Abstract
Aziridine-based cofactor mimics have been synthesized and are shown to undergo methyltransferase-dependent DNA alkylation. Notably, each cofactor mimic possesses an azide functionality, to which can be attached an assortment of unnatural groups following methyltransferase-dependent DNA delivery. DNA duplexes modified with these cofactor mimics are capable of undergoing the Staudinger ligation with phosphines tethered to biological functionalities following enzymatic modification. This methodology provides a new tool by which to selectively modify DNA in a methyltransferase-dependent way. The conversion of biological methyltransferases into azidonucleosidyl transferases demonstrated here also holds tremendous promise as a means of identifying, as yet, unknown substrates of methylation.
INTRODUCTION
S-adenosyl-l-methionine (SAM)-dependent methylation of nucleic acids and proteins plays an absolutely vital role in the regulation of gene transcription (1–6). Flaws in the activity and expression levels of the eukaryotic DNA methyltransferase (MTase) DNMT1 have been integrally linked to oncogenic potential (7–9). Thus, agents capable of undergoing DNMT1-dependent transfer to DNA might represent an attractive new chemotherapeutic strategy by virtue of altered transcriptional repression mechanisms so often associated with promoter methylation. Such an approach is significantly different from those exemplified by simple inhibition of MTases with SAM analogs. The focal point of efforts described here, substances capable of undergoing transfer to nucleic acids in an MTase-dependent way also hold tremendous promise as biochemical tools by which to dissect and understand biological methylation. Although not elaborated here, such agents might also be used by MTases whose natural substrates are not nucleic acids. For instance, posttranslational protein methylation plays a large role in transcription regulation and constitutes an important facet of proteomics. The absence of functionality, however, renders the methyl group difficult to identify and isolate from complex biological mixtures. Thus, substances that take part in SAM-dependent MTase pathways may be important proteomic tools in addition to DNA modifying agents. Our interests in the area of cofactor mimicry are reflected by investigations into the synthesis and DNA modification chemistry of 1 (Scheme 1) and related congeners.
Scheme 1.
Nucleoside transfer by M.TaqI.
The 5′-aziridine adenylate 1 is a substitute for SAM in the M.TaqI catalyzed alkylation of adenine within the recognition sequence d(TCGA) as depicted in Scheme 1 (10–12). Instead of generating the N6-methyladenine, substrate adenylation is accomplished via ring-opening of the aziridine to yield 3. This chemistry is tolerant of cofactor C8 modification and has been successfully used to fluorescently tag short oligonucleotides and large plasmid substrates in an M.TaqI-dependent fashion (13). The aziridine nucleoside 1 also undergoes M.HhaI-dependent DNA attachment within the M.HhaI recognition sequence d(GCGC) (11). These findings provide clear evidence of the importance of 5′ aziridine adenylates as ‘cofactor mimics’ of SAM as tools for biology in the short term and potential therapeutic agents in the long term. The further development of these substances requires not only new and more efficient ways by which to construct them, but also an understanding of how these materials might be made compatible with already existing technologies.
Our interest in the development of cofactors related to 1 as universal cofactors dictated that the core structure of 1 be equipped with a handle through which any desired molecule (DNA damaging moiety, affinity matrix handle, fluorophore, etc.) could be appended following MTase-dependent anchoring. Such a handle would need to present a minimal disruption to cofactor–MTase interactions, and be capable of rapid couplings to other reagents under biological conditions. Thus, both the cofactor and its ligation partner would need to be abiotic. As highlighted by Bertozzi, Zhou and others, the Staudinger ligation of azides and o-methoxycarbonyl functionalized triarylphosphines represents an incredibly powerful reaction in which all our criteria could be met (14–17). Moreover, many azides have been shown to be highly active participants in [2 + 3] Huisgen cycloadditions; these ‘Click chemistry’ reactions also are compatible with biological conditions as most appropriately highlighted by Cravatt and co-workers (18–20). Our program to develop these synthetic cofactors into useful biochemical tools motivated us to pursue chemistry depicted by Scheme 2.
Scheme 2.
Azidonucleoside transfer by M.TaqI and envisioned biotin:avidin associations.
The ability of 8-azidoadenosine (and aryl azides in general) to undergo the Staudinger ligation with triarylphosphines (21) coupled with the ability of C8 azido-SAM to retain its cofactor function (22,23), led to our previously reported synthesis of azido cofactor 4a. However, until now, we have not shown that such a cofactor could be used by DNA methyltransferases or used as an anchoring point for biomolecule biotinylation. We report here that 4a is very effective in its role as a synthetic cofactor and that DNA modified with this substance undergoes facile Staudinger ligation with a biotinylated reagent as shown in rather generic fashion by Scheme 2. We also demonstrate here the facile construction and utility of an alkyl azide bearing cofactor 4b. It is significant that both 4a and 4b serve as effective cofactors with multiple DNA methyltransferases and that the lesions created (MTase-dependent) can be biotinylated under biological conditions, and thus also immobilized by virtue of biotin:streptavidin associations. The power of organic synthesis to afford azide-bearing cofactors allows the conversion of DNA MTases into azidonucleosidyl transferases.
