Abstract
The effect of nitrogen source on methane-oxidizing bacteria with respect to cellular growth and trichloroethylene (TCE) degradation ability were examined. One mixed chemostat culture and two pure type II methane-oxidizing strains, Methylosinus trichosporium OB3b and strain CAC-2, which was isolated from the chemostat culture, were used in this study. All cultures were able to grow with each of three different nitrogen sources: ammonia, nitrate, and molecular nitrogen. Both M. trichosporium OB3b and strain CAC-2 showed slightly lower net cellular growth rates and cell yields but exhibited higher methane uptake rates, levels of poly-β-hydroxybutyrate (PHB) production, and naphthalene oxidation rates when grown under nitrogen-fixing conditions. The TCE-degrading ability of each culture was measured in terms of initial TCE oxidation rates and TCE transformation capacities (mass of TCE degraded/biomass inactivated), measured both with and without external energy sources. Higher initial TCE oxidation rates and TCE transformation capacities were observed in nitrogen-fixing mixed, M. trichosporium OB3b, and CAC-2 cultures than in nitrate- or ammonia-supplied cells. TCE transformation capacities were found to correlate with cellular PHB content in all three cultures. The results of this study suggest that the nitrogen-fixing capabilities of methane-oxidizing bacteria can be used to select for high-activity TCE degraders for the enhancement of bioremediation in fixed-nitrogen-limited environments.
Optimal bioremediation conditions within contaminated aquifers are often found to be limited by the availability of nutrients, including nitrogen. Consequently, microorganisms that are capable of degrading contaminants as well as fixing molecular nitrogen as their sole nitrogen source could have a growth advantage in fixed-nitrogen-deficient environments that would be favorable for promoting in situ bioremediation.
Trichloroethylene (TCE) is a major groundwater contaminant of concern in the United States due to its suspected carcinogenity and persistence in subsurface environments (31). However, a number of laboratory (1, 4, 13, 16, 18, 19, 22, 23, 26–28, 34) and field studies (3, 15, 24, 25) have shown that TCE can be cometabolically transformed into nontoxic end products (CO2 and Cl−) by methane-oxidizing bacteria at the expense of reducing energy in the form of NADH. Many studies have also reported that some methane-oxidizing cultures (type II) are able to utilize different sources of nitrogen (N) for cellular growth (32, 33), including molecular nitrogen at reduced oxygen partial pressures (11, 12, 20, 33). The types of methanotrophs that are capable of nitrogen fixation also produce a type of oxygenase (i.e., soluble methane monooxygenase [sMMO]) which exhibits high activity with respect to the oxidation of TCE.
Poly-β-hydroxybutyrate (PHB) is an internal reducing-energy storage polymer that can be used as an alternative reducing-energy source by a number of methane-oxidizing cultures under starvation conditions (9). Recently, a number of studies observed a correlation between TCE transformation capacities (Tc; mass of TCE transformed per mass of cells inactivated) and microbial PHB content (7, 16, 17), suggesting that PHB might be used as an alternative NADH source for TCE oxidation by methane-oxidizing bacteria in the absence of growth substrate. It has also been shown that the synthesis of PHB is stimulated in cells grown under nutrient-limited conditions, including nitrogen-fixing conditions (2, 9, 10, 21). As a result of the characteristics of methane-oxidizing microorganisms described above, it may be possible to select for nitrogen-fixing methane oxidizers in fixed-nitrogen-limited subsurface environments such that the burden of nutrient addition to the subsurface for the sustained growth of these contaminant degraders is diminished while contaminant degradation is enhanced during in situ bioremediation.
A recent study conducted by us (7) explored the feasibility of using the nitrogen-fixing capabilities of methane oxidizers for the enhancement of bioremediation. Our results suggested that nitrogen-fixing mixed cultures were able to degrade TCE as effectively as nitrate-supplied cultures. Further, higher Tc and higher cellular PHB contents were observed in nitrogen-fixing cultures. Of particular interest were observations of lower TCE product toxicity, measured in terms of methane uptake rates following TCE exposure, for nitrogen-fixing cultures than for nitrate- or ammonia-supplied cultures. Since that study was conducted with mixed cultures, it was difficult to elucidate the reasons for the enhanced degradation performance of the nitrogen-fixing methane oxidizers. An understanding of the effects of nitrogen source on cell growth and TCE degradation ability will be particularly beneficial for designing, operating, and implementing in situ- or ex situ-engineered bioremediation systems. This study evaluates nitrogen source effects on methane-oxidizing bacteria, using two pure strains and one mixed chemostat culture. Nitrogen source effects are examined with regard to cellular growth, specific methane uptake rates, specific naphthalene oxidation rates, and TCE degradation ability.
MATERIALS AND METHODS
Chemicals.
Stock solutions of TCE-saturated water were prepared in 26-ml vials by adding 5 ml of pure TCE (99+% pure American Chemical Society [ACS] reagent; Aldrich Chemical Co., Milwaukee, Wis.) and filling with distilled water. The vials were capped with Mininert valves (Alltech Co., Deerfield, Ill.), shaken vigorously for 1 min, and then allowed to separate into two distinct liquid phases (water and TCE) for at least 24 h prior to use. The solubility of TCE in the aqueous solutions averaged 1,390 mg/liter. Stock solutions of naphthalene (243 μM at 25°C) were prepared similarly to TCE stock solutions. Excess naphthalene crystals (99+% pure, scintillation grade; Aldrich Chemical Co.) were added into 100-ml bottles filled with distilled water. The bottles were sealed with Teflon-lined screw caps. The stock solutions were stirred by magnetic stirring bars for 2 h and then allowed to settle quiescently overnight prior to use. Tetrazotized o-dianisidine was purchased from Fluka Chemical Corp. (Ronkonkoma, N.Y.), and acetylene (97% pure) and ethylene (99% pure, Scotty-certified gas) were purchased from Alltech Co.
