of COVID-19-related acute respiratory distress syndrome (ARDS) (Table 1) [31]. Additionally, Harb et al. found that increased expression of Notch4 in circulating Tregs is associated with disease severity and can predict mortality, with decreased expression observed after recovery. Increased Notch expression can restrict Treg-mediated tissue repair and promote severe lung inflammation during viral infections [32]. Inhibiting Notch expression may be a potential approach for treating COVID-19. Currently, there are four clinical trials for Treg cell therapy in COVID-19, including two completed and two terminated (Table 1).
4. NK Cell Therapy
NK cells, also known as natural killer cells, are a type of large granular lymphocyte belonging to the innate immune system. They constitute approximately 5–20% of the total lymphocyte population in the human body [33]. NK cells originate from lymphoid progenitor cells and undergo maturation at various locations, such as the bone marrow, thymus, and spleen, before entering the bloodstream [34]. In response to viral infection, NK cells can directly or indirectly eliminate virus-infected cells through the release of perforin and granzymes or by exerting antibody-dependent cellular cytotoxicity (ADCC) [35]. Cytokines like IL-2, IL-12, and IL-15 can induce NK cells to secrete pro-inflammatory cytokines, such as TNF-α and IFN-γ, activating other immune responses to control viral infections [36]. Notably, COVID-19 patients often experience a significant decrease in NK cell numbers [37].
Building upon the understanding of NK cell biology, many researchers have utilized NK cell therapy to treat viral infections caused by key human pathogens, including SADS, influenza, and the SARS-CoV-2 virus [38,39,40]. NK cell therapy has demonstrated promising results in the treatment of COVID-19 infection in animal studies. Lu et al. developed mACE2-CAR, a fusion protein combining a mutated fragment of ACE2 (mACE2) with intracellular signaling domains CD28 and CD3ζ, and incorporated it into NK cells isolated from umbilical cord blood, resulting in mACE2-CAR-sIL15 NK cells. The study revealed that these NK cells exhibited strong protective effects against SARS-CoV-2 infection in K18-hACE2 mice, a transgenic mouse model expressing human ACE2 in respiratory epithelial cells [41].
NK cell therapy for the treatment of COVID-19 has progressed to the clinical trial stage (Table 1). CAR-NK cells, derived from umbilical cord blood, have been genetically engineered to target two specific molecules, MKG2D and ACE2. By binding to these molecules, CAR-NK cells can prevent SARS-CoV-2 viral infection of ACE2-expressing host cells and enhance the cytotoxicity of CAR-NK cells, enabling rapid elimination of virus-infected cells [42]. Liu et al. constructed and prepared universal IL15 super-antagonist and GM-CSF neutralizing scFv-secretory NKG2D-ACE2 CAR-NK cells from umbilical cord blood. These CAR-NK cells target the S protein of SARS-CoV-2 and NKG2DL on the surface of infected cells while also providing a preventive effect against cytokine release syndrome (CRS) through IL15 super-antagonist and GM-CSF neutralizing scFv. This approach facilitates viral particle clearance. Furthermore, ACE2 CAR-NK cells can competitively inhibit SARS-CoV-2 infection of type II alveolar epithelial cells and other vital organs or tissues, effectively terminating the SARS-CoV-2 infection. That study is currently in phase 1/2 of clinical trials [43]. Another approach, known as CYNK-001 therapy, utilizes non-genetically-modified natural killer (NK) cells derived from cryopreserved human placental hematopoietic stem cells. CYNK-001 cells are obtained by expanding and differentiating placental hematopoietic stem/progenitor CD34+ cells through a 35-day culture process [44]. CYNK-001 cells express NKG2D and CD94, as well as NK activation receptors DNAM1, NKp30, NKp46, and NKp44. In preclinical studies, CYNK-001 has demonstrated a range of biological activities expected from NK cells, including perforin and granzyme B expression. Currently, CYNK-001 is undergoing phase 1/2 clinical trials for the treatment of COVID-19 infection [45].
5. DC Cell Therapy
Dendritic cell (DC) therapy has emerged as a promising approach in the field of immunotherapy. DCs are considered the most effective antigen-presenting cells and play crucial roles in both innate and adaptive immunity, as well as in the induction of immune tolerance [46]. When exposed to viruses, DCs undergo stimulation and differentiate into two distinct types. Uninfected DCs exert antiviral immune functions, while infected DCs participate in immune evasion processes [47]. SARS-CoV-2 has the ability to infect DCs, and studies have shown that this infection can lead to a decrease in the number and function of DCs, resulting in acute immune dysfunction [48]. This immune dysfunction contributes to the immune evasion of SARS-CoV-2.
