Abstract
Demonstrating biosimilarity entails comprehensive analytical assessment, clinical pharmacology profiling, and efficacy testing in patients for at least one medical indication, as required by the U.S. Biologics Price Competition and Innovation Act (BPCIA). The efficacy testing can be waived if the drug has known pharmacodynamic (PD) markers, leaving most therapeutic proteins out of this concession. To overcome this, the FDA suggests that biosimilar developers discover PD biomarkers using omics technologies such as proteomics, glycomics, transcriptomics, genomics, epigenomics, and metabolomics. This approach is redundant since the mode-action-action biomarkers of approved therapeutic proteins are already available, as compiled in this paper for the first time. Other potential biomarkers are receptor binding and pharmacokinetic profiling, which can be made more relevant to ensure biosimilarity without requiring biosimilar developers to conduct extensive research, for which they are rarely qualified.
Keywords: FDA, omics technology, pharmacodynamic biomarkers, biosimilars, proteomics, glycomics, receptor binding, pharmacokinetics
1. Introduction
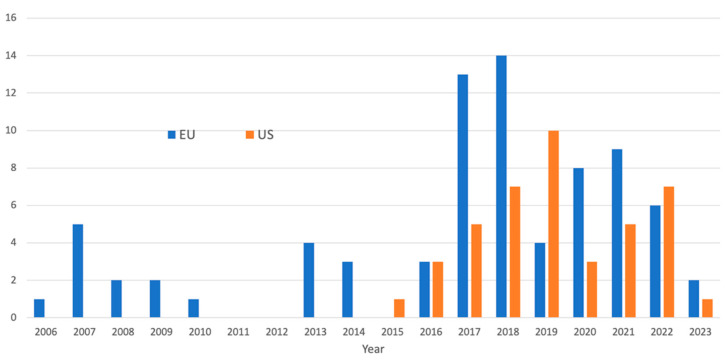
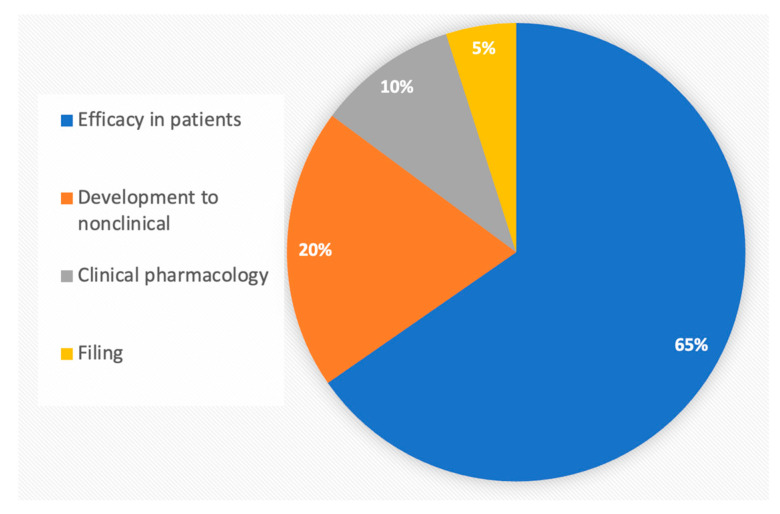
Biosimilars require more extensive testing than generic chemical drugs, owing to the structural variability of recombinant proteins resulting from innate in vivo translation variations. As of August 2023, 42 biosimilars were approved in the United States of America (US), and 74 were approved in the European Union (EU), accounting for approximately 11 molecules and 18 in the EU out of 266 FDA-licensed choices, the majority of which are off-patent [1]. The higher number of approvals in the EU comes from a longer time and the classification of peptides as proteins in the EU. Despite much anticipation, the current downward trend in approving biosimilars (Figure 1) [2] is alarming and is attributed to the high development cost, predominantly towards clinical efficacy testing (Figure 2).
Figure 1.
The number of biosimilars approved in the EU and US (as of June 2023) shows a downward trend (source: FDA and EMA).
Figure 2.
Cost distribution of testing of 246 biosimilars approved in the US, EU, and Japan from 2006 to 2021 [3].
When the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) issued their first guidelines, biosimilars were treated as new biological drugs. They were expected to demonstrate clinical efficacy and safety. With the availability of new data, these guidelines have undergone many revisions, reducing, or eliminating testing where justified [4]. In 2005, the FDA withdrew its pivotal guideline, “Statistical Approaches to Evaluate Analytical Similarity” [5] and replaced it with another guideline that has reduced stringency in testing critical quality attributes [6]. In 2019, the FDA issued guidelines suggesting that the immunogenicity testing of biosimilars is unnecessary if differences in immune responses do not alter the pharmacokinetic (PK) profile [7], specifying that in silico approaches can be used to establish immunogenicity profiles [8]. In 2023, an amendment to the US Biological Products Competition and Innovation Act (BPCIA) replaced the term “animal toxicology” with “nonclinical” testing [9,10]. While it is not the purview of regulatory agencies to amend guidelines for reducing developmental costs, their responsibility is to avoid unnecessary exposure to humans [11].
However, one major hurdle in rationalizing the regulatory pathway for biosimilars comes from the requirements mandated in the following statute governing the BPCIA:
“(cc) a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics or pharmacodynamics) that are sufficient to demonstrate safety, purity, and potency in 1 or more appropriate conditions of use for which the reference product is licensed and intended to be used and for which licensure is sought for the biological product.” [12]
While the FDA guidelines have suggested that if residual uncertainty exists after analytical and clinical pharmacology assessments, “additional clinical studies” may be required, whether additional studies indicate efficacy testing in patients is still unclear. This could refer to further clinical pharmacology studies, but this perception is clouded by mentioning “…in 1 or more appropriate conditions of use”; this could only mean testing in patients, which is considerably less sensitive in differentiating a biosimilar candidate from its reference product [13].
This misconception has generally led to the clinical efficacy testing of biosimilars in patients, enrolling a median of 538 participants (interquartile range, 372–644 patients) at a median cost of USD 27.6 million each (USD 18.0 million–USD 36.7 million), with an average price per enrollee of approximately USD 55,000. Moreover, these trials last a median of 55 weeks (46–78 weeks) [14]. Oncology drugs have the highest testing costs. More complex trial protocols take longer to design, obtain approval for (institutional and FDA), recruit patients from contracted providers, analyze the resulting data, and submit the results.
As of April 2023, 94,910 participants had been enrolled in 170 active or completed phase 3 biosimilar trials with study sizes ranging from 3 to 4994 participants; among these, 100 studies were marked for cancer (26, 34, 25, 21, and 16 studies for lymphoma, breast cancer, metastatic, HER2, and adenocarcinoma, respectively), 18 for macular degeneration, 31 for rheumatoid arthritis, 24 for psoriasis, and 17 for osteoporosis. All completed trials met the equivalence criteria [15]. Current studies cost more than USD 5 billion based on the average cost per enrollee. Reducing these costs may significantly impact the affordability of biosimilars.
To justify this stage of the development process, the FDA has already proposed that under specific conditions, clinical pharmacokinetic (PK) and pharmacodynamic (PD) data that establish comparable exposure and response between a proposed biosimilar product and the reference product may be satisfactory for a comprehensive evaluation of any clinically significant distinctions between the products. This is despite the requirement for a thorough assessment of immunogenicity [16]. However, this concession excludes most biological drugs, such as monoclonal antibodies, which do not exhibit traditional pharmacodynamic (PD) responses.
In 2018, the FDA embarked on a scientific plan to simplify the approval process for biosimilars under the Biosimilars Action Plan [17]. The primary element of this plan involved the creation of information resources and development tools aimed at aiding biosimilar sponsors in the production of biosimilar and interchangeable goods of superior quality, utilizing cutting-edge techniques. Furthermore, the commitment letter for Biosimilar User Fee Amendments III explicitly addresses the inclusion of PD biomarker utilization as a component of the regulatory science pilot program [18].
In September 2022, the FDA organized its first program to expedite biosimilars’ entry, “FDA Workshop: Increasing the Efficiency of Biosimilar Development Programs” [19]. A subsequent significant change was detailed in a publication by the FDA’s Division of Applied Regulatory Science (DARS) [20], which recommended waiving clinical efficacy testing [21] for molecules with prominent PD biomarkers, which need not correlate with clinical efficacy [22]. Examples include the absolute neutrophil count area under the effect–time curve, which is a more reliable endpoint than the clinical-efficacy endpoint for the duration of severe neutropenia [23].
The DARS made these conclusions based on investigations [24] and clinical studies [25,26,27] to identify the best practices for characterizing PD biomarkers for various drug classes. These studies evaluated the use of human plasma proteomic and transcriptomic analyses to identify novel biomarkers that could be used to secure a waiver for efficacy testing in patients [28].
The FDA has also suggested that PD biomarkers can be identified using technologies such as large-scale proteomic approaches [29], which are not readily available. The FDA has also confirmed that PD biomarkers need not correlate with a clinical response to allow their use for establishing biosimilarity. This conclusion is based on the understanding that similar PD responses lead to identical efficacy responses.
In September 2023 [30], the FDA held a workshop with major regulatory agencies to discuss how patient efficacy testing requirements can be rationalized and harmonized. One regulatory agency, the MHRA, clearly declared that no such testing is required; others suggested that the developers can present arguments to seek such waivers.
2. Understanding Pharmacodynamic Biomarkers
A pharmacodynamic marker is a measurable biochemical, physiological, or molecular variable that provides information on a drug’s mechanism of action, efficacy, and safety. In drug development and medical research, pharmacodynamic markers help understand how a drug affects a target organism, which can be at the cellular, tissue, or systemic level.
Without a comparative clinical efficacy study, the FDA guidance documents outline how biosimilars may be approved based on pharmacokinetic (PK) and PD biomarker data. Reliance on PK and PD data allows for shorter and less costly clinical studies that can often be conducted in healthy participants. The PD biomarkers are indicators of a drug’s pharmacological effect on its target or targets. For example, the target might be a receptor molecule that initiates a complex signaling cascade. Changes in the levels of proteins along the signaling cascade or modifications to them could be considered pharmacodynamic responses. Therefore, these proteins could be regarded as PD biomarkers and used to help establish biosimilarity.
Pharmacodynamic markers are crucial for various phases of drug development, including:
Dose determination—they can help determine the optimal dosage of a new drug by showing its effects at different concentrations;
Mechanism of action—understanding the changes in pharmacodynamic markers can help elucidate how a drug exerts its therapeutic or adverse effects;
Efficacy—these markers can provide early indications of a drug’s effectiveness, often before clinical endpoints can be measured;
Safety—monitoring pharmacodynamic markers can give insights into potential side effects or toxicities, enabling researchers to make informed decisions during clinical trials;
Personalized medicine—in some cases, pharmacodynamic markers can also help in patient stratification, identifying which subgroups of patients are most likely to benefit from a particular treatment.
A functional PD marker is a specific type of biomarker that reveals a drug’s biochemical or physiological effects on its target or a downstream pathway. Unlike general biomarkers, that might indicate the presence or risk of a disease, a functional PD marker specifically illustrates the drug’s mechanism of action in the body and how the body reacts to it.
PD biomarkers, like hematocrit or WBC count, are pharmacological markers. Since the FDA does not require a correlation between the PD biomarker and the clinical response, a significant issue is created; if a product meets the biomarker profile, does this mean it will also yield the same clinical response? The answer is no; it has been established. Additionally, if a product fails to meet the biomarker profile due to the nonlinearity of the marker vs. the PK profile, does this mean that a product is not equivalent? The answer is no; it has not been established. Both possibilities lead to the limited utility of newly discovered biomarkers but do not apply to pharmacological markers related to response.
PD biomarkers indicate the effects of a drug on its intended target or a downstream pathway. In simpler terms, they give insights into how the drug works in the body and how the body responds to the drug. The term “functional,” in the context of a PD marker, usually suggests that the marker has a direct link or relevance to the drug’s therapeutic response or mechanism of action, as shown in Table 1.
Table 1.
Biomarker functions for biological drugs.
| Function | Usage | Example |
|---|---|---|
| Demonstrate drug activity | To provide early evidence of a drug’s effect before overt clinical outcomes manifest. | In treating chronic myeloid leukemia (CML), the BCR-ABL tyrosine kinase inhibitor imatinib is used. The decline in BCR-ABL transcript levels in patients’ blood is a functional PD marker of the drug’s activity on its target [31]. |
| Guide dosing | To ensure optimal drug dosing using the dose–response relationship. | For cholesterol-lowering drugs like statins, the low-density lipoprotein cholesterol (LDL-C) levels in the blood serve as a PD marker to guide dosing and assess efficacy [32]. |
| Select patients | To identify patients likely to benefit from a specific treatment. | In some breast cancers, overexpression of the HER2 protein is observed. HER2 status serves as a functional PD marker to select patients who might benefit from trastuzumab, which targets HER2 [33]. |
| Monitor resistance | To track the development of resistance to treatments. | In HIV treatment, the emergence of specific viral mutations can serve as PD markers indicating resistance to specific antiretroviral drugs [34]. |
| Determine the drug mechanism of action | To confirm action through its intended mechanism. | In Alzheimer’s disease, the buildup of beta-amyloid plaques is considered a hallmark. Drugs designed to reduce beta-amyloid levels in the brain might use CSF (cerebrospinal fluid) levels of beta-amyloid as a PD marker to show the drug’s effect [35]. |
| Validate target engagement | To demonstrate that a drug is successfully engaging with and modulating its target. | For multiple sclerosis drugs like fingolimod, a PD marker such as the number of circulating lymphocytes can indicate the drug’s effect on immune cell egress from lymph nodes [36]. |
| Evaluate drug-induced toxicity | To monitor potential adverse effects of a drug. | In chemotherapy, monitoring the levels of liver enzymes like AST and ALT in the blood can serve as PD markers for drug-induced liver damage [37]. |
| Optimize therapeutic window | To establish the range between the minimum effective dose and the onset of adverse effects. | For anticoagulant drugs like warfarin, the INR (International Normalized Ratio) serves as a PD marker to ensure the drug’s effect is within a therapeutic range, minimizing the risk of bleeding and clot formation [38]. |
| Predict long-term drug effects | To predict longer-term therapeutic or adverse effects using early changes in PD markers. | In osteoporosis treatments, reducing bone resorption markers like CTX (C-terminal telopeptide) can predict longer-term benefits in bone mineral density and fracture risk [39]. |
| Assess immune response | For immunotherapies, to gauge the body’s immune response to the treatment. | In cancer immunotherapy, the presence and proliferation of tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment can serve as a PD marker to indicate the activation and targeting of the immune system against tumor cells [40]. |
| Indicate drug combination efficacy | In combination therapies, to show the synergistic or additive effects of the combined drugs. | In treatments for tuberculosis, monitoring bacterial load in sputum samples can serve as a PD marker for the combined efficacy of multiple antimicrobial agents [41]. |
| Track reversal of disease progression | To indicate whether a drug is not just halting but reversing disease progression. | In fibrotic diseases like idiopathic pulmonary fibrosis, measuring levels of collagen-derived peptides in blood or bronchoalveolar lavage fluid can act as PD markers, indicating the repair or degradation of fibrotic tissue [42]. |
| Evaluate neural activity and plasticity | To track neural activity or connection changes in neurologic disorders and treatments. | For treatments aimed at Alzheimer’s or other neurodegenerative conditions, the levels of synaptic proteins or neuronal activity markers in CSF can indicate neural activity and synaptic plasticity [43]. |
| Monitoring metabolic responses | To help track changes in metabolic pathways. | In diabetes management, measuring C-peptide levels alongside insulin can give insights into endogenous insulin production and pancreatic function [44]. |
| Monitoring cellular senescence and aging | In treatments aiming to affect aging processes or cellular health, to track cellular senescence. | Measured levels of senescence-associated beta-galactosidase or p16^INK4a expression can act as PD markers for cellular aging or the efficacy of anti-aging treatments [45]. |
| Evaluating epigenetic changes | To track changes in DNA methylation, histone modification, or other epigenetic markers. | In oncology, when treating with drugs targeting DNA methyltransferases, the global or gene-specific changes in DNA methylation levels can serve as PD markers [46]. |
| Assessing drug-induced autophagy | For therapies inducing autophagy as a mechanism, to monitor the process. | When monitoring LC3B lipidation, a critical step in autophagosome formation can serve as a PD marker for autophagy activation [47]. |
| Monitoring immune checkpoint inhibition | In cancer immunotherapy, target immune checkpoints to gauge the effectiveness of checkpoint inhibition. | In patients receiving PD-1 or PD-L1 inhibitors, monitored circulating tumor DNA (ctDNA) levels can serve as a PD marker to indicate response to therapy [48]. |
3. Omics Technologies
Omics technologies refer to the comprehensive, high-throughput characterization of biological molecules in an organism, cell, or tissue. Genomics is the most widely known “omics” technology, which studies an organism’s entire set of genes. However, there are several other “omics” technologies, each focusing on different types of molecules. Omics studies can be broadly categorized into targeted and untargeted approaches. (Table 2).
Table 2.
Description of omics technologies and related analytical methods.
| Analytical Method | Description |
|---|---|
| Genomics | |
| DNA sequencing | Determines the order of nucleotides in DNA [49]. |
| Microarray analysis | Measures gene expression using hybridization to microarrays [50]. |
| Whole genome sequencing | Sequences the entire genome of an organism [51]. |
| Comparative genomics | Compares genomes of different species to identify similarities and differences [52]. |
| Transcriptomics | |
| RNA-Seq | Sequences and quantifies RNA transcripts to study gene expression [53]. |
| Microarray analysis | Measures gene expression using hybridization to microarrays [54]. |
| Single-cell RNA-Seq | Analyzes gene expression at the single-cell level for cell heterogeneity [55]. |
| Isoform sequencing | Focuses on the identification and quantification of alternative RNA-splicing events [56]. |
| Proteomics | |
| Mass spectrometry | Identifies and quantifies proteins based on their mass-to-charge ratio [57]. |
| 2D gel electrophoresis | Separates proteins based on charge and size, allowing protein profiling [58]. |
| Liquid chromatography (LC) | Separates proteins before mass spectrometry analysis [59]. |
| Protein microarrays | Allows high-throughput screening of protein interactions and activities [60]. |
| Metabolomics | |
| Nuclear magnetic resonance (NMR) | Measures metabolite concentrations and elucidates chemical structures [61]. |
| Gas chromatography–mass spectrometry (GC-MS) | Separates and quantifies metabolites in the gas phase before mass spectrometry [62]. |
| Liquid chromatography–mass spectrometry (LC-MS) | Separates and quantifies metabolites in the liquid phase before mass spectrometry [63]. |
| Targeted metabolomics | Focuses on specific metabolites of interest for quantification [64]. |
| Epigenomics | |
| DNA methylation analysis | Studies DNA methylation patterns to understand epigenetic regulation [65]. |
| ChIP-Seq | Maps protein-DNA interactions, such as histone modifications [66]. |
| Bisulfite sequencing | Analyzes DNA methylation status by treating DNA with bisulfite [67]. |
| Lipidomics | |
| Mass spectrometry | Identifies and quantifies lipids, elucidating lipid profiles in biological samples [68]. |
| Liquid chromatography (LC) | Separates lipids before mass spectrometry analysis [69] |
| Thin-layer chromatography (TLC) | Separates and identifies lipids based on their mobility on a thin layer [70]. |
The omics technologies compare chemical profiles to identify, characterize, and profile peptides, proteins, glycans, lipids, and other unknown entities resulting from administering a therapeutic protein. The targeted approaches in omics applications involve the analysis of a pre-defined set of molecules or pathways. The researcher knows in advance which specific molecules or features they are interested in, such as targeted genomics (PCR assays for gene sequences) and targeted proteomics (multiple reaction monitoring (MRM), where specific proteins or peptides of interest are quantified). Targeted metabolomics [71] focuses on a particular class of metabolites, such as lipidomics, using methods like selected reaction monitoring (SRM).
The untargeted approaches broadly analyze as many molecules as possible in a sample without a pre-defined focus. The aim is often discovery or to obtain a global overview of a system, for example, untargeted genomics (whole genome sequencing), untargeted transcriptomics (RNA sequencing which quantifies all mRNA transcripts in a sample), untargeted proteomics (mass-spectrometry-based approaches like shotgun proteomics whereas many proteins as possible are identified and quantified), and untargeted metabolomics (high-resolution mass spectrometry or nuclear magnetic resonance (NMR) spectroscopy, aiming to detect as many metabolites in a sample as possible). Table 3 lists the common analytical technologies used in omics technology applications.
Table 3.
List of examples of analytical technologies broadly used.
| Technology | Application |
|---|---|
| Affinity chromatography | Purification and target binding analysis. |
| Biacore (SPR-based technology) | Label-free interaction analysis [72]. |
| Bottom-up MS | Mass spectrometric analysis for primary sequence analysis, evaluation of N/O-glycosylation sites, and quantification of methionine oxidation [73]. |
| Capillary electrophoresis (CE) | Biosimilar comparability studies [74]. High-resolution separation of glycans [75]. |
| Capillary electrophoresis–mass spectrometry | Quality control and stability of recombinant proteins in biopharmaceuticals [76,77]. |
| Chromatography coupled with multi-angle light scattering | Absolute molar mass, size, and conformation [78]. |
| Chemical cross-linking coupled with MS | Studying spatial arrangement and interactions within protein complexes [79]. |
| Circular dichroism | In vivo and in vitro stability analyses [79]. |
| Differential scanning calorimetry | Thermal stability analysis [80]. |
| DLS | Size distribution analysis of glycoproteins [81]. |
| Dynamic light scattering (DLS) | Size and stability analysis [82] |
| Electron microscopy | Visualizing glycan structures and localization [83] |
| Enzyme-linked immunosorbent assay (ELISA) | Detection of specific glycan–protein interactions [84]. Specific protein quantification and immunogenicity studies [85]. |
| Enzyme-linked lectin assay | Quantitative glycan analysis [86]. |
| Exoglycosidase sequencing | Structural characterization of glycans [87]. |
| Flow cytometry | Cell line development and monitoring of protein expression [88]. |
| Fluorescence spectroscopy | Sensitivity enhancement in glycan analysis [89]. Folding and conformational analysis [90]. |
| Fourier transform infrared spectroscopy (FTIR) | Secondary structure analysis and stability monitoring [91,92]. Designing more effective protein-based therapies through metabolite profiling [82]. Analysis of glycan structure [93]. |
| Gas chromatography–mass spectrometry | Analysis of volatile derivatives of glycans [94]. Understanding host-pathogen interactions for recombinant vaccine development [95,96] |
| Gel electrophoresis, 2D | Separation and identification of proteins [97,98]. |
| Glycan microarrays | High-throughput analysis of protein–glycan interactions [99]. |
| Glycan sequencing using MS/MS | Sequential identification [100]. |
| Glycoproteomic analysis | Integrative approach for comprehensive glycoprotein study [101]. |
| High-performance liquid chromatography (HPLC) | Separation of glycan structures [102]. |
| Hydrogen–deuterium exchange mass spectrometry | Conformational dynamics and higher-order structure analysis [103]. |
| Hydrophilic interaction liquid chromatography (HIC) | Separation of polar glycans [88]. Analysis of hydrophobicity and aggregation [104]. |
| IEF | Separation of glycoproteins by isoelectric point [105]. |
| Immunoassays | Pharmacokinetic and pharmacodynamic studies [106]. |
| Immunohistochemistry | Localization of specific glycans in tissues [107]. |
| Immunoprecipitation and pull-down assays | Protein interaction studies [108]. |
| Ion exchange chromatography | Charge heterogeneity analysis [109]. |
| Isoelectric focusing (IEF) | Protein separation based on isoelectric point [110]. |
| Lectin affinity chromatography | Separation of glycans using specific binding proteins [111]. |
| Liquid chromatography–mass spectrometry | Comprehensive protein characterization [112]. Improving industrial protein production yields Environmental stress response in recombinant protein production [113]. |
| Mass spectrometry (MS) | Characterization and post-translational modification analysis [114]. Structural analysis of glycan [115]. |
| Mass spectrometry and NMR | The optimization of expression systems for protein production [116]. |
| Mass spectrometry selected reaction monitoring | Targeted protein quantification in biosimilar development [117]. |
| Mass spectrometry, tandem | Sequential fragmentation for glycan structure elucidation [118]. |
| Mass spectrometry, ultra-performance liquid chromatography | Assessing the impact of protein therapeutics on metabolic pathways [119]. |
| Matrix-assisted laser desorption/ionization (MALDI-MS) | Mass determination of glycan [120]. Rapid identification and characterization of proteins [121]. |
| Multi-angle light scattering (MALS) | Molar mass and size distribution [122]. Characterizing size and composition [123]. |
| Multi-angle light scattering (MALS) | Molar mass and size distribution [122]. |
| N-Terminal sequencing | Analysis of protein sequence and modifications [124]. |
| Mass spectrometry, native | Structural characterization and complex formation analysis [125]. |
| NMR spectroscopy | Detailed structural analysis of glycan [126]. Conformational dynamics and structural analysis [127]. Investigating protein–metabolite interactions [128]. |
| Optical glycan biosensors | Real-time monitoring of glycan–protein interactions [129]. |
| Peptide mapping and fingerprinting | Identification and characterization of proteins [130]. |
| Protein microarrays | High-throughput analysis of protein functions and interactions [131]. |
| Reverse-phase liquid chromatography | Separation of glycopeptides and glycoproteins [132]. Analysis of protein purity and heterogeneity [133]. |
| Size-exclusion chromatography | Protein aggregation and purity assessment [134]. |
| SPR | Real-time glycan–protein interaction analysis [135]. |
| Stable isotope labeling | Quantitative glycomics [136]. Quantitative proteomics for expression analysis [137]. |
| Surface plasmon resonance (SPR) | Protein interaction studies [138]. |
| X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy | 3D structure determination of glycoproteins [139]. Structural analysis and 3D modeling [140]. |
| Yeast two-hybrid system | Protein–protein interaction mapping [141]. |
Proteomics
Proteomics is the most relevant application for characterizing and quantifying biomarkers based on the action of proteins as functional molecules in the cell: performing most of the cellular functions and contributing to most of the cell’s structure. Proteomics aims to identify a chemical, such as a protein, carbohydrate, or another entity, which becomes evident only when the reference product is administered compared to a placebo. Detecting these chemical entities requires sophisticated technologies, as listed in Table 4, along with examples of these technologies used to characterize proteins, unknown proteins, and unusual protein structures [57,142].