Microorganisms and culture conditions.
Methylosinus trichosporium OB3b (referred to below as OB3b) was purchased from the American Type Culture Collection (ATCC 35070), and strain CAC-2 was isolated from a mixed chemostat culture grown at 20°C in nitrate mineral salts (NMS) medium (7).
Experiments to compare the growth of methane oxidizers on three different N sources were conducted in 500-ml side-armed Nephelo culture flasks (Bellco Glass Inc., Vineland, N.J.). The three modified mineral media used in this study have been described previously (7): NMS medium, ammonia mineral salts (AMS) medium, and nitrogen-free mineral salts (NFMS) medium. The media were identical except for 11.76 mM NaNO3 added to NMS medium and 5.88 mM (NH4)2SO4 added to AMS medium. Growth flasks were amended with 50 ml of one modified mineral medium and 1 ml of culture inoculum, along with 10% CH4 and 20% O2 (initial concentrations). For those flasks receiving NFMS medium, the headspace was purged with filtered nitrogen gas before introduction of 10% CH4 and 5% O2. All inoculated flasks were then incubated at 20°C with shaking at 150 rpm. Headspace oxygen was maintained above 10% for nitrate- and ammonia-supplied cells and above 2% for nitrogen-fixing cells by repeated addition of oxygen during cell growth. Cell-free flasks were used as negative controls, and gas losses due to leakage were less than 5%.
Cell growth was monitored by measuring gaseous composition in the headspace along with optical densities of cell suspensions. Optical densities of cell suspensions were determined at A600 with a spectrophotometer (Spectronic 20D+; Milton Roy Company) and were correlated with dry cell mass (in terms of volatile suspended solids [VSS]), which was measured from the difference in cell weights after drying at 105°C overnight and after combustion at 550°C for 2 h. Cell suspensions were harvested at an optical density of approximately 0.7 for all experimental uses, while a subsample was removed, acidified with concentrated sulfuric acid, and stored in a 4°C refrigerator for later cellular PHB measurements.
Methane uptake rate analysis.
Methane uptake rates were measured when cells were in the exponential-growth phase. Fresh cell suspensions were withdrawn from flasks, diluted with appropriate medium to an A600 of 0.2, and then purged with N2 for 2 min to remove any remaining volatile organics in the liquid phase prior to use. The purged fresh cell suspensions (15 ml) were then transferred into 26-ml vials and amended with a small amount of pure methane (0.03 ml). The vials were vigorously shaken by hand for 30 s before the first headspace sample was taken and then were incubated on a rotary shaker at 200 rpm. The disappearance of methane in the vial was monitored over time by headspace gas analysis. The specific methane uptake rates of cells (in micromoles of CH4 per milligram of VSS per minute) were determined by dividing the initial uptake slope from the linear regression of the first three to four data points (taken over 1 h) by the total dry cell mass (VSS).
Naphthalene oxidation assay.
The activity of sMMO enzyme was evaluated by naphthalene assays modified from the method described by Brusseau et al. (5). The assay is based on the assumption that only sMMO can oxidize naphthalene to 1- or 2-naphthol in methane-oxidizing bacteria. The production of 1- or 2-naphthol is measured by reaction with tetrazotized o-dianisidine to form a purple naphthol diazo complex. The intensity of the naphthol diazo complex is measured as A530. The assay was conducted by adding 1 ml of cell suspensions (with an A600 of 0.2) along with 1 ml of naphthalene stock solution (234 μM at 25°C) and 20 mM formate into a 3-ml vial sealed with 13-mm Teflon-lined rubber septa (Alltech Co.). Formate (added as sodium formate) was added as a readily available reducing substrate to maximize the naphthalene oxidation rates. The mixture was incubated at 35°C on a shaker at 150 rpm for 60 min before addition of 100 μl of freshly made 0.2% (wt/vol) tetrazotized o-dianisidine. The A530 of naphthol diazo dye was measured by a Perkin-Elmer Coleman 55 spectrophotometer within 2 min, since the absorbance starts to increase after 2 min. The concentrations of naphthol in aqueous solution are known to be proportional to the intensity of the naphthol diazo dye and were calculated by using the extinction coefficient of 38,000 M cm−1 (29). All samples were measured in duplicate. Reaction mixtures containing only cells and formate (no naphthalene) were used as blank controls.
Nitrogenase assay.
The nitrogen-fixing abilities of methane-oxidizing bacteria are indicated by the activity of nitrogenase, which converts acetylene into ethylene in the presence of formate (7, 8). The assay was conducted by adding 2 ml of acetylene along with 20 mM formate into 26-ml vials containing 5 ml of actively growing cells using ammonia, nitrate, or N2 as their sole N source. The treated vials were incubated at room temperature at 150 rpm for 2 days, and the headspace gaseous compositions were analyzed by an HP 5890A gas chromatograph (GC)-mass spectrometer equipped with a flame ionization detector (FID) to detect the decrease of acetylene and the production of ethylene in vials. No ethylene production was observed in acetylene-free controls. When significant levels of ethylene were detected in the vials, reactions were considered positive.
PHB analysis.