Current research has identified various mechanisms through which SARS-CoV-2 can invade host cells, including binding with angiotensin-converting enzyme 2 (ACE2), interacting with DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), interacting with CD147, and interacting with the dipeptidyl peptidase-4 (DPP4) receptor [49,50]. DC-SIGN and CD147 are expressed on DCs, while ACE2 is expressed on pulmonary interstitial DCs. Studies suggest that SARS-CoV-2 can replicate within DCs, although DCs do not transmit the newly generated virus, indicating the ability of SARS-CoV-2 to infect DCs. Clinical data further indicate a decrease in the proportion of conventional DCs (cDCs) and plasmacytoid DCs (pDCs) during the acute and recovery phases of the disease [48], as well as a reduction in the expression of IFN-α, IFN-β [51,52], and co-stimulatory molecules. The impaired and disrupted function of DCs hampers innate and adaptive immune responses, aiding in the viral escape from the host’s immune mechanisms [53].
Currently, COVID-19 vaccine development primarily focuses on viral vectors, nucleic acids (RNA), protein subunits, and inactivated or killed viruses. However, DCs have emerged as potential candidates for vaccine development, as they can process antigens and present them to immune cells. Clinical trials are currently underway to explore the application of DCs in generating COVID-19 vaccines (Table 1). Strategies for vaccine preparation based on DCs include the adoption of chimeric antigen receptor (CAR) strategies, in vitro loading of autologous DCs with SARS-CoV-2 S protein, combining DCs with nanotechnology, and targeting vaccine antigens to DCs through surface receptors, among others [54]. Currently, there are a limited number of vaccines based on DC or DC-enhancing strategies, as well as nanoparticle (NP)-based vaccines to enhance DC function against SARS-CoV-2, with approximately five in total. The highest clinical stage reached is phase III [55]. Developing vaccines that target the role of DCs in immunity offers unique advantages due to the pivotal role of DCs in innate and adaptive immunity, as well as their susceptibility during infection. Vaccination targeting DCs has the potential to address defects in innate and adaptive immune responses, leading to improved outcomes and contributing to long-term and widespread immunity.
6. Monocyte–Macrophage Cell Therapy
The monocyte–macrophage system consists of monocytes in the bloodstream and tissue-resident macrophages [56]. In the lung tissue of mild COVID-19 patients, the number of monocyte–macrophages is low [57]. However, in severe COVID-19 patients, the infection of alveolar epithelial cells by SARS-CoV-2 induces the production of chemokine CCL2, which attracts a large number of circulating monocytes and tissue-resident alveolar macrophages to the site of infection [58]. Once in the tissue, monocytes enhance their phagocytic capacity and gradually differentiate into macrophages, which further secrete pro-inflammatory cytokines (IL-6, IL-7, TNF, etc.) and chemokines (CCL2, CCL3, CCL7, CCL10, etc.), mediating immune responses and engulfing pathogens [59]. It is noteworthy that the substantial amount of cytokines produced by these macrophages in the lung tissue of severe patients can promote pulmonary fibrosis, which is a significant cause of death in COVID-19 patients [60].
Although monocyte–macrophages have long been implicated in promoting tissue fibrosis, due to the plasticity of monocyte–macrophages’ function, recent studies have shown that they also play a pivotal role in fibrosis regression, in part through the expression of matrix-degrading metalloproteinase enzymes (MMPs) [61]. Thus, increasing anti-fibrotic monocyte/macrophage populations is important for clearing partially degraded collagen fragments of the extracellular matrix, especially fibrillar collagen. An attractive therapeutic strategy would be the use of monocytes modified in vitro as a cell therapy to induce fibrosis regression. MON002 is an autologous monocyte cell product that undergoes ex vivo culture before being intravenously injected into patients with post-COVID-19 pulmonary fibrosis [20]. The MONACO Cell Therapy Study is a prospective, non-randomized, open-label phase I/II clinical trial aimed at evaluating the safety of MON002 in five adult patients diagnosed with interstitial lung disease (pulmonary fibrosis) recovering from acute COVID-19 infection (Table 1).
7. MSC Therapy
Mesenchymal stem cells (MSCs) are a type of adult stem cell that originate from the mesoderm and have the potential for self-renewal and multidirectional differentiation. They can differentiate into various mesenchymal tissues, such as bone, cartilage, adipose tissue, and bone marrow hematopoietic tissue. During the process of COVID-19 infection, MSCs reduce cytokine storms, limit organ inflammation and fibrotic damage, and regulate innate and adaptive immune responses through the release of bioactive molecules and direct cell–cell contact. Additionally, MSCs can modulate the clearance of alveolar fluid and the permeability of pulmonary endothelial cells, thereby improving lung injury and associated inflammatory reactions. It is worth noting that most MSCs do not express the ACE2 receptor, and, after intravenous injection, MSCs are distributed predominantly in the pulmonary microvasculature, enhancing the persistence of their effects through this unique immune evasion mechanism, making them potential candidates for COVID-19 treatment [62,63]. The safety of MSC-based therapeutic approaches has been confirmed in various clinical trials conducted over the past few decades, and multiple products have been approved for different diseases [64].
