Abstract
A survival strategy operating in the absence of the host was shown in obligately biotrophic arbuscular mycorrhizal (AM) symbionts. When no host-derived signals from the surrounding environment were perceived by germinating spores, fungal hyphae underwent a programmed growth arrest and resource reallocation, allowing long-term maintenance of viability and host infection capability. The early stages of mycelial growth of AM fungi were studied by a combination of time-lapse and video-enhanced light microscopy, image analysis, and immunodetection, with the aim of acquiring knowledge of cell events leading to the arrest of mycelial growth. The time-course of growth arrest was resolved by precisely timing the growth rate and magnitude of the mycelium originating from individual spores of Glomus caledonium. Extensive mycelial growth was observed during the first 15 days; thereafter, fungal hyphae showed retraction of protoplasm from the tips, with formation of retraction septa separating viable from empty hyphal segments. This active process involved migration of nuclei and cellular organelles and appeared to be functional in the ability of the fungus to survive in the absence of a host. Immunodetection of cytoskeletal proteins, metabolic activity, and the retention of infectivity of germinated spores confirmed the developmental data. The highest amounts of tubulins were detected when hyphal growth had ceased but when retraction of protoplasm was most active. This was consistent with the role of the cytoskeleton during protoplasm retraction. Succinate dehydrogenase activity in hyphae proximal to the mother spore was still detectable in 6-month-old mycelium, which remained viable and able to form appressoria and produce symbiotic structures.
Arbuscular mycorrhizal (AM) fungi are obligately biotrophic organisms, widespread in soil, which live symbiotically with most plant roots. It has been calculated that they are able to colonize the roots of 80% of plant species belonging to all phyla of land plants (29).
Fossil records and DNA sequence data have provided evidence that arbuscular mycorrhizas were established as early as 410 million to 360 million years ago (24, 25, 27). Because of this long-lasting coevolution with their host plants, AM fungi have evolved unequivocal mechanisms of host recognition which play a fundamental role in their life cycle. Only in the presence of signals released by host roots does morphogenetic differentiation of infection structures occur, allowing a functional symbiosis to be established (8, 11). Despite this, the recognition process does not involve specific regulation of the germination event by host-derived signals. AM fungal spores are capable of germination and growth from a quiescent-like state in response to different edaphic and environmental conditions, irrespective of the presence of host plants (7, 12, 15, 19, 20, 30). This is contrary to findings described for other biotrophs, such as some pathogenic fungi, whose resting structures germinate only as a direct response to the presence of host plants (4, 5). Such behavior by AM fungal spores is apparently inconsistent, given that in the absence of the host these fungi are not capable of extensive independent mycelial growth and consequently of completion of the life cycle (8, 13, 19, 21). Thus, many authors have observed that germlings of different AM fungal species ceased growth within 15 days of germination (2, 9, 13, 26).
The fate and behavior of individual AM fungal spores that germinate in the absence of appropriate hosts may affect the survival of individuals and populations. However, this fundamental event in the life cycle of AM fungi has so far not been adequately investigated. In this work we studied the early stages of mycelial growth of AM fungi, adopting a combination of time-lapse and video-enhanced light microscopy, image analysis, and immunodetection in order to acquire knowledge of cell events leading to the arrest of mycelial growth in the absence of host plants.
Our work was aimed at investigating (i) the time course of fungal growth in the absence of the host, from spore germination to growth arrest; (ii) cell events involved in developmental arrest; (iii) metabolic activity of the mycelium after growth arrest and its ability to restore growth and to differentiate infection structures; and (iv) differential expression of cytoskeletal proteins during the various growth phases.
MATERIALS AND METHODS
Fungal material.
Spores of Glomus caledonium (Nicolson et Gerdemann) Trappe et Gerdemann (Rothamsted isolate) and spores or sporocarps of Glomus mosseae (Nicolson et Gerdemann) Gerdemann et Trappe (Kent isolate) were obtained from the collection of the Dipartimento di Chimica e Biotecnologie Agrarie, University of Pisa, Italy. Voucher specimens of these isolates were deposited in the Herbarium of the Department of Botanical Sciences, University of Pisa, Herbarium Horti Botanici Pisani (PI), respectively, as PI-HMZ 8 and PI-HMZ 4.
Time course of hyphal growth in the absence of the host plant.
