Abstract
Obesity has become a major health issue worldwide and obese individuals possess higher levels of adipose tissue when compared with healthy individuals. Obesity is highly associated with the development of different chronic diseases, such as diabetes, cardiovascular diseases, hypertension, cancers, etc. Previous studies established that anthocyanin compounds play an important role in attenuating obesity-related consequences. Among various anthocyanin compounds, cyanidin-3-O-β-glucoside (C3G) is the most important component and is widely distributed in various colored edible plant materials, especially berries, cherries, black rice, purple corn, etc. In recent decades, several studies have reported the therapeutical properties of C3G. C3G has various biological properties and health benefits, such as antioxidant, antimicrobial, anti-inflammatory, antidiabetic, anti-obesity, neuroprotective, anticancer, etc. In this review, we summarized the in vitro and in vivo studies in relation to the role of C3G in obesity-related complications. Several mechanistic studies demonstrated that C3G maintains the metabolism of glucose, fatty acids, and lipids by regulating different genes and signaling pathways. It could be concluded that the consumption of C3G protects healthy individuals from obesity-related issues by maintaining body weight and regulating their metabolism and energy balance. This review provides some important signaling pathways/targets of C3G to facilitate the prevention and treatment of obesity, leading to the development of important food supplements.
Keywords: anthocyanin, cyanidin-3-O-β-glucoside, cyanidin-3-glucoside, obesity, adipogenesis, adipocyte
1. Introduction
Obesity is one of the most important health problems worldwide with considerable illness and mortality. According to the WHO, more than 2.8 million deaths occur every year due to obesity-related consequences. In 2016, approx. 1.9 billion adults were obese [1,2]. If secular trends continue, it is estimated that 38% of the world’s adult population will be overweight by 2030 and an additional 20% will be obese exclusively in underdeveloped countries [3]. Genetic mechanisms, endocrine, appetite disorders, and diabetes-associated diseases are major factors that trigger obesity [4,5]. Obesity is a high-risk factor for developing numerous chronic diseases, including diabetes, heart disease, liver disease, and hypertension [6]. Lipid and energetic metabolisms comprise a sequence of biosynthetic and oxidative reactions required for the energy supply and differentiation of adipocytes. In these, lipogenesis activates mitochondrial biogenesis, the production of reactive oxygen species, and inflammatory responses that are highly correlated with obesity [5]. The major causative factor for obesity is the consumption of more calories accompanied by the accumulation of excessive fat. Although lifestyle changes and improving physical activity are the best strategies for preventing obesity, it can be difficult to continue these activities in the long term [7]. Some treatment strategies that can be followed to prevent obesity are thermogenesis of brown adipose tissue, genetics and epigenetics, physical exercise, and diet, including foods with bioactive components [8]. Therefore, developing a novel effective approach to prevent obesity-related complications is essential for the scientific community [6,7].
Anthocyanins are widely found in colored fruits and vegetables. [4]. They are important flavonoid compounds in terms of food as well as medicine due to the availability of numerous structurally different bioactive components [9,10]. Anthocyanin components offer potential health-promoting functions due to their strong antioxidant effects [9,11]. Anthocyanin components are rich in colored fruits, vegetables, and cereals, including berries, cherries, grapes, black beans, purple cabbage, purple corn, black rice, etc. In addition, anthocyanins are generally treated as colorants in beverages, fruit fillings, and dairy products. Hence, anthocyanins play a significant role in the diet of humans [5,12]. Diets supplemented with anthocyanin-rich berries can significantly affect gastrointestinal bacterial communities with respect to obligate anaerobes by decreasing the gastrointestinal luminal oxygen and oxidative stress [13]. In particular, cyanidin and its derivatives can exhibit a protective effect on DNA cleavage [14]. The administration and consumption of cyanidin glycosides are effective in reducing reactive oxygen species (ROS)-mediated cell and tissue damage [15,16].
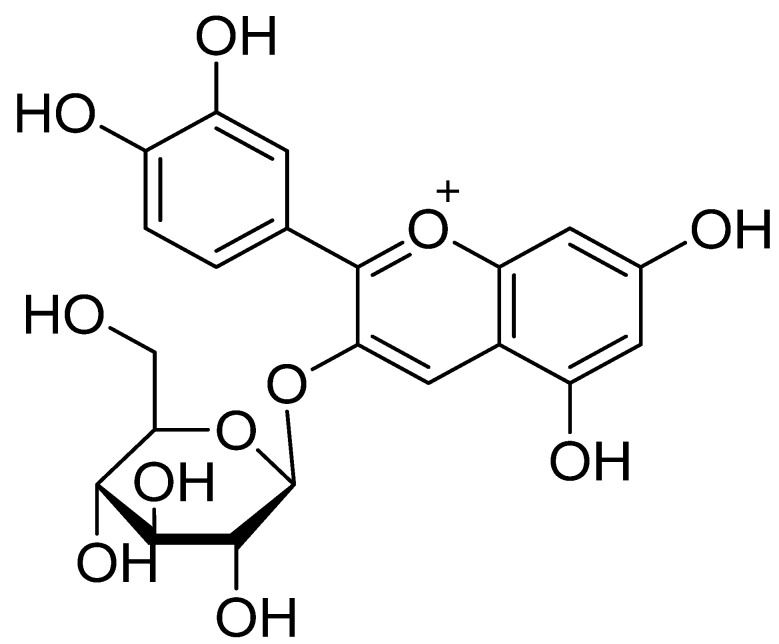
In recent times, there has been significant attention in the research of anthocyanin derivatives. Approx. 635 different anthocyanin components have been identified in various plants. In particular, pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin are important aglycones in foods [5]. Among them, cyanidin-3-O-β-glucoside (C3G) is an important biologically active component (Figure 1). Numerous studies have confirmed that anthocyanins provide various therapeutic effects, especially C3G, which significantly increases energy expenditure as well as decreases weight gain [10,17]. Further, C3G is considered to be the main component in terms of the bioavailability and active form of cyanidin in human tissues [18]. C3G markedly reduces lipogenesis, oxidative stress, and inflammation, thereby improving the obesity-mediated dysregulation of metabolism. C3G has the potential to speed up lipolysis and thermogenesis and to reduce body fat accumulation. A study found that a master regulator of energy metabolism, namely peroxisome proliferator-activated receptors (PPARs), is activated by C3G [19].
Figure 1.
The chemical structure of cyanidin-3-O-β-glucoside.
C3G and its metabolites have better absorption and bioavailability, and their interaction with gut microbiota may improve their positive health effects. Several studies on C3G have been published in the past decades. However, no detailed review has been published regarding the role of C3G in obesity-related complications. In this context, the present review aims to discuss the possible therapeutical properties of C3G for preventing and treating obesity based on in vitro and in vivo studies. For this purpose, a literature search was performed in electronic databases using “obesity” as the major term, together with “C3G”, and mainly comprised articles in English. This review mainly focused on the role of C3G in obesity with its underlying mechanisms, leading to the development of potent food supplements for preventing obesity-related health issues.
2. The Occurrence and Color Characteristics of C3G
Anthocyanin content in colored fruits and vegetables varies remarkably, according to different species. In addition, numerous factors influence the level of anthocyanin content, such as the cultivar, geographical location, environmental conditions, cultural practices, harvest time and methods, processing and storage conditions, etc. [20,21,22]. Berries are a rich source of anthocyanins, including strawberries, blueberries, blackberries, black elderberries, bilberries, blackcurrants, chokeberries, redcurrants, and raspberries [22]. In these, the highest anthocyanin content is found in elderberries (1.4 g) and chokeberries (1.8 g) per 100 g of product [23,24]. Moreover, purple corn, cherries, plums, pomegranates, eggplant, wine, grapes, and colored vegetables such as black carrots, red cabbage, and purple cauliflower contain appreciable amounts of anthocyanin compounds [24,25]. Among the different anthocyanins, C3G is widely found in colorful berries, blue and red fruits, and vegetables. In particular, pomegranates, blackberries, bilberries, elderberries, and mulberries possess a higher bioavailability of C3G. In addition, C3G is commonly found in black rice, black beans, and purple potatoes [26,27].