Table 4.
Examples of applications of proteomic biomarkers.
| Examples of Use of Proteomics Biomarkers | |
|---|---|
| Antibody-drug conjugates (ADCs) | Brentuximab vedotin, an ADC used for Hodgkin’s lymphoma and systemic anaplastic large cell lymphoma, delivers the cytotoxic drug monomethyl auristatin E (MMAE) to CD30-expressing cells, and the measurement of MMAE can serve as a marker of target engagement [143]. |
| Antigen-antibody complex | The formation of antigen–antibody complexes provides direct evidence of target engagement. For example, in the case of adalimumab, an anti-tumor necrosis factor (TNF)-α antibody, the serum levels of the adalimumab-TNFα complex can be measured as evidence of the drug binding to its target [144]. |
| Antigenic modulation | This refers to the downregulation or loss of antigen expression on the cell surface in response to antibody binding and can be used as a marker of monoclonal antibody (mAb) engagement. Rituximab, a monoclonal antibody against the CD20 antigen on B cells, causes antigenic modulation, decreasing CD20 expression and indicating rituximab engagement [145]. |
| Binding of mAbs to Fc receptors | The Fc region of mAbs can bind to Fc receptors on immune cells. This binding can modulate the activity of these cells, making Fc-receptor occupancy a valuable PD marker. The occupancy of RIIIa on natural killer cells by rituximab can be used as a PD marker [146]. |
| Cell proliferation markers | mAbs may also be designed to inhibit cell proliferation. Here, decreased cell proliferation markers, such as Ki-67, can indicate successful target engagement [147]. |
| Circulating tumor antigen levels | In cancer therapy, mAbs are often designed for binding to specific tumor antigens. A reduction in the levels of these circulating antigens following mAb therapy can serve as a marker of target engagement. For instance, CA-125 levels in patients with ovarian cancer have been treated with mAbs targeting the CA-125 antigen [148]. |
| Complement system alterations | mAbs can modulate the complement system. Eculizumab, a mAb that inhibits complement component C5, reduces hemolytic activity and can be used as a PD marker [149]. |
| Cytokine release syndrome | mAbs, particularly those targeting immune cells, increase the release of specific cytokines. For instance, administration of the anti-CD28 mAb TGN1412 releases many cytokines, such as interleukin (IL)-2 and interferon (IFN)-γ, which could be monitored as PD markers. Measuring cytokines, such as IL-2, IL-6, or TNF-α, can estimate target engagement. This is particularly relevant for immunomodulatory mAbs such as ipilimumab, which can increase circulating cytokine levels upon engagement with its target, cytotoxic T lymphocyte-associated (CTLA-4) [150]. |
| Fluorescent tag | Flow cytometry can be a valuable tool for assessing target engagement when the target of a mAb is expressed on cell surfaces. Labeling the mAb with a fluorescent tag confirms its binding to the target cells in a sample. This has been utilized in therapies, such as those using rituximab, wherein binding to CD20+ B cells can be confirmed using flow cytometry [151]. |
| Gut microbiota alterations | Specific mAb therapies can alter gut microbiota, serving as functional response markers. Vedolizumab, a mAb against the α4β7 integrin used in treating inflammatory bowel disease, can restore gut microbial diversity, indicating a functional response to therapy [152]. |
| Immune response markers | Some mAbs stimulate immune responses against specific antigens. Hence, increased antibodies against the target antigen in the patient’s serum can serve as a target engagement marker. For instance, palivizumab, a mAb that prevents respiratory syncytial virus (RSV) infection in high-risk infants, engages its target through anti-RSV antibodies in the patient’s serum [153]. Immune response can be measured as a functional response marker. For instance, ipilimumab, a mAb that targets the immune checkpoint protein CTLA-4, is the most widely used mAb. |
The technologies for proteomics research, especially the nano-LC and mass spectrometry, have experienced unprecedented development; currently, the proteome of the single cell can be identified in its totality, despite many shortcomings that are fast resolved, leaving a great future for proteomics in drug development; whether they are suitable for biosimilars is another matter that is discussed later in the article.
4. Glycomics
Glycomics is a comprehensive study of all glycan structures (sugars and carbohydrates) in each cell type or organism, including the identity of individual glycans and the overall glycan profile. Glycans are crucial components of various biological systems. They play roles in numerous biological processes, including cell–cell communication, immune responses, infection, inflammation, and cancer progression. Glycans are often added post-translationally to proteins and lipids, altering their functions and properties. They also play a critical role in protein folding and stability [154,155]. These patterns can affect therapeutic proteins’ stability, solubility, and biological activity. Understanding glycosylation is vital for developing, producing, and approving biosimilars because even slight variations in glycosylation can result in different clinical outcomes. The evaluation of glycosylation is the second most frequently applied ‘omics’ technology to compare molecules for biosimilarity.
Table 5 lists common analytical approaches to identify and qualify glycans.
Table 5.
Methods of identifying glycans and their applications.
| Application | Example |
|---|---|
| Bioanalytical and bioinformatics | Data integration at different structural levels to identify various glycoforms of recombinant human chorionic gonadotropin (r-hCG), urinary hCG (u-hCG), and recombinant follicle stimulating hormone (r-hFSH) revealed that these biopharmaceuticals differ considerably in their glycosylation patterns [156]. |
| Resolution discrepancies | Between high-resolution native and glycopeptide-centric mass spectrometric approaches for the glycosylation of erythropoietin variants [157]. |
| Glycan microheterogeneity | To identify multiple glycosylation sites in the vascular endothelial growth factor IgG (VEGFR-IgG) fusion protein to understand the functional significance of each glycosylation pattern [158]. |
| Glycoforms | Several glycoforms using hybrid high-performance liquid chromatography–MS approaches [159], such as 24 glycoengineered erythropoietin variants with varying glycan branching and sialylation levels, are crucial parameters for biotherapeutic efficacy. |
| NMR | Identification of glucose-induced glycation in mAbs and other proteins using NMR spectroscopy. [160]. |
| Novel glycoforms | Identification of novel glycosylations in human-serum-derived factor IX. [161]. |
| Mass spectral profiling | The N-linked, O-linked, ganglioside, and glycosaminoglycan compound classes and the tandem mass spectrometry of glycans have led to spectral glycoproteomics [162]. |
| N-glycosylation profile | Analysis of trastuzumab biosimilar candidates using normal-phase liquid chromatography and matrix-assisted laser desorption/ionization time-of-flight MS [163]. |
| Microheterogeneity | Composite glycosylation profiles and other microheterogeneities in intact mAbs via high-resolution native MS using a modified Orbitrap [164]. |
| Targeted site-specific quantitation | N-and O-glycopeptides using 18O-labeling and product ion-based MS [165]. |
| Hybrid MS | Approaches in glycoprotein analysis and their usage in scoring biosimilarity [166]. |
Understanding the complex structures of glycans is essential for understanding glycomics. Techniques such as MS and nuclear magnetic resonance (NMR) have been used for this purpose. Glycans often bind to proteins, affecting their functions. Studying these interactions can provide insights into numerous biological processes and diseases to learn how cells synthesize glycans and how these structures contribute to cellular functions and broader physiological processes. Changes in glycosylation patterns are often associated with diseases, including cancer, making them potential biomarkers for disease diagnosis and progression. The glycan profile also establishes high similarity for protein function, stability, and efficacy.
5. Transcriptomics
Metabolomics and transcriptomics are branches within systems biology, focusing on comprehensive analyses of biological molecules in an organism, cell, or tissue. However, they target different levels of the cellular molecular hierarchy. Metabolomics studies the complete set of small-molecule metabolites within a biological sample. Its primary focus is on metabolites, including amino acids, sugars, lipids, and other small molecules that are products or intermediates of cellular metabolism [167].
Transcriptomics involves studying the complete set of RNA transcripts produced by the genome under specific circumstances. Its primary focus is messenger RNA (mRNA), but other non-coding RNAs can also be studied. It provides insights into the gene expression patterns, revealing which genes are upregulated or downregulated in particular conditions [168].
Transcriptomics is the scientific discipline investigating the comprehensive collection of RNA transcripts generated by the genetic material (genome) of a cell, tissue, or organism during distinct circumstances or at stages of development. RNA transcripts, encompassing various types such as mRNA, rRNA, tRNA, and non-coding RNA, serve as intermediaries in transmitting genetic information from DNA to proteins. The transcriptome composition exhibits variability based on cell type, developmental stage, environmental circumstances, or pathological conditions. Hence, it is imperative to comprehend the transcriptome to interpret the functional components of the genome, unveil the molecular constituents of cells and tissues, and gain insights into the progressions of diseases. Transcriptomics encompasses many methodologies, such as microarray analysis, RNA sequencing (RNA-Seq), and serial gene expression analyses, employed to investigate gene expression patterns. RNA-Seq has emerged as the predominant technique in current scientific research due to its substantial capacity for high-throughput analysis and comprehensive data acquisition [169] (Table 6).
Table 6.
Applications of transcriptomics.
| Application | Example |
|---|---|
| Gene expression profiling | Simultaneous measurement of the expression levels of several genes to create a global picture of cellular functions. |
| Optimization of host cells for protein expression | Optimize host cells to improve recombinant protein expression [170]. |
| Identifying suitable expression systems | Selection of optimal expression system [171]. |
| Understanding protein function and interactions | How proteins function and interact within cellular networks [172]. |
| Monitoring quality control in biopharmaceutical production | Manufacturing QC [173]. |
| Improving protein solubility and folding | Enhance the solubility and folding of recombinants [174]. |
| Tailoring protein post-translational modifications (PTMs) | To produce proteins with the desired PTMs [175]. |
| Enhanced yield in industrial protein production | To enhance the yield of recombinant proteins for industrial applications [176]. |
| Studying protein stability and degradation | Design recombinant proteins with enhanced stability [177]. |
| Personalized medicine and therapeutics | Patient-specific proteins based on individual gene expression profiles [178]. |
6. Genomics
Genomics is the comprehensive study of an organism’s genes or genomes. It involves sequencing and analysis of genomes’ structure, function, and evolution and provides insights into gene expression, function, regulation, and interactions. Genomics incorporates elements from genetics, but its primary focus is the collective characterization and quantification of genes that direct protein production assisted by enzymes and messenger molecules. Genomic techniques vary from the traditional polymerase chain reaction (PCR) and gene sequencing methods to modern next-generation sequencing (NGS) technologies. These methods have allowed the sequencing of entire genomes, such as in the Human Genome Project, which sequenced the whole human genome and revolutionized biomedical research [179,180].
For recombinant proteins and biosimilars, genomics aids in identifying the genes responsible for desired protein functions, optimizing gene expression, and ensuring quality and consistency in production.
One characteristic feature of eukaryotic aging is the degradation of epigenetic information. This phenomenon can be counteracted through a process known as ectopic induction. In mammals, the Yamanaka factors OCT4, SOX2, and KLF4 (OSK) have been found to restore DNA methylation patterns, transcript profiles, and tissue function associated with youthfulness. Importantly, this restoration occurs without compromising the cellular identity, necessitating the active removal of DNA methylation. High-throughput cell-based assays, such as transcription-based aging clocks and real-time nucleocytoplasmic compartmentalization assays, can differentiate between young and old and senescent cells. These assays can be employed to identify compounds that have the potential to reverse cellular aging and rejuvenate human cells while maintaining the integrity of the genome. Consequently, six chemical cocktails have been identified in less than a week, restoring a youthful genome-wide transcript profile and reversing transcriptomic age without compromising cellular identity; thus, genetics and chemical means can achieve rejuvenation by age reversal [181].
The following are some aspects of the use of genomics:
Structural genomics—this area of genomics involves the characterization and mapping of genomic structures, including the sequencing of whole or significant parts of the genome;
Functional genomics—the study of gene and protein functions and their interactions. The techniques used in functional genomics include transcriptomics (gene expression analysis), proteomics (protein expression analysis), and metabolomics (metabolic profile analysis);
Comparative genomics—comparative genomics involves comparing the genomes of different species to understand the similarities and differences in structure and function. This can be used to infer evolutionary relationships among organisms;
Genomic medicine—the application of genomics in health and disease research can help identify disease-susceptibility genes, develop diagnostic tests, and enable personalized treatment strategies based on a patient’s genetic makeup, often called precision medicine.
The following techniques highlight genomics’ broad and powerful impact on recombinant protein research, including improvements in design, production, stability, and therapeutic applications:
Whole genome sequencing;
RNA-Seq—analyzing the quantity and sequences of RNA [53];
Quantitative PCR—quantitative measurement of specific DNA or RNA levels [181];
Microarrays—high-throughput gene expression analysis [54];
Comparative genomic hybridization—detecting and mapping chromosomal imbalances [182].
Combining traditional monitoring techniques with omics technologies represents a unique opportunity to characterize the host cell culture state better and shift from an empirical to a rational approach for process development and optimizing bioreactor cultivation processes. A few examples of genomics applications in the field of recombinant proteins are presented in Table 7.
Table 7.
Examples of genomics applications in recombinant therapeutic protein development.
| Application | Example |
|---|---|
| Improving expression systems | Researchers can optimize expression systems for recombinant protein production by studying host cell genomes [183]. |
| Designing recombinant proteins | Genomics can be used to identify and design recombinant proteins with the desired functions [184]. |
| Enhancing protein stability | Understanding the genomic context can aid in designing recombinant proteins with enhanced stability and activity [185]. |
| Personalized medicine | Personalized genomics allows the development of recombinant proteins tailored to the genetic profiles of individual patients [186]. |
| Metabolic engineering | Genomic insights can guide the redesign of metabolic pathways to efficiently produce recombinant proteins in microbial systems [187]. |
| Biomarker discovery | Genomics aids in the identification of biomarkers that can be targeted with recombinant proteins for diagnostic or therapeutic purposes [188]. |
| Understanding protein function | Comparative genomics can elucidate the functions of proteins by identifying conserved sequences and structures [189]. |
| Enhanced protein folding | Genomic data can be used to understand and improve the folding of recombinant proteins [190]. |
| Development of novel therapeutics | Genomics is used to identify potential targets of therapeutic recombinant proteins [191]. |
| Cell line optimization | Cell line optimization results from: (i) research applied to parental, non-recombinant cell lines; (ii) systems-level datasets generated with recombinant cell lines; (iii) datasets linking phenotypic traits to relevant biomarkers; (iv) data depositories and bioinformatics tools; and (v) in silico model development [192]. |
7. Epigenomics
Epigenomics studies a complete set of epigenetic modifications in DNA or associated proteins other than the DNA sequence, termed the epigenome, that cells use to control gene expression. Epigenetic modifications can influence gene expression without altering the underlying DNA sequence. These modifications play critical roles in cell differentiation and disease, and unlike DNA sequences, they can be changed by environmental conditions. Epigenomics often involves NGS technologies such as whole-genome bisulfite sequencing for DNA methylation and chromatin immunoprecipitation sequencing for histone modifications [193,194,195].
The following are the major types of epigenetic modifications:
DNA methylation—in mammals, DNA methylation typically occurs at cytosine residues in the cytosine-phosphate-guanine context and is an essential process for normal development. Changes in DNA methylation patterns are associated with several key processes, including carcinogenesis;
Histone modification—histone proteins can be modified post-translationally by methylation, acetylation, and ubiquitination. These modifications can alter the chromatin structure and affect gene expression;
Studying the extent to which combinations of DNA-, RNA-, and PTM-level variations contribute to the complexity of the human proteome.
The application of epigenomics to recombinant proteins is an evolving area of research. Table 8 lists a few examples of the applications of epigenomics.
Table 8.
Applications of epigenomics in the development of therapeutic proteins.
| Application | Example |
|---|---|
| Optimization of expression systems | Understanding and manipulating epigenetic markers can enhance recombinant proteins’ expression in host cells [196]. |
| Production of recombinant proteins with specific modifications | Epigenomic control enables the production of recombinant proteins with functionally essential PTMs [197]. |
| Development of recombinant proteins for epigenetically targeted therapies | Epigenomics guides the discovery and development of recombinant proteins that target specific epigenetic modifications involved in various diseases [198]. |
| Studying the epigenetic control of protein function | Recombinant proteins can be used to study how epigenetic modifications regulate endogenous proteins, providing insights into their functions and control [199]. |
| Recombinant epigenetic modifiers for research | Producing recombinant proteins involved in epigenetic modifications, such as methyltransferases, may be helpful for research and drug development [200]. |
| Modeling diseases | Using epigenomic information for modifying host cells to produce recombinant proteins allows the creation of more accurate disease models, particularly for conditions in which epigenetic alterations play a crucial role [201]. |
| Quality control and stability of biopharmaceuticals | Epigenomic control may improve the quality and stability of recombinant proteins for biopharmaceutical applications [75]. |
8. Metabolomics
Metabolomics is the large-scale study of small molecules within cells, biofluids, tissues, or organisms, commonly known as metabolites. These metabolites are the end products of cellular processes and form a significant part of the metabolome, which comprises all metabolites in a biological organism. Metabolomics is a compelling approach in systems biology, as metabolites are often the end products of cellular processes, and changes in their concentrations can be more representative of the current biological status than changes in other biomolecules. Metabolomics is used for various purposes, including studying disease mechanisms, biomarker discovery, drug discovery, and understanding drug effects. This is a critical field in precision medicine and personalized healthcare [202,203,204]. Integrating metabolomics with recombinant protein research offers a multifaceted approach to improving protein production, understanding protein function, and creating more effective therapies. These applications are continually evolving with advancements in analytical techniques and technologies (Table 9).
Table 9.
Examples of metabolomics applications.
| Application | Example |
|---|---|
| Immune response against tumor cells in patients with melanoma | Increases in absolute lymphocyte counts are observed in response to therapy and can be a marker of functional immune response [205]. |
| Lab values alteration | Changes in specific laboratory values can serve as functional response markers for certain conditions. For example, in patients with rheumatoid arthritis treated with the anti-TNFα mAb, adalimumab, reductions in serum C-reactive protein levels and erythrocyte sedimentation rate, which are markers of inflammation, indicate a positive response to therapy [206]. |
| Modulation of T-cell response | Some mAbs, particularly immune checkpoint inhibitors such as pembrolizumab, function by modulating the T-cell response, which can be measured using T-cell activation markers, such as CD137, or by enumerating antigen-specific T-cells [207]. |
| Alteration in serum immunoglobulin levels | Some mAbs can cause changes in the serum levels of immunoglobulins, which can serve as PD markers. Rituximab, an anti-CD20 mAb, decreases serum immunoglobulin levels, which can be monitored clinically [208]. |
| PK and PD correlation | The correlation between PK properties, such as serum drug concentration and PD markers, is a significant component of mAb engagement. This elucidates the dose–response relationship for adjusting the dosing regimens. For instance, with infliximab, an anti-TNFα mAb used in treating autoimmune diseases, measuring the serum drug concentration and correlating this with the clinical response and anti-TNFα activity can indicate effective drug-target engagement [209]. |
9. FDA Omics Perspective
The FDA recommends using omics technologies to identify PD markers for biologicals that do not possess known PD markers, such as hematocrit content on exposure to erythropoietin or white blood cell count on the administration of filgrastim, to waive efficacy testing in patients. Omics refers to comprehensive testing involving high-throughput, large-scale, and integrative approaches for studying and analyzing various components of biological systems. The major omics technologies include genomics, transcriptomics, proteomics, metabolomics, and epigenomics. The FDA has recently conducted investigations and demonstrated how to apply omics technologies to identify novel PD biomarkers without known biomarkers, which can be used for the similarity assessment of biosimilars to waive the need for efficacy testing in patients [28].
9.1. FDA Research
The FDA has undertaken empirical research on biomarkers associated with Parkinson’s disease (PD) to expedite the development of biosimilars [210]. This study encompasses clinical pharmacology investigations wherein participants are administered different biological dosages of a drug, and researchers assess the corresponding biomarker response. The correlation between the dose administered and the response of the biomarker may suggest that the biomarker is suitable for a pharmacodynamic similarity investigation.
The omics exercises can result in functional biomarkers that are part of the mechanism of action, as shown below, or a result of the activity of the administered protein, whether associated or not with the mechanism of action.
9.1.1. PCSK9 Inhibitor Markers (Lipidomic Exercise)
The FDA has approved two cholesterol-lowering drugs, alirocumab, and evolocumab, in a class of biologics known as PCSK9 inhibitors. These inhibitors block PCSK9, which prevents the body from removing excess cholesterol. In an FDA study, 72 healthy participants received different doses of evolocumab (21, 35, 70, or 140 mg), alirocumab (15, 25, 50, or 100 mg), or a placebo [211]. The researchers analyzed two serum biomarkers: low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B (apoB). The primary outcome measures consisted of the areas under the effect curve (AUEC), which quantified the temporal biomarker response and the most significant deviation from baseline values of LDL-C and apoB in serum. The study’s findings exhibited a clear and direct relationship between the dosage of alirocumab and evolocumab and the pharmacodynamics-associated biomarkers. This relationship was evidenced by a substantial alteration in LDL-C and apoB levels compared to the initial measurements as the doses were escalated. Nevertheless, it was shown that apoB demonstrated greater variability in response compared to LDL-C in the study subjects. Therefore, low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B (apoB) have the potential to serve as appropriate biomarkers for comparative investigations of alirocumab and evolocumab in the context of Parkinson’s disease (PD) [212].
9.1.2. IL-5 Antagonists Biomarkers [26] [Functional Markers]
The class of biologics known as anti-IL-5 has been authorized to treat severe eosinophilic asthma, a condition distinguished by an overabundance of eosinophils, a type of white blood cell. Interleukin-5 (IL-5) is the primary cytokine responsible for activating eosinophils, thus leading to airway inflammation. Interleukin-5 (IL-5) antagonists effectively inhibit the activation of eosinophils by preventing IL-5 from binding to its receptors. Three biologics, namely benralizumab, mepolizumab, and reslizumab, have received approval within the IL-5 class of anti-asthma drugs. In a research investigation, a total of 72 individuals who were in good health were randomly assigned to one of two groups. The first group received a placebo, while the second group received a dosage of either mepolizumab (ranging from 3 to 24 mg) or reslizumab (ranging from 0.1 to 0.8 mg/kg), both of which were administered at a lower level than the therapeutic dose. In this investigation, eosinophils in circulation were utilized as biomarkers for Parkinson’s disease (PD). The study’s primary objectives were the highest change observed from the baseline measurement and the area under the effect curve (AUEC). A dose-response association for eosinophil counts was not identified due to the significant heterogeneity in data across the study participants. The administration of the maximum dosage of mepolizumab at 24 mg and reslizumab at 0.8 mg/kg demonstrated observable therapeutic effects. The researchers refrained from doing the intended dose–response analysis due to the significant variability observed in the data. The present work employed pre-existing models and simulations to investigate the impact of various dosages of IL-5 antagonists, including doses up to the therapeutic threshold, on the levels of circulating eosinophils. The findings indicated that administering greater doses, namely therapeutic dosages, might sufficiently discern the distinctions between products using circulating eosinophils as biomarkers in research assessing pharmacodynamic similarities.
9.1.3. IFNβ-1a Biomarkers [213] [Proteomics Markers]
In a particular investigation, the FDA examined the application of proteomics, which involves the comprehensive analysis of proteomes or sets of proteins, to uncover biomarkers for Parkinson’s disease (PD) in biological treatments such as IFNβ-1a and pegylated-IFNβ-1a (pegIFNβ-1a). These products are used to treat multiple sclerosis; however, their mechanisms of action and the relevant PD biomarkers are still unclear, although there are potentially reported candidates. To address this issue, proteomics evaluated circulating biomarkers by assessing more than 7000 proteins from plasma samples to identify potential PD biomarkers. In the present investigation, a total of 84 individuals who were in good health were administered a therapeutic dosage of IFNβ-1a, pegIFNβ-1a, or a placebo. During the preliminary examination, data exclusively from the 36 participants who were administered either a placebo or the highest dosage (30 µg IFNβ-1a or 125 µg pegIFNβ-1a) were assessed. Baseline blood samples were obtained, followed by further collections at many time points. The proteins were evaluated using the proteome test SOMAscan (version 4.1). This investigation successfully identified 248 differentially expressed proteins in the treatment group compared to the placebo group for IFNβ-1a and 528 differentially expressed proteins for pegIFNβ-1a. The researchers prioritized 31 proteins that exhibited the most notable distinctions from the placebo group. Among these proteins, eight had a maximal change from the baseline that exceeded a factor of four. The identification of candidates, both previously reported and newly discovered, was accomplished by researchers. This study showcases the utility of proteomics in identifying potential biomarkers for Parkinson’s disease (PD). These biomarkers can be employed in clinical pharmacology studies to facilitate the creation of biosimilars. This is especially valuable when the products exhibit intricate mechanisms of action or when only a limited number of PD biomarkers have been identified.
