Abstract
Few studies have been conducted in the cooling systems of power plants; they have focused on Naegleria fowleri, leaving a gap in the knowledge of other pathogenic free-living amoebae in this environment. The objective of this study was to determine the occurrence of saline-tolerant pathogenic Acanthamoeba in a geothermal power plant. The identification of isolated amoebae at genus level was carried out, observing their morphological characteristics; the determination of genotype and species of Acanthamoeba was performed via molecular biology (PCR). Water temperature ranged from 18 to 43 °C and conductivity from 4.0 × 104 to 8.7 × 104 μS/cm; this last value was greater than the seawater value. Only five amoeba genera were found. Acanthamoeba was in all the sampling sites, showing high saline tolerance. The high temperature, but mainly high conductivity, were the environmental conditions that determined the presence of pathogenic free-living amoebae in the hot water. All the strains of Acanthamoeba culbertsoni killed the mice, having a mortality of 40 to 100%. Acanthamoeba genotypes T10 and T5 were identified, T10 is rarely isolated from the environment, while T5 is more frequent. This is the first time that genotypes T5 and T10 have been reported in the environment in Mexico.
Keywords: pathogenic free-living amoebae, hot water, cooling system, conductivity, salinity
1. Introduction
Free-living amoebae (FLA) are widely distributed in the environment and have been found throughout the world. Naegleria fowleri, Acanthamoeba spp., Balamuthia mandrillaris, and Sappinia pedata can be pathogenic to humans and animals [1]. Pathogenic, and non-pathogenic FLA also have medical relevance because of their possible role as hosts or vectors for other pathogenic organisms [2,3]. N. fowleri causes a fatal infection of the central nervous system called Primary Amoebic Meningoencephalitis (PAM) in healthy people. Some species of Acanthamoeba, Balamuthia mandrillaris, and Sappinia pedata can cause a central nervous system infection called Granulomatous Amoebic Encephalitis (GAE). Acanthamoeba is an opportunistic amoeba that affects immunosuppressed individuals such as patients of AIDS; moreover, it can cause a corneal infection in contact lens wearers. This is aggravated by the lack of effective treatments [1].
Hot water is a suitable environment for thermophilic FLA [4,5,6,7,8,9]; they can tolerate temperatures over 30 °C. Naegleria fowleri and Acanthamoeba can grow well at high temperatures, even to 45 °C [5]. Thermophilic FLA can survive thanks to the resistance of their cyst or the potential to adapt to adverse conditions. Especially, Acanthamoeba can withstand extreme environmental conditions due to the presence of cellulose in the wall of their cyst [1]. Reports have mentioned that Acanthamoeba cysts can survive in extremely hostile environments; cysts stored at 4 °C can come out of the cyst even after 50 years [10]. Chlorine and pH can have an impact on FLA presence; these conditions could indirectly favor the presence of the more resistant amoebae such as Acanthamoeba [11]: these amoebae were isolated from hot sanitary water systems with a level of chlorine of 8 mg/L [12].
Geothermal power plants use the steam produced by geothermal energy inside the ground to move turbines and to produce electricity. As a result, hot water is produced by steam condensation; hence, a system to cool the hot water is required before the water is returned to the ground [13]. The water is saline, with high concentrations of chlorides, sodium, calcium, and potassium [14].
Salinity is a measure of the concentration of salts dissolved in water; salts dissolved in water decompose into positively and negatively charged ions. Conductivity measures the ability of water to conduct an electric current through dissolved ions. Therefore, salinity and conductivity are related; thus, when the concentration of dissolved ions increases, the values of both increase [15].
Conductivity has hardly been considered in studies of pathogenic FLA in aquatic environments [16,17,18], and few studies have been conducted on water with high salinity [19,20,21,22,23]. On the other hand, studies on the cooling systems of power plants have focused mainly on Naegleria fowleri [4,5,6,9], leaving a gap in the knowledge about the distribution of other FLA in this environment. For these reasons, the objective of this study was to determine the presence of saline-tolerant pathogenic Acanthamoeba in a geothermal power plant.
2. Materials and Methods
2.1. Sampling
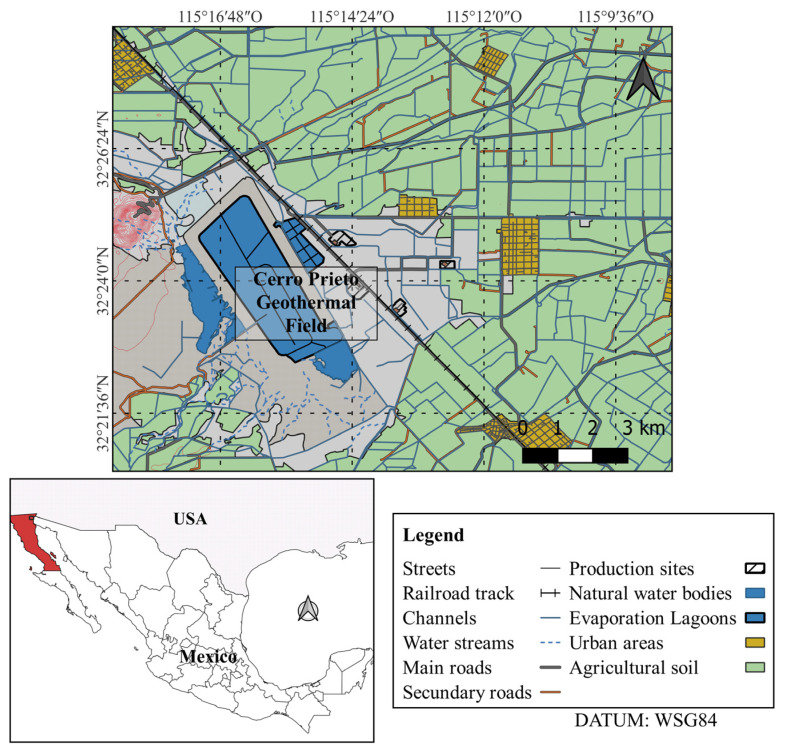
The study was carried out in the Geothermal Power Plant of Cerro Prieto; this is the second largest plant in the world, with an installed capacity of 820 MW and an area of 38.33 km2. The groundwater of the Cerro Prieto geothermal plant is extracted from 1500 to 3100 m deep geothermal wells that are in constant operation to generate electricity. The plant is in the Mexicali Valley, Baja California (Mexico) (Figure 1). The prevailing climate of the area is very warm and dry, with light rainfall in winter; in summer, the temperature can reach 50 °C [24]. The hot water produced during electric power generation is discharged into a cooling system that consists of secondary channels, a primary channel, and an evaporation lagoon.