The PHB contents of cells were measured as described earlier (7, 30). Cell suspensions (0.2 ml) were applied to Whatman glass fiber disks (GF/C, 2.1 cm) which were pretreated with hot concentrated sulfuric acid in a boiling-water bath for 2 h, then sequentially washed several times with distilled water, ethanol, and acetone, and finally air dried overnight. The fiber disks mounted on glass pins were dried at 105°C for 10 min before treatment with 0.15 ml of sodium hypochlorite for 1 h to lyse the attached cells. After the disks were dried, three applications of 0.2-ml warm chloroform (70°C) were applied onto the disks. The disks were then transferred to individual test tubes, sequentially washed twice with distilled water, ethanol, and acetone, and dried again in a 105°C oven. Three milliliters of concentrated sulfuric acid was added to the test tubes, which were then sealed with Teflon-lined caps and heated in a water bath at 100°C for 10 min. The absorbances of the reacted solutions were measured at 234 nm. All samples were measured in duplicate, and cell-free blanks were treated with the same procedure. Pure PHB (Sigma, St. Louis, Mo.) was used for standards and analyzed by the same procedure.
TCE oxidation rates and Tc analysis.
The tests for TCE oxidation and Tc were performed as described previously (7). Six milliliters of purged fresh cell suspension with an A600 of 0.5 (diluted from an optical density of approximately 0.7) was added to 26-ml vials capped with Mininert valves, and the vials were amended with TCE stock solution to achieve an initial aqueous-phase TCE concentration of 0.8 mg/liter. The TCE-amended vials were vigorously shaken by hand for 10 s before the first TCE headspace measurement and then were incubated on a rotary shaker at 200 rpm. Headspace samples were taken over time to monitor the disappearance of TCE in vials. The specific TCE oxidation rates of the cultures were calculated by a linear regression of at least four data points measured within the first 20 min of the experiments divided by total dry cell mass (VSS). Similar treatments were carried out for formate-amended vials, where 20 mM formate was added to provide an exogenous source of reducing energy. Cell-free controls exhibited TCE losses of less than 5% during the course of the experiments. Similar results were observed for at least three repeated experiments.
Experiments for the measurement of Tc with and without formate addition were performed similarly to TCE oxidation tests. The purged cell suspensions, with an optical density of 0.5 at A600, were transferred into 26-ml vials amended with initial liquid-phase TCE concentrations of 18 mg/liter. Vials were incubated on a rotary shaker at 200 rpm for 24 h (a period during which TCE degradation was experimentally determined to have ceased). The liquid-phase TCE concentrations were maintained by adding TCE stock solution repeatedly into vials whenever the headspace TCE concentrations fell below 1 mg/liter. The Tc (mass of TCE degraded/mass of biomass inactivated) were calculated from the change in total TCE mass in the vials over 24 h divided by the total dry cell mass (VSS). A dimensionless Henry’s constant of 0.3 at 20°C (14) was used for calculating aqueous and gaseous changes in the total mass of TCE in the vials. Total cell inactivation after the 24-h incubation with TCE was confirmed for formate-amended cells by purging the remaining TCE and measuring methane uptake following subsequent 3- and 7-day methane incubations. Duplicate vials were used for both TCE oxidation tests and Tc measurements.
Analytical methods.
The gaseous concentrations of CH4, O2, and CO2 in the headspaces of the flasks were measured by GC headspace analysis. Headspace gas (0.25 ml) was injected through a 0.5-ml gas-tight pressure-lok Dynatech-Precision syringe (Alltech Co.) into a Hewlett-Packard HP 5890 series II GC equipped with a thermal conductivity detector and a CTR1 column (Alltech Co.). The temperatures of oven, injector, and detector were maintained at 25, 25, and 30°C, respectively. The flow rate of helium carrier gas was set at 130 ml/min. Scotty-certified multicomponent gas mixtures (Alltech Co.) were used for calibration.
Headspace concentrations of organics, including TCE, CH4, acetylene, and ethylene, were determined by injecting 20- or 50-μl headspace gaseous samples into a Hewlett-Packard HP 5890A GC equipped with a fused silica VOCOL glass capillary column (inside diameter, 0.53 mm; length, 60 m; Supelco, Inc., Bellefonte, Pa.) maintained at 80°C with N2 at 15 ml/min as the carrier gas. The temperatures of the injector and both detectors, the electron capture detector (ECD) and the FID, were maintained at 250°C. The ECD was used for TCE analysis, and the FID was used for the CH4, acetylene, and ethylene measurements. The calibration curves of TCE were obtained by headspace gaseous-sample analysis in vials containing known amounts of TCE. The vials were prepared by adding known amounts of TCE-saturated aqueous solution or of TCE-methanol mixture to 26-ml vials containing known amounts of distilled water. The TCE-amended vials were then vigorously shaken by hand for 30 s before shaking at 200 rpm at ambient temperature. TCE headspace analysis was performed after 1 h of shaking. Methane calibration curves were obtained by analyzing known amounts of pure methane gas.
RESULTS
Nitrogen source effects on cellular growth.