MATERIALS AND METHODS
General
pUC19 and all enzymes (unless indicated) were obtained from New England Biolabs. The DNA MTase reactions with M.TaqI were run in buffer A [20 mM Tris–OAc (pH 6.0), 50 mM KOAc, 10 mM Mg(OAc)2, 0.01% Triton X-100]. The DNA MTase reactions with M.EcoRI were ran in buffer B [10 mM Tris–HCl (pH 7.4), 50 mM NaCl, 0.5 mM EDTA, 0.01% Triton X-100] (11). All agarose gels were prepared with a high-melt agarose in 1× TAE containing 0.05 μg/μl ethidium bromide. Following electrophoresis, gels were de-stained for 20 min (1 mM MgSO4, 10 mM 2-mercaptoethanol) prior to visualization. Bands were visualized at 300 nm using a photo-documentation system. Synthetic oligonucleotides were obtained from Sigma-Genosys and gel-purified prior to use. The concentration of the oligonucleotide was determined at 260 nm using the following molar extinction coefficients: 15 400 (A), 11 500 (G), 8700 (T), 7400 (C). The immobilized streptavidin pull-down assay was performed in buffer C (1 M NaCl, 1 mM EDTA and 10 mM sodium phosphate, pH 7.5) (24).
Synthesis of the azido-based cofactor mimics
The synthesis of the aryl azide cofactor mimic, 4a, has been previously described (25). The experimental procedures and corresponding spectral data for 4b and the intermediates 7–12 can be found in Supplementary Material. Stock concentrations were determined using UV/Vis spectroscopy (260 nm). The concentration of 1 was determined as previously described (10). The molar extinction coefficients were determined to be 6633 for the aryl azide cofactor mimic, 4a, and 8900 for the alkyl azide cofactor mimic, 4b.
Restriction/protection assay
Commercially available pUC19 was linearized with R.EcoRI according to manufacturer's protocol (final concentration of 0.2 μg/μl or 114 nM). R.EcoRI was heat-inactivated at 65°C for 15 min prior to further plasmid use. Reaction mixtures were prepared by the addition of appropriate stock solutions to a total volume of 20 μl (in buffer A). The final DNA concentration was 14.3 nM; the final concentrations of the cofactors and the M.TaqI varied upon the specific reaction sequence. All reactions were heated at 65°C for 4 h, followed by cooling to 0°C. The extent of methyltransferase-dependent DNA alkylation was analyzed by the addition of R.TaqI (2 U in an additional 10 μl buffer A), followed by incubation at 65°C for 1 h. Upon cooling to 0°C, Proteinase K (Ambion) (0.02 U in 5 μl H2O) was added to each reaction and incubated at 37°C for 1 h. The extent of alkylation (as indicated by protection from endonuclease cleavage) was visualized by electrophoresis on a 2% agarose gel.
DNA labeling and duplex formation
The synthetic oligonucleotide utilized for M.TaqI reactions contained the sequence d(TGAATCTCGAGCACCC). The 5′ 32P-labeled oligonucleotide was prepared with T4 polynucleotide kinase and [γ-32P]ATP using standard methods (26). The labeled oligonucleotide was desalted and unincorporated [γ-32P]ATP removed via Sephadex G-25 spin column (Amersham). The labeled strand was annealed to its complement d(GGGTGCTCGAGATTCAAA) in 1× TE buffer by heating to 80°C (5 min) and cooling to 4°C over 4 h. A similar procedure to prepare the synthetic oligonucleotide for M.EcoRI was followed, but utilized the sequence d(TGAATGAATTCGACCC) and its complement d(GGGTCGAATTCATTCAAA).
M.TaqI and M.EcoRI reactions with synthetic oligonucleotide
Reaction mixtures were prepared by the addition of appropriate stock solutions to a total volume of 20 μl in either buffer A or buffer B. The final DNA concentration was 1 μM; final concentration of cofactor was 100 μM; the final concentrations of M.TaqI and M.EcoRI were 6 and 2 μM, respectively. All reactions were incubated at 37°C for 18 h, followed by cooling to 0°C. Proteinase K (0.02 U in 2 μl H2O) was added to each reaction and digestions were carried out for 1 h at 37°C. The resulting alkylated and proteolyzed samples were then processed as indicated below.
Staudinger ligation with cofactor-linked 32P-labeled oligonucleotide
An aliquot (5.5 μl, 5 pmol) of cofactor-linked 32P-labeled duplex was combined with 2.5 μl H2O, 1 μl 50 mM NaOH and 1 μl 10 mM biotin-linked phosphine 13 or 15 (in DMF). The final concentration of DNA in the ligation reaction was 500 nM. Additional control reactions were also prepared (containing DMF only). The samples were incubated at 37°C for 14 h. The ligation reaction was either analyzed by DPAGE or ethanol-precipitated (26) with 39 μg tRNA (E.coli, Type XX, Strain W) before subsequent processing and data acquisition.