Animal studies have shown that MSCs can improve lung function in acute lung injury (ALI) mouse models and reduce the secretion of inflammatory cytokines [65]. Furthermore, preliminary observational studies suggest that MSCs may provide therapeutic benefits in COVID-19-related acute respiratory distress syndrome (ARDS) [66,67]. In severe or critical cases of COVID-19, ARDS remains a highly fatal condition that can result in pulmonary edema, arterial hypoxemia, and impaired lung function [68]. Unfortunately, treatment options for ARDS are still limited. However, many ongoing clinical trials involving over 1600 subjects have found the safety and efficacy of using MSCs in the treatment of severe COVID-19 cases [69,70,71,72,73,74,75,76,77].
Leng et al. found that treatment with an intravenous infusion of 1 × 106 umbilical cord mesenchymal stem cells (UC-MSCs)/kg for 14 days reduced inflammation and promoted lung tissue regeneration [76,78]. Sadeghi et al. found that MSC therapy improved oxygenation, cleared lung infiltrates, and reduced peripheral blood levels of inflammatory cytokines, with 80% of patients recovering and leaving the ICU within a median of 6 days after receiving one to two doses of intravenous MSCs [79]. Similar findings were reported by Lanzoni et al. [70,77]. In addition, a phase 2 clinical trial with a large number of patients showed that UC-MSC therapy significantly improved lung lesion volume on day 28. Data collected every 3 months by Shi et al. showed continued improvement in lung lesions at the 3-month mark in patients treated with UC-MSCs [72,73].
Clinical trial results have revealed that the use of MSCs for treating COVID-19 patients is not an unrealistic therapeutic approach. Furthermore, the use of MSC-based therapy is considered a suitable treatment option for severely ill COVID-19 patients [77,80,81]. However, further research is needed to evaluate the safety, efficacy, and long-term outcomes of using different types of MSCs [82]. Although clinical studies related to MSCs are still in their early stages, MSCs hold great promise as clinical tools for the future.
8. Discussion
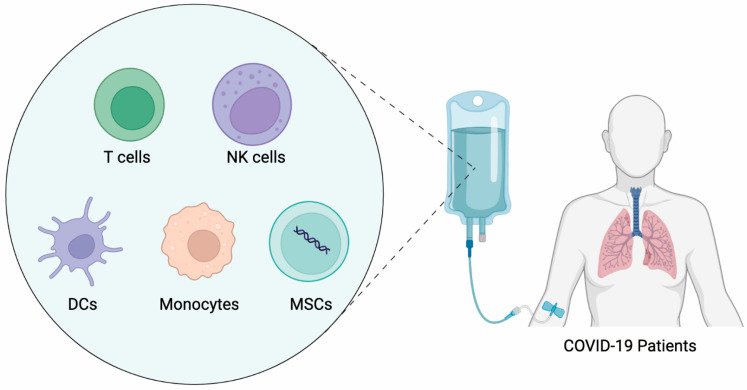
Over the past years, immune cell therapy for cancer has continued to be enthusiastic, showing the great potential of immune cells for the treatment of human diseases. COVID-19 invasion has caused the concept of immune cell therapy to become highly popular once again. Several articles have focused on this field [83,84]. In this article, we summarized the types of cell therapies currently in the clinical trial stage (Figure 2) and listed the latest representative clinical trial programs for COVID-19 immune cell therapy (Table 1).
Figure 2.
Different types of immune cell therapies for treating COVID-19.
However, COVID-19 immune cell therapy is still in the early stages of development, and the key to its development will be the availability of high-quality immune cells and a standardized cell preparation process. The first key point is the high quality of immune cells. Research has confirmed that viral infections can lead to a decrease in the number of immune cells in the body, and even lead to a decline in cellular immune function [5]. Therefore, in order to carry out immune cell therapy, first, it is necessary to guarantee a sufficient number of these immune cells, especially for those therapies aimed at treating solid tissue diseases. Both cell migration and localization to the target site are important considerations, and these may depend heavily on the initial delivery route. Currently, other alternatives to intravenous administration are being investigated, including intradermal, intra-lymph node, subcutaneous, and others. These may provide solutions to this long-standing biological challenge. Secondly, it is important to ensure that these immune cells are of high quality. The efficacy of cell therapy depends heavily on the ability of the cells to maintain their functionality in vivo. Processes of in vitro cell pre-treatment, such as freezing and thawing, may have an impact on the function of the cells [85]. And, changes in the function of the living cells, such as depletion and lack of persistence, can occur after administration of the drug. The solutions to these challenges are often complex. It can be achieved through genetic modification methods, but also through pretreatment with cytokines, small molecules, hypoxia, and/or biomaterials in order to adjust their function to the desired application. The second key point is to have a standardized immune cell preparation process or production process. The development of closed automated production processes is a necessary and challenging task to produce safe, high-quality cell therapy products. Current areas of focus include manufacturing processes for cryopreservation, cell selection and cell activation, as well as automated batch monitoring for quality assurance purposes and the adoption of electronic recording systems. These interventions have the potential to reduce the heterogeneity of cell therapies and shorten production times. In the future, decentralized manufacturing processes will scale up the global availability of cell therapies, making them accessible to patients who are currently hard to reach.