9.1.4. Model-based Testing Markers [214] [Functional Markers]
The FDA has also presented results on a model-based approach for the dose selection (MBADS) of pegfilgrastim, which treats neutropenia (low white blood cell count) caused by anticancer medications. Multiple biosimilars have received approval, and to support their approval, pharmacokinetic (PK) and pharmacodynamic (PD) approaches have been employed. These approaches have utilized the biomarker of absolute neutrophil count. Several projects utilized a dosage of 6 mg for pharmacokinetic (PK) and pharmacodynamic (PD) similarity investigations, while alternative programs employed a dosage of 2 mg. Pegfilgrastim exhibits a non-linear pharmacokinetic profile, indicating that the elimination of the drug from the body is influenced by the dosage administered. This factor makes calculating the optimum dose for PK and PD similarity tests more complex—the present work employed model-informed drug development (MIDD) methodologies to address this issue. MIDD approaches employ quantitative methods and data sources to facilitate drug development and regulatory decision-making. In this work, the researchers modified an existing model by incorporating data from two phase I trials. These trials involved administering a single dosage of either 30, 60, or 100 μg/kg of pegfilgrastim to healthy volunteers. The researchers conducted simulations with two doses, specifically 2 and 6 mg. The findings from the simulation analyses indicate that the administration of 6 mg of pegfilgrastim is adequate for detecting distinctions between a proposed pegfilgrastim biosimilar and the reference product in studies examining pharmacokinetic (PK) and pharmacodynamic (PD) similarities. The findings of this study demonstrate that the utilization of simulations can effectively facilitate the process of dose determination for biosimilars exhibiting non-linear pharmacokinetics.
The FDA has demonstrated the use of omics technologies to discover pharmacodynamic markers for drugs like the mAbs that do not demonstrate functional pharmacodynamic biomarkers. To enable applications of the FDA suggestions, we need to review the role of omics technologies, how they are used in the development of new drugs, and their current status, and to analyze whether there is a need to use omics technologies to demonstrate biosimilarity, given the available biomarkers, the difficulties in applying omics technology for the development of biosimilars, the complexities created by individualized omics applications and finally, the scientific rationale of using the omics approach.
9.2. The Practicality of Omics Technologies
While the FDA’s suggestions for engaging in omics technologies to identify PD markers are scientifically sound, their application is complex, and it is questionable if this should be the responsibility of developers. The selection of a PD biomarker requires several considerations:
Relevance to the mechanism of action;
Sensitivity to differences between the proposed biosimilar and the reference product;
Analytical validity;
Time of onset correlated with dosing;
The dynamic range over the exposure range.
Large-scale proteomic methods allow for simultaneous study of the expression of several proteins after administering the reference product; however, it will always be a random choice, varying among developers. Without a specific criterion to compare one PD marker against another, it will always be uncertain if biosimilarity based on one set of PD markers is more robust than that with another set of PD markers. The same applies to other omics platforms like glycomics, transcriptomics, and metabolomics.
Because developers plan these studies separately, their findings may differ, although all will be relevant. Additionally, these considerations for qualifying biomarkers require extensive testing and validation, which will likely consume more time and investment than conducting efficacy testing on patients, leaving little incentive for developers to engage in omics technology [215].
However, it is inappropriate to expect developers to identify proteomic profiles for several reasons:
Proteins located remain unidentified, and these may well be testing-process dependent;
Proteins may not be related to clinical efficacy and only represent a clinical pharmacological profile;
The expertise of proteomic technology is limited to biosimilar developers who are not necessarily research entities; expecting them to use these technologies may result in conclusions that may be less reliable than the standard technologies.
9.3. Biosimilars and Omics Technologies
With their comprehensive and high-resolution data outputs, omics technologies can be crucial in biosimilars’ efficacy testing and characterization (Table 10).
Table 10.
Role of omics technologies and their rationale in the development of biosimilars.
| Omics Technology | Role | Rationale |
|---|---|---|
| Proteomics | Determining the protein expression profile, post-translational modifications (like glycosylation), and protein–protein interactions of the biosimilar compared to the reference product. | Minor differences in protein structure or modifications can impact the efficacy. |
| Transcriptomics | Analyzing the gene expression profile of cells producing the biosimilar ensures that the cellular machinery has the therapeutic protein in a manner consistent with the reference product. | Differences in gene expression may hint at differences in protein product production, folding, or modification. |
| Metabolomics | Examining the metabolic profile of the biosimilar-producing cells. | The metabolic state of a cell can influence the final product’s quality and consistency. For instance, changes in nutrient levels can impact the glycosylation patterns of proteins. |
| Genomics | Ensuring the genetic stability of the cell line producing the biosimilar. | Over time, cell lines might undergo genetic drift, which can impact the product’s quality, consistency, and efficacy. |
| Microbiomics | Understanding the microbiome can be essential if the biological product has a microbial origin (like some recombinant proteins produced in bacteria). | Microbial contaminants or shifts in the microbial population can influence the final product’s quality and safety. |
| Phosphoproteomics | Analyzing phosphorylation patterns on proteins can be critical for some biologics’ function or stability. | Changes in phosphorylation can affect protein activity, stability, or interaction with other proteins. |
10. Omics Alternatives
These PD biomarkers, like hematocrit or WBC count, are pharmacological markers that correlate with clinical response, but the precursors to the pharmacological markers, the PD markers, need not correlate with the clinical response, creating a major issue; if a biosimilar meets the PD biomarker profile, does this mean it is also going to yield the same clinical response? The answer is not necessarily; it has been established. Additionally, if a product fails to meet the biomarker profile due to the nonlinearity of the marker vs. the PK profile, does this mean that a product is not equivalent? The answer is no; it has not been established. These possibilities show the limited utility of newly discovered biomarkers but do not apply to response-related pharmacological markers.
While FDA efforts have introduced many scientific ideas to improve the assessment of biosimilarity, these may not be the most practical, especially when several other more straightforward comparative testing possibilities are available [216,217]. New robust analytical tests, such as MS, can be used to perform orthogonal biosimilarity testing, such as multiple-receptor binding comparisons, as this is the primary mechanism that triggers PD biomarkers (if available).
10.1. Receptor Binding
In the cascade of events, before a PD response is triggered, the protein molecule first binds to its receptors, a well-known and established mechanism of action. The clinical response to biological drugs is based on their mechanism of action, which begins with receptor binding. Current science has made this testing highly accurate and objective. mAbs can also interact with multiple receptors and can be evaluated using orthogonal analytical methods. The primary receptors involved in the activity of the therapeutic proteins include (parenthetical entry shows the number of such receptors). The primary receptors for approved protein therapeutics include: glucagon-like peptide 1 receptor (3), insulin receptors (3), heat-stable enterotoxin receptors (2), adrenocorticotropic hormone receptor, angiotensin II type 2 (AT-2) receptor, corticotropin-releasing factor receptor 1, glucagon-like peptide 2 receptor, gonadotropin-releasing hormone receptor, notch signaling pathway, oxytocin receptor, parathyroid hormone receptor, parathyroid hormone/parathyroid hormone-related peptide receptor, prothrombin, receptor tyrosine-protein kinase erbB-2, secretin receptor, somatostatin receptor 2, somatostatin receptor 5, type-1 angiotensin II receptor, vasopressin V1a receptor, vasopressin V1b receptor, vasopressin V1a receptor, vasopressin V1b receptor, vasopressin V2 receptor [218] (Table 11).
Table 11.
Binding receptors for pharmacodynamic markers.
| mAb (Brand) | Receptor |
|---|---|
| Abciximab (ReoPro) [219] | GPIIb/IIIa |
| Adalimumab (Humira) [220] | TNFα |
| Alemtuzumab (Lemtrada) [221] | CD52 |
| Atezolizumab (Tecentriq) [222] | PD-L1 |
| Basiliximab (Simulect) [223] | CD25 |
| Belimumab (Benlysta) [224] | BLyS |
| Bevacizumab (Avastin) [225] | VEGF |
| Cetuximab (Erbitux) [226] | EGFR |
| Daclizumab (Zinbryta) [227] | CD25 |
| Daratumumab (Darzalex) [228] | CD38 |
| Denosumab (Prolia) [229] | RANKL |
| Dupilumab (Dupixent) [230] | IL-4Rα |
| Eculizumab (Soliris) [149] | C5 |
| Infliximab (Remicade) [231] | TNFα |
| Ipilimumab (Yervoy) [232] | CTLA-4 |
| Nivolumab (Opdivo) [233] | PD-1 |
| Obinutuzumab (Gazyva) [234] | CD20 |
| Ofatumumab (Arzerra) [235] | CD20 |
| Omalizumab (Xolair) [236] | IgE |
| Palivizumab (Synagis) [237] | RSV F protein |
| Pembrolizumab (Keytruda) [238] | PD-1 |
| Rituximab (Rituxan) [239] | CD20 |
| Sarilumab (Kevzara) [240] | IL-6R |
| Secukinumab (Cosentyx) [241] | IL-17A |
| Tocilizumab (Actemra) [242] | IL-6R |
| Trastuzumab (Herceptin) [33] | HER2/neu |
| Vedolizumab (Entyvio) [243] | α4β7 integrin |
An orthogonal approach to establish comparable receptor binding would be substantially more robust and objective than finding an unknown PD marker, qualifying it, and testing it to establish biosimilarity. This powerful test demonstrates functional similarity when PD biomarkers such as mAbs are unavailable.
10.2. Pharmacokinetic Profiling
While receptor binding leads to PD response, determining the clinical response, the PK profile is one of the strongest PD- and clinical-biomarker surrogates.
The use of pharmacokinetics (PK) as a surrogate for pharmacodynamic (PD) markers in the development and evaluation of biological drugs is a topic of increasing interest. Traditionally, PK and PD have been viewed as separate, yet interconnected, disciplines: PK focuses on the absorption, distribution, metabolism, and excretion of a drug, while PD investigates the drug’s physiological and biochemical effects [244]. However, numerous studies suggest that PK parameters can serve as surrogate PD markers, particularly in establishing the biosimilarity of a candidate drug to a reference product [244].
For instance, the PK profile provides a comprehensive overview of a drug’s disposition kinetics, often indicating its effectiveness or safety [245]. Key PK parameters like Cmax (peak serum concentration of the drug) and AUC (area under the curve, reflecting overall drug exposure) are frequently employed as surrogate endpoints in clinical studies for biological drugs [246].
In monoclonal antibodies (mAbs), PK measures such as clearance rate and volume of distribution have shown strong correlations with efficacy markers like tumor-size reduction [247]. Likewise, PK parameters are commonly used as surrogates for PD endpoints in other classes of biological drugs, including erythropoiesis-stimulating agents and interferons [248].
Regulatory agencies like the FDA are increasingly recognizing the potential of PK as a surrogate for PD markers. For example, the FDA’s guidance for biosimilars acknowledges that comparative PK and PD data may suffice to demonstrate biosimilarity without additional clinical studies under certain conditions [249].
It is essential, however, to recognize that the utility of PK as a surrogate for PD markers is not universally applicable. Its appropriateness depends on the complexity of the biological drug and the robustness of the PK/PD relationship [250]. Therefore, employing PK as a surrogate for PD markers should be predicated on a comprehensive understanding of both the drug’s mechanism of action and its PK/PD correlation.
The burgeoning body of evidence supports the notion that PK can serve as a viable surrogate for PD markers, streamlining the development of biosimilar and biobetter treatments. For example, PK metrics have been employed as predictors for long-term outcomes in treatments for rheumatoid arthritis [251]. PK parameters like Cmax and Tmax also predict toxicity in specific cancer treatments [252].
Nonetheless, limitations exist. One challenge is the often-unclear relationship between PK variables and complex biological responses, particularly for drugs with intricate mechanisms of action or those used in polytherapy [253].
The volume of distribution (Vd) as a function of time offers another exciting dimension. Unlike in traditional PK, where Vd is often treated as a constant, recognizing Vd as a time-dependent function allows for incorporating factors like tissue perfusion rates, varying clearance rates, and organ-specific uptake or release over time [254]. Such considerations are particularly pertinent for biological drugs, which may exhibit nonlinear kinetics and more complex distribution patterns than small molecule drugs [255].
Several applications have been noted in both pre-clinical and clinical research:
Cancer chemotherapy—modeling Vd(t) can lead to better predictions of drug concentrations in tumor tissue versus surrounding tissues, potentially optimizing dosing schedules for maximum efficacy and minimal toxicity [256];
Infectious diseases—understanding Vd(t) can help in designing dosage regimens that ensure sufficient drug concentrations at the infection site while minimizing systemic exposure [257];
Autoimmune diseases—for monoclonal antibodies used in conditions like rheumatoid arthritis, Vd can change over time due to factors like target-mediated drug disposition. Understanding Vd(t) can inform individualized dosing [258];
Geriatric pharmacology—age-related physiological changes can impact Vd, and considering Vd as a function of time provides insights into drug disposition in elderly patients [259];
Drug development—during the pre-clinical phase, understanding Vd(t) can guide decisions about advancing a drug candidate to the next stage, potentially saving time and resources [260].
In conclusion, the role of PK as a surrogate for PD markers is increasingly supported by scientific evidence and accepted in both academic and regulatory settings. While its application depends on various factors, the potential advantages—including accelerated approval processes and reduced development costs—are significant. As the biosimilar landscape evolves, regulatory agencies like the FDA should consider expanding the contexts where PK can be a surrogate for PD markers.
So far, none of those mentioned above technologies—receptor binding, PK profiling, or the long list of potential biomarkers listed in Appendix A that biosimilar developers have used to claim biosimilarity; the reason for this lack of innovation comes from the absence of recognition of these biomarkers by the FDA.
11. Finding Biomarkers for Biosimilars
Understanding their PD markers is a significant aspect of developing and validating antibodies [261]. These markers, representing a drug’s bioactivity and physiological effects, are critical for guiding dosage regimens, monitoring efficacy, and predicting adverse responses [262]. However, these biomarkers have not yet been used to establish biosimilarity.
The approval of biosimilars predominantly relies on a restricted set of evidence about pharmacokinetic (PK) and pharmacodynamic (PD) similarities. Biomarkers in pharmacodynamic (PD) studies, which accurately reflect the underlying mechanisms of action of biological products, hold promise in serving as more sensitive endpoints for detecting clinically significant differences between two such products. This presents potential avenues for biomarkers previously employed as secondary and exploratory endpoints to assume crucial functions in biosimilar development initiatives. Additionally, creative approaches can be employed to develop new biomarkers for Parkinson’s disease (PD) in cases where information regarding a relevant PD biomarker is unavailable [263].
Pharmacodynamic biomarkers play an essential role in drug development as they can demonstrate the biological response of a drug on a target. For protein drugs, several biomarkers can help understand the drug’s mechanism of action, efficacy, and, sometimes, safety profile. The selection of appropriate pharmacodynamic biomarkers is essential for accurately evaluating a drug’s mechanism of action and therapeutic efficacy. Moreover, combining several biomarkers might offer a more comprehensive understanding of the drug’s effects, especially in multifactorial conditions. Harnessing these biomarkers for therapeutic and diagnostic applications requires robust experimental design, reliable analytical techniques, and a thorough understanding of the studied disease or condition. As the understanding of cellular processes deepens, the catalog of potential biomarkers will continue to expand, offering increased precision in drug development and therapeutic monitoring.
Incorporating these biomarkers effectively requires a multidisciplinary approach, where clinicians, molecular biologists, pharmacologists, and statisticians work together to evaluate the pharmacodynamic impacts of a given protein drug. Additionally, with advances in technology and an increased understanding of biology at the molecular level, newer biomarkers are continually being identified and validated.
It is essential to note that the appropriateness of a biomarker is highly dependent on the specific drug and its mechanism of action. Always, thorough validation of a biomarker for a specific context of use is critical to ensure its utility in drug development and clinical application.
The selection and validation of a pharmacodynamic biomarker will vary based on the specific protein drug and its intended therapeutic indication. The above list provides a diverse range of potential biomarkers. Still, the key is always to match the biomarker’s sensitivity and specificity with the drug’s mechanism of action and the intended clinical application. A biomarker that works well for one protein drug in a specific disease state might not be suitable for another, even if they target the same pathway. Hence, rigorous validation is essential for each specific scenario.
A broad approach to biomarker discovery, validation, and implementation is crucial. Technological advancements, particularly in omics fields (genomics, proteomics, metabolomics), enable a more comprehensive evaluation of drug impacts. Integration of these technologies and data types, often termed “systems pharmacology,” holds promise for a deeper understanding of drug action and individualized therapeutic interventions.
A practical example of a functional pharmacodynamic marker is the phosphorylation of a protein in a signaling pathway targeted by a kinase inhibitor drug. If the drug’s intended action is to inhibit a kinase, then decreased phosphorylation of its substrates after treatment would be a functional PD marker, showing that the drug is effectively inhibiting its target kinase.
It is essential to differentiate between a functional pharmacodynamic marker and other types of biomarkers. Not all biomarkers are functional in the sense of directly reflecting drug action. Some might be prognostic (indicating the likely course of a disease) or predictive (indicating the likelihood of responding to a specific treatment) but do not necessarily show the drug’s effect on the body.
New biological drug development involves extensive studies of the mode of action, the identity and function of PD biomarkers, and the factors that can alter the dose–response relationship. Table 12 lists the types of biological drugs licensed by the FDA.
Table 12.
Current licensed biological drugs by the FDA (https://drugs.ncats.io/ (accessed on 12 August 2023).
| Type | Count |
|---|---|
| Fab | 1 |
| Toxin | 1 |
| Carrier protein | 1 |
| Single-domain antibody | 1 |
| Fusion proteins | 1 |
| Bispecific antibody | 3 |
| Coagulation factor | 4 |
| Cytokine | 4 |
| Peptide | 4 |
| Growth factor | 4 |
| Enzyme | 9 |
| Enzyme inhibitor | 11 |
| Hormone | 11 |
| Monoclonal antibody conjugate | 13 |
| Monoclonal antibody | 96 |
| Total | 164 |
More specifically, the modes of action and prospective pharmacodynamic markers identified for all FDA-licensed drugs are compiled and reported for the first time in the literature. These data are reported in Appendix A.
12. Conclusions
The FDA is actively advancing science-based approaches, leading to significant modifications in the approval process for biosimilars. These changes include streamlining analytical assessments, eliminating animal toxicology testing, dispensing with immunogenicity testing where it has no impact on disposition kinetics, and waiving patient testing when pharmacodynamic (PD) markers are present. As a result, the largest category of potential biosimilars, primarily monoclonal antibodies (mAbs), may still require extensive patient testing, as mandated by the BPCIA.
While novel ‘omics’ methodologies offer considerable value in developing new products, their utility for establishing biosimilarity is limited. These approaches often necessitate exhaustive research, potentially making them more cumbersome than traditional efficacy testing in patients. Nearly all biological drugs undergo investigations into their mechanisms of action when developed as new entities. In most cases, these findings can serve as potential PD biomarkers. This paper enumerates these potential PD biomarkers for all FDA-approved therapeutic proteins.
However, the FDA should select these PD biomarkers as appropriate for establishing biosimilarity rather than leaving the choice to individual developers. Such regulatory oversight would ensure a standardized and consistent assessment of biosimilar efficacy. Receptor binding, often considered a precursor to the PD response, provides a more sensitive, objective, and readily available method for evaluating the similarity between a biosimilar candidate and its reference product. Moreover, the pharmacokinetic (PK) profile often serves as a more reliable “PD biomarker” than any other identified PD markers since all PD markers are initiated in response to the disposition profile.
It is anticipated that the FDA will take decisive action—initially declaring that efficacy testing in patients is unnecessary and subsequently recommending specific PD markers, if available, for comparison in PK/PD studies. These steps would serve as the final criteria for establishing biosimilarity. This shift in scientific perspective would eliminate barriers to approving numerous biosimilars without compromising patient safety. With approximately 200 molecules identified in this paper, poised to enter the market as biosimilars, such progress would remain unattainable unless the FDA implements the suggested changes, which it has the authority to do.
Appendix A
Table A1.
Mode of action of therapeutic proteins.