Immobilized streptavidin pull-down assay
ImmunoPure® Immobilized Streptavidin (Pierce) was prepared by washing twice with 1 M NaCl (total slurry volume of 30 μl) (24). The washed agarose was added to the ligation reaction in a total volume of 20 μl buffer C. The slurry was gently mixed at room temperature for 1 h. The material was transferred to a micro-spin column and unbound DNA was washed away by subsequent resuspension in 1 M NaCl and centrifugation (3 × 50 μl). The extent of radiolabeled DNA retention on the streptavidin-linked agarose was determined by liquid scintillation counting of the micro-spin column matrices.
RESULTS
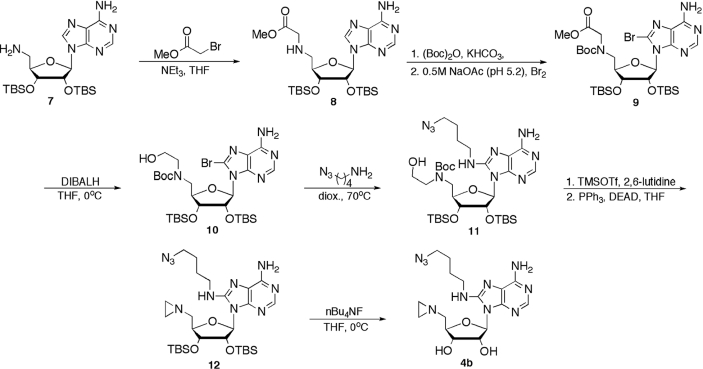
Synthesis of alkyl azide cofactor
The structure and corresponding construction of alkyl azide cofactor 4b differs significantly from that of aryl azide 4a (Scheme 3). Installation of the azide-capped butane moiety dictated C5′ elaboration prior to base modification. Beginning with an intermediate previously described (27), the primary amine 7 was alkylated with the methyl bromoacetate to provide 8 in 85% yield. Due to anticipated difficulties in achieving effective C8 bromination, the secondary amine functionality of 8 was protected as the t-butyl carbamate (28). Subsequent bromination under mildly acidic conditions (29) yielded 9 in 89% yield from 8. Reduction of the methyl ester to the primary alcohol 10 with DIBALH (30) occurred with a yield of 81%, followed by SNAr reaction with 4-azido-butylamine (31), to yield guanidine 11 in 49% yield (32). Following Boc deprotection using TMSOTf (33), the crude 5′ ethanolamine was converted to aziridine 12 utilizing Mitsunobu conditions (34) in 54% over the two steps. Finally, the silyl ethers were removed with nBu4NF (35) to yield the desired alkyl azide cofactor mimic 4b, in 60% yield.
Scheme 3.
Azidonucleoside construction.
M.TaqI-mediated DNA alkylation
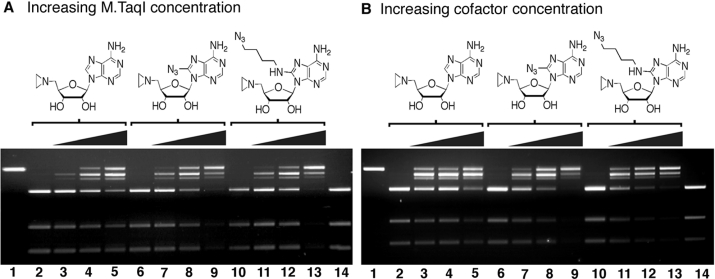
A restriction/protection assay previously described was utilized to analyze the extent of MTase-dependent DNA alkylation by the unsubstituted aziridine cofactor 1 and the two azido-based cofactor mimics 4a and 4b (13). Figure 1A illustrates the effect of increasing M.TaqI concentration on the extent of DNA alkylation. In comparing lanes 3–5, 7–9 and 11–13 for the three cofactor mimics at 100 μM, an increase in the amount of DNA alkylation is observed with a gradient ranging from 20 to 200 nM M.TaqI as reflected by the increased protection from the R.TaqI digestion. Significantly, in the absence of M.TaqI, no protection of the DNA is observed (lanes 2, 6 and 10); none of the cofactors tested thus appeared to inhibit R.TaqI nor was non-specific DNA alkylation by any of the cofactors sufficient to render protection from R.TaqI-mediated plasmid scission. Similar trends were observed with an increase in the amount of cofactor (Figure 1B). Increasing cofactor concentration from 10 to 100 μM coincided with increased protection from R.TaqI as seen in lanes 3–5, 7–9 and 11–13. As indicated by lanes 2, 6 and 10, R.TaqI is not inhibited by the methyltransferase.
Figure 1.
