Abstract
Four strains of Butyrivibrio fibrisolvens, transformed with a gene encoding fluoroacetate dehalogenase, maintained a combined population of 106 to 107 cells ml−1 in the rumens of test sheep. Five inoculated sheep showed markedly reduced toxicological symptoms after fluoroacetate poisoning when behavioral, physiological, and histological effects were compared with those of five uninoculated control sheep.
Monofluoroacetate occurs in some Australian plants at levels of up to 5 g kg (dry weight)−1 (8) and has a 50% lethal dose in ruminants of around 0.3 mg kg of body weight−1 (2). The toxin appears to be cumulative, and a series of sublethal doses can result in fatal poisoning (2). It is not surprising, therefore, that loss of livestock from fluoroacetate poisoning is economically significant (12). The work described here is part of a project to develop genetically modified ruminal bacteria that will provide protection against fluoroacetate poisoning.
Three Butyrivibrio fibrisolvens strains, OB156, OB291, and 10/1, were transformed with plasmid pBHf by electroporation (3, 7) and expressed fluoroacetate dehalogenase activity at 10 nmol of fluoride released min−1 mg of bacterial protein−1 (7). Strain OR85 expressed dehalogenase activity at (20 ± 1.7) nmol min−1 mg−1 when transformed with a pBHf derivative with four base pairs removed from between the putative −10 and −35 regions of the gene promoter (pBHf−4) (unpublished data). OB156, OB291, and OR85 were supplied by R. Forster and R. M. Teather (4) (CFAR, Agriculture and Agrifood Canada, Ottawa, Ontario, Canada). B. fibrisolvens 10/1 was isolated from sheep (11) at the Institute of Biotechnology (University of New England, Armidale, New South Wales, Australia).
Recombinant bacteria were grown in ruminal fluid medium (10) containing yeast extract in place of peptone. Erythromycin was added (50 μg ml−1) except in subcultures prepared for inoculation into the rumen. In previous observations (5, 6), the population of individual bacterial strains within the rumen fluctuated within the range <103 to 107 cells ml−1. Therefore, a combination of four modified strains was used to ensure that effective numbers of detoxifying bacteria would be present consistently. Fifty-milliliter stationary-phase cultures of each strain (108 to 109 cells ml−1) were pooled, and 60 ml of the mixture was inoculated into the rumen of each test animal.
Trial I used four Merino/Border-Leicester crossbred sheep, and trial II used six pure-bred Merino sheep; all sheep were housed in metabolism crates in a PC2-classified room. Sheep received four meals daily (150 g of oat chaff plus 50 g of alfalfa chaff) between 10 a.m. and 4 p.m. Water was provided ad libitum.
Sheep rumens were surgically cannulated for removal of samples. These included particles <1 mm in diameter. Samples were heated (95°C for 10 min) to inactivate nucleases and centrifuged (12,000 × g for 90 s), and the supernatant was removed. The pellet was resuspended in water (10 times the volume of the original sample) and recentrifuged. This procedure was repeated once, and the final pellet was suspended in 10 volumes of water. Dilutions of 10−2, 10−3, 10−4, and 10−5 were prepared and tested by PCR for the presence of dehalogenase gene sequences (1).
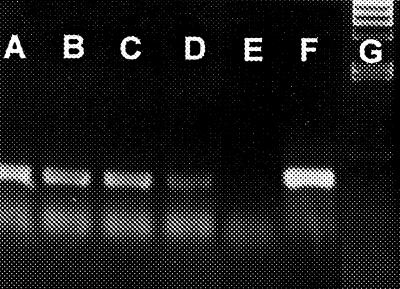
PCR primers used for dehalogenase gene detection were CC5 (5′-TGCGAGGCTATGGCGATTCGGACA-3′) and CC6.2i (5′-CTGACCGATCATGTGCTCGGGGAA-3′), both of which amplified a 322-bp fragment from the coding region of the dehalogenase gene (9). PCR conditions were as follows: 2 cycles of 95°C for 5 min, 55°C for 40 s, and 72°C for 60 s followed by 28 cycles of 95°C for 60 s, 55°C for 40 s, and 72°C for 60 s. Tests on ruminal samples to which transformed bacteria had been added showed that pBHf was detectable in the presence of a single target bacterium. Inclusion of 1 μl of sample in the PCR mixture allowed a threshold of detection of 103 cells ml−1. Detection of the plasmid at a dilution of 10−4 (for example) indicated a ruminal population of ≥107 cells ml−1 in the original sample (Fig. 1). Preinoculation ruminal samples yielded no dehalogenase gene PCR products and were routinely used as negative controls.
FIG. 1.
Agarose gel with PCR products from 10-fold serial dilutions of ruminal particles from sheep T1 (trial I). Lanes and fold dilutions of ruminal samples are as follows: A, 10−1; B, 10−2; C, 10−3; D, 10−4; E, 10−5. Aliquots of 1 μl were used in each reaction. Lane F is the positive control product, and lane G contains λ HindIII size markers. The diffuse lower band was a commonly observed artifact in PCR that used complex biological mixtures as a source of template.
