Abstract
Acylated homoserine lactones (AHLs) are chemical signals that mediate population density-dependent (quorum-sensing) gene expression in numerous gram-negative bacteria. In this study, gram-negative bacilli isolated from catheters were screened for AHL production by a cross-feeding assay utilizing an AHL-responsive Agrobacterium tumefaciens reporter strain. Positive reactions were obtained from 14 isolates of Pseudomonas aeruginosa; negative or weakly positive reactions were recorded for isolates of five other species. P. aeruginosa biofilms were then produced on catheters in a physical model of the bladder. Sections of colonized all-silicone catheters gave positive reactions for the quorum-sensing signal molecules as did sections that had been cleaned of biofilm and autoclaved. Control sections of unused catheters were negative in the tests. Sections from four of nine catheters that had been freshly removed from patients gave positive reactions for AHLs. Cleaned autoclaved sections of three of these catheters also gave strongly positive reactions for AHLs. These results demonstrate that AHLs are produced by biofilms as they develop on the catheters both in vitro in the model and in vivo in the patient’s bladder. They represent the first demonstration of AHL production by biofilms in a clinical setting.
In the many patients undergoing long-term indwelling bladder catheterization, infection of the urinary tract is inevitable (13). While the catheter remains in place, these infections are difficult to eliminate by antibiotic therapy (1) and it is common practice not to intervene with therapeutic agents unless clinical symptoms suggest that the bloodstream or the kidneys have become infected (22). In patients on permanent catheterization, the catheters are generally changed at 8- to 12-week intervals, so infected urine can be flowing through a catheter for periods of up to 3 months. Under these circumstances, substantial bacterial biofilms form on the lumenal surfaces of the catheter and can even completely block the flow of urine from the bladder (9, 15, 17, 19).
The phenotypes of cells in established biofilms are profoundly different from those of cells growing in a planktonic mode (2). The increased resistance of bacteria in biofilms to antibacterial agents, for example, contributes to the difficulties of eliminating these infections from the catheterized urinary tract by both systemic antibiotics and antiseptic bladder instillations (1, 9, 20).
Acylated homoserine lactones (AHLs) are membrane-permeant signal molecules that accumulate in bacterial cultures as a function of cell density. At a threshold population density, described as a bacterial quorum, the accumulated AHLs interact with cellular receptors that control the expression of a set of specific target genes. Expression of these target genes is therefore controlled in response to local cell density (7, 18). The high density of bacteria within biofilms has led to speculation that quorum-sensing genes and AHLs may play important roles in the development of the biofilm-specific physiology (12). Evidence in support of this hypothesis was recently provided by a study of a Pseudomonas aeruginosa mutant unable to make quorum-sensing signals (3). This strain produced thin atypical biofilms which unlike those formed by the wild type did not differentiate into characteristic microcolonies separated by water channels and were sensitive to the biocide sodium dodecyl sulfate. A study of natural biofilms growing on submerged stones taken from the San Marcos river in Texas provided the first evidence that AHLs are produced in biofilms (14). Proof of AHL production by biofilms in a clinical setting, however, has heretofore been lacking. The objective of this study was to determine whether bacterial biofilms on urethral catheters produce AHLs.
MATERIALS AND METHODS
Strains and growth conditions.
Agrobacterium tumefaciens A136 (Ti−)(pCF218)(pCF372) (6) was used as an indicator strain for the detection of AHLs. The genetic element pCF218 codes for the TraR protein, an AHL-responsive transcription factor that recognizes N-3-(oxooctanoyl)-l-homoserine lactone as well as a wide range of related AHLs. A TraR-regulated traI-lacZ reporter is carried on pCF372. A. tumefaciens KYC6 (traM::Tn5-gusA harboring pCF218) was used as an endogenous AHL overproducer (8). The cultures were stored suspended in a mixture of LB broth (Difco Laboratories, Detroit, Mich.) and glycerol and frozen at −70°C. Prior to use, frozen cultures were removed from storage and incubated on LB agar (Difco) (strain KYC6) or LB agar supplemented with spectinomycin and tetracycline (strain A136) (6). All cultures were incubated at 30°C.