DNA alkylation reactions with pUC19. DNA alkylation reactions of R.EcoRI linearized pUC19 by aziridine cofactor mimics 1, 4a and 4b. Reaction mixtures were prepared by addition of appropriate stock solutions to a total volume of 20 μl containing 14.3 nM DNA buffered with 20 mM Tris–OAc (pH 6.0), 50 mM KOAc, 10 mM Mg(OAc)2, 0.01% Triton X-100. The mixtures were analyzed on a 2% agarose gel run at 120 V for 2 h. (A) Increase in M.TaqI concentration: (1) DNA; (2) DNA, 100 μM cofactor 1, R.Taqα I; (3) DNA, 100 μM 1, 20 nM M.TaqI, R.Taqα I; (4) DNA, 100 μM 1, 100 nM M.TaqI, R.Taqα I; (5) DNA, 100 μM 1, 200 nM M.TaqI, R.Taqα I; (6–9) same as 2–5, but with aryl azide cofactor 4a; (10–13) same as 2–5, but with alkyl azide cofactor 4b; (14) DNA, R.Taqα 1. (B) Increase in cofactor concentration. (1) DNA; (2) DNA, 200 nM M.TaqI, R.Taqα I; (3) DNA, 10 μM cofactor 1, 200 nM M.TaqI, R.Taqα I; (4) DNA, 50 μM 1, 200 nM M.TaqI, R.Taqα I; (5) DNA, 100 μM 1, 200 nM M.TaqI, R.Taqα I; (6–9) same as 2–5, but with aryl azide cofactor 4a; (10–13) same as 2–5, but with alkyl azide cofactor 4b; (14) DNA, R.Taqα I.
Staudinger ligation with enzymatically azidated oligonucleotide
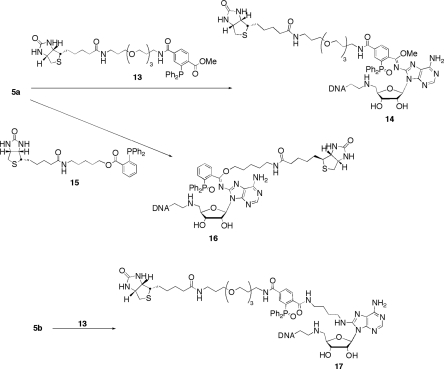
We synthesized the two biotin-linked triarylphosphines (13 and 15, Scheme 4) to assess the effectiveness of Staudinger ligations with cofactor-modified DNA (see Supplementary Material for experimental procedures and corresponding spectral data). Based upon previous results, the o-methoxycarbonyl-functionalized triarylphosphine (13) was expected to ligate with both the aryl and alkyl azide adducts, as depicted in Scheme 4. Alternatively, ester-linked phosphine 15 was anticipated to couple only with the aryl azide-modified DNA based upon previous findings in our lab (21). These expectations were predicated on the knowledge that aryl azides react with o-alkoxycarbonyl triarylphosphines to afford highly stable imidate structures such as 14 or 16. In marked contrast, alkyl azides react with such phosphines to produce amide-linked materials. Alkyl azide reactions with such phosphines invoke dissociation of the ester-derived alkoxide moiety from the ligated species.
Scheme 4.
Biotinylation of azidonucleoside-linked DNA substrates.
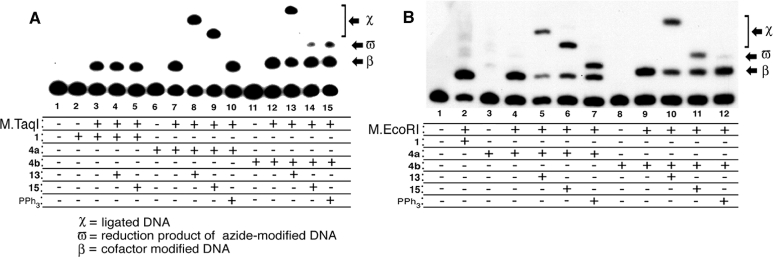
As shown in Figure 2A, a pronounced shift of the DNA mobility in the presence of the three cofactor mimics and M.TaqI (lanes 3, 7 and 12) highlights the covalent modification of interest on the synthetic oligonucleotide. It is important to note that non-specific alkylation is not visible for the three cofactor mimics tested here (lanes 2, 6 and 11). Subjection of 5a to biotinylated triarylphosphine 13 produced a significant reduction in DNA mobility (lanes 8 and 13) consistent with triarylphosphine ligation. Additionally, the biotinylated triarylphosphine 15 only ligated to the product derived from 5a, as seen in lane 9 versus 14. The observation of a new band is evident in lane 14 (reaction of 5b with 15) and is probably the product resulting from the reduction of the azide to the primary amine. This is consistent with the fact that 15 does not ligate to alkyl azides, but rather effects their water-dependent reduction (21). Indeed, the product obtained from treatment with 15 migrates identically to that formed by treatment of 5b with triphenylphosphine (lane 15). Importantly, no shift of 3 occurs in the presence of either phosphine (lanes 4 and 5) and indicates that the observed shift resulting from the addition of the triarylphosphines is, in fact, due to the Staudinger ligation involving the azides of 4a and 4b. In the presence of the phosphines tested here, no alteration in the band mobility occurs for either the DNA or the non-specific alkylation controls (data not shown).
Figure 2.