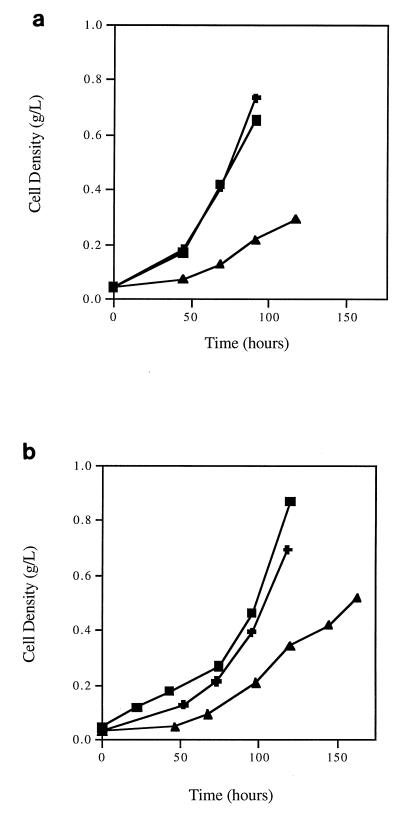
The effects of N sources on the cellular growth of methane-oxidizing cultures were measured in terms of growth curves (Fig. 1), net cellular growth rates, cell yields, nitrogenase enzyme activity, and cellular PHB contents (Table 1). Only cells grown in NFMS medium at low O2 pressures (about 5%) were able to fix molecular nitrogen with detectable nitrogenase enzyme activity. As shown in Fig. 1, both OB3b and CAC-2 were able to grow under ammonia-supplied, nitrate-supplied, or nitrogen-fixing conditions. OB3b and CAC-2 cultures exhibited similar growth patterns; i.e., cells grew at a lower rate when fixing molecular nitrogen and at higher but comparable rates when supplied with nitrate or ammonia as the N source. This trend was consistent with our previous observations with mixed cultures (7).
FIG. 1.
Effects of nitrogen source on growth curves of M. trichosporium OB3b (a) and strain CAC-2 (b). Symbols: ▴, N2; ✚, NO3−; ■, NH4+.
TABLE 1.
Summary of cell characteristics of methane-oxidizing cultures grown with different nitrogen sources
| Nitrogen source and culture | Net cellular growth ratea (day−1) | Net cell yieldb (mg of cells/ mg of CH4) | Nitrogenase activityc | PHB content (μg of PHB/ mg of cells) |
|---|---|---|---|---|
| Molecular nitrogen | ||||
| OB3b | 0.50 ± 0.02 | 0.45 ± 0.02 | Positive | 68.4 ± 3.9 |
| CAC-2 | 0.46 ± 0.03 | 0.24 ± 0.02 | Positive | 29.4 ± 2.2 |
| Mixedd | 0.32 ± 0.01 | 0.37 ± 0.01 | Positive | 25.9 ± 0.07 |
| Nitrate | ||||
| OB3b | 0.60 ± 0.01 | 0.52 ± 0.03 | Negative | 17.7 ± 4.4 |
| CAC-2 | 0.52 ± 0.01 | 0.53 ± 0.01 | Negative | 9.8 ± 1.1 |
| Mixed | 0.41 ± 0.01 | 0.45 ± 0.01 | Negative | 18.8 ± 0.03 |
| Ammonia | ||||
| OB3b | 0.63 ± 0.02 | 0.59 ± 0.03 | Negative | 5.8 ± 1.4 |
| CAC-2 | 0.53 ± 0.01 | 0.56 ± 0.01 | Negative | 4.7 ± 0.3 |
| Mixed | 0.46 ± 0.01 | 0.47 ± 0.01 | Negative | 20.3 ± 0.01 |
Calculated from growth curves. Data are averages ± range from three individual experiments.
Calculated from cell production during exponential growth. Data are averages ± range from three individual experiments.
Results of acetylene assay for nitrogenase activity. Data were obtained from three individual experiments. Positive, ethylene was detected; negative, ethylene was not detected.
Data for mixed cultures are taken from reference 7 and are averages ± range from duplicate experiments.
The net cellular growth rates (biomass produced per biomass per time) and cell yields (biomass produced per mass of methane consumed) of each culture were calculated during the exponential-growth phase and are listed in Table 1. The net cellular growth rate of nitrogen-fixing OB3b was 17 and 20% lower than those of nitrate- and ammonia-supplied OB3b, respectively. However, a much smaller effect was observed in the net cellular growth rates of CAC-2 (4 to 5%). Not surprisingly, nitrogen-fixing OB3b showed 12 and 25% lower cell yields than nitrate- and ammonia-supplied OB3b, respectively, while CAC-2 exhibited the same pattern, with a more exaggerated decrease in yield (55% to 57%) for the nitrogen fixers.
A wide range of cellular PHB contents were measured in mixed and pure cultures growing with three different N sources. As cellular PHB content has been reported to increase under nitrogen-fixing conditions, the observation of much higher levels of PHB in the nitrogen-fixing mixed and pure cultures was not unexpected. The cellular PHB content (68.4 ± 3.9 μg of PHB/mg of VSS) exhibited by nitrogen-fixing OB3b was 3.9- and 12-fold higher than that exhibited by nitrate- and ammonia-supplied OB3b, respectively, while nitrogen-fixing CAC-2 exhibited 3- and 6.3-fold-higher levels of PHB than the nitrate- and ammonia-supplied CAC-2 cells. The mixed culture exhibited a much smaller effect, with only 1.3- to 1.4-fold-higher levels of PHB for the nitrogen-fixing cells. The ammonia-supplied pure strains produced significantly lower PHB contents than either the nitrogen-fixing or nitrate-supplied cells, but this was not the case for the mixed culture.
Nitrogen source effects on methane uptake rates and naphthalene oxidation rates.
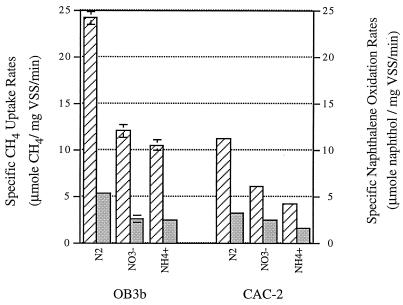
The effects of nitrogen source on the activity of sMMO enzymes were evaluated by measuring methane uptake rates and naphthalene oxidation rates. Since methane disappearance, rather than methanol production, was measured during the course of the experiments, the measured rates are referred to as methane uptake rates rather than methane oxidation rates. The specific methane uptake rates (micromoles of CH4 per milligram of VSS per minute) and specific naphthalene oxidation activities (micromoles of naphthol per milligram of VSS per minute) of OB3b and CAC-2 under three different N-source conditions were measured (Fig. 2). As shown in Fig. 2 and Table 2, the specific methane uptake rates of both cultures (OB3b and CAC-2) were highly correlated with their specific naphthalene oxidation activities. The trends with respect to these two rates for OB3b and CAC-2 with different N sources were similar in that the nitrogen fixers had the highest rates, followed by the nitrate- and ammonia-supplied cells. Furthermore, OB3b exhibited higher rates than CAC-2 for nitrogen-fixing and ammonia-supplied conditions, while rates for nitrate-supplied cells were similar for the two strains.