| Mode of Action | Biomarker Potential |
|---|---|
| AMPK and mTORC1 Signaling | Monitoring these central energy sensors and regulators can be vital for drugs targeting cellular energy status or metabolic health. [264] |
| Angiogenesis Indicators | If a protein drug affects blood vessel formation, angiogenic factors like VEGF can be used as biomarker. [265] |
| Apoptosis Markers | Evaluation of cell death can be instrumental for drugs designed to induce or inhibit apoptosis. Markers such as caspase activation or phosphatidylserine externalization can be employed. [266] |
| Autophagy Markers | LC3-II and p62/SQSTM1, for drugs modulating autophagic activity. [267] |
| Autophagy-lysosomal Pathway Markers | Monitoring markers like p62/SQSTM1 or LAMP1 can give insights into the autophagy-lysosomal activity upon drug treatment. [268] |
| Blood Coagulation Factors | For protein drugs affecting hemostasis, measurement of specific clotting factors or clotting times might be used. [269] |
| Bone Turnover Markers | For protein drugs acting on the skeletal system, bone resorption or formation markers can provide insight into their effect. [270] |
| Calcium Signaling | Monitoring intracellular calcium flux and associated proteins can be important for drugs that modulate calcium homeostasis or signaling pathways. [271] |
| Cell Cycle Regulators | Drugs aiming at modulating the cell cycle might alter levels or activities of cyclins, cyclin-dependent kinases, or associated inhibitors. [272] |
| Cell Metabolism | Assessing the metabolic profile of cells or tissues after drug treatment, for instance, glucose uptake, lactate production, or ATP level. [273] |
| Cell Surface Markers | These markers can be evaluated for drugs targeting cell surface proteins or for those that induce phenotypic changes in cells. [274] |
| Cellular Apoptosis or Proliferation | Some protein drugs may induce or inhibit apoptosis or cell proliferation, which can be quantified. [275] |
| Cellular Signaling Pathways | Assessment of downstream or upstream signaling pathways that might be affected by the protein drug. MAPK, PI3K/AKT, or JAK/STAT pathways. [276] |
| Changes in specific cell populations | Especially in immunology, a protein drug can lead to the proliferation or reduction of specific cell populations. [277] |
| Circadian Rhythms | For protein drugs affecting cellular or physiological rhythms, markers related to circadian clock genes such as PER, CRY, or CLOCK might be relevant. [278] |
| Complement Activation | For specific therapeutic proteins, activation or inhibition of the complement system can serve as a pharmacodynamic readout. [279] |
| Cytokine Levels | Many protein drugs target specific cytokines or have effects on cytokine levels. [280] |
| DNA Damage and Repair Markers | γH2AX and other proteins associated with DNA damage response can be relevant for drugs targeting genomic stability. [281] |
| Drug Concentration | Although this is more of a pharmacokinetic parameter, the concentration of the drug in the bloodstream can sometimes serve as a surrogate for its pharmacodynamic effects, significantly when the concentration closely correlates with the drug’s effect. [282] |
| Endocannabinoid System Markers | Components include CB1 and CB2 receptors or endocannabinoids (anandamide, 2-AG) for drugs affecting this system. [283] |
| Endocrine Biomarkers | For protein drugs affecting the endocrine system, hormones or hormone precursors might be potential pharmacodynamic indicators. [284] |
| Endocytosis and Exocytosis Metrics | Protein drugs targeting cell trafficking mechanisms might alter the rates of endocytosis or exocytosis, which can be tracked using various cellular assays. [285] |
| Endogenous Antioxidant Enzymes | Superoxide dismutase (SOD), catalase, and glutathione peroxidase levels can be tracked for oxidative stress modulation. [286] |
| Endoplasmic Reticulum (ER) Stress Markers | GRP78/BiP, CHOP, XBP1, ATF6 for drugs influencing ER homeostasis or targeting diseases related to protein misfolding. [287] |
| Endosome Trafficking | Protein drugs that interfere with endosomal pathways can be monitored for their effects using markers of early, late, and recycling endosomes. [288] |
| Endothelial Activation Markers | For drugs impacting vascular inflammation or barrier function, such as E-selectin, ICAM-1, and VCAM-1. [289] |
| Enzyme Activity | If the protein drug targets an enzyme, measuring the change in enzyme activity can be an effective biomarker. [290] |
| Epigenetic Markers | Changes in DNA methylation, histone modification, or other epigenetic markers might indicate a response to certain protein drugs. [290] |
| Exosome Release and Composition | Certain protein drugs can influence exosomes and their cargo (RNA, protein, lipids), especially those impacting intercellular communication. [291] |
| Extracellular Matrix (ECM) Components | Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are relevant for tissue remodeling or cancer invasion. [292] |
| Fatty Acid Oxidation (FAO) Rates | Drugs targeting metabolic states might shift cells between carbohydrate and fatty acid metabolism. [293] |
| Flow Cytometry | This is particularly relevant for drugs that target cells of the immune system. Flow cytometry can provide insights into cell numbers, phenotypes, and functions. [273] |
| Functional Assays | Depending on the intended drug action, functional assays can be developed. For instance, if a protein drug aims to inhibit a specific cellular function, assays can be set up to measure that specific function. [155] |
| Gene Expression Profiles | Transcriptomics can reveal the downstream effects of a protein drug on cellular gene expression. [294] |
| Glycolytic versus Oxidative Metabolism | Assessing the switch between glycolytic and oxidative metabolism can be crucial for drugs targeting metabolic diseases or cancer. [295] |
| Glycosylation Patterns | Alterations in the glycosylation patterns of cells or proteins can directly or indirectly affect some protein drugs. [296] |
| Gut Microbiota Composition | Sequencing or metabolomic profiles of gut bacteria can be helpful for drugs impacting the gut environment. [297] |
| Heat Shock Proteins (HSPs) | As molecular chaperones, changes in HSP levels can indicate cellular stress responses or protein homeostasis disruptions. [298] |
| Heat Shock Proteins (HSPs) | These proteins respond to cellular stress and can be targets or indicators for several drugs, especially in protein-misfolding diseases. [298] |
| Histone Modifications | Epigenetic changes, like histone acetylation or methylation, can be markers for drugs targeting chromatin remodeling or gene expression. [299] |
| Hormone Levels | Assessment of specific hormone levels, like insulin, glucagon, or thyroid hormones, can indicate drug impact on endocrine systems. [300] |
| Hypoxia Indicators | For protein drugs affecting cellular responses to oxygen deprivation, markers like HIF-1α can be interesting. [301] |
| Imaging Biomarkers | Techniques like MRI, PET, and CT can be used to measure the effects of protein drugs at the tissue or organ level. [302] |
| Immune Response Markers | The immune system might mount an antibody response for protein drugs, especially those foreign to the human body. Monitoring anti-drug antibodies (ADAs) can be a biomarker for potential immunogenicity issues. [303] |
| Inflammasome Activation | Monitoring inflammasome components can be helpful in drugs targeting inflammatory conditions or diseases like Alzheimer’s. [304] |
| Ion Channel Activity | For protein drugs targeting ion channels, the measurement of ion flux or electrical properties of cells could directly indicate drug action. [305] |
| Iron Metabolism Markers | Ferritin, transferrin, and hepcidin for drugs modulating iron homeostasis. [306] |
| Levels of circulating drug target | If the target of the protein drug circulates in the bloodstream (like a soluble receptor or ligand), measuring its levels can serve as a biomarker. [246] |
| Ligand-Receptor Interactions | Investigating a protein drug’s binding dynamics and affinity to its target receptor can provide insights into its effectiveness. [307] |
| Lipidomic Profile | Analyzing the cellular lipid composition can be informative, especially for drugs impacting lipid metabolism or signaling. [308] |
| Lipophagy Markers | Indicators of lipid droplet autophagy crucial for lipid metabolism-related conditions. [309] |
| Lysosomal Enzymes | The levels and activity of the specific lysosomal enzymes can be essential biomarkers for enzyme replacement therapies in lysosomal storage disorders. [310] |
| Markers of Fibrosis | In conditions like liver or lung fibrosis, protein drugs might target fibrogenesis, and thus, markers such as tissue collagen or specific matrix proteins can serve as indicators. [311] |
| Metabolic Enzymes | Monitoring the levels or activities of critical metabolic enzymes, such as those involved in glycolysis or the TCA cycle, can provide insights into the metabolic state of cells upon drug treatment. [312] |
| MicroRNAs (miRNAs) | Changes in the expression of specific miRNAs can serve as biomarkers since they play pivotal roles in gene regulation and might be influenced by protein drugs. [313] |
| Mitochondrial Dynamics | Assessing mitochondrial morphology and dynamics can indicate cellular health and metabolism, especially for drugs targeting these organelles. [314] |
| Mitophagy Indicators | Monitoring mitophagy, a process to degrade damaged mitochondria, can be helpful in drugs targeting cellular health. [315] |
| mRNA Splicing Markers | Such as components of the spliceosome for drugs modulating RNA splicing or targeting splicing-related diseases. [316] |
| mTOR Signaling | The mechanistic target of the rapamycin (mTOR) pathway, central to cell growth and metabolism, might be affected by certain protein drugs. Monitoring components like p70S6 kinase or 4E-BP1 can be informative. [317] |
| Myelination Markers | Proteins like MBP or PLP can be tracked for drugs targeting neurodegenerative diseases or demyelinating conditions. [318] |
| NAD+/NADH Ratio | A marker for cellular redox state and metabolism, especially relevant for aging |
| Neural Activity Markers | c-Fos, Arc, or immediate early genes can be indicators of neural activity and synaptic plasticity. [319] |
| Neurotransmitter Levels | If the drug has a neurological target, neurotransmitter levels in the central nervous system or peripheral tissues can be evaluated. [320] |
| Neurotrophic Factors | In neurodegenerative diseases, protein drugs might aim to modulate the levels of neurotrophic factors like BDNF, NGF, or GDNF. [321] |
| NO (Nitric Oxide) Production | Relevant for cardiovascular or immunomodulatory drugs, NO levels can indicate endothelial function and inflammatory states. [322] |
| Nrf2-Keap1 Pathway | Tracking the Nrf2-Keap1 pathway components can be vital for drugs that modulate oxidative stress. [323] |
| Nucleotide Metabolites | Monitoring the levels of specific nucleotide metabolites can indicate cellular activity or stress in response to certain protein drugs. [324] |
| Oxidative Phosphorylation (OXPHOS) Metrics | OXPHOS or mitochondrial health markers can be relevant for protein drugs targeting mitochondrial function. [325] |
| Oxidative Stress Markers | Oxidative stress plays a role in numerous diseases, and markers like reactive oxygen species (ROS) or antioxidant levels can be used to assess drug effects. [326] |
| Oxysterols | Such as 24(S)-hydroxycholesterol 27-hydroxycholesterol for drugs targeting cholesterol metabolism or diseases like Niemann-Pick type C. [327] |
| Peroxisome Proliferators-Activated Receptors (PPARs) | As metabolic regulators, PPARs can be markers for drugs impacting lipid metabolism or inflammation. [328] |
| Pharmacogenomic Biomarkers | Some patients might respond differently to protein drugs based on genetic variations. Exploring these can provide insights into efficacy and safety. [329] |
| Phosphorylation status of proteins | The activation or deactivation of specific signaling pathways can be tracked by looking at the phosphorylation status of essential proteins. [330] |
| Proteasome Activity | Assessing proteasomal activity can be insightful for protein drugs that modulate protein degradation. [331] |
| Proteomic Analysis | To assess broader proteome for changes in protein levels or post-translational modifications upon drug treatment. [57] |
| Reactive Oxygen Species (ROS) Levels | As an indicator of oxidative stress, ROS can be monitored for drugs that either induce or counteract cellular stress. [332] |
| Receptor Occupancy | Measuring the degree to which a protein drug binds to its target receptor can be a direct biomarker of its activity. [333] |
| Senescence-associated Secretory Phenotype (SASP) Factors | Monitoring factors associated with cellular senescence might be relevant for drugs targeting aging or oncogenesis. [334] |
| Sirtuin Activity | Monitoring sirtuin proteins can be essential for drugs modulating cellular longevity or metabolic health. [335] |
| Telomerase Activity | Telomerase activity or telomere length might be relevant biomarkers for drugs targeting cancer or aging processes. [336] |
| Tight Junction Proteins | Markers like claudins, occludin, and ZO-1 are relevant for drugs targeting barrier integrity, such as in gut or blood-brain barrier conditions. [337] |
| Tissue Repair and Regeneration Markers | For protein drugs that facilitate tissue healing, markers of tissue repair or stem cell activation might be relevant. [338] |
| Tumor Microenvironment Components | Factors like TGF-beta, PD-L1, and various cytokines/chemokines for drugs targeting cancer immune evasion. [339] |
| Unfolded Protein Response (UPR) in the ER | For protein drugs that might induce ER stress, tracking components of the UPR can be informative. [340] |
| Wnt Signaling Pathway Components | Tracking this pathway can be crucial for drugs that modulate developmental processes, tissue regeneration, or certain cancers. [341] |
Table A2.
Reported PD Biomarkers of approved therapeutic proteins.
| No | Protein Drug | Pharmacodynamic Marker |
|---|---|---|
| 1. | Abatacept (Orencia) | T cell proliferation & co-stimulation [342] |
| 2. | Adalimumab (Humira) | TNF-alpha levels & inflammatory cytokine reduction [343] |
| 3. | Aducanumab (Aduhelm) | Beta-amyloid plaques in the brain [344] |
| 4. | Aflibercept (Eylea) | VEGF levels, Central retinal thickness [345] |
| 5. | Agalsidase Alfa (Replagal) | Lyso-Gb3 levels, Kidney function [346] |
| 6. | Agalsidase Beta (Fabrazyme) | Lyso-Gb3 levels, Kidney function [347] |
| 7. | Albiglutide (Tanzeum) | Blood glucose & GLP-1 levels [348] |
| 8. | Albutrepenonacog Alfa (Idelvion) | Factor IX activity levels [349] |
| 9. | Aldesleukin (Proleukin) | T cell count, IL-2 levels [350] |
| 10. | Alefacept (Amevive) | CD4 and CD8 memory T-cell count [351] |
| 11. | Alemtuzumab (Lemtrada) | CD52-expressing cell count [352] |
| 12. | Alglucerase (Ceredase) | Gaucher cell count, Chitotriosidase levels [353] |
| 13. | Alirocumab (Praluent) | LDL cholesterol levels [354] |
| 14. | Alpha-1-proteinase inhibitor (Prolastin, etc.) | Alpha-1 antitrypsin levels, Neutrophil elastase activity [355] |
| 15. | Alteplase (Activase) | Fibrinolytic activity, clot dissolution [356] |
| 16. | Amivantamab (Rybrevant) | EGFR and MET signaling inhibition [357] |
| 17. | Anakinra (Kineret) | IL-1β levels [358] |
| 18. | Ancestim (Stemgen) | CD34+ cell count in peripheral blood [359] |
| 19. | Andexanet Alfa (Andexxa) | Reversal of factor Xa inhibitors [360] |
| 20. | Anifrolumab (Saphnelo) | Type I interferon gene signature [361] |
| 21. | Anistreplase (Eminase) | Fibrinolytic activity [362] |
| 22. | Ansuvimab (Ebanga) | Reduction in viral load of Ebola virus [363] |
| 23. | Atezolizumab (Tecentriq) | PD-L1 expression on tumor & immune cells [364] |
| 24. | Avelumab (Bavencio) | PD-L1 expression & T cell activation [365] |
| 25. | Benralizumab (Fasenra) | Reduction in eosinophil counts [366] |
| 26. | Bermekimab (Xilonix) | IL-1α levels [367] |
| 27. | Bevacizumab (Avastin) | VEGF level & microvessel density [368] |
| 28. | Bezlotoxumab (Zinplava) | Reduction in C. difficile infection recurrence [369] |
| 29. | Bimekizumab (Bimzelx) | IL-17A and IL-17F levels [370] |
| 30. | Bivalirudin (Angiomax) | Thrombin activity [371] |
| 31. | Blinatumomab (Blincyto) | CD19+ B cell count [372] |
| 32. | Bone Morphogenetic Proteins (BMPs) | Bone density or new bone formation [373] |
| 33. | Botulinum Toxin Type A (Botox) | Neuromuscular transmission inhibition [374] |
| 34. | Botulinum Toxin Type B (Myobloc) | Neuromuscular transmission inhibition [375] |
| 35. | Brodalumab (Siliq) | IL-17 receptor A occupancy [376] |
| 36. | Brolucizumab (Beovu) | VEGF levels, Central retinal thickness [377] |
| 37. | Burosumab (Crysvita) | Serum phosphorus levels [378] |
| 38. | Calaspargase pegol (Asparlas) | Asparagine levels [379] |
| 39. | Canakinumab (Ilaris) | IL-1β levels & CRP [380] |
| 40. | Cetuximab (Erbitux) | EGFR expression & phosphorylation [226] |
| 41. | Chymopapain (Chymodiactin) | Disc volume reduction [381] |
| 42. | Coagulation factor IX (BeneFIX) | Factor IX clotting activity [382] |
| 43. | Coagulation Factor VIIa (NovoSeven) | Clotting activity [383] |
| 44. | Collagenase (Santyl) | Degrades necrotic tissue [384] |
| 45. | Conestat alfa (Ruconest) | Bradykinin levels [385] |
| 46. | Corticotropin (Acthar) | Adrenal gland stimulation [386] |
| 47. | Cosyntropin-ACTH(1-24) (Cortrosyn) | Adrenal gland stimulation [387] |
| 48. | Crizanlizumab (Adakveo) | P-selectin inhibition [388] |
| 49. | Darbepoetin alfa (Aranesp) | Hemoglobin or hematocrit level [389] |
| 50. | Denosumab (Prolia, Xgeva) | RANKL inhibition & bone turnover markers [390] |
| 51. | Denosumab (Prolia) | Bone mineral density & serum C-telopeptide [390] |
| 52. | Dupilumab (Dupixent) | IL-4 and IL-13 signaling pathways [391] |
| 53. | Durvalumab (Imfinzi) | PD-L1 expression in tumor cells [392] |
| 54. | Eculizumab (Soliris) | Complement component C5 activity [393] |
| 55. | Edrecolomab (Panorex) | EpCAM expression [394] |
| 56. | Efalizumab (Raptiva) | CD11a expression [395] |
| 57. | Efgartigimod alfa | IgG reduction [396] |
| 58. | Elapegademase (Revcovi) | ADA enzyme activity [397] |
| 59. | Elosulfase Alfa (Vimizim) | GAG reduction [398] |
| 60. | Elotuzumab (Empliciti) | SLAMF7 expression in myeloma cells [399] |
| 61. | Emapalumab (Gamifant) | IFNγ levels [400] |
| 62. | Emicizumab (Hemlibra) | Factor IXa and factor X bridging [401] |
| 63. | Enfortumab vedotin (Padcev) | Nectin-4 expression [402] |
| 64. | Erenumab (Aimovig) | CGRP receptor binding and inhibition [403] |
| 65. | Erythropoietin (EPO) | Hemoglobin or hematocrit level [404] |
| 66. | Eteplirsen (Exondys 51) | Dystrophin production in muscle tissue [405] |
| 67. | Evolocumab (Repatha) | LDL cholesterol levels [406] |
| 68. | Fibrinolysin (Elase) | Fibrin degradation [407] |
| 69. | Filgrastim (Neupogen) | Neutrophil count [408] |
| 70. | Follitropin (Follistim, Gonal-f) | Follicular development, Estradiol levels [409] |
| 71. | Fremanezumab (Ajovy) | CGRP levels [410] |
| 72. | Galcanezumab (Emgality) | CGRP levels [411] |
| 73. | Galcanezumab (Emgality) | CGRP binding [412] |
| 74. | Galsulfase (Naglazyme) | Urinary glycosaminoglycan levels [413] |
| 75. | Gemtuzumab ozogamicin (Mylotarg) | CD33 antigen expression [414] |
| 76. | Girentuximab | CAIX expression [415] |
| 77. | Glatiramer acetate (Copaxone) | Immune modulation; T-cell response [416] |
| 78. | Glucagon recombinant (GlucaGen) | Blood glucose elevation [417] |
| 79. | Glucarpidase (Voraxaze) | Methotrexate levels reduction [418] |
| 80. | Golimumab (Simponi) | TNFα inhibition [419] |
| 81. | Growth Hormone | IGF-1 (Insulin-like Growth Factor 1) level [420,421] |
| 82. | Guselkumab (Tremfya) | IL-23 levels & PASI score [422] |
| 83. | Human C1-esterase inhibitor (Berinert, Cinryze) | C1-INH levels and activity [423] |
| 84. | Ibalizumab (Trogarzo) | HIV-1 viral load & CD4+ T-cell count [353] |
| 85. | Imiglucerase (Cerezyme) | Glucocerebroside levels, macrophage activity [424] |
| 86. | Inebilizumab (Uplizna) | B-cell depletion [425] |
| 87. | Infliximab (Remicade) | TNF-alpha levels & CRP [426] |
| 88. | Inotuzumab ozogamicin (Besponsa) | CD22 expression [427] |
| 89. | Insulin Regular (Humulin R, etc) | Glucose levels [428] |
| 90. | Interferons | Expression of interferon-responsive genes [429] |
| 91. | Ipilimumab (Yervoy) | T-cell activation [430] |
| 92. | Isatuximab (Sarclisa) | CD38 expression [431] |
| 93. | Itolizumab (Alzumab) | CD6 expression [432] |
| 94. | Ixekizumab (Taltz) | PASI score, serum IL-17A levels [433] |
| 95. | Lanadelumab (Takhzyro) | Plasma kallikrein activity [434] |
| 96. | Lanadelumab (Takhzyro) | Plasma kallikrein inhibition [435] |
| 97. | Laronidase (Aldurazyme) | Reduction of glycosaminoglycans [436] |
| 98. | Lepirudin (Refludan) | Inhibition of thrombin [437] |
| 99. | Leuprolide (Lupron) | Reduction in testosterone or estradiol [438] |
| 100. | Liraglutide (Victoza) | Blood glucose & GLP-1 levels [439] |
| 101. | Lixisenatide (Adlyxin) | GLP-1 receptor activation [440] |
| 102. | Loncastuximab tesirine (Zynlonta) | CD19 expression [441] |
| 103. | Lucinactant (Surfaxin) | Improved lung compliance [441] |
| 104. | Luspatercept-aamt (Reblozyl) | Erythroid maturation [441] |
| 105. | Lutropin alfa (Luveris) | LH receptor activation [442] |
| 106. | Margetuximab (Margenza) | HER2 expression [443] |
| 107. | Mecasermin (Increlex) | IGF-1 receptor activation [444] |
| 108. | Menotropins (Menopur) | FSH and LH receptor activation [445] |
| 109. | Mepolizumab (Nucala) | IL-5 neutralization [446] |
| 110. | Metreleptin (Myalept) | Leptin receptor activation [447] |
| 111. | Mirvetuximab Soravtansine | Folate receptor alpha targeting [448] |
| 112. | Mogamulizumab (Poteligeo) | CCR4 targeting [449] |
| 113. | Moxetumomab pasudotox (Lumoxiti) | CD22 expression [450] |
| 114. | Muromonab (Orthoclone OKT3) | CD3 expression [451] |
| 115. | Natalizumab (Tysabri) | α4-integrin saturation [452] |
| 116. | Naxitamab (Danyelza) | GD2 expression [453] |
| 117. | Necitumumab (Portrazza) | EGFR targeting [454] |
| 118. | Nesiritide (Natrecor) | Natriuretic peptide receptor A activation [455,456] |
| 119. | Netakimab (Netakimab) | IL-17A inhibition [457,458] |
| 120. | Nimotuzumab (Theraloc, h-R3) | EGFR targeting [459,460] |
| 121. | Nivolumab (Opdivo) | PD-1 receptor occupancy & T cell function [233] |
| 122. | Nofetumomab fmerpentan (Verluma) | Carcinoembryonic antigen (CEA) targeting [461] |
| 123. | Obiltoxaximab (Anthim) | Protective antigen (PA) binding of Bacillus anthracis [462] |
| 124. | Obinutuzumab (Gazyva) | CD20 targeting [234] |
| 125. | Ocrelizumab (Ocrevus) | CD20 targeting [463] |
| 126. | Ocriplasmin (Jetrea) | Vitreomacular adhesion dissolution [461] |
| 127. | Ofatumumab (Arzerra) | CD20 targeting [235] |
| 128. | Olaratumab (Lartruvo) | PDGFRα phosphorylation levels [376] |
| 129. | Olipudase alfa (Xenpozyme) | Acid sphingomyelinase replacement [464] |
| 130. | Omalizumab (Xolair) | Free serum IgE levels & FcεRI expression on basophils [465] |
| 131. | Oportuzumab monatox (Vicineum) | N-acetylgalactosamine-4-sulfatase targeting [466] |
| 132. | Oprelvekin (Neumega) | Thrombopoietin receptor activation [467] |
| 133. | Oxytocin (Pitocin) | Oxytocin receptor activation [468] |
| 134. | Palifermin (Kepivance) | Keratinocyte growth factor receptor activation [469] |
| 135. | Palivizumab (Synagis) | RSV neutralization in serum [154] |
| 136. | Pancrelipase amylase (Creon) | Pancreatic enzyme replacement [470] |
| 137. | Panitumumab (Vectibix) | EGFR receptor occupancy & phosphorylation [471,472] |
| 138. | Parathyroid/Preotact (Preos) | Parathyroid hormone (PTH) receptor activation [473] |
| 139. | Pegademase bovine (Adagen) | ADA enzyme replacement [474] |
| 140. | Pegaspargase (Oncaspar) | Asparagine depletion [475] |
| 141. | Pegcetacoplan (Empaveli) | Complement C3 inhibition [476] |
| 142. | Peginterferon alfa-2a (Pegasys) | Interferon alpha receptor activation [477] |
| 143. | Peginterferon alfa-2b (PegIntron) | Interferon alpha receptor activation [478] |
| 144. | Pegloticase (Krystexxa) | Uric acid metabolism [479] |
| 145. | Pegvisomant (Somavert) | Growth hormone receptor antagonist [480] |
| 146. | Pembrolizumab (Keytruda) | PD-1 receptor occupancy & PD-L1 expression [481] |
| 147. | Pertuzumab (Perjeta) | HER2 receptor dimerization inhibition [481] |
| 148. | Pertuzumab (Perjeta) | HER2/neu targeting [482] |
| 149. | Pramlintide (Symlin) | Amylin analogue [483] |
| 150. | Protein S human (PROS) | Protein S supplementation [484] |
| 151. | Ramucirumab (Cyramza) | VEGFR2 targeting [485] |
| 152. | Ranibizumab (Lucentis) | VEGF level & macular thickness [486] |
| 153. | Rasburicase (Elitek) | Uric acid conversion to allantoin [487] |
| 154. | Reteplase (Retavase) | Plasminogen activation [488] |
| 155. | Rilonacept (Arcalyst) | Interleukin-1 blockade [489] |
| 156. | Rituximab (Rituxan) | CD20+ B cell depletion [490] |
| 157. | Romiplostim (Nplate) | Thrombopoietin receptor stimulation [491] |
| 158. | Romosozumab (Evenity) | Sclerostin levels & bone mineral density [492] |
| 159. | Sacrosidase (Sucraid) | Sucrase replacement for sucrose digestion [493] |
| 160. | Sargramostim (Leukine) | GM-CSF receptor activation [494] |
| 161. | Sarilumab (Kevzara) | IL-6 receptor blockade & CRP [495] |
| 162. | Sebelipase alfa (Kanuma) | Lysosomal acid lipase replacement [496] |
| 163. | Secretin (SecreFlo) | Secretin receptor activation [497] |
| 164. | Secukinumab (Cosentyx) | PASI score, serum IL-17A levels [241] |
| 165. | Sermorelin (Geref) | GHRH receptor activation [498] |
| 166. | Siltuximab (Sylvant) | IL-6 targeting [499] |
| 167. | Somatotropin (Genotropin) | GH receptor activation [500] |
| 168. | Streptokinase (Streptase) | Plasminogen activation [501] |
| 169. | Tagraxofusp (Elzonris) | CD123-directed cytotoxin [502] |
| 170. | Taliglucerase alfa (Elelyso) | Glucocerebrosidase enzyme replacement [503] |
| 171. | Teduglutide (Gattex) | GLP-2 receptor activation [504] |
| 172. | Tenecteplase (TNKase) | Plasminogen activation [505] |
| 173. | Teriparatide (Forteo) | Bone formation stimulation [472] |
| 174. | Terlipressin (Varpress) | Vasoconstriction through V1 receptor activation [506] |
| 175. | Tesamorelin (Egrifta) | GH-releasing hormone receptor activation [507] |
| 176. | Thymalfasin (Zadaxin) | Immunomodulation, T-cell stimulation [508] |
| 177. | Thyrotropin Alfa (Thyrogen) | Thyroid-stimulating hormone receptor activation [509] |
| 178. | Tirzepatide (Mounjaro) | Dual GLP-1 and GIP receptor agonism [510] |
| 179. | Tisotumab vedotin (Tivdak) | ADC targeting tissue factor [511] |
| 180. | Tocilizumab (Actemra) | IL-6 receptor blockade [512] |
| 181. | Tositumomab (Bexxar) | CD20 targeting [513] |
| 182. | Trastuzumab (Herceptin) | HER2/neu receptor expression [514] |
| 183. | Urofollitropin (Bravelle) | Follicle-stimulating hormone stimulation [515] |
| 184. | Urokinase (Abbokinase) | Plasminogen activation [516] |
| 185. | Ustekinumab (Stelara) | Inhibition of the p40 subunit of interleukin-12 (IL-12) and interleukin-23 (IL-23) [517] |
| 186. | Vasopressin (Vasostrict) | V1 and V2 receptor activation [518] |
| 187. | Vedolizumab (Entyvio) | α4β7 integrin receptor occupancy [519] |
| 188. | Velaglucerase alfa (Vpriv) | Glucocerebrosidase enzyme replacement [520] |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Appendix A.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.FDA Registered Drugs Inxight Drugs. [(accessed on 10 July 2023)]; Available online: https://drugs.ncats.io.
- 2.Biosimilars in the United-States. [(accessed on 10 July 2023)]. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-united-states-2023-2027.
- 3.Mckinsey R&D Biosimilars. [(accessed on 10 July 2023)]. Available online: https://www.mckinsey.com/industries/life-sciences/our-insights/three-imperatives-for-r-and-d-in-biosimilars.
- 4.FDA. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/media/114574/download.
- 5.FDA Drug Safety. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-withdraws-draft-guidance-industry-statistical-approaches-evaluate-analytical-similarity.