The color of the anthocyanin pigments usually ranges from pale yellow to blue, but are primarily responsible for the red, purple, and blue colors of different plant organs. In general, C3G is normally found in vegetables and fruits as a red pigment. However, the pH, light, temperature, and structure play a critical role in the color and stability of anthocyanins. At an alkaline pH, anthocyanins are blue pigments, whereas they are red at an acidic pH. However, these pigments are highly unstable and oxidized into dark brown compounds at alkaline conditions due to degradation [28]. In their structure, the B-ring and the presence of hydroxyl or methoxyl groups also influence the stability of anthocyanins. Further, the presence of an oxonium ion adjacent to carbon 2, metal ions, temperature, light, and oxygen may also influence the stability of the anthocyanin pigments [22,28]. In the flowers and fruits of some plants, the colors may depend on the combined effect of the light absorbance of chlorophylls and anthocyanins [29,30].
3. The Chemistry of C3G
Anthocyanins are anthocyanidin glycosides (aglycones). They are formed by a flavylium cation backbone that is hydroxylated in different positions to produce diverse anthocyanidins [29]. Cyanidin is one of the extensively distributed pigments in plants. In these, C3G is the most characterized anthocyanin in edible plants, followed by delphinidin, pelargonidin, and peonidin glucosides. These aglycones differ according to their B-ring substitution pattern [28]. C3G is a monomeric anthocyanin and a water-soluble pigment with a molecular weight of 449.4 g/mol. It is derived from the aglycone cyanidin and is more hydrophilic than cyanidin. C3G consists of an O-glycosylated anthocyanidin with two hydroxyls on the third aromatic ring. In acidic conditions, C3G occurs in the flavylium form. On the other hand, in alkaline conditions, C3G occurs in the carbinol form through the hydration of the flavylium cation or in the quinoidal form through the loss of a proton [31]. In plants, C3G is biosynthesized through the flavan-3-ol pathway. Previously, pharmacokinetics studies in humans demonstrated that >20 kinds of C3G metabolites have been identified [32]. The main bioactive metabolites of C3G are protocatechuic acid, phloroglucinaldehyde, vanillic acid, and ferulic acid. Furthermore, the stability of C3G is improved when it is acylated with lauric acid, owing to its ester group [33,34].
4. In Vitro Studies on the Role of C3G in Obesity-Related Complications
The excessive consumption of energy-rich foods and an inactive lifestyle are believed to be the main causes of obesity-related insulin resistance and abnormal glucose metabolism. Scientific studies confirmed that anthocyanins may lower the metabolic risks linked with obesity due to their potent antioxidant and anti-inflammatory properties. The dysfunction of adipocytes is highly correlated with the onset of obesity and insulin resistance. It is believed that obesity and the improvement of insulin sensitivity can be prevented by regulating the secretion of adipocytokine and the expression of adipocyte-specific genes. Table 1 shows the mechanisms of C3G for preventing obesity-related complications under different in vitro conditions.
Table 1.
The effects of cyanidin-3-O-β-glucoside on the obesity-associated mechanisms under in vitro conditions.
| Model | Concentration | Mechanism(s) | Year of Publication | References |
|---|---|---|---|---|
| Rat adipocytes | 100 µM |
|
2004 | [35] |
| Human preadipocytes | 100 µM |
|
2006 | [36] |
| 3T3-L1 adipocytes and RAW 264.7 cells | 10, 50, and 100 μM |
|
2007 | [37] |
| H2O2- and TNF-α-induced insulin resistance in 3T3-L1 adipocytes | 10, 20, and 40 µM |
|
2008 | [38] |
| Primary brown preadipocytes | 100 µM |
|
2010 | [39] |
| 3T3-L1 adipocytes | 20 and 100 μM |
|
2011 | [40] |
| Skeletal muscle cells and adipocytes from female KK-Ay mice | 10, 50, and 100 μmol/L |
|
2011 | [41] |
| Human omental adipocytes and 3T3-L1 cells. | Human omental adipocytes—50 and 100 µmol/L; 3T3-L1 cells—10 and 100 µmol/L |
|
2011 | [42] |
| Preadipocytes from human adipose explant tissue | 50 μM |
|
2012 | [43] |
| Human HepG2 cells | 100 μM |
|
2012 | [44] |
| 3T3-L1 adipocytes | 50 µM |
|
2012 | [45] |
| Preadipocyte 3T3-L1 cells | Black soybean anthocyanins; 12.5 and 50 µg/mL |
|
2012 | [46] |
| 3T3 adipocytes | 12.5, 25, and 50 µM |
|
2014 | [47] |
| 3T3-L1 fibroblasts | 5 or 20 µM |
|
2015 | [48] |
| 3T3-L1 adipocytes | 20 and 100 μM |
|
2015 | [49] |
| Primary human preadipocytes | 100 μM |
|
2016 | [50] |
| 3T3-L1 adipocytes | 10 and 50 μM |
|
2017 | [51] |
| 3T3-L1 adipocytes | 50 μM |
|
2017 | [52] |
| 3T3-L1 adipocytes | 50 and 100 μM |
|
2017 | [53] |
| Brown adipose tissue C3H10T1/2 clone 8 cells induced by palmitate |
Isolated from mulberry; 100 and 200 μg/mL |
|
2018 | [54] |
| Palmitic acid-induced 3T3-L1 adipocytes | Anthocyanin-rich extract; 10 and 20 μg/mL |
|
2019 | [55] |
| Macrophage–adipocyte interaction, using mono- and co-culture | 1.0 mg/mL of the anthocyanin-rich extracts of purple and red maize or 50 μM of pure anthocyanins |
|
2019 | [2] |
| Pancreatic lipase and cholesterol esterase Caco-2 cells |
12.5–100 μM |
|
2019 | [56] |
| Pancreatic lipase activity | 50 to 350 µM |
|
2019 | [57] |
| Human adipose tissue | 25 μM |
|
2019 | [58] |
| RAW 264.7 macrophages and 3T3-L1 adipocytes |
Anthocyanin-rich water extracts (PMWs) from purple maize; 1 mg/mL |
|
2019 | [59] |
| 3T3-L1 hypertrophic adipocytes exposed to palmitic acid | 5–10 μM |
|
2020 | [60] |
| Palmitic acid-induced proximal tubular cells |
2, 10, and 20 μM |
|
2020 | [61] |
| HepG2 cells and C2C12 myotubes |
10 and 50 μM |
|
2020 | [19] |
| C3H/10T1/2 brown adipose cells | 10–40 µM |
|
2021 | [62] |
| Human SGBS adipocytes and murine 3T3-L1 cells. | 1–20 μM |
|
2021 | [63] |
| Human amniotic epithelial cells (hAECs) | 20 µM |
|
2021 | [64] |
| 3T3-L1 preadipocytes | 50–200 μM |
|
2022 | [65] |
| 3T3-L1 preadipocytes | 30–100 μM |
|
2023 | [66] |
| Pancreatic lipase inhibitory assay | 0.05 to 0.35 mg/mL |
|
2023 | [67] |
| 3T3-L1 preadipocytes | 5 and 10 μM |
|
2023 | [68] |
An excessive amount of circulating free fatty acids has been proven to be potentially associated with obesity, insulin resistance, and diabetes. In this context, lipolysis inhibition is the main target for reducing free fatty acids and improving insulin sensitivity. A study reported that C3G effectively suppressed the release of free fatty acids and glycerol from 3T3-L1 adipocytes during hyperglycemia. C3G treatment downregulated the hexosamine biosynthetic pathway by increasing the AMP-activated protein kinase activity, decreasing the glutamine:fructose 6-phosphate aminotransferase activity (GFAT), and reducing the production of cellular UDP-N-acetylglucosamine. Furthermore, C3G downregulated the adipose triglyceride lipase expression by attenuating the forkhead box O1 (FoxO1) transcription factor [45]. C3G efficiently improved insulin resistance in 3T3-L1 adipocytes by upregulating the expression of the glucose transporter type 4 (GLUT4) gene [40].