Spores of G. caledonium were surface sterilized with 2% chloramine T supplemented with streptomycin (400 μg ml−1) for 20 min, rinsed five times in sterile water, put on 20- by 20-mm cellophane membranes (Hoefer, San Francisco, Calif.), and placed on 1% water agar (WA) in 9-cm-diameter petri dishes. After 4 days’ incubation in the dark at 28°C, spores were checked for germination. Cellophane membranes bearing germinated spores were transferred to 5.5-cm-diameter petri dishes on a thin layer of 1% WA for growth measurements, which were carried out under a Reichert-Jung Polyvar light microscope. Hyphal length was assessed by using Quantimet 500 image analysis software (Leica, Milan, Italy).
The lengths of both protoplasm-containing and empty mycelium were measured daily on a group of 12 spores, up to the time of growth arrest. The mean growth rate of the mycelium during the early phase was calculated on eight spores by dividing the mycelial length by the elapsed time (about 6 days). The length of total mycelium of a group of 12 spores was measured monthly, up to 6 months.
Time-lapse video microscopy.
Surface-sterilized spores of G. caledonium and G. mosseae were germinated as previously described. After 1 week of incubation, individual spores were mounted on microscope slides in sterile distilled water (SDW). The coverslips were sealed with 1% WA, which was periodically wetted with SDW. With this method, individual spores could be held for more than 6 h, allowing the entire process of septum formation to be monitored. Spores were observed under a Polyvar light microscope equipped with differential interference contrast optics and with a 3 CCD color video camera connected to a videocassette recorder (Hi 8, EV-C2000E; Sony Co., Tokyo, Japan) and to a videographic printer (UP-890 CE; Sony Co.).
To observe vacuoles and vacuolar strands in hyphal tips at different stages of protoplasm retraction, 1-month-old germinated spores were stained with the fluorescent dye MDY-64 (yeast vacuole membrane marker; Molecular Probes, Inc., Eugene, Oreg.) (10 μM) in dimethyl sulfoxide–10 mM HEPES buffer, pH 7.4. Other spores were stained with the cyanine dye 3,3′-dihexyloxacarbocyanine iodide (Sigma Chemical Co., St. Louis, Mo.) (0.87 μM) in dimethyl sulfoxide-water. All spores were observed under epifluorescence by using a Polyvar microscope equipped with an HBO 200 mercury lamp, with filter combination B1 (BP 450-495, LP 520, DS 510).
To visualize the occurrence and location of nuclei and mitochondria in hyphae during the phase of protoplasm retraction, 1-month-old germinated spores were mounted on microscope slides in diamidinophenylindole (DAPI) (5 μg/ml) in a 1:1 water-glycerol solution and observed under epifluorescence, with filter combination U1 (BP 330-380, LP 418, DS 420).
Immunoblotting of cytoskeletal proteins.
To yield comparable amounts of total proteins from mycelium of different ages and from ungerminated spores, different numbers of fungal propagules were used: 300 sporocarps of G. mosseae and 1,000 spores of G. caledonium were incubated for 30 days; 350 sporocarps of G. mosseae and 2,000 spores of G. caledonium were incubated for 15 days; and 400 sporocarps of G. mosseae and 2,000 spores of G. caledonium were utilized as nongerminated controls.
One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out with a discontinuous buffer system (18). Extracts (4 μg of protein) were loaded onto linear 12% polyacrylamide gels (10 by 8 by 0.75 mm) and run in a Bio-Rad (Richmond, Calif.) Mini Protean II slab cell system.
Immunodetection of cytoskeletal proteins was carried out as previously described (1). After electrophoresis, the proteins were transferred to nitrocellulose membranes by using a Trans-Blot apparatus (Bio-Rad). Electrotransfer was performed first overnight at 100 mA, 20 V, and then for 1 h at 350 mA, 100 V, at 0°C. Nitrocellulose sheets were then blocked for 1 h in 2% bovine serum albumin in phosphate-buffered saline (PBS) (pH 7.3) and incubated for 1 h at room temperature with mouse monoclonal anti-α- and anti-β-tubulin and antiactin antibodies, which were diluted 1:500 in PBS containing 0.1% bovine serum albumin and 0.1% Triton X-100. Bound antibodies were visualized with rabbit anti-mouse immunoglobulin G antibody conjugated to peroxidase and diluted 1:3,000 in PBS containing 0.1% Triton X-100. The peroxidase reaction was carried out with 0.3 mg of 3,3′-diaminobenzidine per ml in 1:1 (vol/vol) Tris-HCl (100 mM) (pH 7.6)–water, supplemented with 25 μl of H2O2 immediately prior to use. Color development was stopped by rinsing the membranes with SDW.
Metabolic activity and infectivity of 1- to 6-month-old mycelium.
