Abstract
Background: Our aim was to estimate the rates of not achieving a robust/above-average humoral response to the COVID-19 mRNA vaccine in people living with HIV (PLWH) who received ≥2 doses and to investigate the role of the CD4 and CD4/CD8 ratio in predicting the humoral response. Methods: We evaluated the humoral anti-SARS-CoV-2 response 1-month after the second and third doses of COVID-19 mRNA vaccine as a proportion of not achieving a robust/above-average response using two criteria: (i) a humoral threshold identified as a correlate of protection against SARS-CoV-2 (<90% vaccine efficacy): anti-RBD < 775 BAU/mL or anti-S < 298 BAU/mL, (ii) threshold of binding antibodies equivalent to average neutralization activity from the levels of binding (nAb titer < 1:40): anti-RBD < 870 BAU/mL or anti-S < 1591 BAU/mL. PLWH were stratified according to the CD4 count and CD4/CD8 ratio at first dose. Logistic regression was used to compare the probability of not achieving robust/above-average responses. A mixed linear model was used to estimate the mean anti-RBD titer at various time points across the exposure groups. Results: a total of 1176 PLWH were included. The proportions of participants failing to achieve a robust/above-average response were significantly higher in participants with a lower CD4 and CD4/CD8 ratio, specifically, a clearer gradient was observed for the CD4 count. The CD4 count was a better predictor of the humoral response of the primary cycle than ratio. The third dose was pivotal in achieving a robust/above-average humoral response, at least for PLWH with CD4 > 200 cells/mm3 and a ratio > 0.6. Conclusions: A robust humoral response after a booster dose has not been reached by 50% of PLWH with CD4 < 200 cells mm3. In the absence of a validated correlate of protections in the Omicron era, the CD4 count remains the most solid marker to guide vaccination campaigns in PLWH.
Keywords: HIV, PLWH, CD4 count, CD4/CD8 ratio, SARS-CoV-2 mRNA vaccine, humoral response
1. Introduction
Since the beginning of the COVID-19 pandemic, concerns regarding the higher susceptibility of PLWH to SARS-CoV-2 infection and worse clinical outcomes have been raised, considering long-lasting HIV-driven immune dysfunction, immune-senescence and the higher prevalence and earlier incidence of age-associated comorbidities. Over time, contradictory results on the clinical outcomes of COVID-19 have emerged [1,2], but collectively, the scientific literature agrees that a lack of viro-immunological control (low CD4+T lymphocyte cell counts and unsuppressed viral load) should be considered as a risk factor for the clinical progression of COVID-19 in PLWH [3]. Hence, since 2021, PLWH have been prioritized for COVID-19 vaccination, the main measure used to reduce the severity and mortality of COVID-19. It is therefore critical to quantify the COVID-19-vaccine-induced humoral response, the peak and subsequent decline of response, particularly in the group with a higher risk of progression, in order to also further understand the potential benefits of offering additional vaccine doses.
The immunogenicity of mRNA vaccines (mRNA-1273 and BNT162b2) has been extensively studied in people living with HIV (PLWH) and has been reported to be similar to that of the general population, particularly in people with well-controlled HIV infection [4,5]. In contrast, PLWH with low CD4+T lymphocyte cell counts, detectable viremia and/or previous AIDS have weaker humoral responses [4,5,6,7,8,9,10,11,12], suggesting that they might benefit from additional vaccine doses. For this reason, the CD4 count level has been used to prioritize vaccination in PLWH.
The CD4/CD8 ratio is another well-studied marker of immune dysfunction in HIV in addition to the absolute CD4 count [13] and has also been implicated in predicting the magnitude of the T-cell immune response after natural SARS-CoV-2 infection [14]. There is also some evidence that the CD4/CD8 ratio might also have a role in predicting the vaccine response in PLWH. This was documented in a study with inactivated vaccine showing that the humoral response of PLWH with a low CD4/CD8 ratio was weaker than those with a medium or high ratio [15]. In addition, it has been shown that the inversion of the CD4/CD8 ratio (<1), despite CD4 restoration, reflects underlying immune activation and immune-senescence, deeply related to a poor vaccine response [16]. However, the role of the CD4/CD8 ratio in predicting humoral immunity remains unclear [14], and currently, the CD4/CD8 ratio is not considered in guidelines as a criterion for vaccine prioritization.
Several studies have suggested the designation of vaccine-elicited neutralizing antibody (nAbs) titers as a correlate of protection (CoP) against SARS-CoV-2 infection, and nAbs have been accepted by regulatory authorities; however, a reliable threshold has not yet been established to date [17].
Gilbert et al. attempted to derive a humoral threshold for CoPs by identifying the humoral response level that was associated with preventing 90% of symptomatic SARS-CoV-2 infections (VE90%) in the Moderna COVE phase 3 clinical trial (mRNA 1273 vaccine); this was suggested to be 775 BAU/mL (binding antibody unit/milliliter) for anti-RBD and 298 BAU/mL for anti-S on day 57. However, participants in this trial received the vaccine when Omicron was not the major circulating variant of concern (VoC) [18].
An attempt to derive the neutralization activity from the levels of binding antibodies came from a study conducted by Matusali et al. [19], which identified 870 BAU/mL for anti-RBD and 1591 BAU/mL for anti-S as the optimal cut-offs for predicting the average neutralization activity (nAbs titer ≥ 1:40) measured against the ancestral D614G strain, with 99% specificity (i.e., OCOnAbs, optimal cut-off neutralization antibody).
In this analysis, we planned to estimate the rates of not achieving a robust/above-average humoral response to COVID-19 mRNA vaccines in PLWH who received ≥2 doses, using two different endpoints indicative of the CoP/in-laboratory average response derived from binding antibodies, and to investigate the role of CD4 and the CD4/CD8 ratio in predicting a humoral response in this setting.
2. Materials and Methods
2.1. VAXICONA-ORCHESTRA Cohort
The VAXICONA-ORCHESTRA cohort is a prospective observational multicenter cohort that began in January 2021 and includes adults (≥18 years) with HIV-1 infection who have received at least two doses of COVID-19 mRNA-based vaccine. The cohort is conducted in 16 institutions, 14 from Italy, 1 from Spain and 1 from Argentina. A full list of hospitals and contributors is detailed in the acknowledgments. The cohort is included in Work Package 4 (WP4), focusing on different fragile populations, of the Horizon2020 funded ORCHESTRA project (https://orchestra-cohort.eu/; Access date: 1 September 2023), which aims to establish a pan-European cohort to rapidly advance knowledge on COVID-19. Study data were registered using the electronic case report form of the ICONA cohort (www.icona.org; Access date: 1 September 2023) and the centralized REDCap capture tool for ORCHESTRA. A common standard protocol based on the ORCHESTRA harmonization and standardization procedure for data collection was used for data exchange.
2.2. Study Population
PLWH of the VAXICONA-ORCHESTRA cohort who received ≥2 doses of SARS-CoV-2 mRNA vaccine and for whom ≥1 measure of anti-RBD/anti-S serology was available, together with CD4 and CD8 cell counts before 1st dose or within 1-month after 1st dose. PLWH who naturally acquired SARS-CoV2 before vaccination and during follow-up were excluded.
2.3. Humoral Anti-SARS-CoV-2 Response Quantification and Time Points
Vaccine-induced humoral responses were measured using a range of different assays across the centers: Elecsys® Anti-SARS-CoV-2 S ECLIA assay (Roche Diagnostics, Rotkreuz, Switzerland), LIAISON® SARS-CoV-2 TrimericS IgG assay and LIAISON® SARS-CoV-2 S1/S2 IgG (DiaSorin, Saluggia, Italy), ARCHITECT® SARS-CoV-2 IgG II Quantitative (Abbott Laboratories, Abbott Park, IL, USA) and V-PLEX SARS-CoV-2 Panel 6 Kit IgG (Meso Scale Discovery, Rockville, MD, USA).