Figure 1.
Localization of Cerro Prieto Geothermal Power Plant, Baja California, Mexico.
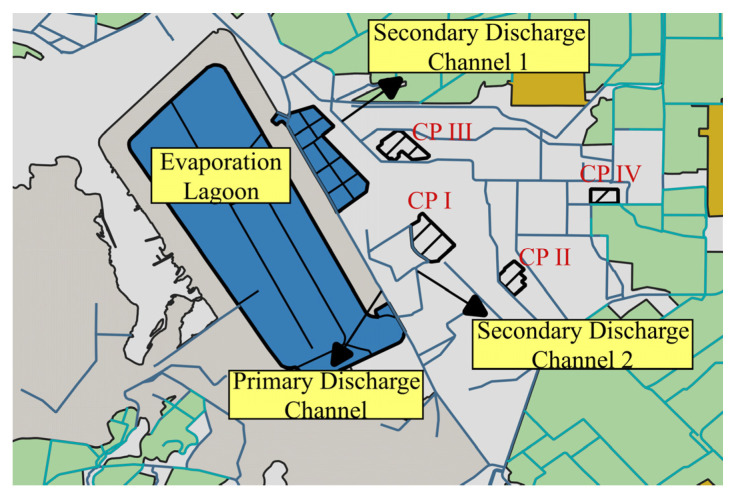
Samples of 500 mL each were collected from different sites at the plant: the Primary Discharge Channel (PDC), Secondary Discharge Channels (SDC), and Evaporation Lagoon (EvL) (Figure 2). Water samples were placed in sterile containers and kept at room temperature until analysis. Physicochemical parameters were measured in situ. Water temperature, conductivity (K25), and pH were measured using a conductimeter model PC18 (Conductronic Instruments, Puebla, Mexico); dissolved oxygen was measured with an oxymeter YSI Model 51 B. These analyses were carried out using standard methods [15].
Figure 2.
Sampling sites at Cerro Prieto Geothermal Power Plant. Yellow boxes, sampling sites; blue lines, water channels; CPI, CPII, CPIII, and CPIV production units.
2.2. Processing Samples
Water samples were homogenized and filtered through a 2.0 µm-diameter Millipore filter (Millipore, Bedford, MA, USA); membranes were placed on Petri dishes with a non-nutrient agar medium with Enterobacter aerogenes ATCC-13048 (NNA). This procedure was carried out in duplicate to incubate at 30 °C and 37 °C. NNA plates were examined through an inverted microscope (Zeiss model 473028) to detect the growth of free-living amoebae.
Amoebae were sub-cultivated in a fresh NNA medium several times to clean and separate them from other microorganisms. Blocks (1 cm2) were taken from the NNA medium containing trophic amoebae and transferred to axenic culture, modified Chang medium, and 2% (w/v) Bacto™Casitone medium (Difco, Le Pont de Claix, France), supplemented with 10% (v/v) fetal bovine serum (Gibco, Grand Island, NY, USA) and 100 U penicillin and 100 µg of streptomycin per milliliter. Cultures were incubated again at 30 °C or 37 °C to obtain pure isolates of the amoebae [25].
2.3. Morphological Identification
The traditional identification based on the morphological characteristics of FLA was used to identify the amoebae at genus level, and molecular biology techniques were used to identify at species level the pathogenic FLA. In vivo preparations of the amoebae from axenic and monoxenic cultures were examined through a phase-contrast optical microscope at magnifications of 40× and 100× (Zeiss K7, Germany). The morphological identification was made, considering the trophozoite and cyst characteristics of amoebae using taxonomical keys for FLA [26].
2.4. Pathogenicity Test
Pathogenicity tests using mice were in accordance with the Mexican federal regulations for animal experimentation and care (NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City, Mexico), and approved by the ethical standards of the Facultad de Estudios Superiores Iztacala, UNAM (Number of Approval CE/FESI/022020/1317). In all experiments, 6- to 8-week-old male BALB/c mice were used. Five mice were maintained per cage and fed ad libitum. This test was carried out on mice using axenic amoeba cultures. Trophozoites were concentrated at 3000 rpm for 10 min., and were adjusted to a concentration of 1 × 105 per ml. A volume of 0.02 mL was taken and was inoculated intranasally. Five mice were inoculated with an amoeba-free culture medium for controls. Mice that were about to die, and that survived after the 21 days, were euthanized; these and mice that died during the test period were opened to remove their brain, liver, lungs, and kidneys and placed on plates with non-nutrient agar medium that was incubated at isolation temperature (30 or 37 °C). Cultures were observed every day for a week to monitor the development of amoebae. The mortality rate was determined, taking in account the dyed mice [27].
2.5. DNA Isolation and PCR
Genomic DNA was extracted from those morphologically identified isolates such as Acanthamoeba. Amoebic cultures were placed directly into a Maxwell 16 tissue DNA purification kit sample cartridge (Promega, Madrid, Spain). The amoebic genomic DNA was purified using the Maxwell 16 instrument in according to the manufacturer recommendations. DNA yield and purity were determined with a NanoDrop spectrophotometer (DeNovix®, USA DS-11, Wilmington, DE, USA).