Another avenue to consider for future immune cell therapy in COVID-19 is the use of a combination of immune cell therapies. For example, the synergistic effects of MSCs with other cell types, including lung endothelial cells and epithelial cells, have been studied [86]. These findings support the investigation of these two cell types as combination cell therapies.
Immune cell therapy is considered a highly active area of research because of the current positive clinical advances, and it is expected that more disruptive cell therapy products for the treatment of viral infections, such as COVID-19, will emerge in the near future.
Author Contributions
Conceptualization, Y.W. and X.L.; data curation, F.C.; writing—original draft preparation, J.Z., Y.C., Z.C. and R.L.; writing—review and editing, Y.W., Q.L. and X.L.; visualization, Q.L.; supervision, X.L.; project administration, X.L.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82173825, 81973335).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Carabelli A.M., Peacock T.P., Thorne L.G., Harvey W.T., Hughes J., Peacock S.J., Barclay W.S., de Silva T.I., Towers G.J., Robertson D.L., et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023;21:162–177. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markov P.V., Ghafari M., Beer M., Lythgoe K., Simmonds P., Stilianakis N.I., Katzourakis A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023;21:361–379. doi: 10.1038/s41579-023-00878-2. [DOI] [PubMed] [Google Scholar]
- 3.Singh S.J., Baldwin M.M., Daynes E., Evans R.A., Greening N.J., Jenkins R.G., Lone N.I., McAuley H., Mehta P., Newman J., et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir. Med. 2023;11:709–725. doi: 10.1016/S2213-2600(23)00159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koutsakos M., Ellebedy A.H. Immunological imprinting: Understanding COVID-19. Immunity. 2023;56:909–913. doi: 10.1016/j.immuni.2023.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altmann D.M., Whettlock E.M., Liu S., Arachchillage D.J., Boyton R.J. The immunology of long COVID. Nat. Rev. Immunol. 2023 doi: 10.1038/s41577-023-00904-7. ahead of print . [DOI] [PubMed] [Google Scholar]
- 6.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 7.Finck A.V., Blanchard T., Roselle C.P., Golinelli G., June C.H. Engineered cellular immunotherapies in cancer and beyond. Nat. Med. 2022;28:678–689. doi: 10.1038/s41591-022-01765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labanieh L., Mackall C.L. CAR immune cells: Design principles, resistance and the next generation. Nature. 2023;614:635–648. doi: 10.1038/s41586-023-05707-3. [DOI] [PubMed] [Google Scholar]
- 9.Ringquist R., Ghoshal D., Jain R., Roy K. Understanding and improving cellular immunotherapies against cancer: From cell-manufacturing to tumor-immune models. Adv. Drug Deliv. Rev. 2021;179:114003. doi: 10.1016/j.addr.2021.114003. [DOI] [PubMed] [Google Scholar]
- 10.Li C.K., Wu H., Yan H., Ma S., Wang L., Zhang M., Tang X., Temperton N.J., Weiss R.A., Brenchley J.M., et al. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller M.D., Harris K.M., Jensen-Wachspress M.A., Kankate V.V., Lang H., Lazarski C.A., Durkee-Shock J., Lee P.H., Chaudhry K., Webber K., et al. SARS-CoV-2-specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood. 2020;136:2905–2917. doi: 10.1182/blood.2020008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper R.S., Fraser A.R., Smith L., Burgoyne P., Imlach S.N., Jarvis L.M., Turner D.M., Zahra S., Turner M.L., Campbell J.D.M. Rapid GMP-Compliant Expansion of SARS-CoV-2-Specific T Cells from Convalescent Donors for Use as an Allogeneic Cell Therapy for COVID-19. Front. Immunol. 2021;11:598402. doi: 10.3389/fimmu.2020.598402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basar R., Uprety N., Ensley E., Daher M., Klein K., Martinez F., Aung F., Shanley M., Hu B., Gokdemir E., et al. Generation of glucocorticoid-resistant SARS-CoV-2 T cells for adoptive cell therapy. Cell Rep. 2021;20:109432. doi: 10.1016/j.celrep.2021.109432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caccamo N., Sullivan L.C., Brooks A.G., Dieli F. Harnessing HLA-E-restricted CD8 T lymphocytes for adoptive cell therapy of patients with severe COVID-19. Br. J. Haematol. 2020;190:e185–e187. doi: 10.1111/bjh.16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonifacius A., Tischer-Zimmermann S., Santamorena M.M., Mausberg P., Schenk J., Koch S., Barnstorf-Brandes J., Gödecke N., Martens J., Goudeva L., et al. Rapid Manufacturing of Highly Cytotoxic Clinical-Grade SARS-CoV-2-specific T Cell Products Covering SARS-CoV-2 and Its Variants for Adoptive T Cell Therapy. Front. Bioeng. Biotechnol. 