Quantitative serology results were measured and converted where necessary to WHO binding antibody units BAU/mL, using a conversion factor provided in each manufacturer’s instructions (Supplementary Table S1).
The anti-SARS-CoV-2 serological response was evaluated at the following time points: the day of 1st vaccine dose administration (T1); the day of 2nd dose (T2, 21 days after T1 for BNT162b2 or 28 days after for mRNA-1273); 1 month after 2nd dose (T3); the day of 3rd dose (T4), 1 month from 3rd dose (T5), and 6 ± 2 months from 3rd dose (T6).
The occurrence of SARS-CoV-2 infection and the clinical course of the infection were collected at baseline and during follow-up visits using clinical information and anti-N SARS-CoV-2 data (for a subgroup of PLWH) using the six time points mentioned above and the following assays: Elecsys® Anti-SARS-CoV-2 anti-N (Roche Diagnostics, Rotkreuz, Switzerland), ARCHITECT® SARS-CoV-2 anti-N IgG (Abbott Laboratories, Abbott Park, IL, USA) and V-PLEX SARS-CoV-2 Panel 6 Kit IgG (Meso Scale Discovery, Rockville, MD, USA).
2.4. Endpoints
The Ig-G anti-SARS-CoV-2 response was evaluated at the different time points both as a continuous factors (log2 scale) and as a proportion of participants not achieving a robust/above-average response. A two-fold definition for a robust/above-average humoral response was used: (i) VE90%: anti-RBD level > 775 BAU/mL or anti-S > 298 BAU/mL, which corresponds to 90% vaccine efficacy according to Gilbert et al. [18] and (ii) OCOnAbs: anti-RBD level > 870 BAU/mL or anti-S > 1591 BAU/mL, corresponding to the optimal cut-off for circulating binding antibodies to identify medium neutralization activity according to Matusali et al. [19].
PLWH were stratified according to exposure groups, defined according to the CD4 count and the CD4/CD8 ratio measured when receiving their first dose: low CD4 (LCD4) if <200 cell/mm3, intermediate CD4 (ICD4) if >201–500 cell/mm3 and high CD4 (HCD4) if >500 cell/mm3; similarly we defined the following cut-offs, for the CD4/CD8 ratio, after calculating the tertiles of the distribution: low ratio (LR) if 0.0–0.59, intermediate ratio (IR) if 0.60–0.99 and high ratio (HR) if ≥1.0.
2.5. Statistical Analysis
Logistic regression was used to compare the probability of not achieving at T3 and T5 a robust/above-average anti-S/anti-RBD response above the thresholds described, according to CD4 and CD4/CD8 at the first dose as strata and as continuous measurements (per 1 standard deviation (SD) decrement). A mixed linear model with a random intercept and slope was also used to estimate the mean anti-RBD titers (using a log2 scale) at the six time points of the study, which were then compared across exposure groups of CD4 and the CD4/CD8 ratio. All multivariable models included the following key confounders: age, HIV-RNA ≤ 50 copies/mL at baseline, number of comorbidities and CD4 nadir. The logistic regression and linear mixed models were repeated in a sensitivity analysis which excluded values measured using the Elecsys® Anti-SARS-CoV-2 ECLIA assay (which detects the total antibodies against SARS-CoV-2 S RBD and not only IgG, like the other immuno-assays). Median response anti-S/anti-RBD response was also evaluated according to the assay used for quantification.
2.6. Ethics
The WP4 study of ORCHESTRA was approved for the Italian centers by the ‘Agenzia Italiana del Farmaco’ and centrally by the Ethics Committee of ‘INMI Lazzaro Spallanzani’, according to the Italian legislation for SARS-CoV-2 studies, and by each Ethics Committee of the participating centers outside Italy. The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all of the subjects involved.
3. Results
3.1. Study Population
A total of 1302 PLWH met the inclusion criteria of the study; 126 PLWH had evidence of natural SARS-CoV-2 infection (10.3%) and were excluded. The remaining 1176 PLWH were distributed among the exposure groups as follows: LCD4: n = 77, ICD4: n = 298 and HCD4: n = 801 for CD4 count at baseline and LR: n = 381, IR: n = 375 and HR: n = 420 for the CD4/CD8 ratio. The general characteristics of the study population according to these groups are shown in Table 1.
Table 1.
General characteristics of the target population according to CD4 count (left panel) and CD4/CD8 ratio (right panel) at the time of first vaccination.
| CD4 Count at the Time of First Vaccination | CD4/CD8 Ratio at the Time of First Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | LCD4 N = 77 |
ICD4 N = 298 |
HCD4 N = 801 |
p * | LR N = 381 |
IR N = 375 |
HR N = 420 |
p * | Total N = 1176 |
| Female, n (%) | 14 (18.2) | 55 (18.5) | 175 (21.8) | 0.397 | 66 (17.3) | 71 (18.9) | 107 (25.5) | 0.010 | 244 (20.7) |
| Age, years, median (IQR) | 56 (48, 59) | 54 (45, 61) | 52 (44, 58) | 0.013 | 53 (44, 60) | 53 (45, 59) | 52 (44, 59) | 0.485 | 53 (44, 59) |
| Caucasian, n (%) | 57 (74.0) | 240 (80.5) | 747 (93.3) | <0.001 | 307 (80.6) | 348 (92.8) | 389 (92.6) | <0.001 | 1044 (88.8) |
| ≥1 comorbidity, n (%) | 34 (44.2) | 108 (36.2) | 224 (28.0) | 0.001 | 129 (33.9) | 130 (34.7) | 107 (25.5) | 0.008 | 366 (31.1) |