Polymerase Chain Reaction (PCR) was conducted as previously described [28] where Genus-specific PCRs were run for Acanthamoeba using the following primers: JDP1 (5′-GCCCAGATCGTTTACCGTGAA-3′) and JDP2 (5′-TCTCACAAGCTCTAGGGAGTCA-3′). Depending on the genotype, the primers amplify 423 to 551 bp of the 18S ribosomal DNA (rDNA) between reference bp 936 and 1402.
A mix PCR was prepared with 50 µL volume, containing 1.25 U Taq DNA polymerase (Ecogen), 40–100 ng DNA, 1.5 mM MgCl2, 200 µM deoxynucleoside triphosphate (dNTP), and 0.2 µM each primer.
Once the mix had been prepared, samples were placed in an Artik Thermal Cycler (Thermo Scientific, Waltham, MA, USA) and run under the following conditions: an initial 5 min denaturation at 95 °C, followed by 35 cycles of amplification at 95 °C, with 30 s denaturing at 95 °C, 30 s annealing at 60 °C, 30 s extension at 72 °C, and a final extension of 7 min at 72 °C.
Amplicons (5 μL aliquot of the PCR reaction) were analyzed by electrophoresis on 2% agarose gel (SIGMA, St. Louis, MO, USA) in TBE buffer, stained with Safe-Green. The gel was then displayed on a UV transilluminator-Gel Doc XR (Bio-Rad) at a wavelength of 260 nm.
2.6. Sequencing and Phylogenetic Analysis
The PCR products of isolates were then used for sequencing, using an automated fluorescent sequencing system (Macrogen Spain service, Av. Sur del Aeropuerto, Madrid, España). The obtained sequences were aligned using the Mega 3.0 software program. Acanthamoeba genotype identification was based on sequence analysis of the DF3 region as previously described and the comparison to the available DNA sequences in GenBank.
3. Results
3.1. Physicochemical Parameters
The temperature distribution in the cooling system presented a cooling in accordance with the path of water circulation (secondary channels, primary channel, and evaporation lagoon). This behavior had been reported before in cooling systems [29]. The temperature was lowered from 43 to 18 °C (Table 1), fulfilling the objective of the cooling process. The water in the discharge channels (SDC and PDC) had a conductivity of 4.0 to 4.3 × 104 µS/cm, and in the Evaporation Lagoon (EvL) it increased to 8.7 × 104 μS/cm. The pH of the water was nearly neutral, and the values of dissolved oxygen ranged from 1.8 to 5.0 mg/L (Table 1).
Table 1.
Physicochemical parameters of sampling sites.
| Sampling Sites | Site ID | pH | Water Temperature (° C) |
Dissolved Oxygen (mg/L) |
Conductivity (µS/cm) | * Salinity (%) |
|---|---|---|---|---|---|---|
| Secondary Discharge Channel 1 | SDC1 | 7.9 | 42.5 | 2.0 | 4.3 × 104 | 2.75 |
| Secondary Discharge Channel 2 | SDC2 | 8.1 | 43.0 | 1.8 | 4.2 × 104 | 2.68 |
| Primary Discharge Channel | PDC | 7.3 | 34.0 | 2.2 | 4.0 × 104 | 2.56 |
| Evaporation Lagoon 1 | EvL1 | 6.6 | 22.0 | 5.0 | 4.1 × 104 | 2.62 |
| Evaporation Lagoon 2 | EvL2 | 6.7 | 18.0 | 5.0 | 8.7 × 104 | 5.56 |
* Conductivity was converted to percentage of salt concentration using Lenntech online converter and the equivalence of Lam.
3.2. Presence of Free-Living Amoebae in the Sampling Sites
Five genera of amoebae were found in the hot water. Acanthamoeba was present at all the sites, followed by Vannella and Vermamoeba, while Saccamoeba and Thecamoeba were only in one site (Table 2).
Table 2.
Free-living amoebae isolated from Geothermal Power Plant.
| Site | Genus |
|---|---|
| SDC1 |
Acanthamoeba (Volkonsky 1931) Vannella (Bovee 1965) Vermamoeba (Cavalier-Smith and Smirnov 2011) |
| SDC2 |
Acanthamoeba (Volkonsky 1931) Saccamoeba (Frenzel 1982 emend. Bovee 1972) Thecamoeba (Fromentel 1874) Vannella (Bovee 1965) |
| PDC |
Acanthamoeba (Volkonsky 1931) Vannella (Bovee 1965) Vermamoeba (Cavalier-Smith and Smirnov 2011) |
| EvL1 | Acanthamoeba (Volkonsky 1931) |
| EvL2 | Acanthamoeba (Volkonsky 1931) |
3.3. Morphological Description of Acanthamoeba
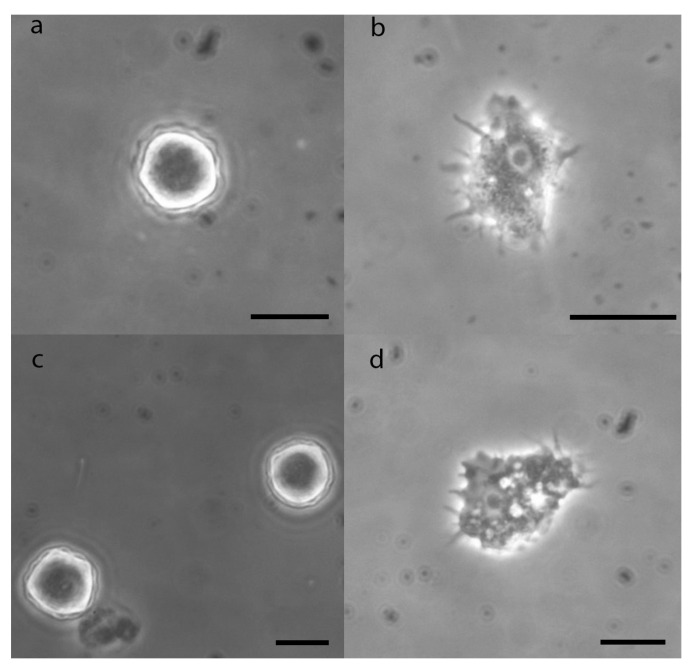
Acanthamoeba isolates were identified at the genus level via morphological criteria; all the isolated strains belonged to Acanthamoeba group III [26]. The trophozoite of Acanthamoeba presents fine pseudopods called acanthopods, which gives the amoeba a “thorny” appearance. It has a spherical nucleus with a central nucleolus that occupies much of the nucleus. A large contractile vacuole and several digestive vacuoles are usually seen in the cytoplasm. The cyst of Acanthamoeba species has two layers on its wall, the ectocyst and the endocyst. In morphological group III, the ectocyst is a thin layer and the endocyst is rounded without forming arms. The two layers are close together around most of the circumference (Figure 3).