FIG. 2.
Specific methane uptake rates and specific naphthalene
oxidation rates (in terms of naphthol production rates) of fresh cells
of M. trichosporium OB3b and strain CAC-2. Error bars are
included in each data set (some fall within the width of the line).
Symbols: ▨, specific methane uptake rates;
 ,
specific naphthalene oxidation rates. N sources are shown below the
bars.
,
specific naphthalene oxidation rates. N sources are shown below the
bars.
TABLE 2.
Correlation matrix of methane uptake rates, naphthalene oxidation rates, initial TCE oxidation rates, and Tc of OB3b and CAC-2 cultures grown with three different nitrogen sources
| Culture and parameter | Correlation
|
|||||
|---|---|---|---|---|---|---|
| CH4a | Naphtholb | TCE
oxidationc
|
Tcd
|
|||
| With formate | Without formate | With formate | Without formate | |||
| OB3b | ||||||
| CH4 | 1 | |||||
| Naphthol | 0.998 | 1 | ||||
| TCE oxidation | ||||||
| With formate | 0.921 | 0.942 | 1 | |||
| Without formate | 0.871 | 0.897 | 0.993 | 1 | ||
| Tc | ||||||
| With formate | 0.988 | 0.978 | 0.851 | 0.785 | 1 | |
| Without formate | 0.823 | 0.789 | 0.538 | 0.437 | 0.900 | 1 |
| CAC-2 | ||||||
| CH4 | 1 | |||||
| Naphthol | 0.953 | 1 | ||||
| TCE oxidation | ||||||
| With formate | 0.906 | 0.736 | 1 | |||
| Without formate | 0.954 | 0.818 | 0.991 | 1 | ||
| Tc | ||||||
| With formate | 0.909 | 0.992 | 0.647 | 0.741 | 1 | |
| Without formate | 0.996 | 0.977 | 0.863 | 0.922 | 0.944 | 1 |
Methane uptake rates in micromoles of CH4 per milligram of VSS per minute.
Naphthalene oxidation rates (in the presence of formate), expressed as micromoles of naphthol per milligram of VSS per minute.
Initial TCE oxidation rates, expressed as micromoles of TCE per milligram of VSS per minute.
Mass of TCE transformed per mass of cells inactivated, expressed as micromoles of TCE per milligram of VSS.
Nitrogen source effects on TCE degradation ability.
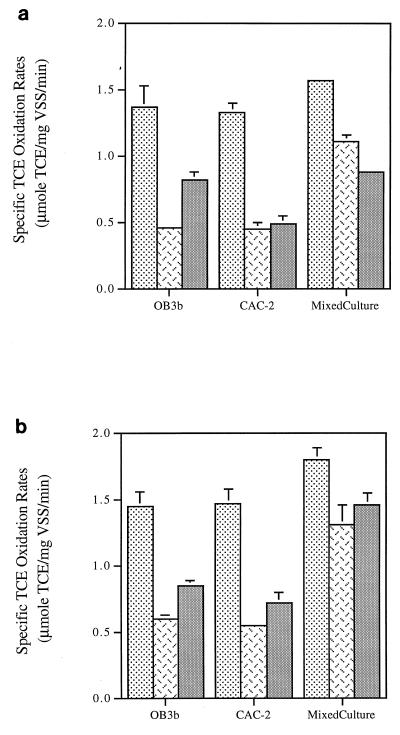
The TCE degradation ability of each culture with each of three different N sources was evaluated by measuring initial TCE oxidation rates (TCE mass degraded per initial biomass per time) (Fig. 3) and Tc (TCE mass transformed per biomass inactivated) (Fig. 4) with and without the addition of formate. The nitrogen-fixing pure and mixed cultures showed the highest initial TCE oxidation rates with and without the addition of formate. However, the ammonia-supplied cells of the pure cultures generally exhibited higher initial TCE oxidation rates than the nitrate-supplied cells both in the absence and in the presence of formate. The most significant exception to this trend was the mixed culture, which, in the absence of formate, exhibited a higher TCE oxidation rate when supplied with nitrate than when supplied with ammonia.
FIG. 3.
Comparison of initial TCE oxidation rates of fresh cells
of M. trichosporium OB3b, strain CAC-2, and the mixed
culture in the absence (a) and in the presence (b) of formate. Error
bars are included in each data set (some fall within the width of the
line). Symbols: ░⃞, N2;
 ,
NO3−;
,
NO3−;
 ,
NH4+.
,
NH4+.
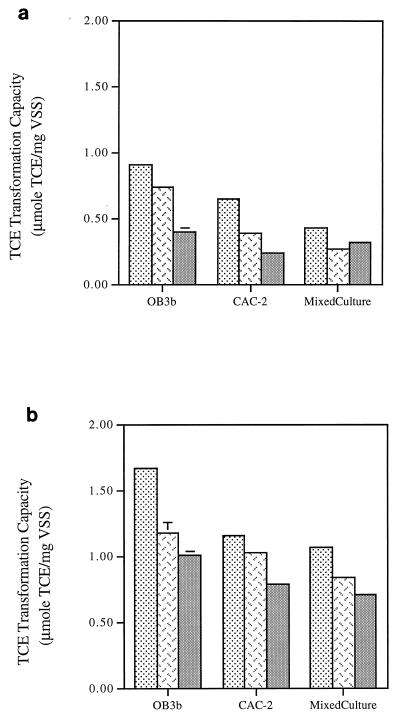
FIG. 4.