2022;10:867042. doi: 10.3389/fbioe.2022.867042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gil-Bescós R., Ostiz A., Zalba S., Tamayo I., Bandrés E., Rojas-de-Miguel E., Redondo M., Zabalza A., Ramírez N. Potency assessment of IFNγ-producing SARS-CoV-2-specific T cells from COVID-19 convalescent subjects. Life Sci. Alliance. 2023;6:e202201759. doi: 10.26508/lsa.202201759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim N., Lee J.M., Oh E.J., Jekarl D.W., Lee D.G., Im K.I., Cho S.G. Off-the-Shelf Partial HLA Matching SARS-CoV-2 Antigen Specific T Cell Therapy: A New Possibility for COVID-19 Treatment. Front. Immunol. 2021;12:751869. doi: 10.3389/fimmu.2021.751869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Martínez A., Mora-Rillo M., Ferreras C., Guerra-García P., Pascual-Miguel B., Mestre-Durán C., Borobia A.M., Carcas A.J., Queiruga-Parada J., García I., et al. Phase I dose-escalation single centre clinical trial to evaluate the safety of infusion of memory T cells as adoptive therapy in COVID-19 (RELEASE) EClinicalMedicine. 2021;39:101086. doi: 10.1016/j.eclinm.2021.101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulou A., Karavalakis G., Papadopoulou E., Xochelli A., Bousiou Z., Vogiatzoglou A., Papayanni P.G., Georgakopoulou A., Giannaki M., Stavridou F., et al. SARS-CoV-2-specific T cell therapy for severe COVID-19: A randomized phase 1/2 trial. Nat. Med. 2023 doi: 10.1038/s41591-023-02480-8. ahead of print . [DOI] [PubMed] [Google Scholar]
- 20.Zavvar M., Yahyapoor A., Baghdadi H., Zargaran S., Assadiasl S., Abdolmohammadi K., Hossein Abooei A., Reza Sattarian M., JalaliFarahani M., Zarei N., et al. COVID-19 immunotherapy: Treatment based on the immune cell-mediated approaches. Int. Immunopharmacol. 2022;107:108655. doi: 10.1016/j.intimp.2022.108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meckiff B.J., Ramírez-Suástegui C., Fajardo V., Chee S.J., Kusnadi A., Simon H., Eschweiler S., Grifoni A., Pelosi E., Weiskopf D., et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell. 2020;183:1340–1353. doi: 10.1016/j.cell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K., Yang T., Peng X.F., Lv S.M., Ye X.L., Zhao T.S., Li J.C., Shao Z.J., Lu Q.B., Li J.Y., et al. A systematic meta-analysis of immune signatures in patients with COVID-19. Rev. Med. Virol. 2021;31:e2195. doi: 10.1002/rmv.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonçalves-Pereira M.H., Santiago L., Ravetti C.G., Vassallo P.F., de Andrade M.V.M., Vieira M.S., de Oliveira F.d.F.S., Carobin N.V., Li G., de Paula Sabino A., et al. Dysfunctional phenotype of systemic and pulmonary regulatory T cells associate with lethal COVID-19 cases. Immunology. 2023;168:684–696. doi: 10.1111/imm.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoud Salehi Khesht A., Karpisheh V., Qubais Saeed B., Olegovna Zekiy A., Yapanto L.M., Nabi Afjadi M., Aksoun M., Nasr Esfahani M., Aghakhani F., Movahed M., et al. Different T cell related immunological profiles in COVID-19 patients compared to healthy controls. Int. Immunopharmacol. 2021;97:107828. doi: 10.1016/j.intimp.2021.107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasrollahi H., Talepoor A.G., Saleh Z., Eshkevar Vakili M., Heydarinezhad P., Karami N., Noroozi M., Meri S., Kalantar K. Immune responses in mildly versus critically ill COVID-19 patients. Front. Immunol. 2023;14:1077236. doi: 10.3389/fimmu.2023.1077236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seepathomnarong P., Ongarj J., Sophonmanee R., Seeyankem B., Chusri S., Surasombatpattana S., Pinpathomrat N. Regulatory T Cells Decreased during Recovery from Mild COVID-19. Viruses. 2022;14:1688. doi: 10.3390/v14081688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vick S.C., Frutoso M., Mair F., Konecny A.J., Greene E., Wolf C.R., Logue J.K., Franko N.M., Boonyaratanakornkit J., Gottardo R., et al. A regulatory T cell signature distinguishes the immune landscape of COVID-19 patients from those with other respiratory infections. Sci. Adv. 2021;7:eabj0274. doi: 10.1126/sciadv.abj0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galván-Peña S., Leon J., Chowdhary K., Michelson D.A., Vijaykumar B., Yang L., Magnuson A.M., Chen F., Manickas-Hill Z., Piechocka-Trocha A., et al. Profound Treg perturbations correlate with COVID-19 severity. Proc. Natl. Acad. Sci. USA. 2021;118:e2111315118. doi: 10.1073/pnas.2111315118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z., Jiang X., Dai X., Li B. The Dynamic Role of FOXP3(+) Tregs and Their Potential Therapeutic Applications During SARS-CoV-2 Infection. Front. Immunol. 