Abstract
Immunoassay procedures were used to investigate the symbiotic relationship of Thiothrix spp. in the intestinal cecum of the spatangoid species Echinocardium cordatum. Thiothrix spp. were identified in nodule samples from E. cordatum digestive tubes based on microscopic examination, enzyme-linked immunosorbent assay, and indirect immunofluorescence. Thiothrix spp. protein made up as much as 84% of the total protein content of the nodules. This is the first identification of Thiothrix spp. internally symbiotic with marine invertebrates.
Thiothrix-like bacteria have been reported as symbionts in invertebrates from sulfide-rich habitats (26). Isolation of these symbiotic Thiothrix-like bacteria has failed, and the organisms have not been previously identified with certainty. The genus Thiothrix was created for ensheathed filamentous bacteria that oxidize sulfide and deposit sulfur granules internally, attach to substrates, produce gliding gonidia, and form rosettes. In nature, Thiothrix spp. have been associated with leaves, rocks, or algae in sulfide-containing flowing water (1, 2). The occurrence of Thiothrix as an ectosymbiont has been demonstrated for both freshwater (19) and saltwater (22) organisms. However, none of the previous studies concern bacteria in digestive tracts. Symbiotic Thiothrix-like bacteria have been found in the digestive tube of Echinocardium cordatum and in that of several other species of spatangoids (deposit-feeder echinoids) (7, 9, 26). In this association, the Thiothrix-like bacteria are most frequently confined to the intestinal cecum (9). Although Thiothrix-like filaments have been observed on the cecum wall, the majority are incorporated into nodules in the cecum lumen (9). These nodules are formed through an intricate process that combines a central detrital particle with a coating consisting of a layer of interlacing bacterial filaments (outer layer) and empty bacterial sheaths (middle layer) (9). Detrital particles from the gut content regularly penetrate the cecum; once in the cecum, these particles are colonized by Thiothrix-like bacteria (26). The filamentous bacteria form a layered coat where empty bacterial sheaths accumulate below a peripheral mat of living Thiothrix-like bacteria (9).
Ectosymbioses involving Thiothrix/Leucothrix-like bacteria occur with marine invertebrates from sulfide-rich environments (7). Epiphytic Thiothrix/Leucothrix-like bacteria have been described in three hydrothermal vent organisms: the polychaetes Alvinella pompejana and Alvinella caudata (14, 23); a mytilid bivalve, Bathymodiolus sp. (18, 20); and a gastropod limpet (5). Epiphytic Thiothrix/Leucothrix-like bacteria have also been found in association with burrowing organisms from anoxic sediment. This is the case of the priapulid Halicryptus spinulosus (21), the oligochaete Tubificoides benedii (11–13), and the bivalve mollusc Montacuta ferruginosa (16).
Previous work (26) has demonstrated that Thiothrix-like bacteria associated with E. cordatum are sulfur oxidizers. Optimal growth conditions occur in this environment for Thiothrix spp. Hydrogen sulfide can be supplied from the digestive activity of E. cordatum and ingested organic matter in sediments (6, 10, 27). Oxygen is provided through the coelomic fluid that surrounds the cecum (7, 8) and from water flow that sporadically passes through the digestive tube (6). The accumulation of sulfide can be toxic, and physiological adaptations to sulfide are of ecological significance (27). Through their particular location and their ability to oxidize reduced sulfur compounds, the Thiothrix-like bacteria may prevent critical accumulation of hydrogen sulfide within the intestine of their host (26).
The present report describes an enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence assay (IIF) which use a Thiothrix spp.-specific mouse monoclonal antibody (MAb), T3511, to detect Thiothrix spp. directly in samples of bacterial nodules from E. cordatum cecum.
Nodules were taken from the intestinal cecum of the spatangoid species E. cordatum. Six E. cordatum individuals were collected from the intertidal zone at Wimereux, Nord Pas-de-Calais, France. A single cecum nodule was sampled from each individual. Three samples, designated 1 to 3, of bacteria were taken from aseptically dissected fragments of the outer coat from three nodules. Three samples (4 to 6) were of entire bacterial nodules. All samples were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and then stored in PBS with 0.1% sodium azide.