| N. of comorbidities &, median (IQR) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 0.964 | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 0.376 | 1 (1, 2) |
| AIDS, n (%) | 30 (39.0) | 130 (43.8) | 208 (26.1) | <0.001 | 164 (43.0) | 110 (29.5) | 94 (22.5) | <0.001 | 368 (31.4) |
| Time from HIV diagnosis, years, median (IQR) | 11 (1, 24) | 9 (4, 21) | 11 (6, 21) | 0.005 | 7 (3, 21) | 12 (7, 21) | 11 (7, 21) | <0.001 | 11 (5, 21) |
| Time from AIDS diagnosis, years, median (IQR) | 4 (2, 9) | 5 (2, 9) | 8 (5, 12) | 0.019 | 5 (2, 7) | 7 (4, 10) | 11 (7, 19) | <0.001 | 6 (3, 10) |
| Nadir CD4 count, cells/mm3, median (IQR) | 39 (12, 72) | 87 (34, 177) | 272 (115, 412) | <0.001 | 68 (28, 166) | 217 (92, 320) | 312 (155, 464) | <0.001 | 189 (57, 348) |
| CD4 count at first vaccination, cells/mm3, median (IQR) | 116 (61, 154) | 357 (275, 436) | 746 (587, 944) | <0.001 | 349 (206, 525) | 622 (479, 790) | 807 (628, 1015) | <0.001 | 606 (397, 838) |
| CD4 count at first booster dose, cells/mm3, median (IQR) | 160 (109, 210) | 378 (296, 443) | 749 (596, 944) | <0.001 | 385 (228, 509) | 678 (477, 837) | 798 (600, 1034) | <0.001 | 597 (391, 832) |
| CD4/CD8 ratio at first vaccination, cells/mm3, median (IQR) | 0.2 (0.1, 0.2) | 0.5 (0.3, 0.8) | 1.0 (0.7, 1.3) | <0.001 | 0.4 (0.2, 0.5) | 0.8 (0.7, 0.9) | 1.3 (1.1, 1.6) | <0.001 | 0.8 (0.5, 1.2) |
| CD4/CD8 ratio at first booster dose, cells/mm3, median (IQR) | 0.2 (0.1, 0.3) | 0.5 (0.4, 0.7) | 0.9 (0.7, 1.3) | <0.001 | 0.4 (0.3, 0.5) | 0.8 (0.7, 0.9) | 0.0 (0.0, 1.3) | <0.001 | 0.0 (0.0, 1.5) |
| HIV-RNA at first vaccination ≤ 50 cps/mL, n (%) | 50 (64.9) | 268 (90.2) | 778 (97.3) | <0.001 | 328 (86.1) | 361 (96.5) | 407 (97.1) | <0.001 | 1096 (93.4) |
| HIV-RNA at first booster dose ≤ 50 cps/mL, n (%) | 33 (84.6) | 111 (94.1) | 282 (95.3) | 0.031 | 147 (93.0) | 141 (93.4) | 138 (95.8) | 0.542 | 426 (94.0) |
| Having Antiretroviral Therapy | 73 (94.8) | 293 (98.3) | 788 (98.5) | 0.064 | 373 (97.9) | 368 (98.4) | 413 (98.3) | 0.853 | 1154 (98.2) |
| Vaccination times (days), medians (IQR) | |||||||||
| From second dose to response | 30 (28.0, 31.0) | 31 (30.0, 35.0) | 31 (29.0, 39.0) | 0.009 | 31 (30.0, 35.0) | 31 (29.0, 37.0) | 31 (29.0, 39.0) | 0.805 | 31 (29.0, 37.0) |
| From second dose to third dose | 158 (154.0, 166.0) | 177 (152.0, 209.0) | 189 (168.0, 216.0) | <0.001 | 167 (154.0, 201.5) | 187 (166.0, 217.0) | 190 (171.0, 213.0) | <0.001 | 183 (160.0, 212.0) |
| From third dose to response | 17 (15.0, 20.5) | 16 (14.0, 20.0) | 16 (14.0, 19.0) | 0.094 | 16 (14.0, 20.0) | 16 (14.0, 20.0) | 16 (14.0, 19.0) | 0.339 | 16 (14.0, 20.0) |
& Comorbidities considered: chronic kidney disease, chronic obstructive pulmonary disease, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic cognitive deficit, connective tissue disease, peptic ulcer disease, chronic liver disease and end-stage liver disease, diabetes mellitus and diabetes with organ damage, hemiplegia, solid cancers with or without metastasis, hematological cancer; * chi-square or Kruskal–Wallis test, as appropriate.
Overall, the median age was 53 years (IQR 44–59), and 31% of participants had at least one comorbidity. The median time since HIV diagnosis was 11 years (IQR 5–21), the CD4 nadir was 189 cells/mm3 (IQR 57–348), the median of CD4 count at baseline was 606/mm3 (IQR 397–838) and that of the CD4/CD8 ratio was 0.8 (IQR 0.5–1.1), and 93% of participants had HIV-RNA < 50 copies/mL at baseline. The median time from the completion of the primary cycle (second dose date) to the first booster dose was 183 days (IQR 160, 212).
According to the CD4 count, LCD4 had a higher median age (p = 0.013) and more frequently had at least one comorbidity compared to ICD4 and HCD4 (p = 0.001), whereas a higher proportion of HCD4 vs. ICD4 and LCD4 were virologically suppressed at the first vaccine dose and at booster dose administration (p < 0.001 and p = 0.031, respectively). According to the CD4/CD8 ratio, no differences in age and proportions of participants with HIV-RNA < 50 cps/mL across the groups were found at the time of the booster doses.
3.2. Binary Humoral Response
After a median of 31 days (IQR 29–37) from the second dose, the overall proportions of PLWH not reaching the VE90% and OCOnAbs thresholds were 59.3% and 71.0%, respectively.
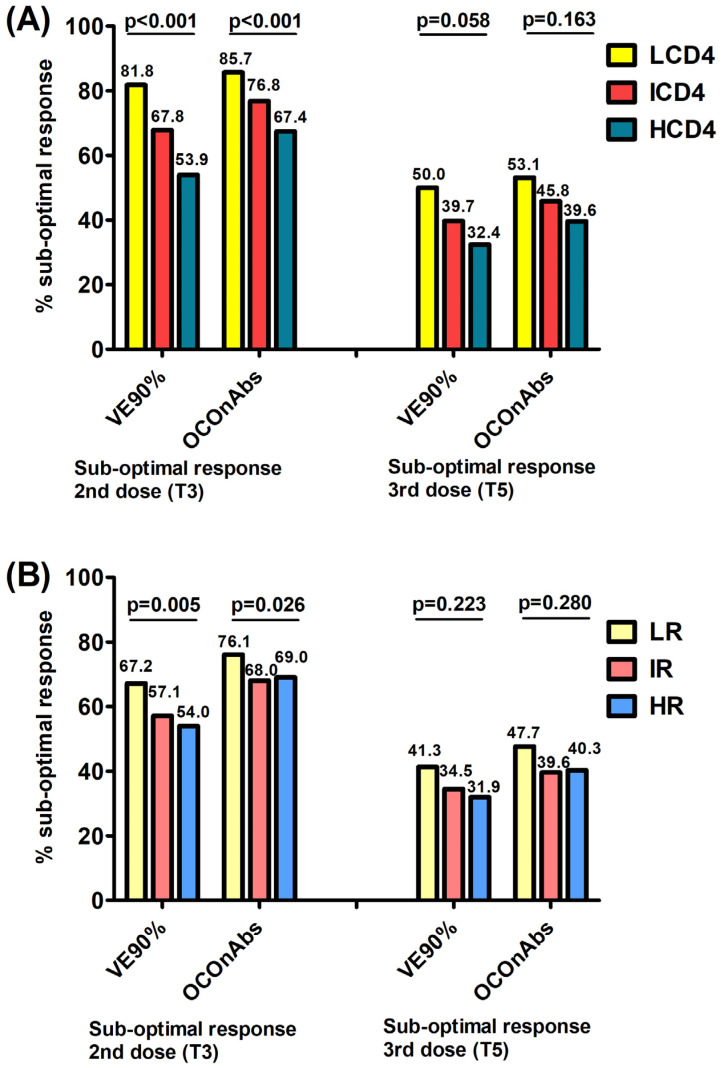
When investigating the association with the exposure groups, the proportions of participants failing to achieve a robust/above-average response (VE90%/OCOnAbs) 1 month after the second dose were significantly higher in participants with a lower CD4 count and CD4/CD8 ratio at baseline, following a dose–response trend. Specifically, a clearer gradient was observed for the CD4 count vs. the CD4/CD8 ratio, with the following percentages: 81.8% (not reaching the VE90% level) and 85.7% (not reaching the OCOnAbs level) for LCD4, 67.8% and 76.8% for ICD4 and 53.9% and 67.49% for HCD4 (Figure 1A, chi-square p < 0.0001/p < 0.0001); similarly, for the CD4/CD8 ratio, it was 67.2% and 76.1% for LR, 57.1% and 68.0% for IR and 54.0% and 69.0% for HR (p = 0.005/p = 0.026), respectively (Figure 1B).
Figure 1.