Succinate dehydrogenase (SDH) activity was assessed, as described by Smith and Gianinazzi-Pearson (28), on 1- to 6-month-old germinated spores of G. caledonium.
Surface-sterilized spores were prepared as described previously, and at least five germinated spores were utilized for each assay. The lengths of hyphae showing formazan salt depositions on 6-month-old mycelium and the numbers of stained and unstained hyphal tips on 1- to 6-month-old mycelium were recorded by using a Polyvar light microscope equipped with Quantimet 500 image analysis software.
To test infectivity by the mycelium, surface-sterilized spores were germinated as previously described and individually used to inoculate the roots of six 2-week-old Ocimum basilicum L. (basil) plants placed on a 47-mm-diameter Millipore membrane (0.45-μm-diameter pores). Another Millipore membrane was used to make up a sandwich system. Ten replicates were set up for each trial, which consisted of 1-, 2-, 3-, 4-, 5-, and 6-month-old germinated spores. One month after inoculation, sandwiches were carefully opened and roots were stained to reveal fungal structures (23). The number of entry points formed by each germinated spore was recorded for each plant root system and for each sandwich.
RESULTS
Time course of hyphal growth in the absence of the host plant.
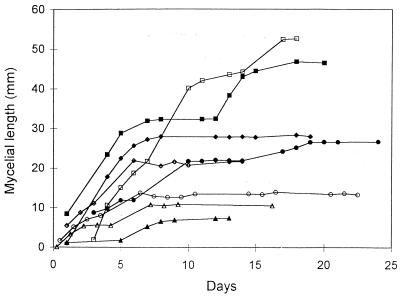
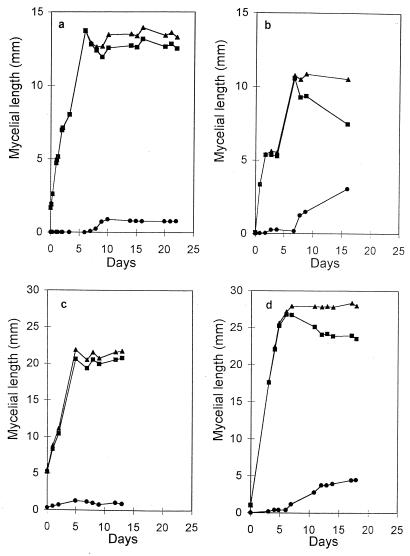
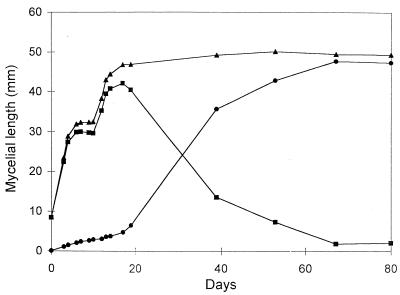
Observations carried out for 15 to 20 days on spores of G. caledonium growing in the absence of the host showed that within a few days the elongating germ tubes gave rise to a mycelial network whose extension was highly variable between individuals. After a period ranging between 5 and 10 days postgermination, total hyphal length reached a plateau and the mycelium entered a state of developmental arrest (Fig. 1). The mean growth rate of the mycelium during this early phase was 1.97 ± 0.39 μm/min (mean ± standard error). An interesting feature of the mycelial net was the occurrence of many hyphae completely devoid of protoplasm. Time course observations showed that during the plateau growth phase, corresponding to arrest of hyphal elongation, fundamental modifications occurred in the mycelium: protoplasm was retracted from most hyphae, leading to the formation of cross walls delimiting an empty mycelial network. This phenomenon was further studied by daily measurements of the extension of protoplasm-containing hyphae and empty hyphae. These showed that when total hyphal growth reached the plateau, the length of protoplasm-containing mycelium remained constant (Fig. 2a and c) or tended to decrease (Fig. 2b and d). Long-term observations, up to 80 days, showed that this decrease was paralleled by rising values of the length of empty hyphae (Fig. 3).
FIG. 1.
Growth curves of mycelia developing from eight individual spores of G. caledonium.
FIG. 2.
Time courses of mycelial growth of four individual spores of G. caledonium. Lengths of total (▴), protoplasm-containing (■), and empty (•) mycelium are shown.
FIG. 3.
Long-term time course of mycelium developing from an individual spore of G. caledonium. Lengths of total (▴), protoplasm-containing (■), and empty (•) mycelium are shown.