The clinical strains of P. aeruginosa, Providencia stuartii, Proteus mirabilis, Morganella morganii, Escherichia coli, and Klebsiella pneumoniae were all isolated from catheters taken from patients undergoing long-term indwelling catheterization. The strains were not categorized on the basis of virulence but were representative of those found in natural catheter biofilms. For experimental purposes, cells were freshly subcultured onto CLED agar (Oxoid Ltd., Basingstoke, United Kingdom) from stock suspensions in 5.0% (vol/vol) glycerol in distilled water stored at −70°C.
Cross-feeding assay for AHL detection.
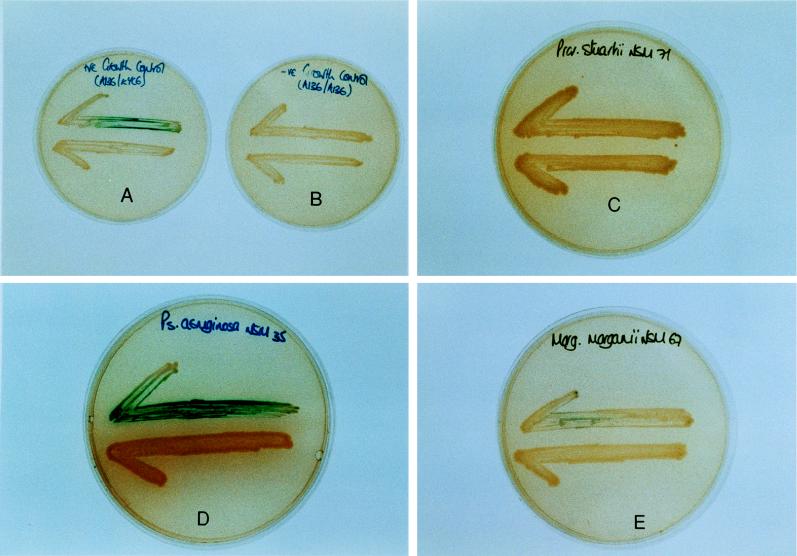
LB agar covered with 40 μl of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (20-mg/ml stock solution in dimethyl formamide) was used for the cross-feeding assays. These assays consisted of streaking the AHL reporter strain, A. tumefaciens A136(pCF218)(pCF372), on the plate and then placing the culture, catheter section, or biofilm extract to be tested approximately 1 cm distant (see Fig. 1). If AHLs are produced or contained within the source material, they will diffuse through the agar and result in activation of the traI-lacZ fusion in the reporter strain (6). Positive and negative controls consisted of culturing the reporter strain with A. tumefaciens KYC6 (AHL overproducer) and with itself.
FIG. 1.
Results of cross-feeding tests. Evidence for the production of AHLs is indicated by the expression of β-galactosidase activity (blue coloration) in the reporter strain, A. tumefaciens A136, which in each test is streaked across the top half of the plate. Production of AHLs by P. aeruginosa NSM35 (D) and A. tumefaciens KYC6 (positive control) (A) is visible. There is no evidence of AHL production by Providencia stuartii NSM71 (C) or by A. tumefaciens A136 when it was incubated with itself (negative control) (B). A weakly positive response can be seen with M. morganii NSM67 (E).
Production of catheter biofilms.
The bacterial biofilms were produced in a simple physical model of the catheterized bladder (21). In essence, this model consists of a glass fermentation flask maintained at 37°C by a water jacket. After sterilization of the model by autoclaving (121°C for 15 min), a size 14 all-silicone catheter (Bard) was inserted into the flask through a section of silicone tubing (a “urethra”) attached to a glass outlet at the base of the flask. The catheter balloon was then inflated in the usual way, which secured the catheter in position and sealed the outlet from the “bladder.” Sterile artificial urine was then supplied to the bladder at 0.5 ml min−1. In this way, a residual volume of 30 ml collects in the bladder below the level of the catheter eyelet and then flows through the catheter and drainage tube to a collecting bag. The artificial urine was based on that devised by Griffith et al. (11) and has been described previously (21).