Fluoroacetate solution was injected into snow peas or absorbed into feed pellets or alfalfa biscuits. These were usually eaten voluntarily, even when chaff was refused. The biscuits were ≈0.5 g each and were made from alfalfa which had been blended to a coarse slurry with a 10% sucrose solution, pressed into discs to remove most of the fluid, and dried overnight at 60°C. On the three occasions that control sheep 2 (C2) (trial II) refused to eat them, the biscuits were placed into the rumens of both C2 and test sheep 2 (T2) via the cannula. Continuous assessments were made of feeding, drinking, response to external stimuli, stance, movement, and sleeping behavior. In trial II, heart rates were measured with an electrocardiograph.
In trial I, sheep were challenged with fluoroacetate 10 days after inoculation (Table 1), when the modified strains had established a stable combined population of >107 cells ml−1 in T1 and >106 cells ml−1 in T2. In trial II, the recombinant bacteria were allowed five weeks to stabilize their population to >106 cells ml−1 in all three sheep before challenge with fluoroacetate. Recombinant bacteria were undetectable in control sheep.
TABLE 1.
Fluoroacetate dosage schedule for toxicity trials I and II
| Toxicity trial | Time period | Dose every: | Dose increment(s), mg kg−1 | Accumulated dose, mg kg−1 |
|---|---|---|---|---|
| I | 0–10 h | 2 h | 1 × 0.045, 5 × 0.03 | 0.195 |
| 20–22 h | 2 h | 2 × 0.03 | 0.255 | |
| 45–57 h | 6 h | 2 × 0.015, 1 × 0.012 | 0.297 | |
| 140–152 h | 1 h | 13 × 0.015 | 0.492 | |
| II | 0–12 h | 2 h | 6 × 0.03, 1 × 0.015 | 0.195 |
| 22–24 h | 2 h | 2 × 0.03 | 0.255 | |
| 38–42 h | 2 h | 3 × 0.015 | 0.300 | |
| 46 h | NA | 1 × 0.03 | 0.330 |
NA, not applicable.
Trial I.
At a cumulative dose of 0.255 mg of fluoroacetate kg−1, control sheep exhibited alarm at familiar noises, compulsive agitated feeding, periodic muscle spasms, “paddling” movements of the legs, skin flushing, panting, and frequent attempts to escape from their crates by jumping. Inoculated test sheep showed occasional head twitching and rapid breathing and periodically increased alertness but remained calm, eating and drinking normally.
At 0.492 mg of fluoroacetate kg−1, control sheep showed intensification of all symptoms and died. Behavior of both test sheep remained unchanged. T2 displayed no signs of acute toxicity but, four hours after the death of the control sheep, died of pulmonary edema, which is common in chronic fluoroacetate poisoning (3a). Sheep T1 survived the test.
Trial II.
At 0.255 mg of fluoroacetate kg−1, control sheep became lethargic and refused food and water. Hyperexcitability and compulsive eating did not occur, but muscle weakness was evident from their faltering stance. Test sheep showed no toxicity symptoms.
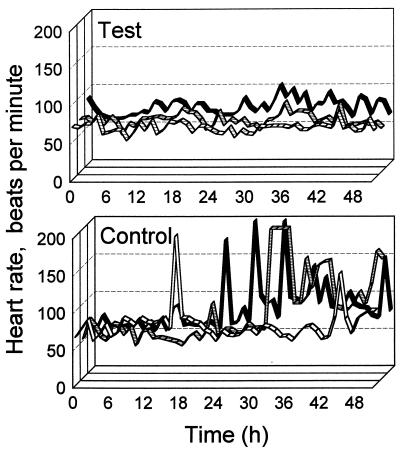
At 0.33 mg of fluoroacetate kg−1, control sheep continued to refuse food and remained lethargic, with pronounced unsteadiness when standing. Electrocardiograms showed heart rates rising to 140 to 200 beats min−1. Test sheep reduced their feed intake approximately 30% but otherwise behaved normally, with maximum heart rates of 80 to 100 beats min−1 (T3 only [Fig. 2]). Prior to administration of fluoroacetate, heart rates of all sheep were in the range of 60 to 80 beats min−1. All sheep survived trial II.
FIG. 2.
Heart rate measurements from sheep in trial II commencing 12 h after the first dose of fluoroacetate in the series (Table 1). Heart rates measured for these sheep the day before dosing were in the range of 60 to 80 beats min−1. The three lines (from the front to the back of the three-dimensional diagram) represent measurements from sheep T1 to T3 and C1 to C3, respectively.
Control sheep displayed reduced appetite to day 6, continued unsteadiness on standing up to day 5, and involuntary muscle activity (head twitching) through day 4. T1 and T2 returned to normal eating habits by day 4, and T3 continued to eat approximately 30% less than usual up to day 6. In all other respects, test sheep remained asymptomatic.
Trial II sheep were euthanized with pentobarbitone (Lethobarb), and necropsies were performed by Rob Woodgate and Bruce Chick of the Veterinary Health Research Laboratory, Armidale, New South Wales, Australia. Tissue samples were fixed in 10% formaldehyde for histological examination (S. Hum, Regional Veterinary Laboratory, Menangle, New South Wales, Australia). The control sheep displayed measurably greater tissue damage (Table 2).