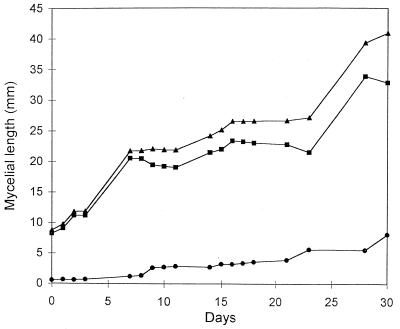
In some spores, a renewal of mycelial elongation following growth arrest was observed. An example is shown in Fig. 4: the spore, 23 days old, resumed growth after 16 days of stasis. Accordingly, assessment of spore growth was carried out monthly, for a period ranging from 1 to 6 months after germination. The results showed that total mycelial length increased from the 1st to the 3rd month (from 45.13 ± 2.94 to 68.19 ± 6.05 mm, respectively), remaining constant up to the 6th month (70.83 ± 7.08 mm).
FIG. 4.
Long-term time course of mycelium developing from an individual spore of G. caledonium. Lengths of total (▴), protoplasm-containing (■), and empty (•) mycelium are shown.
Formation of retraction septa.
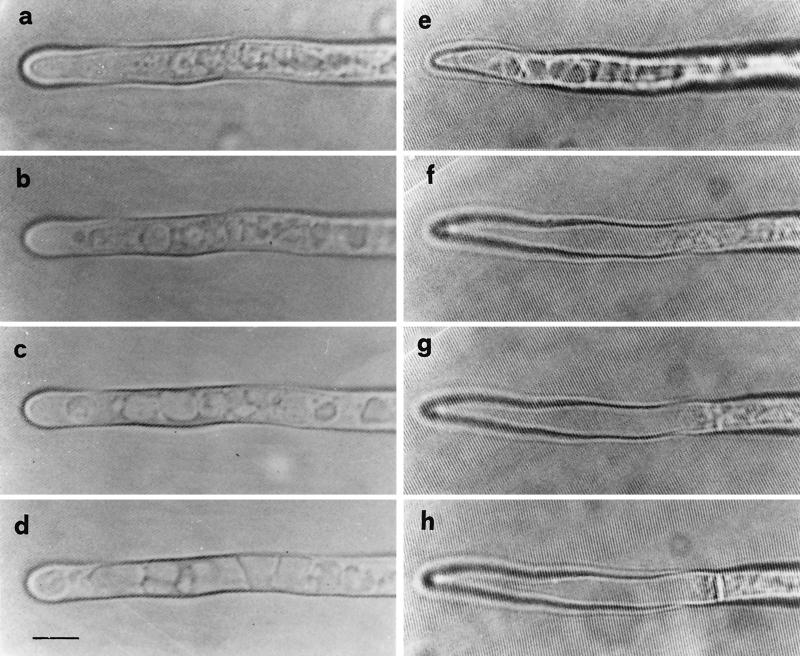
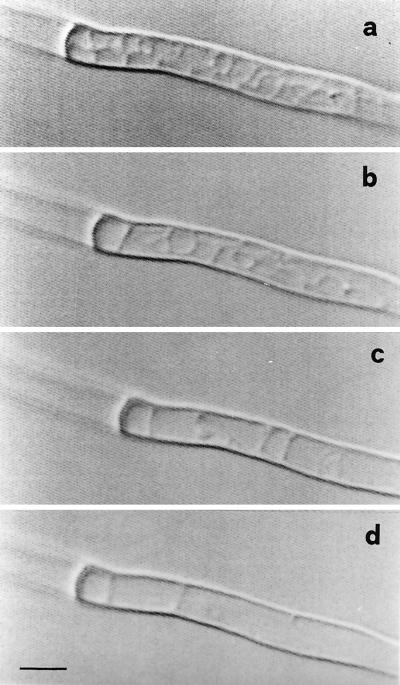
Time-lapse video microscopy provided evidence of the phenomenon of withdrawal of protoplasm from the hyphal tip and the formation of retraction septa. The tip, usually rich in dense granular protoplasm, initiated vacuolization from the apical zone. Vacuoles, usually small when observed in protoplasm of growing hyphae, became progressively enlarged in protoplasm-retracting hyphae, leading to the formation of empty areas (Fig. 5). In the distal zone of the apex, a steady dense area of protoplasm became evident where a cross wall was eventually formed. Protoplasm retraction followed by the formation of further septa occurred in the same way, and the completion of each empty portion (delimited by septa) was carried out in about 2 to 3 h (Fig. 6).
FIG. 5.
Time-lapse video microscopy showing protoplasm retraction and septum formation in apical segments of two G. caledonium hyphae. Note the vacuole extension process (a to d), leading to the formation of empty tips (e to h). Bar, 6.25 μm.