C3G markedly inhibited basal adipocyte lipolysis in differentiated human fat cells. A mixture of docosahexaenoic acid and C3G reduced the tumor necrosis factor-α (TNF-α) secretion and enhanced insulin-induced lipogenesis [50]. Matsukawa et al. [49] demonstrated that C3G treatment differentiated the 3T3-Ll cells into smaller adipocytes by upregulating the gene expressions of PPARγ and C/EBPα, increasing the adiponectin secretion, decreasing the TNF-α secretion, activating insulin signaling, and increasing the glucose uptake. Further, C3G effectively upregulated the expression of PGC-1α, sirtuin-1 (SIRT1), and the uncoupling protein (UCP)-3 genes in C2C12 myotubes. Jia et al. [19] found that C3G effectively enhanced the uptake of glucose in HepG2 cells as well as C2C12 myotubes. Further, C3G stimulated the hepatic fatty acid oxidation rate. The results revealed that C3G upregulated the main regulators of energy metabolism, i.e., PPARs. In rat adipocytes, C3G increased the secretion of adipocytokines, such as adiponectin and leptin. Further, the adipocyte-specific gene expression was upregulated by C3G treatment without PPARγ activation [35]. In human adipocytes, C3G or cyanidin treatment significantly upregulated the gene expression of adiponectin as well as downregulated plasminogen activator inhibitor-1 and IL-6. C3G or cyanidin treatment also activated the lipid metabolism-associated genes, such as uncoupling protein2, acyl-CoA oxidase1, and perilipin [36].
One of the promising approaches for treating obesity is to convert white adipocytes into brown-like adipocytes. In 3T3-L1 adipocytes, C3G stimulated phenotypic modifications into white adipocytes, such as higher levels of multilocular lipid droplets and mitochondrial content. In addition, C3G treatment increased the expression of the mitochondrial genes in 3T3-L1. Moreover, C3G improved the differentiation of preadipocytes by activating the CCAAT/enhancer-binding protein β (C/EBPβ) via increasing the level of intracellular cyclic adenosine 3′5′-monophosphate (cAMP) [53]. Recently, Han et al. [62] found that Prdm16 bound to the promoter region (−500 to −150 bp) of UCP1 to stimulate its transcription with the presence of C3G in C3H10T12 brown adipose cells, thereby facilitating brown adipose tissue programming. In differentiated palmitate-induced C3H10T1/2 clone8 cells, C3G suppressed the release of adipokines, such as extracellular nicotinamide phosphoribosyltransferase and fibroblast growth factor 21 [54].
A study indicated that C3G treatment significantly regulated protein kinase B (Akt) phosphorylation, the sensitivity of adipocytes to palmitic acid, GLUT-1 and GLUT-4 glucose transporters, and hexokinase-II in palmitic acid-induced human SGBS adipocytes. In addition, the cells pretreated with C3G exhibited a significant reduction in the mRNA levels of pro-inflammatory cytokines (TNF-α, interleukin (IL)-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1)) in palmitic acid-induced human SGBS adipocytes [63]. C3G markedly reduced the accumulation of lipids, PPARγ, and the nuclear factor kappa B (NF-κB) pathways in palmitic acid-induced 3T3-L1 adipocytes, and enhanced insulin sensitivity via the restoration of the insulin receptor substrate 1 (IRS-1)/phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Moreover, C3G improved the mRNA levels of adiponectin in palmitic acid-induced 3T3-L1 and SGBS human adipocytes [60]. C3G treatment efficiently triggered the expression and secretion of adiponectin in 3T3 adipocytes through the transcription factor forkhead box O1 (FoxO1). The authors found that C3G upregulated FoxO1 by enhancing its deacetylation through silent mating type information regulation 2 homolog 1 [47].
In 3T3-L1 adipocytes, a study found that C3G treatment effectively reverted the insulin resistance induced by H2O2- or TNF-α via the downregulation of the c-Jun N-terminal kinases (JNK) signaling pathway [38]. C3G and its metabolite protocatechuic acid improved the uptake of adipocyte glucose and GLUT4 membrane translocation in human omental adipocytes and 3T3-L1 cells. Further, these components markedly increased the nuclear PPARγ activity and the expression of GLUT4 [42]. In macrophage-conditioned media-treated adipocytes, C3G and pelargonidin-3-O-glucoside suppressed the activation of the NF-κB and JNK pathways by regulating the phosphorylation of IκBα and JNK, respectively [2]. In 3T3-L1 adipocytes, anthocyanins extracted from colored corn blocked the adipocyte differentiation and the accumulation of lipids, and attenuated the transcription of PPAR-γ. In 3T3-L1 cells, black soybean anthocyanins decreased the accumulation of lipids and inhibited the expression of PPARγ [46]. Further, anthocyanins improved inflammation induced by TNF-α and insulin resistance in adipocytes by activating insulin signaling and enhanced translocation of GLUT4 [52].
Further, AMPK plays a key role in preventing hepatic lipid metabolism by regulating the downstream acetyl CoA carboxylase and carnitine palmitoyl transferase 1 pathways. A study indicated that C3G regulated hepatic lipid homeostasis through an AMPK-dependent signaling pathway [44]. In 3T3-L1 preadipocytes, cyanidin treatment suppressed adipogenesis via the downregulation of gene expressions, such as PPARγ, C/EBPα, adiponectin, and aP2. In addition, intracellular Ca2+ was increased in the cyanidin-stimulated cells [66]. Cyanidin-3-rutinoside (C3R) significantly upregulated the uptake of glucose and the expression of plasma membrane glucose transporter type 4 (GLUT4) by triggering the PI3K/Akt pathways [51]. In mesenteric adipose tissue-cultured medium-induced RAW 264.7 cells, cyanidin and C3G markedly inhibited the migration and production of inflammatory chemokines, such as MCP-1 and MRP-2 [37]. Zhang et al. [59] investigated the effect of anthocyanin-rich water extracts obtained from 20 different purple corn genotypes on RAW 264.7 macrophages and 3T3-L1 adipocytes. These extracts efficiently decreased the production of the pro-inflammatory mediators, regulated the diabetes-associated key enzymes, and improved insulin sensitivity. In the early phase of adipogenesis, C3G exposure markedly suppressed the expression of (C/EBPβ), PPARɣ, and FAS, and activated the AMPK pathway when compared to late-phase exposure [68].
Saulite et al. [58] investigated the effect of malvidin, cyanidin, and delphinidin against adipose tissue-derived human mesenchymal stem cells. The results revealed that delphinidin suppressed adipogenesis and decreased fatty acid-binding protein 4 (FABP4) and the adiponectin genes. Malvidin stimulated a higher level of calcium accumulation in the cells. Further, the osteocyte-specific gene BMP-2 and Runx-2 expression were upregulated in addition to the stimulation of BMP-2 secretion by malvidin. According to suitable stimuli, adipocytes can be proliferated and differentiated. The important transcription factor responsible for lipogenesis is the carbohydrate response element-binding protein (ChREBP) chiefly found in lipogenic organs. Pompei et al. [43] found that cyanidin diminished the differentiation by 20% as well as the ChREBP expression in preadipocytes. The authors suggested that cyanidin exhibited an inhibitory activity on adipogenesis by interfering with the extracellular matrix [43]. Takahashi et al. [64] reported that C3G showed significant induction in adipocyte differentiation in human amniotic epithelial cells.
Pancreatic lipase is one of the most essential digestive enzymes for metabolism as well as for the absorption of triglycerides to monoglycerides and free fatty acids. In the body, the level of total cholesterol is decreased when inhibiting the lipase enzyme activity [69]. In this context, different authors investigated the inhibitory potential of C3G against lipase enzymes. Their studies showed that C3G markedly inhibited the pancreatic lipase activity with IC50 values of 188.28 µM [57] and 0.268 mg/mL [67]. A recent study demonstrated that the development of insulin fibrils was attenuated by anthocyanins, such as cyanidin, C3G, C3R, malvidin, and malvidin-3-glucoside [65]. In another study, the results exhibited that C3R effectively inhibited pancreatic lipase (IC50 at 59.4 μM). Further, C3R showed a considerable reduction in the cholesterol uptake in free cholesterol as well as mixed micelles in addition to the suppression of the NPC1L1 expression in Caco-2 cells [56]. Chen et al. [70] found that C3G from Aronia melanocarpa exhibited the strongest inhibitory activity against α-amylase and lipase. However, cyanidin 3-galactoside, cyanidin-3-arabinoside, and cyanidin 3-xyloside did not show any effect on the α-amylase and lipase enzymes. Further, Xie et al. [71] suggested that the structures of the B-ring and glycosyl group were mainly associated with the inhibitory potentials of monomeric anthocyanins.