Figure 3.
Micrographs of Acanthamoeba isolates. A. lenticulata: (a) cyst, (b) trophozoite. A. culbertsoni: (c) cyst, (d) trophozoite. Bar: 10 µm. Phase-contrast microscopy.
3.4. Morphological and Molecular Biology Identification of Acanthamoeba Strains
Based on molecular techniques, four strains (SDC1, SDC2a, PDC, and EvL1) were identified as Acanthamoeba culbertsoni belonging to genotype T10, and two strains (SDC2a and EvL2) were identified as Acanthamoeba lenticulata belonging to genotype T5 (Table 3).
Table 3.
Morphological and molecular biology identification of Acanthamoeba strains isolated from Geothermal Power Plant.
| Strain | Morphology | Molecular Biology (JDP1/2) | ||
|---|---|---|---|---|
| Species | Genotype | GenBank Accession Number |
||
| SDC1 | Acanthamoeba Group III | Acanthamoeba culbertsoni | T10 | OR767829 |
| SDC2a | Acanthamoeba Group III | Acanthamoeba culbertsoni | T10 | OR767828 |
| SDC2b | Acanthamoeba Group III | Acanthamoeba lenticulata | T5 | OR767826 |
| PDC | Acanthamoeba Group III | Acanthamoeba culbertsoni | T10 | OR767830 |
| EvL1 | Acanthamoeba Group III | Acanthamoeba culbertsoni | T10 | OR767831 |
| EvL2 | Acanthamoeba Group III | Acanthamoeba lenticulata | T5 | OR767827 |
3.5. Pathogenicity Test
All the strains of A. culbertsoni were pathogenic in mice, presenting a mortality of 40 to 100%. SDC2a had the highest mortality (100%), killing mice in a few days; SDC1 and PDC had 60% and 80% mortality, respectively, killing the mice in a few further days; and EVL1 had the lowest mortality (40%), killing the mice in more days. In contrast, only one of the strains of A. lenticulata had 20% mortality, and the other did not kill any mice. The optimal growth temperature of pathogenic amoebae of A. culbertsoni was 37 °C, while the non-pathogenic amoebae of A. lenticulata grow better at 30 °C (Table 4).
Table 4.
Pathogenicity and temperature tolerance of Acanthamoeba strains isolated from Geothermal Power Plant.
| Strain | Mortality (%) | Death Days | Organs from FLA Were Recovered | Temperature Tolerance (°C) |
|---|---|---|---|---|
| SDC1 | 60 | 15–17 | Brain and Lung | 30, 37 *, 42 |
| SDC2a | 100 | 2–11 | Brain | 30, 37 *, 42 |
| SDC2b | 20 | 16 | Brain and Lung | 30 *, 37, 42 |
| PDC | 80 | 12–13 | Brain and Lung | 30, 37 *, 42 |
| EvL1 | 40 | 18–20 | Lung | 30, 37 *, 42 |
| EvL2 | 0 | - | - | 30 *, 37, 42 |
* Optimal growth temperature.
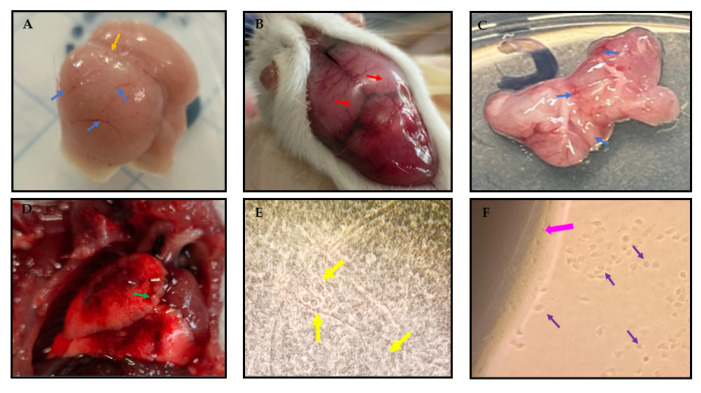
Macroscopic damage was observed in the brain and lung of dead mice, caused by infection with Acanthamoeba culbertsoni. It was proven that the infection was caused by amoebae by inoculating a sample of brain and lung from dead mice on NNA medium, where the amoebae were recovered, and by observing rounded trophozoites in the brain tissue in a brain imprinting from a dead mouse (Figure 4).
Figure 4.
Macroscopic aspects of damage caused by Acanthamoeba culbertsoni infection in mouse brain and lung. (A) Observations were made of the inflammatory process of the brain, the loss of integrity of the central sulcus (orange arrow), and areas with the beginnings of hemorrhagic processes (blue arrows) in a dying mouse brain euthanized 4 days after infection. (B) Mouse brain dead 5 days after infection showed hyperemic meninges (red arrows) that stand out in dorsal view oriented from the central sulcus of the brain and towards the posterior region of the cerebellum. (C) Mouse brain recovered 2 to 4 h after mouse death. Observations were made that the integrity of the brain has been totally lost, the bleeding areas and edema are very evident (blue arrows). (D) Mouse lung dead 12 days after infection; there was abundant hyperemic zone in the anterior lobe; the hemorrhage extended to the central area and in the periphery, and presence of small continuous granules was observed (green arrow). (E) Rounded trophozoites of amoebas (yellow arrows) immersed in brain tissue observed in brain imprinting from a dead mouse. Optical microscope at 40×. (F) Amoeba trophozoites (purple arrows) coming out of the brain of a dead mouse (pink arrow) inoculated on NNA, after 24 h of culture at 37 °C. 10× inverted microscope.