2022;13:916411. doi: 10.3389/fimmu.2022.916411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Candia P., Prattichizzo F., Garavelli S., Matarese G. T Cells: Warriors of SARS-CoV-2 Infection. Trends Immunol. 2021;42:18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gladstone D.E., Kim B.S., Mooney K., Karaba A.H., D’Alessio F.R. Regulatory T Cells for treating patients with COVID-19 and acute respiratory distress syndrome: Two case reports. Ann. Intern. Med. 2020;173:852–853. doi: 10.7326/L20-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harb H., Benamar M., Lai P.S., Contini P., Griffith J.W., Crestani E., Schmitz-Abe K., Chen Q., Fong J., Marri L., et al. Notch4 signaling limits regulatory T-cell-mediated tissue repair and promotes severe lung inflammation in viral infections. Immunity. 2021;54:1186–1199. doi: 10.1016/j.immuni.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langers I., Renoux V.M., Thiry M., Delvenne P., Jacobs N. Natural killer cells: Role in local tumor growth and metastasis. Biologics. 2012;6:73–82. doi: 10.2147/BTT.S23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iannello A., Debbeche O., Samarani S., Ahmad A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J. Leukoc. Biol. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- 35.Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 36.Guidotti L.G., Chisari F.V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 37.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manickam C., Shah S.V., Nohara J., Ferrari G., Reeves R.K. Monkeying Around: Using Non-human Primate Models to Study NK Cell Biology in HIV Infections. Front. Immunol. 2019;10:1124. doi: 10.3389/fimmu.2019.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jegaskanda S., Vanderven H.A., Tan H.X., Alcantara S., Wragg K.M., Parsons M.S., Chung A.W., Juno J.A., Kent S.J. Influenza Virus Infection Enhances Antibody-Mediated NK Cell Functions via Type I Interferon-Dependent Pathways. J. Virol. 2019;93:e02090-18. doi: 10.1128/JVI.02090-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghasemzadeh M., Ghasemzadeh A., Hosseini E. Exhausted NK cells and cytokine storms in COVID-19: Whether NK cell therapy could be a therapeutic choice. Hum. Immunol. 2022;83:86–98. doi: 10.1016/j.humimm.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu T., Ma R., Dong W., Teng K.Y., Kollath D.S., Li Z., Yi J., Bustillos C., Ma S., Tian L., et al. Off-the-shelf CAR natural killer cells secreting IL-15 target spike in treating COVID-19. Nat. Commun. 2022;13:2576. doi: 10.1038/s41467-022-30216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gang M., Marin N.D., Wong P., Neal C.C., Marsala L., Foster M., Schappe T., Meng W., Tran J., Schaettler M., et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood. 2020;136:2308–2318. doi: 10.1182/blood.2020006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonam S.R., Kaveri S.V., Sakuntabhai A., Gilardin L., Bayry J. Adjunct immunotherapies for the management of severely ill COVID-19 patients. Cell Rep. Med. 2020;1:100016. doi: 10.1016/j.xcrm.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spanholtz J., Tordoir M., Eissens D., Preijers F., van der Meer A., Joosten I., Schaap N., de Witte T.M., Dolstra H. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS ONE. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gleason J., Zhao Y., Raitman I., Kang L., He S., Hariri R. Human placental hematopoietic stem cell derived natural killer cells (CYNK-001) mediate protection against influenza a viral infection. Hum. Vaccines Immunother. 2022;18:2055945. doi: 10.1080/21645515.2022.2055945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Z., Li Q., Zhang R., Xie L., Shu Y., Gao S., Wang P., Su X., Qin Y., Wang Y., et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal. Transduct. Target Ther. 2021;6:26. doi: 10.1038/s41392-020-00448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X., Guan F., Miller H., Byazrova M.G., Cndotti F., Benlagha K., Camara N.O.S., Lei J., Filatov A., Liu C. The role of dendritic cells in COVID-19 infection. Emerg. Microbes Infect. 2023;12:2195019. doi: 10.1080/22221751.2023.2195019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winheim E., Rinke L., Lutz K., Reischer A., Leutbecher A., Wolfram L., Rausch L., Kranich J., Wratil P.R., Huber J.E., et al. Impaired function and delayed regeneration of dendritic cells in COVID-19. PLoS Pathog. 2021;17:e1009742. doi: 10.1371/journal.ppat.1009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soilleux E.J., Morris L.S., Leslie G., Chehimi J., Luo Q., Levroney E., Trowsdale J., Montaner L.J., Doms R.W., Weissman D., et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 2002;71:445–457. doi: 10.1189/jlb.71.3.445. [DOI] [PubMed] [Google Scholar]