5. In Vivo Studies on the Role of C3G in Obesity-Related Complications
Similar to the in vitro studies, numerous in vivo studies have been carried out to determine the effect of C3G on obesity-associated mechanisms (Table 2). The C3G-rich purple corn color effectively reduced body weight gain as well as the weights of white and brown adipose tissue in high-fat (HF) diet-induced mice. Further, the purple corn color downregulated the mRNA levels of the enzymes responsible for the synthesis of fatty acid triacylglycerol and reduced the mRNA level of sterol regulatory element-binding protein-1 in white adipose tissue [72]. In another study, the dietary intake of anthocyanin upregulated the adiponectin expression in white adipose tissue and suggested that the activation of AMPK was a possible mechanism for these changes [35].
Table 2.
The effect of cyanidin-3-O-β-glucoside on the obesity-associated mechanisms under in vivo conditions.
| Model | Dose | Mechanism(s) | Year of Publication | References |
|---|---|---|---|---|
| HFD-induced C57BL/6J mice | Purple corn extract; 2 g/kg diet |
|
2003 | [72] |
| HFD-induced C57/Bl6 mice | 90 mg/kg/day |
|
2010 | [39] |
| HFD-induced C57BLK/6J mice | C3G-rich grape pomace extract; 250 mg/kg b.w. per day |
|
2010 | [73] |
| KK-Ay mice | 10, 50, and 100 μmol/L; 1 g/kg |
|
2011 | [41] |
| HFD-induced C57BL/6 mice | 3G-rich black soybean seed coat extract for 14 weeks |
|
2011 | [74] |
| Sprague-Dawley rats—ovariectomized (OVX) | 5% and 10% (w/w) black berries |
|
2012 | [75] |
| HFD-fed C57BL/6J mice and db/db and db/+ mice | 0.2% |
|
2012 | [76] |
| HFD-fed C57BL/6 mice | 40 and 200 mg/kg food for 12 weeks |
|
2013 | [77] |
| HFD-induced rats | 100 mg/kg |
|
2014 | [78] |
| db/db mice | 2 g/kg diet |
|
2014 | [47] |
| Estrogen-deficient animals with diet-induced obesity in OVX rats | Black carrot extract; 2% for 12 weeks |
|
2015 | [48] |
| HFD-induced C57BL/6J mice | Cyanidin-based anthocyanin-rich blackcurrant extract; 1% for 8 weeks |
|
2015 | [79] |
| HFD-induced C57BL/6J mice | Anthocyanin-rich black elderberry extract; 20–40 mg and 100–200 mg/kg b.w. for 16 weeks |
|
2015 | [80] |
| Diabetes model in KK-Ay mice | C3G-rich aronia juice; free intake |
|
2016 | [81] |
| HFD-induced mice | Purple corn anthocyanin; 200 mg/kg |
|
2017 | [82] |
| db/db mice | 1 mg/mL for 16 weeks |
|
2017 | [17] |
| C57BL/6 J mice fed with a high-fat high-cholesterol diet | 200 mg/kg |
|
2018 | [54] |
| HFD-induced obese mice | Haskap Berry; 0.192% C3G-rich extract |
|
2018 | [83] |
| Human primary myotubes derived from obese and obese T2DM participants | 10 and 100 μM |
|
2018 | [84] |
| HFD-fed C57BL/6J mice | AC-rich blend; 2, 20, or 40 mg/kg body weight |
|
2018 | [85] |
| HF and high-fructose diet-induced mice | 1 mg/mL |
|
2018 | [86] |
| Diet-induced mouse model | 0.02 g/kg BW/ day |
|
2019 | [87] |
| HFD-induced C57BL/6N mice | Aronia melanocarpa extract (70% ethanol extract); 50, 100, and 200 mg/kg body weight/day |
|
2019 | [88] |
| C57BL/6 J mice | 50 mg/day/body weight for 8 weeks |
|
2020 | [19] |
| HFD-induced C57BL/6J mice | Lingonberry (5% w/w) and its anthocyanin; C3G for 12 weeks |
|
2020 | [61] |
| HF and high-fructose diet-induced db/db mice | 1 mg/mL |
|
2021 | [62] |
| HFD-induced mice | Purple corn anthocyanin; 400 mg/kg |
|
2021 | [4] |
| High-fat high-sucrose diet-indued mice | 45.2 mg/kg for 10 weeks |
|
2021 | [89] |
| HFD Sprague-Dawley rats | Isolated from Aronia melanocarpa, Haicheng, China; 100 and 200 mg/kg bw/day) for 8 weeks. |
|
2021 | [90] |
| HF-high-carbohydrate diet-induced obesity model | 0.02 g/kg BW/d |
|
2022 | [91] |
| High-fat meal in healthy humans | Cyanidin- and delphinidin-rich extract (1 g consisted of 150 mg bilberry extract, 230 mg black currant extract, and 620 mg black rice extract) |
|
2022 | [92] |
| HFD-fed C57BL/6 mice | Casein/C3G nanoparticles |
|
2023 | [93] |
It is well known that brown adipose tissue burns energy to generate heat. The previous studies found that C3G can improve the thermogenic ability of brown adipose tissue. Han et al. [62] evaluated the effect of C3G on the UCP1 gene expression in db/db mice induced with a HF, high-fructose diet and found that Prdm16 is directly bound to the promoter region of UCP1. In another study, C3G efficiently regulated the transcription of UCP1 in brown adipose tissue as well as in subcutaneous white adipose tissue by enhancing mitochondrial number and function [17]. In the diet-induced obesity mice model, the purple corn cob extract treatment upregulated the M2 markers (ArgI, Fizz1, and TGFβ) and downregulated the inflammatory mediators (TNF-α, IL-6, IL-1β, and COX-2) by suppressing NF-kB signaling [94]. Jia et al. [19] reported that the dietary supplementation of C3G upregulated the PPARs in the HF-diet-induced mice. In the KK-Ay mice, C3G significantly enhanced obesity and triglyceride metabolism by partly activating lipoprotein lipase in the plasma and skeletal muscle. The inhibition of lipoprotein lipase (LPL) in adipose tissue following the activation of pAMPK might have been attributed to the inhibition of lipoprotein lipase [41]. Guo et al. [76] reported that C3G regulated the signaling pathway, c-Jun N-terminal kinase/foxO1, and its associated inflammatory adipocytokines. Ren-Qiang et al. [78] also reported that C3G diminished obesity-related dyslipidemia and insulin resistance in HF-diet-induced rats by enhancing the level of serum adiponectin. Biswas et al. [83] reported that C3G supplementation in the diet reduced weight gain, and improved food intake and metabolism during obesity.
The combination of peptides and C3G markedly improved the glucose uptake with or without insulin. In addition, this combination suppressed the expression of the angiotensin II receptor, type 1 (AGTR-1), and activated the insulin receptor substrate 1 and GLUT4 expressions [84]. In diet-induced obese mice, the mixture of C3G and peptides efficiently decreased systolic as well as diastolic blood pressure. Further, this combination significantly reduced body fat levels and enhanced glucose tolerance [87]. In diet-induced obese mice, the combination of C3G and peptides regulated the expression of the genes associated with glucose metabolism [91]. The administration of anthocyanins from purple corn remarkably reduced body weight gain, fat mass, total cholesterol, and triglyceride levels in the HF-diet-induced mice. The data demonstrated that the anthocyanin extract suppressed the expression of PPARγ, C/EBPα, and SREBP-1c, subsequently activating the expression of PPARα, PGC1α, PRDM16, and FGF21 [4].