Proportions of those failing to achieve robust/above-average responses at T3 and T5, using 2 endpoints VE90% (vaccine efficacy of 90% in preventing symptomatic infections) and OCOnAbs (optimal cut-off for binding Ab calculated for a nAb titer ≥ 1:40) according to the CD4 count (A) and to the CD4/CD8 ratio (B) at the time of first vaccination.
After a median of 16 days (IQR 14–20) post-third dose, the proportion of participants failing to have a robust/above-average response significantly decreased by 36.5% and 43.0% overall for the VE90% and OCOnAbs thresholds, as well as in all exposure groups. To go into more detail, these proportions in the CD4 groups were 50.0% (not reaching the VE90% level) and 53.1% (not reaching the OCOnAbs level) for LCD4, 39.7% and 45.8% for ICD4 and 32.4% and 39.6% for HCD4 (Figure 1A, p = 0.058/p = 0.163); for the CD4/CD8 ratio they were 41.3% and 47.7% for LR, 34.5% and 39.6% for IR and 31.9% and 40.3% for HR (p = 0.223/p = 0.280), respectively, again with a clearer decreasing gradient associated with the CD4 count groups (Figure 1B).
In the multivariable logistic regression models, after adjusting for age, HIV-RNA ≤ 50 copies/mL at the time of the first dose vaccine, number of comorbidities and CD4 nadir, a lower CD4 count were associated with a higher risk of failing to achieve VE90% at T3. The LCD4 group, compared to HCD4, had a higher probability of not achieving VE90% (aOR = 3.36, 95%CI 1.23, 9.16); this was not shown for the ICD4 group in the adjusted model (aOR = 1.61, 95%CI 0.92, 2.81) and not shown using the OCOnAb endpoint. At T3 using the VE90% endpoint and the two exposure factors as continuous variables, per 1 SD lower in CD4 count there was a 71% higher probability of not reaching VE90% (aOR = 1.71, 95%CI 1.24, 2.36) (Table 2), while the corresponding estimate for the CD4/CD8 ratio was aOR = 1.15 (95% CI 0.90, 1.49) (Table 3), indicating a poorer prognostic value to predict the response for this marker as compared to that seen for the absolute CD4 count. The predictive value of the CD4 cell count as a marker of non-reaching a robust/above-average response with the OCOnAbs endpoint at T3 was marginally significant, with an aOR = 1.34 (95%CI 0.96, 1.85) (Table 2)
Table 2.
Association with CD4 count at the time of first vaccination. Odds ratio and adjusted odds ratio, by fitting a logistic regression model, of the probability of failing to achieve a robust/above-average anti-S/RBD response post-vaccination, with VE90% (Panel A) and OCOnAbs (Panel B) endpoints.
| Panel A (VE90% Endpoint) |
Unadjusted Odds Ratio (95% CI) | p-Value | Adjusted * Odds Ratio (95% CI) | p-Value | & Type III p-Value |
|---|---|---|---|---|---|
| CD4 count at T1 | Failed to achieve VE90% after primary cycle (T3) | ||||
| HCD4 | 1 | 1 | 0.029 | ||
| ICD4 | 1.80 (1.36, 2.38) | <0.001 | 1.61 (0.92, 2.81) | 0.097 | |
| LCD4 | 3.84 (2.12, 6.97) | <0.001 | 3.36 (1.23, 9.16) | 0.018 | |
| Per 1 SD lower (log2 scale) | 1.65 (1.42, 1.91) | <0.001 | 1.71 (1.24, 2.36) | 0.001 | |
| CD4 count at T1 | Failed to achieve VE90% after first booster dose (T5) | ||||
| HCD4 | 1 | 1 | 0.937 | ||
| ICD4 | 1.37 (0.89, 2.13) | 0.157 | 0.91 (0.41, 2.03) | 0.814 | |
| LCD4 | 2.00 (1.08, 3.72) | 0.028 | 0.81 (0.24, 2.75) | 0.733 | |
| Per 1 SD lower (log2 scale) | 1.42 (1.16, 1.74) | <0.001 | 1.19 (0.78, 1.83) | 0.421 | |
| Panel B (OCOnAbs Endpoint) |
Unadjusted OR
(95% CI) |
p -Value | Adjusted * OR (95% CI) | p -Value | & Type III p-Value |
| CD4 count at T1 | Failed to achieve OCOnAbs after primary cycle (T3) | ||||
| HCD4 | 1 | 1 | 0.367 | ||
| ICD4 | 1.60 (1.18, 2.18) | 0.003 | 1.53 (0.84, 2.78) | 0.162 | |
| LCD4 | 2.90 (1.51, 5.58) | 0.001 | 1.30 (0.47, 3.54) | 0.612 | |
| Per 1 SD lower (log2 scale) | 1.46 (1.25, 1.72) | <0.001 | 1.34 (0.96, 1.85) | 0.082 | |
| CD4 count at T1 | Failed to achieve OCOnAbs after first booster dose (T5) | ||||
| HCD4 | 1 | 1 | 0.925 | ||
| ICD4 | 1.29 (0.84, 1.98) | 0.244 | 0.87 (0.40, 1.89) | 0.730 | |
| LCD4 | 1.72 (0.93, 3.19) | 0.083 | 0.83 (0.26, 2.67) | 0.759 | |
| Per 1 SD lower (log2 scale) | 1.35 (1.11, 1.65) | 0.003 | 1.19 (0.79, 1.80) | 0.409 | |
* Adjusted for age, VL ≤ 50 cps/mL at 1st vaccination, no. of comorbidities and nadir CD4 count; & from the adjusted model; abbreviations: HCD4, high CD4 count (>500/mm3); ICD4, intermediate CD4 count (200–500/mm3); LCD4, low CD4 count (0–200/mm3); OR, odds ratio.
Table 3.
Association with CD4/CD8 ratio at time of first vaccination. OR and AOR, by fitting a logistic regression model, of the probability of failing to achieve a robust/above-average anti-S/RBD response post-vaccination, with VE90% (Panel A) and OCOnAbs (Panel B) endpoints.
| Panel A (VE 90% Endpoint) |
Unadjusted OR (95% CI) | p-Value | Adjusted * OR (95% CI) | p-Value | & Type III p-Value |
|---|---|---|---|---|---|
| CD4/CD8 ratio at T1 | Failed to achieve VE90% after primary cycle (T3) | ||||
| HR | 1 | 1 | 0.311 | ||
| IR | 1.13 (0.85, 1.50) | 0.393 | 0.76 (0.42, 1.36) | 0.356 | |
| LR | 1.74 (1.31, 2.32) | <0.001 | 1.17 (0.63, 2.17) | 0.612 | |
| Per 1 SD lower (log 2 scale) | 1.28 (1.14, 1.44) | <0.001 | 1.15 (0.90, 1.49) | 0.267 | |
| CD4/CD8 ratio at T1 | Failed to achieve VE90% after first booster dose (T5) | ||||
| HR | 1 | 1 | 0.474 | ||
| IR | 1.12 (0.67, 1.89) | 0.659 | 0.58 (0.23, 1.46) | 0.247 | |
| LR | 1.50 (0.92, 2.45) | 0.106 | 0.82 (0.33, 2.05) | 0.677 | |
| Per 1 SD lower (log 2 scale) | 1.31 (1.09, 1.57) | 0.004 | 0.98 (0.68, 1.41) | 0.921 | |
|
PANEL B
(OCOnAbs Endpoint) |
Unadjusted OR (95% CI) | p -Value | Adjusted OR (95% CI) | p -Value | & Type III p-Value |
| CD4/CD8 ratio at T1 | Failed to achieve OCOnAbs after primary cycle (T3) | ||||
| HR | 1 | 1 | 0.912 | ||
| IR | 0.95 (0.71, 1.29) | 0.751 | 0.91 (0.50, 1.64) | 0.745 | |
| LR | 1.43 (1.04, 1.95) | 0.026 | 1.02 (0.54, 1.93) | 0.949 | |
| Per 1 SD lower (log 2 scale) | 1.18 (1.05, 1.34) | 0.007 | 0.99 (0.76, 1.30) | 0.964 | |
| CD4/CD8 ratio T1 | Failed to achieve OCOnAbs after first booster dose (T5) | ||||
| HR | 1 | 1 | 0.396 | ||
| IR | 0.97 (0.59, 1.60) | 0.900 | 0.65 (0.27, 1.56) | 0.332 | |
| LR | 1.35 (0.84, 2.16) | 0.216 | 1.06 (0.44, 2.55) | 0.898 | |
| Per 1 SD lower (log 2 scale) | 1.27 (1.06, 1.52) | 0.009 | 1.09 (0.77, 1.54) | 0.635 | |
* Adjusted for age, VL ≤ 50 cps/mL at 1st vaccination, no. of comorbidities and nadir CD4 count; & from the adjusted model. Abbreviations: HR, high CD4/CD8 ratio (>1); IR, intermediate CD4/CD8 ratio (0.60–0.99); LR, low CD4/CD8 ratio (0–0.59); OR, odds ratio.