FIG. 6.
Time-lapse video microscopy showing the vacuole extension process accompanying protoplasm retraction (a to c) and leading to the formation of an empty hyphal segment (d) in G. caledonium. Bar, 6.25 μm.
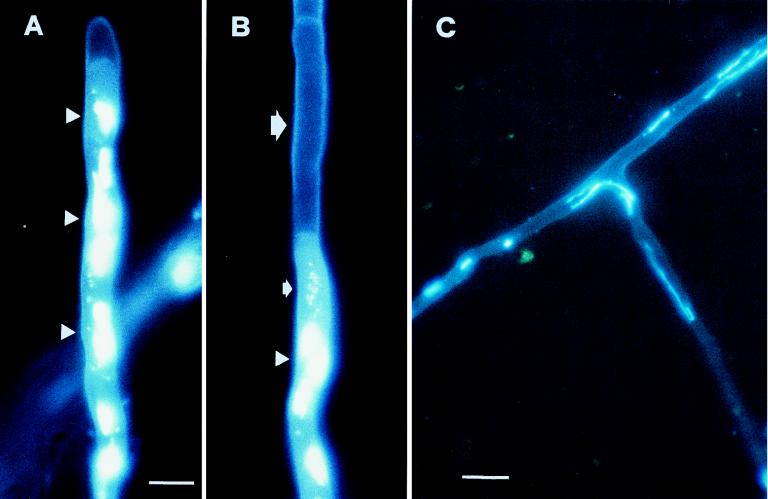
DAPI staining and epifluorescence microscopy showed that the active process of protoplasm retraction also involved the migration of nuclei and cellular organelles (Fig. 7).
FIG. 7.
Epifluorescence microscopy of nuclei (visualized by staining with DAPI) of G. mosseae hyphae. (A) Nuclei (arrowheads) located below the first retraction septum. Note the hyphal tip completely devoid of protoplasm. (B) Empty intercalary hyphal segment (large arrow), separated by a septum from the contiguous tract containing nuclei (arrowhead) and mitochondria (small arrow). (C) Elongated nuclei occurring in hyphae showing protoplasm retraction. Bars, 7.1 μm (A and B) and 12.5 μm (C).
Cytoskeletal proteins.
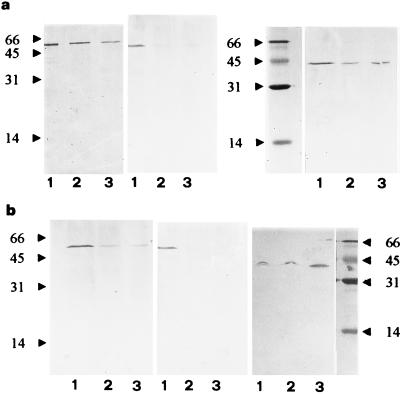
Proteins extracted from ungerminated spores or spore mycelium of the AM fungi G. mosseae and G. caledonium, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunostained after Western blotting for α- and β-tubulins and actin, revealed one band for each cytoskeletal protein in mycelial samples, whereas signals from ungerminated spores were very weak or absent (Fig. 8). A weaker signal was more regularly detected for α-tubulin than for β-tubulin in both fungal species, but their mobilities were similar, with one band of Mr 55.5 × 103 to 56.5 × 103. The actin antibody revealed one band of Mr 41.5 × 103 to 43 × 103, typical of actin. The signals for α- and β-tubulin and actin of mycelium incubated for 30 days appeared stronger than those of mycelium incubated for 15 days in both fungal species (Fig. 8).
FIG. 8.
Immunoblots of β-tubulin, α-tubulin, and actin from 30- (lanes 1) and 15 (lanes 2)-day-incubated mycelium and from ungerminated spores (lanes 3) of G. caledonium (a) and G. mosseae (b). Molecular weight markers were stained with amido black.
Metabolic activity and infectivity of 1- to 6-month-old mycelium.
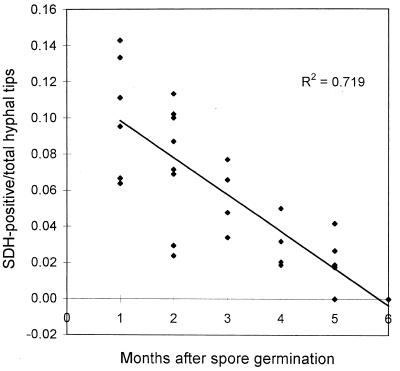
Viability of the mycelium was determined in 6-month-old germinated spores by evaluating SDH activity. The mean percentage of SDH-positive hyphae was 41.98 ± 14.19 (after angular transformation of data). Metabolic activity declined steeply with increasing distance from the spore, with little SDH activity in distal hyphal branches. These results were confirmed by the number of active hyphal tips recorded on 1- to 6-month-old germinated spores. Fractions of SDH-positive hyphal tips were inversely correlated with the age of the mycelium (Fig. 9).