DNA alkylation reaction of synthetic oligonucleotide by aziridine cofactor mimics 1, 4a and 4b, and the Staudinger ligation of resulting alkylation products. (A) Reaction mixtures were prepared by addition of appropriate stock solutions to a total volume of 20 μl containing 1 μM DNA buffered with 20 mM Tris–OAc (pH 6.0), 50 mM KOAc, 10 mM Mg(OAc)2, 0.01% Triton X-100, 100 μM specified cofactor and 6 μM M.TaqI. (B) Reaction mixtures were prepared by addition of appropriate stock solutions to a total volume of 20 μl containing 1 μM DNA buffered with 10 mM Tris–HCl (pH 7.4), 50 mM NaCl, 0.5 mM EDTA, 0.01% Triton X-100, 100 μM specified cofactor and 2 μM M.EcoRI. Samples that were subjected to ligation conditions were brought to a final DNA concentration of 500 nM and contained a 20-fold excess of triarylphosphine. The mixtures were analyzed on a 20% DPAGE ran at 1800 V for 2 h. For the above figure, components that are inclusive in the reaction are denoted by a ‘+’; components that are exclusive are denoted by a ‘−’.
The attachment of cofactors 1, 4a and 4b to DNA is not restricted to the M.TaqI system. As shown in Figure 2B, we briefly investigated the ability of the enzyme M.EcoRI to transfer these cofactors to a radiolabeled synthetic oligo duplex bearing the sequence d(GAATTC). As evidenced by investigation of lane 2, M.EcoRI is clearly capable of transferring 1. As assessed by independent analysis, the slow mobility material observed in lane 2 moves with a different mobility than that observed in the absence of M.EcoRI and the amount of alkylation observed with enzyme is far greater than that observed in the absence of enzyme (data not shown). Perhaps more intriguing is that the mobility pattern of products formed in lanes 3–12 of panel B are very similar to those of lanes 6–15 in panel A. The activity of M.EcoRI with cofactors 4a and 4b closely parallels that observed with M.TaqI. Careful analysis of lane 5 (panel B) clearly indicates however that Staudinger ligation of the 4a-modified M.EcoRI substrate does not proceed with the same efficiency observed with the analogous M.TaqI case. Also noteworthy is that treatment of the 4a-linked M.EcoRI substrate with PPh3, unlike the case seen in lane 10 of panel A, affords a band with altered mobility relative to the 4a-linked strand (lane 7, panel B). We believe these subtle differences to result from DNA sequence moderated hydrogen-bonding networks characteristic of M.TaqI and M.EcoRI products. Although slight differences exist between M.TaqI and M.EcoRI-derived reactions and products, it is highly significant that 4a and 4b are compatible with multiple DNA methyltransferases.
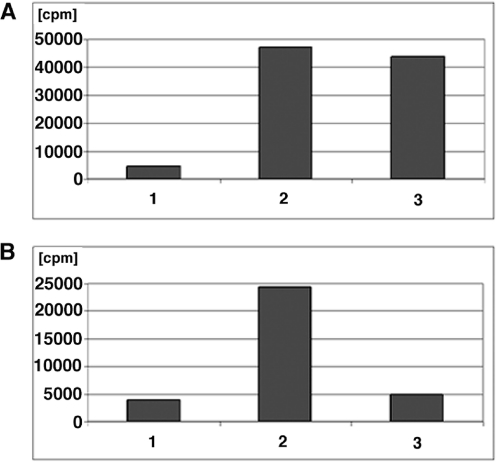
To verify the structural and functional integrity of biotin following the Staudinger ligation, a pull-down assay utilizing agarose-immobilized streptavidin was performed using products derived from M.TaqI reactions. As shown in Figure 3A, scintillation counting of the 5′ end-labeled modified strands revealed a high degree of radioisotopic retention to the streptavidin–agarose in those reactions involving 4a and either 13 or 15 relative to a DMF control devoid of phosphine. Additionally, Figure 3B illustrates the retention of radioactivity for the ligation product of 5b with 13. This observation supports the notion that 5b did not ligate to 15 (lane 14, Figure 2A), consistent with previous efforts in our labs demonstrating that ester-linked triarylphosphines, such as 15, form stable ligation products with aryl azides but not alkyl azides.
Figure 3.
Immobilization of DNA–biotin conjugates on streptavidin–agarose. Reaction mixtures were incubated for 1 h, followed by washing with 1 M NaCl (×3). The amount of DNA retained was quantitated by scintillation counting. (A) Binding reactions performed on 5a. Bar 1. 5a + DMF, Bar 2. 5a + 13, Bar 3. 5a + 15. (B) Binding reactions performed on 5b. Bar 1. 5b + DMF, Bar 2. 5b + 13, Bar 3. 5b + 15.
DISCUSSION
The conversion of biological methyltransferases into azidonucleosidyl transferases represents a significant advancement by virtue of now widely recognized and useful abiotic chemoselective ligation methods. The production of synthetic cofactors similar in structure to SAM but allowing for elaborate post-enzymatic modifications permits site-specific modification of biomolecules in a way not previously known. Importantly, MTase-dependent azidation of large biomolecular substrates may be used not only to modify known substrates of methylation, but also to aid in the isolation and identification of currently unknown substrates of methylation, be they nucleic acids or perhaps proteins. Indeed, as attention at the chemistry biology interface continues to intensify on understanding the roles of DNA and protein methylation, particularly in the realm of transcriptional regulation, synthetic agents capable of intervening in biosynthetic processes will become attractive tools to more readily answer biological questions.