The ability to predict the T5 sub-response using VE90% and OCOnAbs endpoints, according to the baseline (T1) markers, was smaller and not statistically significant for both the CD4 cell count (Table 2) and the CD4/CD8 ratio (Table 3). In the sensitivity analysis in which Elecsys® Anti-SARS-CoV-2 quantifications were excluded, the results were confirmed to be similar (Supplementary Tables S2 and S3).
3.3. Continuous Humoral Response
We then evaluated the predicted anti-RBD responses, as continuous outcomes, by fitting a linear mixed model; we found a similar dose–response relationship across the CD4 count exposure groups at each of the examined time points, with participants in the LCD4 group showing the lowest mean anti-RDB, those in the ICD4 group showing intermediate mean anti-RDB and the highest response in the HCD4 group (Fisher test p-value < 0.001) (Table 4).
Table 4.
Predicted anti-RBD adjusted means (log2 scale) from fitting a linear mixed model.
| Anti-RBD (log2) Adjusted * Means | T1 95% CI |
T2 95% CI |
T3 95% CI |
T4 95% CI |
T5 95% CI |
T6 95% CI |
p-Value § |
|---|---|---|---|---|---|---|---|
| CD4 at T1 | <0.001 | ||||||
| LCD4 | −4.7 (−5.5, −3.9) | 1.1 (0.3, 1.9) | 5.0 (4.2, 5.8) | 2.5 (1.6, 3.3) | 8.0 (7.1, 8.9) | 5.6 (4.2, 7.0) | |
| ICD4 | −5.2 (−5.7, −4.7) | 3.2 (2.7, 3.7) | 9.1 (8.5, 9.6) | 7.0 (6.5, 7.5) | 8.1 (7.3, 9.0) | 4.4 (3.9, 5.0) | |
| HCD4 | −4.8 (−5.2, −4.4) | 4.4 (4.0, 4.8) | 9.1 (8.6, 9.6) | 7.4 (7.1, 7.8) | 7.7 (6.8, 8.6) | 4.8 (4.3, 5.2) | |
| CD4/CD8 ratio at T1 | 0.009 | ||||||
| LR | −4.9 (−5.3, −4.4) | 2.8 (2.4, 3.3) | 6.6 (6.2, 7.0) | 3.8 (3.3, 4.2) | 8.7 (8.2, 9.2) | 7.3 (6.5, 8.0) | |
| IR | −5.0 (−5.6, −4.4) | 4.0 (3.4, 4.5) | 9.2 (8.5, 9.8) | 7.2 (6.7, 7.7) | 8.0 (6.8, 9.3) | 4.7 (4.1, 5.3) | |
| HR | −4.8 (−5.4, −4.3) | 4.4 (3.9, 5.0) | 9.3 (8.6, 10.0) | 7.4 (6.9, 7.9) | 7.7 (6.4, 9.0) | 5.1 (4.5, 5.8) |
§ F-test p-value for type 3 interaction; * Model adjusted for age, VL ≤ 50 cps/mL at 1st vaccination, no. of comorbidities and nadir CD4 count. time points: T1: before vaccination; T2: at the time of 2nd dose; T3: 1 month after 2nd dose; T4: at the time of the 3rd dose; T5: 1 month after the 3rd dose; T6: 6 months after the 3rd dose.
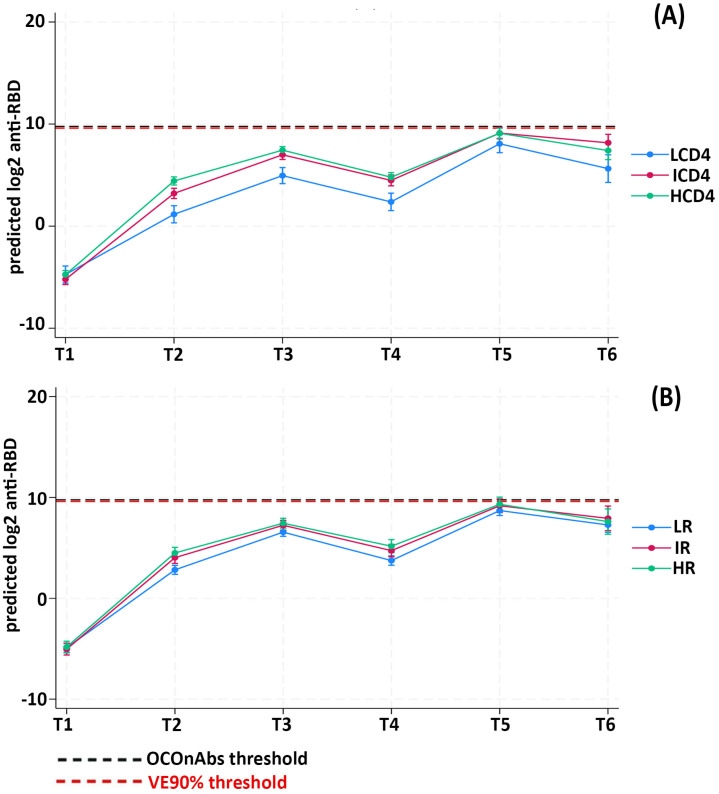
Of note, over T2–T4, the mean predicted vaccine-elicited humoral response remained below both the VE90% and the OCOnAbs thresholds in all of the CD4 groups. Even the HCD4 group that responded the best, on average remained below the robust/above-average response up to T5. The fact that the third dose was crucial for achieving a robust/above-average response, at least for the ICD4 and HCD4 groups, was confirmed after adjusting for age, HIV-RNA ≤ 50 copies/mL at T0, nadir of CD4 count and numbers of comorbidities (Figure 2A).
Figure 2.
Linear mixed model prediction of log2 transformed anti-RBD titers according to CD4 strata (A) and CD4/CD8 strata (B).
Similarly, the CD4/CD8 ratio groups also showed an association with the humoral response at all of the evaluated time points, with lower responses in LR compared to both IR and HR at each time point (p = 0.009) (Table 4). By fitting a multivariable mixed linear model, all of the CD4/CD8 ratio groups remained, on average, below both of the chosen thresholds until the administration of the third dose (Figure 2B).