FIG. 9.
Relationship between the fraction of SDH-positive hyphal tips and time after spore germination in G. caledonium mycelium. The solid line indicates the result of linear regression analysis.
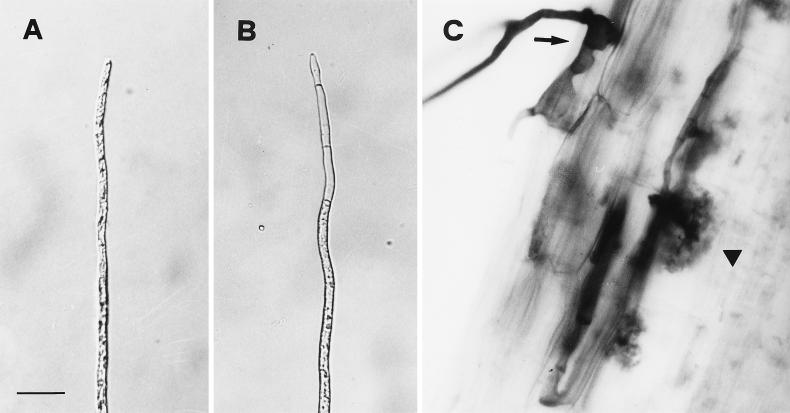
Germinated spores retained the ability to infect plants for several months, but infectivity decreased greatly after the 3rd month. The total number of entry points formed by 1-, 2-, and 3-month-old mycelium (13.10 ± 3.83) was significantly different from that formed by 4-, 5-, and 6-month-old mycelium (1.97 ± 0.50). After 6 months of growth, a low number of protoplasm-containing hyphal tips (Fig. 10A and B) which were still capable of infecting host roots (Fig. 10C) was observed.
FIG. 10.
Light micrographs of hyphae and mycorrhizal infection by G. caledonium of O. basilicum. Viable (A) and empty (B) hyphal tips of 6-month-old mycelium are shown. (C) Viable tip producing appressoria (arrow) capable of root colonization and arbuscule formation (arrowhead). Bar, 25, 25, and 27.8 μm (for panels A, B, and C, respectively).
DISCUSSION
The results of our work led to the identification of a survival strategy in obligately biotrophic AM symbionts operating in the absence of the host. When no host-derived signal from the surrounding environment is perceived by germinating spores, fungal hyphae undergo a programmed growth arrest and resource reallocation, allowing long-term maintenance of viability and host infection capability.
In our study, we resolved the time course of growth arrest by precisely timing the growth length of the mycelium originating from individual spores. In the absence of the host, the AM fungus G. caledonium, maintained in axenic culture, produced extensive mycelial growth during the first 15 days but thereafter underwent developmental arrest, although it proved able to sustain further limited mycelial growth up to 3 months after spore germination. Previous short-term studies showed that in the absence of host roots, hyphal growth of G. mosseae, Gigaspora margarita, and Glomus etunicatum ceased within 15 days of germination (2, 9, 13, 26). Interestingly, growth arrest occurred regardless of the amount of mycelium produced, suggesting that the growth period could be a critical factor involved in growth arrest.
The mycelium produced by all spores examined underwent the process of protoplasm retraction with formation of retraction septa, separating viable from empty hyphal segments. The occurrence of retraction septa in AM fungi has been previously mentioned by a few authors (19, 22), but the cellular events involved in the phenomenon were not described in detail. The dynamics of formation of retraction septa in various other fungal species and genera were described precisely by Ingold (14). In the present study, we documented the same phenomenon occurring in AM fungi by using time-lapse video microscopy. Our studies detected an interesting feature of this phenomenon: in G. caledonium, retraction of protoplasm was initiated from the hyphal tips, which eventually became isolated by the formation of retraction septa; this is in contrast to findings reported for other fungi, where the hyphal segments proximal to the germinating propagule appeared to be empty and septate (14). In both cases, the early cue indicating the initiation of retraction of protoplasm from hyphal tips was the formation and progressive extension of vacuoles, followed by development of a septum.