Specimens from the nodule outer layers were prepared for scanning electron microscopy (SEM) by fixation in 3% glutaraldehyde in cacodylate buffer (0.1 M, pH 7.4) for 24 h. The specimens were then rinsed for 3 days in cacodylate buffer, postfixed in 1% osmium tetroxide in buffer for 1 h, and then briefly rinsed in buffer. Specimens were then dehydrated in graded ethanol, dried by the critical-point method with CO2 as the transition fluid, mounted, sputter coated with gold, and viewed with an ISI DS 130 SEM microscope operating at 20 kV.
To observe bacterial populations taken from cecum nodules, samples were stained with acridine orange (AO). Briefly, fixed bacterial nodule samples (10 μl) were air dried on glass slides for 10 min followed by addition of 10 μl of AO. The samples were then incubated for 2 min and then rinsed with sterile deionized water. Slides were examined with a laser scanning confocal microscope (LSM 310; Carl Zeiss, Inc., Thornwood, N.Y.).
The ELISA procedure was that of Brigmon et al. (2). Briefly, fixed bacterial nodule samples were diluted 1:10 in carbonate-bicarbonate buffer (pH 9.8), vortexed, and pipetted in 100-μl aliquots into Immulon 2 96-well immunoassay plates (Dynatech, Chantilly, Va.). A positive control containing only Thiothrix strain A1 was included on each plate. Negative controls containing PBS and marine samples with no Thiothrix spp. were included on all plates. The ELISA plates were dried overnight at 45°C. After adsorption of bacteria, ELISA plates were treated sequentially for 1 h with 200 μl of PBS containing 1% bovine serum albumin (PBSA) (Sigma Chemical Co., St. Louis, Mo.), 200 μl of Thiothrix spp.-specific mouse MAb T3511, and 200 μl of a 1:1,000 dilution in PBSA of affinity-purified horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulins (Sigma). After incubation with each reagent, ELISA plates were washed six times with PBS containing 0.01% Tween 20 (Sigma). Initially, an ELISA that incorporated alkaline phosphatase as previously described was employed (2). However, due to endogenous alkaline phosphatase in the samples, HRP was substituted for the enzyme in the ELISA. There was no apparent interference with the HRP-linking enzyme. Bound conjugate was observed by addition of enzyme substrate solution o-phenylenediamine in citrate buffer with hydrogen peroxide (Sigma). The plates were read on an ELISA plate reader (Bio-Tek Instruments, Inc., Winooski, Vt.) at 405 nm after 30 min.
The concentration of Thiothrix in marine samples was determined by ELISA. The protein determination method of Williams and Unz (28), developed to eliminate sulfide or sulfur interference, was used to estimate Thiothrix and total microbial biomass. The ELISA was used to compare unknown marine samples with Thiothrix A1 protein standards with concentrations ranging from 0.10 to 100 μg/ml and tested with the ELISA as previously described (2). Thiothrix A1 protein standards were made in 0.2-μm-pore-size-filtered seawater to simulate the environment of the marine samples.
For examination with immunofluorescence, fixed nodule samples (10 μl) (Fig. 1) were dried on slides for 10 min followed by addition of 10 μl of 2% gelatin in PBS and dried for 10 min. Positive and negative controls were the same as those for the ELISA. All samples were washed three times with PBS and incubated with 10 μl of MAb T3511 or PBSA controls for 30 min as previously described (2). Samples were washed three times again and incubated with a secondary antibody, fluorescein isothiocyanate-labeled goat anti-mouse antibody (Sigma), for 30 min. Samples were examined with an LSM 310 laser scanning microscope (Carl Zeiss, Inc.). One drop of SlowFade (Molecular Probes, Inc., Eugene, Oreg.) was added to samples to inhibit photofading of the fluorescein isothiocyanate.
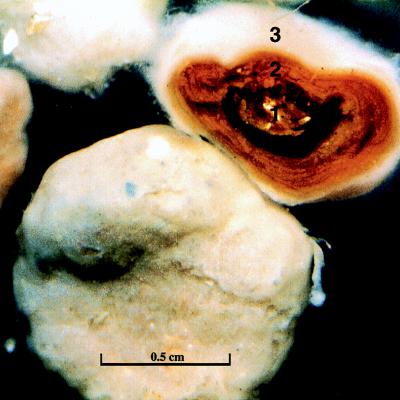
FIG. 1.