4. Discussion
The presence of some FLA in the hot water of the Geothermal Power Plant indicates the endurance of amoebae at high temperatures. It was observed that Acanthamoeba, Vermamoeba, and Vannella could grow at high temperatures up to 43 and 45 °C [3,11,30,31]. Thermotolerant Acanthamoeba and Vermamoeba (previously Hartmannella) had already been isolated from the cooling lagoon of an electric power plant [5]. The capacity of these amoebae to grow at high temperatures explains their frequency in the Geothermal Power Plant of Cerro Prieto. On the other hand, it was a surprise to find amoebae of the genera Saccamoeba and Thecamoeba, because they had not been reported in hot water.
The pH was alkaline in the water of the channels and was slightly acid in the evaporation lagoon. This is due to the underground origin of the water [14]. Dissolved oxygen was in a range of 1.8 to 5.0 mg L−1; these values were appropriate for the presence of the pathogenic FLA [1].
Geothermal water contains many salts, often in varying concentrations [14]. The evaporation lagoon functions as a temporary reservoir for water extracted from the geothermal reservoir during the generation of electrical energy. The water that circulates through the evaporation lagoon undergoes a continuous evaporation until reaching a maximum average value of 58%. In semi-arid climatic conditions, evaporation plays an important role in the concentration of the salts in water [29]. Thus, the ions in the lagoon water, and therefore the conductivity, showed a great increase in the channels to the evaporation lagoon. The conductivity of the water in the discharge channels was near to that of seawater (5.0 × 104 μS/cm) [32] and was higher in the evaporation lagoon. The values of conductivity were much higher than reported in the irrigation channels (average of 1.5 × 103 μS/cm) [17] and swimming pools (average of 2.4 × 103 μS/cm) [16,18].
The high conductivity of the water restricted the presence of the pathogenic free-living amoebae, only five genera of amoebae were found. Acanthamoeba was found at all sites, even in the evaporation lagoon with the highest conductivity. Thus, it showed great tolerance to high concentrations of salts, and reaffirmed its high resistance to extreme conditions [10,11].
It is important to highlight the absence of Naegleria fowleri in the hot water of the geothermal power plant, being a thermophilic amoeba. This amoeba has been reported in different sites with hot water (a thermally polluted channel, geothermal spring, cooling pond of an electric power plant, cooling reservoir of a nuclear power plant, geothermal recreational baths, and cooling lake of a nuclear reactor) [4,5,6,7,8,9]. However, the high conductivity of the water did not allow the growth of the amoeba. It has been reported that trophozoites of N. fowleri are destroyed in saline concentrations >2% [33], equivalent to 3.1 × 104 µS/cm of conductivity, which is lower than the water conductivity of the geothermal power plant.
Acanthamoeba genotype T10 was the most identified in our isolates, followed by genotype T5. These two genotypes were reported in recreational and domestic water sources in Jamaica and West Indies [34]. Genotype T10 is rarely isolated from the environment and is usually associated with Granulomatous Amoebic Encephalitis, while genotype T5 is more frequently isolated from the environment [35]. This is the first time that Acanthamoeba genotypes T5 and T10 have been reported in the environment in Mexico. Four strains were classified as genotype T10 Acanthamoeba culbertsoni, and two strains belonged to genotype T5 Acanthamoeba lenticulata. This last amoeba had already been isolated from the cooling lagoon of an electric power plant [5].
The strains of A. culbertsoni showed a gradient of pathogenicity, from the least pathogenic, with 40% mortality, to the most pathogenic, with 100% mortality; this behavior had also been observed in A. polyphaga strains in swimming pools [16]. This is significant, because the treated water from the Geothermal Power Plant is returned to the ground and can pollute the aquifer of Mexicali Valley, due to that one part of the aquifer being free, which allows the infiltration to groundwater [36]. The pathogenic amoebae of A. culbertsoni were thermotolerant, and grew well at 37 °C and 42 °C. This agrees with the assertion that pathogenic amoebae are thermophilic [1].
Acanthamoeba is widespread in nature. In Mexico, free-living amoebae (FLA) have been isolated from different environments such as air, thermal waters, physiotherapy tubs, groundwater, tap water, rivers, wastewater, springs, swimming pools, and irrigation channels [16,17,25,37,38,39,40,41,42,43].
The Vermamoeba genus, with only one species, Vermamoeba vermiformis (previously described as Hartmannella vermiformis), has been a subject of increased interest in recent times. This amoeba is one of the most prevalent, ubiquitous, and thermotolerant [3,30,31]. It has been isolated from different aquatic environments [16,39,42,43]. In groundwater and thermal springs, it was more frequently found than Acanthamoeba and Naegleria [39,43] and in textile wastewater, it was almost in the same frequency as Acanthamoeba [42]. There is no clear evidence that it can cause brain infections. The only relationship of Vermamoeba vermiformis with a brain infection was a case of GAE, but the role of the amoeba as a causative agent of the disease could not be proven [44]. However, V. vermiformis has demonstrated an ability to produce a cytopathic effect on keratocytes in vitro [45]. The first case of amoebic keratitis caused by V. vermiformis was reported in a soft contact lens wearer in Iran, and there have been reports of cases of keratitis due to V. vermiformis in conjunction with other amoebae [46]. Furthermore, it is known that V. vermiformis can harbor viruses, bacteria such as Legionella pneumophila, and other pathogen microorganisms [3,31].
Vannella is commonly found in aquatic environments such as freshwater, wastewater, tap water, groundwater, springs, and swimming pools [16,39,40,41,42,43,47]. Vannella has not been reported as a pathogen but was isolated from a keratitis patient. It is a natural host and vehicle of transmission for various intracellular organisms. This amoeba can act as a Trojan horse for microsporidian parasites and other pathogens [48].