Comparison of Tc of fresh cells
of M. trichosporium OB3b, strain CAC-2, and the mixed
culture in the absence (a) and in the presence (b) of formate. Error
bars are included in each data set (some fall within the width of the
line). Symbols: ░⃞, N2;
 ,
NO3−;
,
NO3−;
 ,
NH4+.
,
NH4+.
Formate was used in experiments as a means of removing possible NADH limitations during the measurement of initial TCE oxidation rates (Fig. 3b). The addition of formate caused a 5 to 10% increase in the initial TCE oxidation rates for the nitrogen-fixing pure cultures, a 20 to 30% increase for the nitrate-supplied pure cultures, and only a 3% increase for ammonia-supplied OB3b, but a >45% increase for ammonia-supplied CAC-2. However, for the mixed culture, formate addition caused more than a 60% increase in the initial TCE oxidation rates of ammonia-supplied cells but only a moderate increase (20%) in the rates in nitrogen-fixing and nitrate-supplied cells.
Tc with and without formate addition were also examined as a measure of TCE-degrading ability of the cultures with three different N sources (Fig. 4). Tc measured in the absence of formate evaluates TCE degradation under the potential influence of both NADH limitation and TCE product toxicity, while Tc measured in the presence of formate reflects TCE degradation under the influence of TCE product toxicity alone (without NADH limitation). Both in the absence and in the presence of formate, nitrogen-fixing OB3b exhibited the highest Tc values, followed by nitrate- and ammonia-supplied cells. A similar trend was observed for CAC-2. The Tc value trends of both the pure and mixed cultures in the absence of formate were moderately to highly correlated with the cellular PHB content of each culture (0.86 and 0.99 for OB3b and CAC-2, respectively).
When reducing-energy limitations were removed by the addition of formate, nitrogen-fixing methane oxidizers still exhibited the highest Tc (Fig. 4b). The addition of formate increased Tc values in both pure and mixed cultures with each of the three N sources. The Tc values in the presence of formate were about 200% higher than those in the absence of formate under nitrogen-fixing conditions and about 150 to 300% higher than those in the absence of formate under nitrate- and ammonia-supplied conditions.
In this study, formate addition exerted relatively small effects on TCE oxidation rates compared to its effects on Tc. The major reason for this is that the degradation rate experiments were purposely conducted with low TCE concentrations in order to minimize the effects of toxicity and reductant limitation on the initial TCE degradation rates. However, in the Tc experiments, cells were exposed to much higher concentrations of TCE, resulting in much greater NADH requirements and significant reductant limitations.
DISCUSSION
Although the nitrogen-fixing capabilities of methane-oxidizing bacteria have been well documented (11, 12, 20, 33), the concept of capitalizing upon this characteristic for the enhancement of bioremediation is novel. This study evaluated the growth and TCE degradation capabilities of methane-oxidizing bacteria grown with various nitrogen sources. As expected, the type II methane-oxidizing cultures, OB3b and CAC-2, were able to fix molecular nitrogen at reduced oxygen partial pressures with slightly lower net growth rates and cell yields than those of nitrate- or ammonia-supplied cultures. Furthermore, the effects of nitrogen source on growth patterns, net cellular growth rates, cell yields, PHB contents, and nitrogenase enzyme activities were consistent with our previous reports for mixed cultures (7). TCE-degrading abilities measured by initial TCE oxidation rates and Tc were repeatedly observed to be enhanced for nitrogen-fixing cultures compared with those of nitrate- or ammonia-supplied cultures. Reduced oxygen partial pressures have previously been shown to have no significant effect on TCE oxidation rates for nitrogen-fixing, nitrate-supplied, and ammonia-supplied methane oxidizers (7).
In this study, methane, naphthalene, and TCE oxidation reactions were used to gauge the activity of sMMO enzymes. Although each of these reactions requires NADH as reducing energy, only methane oxidation results in products that promote the regeneration of NADH. Therefore, in experiments conducted with no external source of reducing energy, measured naphthalene or TCE oxidation rates may be limited by low levels of reducing energy. However, when reducing-energy limitations are removed by the addition of formate (as an external source of reducing energy), both naphthalene oxidation rates and initial TCE oxidation rates (measured prior to significant product toxicity) should reflect the maximum activity of sMMO in methane-oxidizing cells. Accordingly, the high correlation between methane uptake rates and naphthalene oxidation rates (in the presence of formate) in OB3b and CAC-2 with three different N sources (Fig. 2 and Table 2) was not surprising. It was also not surprising to find that a relatively high correlation existed between methane uptake rates and initial TCE oxidation rates in the presence of formate.
Although a high correlation was observed between naphthalene and TCE oxidation rates in the presence of formate for OB3b (r = 0.94), the observed correlation was much lower for the CAC-2 culture (r = 0.74). Further, although TCE oxidation rates in the presence and in the absence of formate were highly correlated with each other (>0.99), TCE oxidation rates in the absence of formate were poorly correlated with naphthalene oxidation rates in the presence of formate (<0.9). These data suggested that methane uptake rates may serve as slightly better indicators for TCE oxidation rates than naphthalene oxidation rates when it is not possible to measure TCE oxidation rates directly.