Whole and cross-sectioned nodule. The three layers of the cross-sectioned nodule are the inner nucleus (1), the middle layer (2), and the outer layer (3).
The Thiothrix spp.-specific immunoassays demonstrated that the spatangoid E. cordatum does have symbiotic Thiothrix spp. associated with its nodules. The morphology and physiology of the filamentous bacteria found in the nodules of other spatangoid species are similar (7). While the symbiosis between the Thiothrix-like bacteria and spatangoid nodules was previously described, the Thiothrix spp. were not positively identified (7, 9, 25). This is the first report of Thiothrix symbiotic with echinoderms and the first report of symbiotic Thiothrix spp. living in the digestive tube of an animal.
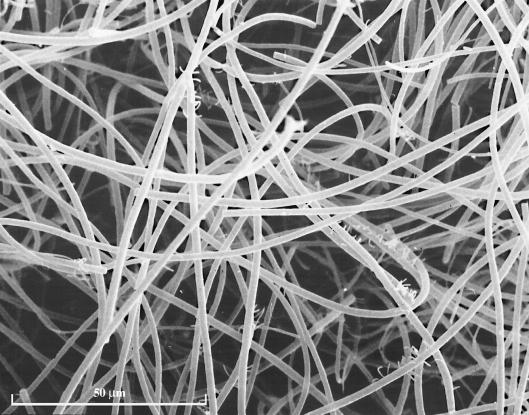
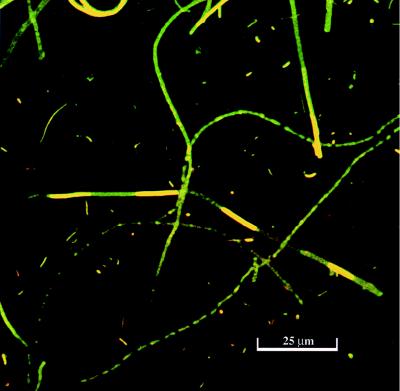
Cecum nodules were typically characterized as spherically shaped with a white filamentous biofilm covering the surface of the nodule (Fig. 1). The nodules consisted of three distinct layers (layers 1 to 3) that can be seen in the sectioned sample (Fig. 1). Examination of the outer layer of the biofilm by SEM revealed a mat of filamentous bacteria (Fig. 2) and revealed that these filamentous species appeared to dominate in all layers of the nodule. The Thiothrix spp. can be seen as large filaments dominating in biomass over other types of bacteria (Fig. 3). Some cyanobacterial filaments were also observed in samples and could be distinguished from bacteria based on morphology.
FIG. 2.
A scanning electron micrograph of the nodule surface.
FIG. 3.
AO-stained sample from a whole bacterial nodule.
Results from the ELISA demonstrated that absorbance of duplicate nodule samples tested was correlated (P < 0.05) with Thiothrix strain A1 protein. Based on results from the ELISA, the concentration of Thiothrix spp. was greater in the whole nodules than in the outer coat. The total protein concentration of the biomass from the whole nodules was 66.7 μg per g (wet weight) of nodule. Immunocytochemical testing results with ELISA indicate that the nodules are 56 μg of Thiothrix spp. per g (wet weight) of nodule. Thus, the percentage of Thiothrix spp. protein in the total protein concentration in the whole nodule was 84%. The total protein concentration of the outer nodule was 10 μg of protein per g (wet weight) of fragment. Further ELISA testing estimates that the dissected outer coat fragments are 3.7 μg of Thiothrix spp. protein per g (wet weight) of fragment. The ratio of Thiothrix spp. protein to total protein in the outer coat nodule fragments was 37%. Dead or inactive Thiothrix spp. cells may be present, since many empty sheaths were noted with microscopy. Bacterial protein was used as an estimate of biomass because the attachment, culture morphology, and filamentous nature of Thiothrix spp. prevent the use of other microbiological methods, including direct counts. The size and structure of the described Thiothrix spp. associated with the nodules appear to be similar to those (Fig. 4) of Thiothrix strain A1 (2). However, it cannot be assumed that the protein concentrations are the same, and so these biomass determinations are estimates until the marine Thiothrix spp. are isolated and characterized.