Again, analyses were repeated after excluding the Elecsys® Anti-SARS-CoV-2 measurements, confirming the main results (Supplementary Table S4). Supplementary Figure S1 reports the unadjusted medians and IQR at different time points after stratifying for the assay used, while the median values of the anti-S/anti-RBD response have only been further stratified according to the CD4 and CD4/CD8 strata, only for ARCHITECT® anti-SARS-CoV-2 IgG II and LIAISON® SARS-CoV-2 IgG (Supplementary Table S5).
4. Discussion
Our data show that after three doses of an mRNA vaccine, 90%VE was not achieved by 50% of PLWH, classified as LCD4, versus 40% in ICD4 and 32% in HCD4. A similar association was observed when using the alternative thresholds based on average neutralization. When analyzing the risk of not achieving robust/above-average responses and the mean level of responses after the primary cycle (but not after the third dose), we found a strong association between the baseline CD4 count and humoral response to the vaccine, after controlling for key confounders.
These findings confirm our hypothesis that, given the immune dysregulation that characterizes chronic HIV infection, despite effective ART, PLWH with a low CD4 count remain at risk of lower immune responses to SARS-CoV-2 vaccination, at least in the short term, with potentially negative implications for clinical outcomes. Mechanistically, it is known that the induction of strong antibody responses is dependent on both CD4+ and CD8+ T-cell responses, and there are reports of immunocompromised individuals, including PLWH, who did not mount antibody responses to vaccination against SARS-CoV-2 and subsequently became infected [5,8,20,21,22,23,24,25].
However, our analysis also shows that in the short term after the primary vaccination cycle, the CD4 count was a stronger predictor of the humoral vaccine response than the CD4/CD8 ratio, thus confirming that the CD4/CD8 ratio has a secondary role. The association was independent of CD4 nadir [26]; however, the role of CD4 nadir has not been investigated in this study and instead was merely treated as a confounder of the main associations of interest. It is well known that HIV infection is characterized by a profound disruption of the cellular and humoral components of the adaptive immune system [27] and, PLWH with low CD4 counts were found to have weaker humoral and T-cell responses to mRNA vaccines [5], suggesting that they may benefit from additional vaccine doses. In this respect, we previously showed that a third dose of an mRNA vaccine following the primary cycle can strongly boost humoral but not T-cell responses in PLWH with advanced disease at the time of HIV diagnosis [28].
In addition, our analysis shows that, regardless of the threshold used, the third mRNA dose was pivotal in achieving a robust/above-average humoral response, at least in participants with a baseline CD4 count > 200 cells/mm3, as shown in previous works with a smaller sample size [29]. It should also be noted that, despite the SARS-CoV-2 humoral response elicited by the vaccine, may not reach the targets for a valid correlate of protection against infection and severe disease, is likely to be prevented by strong cellular immunity; this is also the case for the newly circulating variants of concern [30,31].
There are several factors that are potential common causes of the studied HIV markers and the levels of vaccine humoral responses, but based on our assumptions, all of the known measured potential confounders (age, baseline HIV-RNA, number of comorbidities and nadir CD4 count) were controlled for during the analysis stage. We did not control for time since HIV diagnosis because it is known to be an inaccurate estimate of the duration of HIV infection [32].
Our analysis has a number of limitations. First, this is an observational setting and unmeasured confounding bias cannot be ruled out. Second, this is a large cohort using different serology assays, across centers, with potential problems related to a lack of standardization and interpretability. However, a strong correlation between anti-S and anti-RBD was observed for values < 4000 BAU/mL [19], which are below the thresholds used in our study, and the results of the sensitivity analyses conducted after excluding measurements quantifying the total Ab (instead of IgG only) were consistent with those of the main analyses. Third, the study period covered the waves of the COVID-19 pandemic mainly sustained by Alpha/Delta circulating VoCs; therefore, it is possible that the proportion of PLWH not achieving a robust/above-average response, for both thresholds, would have been even higher for Omicron. Indeed, the vaccine efficacy threshold was defined in the COVE clinical trial, during the circulation of ancestral strains of SARS-CoV-2. During the Omicron era, the binding antibody threshold of vaccine efficacy should supposedly be substantially higher. Fourth, this study only included PLWH, without using a control group of HIV-negative people vaccinated in the same period and with assessments of the SARS-CoV-2 humoral response with the same timelines; we, therefore, cannot estimate the effect of HIV infection per se, but can only compare the response over time according to the HIV immunological markers also related to the vaccine response. Further, cross-reactivity with other common coronavirus was not measured in this study. Nevertheless, because of the established high positive predictive values of the assays used and the low circulations of other coronavirus during the pandemic, cross-reactivity was unlikely to have introduced a large bias in this analysis. Lastly, the use of two different sets of thresholds may be confusing for the reader. It needs to be made clear that the context in which these threshold values were derived and their significance are quite different. The first set of thresholds refers to vaccine efficacy in vivo. In contrast, the second set was derived in a laboratory and refers to the average response seen in the general population. Although a consistent relationship between neutralizing antibodies and clinical protection has been shown, different immunological factors contribute to vaccine efficacy, including innate and adaptive cellular responses, mucosal immunity and non-neutralizing antibody functions. Moreover, an exact protective neutralization titer threshold at which an individual is likely to be protected from getting infected with COVID-19 does not exist [33]. Indeed, the identification of such a threshold has proven difficult for a variety of reasons, such as the diversity of the assays used and the emergence of new and more immune-evasive viral variants, as well as the diversity in the vaccine platforms used [33]. Our analysis is an attempt to provide further data comparing the predictive value of laboratory- and clinically derived thresholds. Of note, the CD4 count was slighter more predictive of the VE than of the laboratory-derived response.
The major strengths of our study are the analysis of a multinational prospective cohort adopting the same protocol with the same time points, and the large sample size of PLWH with CD4 count < 200 cells/mm3, allowing us to better investigate the predictive role of this marker than what has previously been achieved [34].
5. Conclusions
In conclusion, under the assumption that the titer thresholds used are reasonable CoPs in the Omicron era as well, we found that the third mRNA vaccine dose is key in establishing protection against SARS-CoV-2 infection in most PLWH. Furthermore, the CD4 count measured before the primary vaccination cycle strongly predicts the early humoral response in PLWH better than a concomitant measure of the CD4/CD8 ratio; therefore, the absolute CD4 count should still be the HIV marker of choice to guide vaccination and booster campaigns for the more immunosuppressed PLWH. However, similar analyses need to be repeated and the detected associations should be validated in the current setting of the circulating Omicron variants.