Models were assembled, and the bladders were primed with artificial urine. The bladders were inoculated with 10 ml of a 4-h urine culture of the test strains. After 1 h to allow the organisms to establish themselves in the model, the supply of urine was switched on. The models were run for 48 h before the urine supply was switched off. The catheters were then removed from the models, and sections 1 cm in length were cut from regions just below the eyeholes. These sections were then cut in half longitudinally and tested in the cross-feeding assay by standing them upright on the agar 1 cm from the A. tumefaciens reporter strain. Similar sections were placed in sterile deionized water (1 ml), sonicated for 5 min at 33 kHz (Transsonic water bath; Camlab, Cambridge, United Kingdom) to remove the biofilm, and autoclaved at 121°C for 15 min. The cleaned autoclaved catheter sections and the autoclaved biofilm extracts (0.2 ml) were then placed on the cross-feeding assay plates, as were samples (0.2 ml) of the uninoculated urine.
Viable cell counts on the catheter biofilms.
Sections (1 cm) from just below the eyeholes of the catheters that had been incubated in the models for 48 h were suspended in 10 ml of nutrient broth (Oxoid). The biofilm bacteria on these sections were then dispersed in the broth by sonication for 5 min and mixing for 2 min on a Rota mixer (Fisher Scientific Ltd., Loughborough, United Kingdom). The viable cells in the resulting cell suspension were then enumerated by plating onto CLED agar (Oxoid).
Electron microscopy.
Visual confirmation of the presence of biofilms on the catheters was obtained by scanning electron microscopy. Sections of catheters (1 cm in length), taken from the region just below the retention balloon, were plunged into liquid nitrogen-cooled propane and then transferred to liquid nitrogen. Cross sections were produced by freeze-fracturing and freeze-dried for 24 h at −80°C. These samples were then mounted on aluminum stubs, sputtered with gold, and examined in a JEOL LSM5200 scanning electron microscope. To observe the nature of the biofilm surfaces, sections (approximately 1 cm long) from the region just below the retention balloon were cut longitudinally into halves. They were fixed in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 h and then washed overnight in the phosphate buffer before being postfixed in Millonig’s phosphate-buffered osmium tetroxide (1.0%) for 1 h. The samples were dehydrated in a graded series of aqueous ethanol solutions (30 to 100%) and then critical point dried by using liquid carbon dioxide. Finally, the samples were mounted on aluminum stubs sputtered with gold and examined in the scanning electron microscope.
RESULTS
Production of AHLs by catheter isolates.
A collection of isolates of gram-negative bacilli from patient catheters were screened for AHL production in the cross-feeding test. Strong positive reactions were recorded for all 14 isolates of P. aeruginosa tested. Negative or weakly positive reactions were recorded for isolates of M. morganii, Providencia stuartii, K. pneumoniae, Proteus mirabilis, and E. coli. Examples of cross-feeding tests with P. aeruginosa, M. morganii, and Providencia stuartii are shown in Fig. 1. It can be seen that the incubation of P. aeruginosa in the presence of the reporter strain, A. tumefaciens A136, produced a blue coloration in the bioassay medium due to the expression of the lacZ reporter gene. A similar reaction was observed when the AHL-producing strain A. tumefaciens KYC6 was cultivated with A. tumefaciens A136 (positive control). No evidence of AHL activity was observed when A136 was incubated with itself (negative control) or with an isolate of Providencia stuartii. The weak reaction observed with M. morganii after 48 h in the cross-feeding test (Fig. 1) was reproducible and became stronger on continued incubation but did not reach the intensity of the reaction produced by P. aeruginosa.
Production of AHLs by biofilms formed on catheters in the bladder model.
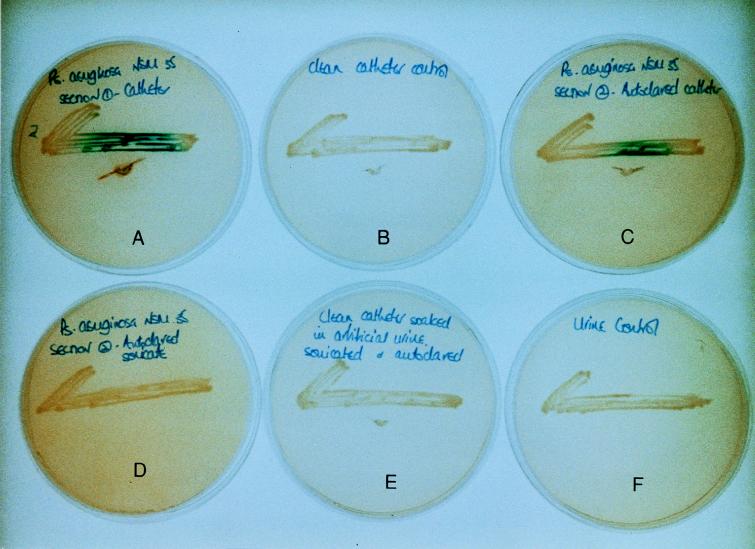
Biofilms were developed over 48 h in bladder models infected with a test strain of P. aeruginosa on all-silicone, silicone-coated latex, and hydrogel-coated latex catheters. The results of the cross-feeding experiments on all-silicone catheters are illustrated in Fig. 2. It can be seen that sections of all-silicone catheters colonized by P. aeruginosa biofilms gave a positive test for the quorum sensor as did sections of P. aeruginosa-colonized all-silicone catheters that had been cleaned of biofilm by sonication and then autoclaved. In contrast, autoclaved biofilm extracts, sections of unused catheter, catheter sections that had been exposed to urine and then sonicated and autoclaved, and samples of the urine medium used to grow the biofilms all produced negative results.