Saccamoeba is usually found in soil and in different aquatic environments such as groundwater, streams, and wastewater [39,41,42]. However, some species (S. marina, S. osseosaccus and S. verrucosa) were found in marine water [20,49]. Saccamoeba have not been reported to cause disease, but S. lacustris was found harboring mutualistic rod-shaped gram-negative bacteria [50].
Thecamoeba is present in fresh water, salt water, and wastewater, and is among the largest protozoa in soil. Thecamoeba feed on a variety of protozoa and algae, as well as bacteria, and occupy an important ecological position amongst the microbiota of both aquatic and terrestrial habitats [39,41,42]. It has not been reported as pathogenic.
The presence of some species of Saccamoeba and Thecamoeba in marine water might be the reason that were found in the Geothermal Power Plant.
5. Conclusions
The high temperature, but mainly high conductivity, were the environmental conditions that determined and restricted the presence of the pathogenic free-living amoebae in the Geothermal Power Plant, finding that few amoeba genera were tolerant to salinity. Acanthamoeba was the only amoeba found at all sites, even in the evaporation lagoon with the highest conductivity, confirming its resistance to extreme environmental conditions. Vannella and Vermamoeba were at several sites in the cooling system, also showing tolerance to high temperatures and salinity. The strains of Acanthamoeba culbertsoni were pathogenic; it is an opportunistic amoeba that may affect immunosuppressed individuals; this is significant because the treated water is returned to the ground and can pollute the aquifer. This is the first time that Acanthamoeba genotypes T5 and T10 have been reported in the environment in Mexico.
Acknowledgments
The authors acknowledge the Comisión Federal de Electricidad for support on sampling within the Geothermal Power Plant at Cerro Prieto.
Author Contributions
Conceptualization, P.B.-L. and E.R.-F.; methodology, E.R.-F., P.B.-L., M.M.C.-Y. and S.R.-H.; investigation, E.R.-F., P.B.-L., M.A.R.-F., K.A.B.-G. and M.R.-B.; resources, P.B.-L., E.R.-F. and J.L.-M.; writing—original draft preparation, E.R.-F., P.B.-L. and M.A.R.-F.; writing—review and editing, E.R.-F., P.B.-L. and M.M.C.-Y.; visualization, P.B.-L. and E.R.-F.; supervision, E.R.-F., P.B.-L. and J.L.-M.; project administration, P.B.-L., M.M.C.-Y. and S.R.-H.; funding acquisition, P.B.-L., M.M.C.-Y. and S.R.-H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Pathogenicity tests using mice were in accordance with the Mexican federal regulations for animal experimentation and care (NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City, Mexico), and approved by the ethical standards of the Facultad de Estudios Superiores Iztacala, UNAM (Number of Approval CE/FESI/022020/1317).
Informed Consent Statement
Not applicable.
Data Availability Statement
Research data are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded at ULL by the Consorcio Centro de Investigación Biomédica (CIBER) de Enfermedades Infecciosas (CIBERINFEC); Instituto de Salud Carlos III, 28006 Madrid, Spain (CB21/13/00100); and Cabildo Insular de Tenerife 2023–2028 and Ministerio de Sanidad, Spain.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Visvesvara G.S. Infections with free-living amoebae. Handb. Clin. Neurol. 2013;114:153–168. doi: 10.1016/B978-0-444-53490-3.00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Magnet A., Peralta R.H.S., Gomes T.S., Izquierdo F., Fernandez-Vadillo C., Galvan A.L., Pozuelo M.J., Pelaz C., Fenoy S., Del Águila C. Vectorial role of Acanthamoeba in Legionella propagation in water for human use. Sci. Total Environ. 2015;505:889–895. doi: 10.1016/j.scitotenv.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 3.Scheid P.L. Vermamoeba vermiformis—A free-living amoeba with public health and environmental health significance. Open Parasitol. J. 2019;7:40–47. doi: 10.2174/1874421401907010040. [DOI] [Google Scholar]
- 4.De Jonckheere J., Van Dijck P., Van de Voorde V. The effect of thermal pollution on the distribution of Naegleria fowleri. J. Hyg. 1975;75:7–13. doi: 10.1017/S0022172400047021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dive D., Delattre J.M., Leclerc H. Occurrence of thermotolerant amoebae in an electric power plant cooling pond. J. Therm. Biol. 1982;7:11–14. doi: 10.1016/0306-4565(82)90013-4. [DOI] [Google Scholar]
- 6.Huizinga H.W., McLaughlin G.L. Thermal ecology of Naegleria fowleri from a power plant cooling reservoir. Appl. Environ. Microbiol. 1990;56:2200–2205. doi: 10.1128/aem.56.7.2200-2205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montalbano D.F., Novelletto M.A., Di Cave D., Berrilli F. Identification and phylogenetic position of Naegleria spp. from geothermal springs in Italy. Exp. Parasitol. 2017;183:143–149. doi: 10.1016/j.exppara.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Moussa M., De Jonckheere J.F., Guerlotté J., Richard V., Bastaraud A., Romana M., Talarmin A. Survey of Naegleria fowleri in geothermal recreational waters of Guadeloupe (French West Indies) PLoS ONE. 2013;8:e54414. doi: 10.1371/journal.pone.0054414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyndall R.L., Ironside K.S., Metler P.L., Tan E.L., Hazen T.C., Fliermans C.B. Effect of thermal additions on the density and distribution of thermophilic amoebae and pathogenic Naegleria fowleri in a newly created cooling lake. Appl. Environ. Microbiol. 