Previous studies reported that prolonged incubation of spores in the soil without host plants induced a progressive increase in the proportion of empty hyphae (31). Decreasing amounts of free sterols and a parallel increase in bound sterols were observed in G. caledonium germinated spores after 14-day growth in axenic culture, suggesting the occurrence of a senescence phase in the mycelium of AM fungi, as described for other fungal species (3, 6).
Systematic observation of mycelial growth in axenic conditions suggested that the formation of retraction septa might be associated not only with mycelial senescence and aging but also with a mechanism involving control over allocation of the limited energy resources of spores. The active process of protoplasmic retraction, involving migration of nuclei and cellular organelles, appears to have a function in enhancing the fungal ability to survive in the absence of a carbon donor.
Data on immunodetection of cytoskeletal proteins and on metabolic activity and retention of infectivity of germinated spores confirmed this behavioral explanation. Immunoblotting of cytoskeletal proteins showed that signal strengths for α- and β-tubulins and actin increased with mycelial age, in contrast to the growth pattern of germinated spores, which showed a growth arrest as early as 10 days after germination (Fig. 1). It is noteworthy that the strongest signals for tubulins were detected in 30-day-incubated mycelium, when hyphal growth had ceased but protoplasmic retraction activity was at its highest, as demonstrated by long-term observations and video microscopy. These results suggest the occurrence of higher amounts of cytoskeletal proteins in old mycelium, consistently with their role in movements of nuclei and organelles and transport during protoplasm retraction (Fig. 7C).
Moreover, SDH activity in hyphae proximal to the mother spore was still detectable in 6-month-old mycelium. These data showed that after the spore had carried out a prolonged exploration of the surrounding environment and reached the phase of growth arrest, a variable length of mycelium with metabolic activity was maintained. This confirmed our previous results showing that 26-day-old mycelium was resting, still viable, and capable of renewed growth in response to host roots (10).
The long-term ability of AM mycelium to retain infectivity in the absence of the host was tested in soil by Tommerup (31), who showed that after a 4-month incubation, germinated spores of G. caledonium and Acaulospora laevis could develop infection structures and colonize Trifolium roots. Experimental evidence presented here indicates that the longevity of individual germinated spores can be even greater, since G. caledonium mycelium remained viable and able to form appressoria and produce symbiotic structures for as long as 6 months after germination. The decreasing ability of germinated spores to form infection structures on host roots could be due to the correlated decrease in the number of protoplasm-containing hyphal tips. The progressive retraction of protoplasm from hyphae and its reallocation proximally to the mother spore caused a marked decrease in the length of viable mycelium and reduced the chances of the fungus locating host roots.
In conclusion, our developmental and spatiotemporal studies provided evidences of a survival strategy evolved by the obligately biotrophic AM fungi—an energy-saving mechanism controlling lifespan and the growth arrest decision, allowing long-term infectivity of mycelium growing in the absence of the host.
This strategy may cooperate with other mechanisms, e.g., a wide host range (29), the ability of multiple germination (16, 17, 19), and the regulation of infection structure differentiation (8, 11), in the survival of individuals and populations of AM fungi.
ACKNOWLEDGMENT
This work was supported by National Research Council Italy, Special Project R.A.I.S.A., and by a University of Pisa grant (Fondo di Ateneo).
REFERENCES
- 1.Ästrom H, Giovannetti M, Raudaskoski M. Cytoskeletal components in the arbuscular mycorrhizal fungus Glomus mosseae. Mol Plant-Microbe Interact. 1994;7:309–312. [Google Scholar]
- 2.Bécard G, Piché Y. Fungal growth stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl Environ Microbiol. 1989;55:2320–2325. doi: 10.1128/aem.55.9.2320-2325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beilby J P, Kidby D K. Biochemistry of ungerminated and germinated spores of the vesicular-arbuscular mycorrhizal fungus, Glomus caledonius: changes in neutral and polar lipids. J Lipid Res. 1980;21:739–750. [PubMed] [Google Scholar]