FIG. 2.
Production of AHLs by biofilms of P. aeruginosa NSM35 growing on all-silicone catheters. Positive reactions for AHLs are visible in colonized catheter sections (A) and in sections that had been cleaned of biofilm by sonication and then autoclaved (C). No evidence of AHL activity is visible in sections of fresh unused catheters (B), catheter sections that had been exposed to urine and then sonicated and autoclaved (E), autoclaved biofilm extracts (D), or samples of urine (F).
Colonized silicone-coated and hydrogel-coated latex catheters both gave positive reactions for the production of AHLs. The cleaned autoclaved sections, however, gave either weakly positive (silicone-coated catheters) or negative (hydrogel-coated catheters) responses in the assay.
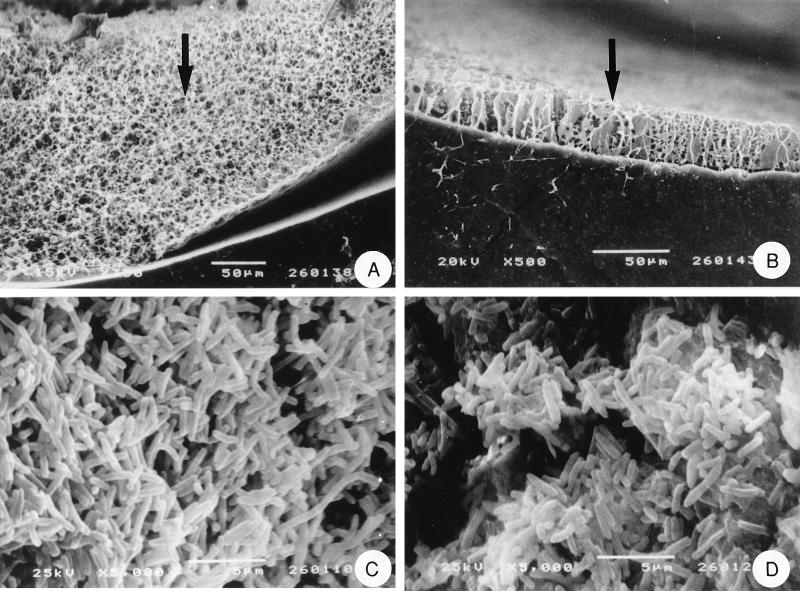
The electron micrographs (Fig. 3) show the formation of biofilm on all-silicone and hydrogel-coated latex catheters. The numbers of P. aeruginosa cells colonizing each catheter type are presented in Table 1.
FIG. 3.
Scanning electron micrographs of P. aeruginosa NSM35 growing on catheters. Freeze-dried, freeze-fractured cross sections of colonized all-silicone (A) and hydrogel-coated latex (B) catheters are shown. The arrows indicate the bacterial biofilms colonizing the lumenal surfaces of the catheters. Critical-point-dried preparations showing the surfaces of these biofilms on all-silicone (C) and hydrogel-coated (D) catheters are also shown.
TABLE 1.