Tc under potential reducing-energy limitation were found to be correlated with PHB content in methane oxidizers with all N sources, in agreement with observations for the mixed cultures (7). A high correlation (r = 0.99) was observed for CAC-2 and the mixed cultures, while a lower correlation (r = 0.86) was observed for OB3b (Table 1 and Fig. 4a), suggesting that internal NADH limitation might be more significant in CAC-2 and the mixed cultures than in OB3b. Although the linkage between PHB and Tc may explain the Tc enhancement observed for nitrogen fixers measured in the absence of formate, it does little to explain why nitrogen-fixing cells also exhibited higher Tc values than ammonia- or nitrate-supplied cells when energy limitation was eliminated by the addition of formate.
Three hypotheses had been proposed in our previous study to explain why nitrogen-fixing mixed cultures of methane oxidizers experienced lower product toxicity following TCE oxidation than nitrate- or ammonia-supplied cells (7). The first hypothesis was that nitrogen fixation selected for methane-oxidizing bacteria that were less susceptible to TCE product toxicity within the mixed cultures. The second hypothesis was that nitrogen-fixing cells produced more MMO per cell to fulfill the high energy demand required for nitrogen fixation. The final hypothesis was that the enzymatic protection mechanisms associated with the oxygen-sensitive nitrogenase enzymes, or the nitrogenase enzymes themselves, may help to protect microorganisms from TCE product toxicity. In this study, conducted primarily with pure cultures, the first hypothesis is not feasible, since there is no selective enrichment with pure cultures. In contrast with results from the mixed-culture study, the second hypothesis is supported in this study by observations of higher specific methane, naphthalene, and TCE oxidation rates in nitrogen-fixing cultures than in nitrate- or ammonia-supplied cells, suggesting that nitrogen-fixing conditions may result in the generation of greater amounts of sMMO enzyme per cell. There are a number of reasons why the second hypothesis may not have been supported in the mixed-culture study, including the possibility of selective pressure causing enrichment of different cells with the different nitrogen sources (the first hypothesis). The final hypothesis was also supported in this study by repeated observations of high Tc values both in the presence and in the absence of formate in nitrogen-fixing OB3b and CAC-2. However, the determination of whether protective mechanisms for the nitrogenase enzyme are responsible for the decreased TCE product toxicity of nitrogen fixers is an interesting question that requires experiments beyond the scope of this work.
Although Tc and initial TCE oxidation rates are commonly used for estimating the TCE-degrading ability of microorganisms, direct correlations between Tc values and initial TCE oxidation rates (or methane uptake rates, or naphthalene oxidation rates) have not been commonly observed (1, 6, 7, 22, 23). This is not surprising in that Tc is not a kinetic measurement like the TCE oxidation rate, but rather a measure of the amount of TCE that can be transformed by a given culture prior to inactivation. Although it may seem reasonable that these two parameters would be related, it is not expected, since the former is a measure of the cumulative effects of product toxicity while the latter is a measure of the maximum enzyme efficiency. In this study, higher correlations were found between Tc values and methane uptake rates (r = 0.99 for OB3b and r = 0.91 for CAC-2) and between Tc values and naphthalene oxidation rates (r = 0.98 for OB3b and r = 0.99 for CAC-2) than between Tc values and TCE oxidation rates (r = 0.85 for OB3b and r = 0.65 for CAC-2) for both cultures when reducing energy limitation was removed by the addition of formate (Table 2). These correlations provide additional support for the second hypothesis stated above, suggesting that the enhanced activity of sMMO in nitrogen-fixing cells might be responsible for their enhanced TCE-degrading abilities.
This study demonstrated the enhanced contaminant-degrading abilities of pure and mixed methane-oxidizing cultures under nitrogen-fixing conditions, suggesting the feasibility of enriching nitrogen-fixing methane oxidizers for the enhancement of bioremediation in fixed-nitrogen-limited environments.
ACKNOWLEDGMENTS
This work was supported in part by a fellowship from the University of California Toxic Substance Research and Teaching Program and in part by the National Institute of Environmental Health Sciences under grant P42-ES04705 and by a National Science Foundation Young Investigator Award (BES-9457246).