TABLE 2.
Pathological and histological observations
| Tissue and feature | Result for sheep:
|
|||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | T1 | T2 | T3 | |
| Liver | ||||||
| Color | Dark | Dark | Dark | Normal | Normal | Normal |
| Congestion | Marked | Marked | Marked | Mild | Mild | Moderate |
| Mononuclear cell infiltration | None | Mild | Mild | Very mild | None | None |
| Lungs | ||||||
| Congestion | Moderate | Severe | Moderate | Mild | Mild | Severe |
| Texture | Consolidated firm | Consolidated firm | Normal | Normal | Normal | Normal |
| Atelectasia and focal emphysema | Mild | Mild | None | None | None | None |
| Perivascular mononuclear cell infiltrate | Mild | Mild | None | None | None | None |
| Kidneys | ||||||
| Color | Dark | Dark | Dark | Normal | Normal | Normal |
| Protein inclusions: proximal tubules | Present | None | Present | None | None | None |
| Heart (multifocal mononuclear cell infiltration) | Mild/moderate | Mild/moderate | Moderate/focally severe | None | Very mild | Very mild |
The intraruminal levels of modified bacteria achieved here allowed us to demonstrate their protective effect upon host animals, but an increased level of protection would be desirable for field trials with larger numbers of animals. This would require additional recombinant strains in the rumen and/or more efficient expression of dehalogenase from each strain. Current work is aimed to achieve these improvements.
Acknowledgments
This work was conducted at the University of New England, Armidale, New South Wales, Australia. Funding was provided by Applied Biotechnology Ltd. (Queensland), The Australian Meat Research Corporation, and The Faculty of the Sciences, University of New England.
We thank B. Entsch for administration of project funds, S. Atkinson for veterinary services, B. Chick and R. Woodgate for veterinary pathology, and staff of the Animal Sciences Department and the University of New England animal house for their assistance.
REFERENCES
- 1.Allen G. Polymerase chain reaction techniques for the study of rumen bacterial ecology and biotechnology. Ph.D. thesis. Armidale, New South Wales, Australia: University of New England; 1998. [Google Scholar]
- 2.Annison E F, Hill K J, Lindsay D B, Peters R A. Fluoroacetate poisoning in sheep. J Comp Pathol. 1960;70:145–155. doi: 10.1016/s0368-1742(60)80014-8. [DOI] [PubMed] [Google Scholar]
- 3.Beard C E, Hefford M A, Forster R J, Sontakke S, Teather R M, Gregg K. A stable and efficient transformation system for Butyrivibrio fibrisolvens OB156. Curr Microbiol. 1995;30:105–109. doi: 10.1007/BF00294191. [DOI] [PubMed] [Google Scholar]
- 3a.Chick, B. Personal communication.
- 4.Forster R B, Gong J, Teather R M. Group-specific 16S rRNA hybridization probes for determinative and community structure studies of Butyrivibrio fibrisolvens in the rumen. Appl Environ Microbiol. 1997;63:1256–1260. doi: 10.1128/aem.63.4.1256-1260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregg K. Engineering gut flora of ruminant livestock to reduce forage toxicity: progress and problems. Trends Biotechnol. 1995;13:418–421. doi: 10.1016/S0167-7799(00)88995-7. [DOI] [PubMed] [Google Scholar]
- 6.Gregg K, Allen G, Bauchop T, Klieve A, Lincoln M. Practical genetic engineering of rumen bacteria. In: Farrell D J, editor. Recent advances in animal nutrition in australia—1993. Armidale, New South Wales, Australia: University of New England; 1993. pp. 13–21. [Google Scholar]
- 7.Gregg K, Cooper C L, Schafer D J, Sharpe H, Beard C E, Allen G, Xu J. Detoxification of the plant toxin fluoroacetate by a genetically modified rumen bacterium. Bio/Technology. 1994;12:1361–1365. doi: 10.1038/nbt1294-1361. [DOI] [PubMed] [Google Scholar]
- 8.Hall R J. The distribution of organic fluorine in some toxic tropical plants. New Phytol. 1972;71:855–871. [Google Scholar]
- 9.Kawasaki H, Tsuda K, Matsushita I, Tonomura K. Lack of homology between two haloacetate dehalogenase genes encoded on a plasmid from Moraxella species strain B. J Gen Microbiol. 1992;138:1317–1323. doi: 10.1099/00221287-138-7-1317. [DOI] [PubMed] [Google Scholar]
- 10.Klieve A V, Hudman J F, Bauchop T. Inducible bacteriophages from ruminal bacteria. Appl Environ Microbiol. 1989;55:1630–1634. doi: 10.1128/aem.55.6.1630-1634.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopecný J, Fliegerová K, Gregg K. Transformation of B. fibrisolvens strains with pBHf plasmid. Reprod. Nutr. Dev. (Suppl. 1) 1998. pp. 72–73. [Google Scholar]
- 12.McCosker T. Ruminal detoxification of fluoroacetate. Agric Sci New Ser. 1989;2:46–47. [Google Scholar]