Titta et al. [39] demonstrated that the anthocyanin-rich extract from Moro oranges marginally affected the accumulation of fats in C57/Bl6 mice induced with a HF diet. Anthocyanin black carrots fermented with Aspergillus oryzae inhibited lipid and glucose metabolism via the activation of hepatic insulin signaling and the AMPK pathways in OVX rats [48]. A study demonstrated that anthocyanins from black rice, black soybeans, and purple corn alleviated oxidative stress and inflammation-related obesity in HF-diet-induced mice by suppressing the expression of TNFα, IL-6, inducible nitric oxide synthase (iNOS), and NF-κB, and increasing the hepatic superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities [82]. Blackberries containing 87% of the C3G diet downregulated the expression levels of hepatic NF-κB and cyclooxygenase-2 in ovariectomized rats [75]. A C3G-enriched Aronia melanocarpa extract inhibited adipogenesis by downregulating the expressions of the CCAAT/enhancer-binding protein, PPAR, sterol regulatory element-binding protein-1c, PPARγ coactivator-1, acetyl-CoA carboxylase 1, ATP-citrate lyase, fatty acid synthase, and adipocyte protein 2 [88]. The anthocyanin-rich extract from aronia fruit reduced the accumulation of visceral fat and hyperglycemia via the inhibition of pancreatic lipase activity and the adsorption of intestinal lipids [95]. In HF-diet-induced rats, C3G isolated from Aronia melanocarpa treatment expressively decreased the levels of TNF-α, IL-6, and IL-1β in the serum. Further, C3G improved HFD-induced obesity by activating AMPK and suppressed HFD-induced inflammation by activating the phosphorylation of STAT3 and suppressing NF-κB p65 in the nucleus [90].
Anthocyanins, such as C3G, C3R, and pelargonidin-3-glucoside, isolated from Chinese mulberry significantly inhibited body weight gain, reduced insulin resistance, lowered the size of adipocytes, and attenuated the accumulation of lipids and the secretion of leptin [77]. The supplementation of cyanidin and delphinidin effectively controlled overweight, obesity, and type 2 diabetes by modulating the activity of inflammatory mediators, oxidative stress, and NF-κB/JNK activation [85]. Esposito et al. [79] suggested that the gut microbiome and the kind of anthocyanin aglycone moiety could influence the preventive role of anthocyanins in obesity and their associated insulin resistance. The supplementation of cyanidin- and delphinidin-rich extracts also exhibited beneficial effects on unhealthy diets [92]. The administration of C3G from Saskatoon berries diminished liver steatosis, insulin resistance, and chronic inflammation in HF and high-sucrose-induced mice [89].
The improvement in the thermogenesis of brown adipose tissue is an efficient strategy for alleviating obesity. A study exhibited that C3G improved the energy expenditure and thermogenesis of brown adipose tissue in mice induced with HF diet obesity. Additionally, C3G upregulated the UCP1 expression as well as other thermogenic genes in inguinal white adipose tissue and brown adipose tissue [86]. In db/db mice, C3G treatment restored the endothelium-dependent relaxation of the aorta [47]. C3G controlled the adipokine expression in brown adipose tissue in high-fat-cholesterol diet-induced mice [54]. A recent study demonstrated that treatment with casein/C3G nanoparticles ameliorated the HFD-induced accumulation of fats and liver oxidative stress in C57BL/6 mice [93]. In a recent study, Meleleo et al. [96] demonstrated that anthocyanins, such as cyanidin, C3G quercetin, and quercetin-O-glucoside, interacted with planar lipid membranes and formed conductive units.
6. Conclusions and Future Perspectives
C3G is an important anthocyanin compound and is widely found in various fruits and vegetables. Previous studies established that C3G supplementation offers numerous health benefits to the consumer. In this review, we discussed the effect of C3G treatment on obesity-associated mechanisms under different in vitro and in vivo models. The mechanisms preventing obesity highly depend on their capacity to control the consumption of food and energy metabolism and improve inflammatory response, insulin resistance, and glucose metabolism. In addition, C3G can effectively inhibit the pancreatic lipase enzyme. A variety of pathways and gene expressions play a crucial role in preventing obesity-related consequences. In particular, AMPK is an important pathway, attributed to the prevention of hepatic lipid metabolism, and PPARs play a key role in energy metabolism. The IRS-1/PI3K/Akt pathway and GLUT4 gene expression are mainly responsible for insulin sensitivity and the NF-κB signaling pathway is for inflammatory response. The published papers clearly showed that C3G could be a potential agent for facilitating the prevention and treatment of obesity-associated complications owing to its multi-targeted activities. Although C3G can effectively attenuate obesity-associated complications, further clinical studies leading to the development of C3G-enriched functional foods are required.
Acknowledgments
This work was supported by the “Quality characteristics of health function and development of breeding material lines of purple corn (PJ015140)” from the Rural Development Administration, Korea.
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| AGTR-1 | Angiotensin II Receptor Type 1 |
| Akt | Protein Kinase B |
| AMPK | Adenosine monophosphate-activated protein kinase |
| aP2 | Adipocyte Protein 2 |
| ATP | Adenosine triphosphate |
| BMP-2 | Bone Morphogenetic Protein-2 |
| C/EBPα | CCAAT/enhancer-binding protein α |
| C3G | Cyanidin-3-O-β-glucoside |
| C3R | Cyanidin-3-rutinoside |
| cAMP | Cyclic adenosine 3′,5′-monophosphate |
| ChREBP | Carbohydrate response element-binding protein |
| COX-2 | Cyclooxygenase 2 |
| CPT-1 | Carnitine palmitoyltransferase I |
| FABP4 | Fatty acid-binding protein 4 |
| FAS | Fatty acid synthase |
| FGF21 | Fibroblast growth factor 21 i |
| FoxO1 | Forkhead box protein O1 |
| GFAT | Glutamine:fructose-6-phosphate aminotransferase |
| GLUT4 | Glucose transporter type 4 |
| GPx | Glutathione peroxidase |
| H2O2 | Hydrogen peroxide |
| hAECs | Human aortic endothelial cells |
| HFD | High-fat diet |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| IRS-1 | Insulin receptor substrate 1 |
| IκBα | Inhibitor of nuclear factor kappa B |
| JNK | c-Jun N-terminal kinase |
| LPL | Lipoprotein lipase |
| malonyl CoA | Malonyl coenzyme A |
| MCP-1 | Monocyte chemoattractant protein-1 |
| mRNA | Messenger RNA |
| MRP-2 | Macrophage inflammatory protein-related protein-2 |
| NF-κB | Nuclear factor kappa B |
| NPC1L1 | Niemann-Pick C1-Like 1 |
| OVX | Ovariectomy |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PGC1α | Peroxisome proliferator-activated receptor-γ coactivator 1-α |
| PI3K | Phosphoinositide 3-kinase |
| PPARs | Peroxisome proliferator-activated receptors |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gammaa |
| PRDM16 | PR/SET Domain 16 |
| PTP1B | Protein tyrosine phosphatase 1B |
| ROS | Reactive oxygen species |
| Runx-2 | Runt-related transcription factor 2 |
| SIRT1 | Sirtuin1 |
| SOD | Superoxide dismutase |
| SOD2 | Superoxide dismutase-2 |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| STAT3 | Signal transducer and activator of transcription 3 |
| TFAM | Mitochondrial transcription factor A |
| TNF-α | Tumor necrosis factor alpha |
| UCP | Uncoupling protein |
| WHO | World Health Organization |
Author Contributions