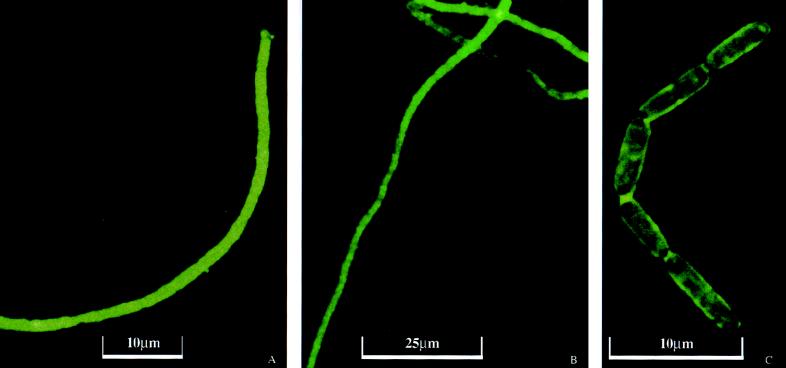
FIG. 4.
IIF of nodule samples: Thiothrix spp. filaments from the nodule outer coat labeled with IIF (A), Thiothrix sp. filaments from the bacterial nodule labeled with IIF (B), and Thiothrix spp. gliding gonidia labeled with IIF (C).
Thiothrix spp. filaments were directly observed by IIF in dissected outer coat nodule fragments, in whole nodules attached to particles, in close association with other bacteria in biofilms, and unattached. Few Thiothrix spp. rosettes were observed in nodule samples. Figure 4A shows an IIF-labeled bacterial filament from the outer coat from a bacterial nodule, while Fig. 4B shows IIF-labeled bacterial filaments from a cecum nodule. Gliding gonidia, which are characteristic of Thiothrix spp., can be seen in the cecum nodule in Fig. 4C.
The detrital particles that form the nucleus of the nodules regularly enter the digestive tract through the ingestion of sediments (15). Particles that enter the cecum are later colonized by bacteria including Thiothrix spp. The oxygen required by Thiothrix spp. is provided by the intake of water by E. cordatum. The seawater where these animals were collected is aerated due to intertidal wave activity. The main source of oxygen is provided by the coelomic fluid. Indeed, the intestinal cecum is located next to the ampullae of the respiratory tube-feet in the general coelomic cavity and oxygen may reach the bacteria by diffusing across the cecal wall (8, 26). The ingested seawater that penetrates the siphon and the intestine can bring oxygen to the bacteria when the intestine is not filled with sediment (6, 8). Hydrogen sulfide forms in the ingested sediment from the intestine and through the activities of sulfur-reducing bacteria in the nodule inner layers (26). Sulfide can also react with iron to form iron sulfide that gives the nodule inner layers their black coloration (10). The sulfur-oxidizing Thiothrix spp. that are symbiotic with spatangoids could have a detoxifying effect by preventing accumulation of sulfide within the intestine of their hosts. By removing sulfide, Thiothrix spp. could facilitate a longer period of sediment digestion and, consequently, a more complete hydrolysis of the organic matter. Thiothrix bacteria symbiotic with spatangoids may influence the mechanism that their hosts use to get nutrients from ingested sediments, including essential fatty acids (24).
Sulfur-oxidizing bacterial ectosymbionts can prevent environmental sulfide from diffusing into their host and act as peripheral defense mechanisms (27). This phenomenon has been also assumed for the Thiothrix spp. symbiotic with spatangoids that are exposed to sulfide in their digestive tubes rather than their habitats (7, 26). In that context, it is important to note that several features considered potential peripheral defense mechanisms against sulfide have been observed along the intestine (27). These mechanisms include mucus secretion (8); periodic water flow via the siphon, when the sediments are eliminated via the anus (6); iron oxide deposits in the connective tissue of the intestine wall (3, 8); and ectosymbiotic sulfur-oxidizing bacteria in the intestinal cecum (9). However, the efficiency of these mechanisms has not been measured in spatangoids and remains speculative. In light of Dubilier and collaborators’ conclusions (13), calculations of the rate of sulfide diffusion into the animal body are required before the effectiveness of peripheral defense mechanisms can be adequately determined. Peripheral defense mechanisms such as mucus coating and external iron sulfide deposition are not sufficient to prevent sulfide diffusion into the oligochaete T. benedii, and thus, the animal must rely on an anaerobic metabolism when internal sulfide concentrations are inhibitory to cytochrome c oxidase (12, 13).