1989;55:22–32. doi: 10.1128/aem.55.3.722-732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd D. Encystment in Acanthamoeba castellanii: A Review. Exp. Parasitol. 2014;145:S20–S27. doi: 10.1016/j.exppara.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Delafont V., Bouchon D., Héchard Y., Moulin L. Environmental factors shaping cultured free-living amoebae and their associated bacterial community within drinking water network. Water. 2016;100:382–392. doi: 10.1016/j.watres.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Canals O., Serrano-Suárez A., Salvadó H., Méndez J., Cervero-Aragó S., Ruiz de Porras V. Effect of chlorine and temperature on free-living protozoa in operational man-made water systems (cooling towers and hot sanitary water systems) in Catalonia. Environ. Sci. Pollut. Res. 2015;22:6610–6618. doi: 10.1007/s11356-014-3839-y. [DOI] [PubMed] [Google Scholar]
- 13.Kagel A., Bates D., Gawell K. Guide to Geothermal Energy and the Environment. United States Department of Energy; Washington, DC, USA: 2005. Technical Report. [DOI] [Google Scholar]
- 14.Morales-Arredondo I., Armienta M.A., Segovia N. Groundwater chemistry and overpressure evidence in Cerro Prieto Geothermal Field. Geofluids. 2017;2017:e2395730. doi: 10.1155/2017/2395730. [DOI] [Google Scholar]
- 15.American Public Health Association. American Water Works Association. Water Environment Federation . Standard Methods for the Examination of Water and Wastewater. 19th ed. American Public Health Association; Washington, DC, USA: 1995. [Google Scholar]
- 16.Robles E., Ramírez E., Sáinz M.G., Martínez B., Ayala R., González M.E. Microbiological and physico-chemical study of swimming pool water. Int. Res. J. Adv. Eng. Sci. 2019;4:15–20. doi: 10.5281/zenodo.3490477. [DOI] [Google Scholar]
- 17.Bonilla-Lemus P., Rojas-Hernández S., Ramírez-Flores E., Castillo-Ramírez D.A., Monsalvo-Reyes A.C., Ramírez-Flores M.A. Barrón-Graciano, K.; Reyes-Batlle, M.; Lorenzo-Morales, J.; Carrasco-Yépez, M.M. Isolation and identification of Naegleria species in irrigation channels for recreational use in Mexicali Valley, Mexico. Pathogens. 2020;9:820. doi: 10.3390/pathogens9100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes-Batlle M., Gabriel M.F., Rodríguez-Expósito R., Felgueiras F., Sifaoui I., Mourão Z., de Oliveira Fernandes E., Piñero J.E., Lorenzo-Morales J. Evaluation of the occurrence of pathogenic free-living amoeba and bacteria in 20 public indoor swimming pool facilities. Microbiol. Open. 2021;10:e1159. doi: 10.1002/mbo3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauer G., Rogerson A., Anderson O.R. Platyamoeba pseudovannellida n. sp., a naked amoeba with wide salt tolerance isolated from the Salton Sea, California. J. Eukaryot. Microbiol. 2001;48:663–669. doi: 10.1111/j.1550-7408.2001.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 20.Hauer G., Rogerson A. Remarkable salinity tolerance of seven species of naked amoebae (Gymnamoebae) Hydrobiologia. 2005;549:33–42. doi: 10.1007/s10750-005-2210-1. [DOI] [Google Scholar]
- 21.Rogerson A., Hauer G. Naked Amoebae (Protozoa) of the Salton Sea, California. Hydrobiologia. 2002;473:161–177. doi: 10.1023/A:1016594021391. [DOI] [Google Scholar]
- 22.Smirnov A.V. Vertical distribution and abundance of Gymnamoebae amoebae (Rhizopoda) in bottom sediments of the Brackish Water Nivå Bay (Baltic Sea, The Sound) Protist. 2002;153:239–250. doi: 10.1078/1434-4610-00101. [DOI] [PubMed] [Google Scholar]
- 23.Smirnov A.V. Cryptic freshwater amoeba species in the bottom sediments of Nivå Bay (Øresund, Baltic Sea) Eur. J. Protistol. 2007;43:87–94. doi: 10.1016/j.ejop.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 24.SEMARNART. CFE . Proyecto Geotermoeléctrico Cerro Prieto. Manifestación de Impacto Ambiental. Modalidad Particular, Secretaría de Manejo de Recursos Naturales; Mexico: 2007. [(accessed on 27 August 2023)]. Available online: http://sinat.semarnat.gob.mx. [Google Scholar]
- 25.Rivera F., Roy-Ocotla G., Rosas I., Ramirez E., Bonilla P., Lares F. Amoebae isolated from the atmosphere of Mexico City and environs. Environ. Res. 1987;42:149–154. doi: 10.1016/S0013-9351(87)80016-6. [DOI] [PubMed] [Google Scholar]
- 26.Page F.C. A New Key to Freshwater and Soil Gymnamoebae: With Instructions for Culture. Freshwater Biological Association; Cumbria, UK: 1988. [Google Scholar]
- 27.De Jonckheere J.F. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl. Environ. Microbiol. 1980;39:681–685. doi: 10.1128/aem.39.4.681-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder J.M., Booton G.C., Hay J., Niszl I.A., Seal D.V., Markus M.B., Fuerst P.A., Byers T.J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoeba from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001;39:1903–1911. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portugal E., Pal Verma M. Hydrochemistry of the evaporation lagoon in Cerro Prieto, Baja California, Mexico. Hidroquímica de la laguna de evaporación en Cerro Prieto, Baja California, México. Ing. Hidráulica México. 2001;XVI:153–173. [Google Scholar]