Conceptualization, S.K.; methodology, S.K. and P.D.; writing—original draft preparation, P.D. and K.S.; writing—review and editing, M.H., K.S. and S.K. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wharton S., Lau D.C.W., Vallis M., Sharma A.M., Biertho L., Campbell-Scherer D. Obesity in adults: A clinical practice guideline. Can. Med. Assoc. J. 2020;192:E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q., Vital D.L., Mejia E.G.D. Anthocyanins from colored maize ameliorated the inflammatory paracrine interplay between macrophages and adipocytes through regulation of NF-κB and JNK-dependent MAPK pathways. J. Funct. Foods. 2019;54:175–186. doi: 10.1016/j.jff.2019.01.016. [DOI] [Google Scholar]
- 3.Hruby A., Hu F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H., Liu M., Liu H., Zhao B., Zheng M., Liu J. Anthocyanins from purple corn ameliorated obesity in high fat diet-induced obese mice through activating hepatic AMPK. J. Funct. Foods. 2021;84:104582. doi: 10.1016/j.jff.2021.104582. [DOI] [Google Scholar]
- 5.Gomes J.V.P., Rigolon T.C.B., Souza M.S.D.S. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: A systematic review. Nutrition. 2019;66:192–202. doi: 10.1016/j.nut.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Wolfenden L., Ezzati M., Larijani B., Dietz W. The challenge for global health systems in preventing and managing obesity. Obes. Rev. 2019;20:185–193. doi: 10.1111/obr.12872. [DOI] [PubMed] [Google Scholar]
- 7.Bluher M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 8.Palou A., Bonet M.L. Challenges in obesity research. Nutr. Hosp. 2013;5:144–153. doi: 10.3305/nh.2013.28.sup5.6930. [DOI] [PubMed] [Google Scholar]
- 9.Lao F., Sigurdson G.T., Giusti M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2017;16:234–246. doi: 10.1111/1541-4337.12249. [DOI] [PubMed] [Google Scholar]
- 10.Cuevas Montilla E., Hillebrand S., Antezana A., Winterhalter P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011;59:7068–7074. doi: 10.1021/jf201061x. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z., Zhai W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.) Innov. Food Sci. Emerg. Technol. 2010;11:169–176. doi: 10.1016/j.ifset.2009.08.012. [DOI] [Google Scholar]
- 12.You Y., Zhou F., Huang D. Eating the right color: Dietary anthocyanins and obesity control. Food Beverage Asia. 2018;12:57–59. [Google Scholar]
- 13.Overall J., Bonney S.A., Wilson M., Beermann A., Grace M.H., Esposito D., Lila M.A., Komarnytsky S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int. J. Mol. Sci. 2017;18:422. doi: 10.3390/ijms18020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acquaviva R., Russo A., Galvano F., Galvano G., Barcellona M.L., Li Volti G., Vanella A. Cyanidin and cyanidin 3-O-beta-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003;19:243–252. doi: 10.1023/B:CBTO.0000003974.27349.4e. [DOI] [PubMed] [Google Scholar]
- 15.Galvano F., La Fauci L., Lazzarino G., Fogliano V., Ritieni A., Ciappellano S., Battistini N.C., Tavazzi B., Galvano G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004;15:2–11. doi: 10.1016/j.jnutbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W., Jia Q., Wang Y., Zhang Y., Xia M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012;52:314–327. doi: 10.1016/j.freeradbiomed.2011.10.483. [DOI] [PubMed] [Google Scholar]
- 17.You Y., Yuan X., Liu X., Liang C., Meng M., Huang Y., Han X., Guo J., Guo Y., Ren C., et al. Cyanidin-3-glucoside increases whole body energy metabolism by upregulating brown adipose tissue mitochondrial function. Mol. Nutr. Food Res. 2017;61:1700261. doi: 10.1002/mnfr.201700261. [DOI] [PubMed] [Google Scholar]
- 18.Zannou O., Oussou K.F., Chabi I.B., Awad N.M.H., Aïssi M.V., Goksen G., Mortas M., Oz F., Proestos C., Kayodé A.P.P. Nanoencapsulation of Cyanidin 3-O-Glucoside: Purpose, technique, bioavailability, and stability. Nanomaterials. 2023;13:617. doi: 10.3390/nano13030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia Y., Wu C., Kim Y.S., Yang S.O., Kim Y., Kim J.S., Jeong M.Y., Lee J.H., Kim B., Lee S., et al. A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun. Biol. 2020;3:514. doi: 10.1038/s42003-020-01231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinardi A., Cola G., Gardana C.S., Mignani I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019;10:1045. doi: 10.3389/fpls.2019.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z., Dong B., Liu C., Zong Y., Shao Y., Liu B., Yue H. Variation of anthocyanin content in fruits of wild and cultivated Lycium ruthenicum. Ind. Crops Prod. 2020;146:11220. doi: 10.1016/j.indcrop.2020.112208. [DOI] [Google Scholar]
- 22.Mattioli R., Francioso A., Mosca L., Silva P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules. 2020;25:3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Pascual-Teresa S., Sanchez-Ballesta M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008;7:281–299. doi: 10.1007/s11101-007-9074-0. [DOI] [Google Scholar]
- 24.Wu X., Beecher G.R., Holden J.M., Haytowitz D.B., Gebhardt S.E., Prior R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 25.Neveu V., Perez-Jimenez J., Vos F., Crespy V., du Chaaut L., Mennen L., Knox C., Eisner R., Cruz J., Wishart D., et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database. 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Z., Si X., Tan H., Zang Z., Tian J., Shu C., Sun X., Li Z., Jiang Q., Meng X., et al. Cyanidin-3-O-glucoside and its phenolic metabolites ameliorate intestinal diseases via modulating intestinal mucosal immune system: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2023;63:1629–1647. doi: 10.1080/10408398.2021.1966381. [DOI] [PubMed] [Google Scholar]
- 27.Frountzas M., Karanikki E., Toutouza O., Sotirakis D., Schizas D., Theofilis P., Tousoulis D., Toutouzas K.G. Exploring the Impact of Cyanidin-3-Glucoside on Inflammatory Bowel Diseases: Investigating New Mechanisms for Emerging Interventions. Int. J. Mol. Sci. 2023;24:9399. doi: 10.3390/ijms24119399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castañeda-Ovando A., de Lourdes Pacheco-Hernández M., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- 30.He J., Giusti M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 31.Olivas-Aguirre F.J., Rodrigo-García J., Martínez-Ruiz N.D.R., Cárdenas-Robles A.I., Mendoza-Díaz S.O., Álvarez-Parrilla E., González-Aguilar G.A., De la Rosa L.A., Ramos-Jiménez A., Wall-Medrano A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules. 2016;21:1264. doi: 10.3390/molecules21091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Ferrars R.M., Czank C., Zhang Q., Botting N.P., Kroon P.A., Cassidy A., Kay C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014;171:3268–3282. doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X., Yuan Z., Feng L., Fang Y. Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 2015;128:687–696. doi: 10.1007/s10265-015-0717-8. [DOI] [PubMed] [Google Scholar]
- 34.Tan J., Li Y., Hou D.X., Wu S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants. 2019;8:479. doi: 10.3390/antiox8100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuda T., Ueno Y., Aoki H., Koda T., Horio F., Takahashi N., Kawada T., Osawa T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004;316:149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 36.Tsuda T., Ueno Y., Yoshikawa T., Kojo H., Osawa T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006;71:1184–1197. doi: 10.1016/j.bcp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 37.Choe M.R., Kang J.H., Yoo H., Choe S.Y., Yang C.H., Kim M.O., Yu R. Cyanidin and Cyanidin-3-O-β-D-glucoside suppress the inflammatory responses of obese adipose tissue by inhibiting the release of chemokines MCP-1 and MRP-2. J. Food Sci. Nutr. 2007;12:148–153. doi: 10.3746/jfn.2007.12.3.148. [DOI] [Google Scholar]