- 6.FDA Guidance. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-therapeutic-protein-biosimilars-comparative-analytical-assessment-and-other-quality.
- 7.Regulatory Information Guidelines. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-immunogenicity-considerations-biosimilar-and-interchangeable-insulin-products.
- 8.Biosimilars Science and Research. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/drugs/biosimilars/biosimilars-science-and-research.
- 9.Congress Bill 5002. [(accessed on 10 July 2023)]; Available online: https://www.congress.gov/bill/117th-congress/senate-bill/5002.
- 10.Niazi S.K. End animal testing for biosimilar approval. Science. 2022;377:162–163. doi: 10.1126/science.add4664. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Trials and Human Subject Protection. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/science-research/clinical-trials-and-human-subject-protection/fda-policy-protection-human-subjects.
- 12.Congress Bill 1695. [(accessed on 10 July 2023)]; Available online: https://www.congress.gov/bill/110th-congress/senate-bill/1695/text.
- 13.Niazi S. Scientific Rationale for Waiving Clinical Efficacy Testing of Biosimilars. Drug Des. Dev. Ther. 2022;16:2803–2815. doi: 10.2147/DDDT.S378813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore T.J., Mouslim M.C., Blunt J.L., Alexander G.C., Shermock K.M. Assessment of Availability, Clinical Testing, and US Food and Drug Administration Review of Biosimilar Biologic Products. JAMA Intern. Med. 2021;181:52–60. doi: 10.1001/jamainternmed.2020.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Trials Database. [(accessed on 25 April 2023)]; Available online: https://clinicaltrials.gov/ct2/results?cond=&term=biosimilar&cntry=&state=&city=&dist=&recrs=e.
- 16.FDA Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product. Guidance for Industry. [(accessed on 10 July 2023)]; Available online: https://www.federalregister.gov/documents/2016/12/29/2016-31511/clinical-pharmacology-data-to-support-a-demonstration-of-biosimilarity-to-a-reference-product#:~:text=The%20Food%20and%20Drug%20Administration%20(FDA%20or%20Agency)%20is%20announcing,other%20investigators%20engaged%20in%20biosimilar.
- 17.FDA Biosimilars Plan. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/media/114574/download?attachment.
- 18.BIOSIMILAR BIOLOGICAL PRODUCT REAUTHORIZATION PERFORMANCE GOALS AND PROCEDURES FISCAL YEARS 2023 THROUGH 2027. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/media/152279/download?attachment.
- 19.Increasing-Efficiency-Biosimilar-Development-Programs. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-workshop-increasing-efficiency-biosimilar-development-programs-09192022.
- 20.Center-Drug-Evaluation-And-Research-Cder. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/division-applied-regulatory-science.
- 21.Chiu K., Racz R., Burkhart K., Florian J., Ford K., Iveth Garcia M., Geiger R.M., Howard K.E., Hyland P.L., Ismaiel O.A., et al. New science, drug regulation, and emergent public health issues: The work of FDA’s division of applied regulatory science. Front. Med. 2023;9:1109541. doi: 10.3389/fmed.2022.1109541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration FDA Guidance: Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product. [(accessed on 14 August 2023)];2016 Available online: https://www.fda.gov/media/88622/download.
- 23.Li L., Ma L., Schrieber S.J., Rahman N.A., Deisseroth A., Farrell A.T., Wang Y., Sinha V., Marathe A. Quantitative Relationship Between AUEC of Absolute Neutrophil Count and Duration of Severe Neutropenia for G-CSF in Breast Cancer Patients. Clin. Pharmacol. Ther. 2018;104:742–748. doi: 10.1002/cpt.991. [DOI] [PubMed] [Google Scholar]
- 24.Li J., Florian J., Campbell E., Schrieber S.J., Bai J.P.F., Weaver J.L., Hyland P.L., Thway T.M., Matta M.K., Lankapalli R.H., et al. Advancing Biosimilar Development Using Pharmacodynamic Biomarkers in Clinical Pharmacology Studies. Clin. Pharmacol. Ther. 2019;107:40–42. doi: 10.1002/cpt.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikhy M., Schrieber S.J., Sun Q., Gershuny V., Matta M.K., Bai J.P.F., Du X., Vegesna G., Shah A., Prentice K., et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development. (I) A randomized trial with PCSK9 inhibitors. Clin. Pharmacol. Ther. 2022;113:71–79. doi: 10.1002/cpt.2769. [DOI] [PubMed] [Google Scholar]
- 26.Gershuny V., Sun Q., Schrieber S.J., Matta M.K., Weaver J.L., Ji P., Sheikhy M., Hsiao C.H., Vegesna G., Shah A. Considerations for use of pharmacodynamic biomarkers to support biosimilar development. (II) A randomized trial with IL-5 antagonists. Clin. Pharmacol. Ther. 2022;113:80–89. doi: 10.1002/cpt.2760. [DOI] [PubMed] [Google Scholar]
- 27.Florian J., Gershuny V., Sun Q., Schrieber S.J., Matta M.K., Hazel A., Sheikhy M., Weaver J.L., Hyland P.L., Hsiao C.H. Considerations for use of pharmacodynamic biomarkers to support biosimilar development—(III) A randomized trial with interferon beta-1a products. Clin. Pharmacol. Ther. 2022;113:339–348. doi: 10.1002/cpt.2784. [DOI] [PubMed] [Google Scholar]
- 28.Hyland P.L., Chekka L.M.S., Samarth D.P., Rosenzweig B.A., Decker E., Mohamed E.G., Guo Y., Matta M.K., Sun Q., Wheeler W., et al. Evaluating the utility of proteomics for the identification of circulating pharmacodynamic biomarkers of IFNbeta-1a biologics. Clin. Pharmacol. Ther. 2023;113:98–107. doi: 10.1002/cpt.2778. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Strauss D.G. Advancing Innovations in Biosimilars. Clin. Pharmacol. Ther. 2022;113:11–15. doi: 10.1002/cpt.2782. [DOI] [PubMed] [Google Scholar]
- 30. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/drugs/news-events-human-drugs/increasing-efficiency-biosimilar-development-programs-reevaluating-need-comparative-clinical.
- 31.Hughes T., Branford S. Molecular monitoring of BCR-ABL as a guide to clinical management in chronic myeloid leukaemia. Blood Rev. 2006;20:29–41. doi: 10.1016/j.blre.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., Goldberg A.C., Gordon D., Levy D., Lloyd-Jones D.M. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., Eiermann W., Wolter J., Pegram M., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 34.Shafer R.W., Schapiro J.M. HIV-1 drug resistance mutations: An updated framework for the second decade of HAART. Antivir. Ther. 2008;13:145–176. [PMC free article] [PubMed] [Google Scholar]
- 35.Blennow K., Zetterberg H., Fagan A.M. Fluid biomarkers in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006221. doi: 10.1101/cshperspect.a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: Therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Björnsson E.S., Gunnarsson B.I., Gröndal G., Jonasson J.G., Einarsdottir R., Ludviksson B.R., Gudbjörnsson B., Olafsson S. Risk of drug-induced liver injury from tumor necrosis factor antagonists. Clin. Gastroenterol. Hepatol. 2015;13:602–608. doi: 10.1016/j.cgh.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 38.Ansell J., Hirsh J., Hylek E., Jacobson A., Crowther M., Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 39.Vasikaran S., Eastell R., Bruyere O., Foldes A.J., Garnero P., Griesmacher A., McClung M., Morris H.A., Silverman S., Trenti T. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 40.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J.M., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallis R.S., Wang C., Doherty T.M., Onyebujoh P., Vahedi M., Laang H., Olesen O., Parida S., Zumla A. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect. Dis. 2013;10:68–69. doi: 10.1016/S1473-3099(10)70003-7. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins R.G., Simpson J.K., Saini G., Bentley J.H., Russell A.-M., Braybrooke R., Molyneaux P.L., McKeever T.M., Wells A.U., Flynn A. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: An analysis from the prospective, multicentre PROFILE study. Lancet Respir. Med. 2012;2:434–440. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 43.Blennow K., Zetterberg H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018;284:643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 44.Jones A.G., Hattersley A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013;30:803–817. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizushima N., Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Gandara D.R., Paul S.M., Kowanetz M., Schleifman E., Zou W., Li Y., Rittmeyer A., Fehrenbacher L., Otto G., Malboeuf C. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 49.Venter J.C., Smith H.O., Adams M.D. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 50.Microarray|Learn Science at Scitable. (n.d.) [(accessed on 10 July 2023)]. Available online: https://www.nature.com/scitable/definition/microarray-202/#:~:text=Following%20hybridization%2C%20the%20microarray%20is,on%20the%20microarray%20appears%20red.
- 51.Lander E.S., Linton L.M., Birren B., Nusbaum C. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 52.Doolittle W.F., Brown J.R. Tempo, mode, the progenote, and the universal root. Proc. Natl. Acad. Sci. USA. 1994;91:6721–6728. doi: 10.1073/pnas.91.15.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z., Gerstein M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schena M., Shalon D., Davis R.W., Brown P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 55.Tang F., Barbacioru C., Wang Y., Nordman E., Lee C., Xu N., Wang X., Bodeau J., Tuch B.B., Siddiqui A. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 56.Steijger T., The RGASP Consortium. Abril J.F., Engström P.G., Kokocinski F., Hubbard T.J., Guigó R., Harrow J., Bertone P. Assessment of transcript reconstruction methods for RNA-seq. Nat. Methods. 2013;10:1177–1184. doi: 10.1038/nmeth.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aebersold R., Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 58.O’Farrell P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975;250:4007–4021. doi: 10.1016/S0021-9258(19)41496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michalski A., Cox J., Mann M. More than 100,000 detectable peptide species elute in single shotgun proteomics runs but the majority is inaccessible to data-dependent LC-MS/MS. J. Proteome Res. 2011;10:1785–1793. doi: 10.1021/pr101060v. [DOI] [PubMed] [Google Scholar]
- 60.Haab B.B., Zhu X. Proteomic analysis of cellular systems. Curr. Opin. Biotechnol. 2001;12:340–345. [Google Scholar]
- 61.Beckonert O., Keun H.C., Ebbels T.M., Bundy J., Holmes E., Lindon J.C., Nicholson J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 62.Fiehn O., Kopka J., Dörmann P., Altmann T., Trethewey R.N., Willmitzer L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000;18:1157–1161. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- 63.Dunn W.B., Ellis D.I. Metabolomics: Current analytical platforms and methodologies. Trends Anal. Chem. 2005;24:285–294. [Google Scholar]
- 64.Wishart D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016;15:473–484. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 65.Feinberg A.P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 66.Johnson D.S., Mortazavi A., Myers R.M., Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 67.Lister R., O’Malley R.C., Tonti-Filippini J., Gregory B.D., Berry C.C., Millar A.H., Ecker J.R. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han X., Gross R.W. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 69.Cajka T., Fiehn O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal. Chem. 2014;61:192–206. doi: 10.1016/j.trac.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lipid Analysis-4th Edition. [(accessed on 10 January 2010)]. Available online: https://shop.elsevier.com/books/lipid-analysis/christie/978-0-9552512-4-5.
- 71.Goodacre R., Vaidyanathan S., Dunn W.B., Harrigan G.G., Kell D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004;22:245–252. doi: 10.1016/j.tibtech.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Bantscheff M., Lemeer S., Savitski M.M., Kuster B. Quantitative mass spectrometry in proteomics: Critical re-view update from 2007 to the present. Anal. Bioanal. Chem. 2012;404:939–965. doi: 10.1007/s00216-012-6203-4. [DOI] [PubMed] [Google Scholar]
- 73.Karlsson R., Katsamba P.S., Nordin H., Pol E., Myszka D.G. Analyzing a kinetic titration series using affinity biosensors. Anal. Biochem. 2006;349:136–147. doi: 10.1016/j.ab.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 74.Fazel R., Guan Y., Vaziri B., Krisp C., Heikaus L., Saadati A., Hidayah S.N., Gaikwad M., Schlüter H. Structural and In Vitro Functional Comparability Analysis of Altebrel™, a Proposed Etanercept Biosimilar: Focus on Primary Sequence and Glycosylation. Pharmaceuticals. 2019;12:14. doi: 10.3390/ph12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beck A., Diemer H., Ayoub D., Debaene F., Wagner-Rousset E., Carapito C., Van Dorsselaer A., Sanglier-Cianférani S. Analytical characterization of biosimilar antibodies and Fc-fusion proteins. TrAC Trends Anal. Chem. 2013;48:81–95. doi: 10.1016/j.trac.2013.02.014. [DOI] [Google Scholar]
- 76.Mechref Y., Novotny M.V. Glycomic analysis by capillary electrophoresis-mass spectrometry. Mass Spectrom. Rev. 2009;28:207–222. doi: 10.1002/mas.20196. [DOI] [PubMed] [Google Scholar]
- 77.Rogers R.S., Nightlinger N.S., Livingston B., Campbell P., Bailey R., Balland A. Development of a quantitative mass spectrometry multi-attribute method for characterization, quality control testing and disposition of biologics. mAbs. 2013;7:881–890. doi: 10.1080/19420862.2015.1069454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarnowski C.P., Bikaki M., Leitner A. Cross-linking and mass spectrometry as a tool for studying the structural biology of ribonucleoproteins. Structure. 2022;30:441–461. doi: 10.1016/j.str.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Wyatt P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta. 1993;272:1–40. doi: 10.1016/0003-2670(93)80373-S. [DOI] [Google Scholar]
- 80.Kelly S.M., Price N.C. The use of circular dichroism in the investigation of protein structure and function. Curr. Protein Pept. Sci. 2000;1:349–384. doi: 10.2174/1389203003381315. [DOI] [PubMed] [Google Scholar]
- 81.Bains G., Freire E. Calorimetric determination of cooperative interactions in high affinity binding processes. Anal. Biochem. 1991;192:203–206. doi: 10.1016/0003-2697(91)90207-A. [DOI] [PubMed] [Google Scholar]
- 82.Provencher S.W., Glöckner J. Analysis of the components present in kinetics (or titration) curves. J. Biochem. Biophys. Methods. 1983;7:331–334. doi: 10.1016/0165-022X(83)90058-1. [DOI] [PubMed] [Google Scholar]
- 83.Pecora R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanoparticle Res. 2000;2:123–131. doi: 10.1023/A:1010067107182. [DOI] [Google Scholar]
- 84.Laine R.A. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 10(12) structures for a reducing hexasaccharide: The Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology. 1994;4:759–767. doi: 10.1093/glycob/4.6.759. [DOI] [PubMed] [Google Scholar]
- 85.Zeng Y., Wang J., Li B., Hauser S., Li H., Wang L.X. Glycopeptide synthesis through endo-glycosidase-catalyzed oligosaccharide transfer of sugar oxazolines: Probing substrate structural requirement. Chemistry. 2006;12:3355–3364. doi: 10.1002/chem.200501196. [DOI] [PubMed] [Google Scholar]
- 86.Crowther J.R. The ELISA Guidebook. Methods Mol. Biol. 2009;149:516. doi: 10.1385/1592590497. [DOI] [PubMed] [Google Scholar]
- 87.Tate E.W., Dixon D.J., Ley S.V. A highly enantioselective total synthesis of (+)-goniodiol. Org. Biomol Chem. 2006;4:1698–1706. doi: 10.1039/b602805e. [DOI] [PubMed] [Google Scholar]
- 88.Royle L., Radcliffe C.M., Dwek R.A., Rudd P.M. Detailed structural analysis of N-glycans released from glycoproteins in SDS-PAGE gel bands using HPLC combined with exoglycosidase array digestions. Methods Mol. Biol. 2006;347:125–143. doi: 10.1385/1-59745-167-3:125. [DOI] [PubMed] [Google Scholar]
- 89.Gossett D.R., Tse H.T., Lee S.A., Ying Y., Lindgren A.G., Yang O.O., Rao J., Clark A.T., Di Carlo D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA. 2012;109:7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anumula K.R., Taylor P.B. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 1992;203:101–108. doi: 10.1016/0003-2697(92)90048-C. [DOI] [PubMed] [Google Scholar]
- 91.Eftink M.R. Fluorescence techniques for studying protein structure. Methods Biochem. Anal. 1991;35:127–205. doi: 10.1002/9780470110560.ch3. [DOI] [PubMed] [Google Scholar]
- 92.Barth A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta. 2007;1767:1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 93.Patti G.J., Yanes O., Siuzdak G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kailemia M.J., Xu G., Wong M., Li Q., Goonatilleke E., Leon F., Lebrilla C.B. Recent Advances in the Mass Spectrometry Methods for Glycomics and Cancer. Anal Chem. 2018;90:208–224. doi: 10.1021/acs.analchem.7b04202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciucanu I., Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984;131:209–217. doi: 10.1016/0008-6215(84)85242-8. [DOI] [Google Scholar]
- 96.Weber J., Kayser A., Rinas U. Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. II. Dynamic response to famine and feast, activation of the methylglyoxal pathway and oscillatory behaviour. Pt 3Microbiology. 2011;157:707–717. doi: 10.1099/mic.0.27482-0. [DOI] [PubMed] [Google Scholar]
- 97.Barteneva N.S., Fasler-Kan E., Bernimoulin M., Stern J.N., Ponomarev E.D., Duckett L., Vorobjev I.A. Circulating microparticles: Square the circle. BMC Cell Biol. 2013;14:23. doi: 10.1186/1471-2121-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Görg A., Obermaier C., Boguth G., Harder A., Scheibe B., Wildgruber R., Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21:1037–1053. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1037::AID-ELPS1037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 99.Cummings R.D., Pierce J.M. The challenge and promise of glycomics. Chem Biol. 2014;21:1–15. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morelle W., Michalski J.C. Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2007;2:1585–1602. doi: 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- 101.Apweiler R., Aslanidis C., Deufel T., Gerstner A., Hansen J., Hochstrasser D., Kellner R., Kubicek M., Lottspeich F., Maser E., et al. Approaching clinical proteomics: Current state and future fields of application in fluid proteomics. Clin. Chem. Lab. Med. 2009;47:724–744. doi: 10.1515/CCLM.2009.167. [DOI] [PubMed] [Google Scholar]
- 102.Lauber M.A., Yu Y.Q., Brousmiche D.W., Hua Z., Koza S.M., Magnelli P., Guthrie E., Taron C.H., Fountain K.J. Rapid Preparation of Released N-Glycans for HILIC Analysis Using a Labeling Reagent that Facilitates Sensitive Fluorescence and ESI-MS Detection. Anal. Chem. 2015;87:5401–5409. doi: 10.1021/acs.analchem.5b00758. [DOI] [PubMed] [Google Scholar]
- 103.Engen J.R. Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS. Anal. Chem. 2009;81:7870–7875. doi: 10.1021/ac901154s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yigzaw Y., Hinckley P., Hewig A., Vedantham G. Ion exchange chromatography of proteins and clearance of aggregates. Curr. Pharm. Biotechnol. 2009;10:421–426. doi: 10.2174/138920109788488842. [DOI] [PubMed] [Google Scholar]
- 105.Apweiler R., Hermjakob H., Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta. 1999;1473:4–8. doi: 10.1016/S0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 106.Xue L., Rup B. Evaluation of pre-existing antibody presence as a risk factor for posttreatment anti-drug antibody induction: Analysis of human clinical study data for multiple biotherapeutics. AAPS J. 2013;15:893–896. doi: 10.1208/s12248-013-9497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomopoulos G.N., Schulte B.A., Spicer S.S. The influence of embedding media and fixation on the post-embedment ultrastructural demonstration of complex carbohydrates. II. Dialyzed iron staining. Histochemistry. 1983;79:417–431. doi: 10.1007/BF00491777. [DOI] [PubMed] [Google Scholar]
- 108.Bruzzone C., Loizaga-Iriarte A., Sánchez-Mosquera P., Gil-Redondo R., Astobiza I., Diercks T., Cortazar A.R., Ugalde-Olano A., Schäfer H., Blanco F.J., et al. 1H NMR-Based Urine Metabolomics Reveals Signs of Enhanced Carbon and Nitrogen Recycling in Prostate Cancer. J. Proteome Res. 2020;19:2419–2428. doi: 10.1021/acs.jproteome.0c00091. [DOI] [PubMed] [Google Scholar]
- 109.Ahrer K., Buchacher A., Iberer G., Jungbauer A. Detection of aggregate formation during production of human immunoglobulin G by means of light scattering. J. Chromatogr. A. 2004;1043:41–46. doi: 10.1016/j.chroma.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 110.Righetti P.G., Gianazza E. New developments in isoelectric focusing. J. Chromatogr. 1980;184:415–456. doi: 10.1016/S0021-9673(00)93873-1. [DOI] [PubMed] [Google Scholar]
- 111.Yamashita K., Watanabe K., Takayama H., Mizuguchi S., Ishibashi M., Miyazaki H., Tanaka W., Umezawa H. Assay of plasma leupeptin using the reversible binding of leupeptin to bovine pancreatic trypsin. Anal. Biochem. 1986;156:503–507. doi: 10.1016/0003-2697(86)90285-X. [DOI] [PubMed] [Google Scholar]
- 112.Doneanu C.E., Xenopoulos A., Fadgen K., Murphy J., Skilton S.J., Prentice H., Stapels M., Chen W. Analysis of host-cell proteins in biotherapeutic proteins by comprehensive online two-dimensional liquid chromatography/mass spectrometry. MAbs. 2012;4:24–44. doi: 10.4161/mabs.4.1.18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tugizimana F., Steenkamp P.A., Piater L.A., Dubery I.A. Multi-platform metabolomics analyses of ergosterol-induced dynamic changes in Nicotiana tabacum cells. PLoS ONE. 2017;12:e0169741. doi: 10.1371/journal.pone.0087846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y., Fonslow B.R., Shan B., Baek M.C., Yates J.R., 3rd Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013;113:2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Varki A., Cummings R.D., Esko J.D., editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2009. [(accessed on 10 July 2023)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1908/ [Google Scholar]