Population densities of P. aeruginosa in catheter biofilms
| Catheter type | No. of viable cells in 48-h biofilms (CFU/cm of catheter [mean ± SD])a |
|---|---|
| All silicone | (1.8 ± 0.2) × 109 |
| Silicone-coated latex | (2.5 ± 0.2) × 108 |
| Hydrogel-coated latex | (2.0 ± 0.9) × 108 |
Calculated from three separate experiments. Analysis of variance on log-transformed data showed significant differences (P > 0.001) between the densities of cells in the biofilms on the all-silicone catheters and those on the coated latex catheters.
Production of AHLs by biofilms on catheters removed from patients.
Nine catheters that had been freshly removed from patients were also tested in the cross-feeding assay. All of these catheters were colonized by biofilm. In four cases, the colonized sections gave positive reactions for AHLs. One of these was a silicone-coated latex catheter, and after cleaning and autoclaving, it gave no response in the cross-feeding assay. The other three were all-silicone catheters, and cleaned autoclaved sections gave clear positive reactions for AHLs.
DISCUSSION
The reporter strain used in the cross-feeding assay has the ability to detect N-3-(oxooctanoyl)-l-homoserine lactone and a range of its analogues (6). Previous studies have shown that P. aeruginosa produces N-3-(oxododecanoyl)-l-homoserine lactone and N-(butyryl)-l-homoserine lactone and that these quorum sensors are required for the expression of the virulence factors toxin A and elastase (16). It is not surprising therefore that all 14 isolates of P. aeruginosa from urethral catheters were strongly positive in the cross-feeding assay. It should be emphasized that a negative response in this assay does not mean that the test strain failed to make quorum-sensing signals; it merely shows an inability to make AHLs that are recognizable to A. tumefaciens or that the levels of the AHLs are very low. Thus, organisms such as M. morganii and Providencia stuartii which gave weak or negative responses could well be producing quorum-sensing signals perceptible to themselves and other species that inhabit catheter biofilms.
The strongly positive reaction produced by the P. aeruginosa biofilms that had been generated on catheters in the bladder model (Fig. 2) could be due, in part, to AHL production by the bacteria growing on the agar during the testing procedure. It is significant, however, that sections of the all-silicone catheters from which biofilm had been removed by sonication and autoclaving also gave strongly positive responses in the assay. This result together with negative reactions observed with unused catheters, and with unused catheters that had been exposed to urine and then sonicated and autoclaved, demonstrates unequivocally that the quorum-sensing signal molecules must have been produced by the biofilm during its development on the catheter. It is also clear that AHLs produced during the development of the biofilm adsorb to the silicone catheter surface. The observation that the autoclaved biofilm extract gave a negative response was surprising in view of the previous positive results with autoclaved biofilm extracts from pebbles taken from a river (14). While the AHLs are clearly not destroyed by autoclaving, it could be that resuspension of the biofilm in sterile deionized water diluted the AHL beyond the detection limit of the assay. It is also possible that the binding of AHLs to the catheter reduces their concentration in the biofilm.
There are several possible explanations of the observation that while biofilm-colonized coated latex catheters produced positive reactions in the cross-feeding tests, the cleaned autoclaved catheters gave weak (silicone-coated latex) or negative (hydrogel-coated latex) responses. It might simply be that the numbers of cells in the biofilms formed on these catheters over the 48-h incubation period in the model did not generate enough AHL to register in the assay. Indeed, it was the case that significantly fewer (P > 0.001) cells colonized the latex catheters (Table 1). Electron microscopy also revealed that the biofilms formed on all-silicone catheters were more substantial than those on hydrogel-coated catheters (Fig. 3). Alternatively, the amounts of AHL adsorbing to the coated latex surfaces during biofilm growth and eluting from the cleaned catheters during the cross-feeding tests may have been insufficient to register positive responses.
The results obtained from the catheters removed from patients show that AHLs are produced by the biofilms while the catheter is in situ in the bladder. These results also confirm that the quorum sensors produced in vivo adsorb to the all-silicone catheters.
Studies with scanning confocal laser microscopy have shown that biofilms are organized communities of cells that have a complex architecture and physiology (2). The precise role of AHLs in biofilms remains to be established. Evidence is emerging (3), however, that as molecules mediating cell-to-cell communication, they have important functions in coordinating the activity of cells in biofilms and ensuring that a social order in these communities is established and maintained. It is possible, therefore, that scrambling the bacterial communication systems by, for example, coating catheters with analogues of AHLs capable of interfering with signalling could have practical applications in preventing the formation and development of biofilms on catheters and many other implanted medical devices. Furanone derivatives produced by the seaweed Delisea pulchra are thought to interfere with AHL-mediated quorum sensing by mimicking AHLs and blocking transcriptional activation of target genes (4, 10). Such inhibition has been directly demonstrated in the AHL-regulated swarming of Serratia liquefaciens (5, 10) and may provide avenues for controlling biofilm formation and dissolution in situ.