- 38.Guo H., Ling W., Wang Q., Liu C., Hu Y., Xia M. Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or TNF-alpha-induced insulin resistance by inhibiting c-Jun NH2-terminal kinase activation. Biochem. Pharmacol. 2008;75:1393–1401. doi: 10.1016/j.bcp.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Titta L., Trinei M., Stendardo M., Berniakovich I., Petroni K., Tonelli C., Riso P., Porrini M., Minucci S., Pelicci P.G., et al. Blood orange juice inhibits fat accumulation in mice. Int. J. Obes. 2010;34:578–588. doi: 10.1038/ijo.2009.266. [DOI] [PubMed] [Google Scholar]
- 40.Inaguma T., Han J., Isoda H. Improvement of insulin resistance by Cyanidin 3-glucoside, anthocyanin from black beans through the up-regulation of GLUT4 gene expression. BMC Proc. 2011;5((Suppl. S8)):P21. doi: 10.1186/1753-6561-5-S8-P21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei X., Wang D., Yang Y., Xia M., Li D., Li G., Zhu Y., Xiao Y., Ling W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011;91:1006–1013. doi: 10.1002/jsfa.4275. [DOI] [PubMed] [Google Scholar]
- 42.Scazzocchio B., Vari R., Filesi C., Archivio M.D., Santangelo C., Giovannini C., Lacovelli A., Silecchia G., Volti G.L., Galvano F., et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes. 2011;60:2234–2244. doi: 10.2337/db10-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pompei A., Toniato E., Innocenti P.D., Alimonte I., Cellini C., Mattoscio D., Cotellese R., Bosco D., Ciccarelli R., Dadorante V.D., et al. Cyanidin reduces preadipocyte differentiation and relative ChREBP expression. J. Biol. Regul. Homeost. Agents. 2012;26:253–264. [PubMed] [Google Scholar]
- 44.Guo H., Liu G., Zhong R., Wang Y., Wang D., Xia M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012;11:10. doi: 10.1186/1476-511X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo H., Guo J., Jiang X., Li Z., Ling W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012;50:3040–3047. doi: 10.1016/j.fct.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Kim H.K., Kim J.N., Han S.N., Nam J.H., Na H.N., Ha T.J. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr. Res. 2012;32:770–777. doi: 10.1016/j.nutres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y., Li D., Zhang Y., Sun R., Xia M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014;306:E975–E988. doi: 10.1152/ajpendo.00699.2013. [DOI] [PubMed] [Google Scholar]
- 48.Park S., Kang S., Jeong D.Y., Jeong S.Y., Park J.J., Yun H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015;10:455. doi: 10.1007/s12263-015-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsukawa T., Inaguma T., Han J., Villareal M.O., Isoda H. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. J. Nutr. Biochem. 2015;26:860–867. doi: 10.1016/j.jnutbio.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Bjork C., Wilhelm U., Mandrup S., Larsen B.D., Bordoni A., Heden P., Ryden M., Arner P., Laurencikiene J. Effects of selected bioactive food compounds on human white adipocyte function. Nutr. Metab. 2016;13:4. doi: 10.1186/s12986-016-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi K.H., Lee H.A., Park M.H., Han J.S. Cyanidin-3-rutinoside increases glucose uptake by activating the PI3K/Akt pathway in 3T3-L1 adipocytes. Environ. Toxicol. Pharmacol. 2017;54:1–6. doi: 10.1016/j.etap.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Luna-Vital D., Weiss M., Mejia E.G.D. Anthocyanins from purple corn ameliorated tumor necrosis factor-α-induced inflammation and insulin resistance in 3T3-L1 adipocytes via activation of insulin signaling and enhanced GLUT4 translocation. Mol. Nutr. Food Res. 2017;61:1700362. doi: 10.1002/mnfr.201700362. [DOI] [PubMed] [Google Scholar]
- 53.Matsukawa T., Villareal M.O., Motojima H., Isoda H. Increasing cAMP levels of preadipocytes by cyanidin-3-glucoside treatment induces the formation of beige phenotypes in 3T3-L1 adipocytes. J. Nutr. Biochem. 2017;40:77–85. doi: 10.1016/j.jnutbio.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Pei L., Wan T., Wang S., Ye M., Qiu Y., Jiang R., Pang N., Huang Y., Zhou Y., Jiang X., et al. Cyanidin-3-O-β-glucoside regulates the activation and the secretion of adipokines from brown adipose tissue and alleviates diet induced fatty liver. Biomed. Pharmacother. 2018;105:625–632. doi: 10.1016/j.biopha.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Muscarà C., Molonia M.S., Speciale A., Bashllari R., Cimino F., Occhiuto C., Saija A., Cristani M. Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Phytother. Res. 2019;33:1888–1897. doi: 10.1002/ptr.6379. [DOI] [PubMed] [Google Scholar]
- 56.Thilavech T., Adisakwattana S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement. Altern. Med. 2019;19:242. doi: 10.1186/s12906-019-2664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vijayaraj P., Nakagawa H., Yamaki K. Cyanidin and cyanidin-3-glucoside derived from Vigna unguiculata act as noncompetitive inhibitors of pancreatic lipase. J. Food Biochem. 2019;43:e12774. doi: 10.1111/jfbc.12774. [DOI] [PubMed] [Google Scholar]
- 58.Saulite L., Jekabsons K., Klavins M., Muceniece R., Riekstina U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine. 2019;53:86–95. doi: 10.1016/j.phymed.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q., Mejia E.G.D., Vital D.L., Tao T., Chandrasekaran S., Chatham L., Juvik J., Singh V., Kumar D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019;289:739–750. doi: 10.1016/j.foodchem.2019.03.116. [DOI] [PubMed] [Google Scholar]
- 60.Molonia M.S., Occhiuto C., Muscara C., Speciale A., Bashllari R., Villarroya F., Saija A., Cimino F., Cristani M. Cyanidin-3-O-glucoside restores insulin signaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020;691:108488. doi: 10.1016/j.abb.2020.108488. [DOI] [PubMed] [Google Scholar]
- 61.Madduma Hewage S., Prashar S., Debnath S.C., Karmin O., Siow Y.L. Inhibition of Inflammatory Cytokine Expression Prevents High-Fat Diet-Induced Kidney Injury: Role of Lingonberry Supplementation. Front. Med. 2020;7:80. doi: 10.3389/fmed.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han S., Yang Y., Lu Y., Guo J., Han X., Gao Y., Huang W., You Y., Zhan J. Cyanidin-3-O-glucoside regulates the expression of Ucp1 in brown adipose tissue by activating Prdm16 gene. Antioxidants. 2021;10:1986. doi: 10.3390/antiox10121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molonia M.S., Quesada-Lopez T., Speciale A., Muscara C., Saija A., Villarroya F., Cimino F. In vitro effects of Cyanidin-3-O-Glucoside on inflammatory and insulin-sensitizing genes in human adipocytes exposed to palmitic acid. Chem. Biodivers. 2021;12:e2100607. doi: 10.1002/cbdv.202100607. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi S., Ferdousi F., Zheng Y.-W., Oda T., Isoda H. Human amniotic epithelial cells as a tool to investigate the effects of cyanidin 3-O-Glucoside on cell differentiation. Int. J. Mol. Sci. 2021;22:3768. doi: 10.3390/ijms22073768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Channuwong P., Salae K., Chongruchiroj S., Cheng H., Suantawee T., Thilavech T., Adisakwattana S. Dietary anthocyanins inhibit insulin fibril formation and cytotoxicity in 3T3-L1 preadipocytes. Int. J. Biol. Macromol. 2022;223:1578–1585. doi: 10.1016/j.ijbiomac.2022.11.077. [DOI] [PubMed] [Google Scholar]
- 66.Kongthitilerd P., Barras E., Rong W., Thibodeaux A., Rigdon M., Yao S., Adisakwattana S., Suantawee T., Cheng H. Cyanidin inhibits adipogenesis in 3T3-L1 preadipocytes by activating the PLC-IP3 pathway. Biomed. Pharmacother. 2023;162:114677. doi: 10.1016/j.biopha.2023.114677. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Chen L., Liu H., Xie J., Yin W., Xu Z., Ma H., Wu W., Zheng M., Liu M., et al. Characterization of the synergistic inhibitory effect of cyanidin-3-O-glucoside and catechin on pancreatic lipase. Food Chem. 2023;404:134672. doi: 10.1016/j.foodchem.2022.134672. [DOI] [PubMed] [Google Scholar]