- 50.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-Gómez A., Vitallé J., Gasca-Capote C., Gutierrez-Valencia A., Trujillo-Rodriguez M., Serna-Gallego A., Muñoz-Muela E., Jiménez-Leon M.L.R., Rafii-El-Idrissi Benhnia M., Rivas-Jeremias I., et al. Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection. Cell Mol. Immunol. 2021;18:2128–2139. doi: 10.1038/s41423-021-00728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park A., Iwasaki A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou R., To K.K., Wong Y.C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.Y., Lau T.T., et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity. 2020;53:864–877. doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young Chung J., Thone M.N., Davies J.E., Gach J.S., Huw Davies D., Forthal D.N., Kwon Y.J. Vaccination against SARS-CoV-2 using extracellular blebs derived from spike protein-expressing dendritic cells. Cell Immunol. 2023;386:104691. doi: 10.1016/j.cellimm.2023.104691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galati D., Zanotta S., Capitelli L., Bocchino M. A bird’s eye view on the role of dendritic cells in SARS-CoV-2 infection: Perspectives for immune-based vaccines. Allergy. 2022;77:100–110. doi: 10.1111/all.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maison D.P., Deng Y., Gerschenson M. SARS-CoV-2 and the host-immune response. Front. Immunol. 2023;14:1195871. doi: 10.3389/fimmu.2023.1195871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merad M., Martin J. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savan R., Gale M., Jr. Innate immunity and interferon in SARS-CoV-2 infection outcome. Immunity. 2023;56:1443–1450. doi: 10.1016/j.immuni.2023.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J.S., Koh J.Y., Yi K., Kim Y.I., Park S.J., Kim E.H., Kim S.M., Park S.H., Ju Y.S., Choi Y.K., et al. Single-cell transcriptome of bronchoalveolar lavage fluid reveals sequential change of macrophages during SARS-CoV-2 infection in ferrets. Nat. Commun. 2021;12:4567. doi: 10.1038/s41467-021-24807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henderson N.C., Rieder F., Wynn T.A. Fibrosis: From mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghahremani Piraghaj M., Soudi S., Ghanbarian H., Bolandi Z., Namaki S., Hashemi S.M. Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function. Life Sci. 2018;212:203–212. doi: 10.1016/j.lfs.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 63.Juárez-Navarro K.J., Padilla-Camberos E., Díaz N.F., Miranda-Altamirano A., Díaz-Martínez N.E. Human mesenchymal stem cells: The present alternative for high-incidence diseases, even SARS-Cov-2. Stem Cells Int. 2020;2020:8892189. doi: 10.1155/2020/8892189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krasnodembskaya A., Samarani G., Song Y., Zhuo H., Su X., Lee J.W., Gupta N., Petrini M., Matthay M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:1003–1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji F., Li L., Li Z., Jin Y., Liu W. Mesenchymal stem cells as a potential treatment for critically ill patients with coronavirus disease. Stem Cells Transl. Med. 2020;9:813–814. doi: 10.1002/sctm.20-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atluri S., Manchikanti L., Hirsch J.A. Expanded Umbilical Cord Mesenchymal Stem Cells (UCMSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020;23:e71–e83. [PubMed] [Google Scholar]