M.TaqI and M.EcoRI both promote DNA azidation upon presentation with an appropriate substrate and either 4a or 4b. Qualitative analysis of M.TaqI promoted alkylation of linearized pUC19 can be made by observing the intensity and location of modified and/or unmodified DNA bands in Figure 1. In comparing the efficiency of M.TaqI-dependent DNA alkylation with the three cofactors, it appears that 4a and 4b are better cofactors for M.TaqI than is the unsubstituted cofactor 1. This can be deduced from the almost complete disappearance of the smaller restriction fragments in reactions containing 4a and 4b, relative to 1. Using a small synthetic oligonucleotide, it was possible to assess the activity of M.EcoRI with 1, 4a and 4b. As noted before, M.EcoRI, like M.TaqI, is highly amenable to DNA modification with all three cofactors. These results implicate both 4a and 4b as potentially powerful tools by which to modify both known and unknown biological substrates of adenine MTases. Notably, azide-bearing cofactors are not restricted to use by adenine methyltransferases. In addition to M.TaqI and M.EcoRI, we examined two cytosine C5 methylases M.HhaI and M.HpaII. Of the two enzymes, M.HhaI was capable of catalyzing the transfer of aryl azide 4a and alkyl azide 4b, albeit with a lower efficiency than M.TaqI. M.HpaII was compatible with the unsubstituted cofactor mimic 1, but was not able to catalyze the transfer of either azido-based cofactor (unpublished results). From these early efforts, it was clear that the nucleic acid chemistry of azide-bearing cofactors is, in reality, not restricted to adenine methyltransferases. But can azide-bearing DNA substrates undergo ligation chemistries as initially hypothesized?
Initial ligation model studies focused on the ability of cofactor precursors with aryl and alkyl azides to undergo the Staudinger ligation and Huisgen [2 + 3] cycloaddition reactions under biological conditions [(21), unpublished results]. HPLC analysis of an aryl azide derivative indicated fast reaction times and high yields with triarylphosphines bearing the core of 13 and 15. The alkyl azide derivative also underwent ligation to a terminal alkyne in a similarly facile manner. However, we found the aryl azide to be incompatible with alkyne ligations under a wide array of reaction conditions. Thus, it was concluded that the aryl azide cofactor mimic, 4a, would be well suited for the Staudinger ligation, whereas the alkyl azide cofactor mimic, 4b, would be compatible with both the Staudinger ligation (although not as fast as 4a) and Click chemistry.
Recent applications of Cu(I)-catalyzed Click chemistry emphasize the reliable Huisgen dipolar cycloaddition between an azide and alkyne. This coupling, in which both components are abiotic, has become an important method for coupling subunits together with a high thermodynamic driving force. Applications have ranged from modifications of enzyme active sites to the cell surfaces of E.coli (18,19), and continue to evolve on an almost daily basis. Although the methodology has been successful with non-nucleic acid substrates, the Cu(I)-catalyzed cycloaddition in the presence of DNA leads to rapid destruction of nucleic acids via Haber Weiss chemistry (36,37). As a result, this ordinarily useful methodology was deemed unsuitable for the purposes highlighted here and emphasis was placed on the Staudinger ligation.
Initial efforts to couple biotinylated reagents 13 and 15 with azide-linked pUC19 failed to yield discernible ligation via the streptavidin pull-down assay. Two hypotheses were developed to explain this shortcoming: the first involved the low DNA concentration and the second took into account the sterics imparted by the larger piece of DNA. Thus, our attention was turned to the use of a synthetic oligonucleotide duplex to perform the MTase-dependent alkylations, followed by the Staudinger ligation. Not only did this ultimately allow us to perform the ligations at a higher DNA concentration, but even subtle structural differences between DNA adducts could be readily visualized with denaturing polyacrylamide gel electrophoresis.
The ability of the o-methoxycarbonyl functionalized triarylphosphine 13 and the triarylphosphine 15 to undergo the Staudinger ligation on DNA modified with the two azido cofactor mimics has been demonstrated. Based upon previous observations and mechanistic rationale (16,21), the ability of 13 to couple with both the alkyl and aryl azide cases was validated. As expected, the extent of ligation to the alkyl azide-modified DNA was lower than that observed with the aryl azide. This is perhaps best rationalized when one considers the significantly greater electron density (and thus, reduced electrophilic character) of alkyl azides relative to the more electron poor aryl azides. Also noteworthy is that formation of the imidate structures characteristic of purported ligation adducts 14 and 16 (Scheme 4) calls for a significantly abbreviated mechanistic pathway than that involved in the formation of amide structures like 17 (21). Although phosphine 15 is compatible only with aryl azides, its ligation to azide-modified DNA is remarkably efficient. The principal advantage to 15 lies in its ease of preparation. Unlike the phosphine developed by Bertozzi, which requires multiple synthetic manipulations and tedious recrystallizations to purify intermediates (14), 15 can be readily produced from a commercially available triphenylphosphinic acid via attachment to one's linker of choice through simple esterification procedures (21) [see Supplementary Material for experimental procedures and corresponding spectral data].