Acknowledgments
Orchestra Project coordinator: Evelina Tacconelli; Orchestra WP4 coordinator: Maddalena Giannella; Vax-Icona Orchestra scientific coordinators: Antonella d’Arminio Monforte, Andrea Antinori; Data coordinators and statisticians: Alessandro Tavelli, Alessandra Rodanò, Iuri Fanti, Alessandro Cozzi-Lepri; Participating centers, in Italy: Andrea Costantini (Ospedali Riuniti, Ancona); Pierluigi Viale, Maddalena Giannella, Lorenzo Marconi, Leonardo Calza, Natascia Carroccia, Michela Di Chiara, Francesca Fanì (IRCCS AOU di Bologna, Bologna); Sergio Lo Caputo, Sergio Ferrara (Ospedali Riuniti, Foggia); Miriam Lichtner, Giulia Mancaralla, Laura Fondaco, Anna Carraro (Ospedale SM Goretti, Latina); Stefania Piconi, Silvia Pontiggia, Chiara Molteni (ASST Lecco, Lecco); Giuseppe Nunnari, Giovanni Pellicanò (AOU Gaetano Martino, Messina); Giuliano Rizzardini, Maria Vittoria Cossu (ASST Fatebenefratelli-Sacco, Milano); Giulia Marchetti, Nicole Gemignani, Valeria Bono, Ylenia D’Errico, Daniela Campisi, Walter De Francesco, Luigi Pantaleo (ASST Santi Paolo e Carlo, Milano); Vincenzo Sangiovanni, Francesco Maria Fusco, Nadia Sangiovanni (AORN Ospedali dei Colli, Napoli); Antonio Cascio, Marcello Trizzino (Policlinico P. Giaccone, Palermo); Stefania Cicalini, Alessandra Vergori, Chiara Salis, Jessica Paulicelli, Valentina Mazzotta, Simone Lanini, Giuseppina Giannico, Angela D’Urso, Marisa Fusto (INMI L. Spallanzani IRCCS, Roma); Alessandra Latini, Aldo Morrone, Fulvia Pimpinelli, Anna Pacifici (IFO-Regina Elena-San Gallicano, Roma); Giordano Madeddu, Andrea De Vito (AOU di Sassari, Sassari); Evelina Tacconelli, Anna Azzini, Elda Righi, Assunta Sartor, Giulia Belli, Concetta Sciammarella (AOUI di Verona, Verona); in Argentina: Gabriel Levy-Hara (Universidad de Buenos Aires, Buenos Aires); in Spain: Jesus Rodriguez Baño, Zaira Palacios, Giulia Caponcello (Hospital Virgen Macarena, Seville).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines11111664/s1. Supplementary Table S1. Conversion factors for the different assays used; Supplementary Table S2. Odds Ratio (OR) and Adjusted Odds Ratio (AOR) from fitting a logistic regression model of the probability of failing to achieve a robust anti-S/RBD response post vaccination VE90% (Panel A) and OCOnAbs (Panel B) endpoints after excluding subjects and data quantified with Elecsys Anti-SARS-CoV2 S (Roche); Supplementary Table S3. OR and AOR from fitting a logistic regression model of the probability of failing to achieve a robust anti-S/RBD response post vaccination VE90% (Panel A) and OCOnAbs (Panel B) endpoints after excluding subjects and data quantified with Elecsys Anti-SARS-CoV2 S (Roche); Supplementary Table S4. Adjusted mean anti-RBD values from fitting a mixed-linear model excluding subjects and data quantified with Elecsys Anti-SARS-CoV2 S (Roche); Supplementary Figure S1. Boxplots of Anti-SARS-CoV-2 titers (log2 transformation) according to the assay used; Supplementary Table S5. Median (IQR) quantification of anti-SARS-CoV-2 titers (log2 transformation) at different time points after vaccination according to immunoassay used and CD4 strata (A) and CD4/CD8 strata (B).
Author Contributions
Conception: A.V., A.T., A.d.M., A.A., A.C.-L., M.G. and E.T. Study design: A.V., A.T., A.d.M., A.A., A.C.-L, G.M. (Giulia Matusali). and G.M. (Giulia Marchetti); Acquisition of data: G.M. (Giulia Matusali), A.M.A., M.A., G.F.P., V.M., A.C. (Andrea Costantini), A.C. (Antonio Cascio), A.D.V., L.M., E.R., A.S., C.P., F.M., F.B., S.L., S.P., G.L.H. and Vax-ICONA-ORCHESTRA Study Group. Statistical analysis: A.C.-L. Interpretation of the data: A.V., A.T., A.d.M., A.A., A.C.-L., G.M. (Giulia Matusali), M.A., G.M. (Giulia Marchetti), A.M.A. and E.T. Draft of the article: A.V., A.T. and A.C.-L. Review of the article and critical revision of important intellectual content: A.V., A.T., G.M. (Giulia Matusali), A.M.A., M.A., V.M., G.F.P., A.C. (Andrea Costantini), A.C. (Antonio Cascio), A.D.V., L.M., E.R., A.S., C.P., F.M., F.B., S.L., S.P., G.L.H., G.M. (Giua Marchetti), M.G., E.T., A.d.M., A.A., A.C.-L. Final approval of the submitted version: A.V., A.T., G.M. (Giulia Matusali), A.M.A., M.A., V.M., G.F.P., A.C. (Andrea Costantini), A.C. (Antonio Cascio), A.D.V., L.M., E.R., A.S., C.P., F.M., F.B., S.L., S.P., G.L.H., G.M. (Giua Marchetti), M.G., E.T., A.d.M., A.A., A.C.-L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The WP4 study of ORCHESTRA was approved for the Italian centers by the Agenzia Italiana del Farmaco and centrally by the Ethics Committee of Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani (no. 359 of Study’s Registry 2020/2021), according to the Italian legislation for SARS-CoV-2 studies, and by each Ethics Committee of the participating centers outside Italy. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
An informed consent for study participation was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during the current study are not publicly available because they contain sensitive data to be treated under data protection laws and regulations. Appropriate forms of data sharing can be arranged after a reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The ORCHESTRA project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 101016167.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vizcarra P., Pérez-Elías M.J., Quereda C., Moreno A., Vivancos M.J., Dronda F., Casado J.L., Moreno S., Fortún J., Navas E., et al. Description of COVID-19 in HIV-infected individuals: A single-centre prospective cohort. Lancet HIV. 2020;7:e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geretti A.M., Stockdale A.J., Kelly S.H., Cevik M., Collins S., Waters L., Villa G., Docherty A., Harrison E.M., Turtle L., et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization Among People With Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin. Infect. Dis. 2021;73:e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandachi D., Geiger G., Montgomery M.W., Karmen-Tuohy S., Golzy M., Antar A.A., Llibre J.M., Camazine M., Santiago D.-D., Carlucci P.M., et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients With Human Immunodeficiency Virus and Coronavirus Disease 2019. Clin. Infect. Dis. 2021;73:e1964–e1972. doi: 10.1093/cid/ciaa1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nault L., Marchitto L., Goyette G., Tremblay-Sher D., Fortin C., Martel-Laferrière V., Trottier B., Richard J., Durand M., Kaufmann D., et al. COVID-19 vaccine immunogenicity in people living with HIV-1. Vaccine. 2022;40:3633–3637. doi: 10.1016/j.vaccine.2022.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antinori A., Cicalini S., Meschi S., Bordoni V., Lorenzini P., Vergori A., Lanini S., De Pascale L., Matusali G., Mariotti D., et al. Humoral and cellular immune response elicited by mRNA vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in people living with human immunodefciency virus receiving antiretroviral therapy based on current CD4 T-lymphocyte count. Clin. Infect. Dis. 2022;75:e552–e563. doi: 10.1093/cid/ciac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sisteré-Oró M., Andrade N., Wortmann D.D., Du J., Garcia-Giralt N., González-Cao M., Güerri-Fernández R., Meyerhans A. Anti-SARS-CoV-2 specifc immunity in HIV immunological non-responders after mRNA-based COVID-19 vaccination. Front. Immunol. 