Although over 60% of echinoderm species have bacterial symbionts, knowledge of the nature of the relationship to the hosts is limited (4). It has been proposed that the bacterial symbionts are transmitted from parent to offspring and are host species specific (4). Environmental transmission may be important for the association between host and free-living symbionts, as has been demonstrated for symbiotic sulfur-oxidizing bacteria in bivalves (17).
From our experience, the identification of Thiothrix spp. in these marine organisms is possible by immunological methods. The symbiotic association between Thiothrix spp. and E. cordatum develops externally on the cecum nodule, located in a particular outpocketing of the digestive system. Thus, the Thiothrix spp. are ectosymbionts (24). Further examination of the host-ectosymbiont relationship is needed to determine the perpetuation of the symbiosis (from one generation of hosts to the next one) and the importance of the symbiosis to the host development, metabolism, and growth.
Acknowledgments
This paper was prepared in connection with work done under a subcontract to contract no. DE-AC09-76SR00001 with the U.S. Department of Energy. Research was supported by an FRFC grant to C. De Ridder (convention n-4510.96).
Footnotes
Contribution of the Centre Interuniversitaire de Biologie Marine (CIBIM), Belgium.
REFERENCES
- 1.Brigmon R L, Martin H W, Morris T, Zam S, Bitton G. Biogeochemical ecology of Thiothrix spp. in underwater limestone caves. Geomicrobiol J. 1994;12:141–159. [Google Scholar]
- 2.Brigmon R L, Bitton G, Zam S G, O’Brien B. Development and application of a monoclonal antibody against Thiothrix spp. Appl Environ Microbiol. 1995;61:13–20. doi: 10.1128/aem.61.1.13-20.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan J B, Brown B E, Coombs T L, Pirie B J S, Allen J A. The accumulation of ferric iron in the guts of some spatangoid echinoderms. J Mar Biol. 1980;60:631–640. [Google Scholar]
- 4.Burnett W J, McKenzie J D. Subcuticular bacteria from the brittle star Ophiactis balli (Echinodermata: Ophiuroidea) represent a new lineage of extracellular marine symbionts in the α subdivision of the class Proteobacteria. Appl Environ Microbiol. 1997;63:1721–1724. doi: 10.1128/aem.63.5.1721-1724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Burgh M E, Singla C L. Bacterial colonization and endocytosis on the gill of a new limpet species from a hydrothermal vent. Mar Biol. 1984;84:1–6. [Google Scholar]
- 6.De Ridder, C. 1987. Mécanique digestive chez l’échinide fouisseur Echinocardium cordatum (Echinodermata). Actes du 5ème Séminaire intern. Echinodermes (Nantes 1986). Bull. Soc. Sci. Nat. Ouest France Suppl. H.S:65–71.
- 7.De Ridder C. Symbioses between spatangoids (Echinoidea) and Thiothrix-like bacteria (Beggiatoales) In: David B, Guille A, Feral J P, Roux M, editors. Proceedings of the 8th International Echinoderm Conference Dijon 1993. Rotterdam, The Netherlands: Balkema Publishers; 1994. pp. 619–625. [Google Scholar]
- 8.De Ridder C, Jangoux M. The digestive tract of the spatangoid echinoid Echinocardium cordatum (Echinodermata): morphofunctional study. Acta Zool (Stockholm) 1993;74:337–351. [Google Scholar]
- 9.De Ridder C, Jangoux M, De Vos L. Description and significance of a peculiar intradigestive symbiosis between bacteria and a deposit-feeding echinoid. J Exp Mar Biol Ecol. 1985;91:65–76. [Google Scholar]
- 10.De Ridder C, Jangoux M, Van Impe E. Food selection and absorption efficiency in the spatangoid echinoid Echinocardium cordatum (Echinodermata) In: Keegan B, O’Connor B, editors. Proceedings of the 5th International Echinoderm Conference Galway 1984. Rotterdam, The Netherlands: Balkema Publishers; 1985. pp. 245–251. [Google Scholar]