- 30.Delafont V., Rodier M.H., Maisonneuve E., Cateau E. Vermamoeba vermiformis: A free-living amoeba of interest. Microb. Ecol. 2018;76:991–1001. doi: 10.1007/s00248-018-1199-8. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui R., Makhlouf Z., Khan N.A. The increasing importance of Vermamoeba vermiformis. J. Eukaryot. Microbiol. 2021;68:e12857. doi: 10.1111/jeu.12857. [DOI] [PubMed] [Google Scholar]
- 32.Mantyla A.W. Comparaciones de estándares de agua de mar. J. Phys. Oceanogr. 1987;17:543–548. doi: 10.1175/1520-0485(1987)017<0543:SSCU>2.0.CO;2. [DOI] [Google Scholar]
- 33.Tiewchaloren S., Junnu V. Factors affecting the viability of pathogenic Naegleria species isolated from Thai patients. J. Trop. Med. Parasitol. 1999;22:172–178. [Google Scholar]
- 34.Todd C.D., Reyes-Batlle M., Piñero J.E., Martínez-Carretero E., Valladares B., Streete D., Lorenzo-Morales J., Lindo J.F. Isolation and molecular characterization of Acanthamoeba genotypes in recreational and domestic water sources from Jamaica, West Indies. J. Water Health. 2015;13:909–919. doi: 10.2166/wh.2015.232. [DOI] [PubMed] [Google Scholar]
- 35.Booton G.C., Visvesvara G.S., Byers T.J., Kelly D.J., Fuerst P.A. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J. Clin. Microbiol. 2005;43:1689–1693. doi: 10.1128/JCM.43.4.1689-1693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CONAGUA . Actualización de la Disponibilidad Media Anual en el Acuífero Valle de Mexicali (0210), Estado de Baja California. CONAGUA, Subdirección General Técnica, Gerencia De Aguas Subterránea; Ciudad de Mexico, Mexico: 2020. [Google Scholar]
- 37.Rivera F., Lares F., Gallegos E., Ramirez E., Bonilla P., Calderon A., Martinez J.J., Rodriguez S., Alcocer J. Pathogenic amoebae in natural thermal waters of three resorts of Hidalgo, Mexico. Environ. Res. 1989;50:289–295. doi: 10.1016/S0013-9351(89)80010-6. [DOI] [PubMed] [Google Scholar]
- 38.Rivera F., Ramirez E., Bonilla P., Calderon A., Gallegos E., Rodriguez S., Ortiz R., Zaldívar B., Ramírez P., Duran A.N. Pathogenic and free-living amoebae isolated from swimming pools and physiotherapy tubs in Mexico. Environ. Res. 1993;62:43–52. doi: 10.1006/enrs.1993.1087. [DOI] [PubMed] [Google Scholar]
- 39.Ramírez E., Robles E., Sáinz M.G., Ayala R., Campoy E. Microbiological quality of the aquifer of Zacatepec, Morelos, Mexico. Calidad microbiológica del acuífero de Zacatepec, Morelos, México. Rev. Int. Contam. Ambient. 2009;5:98–105. [Google Scholar]
- 40.Bonilla-Lemus P., Ramírez-Bautista G.A., Zamora-Muñoz C., Ibarra-Montes M.R., Ramírez-Flores E., Hernández-Martínez M.D. Acanthamoeba spp. in domestic tap water in houses of contact lens wearers in the Metropolitan Area of Mexico City. Exp. Parasitol. 2010;126:54–58. doi: 10.1016/j.exppara.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Bonilla-Lemus P., Caballero A., Carmona J., Lugo A. Occurrence of free-living amoebae in streams of the Mexico Basin. Exp. Parasitol. 2014;145:S28–S33. doi: 10.1016/j.exppara.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Ramírez E., Robles E., Martínez B., Ayala R., Sáinz M.G., Martínez M.E., Gonzalez M.E. Distribution of free-living amoebae in a treatment system of textile industrial wastewater. Exp. Parasitol. 2014;145:S34–S38. doi: 10.1016/j.exppara.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Ramírez E., Robles E., Martínez M.E., Sáinz M.G., Martínez B., Rivas B.I., Rocha A. Distribution of free-living amoebae in springs in Morelos, Mexico. Glob. Adv. Res. J. Microbiol. 2016;5:57–67. [Google Scholar]
- 44.Centeno M., Rivera F., Cerva L., Tsutsumi V., Gallegos E., Calderon A., Ortiz R., Bonilla P., Ramirez E., Suarez G. Hartmannella vermiformis isolated from the cerebrospinal fluid of a young male patient with meningoencephalitis and bronchopneumonia. Arch. Med. Res. 1996;27:579–586. [PubMed] [Google Scholar]
- 45.Kinnear F.B. Cytopathogenicity of Acanthamoeba, Vahlkampfia and Hartmannella: Quantitative and qualitative in vitro studies on keratocytes. J. Infect. 2003;46:228–237. doi: 10.1053/jinf.2002.1116. [DOI] [PubMed] [Google Scholar]
- 46.Abedkhojasteh H., Niyyati M., Rahimi F., Heidari M., Farnia S., Rezaeian M. First Report of Hartmannella keratitis in a cosmetic soft contact lens wearer in Iran. Iran. J. Parasitol. 2013;8:481–485. [PMC free article] [PubMed] [Google Scholar]
- 47.Todd C.H.D., Reyes-Batlle M., Valladares B., Lindo J.F., Lorenzo-Morales J. Vannellid species isolated from freshwater source in a park in Jamaica, West Indies. Microbiol. Insights. 2015;8:7–9. doi: 10.4137/MBI.S30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheid P. Mechanism of intrusion of a microspordian-like organism into the nucleus of host amoebae (Vannella sp.) isolated from a keratitis patient. Parasitol. Res. 2007;101:1097–1102. doi: 10.1007/s00436-007-0590-z. [DOI] [PubMed] [Google Scholar]
- 49.Anderson O.R., Rogerson A., Hannah F. Three new limax amoebae isolated from marine surface sediments: Vahlkampfia caledonica N. Sp., Saccamoeba marina N. Sp., and Hartmannella vacuolata N. sp. J. Eukaryot. Microbiol. 1997;44:33–42. doi: 10.1111/j.1550-7408.1997.tb05688.x. [DOI] [Google Scholar]
- 50.Corsaro D., Michel R., Walochnik J., Müller K.D., Greub G. Saccamoeba lacustris sp. nov. (Amoebozoa: Lobosea: Hartmannellidae), a new lobose amoeba, parisitized by the novel chlamydia ´Candidatus Metachlamydia lacustris´ (Chlamydiae: Parachlamydiaceae) Eur. J. Protistol. 2010;46:86–95. doi: 10.1016/j.ejop.2009.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are included in the article.