Having validated the hypothesis that enzymatically azidated DNA was compatible with the Staudinger ligation, it was essential to demonstrate the utility of this methodology in identifying and isolating biological molecules. The high binding affinity between avidin and biotin prompted us to choose this affinity matrix handle to demonstrate this methodology (38). One could easily exploit the avidin–biotin interaction by performing an electrophoretic mobility shift (gel shift) assay or by one of several technologies based on the strength of this interaction. We opted to use an immobilized streptavidin that would allow us to wash away unbound molecules, leaving the biotinylated product bound to the agarose (24). Selective 5′ end-labeling of the DNA duplex allowed the application of liquid scintillation counting for detection of avidin-bound materials. The results obtained reveal the cofactor, methylase and ligation-dependent retention of radiolabeled DNA to the immobilized agarose relative to the DMF control. Data obtained from ethanol precipitations must be considered relative to necessary controls as the precipitations are not quantitative and ∼20% is lost. This loss is, however, consistent for all samples regardless of conditions to which they have been subjected.
The data presented here not only validates the integrity of the azido-based cofactor mimics following enzymatic transfer to substrate DNA, but also verifies that affinity-tagged triarylphosphines undergo ligation to azide-linked DNA under biological conditions. The conversion of biological methylases into azidonucleosidyl transferases through cofactor mimicry has thus been established. Although demonstrated for nucleic acids here, we project that azide-bearing cofactors hold tremendous promise as new tools for those at the chemical biology interface.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online. Contained in these materials are preparation and characterization details for substances 7–12, 4b, 13 and 15.
Supplementary Material
Acknowledgments
We would like to thank Dr Derek Robinson of New England Biolabs for supplying the M.TaqI. We thank our colleague José A. Restituyo for very helpful discussions. We would also like to acknowledge Ricky R. Savjani for the work done with M.HpaII. L.R.C. would like to thank the American Foundation for Pharmaceutical Education (AFPE) for a Pre-Doctoral Fellowship in the Pharmaceutical Sciences. We gratefully acknowledge the University of Wisconsin Graduate School and School of Pharmacy for generous startup funding. Funding to pay the Open Access publication charges for this article was provided by University of Wisconsin Graduate School.
Conflict of interest statement. None declared.
REFERENCES
- 1.Nakayama J., Rice J.C., Strahl B.D., Allis C.D., Grewal S.I. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 2.Cheung P., Allis C.D., Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Xu W., Chen H., Du K., Asahara H., Tini M., Emerson B.M., Montminy M., Evans R.M. A transcriptional switch mediated by cofactor methylation. Science. 2001;294:2507–2511. doi: 10.1126/science.1065961. [DOI] [PubMed] [Google Scholar]
- 5.Chen D., Ma H., Hong H., Koh S.S., Huang S.M., Schurter B.T., Aswad D.W., Stallcup M.R. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 6.Turner B.M. Cellular memory and the histone code. Cell. 2002;111:285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- 7.Robertson K.D. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20:3139–3155. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 8.Patra S.K., Patra A., Dahiya R. Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochem. Biophs. Res. Comm. 2001;287:705–713. doi: 10.1006/bbrc.2001.5639. [DOI] [PubMed] [Google Scholar]
- 9.Szyf M. Towards a pharmacology of DNA methylation. Trends Pharmacol. Sci. 2001;22:350–354. doi: 10.1016/s0165-6147(00)01713-2. [DOI] [PubMed] [Google Scholar]
- 10.Pignot M., Siethoff C., Linscheid M., Weinhold E. Coupling of a nucleoside with DNA by a methyltransferase. Angew. Chem. Int. Ed. 1998;37:2888–2891. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2888::AID-ANIE2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Pignot M., Siethoff C., Linscheid M., Weinhold E. European patent WO 00/06578. 2000
- 12.Pljevaljcic G., Pignot M., Weinhold E. Design of a new fluorescent cofactor for DNA methyltransferases and sequence-specific labeling of DNA. J. Am. Chem. Soc. 2003;125:3486–3492. doi: 10.1021/ja021106s. [DOI] [PubMed] [Google Scholar]
- 13.Pljevaljcic G., Schmidt F., Weinhold E. Sequence-specific methyltransferase-induced labeling of DNA (SMILing DNA) ChemBioChem. 2004;5:265–269. doi: 10.1002/cbic.200300739. [DOI] [PubMed] [Google Scholar]