- 68.Molonia M.S., Salamone F.L., Muscarà C., Costa G., Vento G., Saija A., Speciale A., Cimino F. Regulation of mitotic clonal expansion and thermogenic pathway are involved in the antiadipogenic effects of cyanidin-3-O-glucoside. Front. Pharmacol. 2023;14:1225586. doi: 10.3389/fphar.2023.1225586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vangoori Y., Dakshinamoorthi A., Kavimani S. Prominent Pancreatic Lipase Inhibition and Free Radical Scavenging Activity of a Myristica fragrans Ethanolic Extract in vitro. Potential Role in Obesity Treatment. Maedica. 2019;14:254–259. doi: 10.26574/maedica.2019.14.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L., Chen W., Li D., Liu X. Anthocyanin and proanthocyanidin from Aronia melanocarpa (Michx.) Ell.: Purification, fractionation, and enzyme inhibition. Food Sci. Nutr. 2023;11:3911–3922. doi: 10.1002/fsn3.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie L., Xie J., Xu Y., Chen W. Discovery of anthocyanins from cranberry extract as pancreatic lipase inhibitors using a combined approach of ultrafiltration, molecular simulation and spectroscopy. Food Funct. 2020;11:8527–8536. doi: 10.1039/D0FO01262A. [DOI] [PubMed] [Google Scholar]
- 72.Tsuda T., Horio F., Uchida K., Aoki H., Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 73.Hogan S., Canning C., Sun S., Sun X., Zhou K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem. 2010;58:11250–11256. doi: 10.1021/jf102759e. [DOI] [PubMed] [Google Scholar]
- 74.Kanamoto Y., Yamashita Y., Nanba F., Yoshida T., Tsuda T., Fukuda I., Nakamura-Tsuruta S., Ashida H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J. Agric. Food Chem. 2011;59:8985–8993. doi: 10.1021/jf201471p. [DOI] [PubMed] [Google Scholar]
- 75.Kaume L., Gilbert W.C., Brownmiller C., Howard L.R., Devareddy L. Cyanidin 3-O-β-D-glucoside-rich blackberries modulate hepatic gene expression, and anti-obesity effects in ovariectomized rats. J. Funct. Foods. 2012;4:480–488. doi: 10.1016/j.jff.2012.02.008. [DOI] [Google Scholar]
- 76.Guo H., Xia M., Zou T., Ling W., Zhong R., Zhang W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012;23:349–360. doi: 10.1016/j.jnutbio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 77.Wu T., Qi X., Liu Y., Guo J., Zhu R., Chen W., Zheng X., Yu T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013;141:482–487. doi: 10.1016/j.foodchem.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 78.Ren-Qiang Y., Xiao-You Q., Xiang Z., Jing Z., Lu-Yi M. Cyanidin-3-glucoside attenuates body weight gain, serum lipid concentrations and insulin resistance in high-fat diet-induced obese rats. Chin. J. Contemp. Pediatr. 2014;16:534–538. [PubMed] [Google Scholar]
- 79.Esposito D., Damsud T., Wilson M., Grace M.H., Strauch R., Li X., Lila M.A., Komarnytsky S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015;63:6172–6180. doi: 10.1021/acs.jafc.5b00963. [DOI] [PubMed] [Google Scholar]
- 80.Farrell N.J., Norris G.H., Ryan J., Porter C.M., Jiang C., Blesso C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015;114:1123–1131. doi: 10.1017/S0007114515002962. [DOI] [PubMed] [Google Scholar]
- 81.Yamane T., Kozuka M., Konda D., Nakano Y., Nakagaki T., Ohkubo I., Ariga H. Improvement of blood glucose levels and obesity in mice given aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. J. Nutr. Biochem. 2016;31:106–112. doi: 10.1016/j.jnutbio.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Wu T., Guo X., Zhang M., Yang L., Liu R., Yin J. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mice. Food Funct. 2017;8:3178–3186. doi: 10.1039/C7FO00449D. [DOI] [PubMed] [Google Scholar]
- 83.Biswas D., Sarkar S., De Silva A.B.K.H., D’Souza K., Kienesberger P., Rupasinghe H.P.V., Pulinilkunnil T. Cyanidin-3-O-Glucoside rich extract from haskap berry improves glucose homeostasis and insulin sensitivity in diet-induced obese mice. Can. J. Diabetes. 2018;42:S24–S61. doi: 10.1016/j.jcjd.2018.08.169. [DOI] [Google Scholar]
- 84.Shi M., O’Keefe L., Simcocks A.C., Su X.Q., McAinch A.J. The effect of cyanidin-3-O-β-glucoside and peptides extracted from yoghurt on glucose uptake and gene expression in human primary skeletal muscle myotubes from obese and obese diabetic participants. J. Funct. Foods. 2018;51:55–64. doi: 10.1016/j.jff.2018.10.012. [DOI] [Google Scholar]
- 85.Daveri E., Cremonini E., Mastaloudis A., Hester S.N., Wood S.M., Waterhouse A.L., Anderson M., Fraga C.G., Oteiza P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018;18:16–24. doi: 10.1016/j.redox.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.You Y., Han X., Guo J., Guo Y., Yin M., Liu G., Huang W., Zhan J. Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue. J. Funct. Foods. 2018;41:62–71. doi: 10.1016/j.jff.2017.12.025. [DOI] [Google Scholar]
- 87.Shi M., Mathai M.L., Xu G., McAinch A.J., Su X.Q. The effects of supplementation with blueberry, cyanidin-3-O-β-glucoside, yoghurt and its peptides on obesity and related comorbidities in a diet-induced obese mouse model. J. Funct. Foods. 2019;56:92–101. doi: 10.1016/j.jff.2019.03.002. [DOI] [Google Scholar]
- 88.Lim S.-M., Lee H.S., Jung J.I., Kim S.M., Kim N.Y., Seo T.S., Bae J.-S., Kim E.J. Cyanidin-3-O-Galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients. 2019;11:1190. doi: 10.3390/nu11051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao R., Xiang B., Dolinsky V.W., Xia M., Shen G.X. Saskatoon berry powder reduces hepatic steatosis and insulin resistance in high fat-high sucrose diet-induced obese mice. J. Nutr. Biochem. 2021;95:108778. doi: 10.1016/j.jnutbio.2021.108778. [DOI] [PubMed] [Google Scholar]
- 90.Jiao X., Shen Y., Deng H., Zhang Q., Zhao J. Cyanidin-3-O-galactoside from Aronia melanocarpa attenuates high-fat diet-induced obesity and inflammation via AMPK, STAT3, and NF-κB p65 signaling pathways in Sprague-Dawley rats. J. Funct. Foods. 2021;85:104616. doi: 10.1016/j.jff.2021.104616. [DOI] [Google Scholar]
- 91.Shi M., Mathai M.L., Xu G., Su X.Q., McAinch A.J. The effect of dietary supplementation with blueberry, cyanidin-3-O-β-glucoside, yoghurt and its peptides on gene expression associated with glucose metabolism in skeletal muscle obtained from a high-fat-high-carbohydrate diet induced obesity model. PLoS ONE. 2022;17:e0270306. doi: 10.1371/journal.pone.0270306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cremonini E., Daveri E., Iglesias D.E., Kang J., Wang Z., Gray R., Mastaloudis A., Kay C.D., Hester S.N., Wood S.M., et al. A randomized placebo-controlled cross-over study on the effects of anthocyanins on inflammatory and metabolic responses to a high-fat meal in healthy subjects. Redox Biol. 2022;51:102273. doi: 10.1016/j.redox.2022.102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyu Q., Deng H., Wang S., El-Seedi H., Cao H., Chen L., Teng H. Dietary supplementation with casein/cyanidin-3-O-glucoside nanoparticles alters the gut microbiota in high-fat fed C57BL/6 mice. Food Chem. 2023;412:135494. doi: 10.1016/j.foodchem.2023.135494. [DOI] [PubMed] [Google Scholar]
- 94.Tomay F., Marinelli A., Leoni V., Caccia C., Matros A., Mock H.P., Tonelli C., Petroni K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019;17:237. doi: 10.1186/s12967-019-1972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi A., Shimizu H., Okazaki Y., Sakaguchi H., Taira T., Suzuki T., Chiji H. Anthocyanin-rich Phytochemicals from Aronia Fruits Inhibit Visceral Fat Accumulation and Hyperglycemia in High-fat Diet-induced Dietary Obese Rats. J. Oleo. Sci. 2015;64:1243–1250. doi: 10.5650/jos.ess15181. [DOI] [PubMed] [Google Scholar]
- 96.Meleleo D., Avato P., Conforti F., Argentieri M.P., Messina G., Cibelli G., Mallamaci R. Interaction of Quercetin, Cyanidin, and Their O-Glucosides with Planar Lipid Models: Implications for Their Biological Effects. Membranes. 2023;13:600. doi: 10.3390/membranes13060600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.