ACKNOWLEDGMENTS
We are grateful to Carole Winters and Mike Turner for their assistance with the electron microscopy.
Nicola Morris was supported by a grant from the Welsh Scheme for the Development of Health and Social Research. Clay Fuqua received grant support from the National Science Foundation (MCB-9723837).
REFERENCES
- 1.Clayton C L, Chawla J C, Stickler D J. Some observations on urinary tract infections in patients undergoing long-term bladder catheterisation. J Hosp Infect. 1982;3:39–47. doi: 10.1016/0195-6701(82)90029-9. [DOI] [PubMed] [Google Scholar]
- 2.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 3.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 4.de Nys R, Steinberg P D, Willemsen P, Dwarjanyn S, Gabelish C L, King R J. Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling. 1995;8:259–271. [Google Scholar]
- 5.Eberl L, Winson M K, Sternberg C, Stewart G S A B, Christiansen G, Chhabra S R, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-acyl-l-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 6.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 8.Fuqua W C, Burbea M, Winans S C. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J Bacteriol. 1995;177:1367–1373. doi: 10.1128/jb.177.5.1367-1373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganderton L, Chawla J, Winters C, Wimpenny J, Stickler D. Scanning electron microscopy of bacterial biofilms on indwelling bladder catheters. Eur J Clin Microbiol Infect Dis. 1992;11:789–797. doi: 10.1007/BF01960877. [DOI] [PubMed] [Google Scholar]
- 10.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith D P, Musher D M, Itin C. Urease: the preliminary cause of infection induced urinary stones. Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 12.Heyes S J D, Gilbert P, Eberhard A, Allison D G. Homoserine lactones and bacterial biofilms. In: Wimpenny J, Handley P, Gilbert P, Lappin-Scott H, Jones M, editors. Biofilms: community interactions and control. Cardiff, United Kingdom: Bioline; 1997. pp. 103–112. [Google Scholar]
- 13.Kunin C M. Detection, prevention and management of urinary tract infections. 4th ed. Philadelphia, Pa: Lea & Febiger; 1987. pp. 245–297. [Google Scholar]
- 14.McLean R J C, Whitely M, Stickler D J, Fuqua W C. Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol Lett. 1997;154:259–263. doi: 10.1111/j.1574-6968.1997.tb12653.x. [DOI] [PubMed] [Google Scholar]
- 15.Nickel J C, Gristina A G, Costerton J W. Electron microscopic study of an infected Foley catheter. Can J Surg. 1985;28:50–52. [PubMed] [Google Scholar]
- 16.Passador L, Cook J M, Gambello M J, Rust L, Inglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay J W A, Garnham A J, Mulhall A B, Crow R A, Bryan J M, Eardley I, Vale J A, Whitfield H N. Biofilms, bacteria and bladder catheters. Br J Urol. 1989;64:395–398. doi: 10.1111/j.1464-410x.1989.tb06050.x. [DOI] [PubMed] [Google Scholar]
- 18.Salmond G P C, Bycroft B W, Stewart S A B, Williams P. The bacterial “enigma”: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 19.Stickler D J. Bacterial biofilms and the encrustation of urethral catheters. Biofouling. 1996;9:293–305. [Google Scholar]
- 20.Stickler D J, Clayton C L, Chawla J C. The resistance of urinary tract pathogens to chlorhexidine bladder washouts. J Hosp Infect. 1987;10:219–228. doi: 10.1016/0195-6701(87)90029-6. [DOI] [PubMed] [Google Scholar]
- 21.Stickler D J, Howe N S, Winters C. Bacterial biofilm growth on ciprofloxacin treated urethral catheters. Cells Mater. 1994;4:387–398. [Google Scholar]
- 22.Warren J W. Catheter associated bacteriuria in long-term care facilities. Infect Control Hosp Epidemiol. 1994;15:557–562. doi: 10.1086/646977. [DOI] [PubMed] [Google Scholar]