- 11.Dubilier N. Association of filamentous epibacteria with Tubificoides benedii (Oligochaeta: Annelida) Mar Biol. 1986;92:285–288. [Google Scholar]
- 12.Dubilier N, Giere O, Grieshaber M K. Concomitant effects of sulfide and hypoxia on the aerobic metabolism of the marine oligochaete Tubificoides benedii. J Exp Zool. 1994;269:287–297. [Google Scholar]
- 13.Dubilier N, Giere O, Grieshaber M K. Morphological and ecophysiological adaptations of the marine oligochaete Tubificoides benedii to sulfidic sediments. Am Zool. 1995;35:163–173. [Google Scholar]
- 14.Gaill F, Desbruyères D, Prieur D. Bacterial communities associated with “Pompei worms” from the East Pacific Rise hydrothermal vents: SEM, TEM observations. Microb Ecol. 1987;13:129–139. doi: 10.1007/BF02011249. [DOI] [PubMed] [Google Scholar]
- 15.Gerdes G, Krumbein W E. Lecture notes in earth sciences. 9. Biolaminated deposits. Berlin, Germany: Springer-Verlag; 1987. [Google Scholar]
- 16.Gillian D, De Ridder C. Morphology of a ferric iron-encrusted biofilm forming on the shell of a burrowing bivalve (Mollusca) Aquat Microbiol Ecol. 1997;12:1–10. [Google Scholar]
- 17.Gros O, Darasse A, Durand P, Frenkiel L, Moueza M. Environmental transmission of a sulfur-oxidizing bacterial gill endosymbiont in the topical Lucind bivalve Codakia orbicularis. App Environ Microbiol. 1996;62:2324–2330. doi: 10.1128/aem.62.7.2324-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jannasch H W, Wirsen C O. Morphological survey of microbial mats near deep-sea thermal vents. Appl Environ Microbiol. 1981;41:528–538. doi: 10.1128/aem.41.2.528-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin J M, Henk M C, Burton S D. Occurrence of a Thiothrix sp. attached to mayfly larvae and presence of parasitic bacteria in the Thiothrix sp. Appl Environ Microbiol. 1990;56:357–361. doi: 10.1128/aem.56.2.357-361.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Pennec M, Hily A. Anatomie, structure et ultrastructure de la branchie d’un Mytilidae des sites hydrothermaux du Pacifique oriental. Oceanol Acta. 1984;7:517–523. [Google Scholar]
- 21.Oeschger R, Schmaljohann R. Association of various types of epibacteria with Halicryptus spinulosus (Priapulida) Mar Ecol Prog Ser. 1988;48:285–293. [Google Scholar]
- 22.Polz M F, Distel D L, Zarda B, Amann R, Felbeck H, Ott J A, Cavanaugh C M. Phylogenetic analysis of a highly specific association between ectosymbiotic, sulfur-oxidizing bacteria and a marine nematode. Appl Environ Microbiol. 1994;60:4461–4467. doi: 10.1128/aem.60.12.4461-4467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prieur D, Chamroux S, Durand P, Erauso G, Fera P, Jeanthon C, Le Borgne L, Mével G, Vincent P. Metabolic diversity in epibiotic microflora associated with the Pompeii worms Alvinella pompejana and A. caudata (Polychaetae: Annelida) from deep-sea hydrothermal vents. Mar Biol. 1990;106:361–367. [Google Scholar]
- 24.Stanier R Y, Ingraham J L, Wheelis M L, Painter P R. General microbiology. London, United Kingdom: Macmillan Education Ltd; 1989. [Google Scholar]
- 25.Temara A, De Ridder C, Kaisin M. Presence of an essential polyunsaturated fatty acid in intradigestive bacterial symbionts of a deposit-feeder echinoid (Echinodermata) Comp Biochem Physiol. 1991;100B:503–505. [Google Scholar]
- 26.Temara A, De Ridder C, Kuenen J C, Robertson L A. Sulfide-oxidizing bacteria in the burrowing echinoid, Echinocardium cordatum (Echinodermata) Mar Biol. 1993;115:179–185. [Google Scholar]
- 27.Vismann B. Sulfide tolerance: physiological mechanisms and ecological implications. Ophelia. 1991;34:1–27. [Google Scholar]
- 28.Williams T M, Unz R F. The nutrition of Thiothrix, type 021N, Beggiatoa, and Leucothrix strains. Water Res. 1989;23:15–22. [Google Scholar]