- 67.Shetty A.K. Mesenchymal Stem Cell Infusion Shows Promise for Combating Coronavirus (COVID-19)-Induced Pneumonia. Aging Dis. 2020;11:462–464. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: The Berlin defnition. JAMA. 2012;301:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 69.Sadeghi B., Roshandel E., Pirsalehi A., Kazemi S., Sankanian G., Majidi M., Salimi M., Aghdami N., Sadrosadat H., Samadi Kochaksaraei S., et al. Conquering the cytokine storm in COVID-19–induced ARDS using placenta-derived decidua stromal cells. J. Cell Mol. Med. 2021;25:10554–10564. doi: 10.1111/jcmm.16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adas G., Cukurova Z., Yasar K.K., Yilmaz R., Isiksacan N., Kasapoglu P., Yesilbag Z., Koyuncu I.D., Karaoz E. The Systematic Effect of Mesenchymal Stem Cell Therapy in Critical COVID-19 Patients: A Prospective Double Controlled Trial. Cell Transplant. 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dilogo I.H., Aditianingsih D., Sugiarto A., Burhan E., Damayanti T., Sitompul P.A., Mariana N., Antarianto R.D., Liem I.K., Kispa T., et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl. Med. 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lanzoni G., Linetsky E., Correa D., Messinger Cayetano S., Alvarez R.A., Kouroupis D., Alvarez Gil A., Poggioli R., Ruiz P., Marttos A.C., et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T., Shi L., Fu J., Jiang T., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct. Target Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S., Zhang C., Yuan X., Xu Z., et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi L., Yuan X., Yao W., Wang S., Zhang C., Zhang B., Song J., Huang L., Xu Z., Fu J.L., et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75:103789. doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shu L., Niu C., Li R., Huang T., Wang Y., Huang M., Ji N., Zheng Y., Chen X., Shi L., et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020;11:361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu X., Jiang W., Chen L., Xu Z., Zhang Q., Zhu M., Ye P., Li H., Yu L., Zhou X., et al. Evaluation of the safety and effcacy of using human menstrual bloodderived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin. Transl. Med. 2021;11:e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muthu S., Jeyaraman M., Kotner M.B., Jeyaraman N., Rajendran R.L., Sharma S., Khanna M., Rajendran S.N.S., Oh J.M., Gangadaran P., et al. Evolution of Mesenchymal Stem Cell Therapy as an Advanced Therapeutic Medicinal Product (ATMP)—An Indian Perspective. Bioengineering. 2022;9:111. doi: 10.3390/bioengineering9030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira Chilima T.D., Moncaubeig F., Farid F.F. Impact of allogeneic stem cell manufacturing decisions on cost of goods, process robustness and reimbursement. Biochem. Eng. J. 2018;137:132–151. [Google Scholar]
- 80.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., et al. Transplantation of ACE2-mesenchymal stem cells improve the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barkama R., Mayo A., Paz A., Solopov A., Mann T., Vadasz Z., Appel T., Ofir R., Shani L., Sheleg M., et al. Placenta-Derived Cell Therapy to Treat Patients with Respiratory Failure Due to Coronavirus Disease 2019. Crit. Care Explor. 2020;2:e0207. doi: 10.1097/CCE.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yudintceva N., Mikhailova N., Fedorov V., Samochernych K., Vinogradova T., Muraviov A., Shevtsov M. Mesenchymal Stem Cells and MSCs-Derived Extracellular Vesicles in Infectious Diseases: From Basic Research to Clinical Practice. Bioengineering. 2022;9:662. doi: 10.3390/bioengineering9110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Couto P.S., Al-Arawe N., Filgueiras I.S., Fonseca D.L.M., Hinterseher I., Catar R.A., Chinnadurai R., Bersenev A., Cabral-Marques O., Moll G., et al. Systematic review and meta-analysis of cell therapy for COVID-19: Global clinical trial landscape, published safety/efficacy outcomes, cell product manufacturing and clinical delivery. Front Immunol. 2023;14:1200180. doi: 10.3389/fimmu.2023.1200180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moll G., Drzeniek N., Kamhieh-Milz J., Geissler S., Volk H.D., Reinke P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front Immunol. 2020;11:1091. doi: 10.3389/fimmu.2020.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cottle C., Porter A.P., Lipat A., Turner-Lyles C., Nguyen J., Moll G., Chinnadurai R. Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics. Curr. Stem Cell Rep. 2022;8:72–92. doi: 10.1007/s40778-022-00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tripathi H., Domingues A., Donahue R., Cras A., Guerin C.L., Gao E., Levitan B., Ratajczak M.Z., Smadja D.M., Abdel-Latif A., et al. Combined Transplantation of Human MSCs and ECFCs Improves Cardiac Function and Decrease Cardiomyocyte Apoptosis After Acute Myocardial Infarction. Stem Cell Rev. Rep. 2023;19:573–577. doi: 10.1007/s12015-022-10468-z. [DOI] [PubMed] [Google Scholar]