2022;13:994173. doi: 10.3389/fimmu.2022.994173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tau L., Turner D., Adler A., Marom R., Ahsanov S., Matus N., Levi I., Gerber G., Lev S., Ziv-Baran T., et al. SARS-CoV-2 humoral and cellular immune responses of patients with HIV after vaccination with BNT162b2 mRNA COVID-19 vaccine in the Tel-Aviv Medical Center. Open Forum. Infect. Dis. 2022;9:ofac089. doi: 10.1093/ofid/ofac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinelli M.A., Peluso M.J., Lynch K.L., Yun C., Glidden D.V., Henrich T.J., Deeks S.G., Gandhi M. Differences in post-mRNA vaccination severeacute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) concentrations and surrogate virus neutralization test response by human immunodefciency virus (HIV) status and type of vaccine: A matched case-control observational study. Clin. Infect. Dis. 2022;75:e916–e919. doi: 10.1093/cid/ciab1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benet S., Blanch-Lombarte O., Ainsua-Enrich E., Pedreño-Lopez N., Muñoz-Basagoiti J., Raïch-Regué D., Perez-Zsolt D., Peña R., Jiménez E., de la Concepción M.L.R., et al. Limited humoral and specifc T-cell responses after SARS-CoV-2 vaccination in PLWH with poor immune reconstitution. J. Infect. Dis. 2022;226:1913–1923. doi: 10.1093/infdis/jiac406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensley K.S., Jongkees M.J., Geers D., GeurtsvanKessel C.H., Mueller Y.M., Dalm V.A.S.H., Papageorgiou G., Steggink H., Gorska A., Bogers S., et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: A nationwide prospective cohort study. PLoS Med. 2022;19:e1003979. doi: 10.1371/journal.pmed.1004159. Erratum in PLoS Med. 2023, 20, e1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corma-Gómez A., Fernández-Fuertes M., García E., Fuentes-López A., Gómez-Ayerbe C., Rivero-Juárez A., Domínguez C., Santos M., Viñuela L., Palacios R., et al. Severe immunosuppression is related to poorer immunogenicity to SARS-CoV-2 vaccines among people living with HIV. Clin. Microbiol. Infect. 2022;28:1492–1498. doi: 10.1016/j.cmi.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano-Villar S., Moreno S., Fuentes-Ferrer M., Sánchez-Marcos C., Ávila M., Sainz T., de Villar N., Fernández-Cruz A., Estrada V. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV Med. 2014;15:40–49. doi: 10.1111/hiv.12081. [DOI] [PubMed] [Google Scholar]
- 14.Alrubayyi A., Gea-Mallorquí E., Touizer E., Hameiri-Bowen D., Kopycinski J., Charlton B., Fisher-Pearson N., Muir L., Rosa A., Roustan C., et al. Characterization of humoral and SARS-CoV-2 specifc T cell responses in people living with HIV. Nat. Commun. 2021;12:5839. doi: 10.1038/s41467-021-26137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y., Zhang Y., He Z., Huang H., Tian X., Wang G., Chen D., Ren Y., Jia L., Wang W., et al. Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: A non-randomized cohort study. EClinicalMedicine. 2022;43:101226. doi: 10.1016/j.eclinm.2021.101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacios-Pedrero M.Á., Jansen J.M., Blume C., Stanislawski N., Jonczyk R., Molle A., Hernandez M.G., Kaiser F.K., Jung K., Osterhaus A.D., et al. Signs of immunosenescence correlate with poor outcome of mRNA COVID-19 vaccination in older adults. Nat. Aging. 2022;2:896–905. doi: 10.1038/s43587-022-00292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert P.B., Donis R.O., Koup R.A., Fong Y., Plotkin S.A., Follmann D. A COVID-19 Milestone Attained—A Correlate of Protection for Vaccines. N. Engl. J. Med. 2022;387:2203–2206. doi: 10.1056/NEJMp2211314. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., Zhou H., Houchens C.R., Martins K., Jayashankar L., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matusali G., Sberna G., Meschi S., Gramigna G., Colavita F., Lapa D., Francalancia M., Bettini A., Capobianchi M.R., Puro V., et al. Differential Dynamics of SARS-CoV-2 Binding and Functional Antibodies upon BNT162b2 Vaccine: A 6-Month Follow-Up. Viruses. 2022;14:312. doi: 10.3390/v14020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Painter M.M., Mathew D., Goel R.R., Apostolidis S.A., Pattekar A., Kuthuru O., Baxter A.E., Herati R.S., Oldridge D.A., Gouma S., et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54:2133–2142.e3. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aleman A., Van Oekelen O., Upadhyaya B., Agte S., Kappes K., Beach K., Srivastava K., Gleason C.R., PVI Study Group. Wang B., et al. Fatal breakthrough infection after anti-BCMA CAR-T therapy highlights suboptimal immune response to SARS-CoV-2 vaccination in myeloma patients. medRxiv. 2021 doi: 10.1101/2021.05.15.21256814. preprint . [DOI] [Google Scholar]
- 22.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., Garonzik-Wang J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deepak P., Kim W., Paley M.A., Yang M., Carridi A.B., El-Qunni A.A., Haile A., Huang K., Kinnett B., Liebeskind M.J., et al. Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.04.05.21254656. Updated in Ann. Intern. Medicine. 2021. [DOI] [Google Scholar]
- 24.Noe S., Ochana N., Wiese C., Schabaz F., Von Krosigk A., Heldwein S., Rasshofer R., Wolf E., Jonsson-Oldenbuettel C. Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection. 2022;50:617–623. doi: 10.1007/s15010-021-01721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agha M., Blake M., Chilleo C., Wells A., Haidar G. Suboptimal Response to Coronavirus Disease 2019 Messenger RNA Vaccines in Patients With Hematologic Malignancies: A Need for Vigilance in the Postmasking Era. Open Forum. Infect. Dis. 2021;8:ofab353. doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso M., Pirola N., Pascoli S., Bragato B., Vinci A., Iannetta M., Colombo F., Geremia N., Martignago L., Rossi M.C., et al. Humoral Response after Two Doses of BNT162b2 mRNA Vaccine Has a Role in Predicting Response after Three Doses That Is Related to Plasma HIV Viremia and Nadir CD4+ Cell Count in HIV-Positive Patients. Vaccines. 2022;11:82. doi: 10.3390/vaccines11010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augello M., Bono V., Rovito R., Tincati C., Marchetti G. Immunologic Interplay between HIV/AIDS and COVID-19: Adding Fuel to the Flames? Curr. HIV/AIDS Rep. 2023;20:51–75. doi: 10.1007/s11904-023-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergori A., Cozzi Lepri A., Cicalini S., Matusali G., Bordoni V., Lanini S., Meschi S., Iannazzo R., Mazzotta V., Colavita F., et al. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nat Commun. 2022;13:4922. doi: 10.1038/s41467-022-32263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cicalini S., Vergori A., Cozzi-Lepri A., Meschi S., Bordoni V., Lanini S., Lapa D., Mariotti D., De Pascale L., D’Aquila V., et al. Durability of SARS-CoV-2 mRNA vaccine immune response in PLWH with advanced disease. CROI. 2022;30:109–110. [Google Scholar]
- 30.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skelly D.T., Harding A.C., Gilbert-Jaramillo J., Knight M.L., Longet S., Brown A., Adele S., Adland E., Brown H., Tipton T., et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat. Commun. 2021;12:5061. doi: 10.1038/s41467-021-25167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gbadamosi S.O., Trepka M.J., Dawit R., Jebai R., Sheehan D.M. A Systematic Review and Meta-analysis to Estimate the Time from HIV Infection to Diagnosis for People with HIV. AIDS Rev. 2022;24:32–40. doi: 10.24875/AIDSRev.21000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoury D.S., Schlub T.E., Cromer D., Steain M., Fong Y., Gilbert P.B., Subbarao K., Triccas J.A., Kent S.J., Davenport M.P. Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection. Emerg. Infect. Dis. 2023;29:381–388. doi: 10.3201/eid2902.221422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou S., Guo W., Wu S., Ming F., Tan Y., Wu M., Tang W., Liang K. Six-month humoral immune response to inactivated COVID-19 vaccine among people living with, H.I.V. Front. Immunol. 2022;13:988304. doi: 10.3389/fimmu.2022.988304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are not publicly available because they contain sensitive data to be treated under data protection laws and regulations. Appropriate forms of data sharing can be arranged after a reasonable request to the corresponding author.