- 116.Sengupta N. Metabolomics Analysis of Recombinant Escherichia coli Expression Systems for Recombinant Protein Production. J. Proteom. Bioinform. 2018;11:110–120. [Google Scholar]
- 117.Lange V., Malmström J.A., Didion J., King N.L., Johansson B.P., Schäfer J., Rameseder J., Wong C.H., Deutsch E.W., Brusniak M.Y., et al. Targeted quantitative analysis of Streptococcus pyogenes virulence factors by multiple reaction monitoring. Mol. Cell Proteom. 2008;7:1489–1500. doi: 10.1074/mcp.M800032-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Domon B., Vath J.E., Costello C.E. Analysis of derivatized ceramides and neutral glycosphingolipids by high-performance tandem mass spectrometry. Anal. Biochem. 1990;184:151–164. doi: 10.1016/0003-2697(90)90028-8. [DOI] [PubMed] [Google Scholar]
- 119.Zhao Y.Y., Lin R.C. UPLC-MS(E) application in disease biomarker discovery: The discoveries in proteomics to metabolomics. Chem. Biol. Interact. 2014;215:7–16. doi: 10.1016/j.cbi.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 120.Harvey D.J. Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates. Mass Spectrom. Rev. 1999;18:349–450. doi: 10.1002/(SICI)1098-2787(1999)18:6<349::AID-MAS1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 121.Hillenkamp F., Karas M., Beavis R.C., Chait B.T. Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal. Chem. 1991;63:1193A–1203A. doi: 10.1021/ac00024a716. [DOI] [PubMed] [Google Scholar]
- 122.Wyatt P.J. Submicrometer Particle Sizing by Multiangle Light Scattering following Fractionation. J. Colloid Interface Sci. 1998;197:9–20. doi: 10.1006/jcis.1997.5215. [DOI] [PubMed] [Google Scholar]
- 123.Wyatt P.J. Differential light scattering and the measurement of molecules and nanoparticles: A review. Anal. Chim. Acta X. 2021;7–8:100070. doi: 10.1016/j.acax.2021.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harris R.P., Kilby P.M. Amino acid misincorporation in recombinant biopharmaceutical products. Curr. Opin. Biotechnol. 2014;30:45–50. doi: 10.1016/j.copbio.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Heck A.J. Native mass spectrometry: A bridge between interactomics and structural biology. Nat. Methods. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 126.Kato K., Sasakawa H., Kamiya Y., Utsumi M., Nakano M., Takahashi N., Yamaguchi Y. 920 MHz ultra-high field NMR approaches to structural glycobiology. Biochim. Biophys. Acta. 2008;1780:619–625. doi: 10.1016/j.bbagen.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 127.Bai Y., Milne J.S., Mayne L., Englander S.W. Primary structure effects on peptide group hydrogen exchange. Proteins. 1993;17:75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bollard M., Murray A., Clarke K., Nicholson J., Griffin J. A study of metabolic compartmentation in the rat heart and cardiac mitochondria using high-resolution magic angle spinning 1H NMR spectroscopy. FEBS Lett. 2005;579:1779–1788. doi: 10.1016/S0014-5793(03)00969-4. [DOI] [PubMed] [Google Scholar]
- 129.Rich R.L., Myszka D.G. Survey of the 2009 commercial optical biosensor literature. J. Mol. Recognit. 2011;24:892–914. doi: 10.1002/jmr.1138. [DOI] [PubMed] [Google Scholar]
- 130.Suckau D., Köhl J., Karwath G., Schneider K., Casaretto M., Bitter-Suermann D., Przybylski M. Molecular epitope identification by limited proteolysis of an immobilized antigen-antibody complex and mass spectrometric peptide mapping. Proc. Natl. Acad. Sci. USA. 1990;87:9848–9852. doi: 10.1073/pnas.87.24.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu H., Bilgin M., Bangham R., Hall D., Casamayor A., Bertone P., Lan N., Jansen R., Bidlingmaier S., Houfek T., et al. Global analysis of protein activities using proteome chips. Science. 2001;293:2101–2105. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]
- 132.Wolters D.A., Washburn M.P., Yates J.R. An automated multidimensional protein identification technology for shotgun proteomics. Anal. Chem. 2001;73:5683–5690. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- 133.Cserháti T., Forgács E. Effect of pH and salts on the binding of free amino acids to the corn protein zein studied by thin-layer chromatography. Amino Acids. 2005;28:99–103. doi: 10.1007/s00726-004-0134-0. [DOI] [PubMed] [Google Scholar]
- 134.Philo J.S. A critical review of methods for size characterization of non-particulate protein aggregates. Curr. Pharm. Biotechnol. 2009;10:359–372. doi: 10.2174/138920109788488815. [DOI] [PubMed] [Google Scholar]
- 135.Houseman B.T., Mrksich M. Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem. Biol. 2002;9:443–454. doi: 10.1016/S1074-5521(02)00124-2. [DOI] [PubMed] [Google Scholar]
- 136.Zhuo H., Lyu Z., Su J., He J., Pei Y., Cheng X., Zhou N., Lu X., Zhou S., Zhao Y. Effect of lung squamous cell carcinoma tumor microenvironment on the CD105+ endothelial cell proteome. J. Proteome Res. 2014;13:4717–4729. doi: 10.1021/pr5006229. [DOI] [PubMed] [Google Scholar]
- 137.Ong S.E., Blagoev B., Kratchmarova I., Kristensen D.B., Steen H., Pandey A., Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell Proteom. 2002;1:376–386. doi: 10.1074/mcp.M200025-MCP200. [DOI] [PubMed] [Google Scholar]
- 138.Ståhl S., Gräslund T., Eriksson Karlström A., Frejd F.Y., Nygren P.Å., Löfblom J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017;35:691–712. doi: 10.1016/j.tibtech.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 139.Joosten R.P., Lütteke T. Carbohydrate 3D structure validation. Curr. Opin. Struct. Biol. 2017;44:9–17. doi: 10.1016/j.sbi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 140.Wüthrich K. NMR studies of structure and function of biological macromolecules (Nobel Lecture) J. Biomol. NMR. 2003;27:13–39. doi: 10.1023/A:1024733922459. [DOI] [PubMed] [Google Scholar]
- 141.Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 142.Cox J., Mann M. Quantitative, high-resolution proteomics for data-driven systems biology. Annu. Rev. Biochem. 2011;80:273–299. doi: 10.1146/annurev-biochem-061308-093216. [DOI] [PubMed] [Google Scholar]
- 143.Francisco J.A., Cerveny C.G., Meyer D.L., Mixan B.J., Klussman K., Chace D.F., Rejniak S.X., Gordon K.A., DeBlanc R., Toki B.E., et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 144.Bendtzen K., Geborek P., Svenson M., Larsson L., Kapetanovic M.C., Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2003;48:3782–3789. doi: 10.1002/art.22214. [DOI] [PubMed] [Google Scholar]
- 145.Beum P.V., Peek E.M., Lindorfer M.A., Beurskens F.J., Engelberts P.J., Parren P.W.H.I., van de Winkel J.G.J., Taylor R.P., Labrijn A.F., Rispens T. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. J. Immunol. 2006;177:8071–8079. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 146.Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 147.Gerdes J., Lemke H., Baisch H., Wacker H.H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the mono-clonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. doi: 10.4049/jimmunol.133.4.1710. [DOI] [PubMed] [Google Scholar]
- 148.Bast R.C., Jr., Klug T.L., St. John E., Jenison E., Niloff J.M., Lazarus H., Berkowitz R.S., Leavitt T., Griffiths C.T., Parker L. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 149.Hillmen P., Hall C., Marsh J.C., Elebute M., Bombara M.P., Petro B.E., Cullen M.J., Richards S.J., Rollins S.A., Mojcik C.F., et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2004;350:552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 150.Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D., Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 151.Wolchok J.D., Hoos A., O’Day S., Weber J.S., Hamid O., Lebbé C., Maio M., Binder M., Bohnsack O., Nichol G., et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2010;16:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 152.Beers S.A., French R.R., Chan H.T., Lim S.H., Jarrett T.C., Vidal R.M., Wijayaweera S.S., Dixon S.V., Kim H., Cox K.L., et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: Implications for antibody selection. Blood. 2010;115:5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
- 153.Ananthakrishnan A.N., Luo C., Yajnik V., Khalili H., Garber J.J., Stevens B.W., Cleland T., Xavier R.J. Gut microbiome function predicts response to anti-integrin biologic therapy in in-flammatory bowel diseases. Cell Host Microbe. 2017;21:603–610. doi: 10.1016/j.chom.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johnson S., Oliver C., Prince G.A., Hemming V.G., Pfarr D.S., Wang S.-C., Dormitzer M., O’Grady J., Koenig S., Tamura J.K. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 155.Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Di Marco F., Blümel G., Blöchl C., Wuhrer M., Huber C.G. A semi-automated hybrid HPLC-MS approach for in-depth char-acterization of intact non-covalent heterodimer glycoforms of gonadotropin biopharmaceuticals. Anal. Chim. Acta. 2023;1274:341574. doi: 10.1016/j.aca.2023.341574. [DOI] [PubMed] [Google Scholar]
- 158.Čaval T., Buettner A., Haberger M., Reusch D., Heck A.J.R. Discrepancies between High-Resolution Native and Glycopeptide-Centric Mass Spectrometric Approaches: A Case Study into the Glycosylation of Erythropoietin Variants. J. Am. Soc. Mass Spectrom. 2021;32:2099–2104. doi: 10.1021/jasms.1c00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hahm Y.H., Lee J.Y., Ahn Y.H. Investigation of Site-Specific Differences in Glycan Microheterogeneity by N-Glycopeptide Mapping of VEGFR-IgG Fusion Protein. Molecules. 2019;24:3924. doi: 10.3390/molecules24213924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Di Marco F., Blöchl C., Esser-Skala W., Schäpertöns V., Zhang T., Wuhrer M., Sandra K., Wohlschlager T., Huber C.G. Glycoproteomics of a single protein: Revealing tens of thousands of Myozyme® glycoforms by hybrid HPLC-MS approaches. Mol. Cell. Proteom. 2023;22:100622. doi: 10.1016/j.mcpro.2023.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moises J.E., Regl C., Hinterholzer A., Huber C.G., Schubert M. Unambiguous Identification of Glucose-Induced Glycation in mAbs and other Proteins by NMR Spectroscopy. Pharm. Res. 2023;40:1341–1353. doi: 10.1007/s11095-022-03454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pegg C.L., Zacchi L.F., Recinos D.R., Howard C.B., Schulz B.L. Identification of novel glycosylation events on human se-rum-derived factor IX. Glycoconj. J. 2020;37:471–483. doi: 10.1007/s10719-020-09922-2. [DOI] [PubMed] [Google Scholar]
- 163.Zaia J., Paschinger K., Wilson I.B.H., Popov R.S., Ivanchina N.V., Kicha A.A., Malyarenko T.V., Dmitrenok P.S., Stonik V.A., Qi Y. Mass spectrometry and glycomics. OMICS A J. Integr. Biol. 2010;14:401–418. doi: 10.1089/omi.2009.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sanchez-De Melo I. N-glycosylation profile analysis of Trastuzumab biosimilar candidates by Normal Phase Liquid Chromatography and MALDI-TOF MS approaches. J. Proteom. 2015;127 Pt B:225–233. doi: 10.1016/j.jprot.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 165.Rosati S. In-depth qualitative and quantitative analysis of composite glycosylation profiles and other mi-croheterogeneity on intact monoclonal antibodies by high-resolution native mass spectrometry using a modified Orbitrap. MAbs. 2013;5:917–924. doi: 10.4161/mabs.26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Srikanth J., Agalyadevi R., Babu P. Targeted, Site-specific quantitation of N-and O-glycopeptides using 18-O-labeling and product ion based mass spectrometry. Glycoconj. J. 2017;34:95–105. doi: 10.1007/s10719-016-9733-8. [DOI] [PubMed] [Google Scholar]
- 167.Yang Y., Liu F., Franc V., Halim L.A., Schellekens H., Heck A.J. Hybrid mass spectrometry approaches in glycoprotein analysis and their usage in scoring biosimilarity. Nat. Commun. 2016;7:13397. doi: 10.1038/ncomms13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Conesa A., Madrigal P., Tarazona S., Gomez-Cabrero D., Cervera A., McPherson A., Szcześniak M.W., Gaffney D.J., Elo L.L., Zhang X. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:13. doi: 10.1186/s13059-016-0881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pereira R., Vilaça P., Maia P., Nielsen J., Rocha I. Turnover Dependent Phenotypic Simulation: A Quantitative Constraint-Based Simulation Method That Accommodates All Main Strain Design Strategies. ACS Synth. Biol. 2019;8:976–988. doi: 10.1021/acssynbio.8b00248. [DOI] [PubMed] [Google Scholar]
- 170.Rosano G.L., Ceccarelli E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014;5:172. doi: 10.3389/fmicb.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krogan N.J., Cagney G., Yu H., Zhong G., Guo X., Ignatchenko A., Li J., Pu S., Datta N., Tikuisis A.P. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 172.Buckle A.M., Bate M.A., Androulakis S., Cinquanta M., Basquin J., Bonneau F., Chatterjee D.K., Cittaro D., Gräslund S., Gruszka A., et al. Recombinant protein quality evaluation: Proposal for a minimal information standard. Stand. Genom. Sci. 2011;5:195–197. doi: 10.4056/sigs.1834511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De Marco A. Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb. Cell Factories. 2009;8:26. doi: 10.1186/1475-2859-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tang S.Y., Cirino P.C. Design and application of a mevalonate-responsive regulatory protein. Angew. Chem. Int. Ed. Engl. 2011;50:1084–1086. doi: 10.1002/anie.201006083. [DOI] [PubMed] [Google Scholar]
- 175.Castrillo J.I., Zeef L.A., Hoyle D.C., Zhang N., Hayes A., Gardner D.C., Cornell M.J., Petty J., Hakes L., Wardleworth L. Growth control of the eukaryote cell: A systems biology study in yeast. J. Biol. 2007;6:4. doi: 10.1186/jbiol54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Geiger T., Velic A., Macek B., Lundberg E., Kampf C., Nagaraj N., Uhlen M., Cox J., Mann M. Initial Quantitative Proteomic Map of 28 Mouse Tissues Using the SILAC Mouse. Mol. Cell. Proteom. 2012;12:1709–1722. doi: 10.1074/mcp.M112.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hasin Y., Seldin M., Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Green E.D., Watson J.D., Collins F.S. Human Genome Project: Twenty-five years of big biology. Nature. 2015;526:29–31. doi: 10.1038/526029a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Goodwin S., McPherson J.D., McCombie W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016;17:333–351. doi: 10.1038/nrg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang J.-H., Petty C.A., Dixon-Mcdougall T., Lopez M.V., Tyshkovskiy A., Maybury-Lewis S., Tian X., Ibrahim N., Chen Z., Griffin P.T. Chemically induced reprogramming to reverse cellular aging. Aging. 2023;15:5966–5989. doi: 10.18632/aging.204896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 182.Kallioniemi A., Kallioniemi O.P., Sudar D., Rutovitz D., Gray J.W., Waldman F., Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258:818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- 183.Lewis A.M., Abu-Absi N.R., Borys M.C., Li Z.J. The use of ‘Omics technology to rationally improve industrial mammalian cell line performance. Biotechnol. Bioeng. 2016;113:26–38. doi: 10.1002/bit.25673. [DOI] [PubMed] [Google Scholar]
- 184.Gustafsson C., Govindarajan S., Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22:346–353. doi: 10.1016/j.tibtech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 185.Edwards A.M., Isserlin R., Bader G.D., Frye S.V., Willson T.M., Yu F.H. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- 186.Goldenzweig A., Goldsmith M., Hill S.E., Gertman O., Laurino P., Ashani Y., Dym O., Unger T., Albeck S., Prilusky J. Automated Structure- and Sequence-Based Design of Proteins for High Bacterial Expression and Stability. Mol. Cell. 2016;63:337–346. doi: 10.1016/j.molcel.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hamburg M.A., Collins F.S. The path to personalized medicine. N. Engl. J. Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 188.Keasling J.D. Manufacturing molecules through metabolic engineering. Science. 2010;330:1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- 189.Hood L., Friend S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011;8:184–187. doi: 10.1038/nrclinonc.2010.227. [DOI] [PubMed] [Google Scholar]
- 190.Gabaldón T., Huynen M.A. Shaping the mitochondrial proteome. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2004;1659:212–220. doi: 10.1016/j.bbabio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 191.Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 192.Hopkins A.L., Groom C.R. The druggable genome. Nat. Rev. Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 193.Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Feinberg A.P. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 195.Lister R., Ecker J.R. Finding the fifth base: Genome-wide sequencing of cytosine methylation. Genome Res. 2009;19:959–966. doi: 10.1101/gr.083451.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mikkelsen T.S., Ku M., Jaffe D.B., Issac B., Lieberman E., Giannoukos G., Alvarez P., Brockman W., Kim T.-K., Koche R.P. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Walsh G. Post-translational modifications of protein biopharmaceuticals. Drug Discov. Today. 2010;15:773–780. doi: 10.1016/j.drudis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 198.Laugesen A., Helin K. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell. 2014;14:735–751. doi: 10.1016/j.stem.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 199.Rothbart S.B., Strahl B.D. Interpreting the language of histone and DNA modifications. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2014;1839:627–643. doi: 10.1016/j.bbagrm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dillon S.C., Zhang X., Trievel R.C., Cheng X. The SET-domain protein superfamily: Protein lysine methyl-transferases. Genome Biol. 2005;6:227. doi: 10.1186/gb-2005-6-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Egger G., Liang G., Aparicio A., Jones P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 202.Nicholson J.K., Lindon J.C., Holmes E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 203.Wishart D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008;19:482–493. doi: 10.1016/j.tifs.2008.03.003. [DOI] [Google Scholar]
- 204.Johnson C.H., Gonzalez F.J. Challenges and opportunities of metabolomics. J. Cell. Physiol. 2012;227:2975–2981. doi: 10.1002/jcp.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ku G.Y., Yuan J., Page D.B., Schroeder S.E., Panageas K.S., Carvajal R.D., Chapman P.B., Schwartz G.K., Allison J.P., Wolchok J.D. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: Lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rau R. Adalimumab (a fully human anti-tumour necrosis factor alpha monoclonal antibody) in the treatment of active rheumatoid arthritis: The initial results of five trials. Ann. Rheum. Dis. 2002;61((Suppl. 2)):ii70–ii73. doi: 10.1136/ard.61.suppl_2.ii70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ribas A., Shin D.S., Zaretsky J., Frederiksen J., Cornish A., Avramis E., Seja E., Kivork C., Siebert J., Kaplan-Lefko P. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol. Res. 2016;4:194–203. doi: 10.1158/2326-6066.CIR-15-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Besada E., Koldingsnes W., Nossent J.C. Serum immunoglobulin levels and risk factors for hypogammaglobulinaemia during long-term maintenance therapy with rituximab in patients with granulomatosis with polyangiitis. Rheumatology. 2014;53:1818–1824. doi: 10.1093/rheumatology/keu194. [DOI] [PubMed] [Google Scholar]
- 209.Ternant D., Mulleman D., Degenne D., Willot S., Guillaumin J.-M., Watier H., Goupille P., Paintaud G. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther. Drug Monit. 2008;30:169–174. doi: 10.1097/01.ftd.0000189901.08684.4b. [DOI] [PubMed] [Google Scholar]
- 210.Van der Graaf P.H. Innovations in biosimilars. [(accessed on 12 August 2023)];Clin. Pharmacol. Ther. 2023 113:1–195. Available online: https://ascpt.onlinelibrary.wiley.com/toc/15326535/2023/113/1. [Google Scholar]
- 211.Schwartz G.G., Gabriel Steg P., Bhatt D.L., Bittner V.A., Diaz R., Goodman S.G., Jukema J.W., Kim Y.U., Li Q.H., Manvelian G., et al. Clinical Efficacy and Safety of Alirocumab After Acute Coronary Syndrome According to Achieved Level of Low-Density Lipoprotein Cholesterol: A Propensity Score-Matched Analysis of the ODYSSEY OUTCOMES Trial. Circulation. 2021;143:1109–1122. doi: 10.1161/CIRCULATIONAHA.120.049447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.FDA Pharmacodynamic-Biomarkers and Their Role in Biosmilar Product Development. [(accessed on 10 July 2023)]; Available online: https://www.fda.gov/drugs/news-events-human-drugs/pharmacodynamic-biomarkers-their-role-biosimilar-product-development#footnote4_55gxd3i.
- 213.Li F., Sun Q., Du S., Florian J., Wang Y., Huang S.M., Zineh I., Wang Y.C. Model-Based Approach to Selecting Pegfilgrastim Dose for Pharmacokinetic and Pharmacodynamic Similarity Studies in Biosimilar Development. Clin. Pharmacol. Ther. 2023;113:62–70. doi: 10.1002/cpt.2722. [DOI] [PubMed] [Google Scholar]
- 214.Schiestl M., Stangler T., Torella C., Čepeljnik T., Toll H., Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat. Biotechnol. 2011;29:310–312. doi: 10.1038/nbt.1839. [DOI] [PubMed] [Google Scholar]
- 215.Sellick C.A., Croxford A.S., Maqsood A.R., Stephens G., Goodacre R. Metabolite profiling of recombinant CHO cells: Designing tailored feeding regimes that enhance recombinant antibody production. Biotechnol. Bioeng. 2015;112:2575–2585. doi: 10.1002/bit.23269. [DOI] [PubMed] [Google Scholar]
- 216.U.S. Food and Drug Administration (FDA) Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product: Guidance for Industry. [(accessed on 10 July 2023)];2020 Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-pharmacology-data-support-demonstration-biosimilarity-reference-product.
- 217.European Medicines Agency (EMA) Guideline on Similar Biological Medicinal Products. 2014. [(accessed on 10 July 2023)]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf.
- 218.FDA. [(accessed on 10 July 2023)]. Available online: https://drugs.ncats.io/substances?facet=Development%20Status%2FUS%20Approved%20Rx&facet=Substance%20Class%2Fprotein&facet=Substance%20Form%2FPrincipal%20Form&page=1.