- 14.Saxon E., Bertozzi C.R. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 15.Saxon E., Luchansky S.J., Hang H.C., Yu C., Lee S.C., Bertozzi C.R. Investigating cellular metabolism of synthetic azidosugars with the Staudinger ligation. J. Am. Chem. Soc. 2002;124:14893–14902. doi: 10.1021/ja027748x. [DOI] [PubMed] [Google Scholar]
- 16.Kiick K.L., Saxon E., Tirrell D.A., Bertozzi C.R. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl Acad. Sci. USA. 2002;99:19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kho Y., Kim S.C., Jiang C., Barma D., Kwon S.W., Cheng J., Jaunbergs J., Weinbaum C., Tamanoi F., Falck J., et al. A tagging-via-substrate technology for detection and proteomics of farnesylated proteins. Proc. Natl Acad. Sci. USA. 2004;101:12479–12484. doi: 10.1073/pnas.0403413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link A.J., Tirrell D.A. Cell surface labeling of Escherichia coli via copper(I)-catalyzed [3+2] cycloaddition. J. Am. Chem. Soc. 2003;125:11164–11165. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- 19.Speers A.E., Adam G.C., Cravatt B.F. Activity-based protein profiling in vivo using a copper(I)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 20.Kolb H.C., Finn M.G., Sharpless K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Restituyo J.A., Comstock L.R., Petersen S.G., Stringfellow T., Rajski S.R. Conversion of aryl azides to O-alkyl imidates via modified Staudinger ligation. Org. Lett. 2003;5:4357–4360. doi: 10.1021/ol035635s. [DOI] [PubMed] [Google Scholar]
- 22.Reich N.O., Everett E.A. Identification of peptides involved in S-adenosylmethionine binding in the EcoRI DNA methylase. J. Biol. Chem. 1990;265:8929–8934. [PubMed] [Google Scholar]
- 23.Kaiser I.I., Kladianos D.M., Van Kirk E.A., Haley B.E. Photoaffinity labeling of catechol O-methyltransferase with 8-azido-S-adenosylmethionine. J. Biol. Chem. 1983;258:1747–1751. [PubMed] [Google Scholar]
- 24.Syvanen A.C., Laaksonen M., Soderlund H. Fast quantification of nucleic acid hybrids by affinity-based hybrid collection. Nucleic Acids Res. 1986;14:5037–5048. doi: 10.1093/nar/14.12.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comstock L.R., Rajski S.R. Efficient synthesis of azide-bearing cofactor mimics. J. Org. Chem. 2004;69:1425–1428. doi: 10.1021/jo035485z. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J., Russell D. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. pp. 9.68–9.69. 10.20–10.21. [Google Scholar]
- 27.Comstock L.R., Rajski S.R. Expeditious synthesis of aziridine based cofactor mimics. Tetrahedron. 2002;58:6019–6026. [Google Scholar]
- 28.Kolb M., Barth J. Synthesis of 5′-[(3-aminooxypropyl)amino]-5′-deoxyadenosine. Liebigs Ann. Chem. 1985:1036–1040. [Google Scholar]
- 29.Urata H., Miyagoshi H., Yumoto T., Akagi M. Racemic synthesis of carbocyclic purine nucleoside analogues with restricted glycosyl conformation. J. Chem. Soc. Perkin Trans. 1999;1:1833–1838. doi: 10.1093/nass/42.1.45. [DOI] [PubMed] [Google Scholar]
- 30.Gano K.W., Monbouquette H.G., Myles D.C. An efficient synthesis of a class of heterobifunctional photo-reactive crosslinkers, labels, and probes. Tetrahedron Lett. 2001;42:2249–2251. [Google Scholar]
- 31.Lee J.W., Jun S.I., Kom K. An efficient and practical method for the synthesis of mono-N-protected α,ω-diaminoalknaes. Tetrahedron Lett. 2001;42:2709–2711. [Google Scholar]
- 32.Roelen H., Veldman N., Spek A.L., von Frijtag Drabe Kunzel J.K., Mathot R.A.A., IJzerman A.P. N6,C8-Disubstituted adenosine derivatives as partial agonists for adenosine A1 receptors. J. Med. Chem. 1996;39:1463–1471. doi: 10.1021/jm950267m. [DOI] [PubMed] [Google Scholar]
- 33.Sakaitani M., Ohfune Y. Syntheses and reactions of silyl carbamates. 1. Chemoselective transformation of amino protecting groups via tert-butyldimethylsilyl carbamates. J. Org. Chem. 1990;55:870–876. [Google Scholar]
- 34.Lindstrom U.M., Somfai P. Aminolysis of vinyl epoxides as an efficient entry to N-H vinylaziridines. Synthesis. 1998:109–117. [Google Scholar]
- 35.Van der Wende E.M., Carnielli M., Roelen H.C.P.F., Lorenzen A., von Frijtag Drabe Kunzel J.K., IJzerman A.P. 5′-Substituted adenosine analogs as new high-affinity partial agonists for the adenosine A1 receptor. J. Med. Chem. 1998;41:102–108. doi: 10.1021/jm970508l. [DOI] [PubMed] [Google Scholar]
- 36.Kanan M.W., Rozenman M.M., Sakurai K., Snyder T.M., Liu D.R. Reaction discovery enabled by DNA-templated synthesis and in vitro selection. Nature. 2004;431:545–549. doi: 10.1038/nature02920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erdem G., Oner C., Onal A.M., Kisakurek D., Oaus A. Free radical mediated interaction of ascorbic acid and ascorbate/Cu(II) with viral and plasmid DNAs. J. Biosci. 1994;19:9–17. [Google Scholar]
- 38.Wilchek M., Bayer E.A. The avidin–biotin complex in bioanalytical applications. Anal. Biochem. 1988;171:1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.