- 4.Coley-Smith J R. Studies of the biology of Sclerotium cepivorum. Berk Ann Appl Biol. 1960;48:8–18. [Google Scholar]
- 5.Coley-Smith J R, Holt R W. The effect of species of Allium on germination in soil of sclerotia of Sclerotium cepivorum. Berk Ann Appl Biol. 1966;58:273–278. [Google Scholar]
- 6.Elliott C G. Sterols in fungi: their functions in growth and reproduction. Adv Microb Physiol. 1977;15:121–172. doi: 10.1016/s0065-2911(08)60316-1. [DOI] [PubMed] [Google Scholar]
- 7.Gerdemann J W. Relation of a large soil-borne spore to phycomycetous mycorrhizal infections. Mycologia. 1955;47:619–632. [Google Scholar]
- 8.Giovannetti M, Avio L, Sbrana C, Citernesi A S. Factors affecting appressorium development in the vesicular-arbuscular mycorrhizal fungus Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. New Phytol. 1993;123:115–122. [Google Scholar]
- 9.Giovannetti M, Sbrana C, Avio L, Citernesi A S, Logi C. Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during preinfection stages. New Phytol. 1993;125:587–593. doi: 10.1111/j.1469-8137.1993.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 10.Giovannetti M, Sbrana C, Citernesi A S, Avio L. Analysis of factors involved in fungal recognition responses to host derived signals by arbuscular mycorrhizal fungi. New Phytol. 1996;133:65–71. [Google Scholar]
- 11.Giovannetti M, Sbrana C, Logi C. Early processes involved in host recognition by arbuscular mycorrhizal fungi. New Phytol. 1994;127:703–709. doi: 10.1111/j.1469-8137.1994.tb02973.x. [DOI] [PubMed] [Google Scholar]
- 12.Green N E, Graham J H, Schenck N C. The influence of pH on the germination of vesicular-arbuscular mycorrhizal spores. Mycologia. 1976;68:929–934. [PubMed] [Google Scholar]
- 13.Hepper C M. Isolation and culture of VA mycorrhizal (VAM) fungi. In: Powell C L, Bagyaraj D J, editors. VA mycorrhizas. Boca Raton, Fla: CRC Press; 1984. pp. 95–112. [Google Scholar]
- 14.Ingold C T. Retraction-septa in various fungi. Trans Br Mycol Soc. 1982;79:373–380. [Google Scholar]
- 15.Koske R E. Gigaspora gigantea: observations on spore germination of a VA-mycorrhizal fungus. Mycologia. 1981;73:288–300. [Google Scholar]
- 16.Koske R E. Multiple germination by spores of Gigaspora gigantea. Trans Br Mycol Soc. 1981;76:320–330. [Google Scholar]
- 17.Koske R E, Gemma J N. Fungal reactions to plants prior to mycorrhizal formation. In: Allen M F, editor. Mycorrhizal functioning: an integrative plant fungal process. New York, N.Y: Chapman & Hall; 1992. pp. 3–27. [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Mosse B. The regular germination of resting spores and some observations on the growth requirements of an Endogone sp. causing vesicular-arbuscular mycorrhiza. Trans Br Mycol Soc. 1959;42:273–286. [Google Scholar]
- 20.Mosse B. Honey-coloured sessile Endogone spores. I. Life history. Arch Microbiol. 1970;70:167–175. [Google Scholar]
- 21.Mosse B. Some studies relating to “independent” growth of vesicular-arbuscular endophytes. Can J Bot. 1988;66:2533–2540. [Google Scholar]
- 22.Nicolson T H. Mycorrhiza in the Gramineae. I. Vesicular-arbuscular endophytes, with special reference to the external phase. Trans Br Mycol Soc. 1959;42:421–438. [Google Scholar]
- 23.Phillips J M, Hayman D J. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158–161. [Google Scholar]
- 24.Phipps C J, Taylor T N. Mixed arbuscular mycorrhizae from the Triassic of Antarctica. Mycologia. 1996;88:707–714. [Google Scholar]
- 25.Remy W, Taylor T N, Hass H, Kerp H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci USA. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiner R P, Koide R T. Stimulation of vesicular-arbuscular mycorrhizal fungi by mycotrophic and nonmycotrophic plant root systems. Appl Environ Microbiol. 1993;59:2750–2752. doi: 10.1128/aem.59.8.2750-2752.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon L, Bousquet J, Levesque R C, Lalonde M. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature. 1993;363:67–69. [Google Scholar]
- 28.Smith S E, Gianinazzi-Pearson V. Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L.: effects of photon irradiance and phosphate nutrition. Aust J Plant Physiol. 1990;17:177–188. [Google Scholar]
- 29.Smith S E, Read D J. Mycorrhizal symbiosis. San Diego, Calif: Academic Press; 1997. pp. 1–605. [Google Scholar]
- 30.Tommerup I C. Spore dormancy in vesicular-arbuscular mycorrhizal fungi. Trans Br Mycol Soc. 1983;81:37–45. [Google Scholar]
- 31.Tommerup I C. Persistence of infectivity by germinated spores of vesicular-arbuscular mycorrhizal fungi in soil. Trans Br Mycol Soc. 1984;82:275–282. [Google Scholar]