- 219.Topol E.J. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet. 1994;344:494–502. doi: 10.1016/S0140-6736(98)85010-1. [DOI] [PubMed] [Google Scholar]
- 220.Weinblatt M.E., Keystone E.C., Furst D.E., Moreland L.W., Weisman M.H., Birbara C.A., Teoh L.A., Fischkoff S.A., Chartash E.K. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 221.Hill-Cawthorne G.A., Button T., Tuohy O., Jones J.L., May K., Somerfield J., Green A., Giovannoni G., Compston D.A.S., Fahey M.T. Long-term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2012;83:298–304. doi: 10.1136/jnnp-2011-300826. [DOI] [PubMed] [Google Scholar]
- 222.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nashan B., Moore R., Amlot P., Schmidt A.-G., Abeywickrama K., Soulillou J.-P. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet. 1997;350:1193–1198. doi: 10.1016/S0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 224.Navarra S.V., Guzmán R.M., Gallacher A.E., Hall S., Levy R.A., Jimenez R.E., Li E.K., Thomas M., Kim H.Y., León M.G. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 225.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 226.Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 227.Kappos L., Wiendl H., Selmaj K., Arnold D.L., Havrdova E., Boyko A., Kaufman M., Rose J., Greenberg S., Sweetser M. Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2015;373:1418–1428. doi: 10.1056/NEJMoa1501481. [DOI] [PubMed] [Google Scholar]
- 228.Lokhorst H.M., Plesner T., Laubach J.P., Nahi H., Gimsing P., Hansson M., Minnema M.C., Lassen U., Krejcik J., Palumbo A. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 229.Cummings S.R., Martin J.S., McClung M.R., Siris E.S., Eastell R., Reid I.R., Delmas P., Zoog H.B., Austin M., Wang A. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 230.Simpson E.L., Bieber T., Guttman-Yassky E., Beck L.A., Blauvelt A., Cork M.J., Silverberg J.I., Deleuran M., Kataoka Y., Lacour J.-P. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 231.Hanauer S.B., Feagan B.G., Lichtenstein G.R., Mayer L.F., Schreiber S., Colombel J.F., Rachmilewitz D., Wolf D.C., Olson A., Bao W. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 232.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Goede V., Fischer K., Busch R., Engelke A., Eichhorst B., Wendtner C.M., Chagorova T., De La Serna J., Dilhuydy M.S., Illmer T. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 235.Wierda W.G., Kipps T.J., Mayer J., Stilgenbauer S., Williams C.D., Hellmann A., Robak T., Furman R.R., Hillmen P., Trneny M. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Busse W., Corren J., Lanier B.Q., McAlary M., Fowler-Taylor A., Della Cioppa G., van As A., Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 237.The IMpact-RSV Study Group Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Re-duces Hospitalization from Respiratory Syncytial Virus Infection in High-risk Infants. Pt 1Pediatrics. 1998;102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 238.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 239.Maloney D.G., Grillo-López A.J., White C.A., Bodkin D., Schilder R.J., Neidhart J.A., Janakiraman N., Foon K.A., Liles T.M., Dallaire B.K. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. doi: 10.1182/blood.V90.6.2188. [DOI] [PubMed] [Google Scholar]
- 240.Fleischmann R., van Adelsberg J., Lin Y., Castelar-Pinheiro G.d.R., Brzezicki J., Hrycaj P., Graham N.M.H., van Hoogstraten H., Bauer D., Burmester G.R. Sarilumab and Nonbiologic Disease-Modifying Antirheumatic Drugs in Patients with Active Rheumatoid Arthritis and Inadequate Response or Intolerance to Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol. 2017;69:277–290. doi: 10.1002/art.39944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E., Papp K., Puig L., Nakagawa H., Spelman L., Sigurgeirsson B. Secukinumab in Plaque Psoriasis—Results of Two Phase 3 Trials. N. Engl. J. Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 242.Maini R.N., Breedveld F.C., Kalden J.R., Smolen J.S., Davis D., MacFarlane J.D., Antoni C., Leeb B., Elliott M.J., Woody J.N. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha mono-clonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheumatol. 2006;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 243.Feagan B.G., Rutgeerts P., Sands B.E., Hanauer S., Colombel J.-F., Sandborn W.J., Van Assche G., Axler J., Kim H.-J., Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 244.Wagner J.G. History of pharmacokinetics. Pharmacol. Ther. 1981;12:537–562. doi: 10.1016/0163-7258(81)90097-8. [DOI] [PubMed] [Google Scholar]
- 245.Jin J.Y., Almon R.R., DuBois D.C., Jusko W.J. Comparative Pharmacodynamics of Rosiglitazone, Pioglitazone, and Troglitazone in Cultured Human and Rat Adipocytes. Drug Metab. Dispos. 2017;45:160–167. [Google Scholar]
- 246.Mager D.E., Jusko W.J. General Pharmacokinetic Model for Drugs Exhibiting Target-Mediated Drug Disposition. J. Pharmacokinet. Pharmacodyn. 2001;28:507–532. doi: 10.1023/A:1014414520282. [DOI] [PubMed] [Google Scholar]
- 247.Lee J.Y., Lee J.H., Kim Y.H. Clinical Pharmacology in Drug Development: An Overview of Phase 1 Studies. Korean J. Physiol. Pharmacol. 2010;14:283–290. [Google Scholar]
- 248.Bruno R., Washington C.B., Lu J.F., Lieberman G., Banken L., Klein P. Population Pharmacokinetics of Trastuzumab in Patients with HER2+ Metastatic Breast Cancer. Cancer Chemother. Pharmacol. 2005;56:361–369. doi: 10.1007/s00280-005-1026-z. [DOI] [PubMed] [Google Scholar]
- 249.Frampton J.E., Peters D.H. Epoetin Alfa: A Review of its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Use in Nonrenal Applications. Drugs. 1999;57:233–260. doi: 10.2165/00003495-199549020-00008. [DOI] [PubMed] [Google Scholar]
- 250.FDA . Considerations in Demonstrating Interchangeability with a Reference Product: Guidance for Industry. U.S. Food and Drug Administration; Silver Spring, MD, USA: 2019. [Google Scholar]
- 251.Turner D.C., Navid F., Daw N.C., Mao S., Wu J., Santana V.M., Neel M. Population Pharmacokinetics of Bevacizumab in Children with Osteosarcoma: Implications for Dosing. Clin. Cancer Res. 2018;24:1480–1487. doi: 10.1158/1078-0432.CCR-13-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mould D.R., Upton R.N., Wojciechowski J. Dashboards for Monitoring Clinical Trials. Clin. Pharmacol. Ther. 2012;91:927–937. [Google Scholar]
- 253.Davies B., Morris T. Physiological Parameters in Laboratory Animals and Humans. Pharm. Res. 2013;10:1093–1095. doi: 10.1023/A:1018943613122. [DOI] [PubMed] [Google Scholar]
- 254.Thompson L., Boudinot F.D., Nagar S. Pharmacokinetics and Pharmacodynamics of Approved and Investigation-al Janus Kinase Inhibitors. Clin. Pharmacokinet. 2019;58:1103–1118. [Google Scholar]
- 255.Mager D.E., Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm. Res. 2005;22:1589–1596. doi: 10.1007/s11095-005-6650-0. [DOI] [PubMed] [Google Scholar]
- 256.Danhof M., de Lange E.C., Della Pasqua O.E., Ploeger B.A., Voskuyl R.A. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol. Sci. 2007;28:186–191. doi: 10.1016/j.tips.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 257.El-Kareh A.W., Secomb T.W. A mathematical model for comparison of bolus injection, continuous infusion, and liposomal delivery of doxorubicin to tumor cells. Neoplasia. 2000;2:325–338. doi: 10.1038/sj.neo.7900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Craig W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 259.Gibiansky L., Gibiansky E. Target-mediated drug disposition model and its approximations for antibody-drug conjugates. J. Pharmacokinet. Pharmacodyn. 2009;36:25–45. doi: 10.1007/s10928-013-9344-y. [DOI] [PubMed] [Google Scholar]
- 260.Turnheim K. When drug therapy gets old: Pharmacokinetics and pharmacodynamics in the elderly. Exp. Gerontol. 2003;38:843–853. doi: 10.1016/S0531-5565(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 261.Bonate P.L. Pharmacokinetic-Pharmacodynamic Modeling and Simulation. Springer; Berlin/Heidelberg, Germany: 2011. [Google Scholar]
- 262.Leach M.W., Ruff D. Biomarkers, Pharmacodynamics and Drug Development. J. Pharmacol. Exp. Ther. 2013;3:153–158. [Google Scholar]
- 263.Sung J.A., Pickeral J., Liu L., Stanfield-Oakley S.A., Lam C.-Y.K., Garrido C., Pollara J., LaBranche C., Bonsignori M., Moody M.A. Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J. Clin. Investig. 2015;125:4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hardie D.G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell. Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 265.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Klionsky D.J., Abdelmohsen K., Abe A. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mizushima N., Levine B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Davie E.W., Kulman J.D. An overview of the structure and function of thrombin. Semin. Thromb. Hemost. 2006;32((Suppl. 1)):3–15. doi: 10.1055/s-2006-939550. [DOI] [PubMed] [Google Scholar]
- 270.Seibel M.J. Biochemical markers of bone turnover part I: Biochemistry and variability. Clin. Biochem. Rev. 2005;26:97–122. [PMC free article] [PubMed] [Google Scholar]
- 271.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 272.Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 273.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Maecker H.T., Ghanekar S.A., Suni M.A., He X.S., Picker L.J., Maino V.C. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J. Immunol. 2001;166:7268–7275. doi: 10.4049/jimmunol.166.12.7268. [DOI] [PubMed] [Google Scholar]
- 275.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 276.Manning B.D., Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Maecker H.T., McCoy J.P., Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ciccia A., Elledge S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gabrielsson J., Weiner D. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications. 5th ed. Swedish Pharmaceutical Press; Stockholm, Sweden: 2016. [Google Scholar]
- 283.Lu H.C., Mackie K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry. 2016;79:516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Newell-Price J., Bertagna X., Grossman A.B., Nieman L.K. Cushing’s syndrome. Lancet. 2006;367:1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 285.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 286.Poljsak B., Šuput D., Milisav I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hetz C., Zhang K., Kaufman R.J. Mechanisms, regulation, and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020;21:421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Huotari J., Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 290.Copeland R.A., Pompliano D.L., Meek T.D. Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discov. 2006;5:730–739. doi: 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- 291.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 292.Lu P., Takai K., Weaver V.M., Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jeong J.H., Chang J.S., Jo Y.H. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front. Endocrinol. 2018;9:384. doi: 10.3389/fendo.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Perfetto S.P., Chattopadhyay P.K., Roederer M. Seventeen-colour flow cytometry: Unravelling the immune system. Nat. Rev. Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 295.Macarron R., Banks M.N., Bojanic D., Burns D.J., Cirovic D.A., Garyantes T., Green D.V., Hertzberg R.P., Janzen W.P., Paslay J.W., et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011;10:188–195. doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- 296.Quackenbush J. Microarray analysis and tumor classification. N. Engl. J. Med. 2006;354:2463–2472. doi: 10.1056/NEJMra042342. [DOI] [PubMed] [Google Scholar]
- 297.Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 298.Kampinga H.H., Hageman J., Vos M.J., Kubota H., Tanguay R.M., Bruford E.A., Cheetham M.E., Chen B., Hightower L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rosen E.D., Spiegelman B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gambhir S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 303.Van Schouwenburg P.A., Rispens T., Wolbink G.J. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 2013;9:164–172. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 304.Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 305.Jan L.Y., Jan Y.N. Structural elements involved in specific K+ channel functions. Nature. 1990;346:672–675. doi: 10.1038/345672a0. [DOI] [PubMed] [Google Scholar]
- 306.Muckenthaler M.U., Rivella S., Hentze M.W., Galy B. A red carpet for iron metabolism. Cell. 2017;168:344–361. doi: 10.1016/j.cell.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Schreiber G., Keating A.E. Protein binding specificity versus promiscuity. Curr. Opin. Struct. Biol. 2011;21:50–61. doi: 10.1016/j.sbi.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wenk M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 309.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Platt F.M., d’Azzo A., Davidson B.L., Neufeld E.F., Tifft C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers. 2018;4:27. doi: 10.1038/s41572-018-0025-4. [DOI] [PubMed] [Google Scholar]
- 311.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.DeBerardinis R.J., Thompson C.B. Cellular metabolism and disease: What do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rupaimoole R., Slack F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 314.Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Scotti M.M., Swanson M.S. RNA mis-splicing in disease. Nat. Rev. Genet. 2016;17:19–32. doi: 10.1038/nrg.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Franklin R.J., Ffrench-Constant C. Regenerating CNS myelin—From mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017;18:753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 319.Minatohara K., Akiyoshi M., Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front. Mol. Neurosci. 2015;8:78. doi: 10.3389/fnmol.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hinz M., Stein A., Uncini T. Monoamine oxidase inhibition and mood. Neurotox. Res. 2011;21:81–89. [Google Scholar]
- 321.Skaper S.D. Neurotrophic factors: An overview. Methods Mol. Biol. 2018;1727:1–17. doi: 10.1007/978-1-4939-7571-6_1. [DOI] [PubMed] [Google Scholar]
- 322.Förstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Suzuki T., Yamamoto M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015;88 Pt B:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 324.Lane A.N., Fan T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43:2466–2485. doi: 10.1093/nar/gkv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wallace D.C. Mitochondria and cancer. Nat. Rev. Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Griffiths W.J., Wang Y. Oxysterol research: A brief review. Biochem. Soc. Trans. 2019;47:517–526. doi: 10.1042/BST20180135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Relling M.V., Evans W.E. Pharmacogenomics in the clinic. Nature. 2015;526:343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Cohen P. The role of protein phosphorylation in human health and disease. Eur. J. Biochem. 2001;268:5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- 331.Bedford L., Paine S., Sheppard P.W., Mayer R.J., Roelofs J. Assembly, structure, and function of the 26S proteasome. Trends Cell. Biol. 2010;20:391–401. doi: 10.1016/j.tcb.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lobo E.D., Hansen R.J., Balthasar J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 334.Coppé J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Houtkooper R.H., Pirinen E., Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Shay J.W., Wright W.E. Role of telomeres and telomerase in cancer. Semin. Cancer Biol. 2011;21:349–353. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Turner J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Watt F.M., Driskell R.R. The therapeutic potential of stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:155–163. doi: 10.1098/rstb.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 341.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 342.Ferrara N., Adamis A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016;15:385. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 343.Keystone E., Heijde D., Mason D., Jr., Landewé R., Vollenhoven R.V., Combe B. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: Findings of a fifty-two–week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–3329. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 344.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. Update in Nature 2017, 546, 564. [DOI] [PubMed] [Google Scholar]
- 345.Heier J.S., Brown D.M., Chong V., Korobelnik J.F., Kaiser P.K., Nguyen Q.D., Kirchhof B., Ho A., Ogura Y., Yancopoulos G.D., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. Erratum in Ophthalmology 2013, 120, 209–210. [DOI] [PubMed] [Google Scholar]
- 346.Schiffmann R., Kopp J.B., Austin H.A., 3rd, Sabnis S., Moore D.F., Weibel T., Balow J.E., Brady R.O. Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 347.Eng C.M., Guffon N., Wilcox W.R., Germain D.P., Lee P., Waldek S., Caplan L., Linthorst G.E., Desnick R.J., International Collaborative Fabry Disease Study Group Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 348.GlaxoSmithKline . Tanzeum (Albiglutide) U.S. Food and Drug Administration; Silver Spring, MA, USA: 2014. [Package Insert] [Google Scholar]
- 349.Santagostino E., Martinowitz U., Lissitchkov T., Pan-Petesch B., Hanabusa H., Oldenburg J., Boggio L., Negrier C., Pabinger I., von Depka Prondzinski M., et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: Results of a phase 3 trial. Blood. 2016;127:1761–1769. doi: 10.1182/blood-2015-09-669234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Atkins M.B., Kunkel L., Sznol M., Rosenberg S.A. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: Long-term survival update. Cancer J. Sci. Am. 2000;6((Suppl. 1)):S11–S14. [PubMed] [Google Scholar]
- 351.Krueger G.G., Langley R.G., Leonardi C., Yeilding N., Guzzo C., Wang Y., Dooley L.T., Lebwohl M., CNTO 1275 Psoriasis Study Group A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N. Engl. J. Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 352.Cohen J.A., Coles A.J., Arnold D.L., Confavreux C., Fox E.J., Hartung H.P., Havrdova E., Selmaj K.W., Weiner H.L., Fisher E., et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 353.Barton N.W., Brady R.O., Dambrosia J.M., Di Bisceglie A.M., Doppelt S.H., Hill S.C., Mankin H.J., Murray G.J., Parker R.I., Argoff C.E., et al. Replacement therapy for inherited enzyme deficiency—Macrophage-targeted glucocerebrosidase for Gaucher’s disease. N. Engl. J. Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 354.Robinson J.G., Farnier M., Krempf M., Bergeron J., Luc G., Averna M., Stroes E.S., Langslet G., Raal F.J., El Shahawy M., et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 355.Hubbard R.C., Sellers S., Czerski D., Stephens L., Crystal R.G. Biochemical efficacy and safety of monthly augmentation therapy for alpha 1-antitrypsin deficiency. JAMA. 1988;260:1259–1264. doi: 10.1001/jama.1988.03410090091037. [DOI] [PubMed] [Google Scholar]
- 356.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 357.Moores S.L., Chiu M.L., Bushey B.S., Chevalier K., Luistro L., Dorn K., Brezski R.J., Haytko P., Kelly T., Wu S.J., et al. A Novel Bispecific Antibody Targeting EGFR and cMet Is Effective against EGFR Inhibitor-Resistant Lung Tumors. Cancer Res. 2016;76:3942–3953. doi: 10.1158/0008-5472.CAN-15-2833. [DOI] [PubMed] [Google Scholar]
- 358.Fleischmann R.M., Tesser J., Schiff M.H., Schechtman J., Burmester G.R., Bennett R., Modafferi D., Zhou L., Bell D., Appleton B. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:1006–1012. doi: 10.1136/ard.2005.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bensinger W.I., Longin K., Appelbaum F., Rowley S., Weaver C., Lilleby K., Gooley T., Lynch M., Higano T., Klarnet J. Peripheral blood stem cells (PBSCs) collected after recombinant granulocyte colony stimulating factor (rhG-CSF): An analysis of factors correlating with the tempo of engraftment after transplantation. Br. J. Haematol. 1994;87:825–831. doi: 10.1111/j.1365-2141.1994.tb06744.x. [DOI] [PubMed] [Google Scholar]
- 360.Connolly S.J., Milling TJJr Eikelboom J.W., Gibson C.M., Curnutte J.T., Gold A., Bronson M.D., Lu G., Conley P.B., Verhamme P., Schmidt J., et al. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med. 2016;375:1131–1141. doi: 10.1056/NEJMoa1607887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Furie R., Khamashta M., Merrill J.T., Werth V.P., Kalunian K., Brohawn P., Illei G.G., Drappa J., Wang L., Yoo S., et al. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69:376–386. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Neuhaus K.L., Tebbe U., Gottwik M., Weber M.A., Feuerer W., Niederer W., Haerer W., Praetorius F., Grosser K.D., Huhmann W. Intravenöse Infusion von recombinant tissue plasminogen activator (rt-PA) und Urokinase beim akuten Myokardinfarkt: Zwischenergebnisse der G.A.U.S.-Studie (German Activator Urokinase Study) [Intravenous infusion of recombinant tissue-type plasminogen activator (rt-PA) and urokinase in acute myocardial infarct: Intermediate results of the G.A.U.S. study (German Activator Urokinase Study)] Klin. Wochenschr. 1988;66((Suppl. 12)):102–108. [PubMed] [Google Scholar]
- 363.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kaufman H.L., Russell J., Hamid O., Bhatia S., Terheyden P., D’Angelo S.P., Shih K.C., Lebbé C., Linette G.P., Milella M., et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Criner G.J., Celli B.R., Brightling C.E., Agusti A., Papi A., Singh D., Sin D.D., Vogelmeier C.F., Sciurba F.C., Bafadhel M., et al. Benralizumab for the Prevention of COPD Exacerbations. N. Engl. J. Med. 2019;381:1023–1034. doi: 10.1056/NEJMoa1905248. [DOI] [PubMed] [Google Scholar]
- 367.Frew J.W., Navrazhina K., Grand D., Sullivan-Whalen M., Gilleaudeau P., Garcet S., Ungar J., Krueger J.G. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. J. Am. Acad. Dermatol. 2020;83:1341–1348. doi: 10.1016/j.jaad.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ferrara N., Chen H., Davis-Smyth T., Gerber H.P., Nguyen T.N., Peers D., Chisholm V., Hillan K.J., Schwall R.H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- 369.Wilcox M.H., Gerding D.N., Poxton I.R., Kelly C., Nathan R., Birch T., Cornely O.A., Rahav G., Bouza E., Lee C., et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017;376:305–317. doi: 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 370.Glatt S., Baeten D., Baker T., Griffiths M., Ionescu L., Lawson A.D.G., Maroof A., Oliver R., Popa S., Strimenopoulou F., et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: Evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann. Rheum. Dis. 2018;77:523–532. doi: 10.1136/annrheumdis-2017-212127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lincoff A.M., Bittl J.A., Harrington R.A., Feit F., Kleiman N.S., Jackman J.D., Sarembock I.J., Cohen D.J., Spriggs D., Ebrahimi R., et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–863. doi: 10.1001/jama.289.7.853. Erratum in JAMA 2003, 289, 1638. [DOI] [PubMed] [Google Scholar]
- 372.Topp M.S., Gökbuget N., Stein A.S., Zugmaier G., O’Brien S., Bargou R.C., Dombret H., Fielding A.K., Heffner L., Larson R.A., et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 373.Katagiri T., Watabe T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016;8:a021899. doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Carruthers A., Carruthers J. Botulinum toxin type A. J. Am. Acad. Dermatol. 2005;53:284–290. doi: 10.1016/j.jaad.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 375.Lew M.F., Adornato B.T., Duane D.D., Dykstra D.D., Factor S.A., Massey J.M., Brin M.F., Jankovic J., Rodnitzky R.L., Singer C., et al. Botulinum toxin type B: A double-blind, placebo-controlled, safety and efficacy study in cervical dystonia. Neurology. 1997;49:701–707. doi: 10.1212/WNL.49.3.701. [DOI] [PubMed] [Google Scholar]
- 376.Tap W.D., Jones R.L., Van Tine B.A., Chmielowski B., Elias A.D., Adkins D., Agulnik M., Cooney M.M., Livingston M.B., Pennock G., et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: An open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Dugel P.U., Koh A., Ogura Y., Jaffe G.J., Schmidt-Erfurth U., Brown D.M., Gomes A.V., Warburton J., Weichselberger A., Holz F.G., et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 378.Carpenter T.O., Whyte M.P., Imel E.A., Boot A.M., Högler W., Linglart A., Padidela R., Van’t Hoff W., Mao M., Chen C.Y., et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N. Engl. J. Med. 2018;378:1987–1998. doi: 10.1056/NEJMoa1714641. [DOI] [PubMed] [Google Scholar]
- 379.Hijiya N., Stewart C.F., Zhou Y., Campana D., Coustan-Smith E., Rivera G.K., Relling M.V., Pui C.H., Gajjar A. Phase II study of topotecan in combination with dexamethasone, asparaginase, and vincristine in pediatric patients with acute lymphoblastic leukemia in first relapse. Cancer. 2008;112:1983–1991. doi: 10.1002/cncr.23395. [DOI] [PubMed] [Google Scholar]
- 380.Ruperto N., Brunner H.I., Quartier P., Constantin T., Wulffraat N., Horneff G., Brik R., McCann L., Kasapcopur O., Rutkowska-Sak L., et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012;367:2396–2406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 381.Fraser R.D. Chymopapain for chemonucleolysis. Orthop. Clin. N. Am. 2002;33:375–382. [Google Scholar]
- 382.Ragni M.V., Pasi K.J., White G.C., Giangrande P.L., Courter S.G., Tubridy K.L., Recombinant FIX Surgical Study Group Use of recombinant factor IX in subjects with haemophilia B undergoing surgery. Haemophilia. 2002;8:91–97. doi: 10.1046/j.1365-2516.2002.00587.x. [DOI] [PubMed] [Google Scholar]
- 383.Hedner U. Recombinant activated factor VII: 30 years of research and innovation. Blood Rev. 2015;29:81–89. doi: 10.1016/S0268-960X(15)30002-3. [DOI] [PubMed] [Google Scholar]
- 384.Falabella A.F., Carson P. The use of enzymatic agents in the treatment of chronic ulcers. Clin. Dermatol. 1996;14:59–65. [Google Scholar]
- 385.Zuraw B.L., Busse P.J., White M., Jacobs J., Lumry W., Baker J., Craig T., Grant J.A., Hurewitz D., Bielory L., et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N. Engl. J. Med. 2010;363:513–522. doi: 10.1056/NEJMoa0805538. [DOI] [PubMed] [Google Scholar]
- 386.Kerrigan J.R., Veldhuis J.D. Dose response of arginine vasopressin to ACTH in man. J. Clin. Endocrinol. Metab. 1990;70:216–220. [Google Scholar]
- 387.Ospina N.S., Al Nofal A., Bancos I., Javed A., Benkhadra K., Kapoor E., Lteif A.N., Natt N., Murad M.H. ACTH Stimulation Tests for the Diagnosis of Adrenal Insufficiency: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2016;101:427–434. doi: 10.1210/jc.2015-1700. [DOI] [PubMed] [Google Scholar]
- 388.Ataga K.I., Kutlar A., Kanter J., Liles D., Cancado R., Friedrisch J., Guthrie T.H., Knight-Madden J., Alvarez O.A., Gordeuk V.R., et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Macdougall I.C., Cooper A.C. The inflammatory response and epoetin sensitivity. Nephrol. Dial. Transplant. 2002;17((Suppl. 1)):48–52. doi: 10.1093/ndt/17.suppl_1.48. [DOI] [PubMed] [Google Scholar]
- 390.Eastell R., Christiansen C., Grauer A., Kutilek S., Libanati C., McClung M.R., Reid I.R., Resch H., Siris E., Uebelhart D., et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011;26:530–537. doi: 10.1002/jbmr.251. [DOI] [PubMed] [Google Scholar]
- 391.Harb H., Chatila T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2020;50:5–14. doi: 10.1111/cea.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., Kurata T., Chiappori A., Lee K.H., de Wit M., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 393.Hillmen P., Young N.S., Schubert J., Brodsky R.A., Socié G., Muus P., Röth A., Szer J., Elebute M.O., Nakamura R., et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 394.Riethmüller G., Schneider-Gädicke E., Schlimok G., Schmiegel W., Raab R., Höffken K., Gruber R., Pichlmaier H., Hirche H., Pichlmayr R. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes’ C colorectal carcinoma. German Cancer Aid 17-1A Study Group. Lancet. 1994;343:1177–1183. doi: 10.1016/S0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 395.Gordon K.B., Papp K.A., Hamilton T.K., Walicke P.A., Dummer W., Li N., Bresnahan B.W., Menter A., Efalizumab Study Group Efalizumab for patients with moderate to severe plaque psoriasis: A randomized controlled trial. JAMA. 2003;290:3073–3080. doi: 10.1001/jama.290.23.3073. Erratum in JAMA 2004, 291, 1070. [DOI] [PubMed] [Google Scholar]