REFERENCES
- 1.Alvarez-Cohen L, McCarty P L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991;57:228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asenjo J A, Suk J S. Microbial conversion of methane into poly-β-hydroxybutyrate (PHB): growth and intracellular product accumulation in a type II methanotroph. J Ferment Technol. 1986;64:271–278. [Google Scholar]
- 3.Brockman F J, Payne W, Workman D J, Soong A, Manley S, Hazen T C. Effect of gaseous nitrogen and phosphorus injection on in situ bioremediation of a trichloroethylene-contaminated site. J Hazard Mater. 1995;41:287–298. [Google Scholar]
- 4.Broholm K, Jensen B K, Christensen T H, Olsen L. Toxicity of 1,1,1-trichloroethane and trichloroethene on a mixed culture of methane-oxidizing bacteria. Appl Environ Microbiol. 1990;56:2488–2493. doi: 10.1128/aem.56.8.2488-2493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brusseau G A, Tsien H C, Hanson R S, Wackett L P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble methane monooxygenase activity. Biodegradation. 1990;1:19–29. doi: 10.1007/BF00117048. [DOI] [PubMed] [Google Scholar]
- 6.Chang H L, Alvarez-Cohen L. Biodegradation of individual and multiple chlorinated aliphatic hydrocarbons by methane-oxidizing cultures. Appl Environ Microbiol. 1996;62:3371–3377. doi: 10.1128/aem.62.9.3371-3377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu K H, Alvarez-Cohen L. Trichloroethylene degradation by methane-oxidizing cultures grown with various nitrogen sources. Water Environ Res. 1996;68:76–82. [Google Scholar]
- 8.Dalton H, Whittenbury R. The acetylene reduction technique as an assay for nitrogenase activity in the methane-oxidizing bacterium Methylococcus capsulatusstrain Bath. Arch Microbiol. 1976;109:147–151. [Google Scholar]
- 9.Davis J B, Coty V F, Stanley J P. Atmospheric nitrogen fixation by methane-oxidizing bacteria. J Bacteriol. 1964;88:468–472. doi: 10.1128/jb.88.2.468-472.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawes E A, Senior P J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 11.de Bont J A M. Nitrogen fixation by methane-utilizing bacteria. Antonie Leeuwenhoek. 1976;42:245–253. doi: 10.1007/BF00394121. [DOI] [PubMed] [Google Scholar]
- 12.de Bont J A M, Mulder E G. Nitrogen fixation and co-oxidation of ethylene by a methane-utilizing bacterium. J Gen Microbiol. 1974;83:113–121. [Google Scholar]
- 13.Fogel M M, Taddeo A R, Fogel S. Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Appl Environ Microbiol. 1986;51:720–724. doi: 10.1128/aem.51.4.720-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gossett J M. Measurement of Henry’s law constants for C1 and C2chlorinated hydrocarbons. Environ Sci Technol. 1987;21:202–208. [Google Scholar]
- 15.Hazen T C, Looney B B, Enzien M, Dougherty J M, Wear J, Fliermans C B, Eddy C A. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. In situ bioremediation of chlorinated-solvents via horizontal wells, abstr. N-77; p. 329. [Google Scholar]
- 16.Henry S M, Grbić-Galić D. Influence of endogenous and exogenous electron donors and trichloroethylene oxidation toxicity on trichloroethylene oxidation by methanotrophic cultures from a groundwater aquifer. Appl Environ Microbiol. 1991;57:236–244. doi: 10.1128/aem.57.1.236-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henrysson T, McCarty P L. Influence of the endogenous storage lipid poly-β-hydroxybutyrate on the reducing power availability during cometabolism of trichloroethylene and naphthalene by resting methanotrophic mixed cultures. Appl Environ Microbiol. 1993;59:1602–1606. doi: 10.1128/aem.59.5.1602-1606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henson J M, Yates M V, Cochran J W, Shackleford D L. Microbial removal of halogenated methanes, ethanes, and ethylenes in an aerobic soil exposed to methane. FEMS Microb Ecol. 1988;53:193–201. [Google Scholar]
- 19.Little C D, Palumbo A V, Herbes S E, Lidstrom M E, Tyndall R L, Gilmer P J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988;54:951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murrell J C, Dalton H. Nitrogen fixation in obligate methanotrophs. J Gen Microbiol. 1983;129:3481–3485. [Google Scholar]
- 21.Nichols P D, White D C. Accumulation of poly-β-hydroxybutyrate in a methane-enriched, halogenated hydrocarbon-degrading soil column: implications for microbial community structure and nutritional status. Hydrobiologia. 1989;176/177:369–377. [Google Scholar]
- 22.Oldenhuis R, Oedzes J Y, van der Waarde J J, Janssen D B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporiumOB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991;57:7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenhuis R, Vink R L, Janssen D B, Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporiumOB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989;55:2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfiffner S M, Palumbo A V, Phelps T J, Hazen T C. Effects of nutrient dosing on subsurface methanotrophic populations and trichloroethylene degradation. J Ind Microbiol Biotechnol. 1997;18:204–212. doi: 10.1038/sj.jim.2900350. [DOI] [PubMed] [Google Scholar]
- 25.Semprini L, Roberts P V, Hopkins G D, McCarty P L. A field evaluation of in-situ biodegradation of chlorinated ethanes. Part 2. Results of biostimulation and biotransformation experiments. Ground Water. 1990;28:715–727. [Google Scholar]
- 26.Speitel G E, Alley E R. Bioremediation of unsaturated soils contaminated with chlorinated solvents. J Hazard Mater. 1991;28:81–90. [Google Scholar]
- 27.Speitel G E, Closmann F B. Chlorinated solvent biodegradation by methanotrophs in unsaturated soils. J Environ Eng. 1991;117:541–548. [Google Scholar]
- 28.Tsien H-C, Brusseau G A, Hanson R S, Wackett L P. Biodegradation of trichloroethylene by Methylosinus trichosporiumOB3b. Appl Environ Microbiol. 1989;55:3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wackett L P, Gibson D T. Rapid method for detection and quantitation of hydroxylated aromatic intermediates produced by microorganisms. Appl Environ Microbiol. 1983;45:1144–1147. doi: 10.1128/aem.45.3.1144-1147.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward A C, Dawes E A. A disk assay for poly-β-hydroxybutyrate. Anal Biochem. 1973;52:607–613. doi: 10.1016/0003-2697(73)90067-5. [DOI] [PubMed] [Google Scholar]
- 31.Westrick J J, Mello J W, Thomas R F. The groundwater supply survey. J Am Water Works Assoc. 1984;5:52–59. [Google Scholar]
- 32.Whittenbury R, Dalton H. The methylotrophic bacteria. In: Starr M P, Stolp H, Trüper H G, Balows A, Schlegel H G, editors. The prokaryotes. New York, N.Y: Springer-Verlag; 1980. pp. 894–902. [Google Scholar]
- 33.Whittenbury R, Phillips K C, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 34.Wilson J T, Wilson B H. Biotransformation of trichloroethylene in soil. Appl Environ Microbiol. 1985;49:242–243. doi: 10.1128/aem.49.1.242-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]