- 396.Howard J.F., Jr., Bril V., Burns T.M., Mantegazza R., Bilinska M., Szczudlik A., Beydoun S., Garrido F.J.R.R., Piehl F., Rottoli M., et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92:e2661–e2673. doi: 10.1212/WNL.0000000000007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Onodera M., Uchiyama T., Ariga T., Yamada M., Miyamura T., Arizono H., Morio T. Safety and efficacy of elapegademase in patients with adenosine deaminase deficiency: A multicenter, open-label, single-arm, phase 3, and postmarketing clinical study. Immun. Inflamm. Dis. 2023;11:e917. doi: 10.1002/iid3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Hendriksz C.J., Burton B., Fleming T.R., Harmatz P., Hughes D., Jones S.A., Lin S.P., Mengel E., Scarpa M., Valayannopoulos V., et al. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): A phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014;37:979–990. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Lonial S., Dimopoulos M., Palumbo A., White D., Grosicki S., Spicka I., Walter-Croneck A., Moreau P., Mateos M.V., Magen H., et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 400.Locatelli F., Jordan M.B., Allen C., Cesaro S., Rizzari C., Rao A., Degar B., Garrington T.P., Sevilla J., Putti M.C., et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- 401.Oldenburg J., Mahlangu J.N., Kim B., Schmitt C., Callaghan M.U., Young G., Santagostino E., Kruse-Jarres R., Negrier C., Kessler C., et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N. Engl. J. Med. 2017;377:809–818. doi: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 402.Rosenberg J.E., O’Donnell P.H., Balar A.V., McGregor B.A., Heath E.I., Yu E.Y., Galsky M.D., Hahn N.M., Gartner E.M., Pinelli J.M., et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019;37:2592–2600. doi: 10.1200/JCO.19.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Goadsby P.J., Reuter U., Hallström Y., Broessner G., Bonner J.H., Zhang F., Sapra S., Picard H., Mikol D.D., Lenz R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017;377:2123–2132. doi: 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- 404.Jelkmann W. Regulation of erythropoietin production. J. Physiol. 2011;589:1251–1258. doi: 10.1113/jphysiol.2010.195057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Mendell J.R., Goemans N., Lowes L.P., Alfano L.N., Berry K., Shao J., Kaye E.M., Mercuri E. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 2016;79:257–271. doi: 10.1002/ana.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A., Kuder J.F., Wang H., Liu T., Wasserman S.M., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 407.Jenkins M., Alexander J.W., MacMillan B.G., Waymack J.P., Kopcha R. Failure of topical steroids and vitamin E to reduce postoperative scar formation following reconstructive surgery. J. Burn Care Rehabil. 1986;7:309–312. doi: 10.1097/00004630-198607000-00002. [DOI] [PubMed] [Google Scholar]
- 408.Crawford J., Becker P.S. Role of the hematologist in the management of G-CSF–associated leukemia. Blood. 2013;121:509–511. [Google Scholar]
- 409.van Wely M., Kwan I., Burt A.L., Thomas J., Vail A., Van der Veen F., Al-Inany H.G. Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles. Cochrane Database Syst. Rev. 2011;2011:CD005354. doi: 10.1002/14651858.CD005354.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Silberstein S.D., Dodick D.W., Bigal M.E., Yeung P.P., Goadsby P.J., Blankenbiller T., Grozinski-Wolff M., Yang R., Ma Y., Aycardi E. Fremanezumab for the preventive treatment of chronic migraine. N. Engl. J. Med. 2017;377:2113–2122. doi: 10.1056/NEJMoa1709038. [DOI] [PubMed] [Google Scholar]
- 411.Stauffer V.L., Dodick D.W., Zhang Q., Carter J.N., Ailani J., Conley R.R. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75:1080–1088. doi: 10.1001/jamaneurol.2018.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Stauffer V.L. A phase 3 placebo-controlled study of galcanezumab in patients with chronic migraine: Results from the 3-month double-blind treatment phase of the REGAIN study. J. Neurol. Neurosurg. Psychiatry. 2018;89:603–611. [Google Scholar]
- 413.Harmatz P., Whitley C.B., Waber L., Pais R., Steiner R., Plecko B., Kaplan P., Simon J., Butensky E., Hopwood J.J. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) J. Pediatr. 2004;144:574–580. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 414.Larson R.A., Sievers E.L., Stadtmauer E.A., Löwenberg B., Estey E.H., Dombret H., Theobald M., Voliotis D., Bennett J.M., Richie M., et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 415.Ashida S., Nishimori I., Tanimura M., Onishi S., Shuin T. Effects of von Hippel-Lindau gene mutation and methylation status on expression of transmembrane carbonic anhydrases in renal cell carcinoma. J. Cancer Res. Clin. Oncol. 2002;128:561–568. doi: 10.1007/s00432-002-0374-x. [DOI] [PubMed] [Google Scholar]
- 416.Johnson K.P., Brooks B.R., Cohen J.A., Ford C.C., Goldstein J., Lisak R.P., Myers L.W., Panitch H.S., Rose J.W., Schiffer R.B. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/WNL.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 417.Heller S.R., Cryer P.E. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 418.Widemann B.C., Balis F.M., Kim A., Boron M., Jayaprakash N., Shalabi A., O’Brien M., Eby M., Cole D.E., Murphy R.F., et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J. Clin. Oncol. 2010;28:3979–3986. doi: 10.1200/JCO.2009.25.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Kavanaugh A., McInnes I., Mease P., Krueger G.G., Gladman D., Gomez-Reino J., Papp K., Zrubek J., Mudivarthy S., Mack M., et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–986. doi: 10.1002/art.24403. Erratum in Arthritis Rheum. 2010, 62, 2555. [DOI] [PubMed] [Google Scholar]
- 420.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm. IGF Res. 2001;11:113–170. doi: 10.1016/S1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 421.McInnes I.B., Rahman P., Gottlieb A.B., Hsia E.C., Kollmeier A.P., Chakravarty S.D., Xu X.L., Subramanian R.A., Agarwal P., Sheng S., et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo-and active comparator–controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 2017;76:405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 422.Zuraw B.L., Kalfus I. Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. Am. J. Med. 2012;125:e1–e7. doi: 10.1016/j.amjmed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 423.Riedl M. Recombinant human C1 esterase inhibitor in the management of hereditary angioedema. Clin. Drug Investig. 2015;35:407–417. doi: 10.1007/s40261-015-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Cree B.A.C., Bennett J.L., Kim H.J., Weinshenker B.G., Pittock S.J., Wingerchuk D.M., Fujihara K., Paul F., Cutter G.R., Marignier R., et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): A double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394:1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 425.Tracey D., Klareskog L., Sasso E.H., Salfeld J.G., Tak P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 426.Kantarjian H.M., DeAngelo D.J., Stelljes M., Martinelli G., Liedtke M., Stock W., Gökbuget N., O’Brien S., Wang K., Wang T., et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016;375:740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 428.Gibson S.J., Imbert-Fernandez Y. Type I interferons and the innate immune response—More than just antiviral cytokines. Mol. Med. Rep. 2014;9:869–877. doi: 10.1016/j.molimm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 429.Attal M., Richardson P.G., Rajkumar S.V., San-Miguel J., Beksac M., Spicka I., Leleu X., Schjesvold F., Moreau P., Dimopoulos M.A., et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096–2107. doi: 10.1016/S0140-6736(19)32556-5. Erratum in Lancet 2019, 394, 2072. [DOI] [PubMed] [Google Scholar]
- 430.Moots R.J., Curiale C., Petersel D., Rolland C., Jones H., Mysler E. Efficacy and Safety Outcomes for Originator TNF Inhibitors and Biosimilars in Rheumatoid Arthritis and Psoriasis Trials: A Systematic Literature Review. BioDrugs. 2018;32:193–199. doi: 10.1007/s40259-018-0283-4. [DOI] [PubMed] [Google Scholar]
- 431.Griffiths C.E., Reich K., Lebwohl M., van de Kerkhof P., Paul C., Menter A., Cameron G.S., Erickson J., Zhang L., Secrest R.J., et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet. 2015;386:541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 432.Banerji A., Riedl M.A., Bernstein J.A., Cicardi M., Longhurst H.J., Zuraw B.L., Busse P.J., Anderson J., Magerl M., Martinez-Saguer I., et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: A randomized clinical trial. JAMA. 2018;320:2108–2121. doi: 10.1001/jama.2018.16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Banerji A., Busse P., Shennak M., Lumry W., Davis-Lorton M., Wedner H.J., Jacobs J., Baker J., Bernstein J.A., Lockey R., et al. Inhibiting Plasma Kallikrein for Hereditary Angioedema Prophylaxis. N. Engl. J. Med. 2017;376:717–728. doi: 10.1056/NEJMoa1605767. [DOI] [PubMed] [Google Scholar]
- 434.Kakkis E.D., Muenzer J., Tiller G.E., Waber L., Belmont J., Passage M., Izykowski B., Phillips J., Doroshow R., Walot I., et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 435.Greinacher A., Janssens U., Berg G., Böck M., Kwasny H., Kemkes-Matthes B., Eichler P., Völpel H., Pötzsch B., Luz M. Lepirudin (recombinant hirudin) for parenteral anticoagulation in patients with heparin-induced thrombocytopenia. Heparin-Associated Thrombocytopenia Study (HAT) investigators. Circulation. 1999;100:587–593. doi: 10.1161/01.CIR.100.6.587. [DOI] [PubMed] [Google Scholar]
- 436.Klotz L., Boccon-Gibod L., Shore N.D., Andreou C., Persson B.E., Cantor P., Jensen J.K., Olesen T.K., Schröder F.H. The efficacy and safety of degarelix: A 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 437.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Hamadani M., Radford J., Carlo-Stella C., Caimi P.F., Reid E., O’Connor O.A., Feingold J.M., Ardeshna K.M., Townsend W., Solh M., et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021;137:2634–2645. doi: 10.1182/blood.2020007512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Moya F.R., Gadzinowski J., Bancalari E., Salinas V., Kopelman B., Bancalari A., Kornacka M.K., Merritt T.A., Segal R., Schaber C.J., et al. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005;115:1018–1029. doi: 10.1542/peds.2004-2183. [DOI] [PubMed] [Google Scholar]
- 440.Oh H.S., Park E.J., Lee T.S., An Y., Kim S.Y., Shin S.Y., Shin B.S. Pharmacokinetics of lixisenatide, a GLP-1 receptor agonist, determined by a novel liquid Chromatography–Tandem mass spectrometry analysis in rats. Separations. 2023;10:282. doi: 10.3390/separations10050282. [DOI] [Google Scholar]
- 441.Piga A., Perrotta S., Gamberini M.R., Voskaridou E., Melpignano A., Filosa A., Caruso V., Pietrangelo A., Longo F., Tartaglione I., et al. Luspatercept improves hemoglobin levels and blood transfusion requirements in a study of patients with β-thalassemia. Blood. 2019;133:1279–1289. doi: 10.1182/blood-2018-10-879247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Mochtar M.H., Danhof N.A., Ayeleke R.O., Van der Veen F., van Wely M. Recombinant luteinizing hormone (rLH) and recombinant follicle stimulating hormone (rFSH) for ovarian stimulation in IVF/ICSI cycles. Cochrane Database Syst Rev. 2017;5:CD005070. doi: 10.1002/14651858.CD005070.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Rugo H.S., Im S.A., Cardoso F., Cortés J., Curigliano G., Musolino A., Pegram M.D., Wright G.S., Saura C., Escrivá-de-Romaní S., et al. Efficacy of Margetuximab vs Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Chernausek S.D., Backeljauw P.F., Frane J., Kuntze J., Underwood L.E. GH Insensitivity Syndrome Collaborative Group. Long-term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-I deficiency due to growth hormone insensitivity. J. Clin. Endocrinol. Metab. 2007;92:902–910. doi: 10.1210/jc.2006-1610. [DOI] [PubMed] [Google Scholar]
- 445.Recombinant Human FSH Product Development Group Recombinant follicle stimulating hormone: Development of the first biotechnology product for the treatment of infertility. Hum. Reprod. Update. 1998;4:862–881. doi: 10.1093/humupd/4.6.862. [DOI] [PubMed] [Google Scholar]
- 446.Ortega H.G., Liu M.C., Pavord I.D., Brusselle G.G., FitzGerald J.M., Chetta A., Humbert M., Katz L.E., Keene O.N., Yancey S.W., et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. Erratum in N. Engl. J. Med. 2015, 372, 1777. [DOI] [PubMed] [Google Scholar]
- 447.Chan J.L., Heist K., DePaoli A.M., Veldhuis J.D., Mantzoros C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003;111:1409–1421. doi: 10.1172/JCI200317490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Moore K.N., Martin L.P., O’Malley D.M., Matulonis U.A., Konner J.A., Perez R.P., Bauer T.M., Ruiz-Soto R., Birrer M.J. Safety and Activity of Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha-Targeting Antibody-Drug Conjugate, in Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer: A Phase I Expansion Study. J. Clin. Oncol. 2017;35:1112–1118. doi: 10.1200/JCO.2016.69.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Mizukami Y., Kono K., Kawaguchi Y., Akaike H., Kamimura K., Sugai H., Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 450.Kreitman R.J., Dearden C., Zinzani P.L., Delgado J., Karlin L., Robak T., Gladstone D.E., le Coutre P., Dietrich S., Gotic M., et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768–1777. doi: 10.1038/s41375-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Ortho Multicenter Transplant Study Group A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. N. Engl. J. Med. 1985;313:337–342. doi: 10.1056/NEJM198508083130601. [DOI] [PubMed] [Google Scholar]
- 452.Polman C.H., O’Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H., Phillips J.T., Lublin F.D., Giovannoni G., Wajgt A., et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 453.Kushner B.H., Cheung I.Y., Modak S., Basu E.M., Roberts S.S., Cheung N.K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing with Granulocyte-Macrophage Colony-Stimulating Factor in Patients with Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018;4:1729–1735. doi: 10.1001/jamaoncol.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Thatcher N., Hirsch F.R., Luft A.V., Szczesna A., Ciuleanu T.E., Dediu M., Ramlau R., Galiulin R.K., Bálint B., Losonczy G., et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): An open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 455.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 456.Erdes S., Nasonov E., Kunder E., Pristrom A., Soroka N., Shesternya P., Dubinina T., Smakotina S., Raskina T., Krechikova D., et al. Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults. Clin. Exp. Rheumatol. 2020;38:27–34. [PubMed] [Google Scholar]
- 457.Reddy B.K., Lokesh V., Vidyasagar M.S., Shenoy K., Babu K.G., Shenoy A., Naveen T., Joseph B., Bonanthaya R., Nanjundappa Bapsy P.P., et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: A randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol. 2014;50:498–505. doi: 10.1016/j.oraloncology.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 458.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Koprowski H., Herlyn M., Steplewski Z., Sears H.F. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 460.Migone T.S., Subramanian G.M., Zhong J., Healey L.M., Corey A., Devalaraja M., Lo L., Ullrich S., Zimmerman J., Chen A., et al. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 2009;361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 461.Stalmans P., Benz M.S., Gandorfer A., Kampik A., Girach A., Pakola S., Haller J.A., MIVI-TRUST Study Group Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N. Engl. J. Med. 2012;367:606–615. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 462.Hauser S.L., Bar-Or A., Comi G., Giovannoni G., Hartung H.P., Hemmer B., Lublin F., Montalban X., Rammohan K.W., Selmaj K., et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 463.Papp K.A., Reich K., Paul C., Blauvelt A., Baran W., Bolduc C., Toth D., Langley R.G., Cather J., Gottlieb A.B., et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 2016;175:273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- 464.Wasserstein M.P., Diaz G.A., Lachmann R.H., Jouvin M.H., Nandy I., Ji A.J., Puga A.C. Olipudase alfa for treatment of acid sphingomyelinase deficiency (ASMD): Safety and efficacy in adults treated for 30 months. J. Inherit. Metab. Dis. 2018;41:829–838. doi: 10.1007/s10545-017-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Holgate S.T., Chuchalin A.G., Hébert J., Lötvall J., Persson G.B., Chung K.F., Bousquet J., Kerstjens H.A., Fox H., Thirlwell J., et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin. Exp. Allergy. 2004;34:632–638. doi: 10.1111/j.1365-2222.2004.1916.x. [DOI] [PubMed] [Google Scholar]
- 466.Dinney C.P., Greenberg R.E., Steinberg G.D. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol. Oncol. 2013;31:1635–1642. doi: 10.1016/j.urolonc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 467.Wilde M.I., Faulds D. Oprelvekin: A review of its pharmacology and therapeutic potential in chemotherapy-induced thrombocytopenia. BioDrugs. 1998;10:159–171. doi: 10.2165/00063030-199810020-00006. [DOI] [PubMed] [Google Scholar]
- 468.Gimpl G., Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 469.Spielberger R., Stiff P., Bensinger W., Gentile T., Weisdorf D., Kewalramani T., Shea T., Yanovich S., Hansen K., Noga S., et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N. Engl. J. Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 470.Whitcomb D.C., Lehman G.A., Vasileva G. Pancrelipase delayed-release capsules (CREON) for exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery: A double-blind randomized trial. Am. J. Gastroenterol. 2010;105:2276–2286. doi: 10.1038/ajg.2010.201. [DOI] [PubMed] [Google Scholar]
- 471.Douillard J.Y., Oliner K.S., Siena S., Tabernero J., Burkes R., Barugel M., Humblet Y., Bodoky G., Cunningham D., Jassem J., et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 472.Neer R.M., Arnaud C.D., Zanchetta J.R., Prince R., Gaich G.A., Reginster J.Y., Hodsman A.B., Eriksen E.F., Ish-Shalom S., Genant H.K., et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 473.Polmar S.H. Enzyme replacement and other biochemical approaches to the therapy of adenosine deaminase deficiency. Ciba Found. Symp. 1978;68:213–230. doi: 10.1002/9780470720516.ch14. [DOI] [PubMed] [Google Scholar]
- 474.Avramis V.I., Sencer S., Periclou A.P., Sather H., Bostrom B.C., Cohen L.J., Ettinger A.G., Ettinger L.J., Franklin J., Gaynon P.S., et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: A Children’s Cancer Group study. Blood. 2002;99:1986–1994. doi: 10.1182/blood.V99.6.1986. Erratum in Blood 2002, 100, 1531. [DOI] [PubMed] [Google Scholar]
- 475.Dmytrijuk A., Robie-Suh K., Cohen M.H., Rieves D., Weiss K., Pazdur R. FDA report: Eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist. 2008;13:993–1000. doi: 10.1634/theoncologist.2008-0086. [DOI] [PubMed] [Google Scholar]
- 476.Manns M.P., McHutchison J.G., Gordon S.C., Rustgi V.K., Shiffman M., Reindollar R., Goodman Z.D., Koury K., Ling M., Albrecht J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 477.Fried M.W., Shiffman M.L., Reddy K.R., Smith C., Marinos G., Gonçales FLJr Häussinger D., Diago M., Carosi G., Dhumeaux D., Craxi A., et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 478.Sundy J.S., Baraf H.S., Yood R.A., Edwards N.L., Gutierrez-Urena S.R., Treadwell E.L., Vázquez-Mellado J., White W.B., Lipsky P.E., Horowitz Z., et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: Two randomized controlled trials. JAMA. 2011;306:711–720. doi: 10.1001/jama.2011.1169. [DOI] [PubMed] [Google Scholar]
- 479.Trainer P.J., Drake W.M., Katznelson L., Freda P.U., Herman-Bonert V., van der Lely A.J., Dimaraki E.V., Stewart P.M., Friend K.E., Vance M.L., et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N. Engl. J. Med. 2000;342:1171–1177. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- 480.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Baselga J., Cortés J., Kim S.B., Im S.A., Hegg R., Im Y.H., Roman L., Pedrini J.L., Pienkowski T., Knott A., et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Ishii K., Morii N., Yamashiro H. Pertuzumab in the treatment of HER2-positive breast cancer: An evidence-based review of its safety, efficacy, and place in therapy. Core Evid. 2019;14:51–70. doi: 10.2147/CE.S217848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Ratner R., Whitehouse F., Fineman M.S., Strobel S., Shen L., Maggs D.G., Kolterman O.G., Weyer C. Adjunctive therapy with pramlintide lowers HbA1c without concomitant weight gain and increased risk of severe hypoglycemia in patients with type 1 diabetes approaching glycemic targets. Exp. Clin. Endocrinol. Diabetes. 2005;113:199–204. doi: 10.1055/s-2005-837662. [DOI] [PubMed] [Google Scholar]
- 484.Marlar R.A., Gausman J.N. Protein S abnormalities: A diagnostic nightmare. Am. J. Hematol. 2011;86:418–421. doi: 10.1002/ajh.21992. [DOI] [PubMed] [Google Scholar]
- 485.Fuchs C.S., Tomasek J., Yong C.J., Dumitru F., Passalacqua R., Goswami C., Safran H., Dos Santos L.V., Aprile G., Ferry D.R., et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 486.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 487.Pui C.H., Mahmoud H.H., Wiley J.M., Woods G.M., Leverger G., Camitta B., Hastings C., Blaney S.M., Relling M.V., Reaman G.H. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients with leukemia or lymphoma. J. Clin. Oncol. 2001;19:697–704. doi: 10.1200/JCO.2001.19.3.697. [DOI] [PubMed] [Google Scholar]
- 488.Cannon C.P., Weintraub W.S., Demopoulos L.A., Vicari R., Frey M.J., Lakkis N., Neumann F.J., Robertson D.H., DeLucca P.T., DiBattiste P.M., et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N. Engl. J. Med. 2001;344:1879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 489.Sundy J.S., Schumacher H.R., Kivitz A., Weinstein S.P., Wu R., King-Davis S., Evans R.R. Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: Results of RESURGE, a phase III, international safety study. J. Rheumatol. 2014;41:1703–1711. doi: 10.3899/jrheum.131226. [DOI] [PubMed] [Google Scholar]
- 490.Chen D.R., Cohen P.L. Living life without B cells: Is repeated B-cell depletion a safe and effective long-term treatment plan for rheumatoid arthritis? Int. J. Clin. Rheumatol. 2012;7:159–166. doi: 10.2217/ijr.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Bussel J.B., Cheng G., Saleh M.N., Psaila B., Kovaleva L., Meddeb B., Kloczko J., Hassani H., Mayer B., Stone N.L., et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N. Engl. J. Med. 2007;357:2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 492.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., Hofbauer L.C., Lau E., Lewiecki E.M., Miyauchi A., et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 493.Treem W.R., McAdams L., Stanford L., Kastoff G., Justinich C., Hyams J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 1999;28:137–142. doi: 10.1097/00005176-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 494.Nemunaitis J., Rabinowe S.N., Singer J.W., Bierman P.J., Vose J.M., Freedman A.S., Onetto N., Gillis S., Oette D., Gold M. Recombinant granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid cancer. N. Engl. J. Med. 1991;324:1773–1778. doi: 10.1056/NEJM199106203242504. [DOI] [PubMed] [Google Scholar]
- 495.Genovese M.C., Fleischmann R., Kivitz A.J., Rell-Bakalarska M., Martincova R., Fiore S., Rohane P., van Hoogstraten H., Garg A., Fan C., et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: Results of a phase III study. Arthritis Rheumatol. 2015;67:1424–1437. doi: 10.1002/art.39093. [DOI] [PubMed] [Google Scholar]
- 496.Burton B.K., Balwani M., Feillet F., Barić I., Burrow T.A., Camarena Grande C., Coker M., Consuelo-Sánchez A., Deegan P., Di Rocco M., et al. A Phase 3 Trial of Sebelipase Alfa in Lysosomal Acid Lipase Deficiency. N. Engl. J. Med. 2015;373:1010–1020. doi: 10.1056/NEJMoa1501365. [DOI] [PubMed] [Google Scholar]
- 497.Vidon N., Pfeiffer A., Chayvialle J.A., Merite F., Maurel M., Franchisseur C., Huchet B., Bernier J.J. Effect of jejunal infusion of nutrients on gastrointestinal transit and hormonal response in man. Gastroenterol. Clin. Biol. 1989;13:1042–1049. [PubMed] [Google Scholar]
- 498.Walker R.F. Sermorelin: A better approach to management of adult-onset growth hormone insufficiency? Clin. Interv. Aging. 2006;1:307–308. doi: 10.2147/ciia.2006.1.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Kurzrock R., Voorhees P.M., Casper C., Furman R.R., Fayad L., Lonial S., Borghaei H., Jagannath S., Sokol L., Usmani S.Z., et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin. Cancer Res. 2013;19:3659–3670. doi: 10.1158/1078-0432.CCR-12-3349. [DOI] [PubMed] [Google Scholar]
- 500.Rudman D., Feller A.G., Nagraj H.S., Gergans G.A., Lalitha P.Y., Goldberg A.F., Schlenker R.A., Cohn L., Rudman I.W., Mattson D.E. Effects of human growth hormone in men over 60 years old. N. Engl. J. Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 501.Yusuf S., Collins R., Peto R., Furberg C., Stampfer M.J., Goldhaber S.Z., Hennekens C.H. Intravenous and intracoronary fibrinolytic therapy in acute myocardial infarction: Overview of results on mortality, reinfarction and side-effects from 33 randomized controlled trials. Eur. Heart J. 1985;6:556–585. doi: 10.1093/oxfordjournals.eurheartj.a061905. [DOI] [PubMed] [Google Scholar]
- 502.Pemmaraju N., Lane A.A., Sweet K.L., Stein A.S., Vasu S., Blum W., Rizzieri D.A., Wang E.S., Duvic M., Sloan J.M., et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019;380:1628–1637. doi: 10.1056/NEJMoa1815105. [DOI] [PubMed] [Google Scholar]
- 503.Pastores G.M., Petakov M., Giraldo P., Rosenbaum H., Szer J., Deegan P.B., Amato D.J., Mengel E., Tan E.S., Chertkoff R., et al. A Phase 3, multicenter, open-label, switchover trial to assess the safety and efficacy of taliglucerase alfa, a plant cell-expressed recombinant human glucocerebrosidase, in adult and pediatric patients with Gaucher disease previously treated with imiglucerase. Blood Cells Mol. Dis. 2014;53:253–260. doi: 10.1016/j.bcmd.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 504.Jeppesen P.B., Pertkiewicz M., Messing B., Iyer K., Seidner D.L., O’keefe S.J., Forbes A., Heinze H., Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473–1481.e3. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 505.White H.D., Cross D.B., Williams B.F., Norris R.M. Safety and efficacy of repeat thrombolytic treatment after acute myocardial infarction. Br. Heart J. 1990;64:177–181. doi: 10.1136/hrt.64.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Moreau R., Durand F., Poynard T., Duhamel C., Cervoni J.P., Ichaï P., Abergel A., Halimi C., Pauwels M., Bronowicki J.P., et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: A retrospective multicenter study. Gastroenterology. 2002;122:923–930. doi: 10.1053/gast.2002.32364. [DOI] [PubMed] [Google Scholar]
- 507.Falutz J., Potvin D., Mamputu J.C., Assaad H., Zoltowska M., Michaud S.E., Berger D., Somero M., Moyle G., Brown S., et al. Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: A randomized placebo-controlled trial with a safety extension. J. Acquir. Immune Defic. Syndr. 2010;53:311–322. doi: 10.1097/QAI.0b013e3181cbdaff. [DOI] [PubMed] [Google Scholar]
- 508.Zeng Z.C., Tang Z.Y., Wu Z.Q., Ma Z.C., Fan J., Qin L.X., Zhou J., Wang J.H., Wang B.L., Zhong C.S. Phase I clinical trial of oral furtulon and combined hepatic arterial chemoembolization and radiotherapy in unresectable primary liver cancers, including clinicopathologic study. Am. J. Clin. Oncol. 2000;23:449–454. doi: 10.1097/00000421-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 509.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Coskun T., Sloop K.W., Loghin C., Alsina-Fernandez J., Urva S., Bokvist K.B., Cui X., Briere D.A., Cabrera O., Roell W.C., et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Coleman R.L., Lorusso D., Gennigens C., González-Martín A., Randall L., Cibula D., Lund B., Woelber L., Pignata S., Forget F., et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:609–619. doi: 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]
- 512.Nishimoto N., Terao K., Mima T., Nakahara H., Takagi N., Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 513.Vose J.M., Wahl R.L., Saleh M., Rohatiner A.Z., Knox S.J., Radford J.A., Zelenetz A.D., Tidmarsh G.F., Stagg R.J., Kaminski M.S. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin’s lymphomas. J. Clin. Oncol. 2000;18:1316–1323. doi: 10.1200/JCO.2000.18.6.1316. [DOI] [PubMed] [Google Scholar]
- 514.Hudis C.A. Trastuzumab—Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 515.Katsikis I., Kita M., Karkanaki A., Prapas N., Panidis D. Anovulation and ovulation induction. Hippokratia. 2006;10:120–127. [PMC free article] [PubMed] [Google Scholar]
- 516.Goldhaber S.Z., Kessler C.M., Heit J., Markis J., Sharma G.V., Dawley D., Nagel J.S., Meyerovitz M., Kim D., Vaughan D.E. Randomised controlled trial of recombinant tissue plasminogen activator versus urokinase in the treatment of acute pulmonary embolism. Lancet. 1988;2:293–298. doi: 10.1016/S0140-6736(88)92354-9. [DOI] [PubMed] [Google Scholar]
- 517.Krueger J.G., Ferris L.K., Menter A., Wagner F., White A., Visvanathan S., Lalovic B., Aslanyan S., Wang E.E., Hall D., et al. Anti–IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2017;140:172–182. doi: 10.1016/j.jaci.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 518.Russell J.A., Walley K.R., Singer J., Gordon A.C., Hébert P.C., Cooper D.J., Holmes C.L., Mehta S., Granton J.T., Storms M.M., et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N. Engl. J. Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 519.Cherry L.N., Yunker N.S., Lambert E.R., Vaughan D., Lowe D.K. Vedolizumab: An α4β7 integrin antagonist for ulcerative colitis and Crohn’s disease. Ther. Adv. Chronic Dis. 2015;6:224–233. doi: 10.1177/2040622315586970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Ida H., Tanaka A., Matsubayashi T., Murayama K., Hongo T., Lee H.M., Mellgard B. A multicenter, open-label extension study of velaglucerase alfa in Japanese patients with Gaucher disease: Results after a cumulative treatment period of 24months. Blood Cells Mol. Dis. 2016;59:140–147. doi: 10.1016/j.bcmd.2015.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article and Appendix A.