Abstract
We highlighted in this current paper similar prolonged respiratory presentation with COVID-19 pneumonia in four severely immunocompromised patients currently being treated with anti-CD20 monoclonal antibodies (mAbs), such as ocrelizumab and rituximab, for multiple sclerosis or rheumatoid polyarthritis. Real-time reverse transcription-polymerase chain reaction on a nasopharyngeal swab specimen was negative in all patients. SARS-CoV-2 infection was confirmed from bronchoalveolar lavage fluid. A high titer of post-vaccine COVID-19 convalescent plasma was administered with complete recovery in all patients.
Keywords: convalescent plasma, COVID-19, humoral immunity, ocrelizumab, rituximab, SARS-CoV-2
1. Key Bullet Points
Immunocompromised patients with B-cell depletion agents are at risk for persistent SARS-CoV-2 infection.
Negative tests for SARS-CoV-2 pneumonia based on RT-PCR cannot rule out this diagnosis.
We reported a case series of prolonged SARS-CoV-2 infection (with negative nasopharyngeal RT-PCR result) in patients receiving anti-CD20 mAbs who recovered after administration of high-titer post-vaccine COVID-19 convalescent plasma.
COVID-19 convalescent plasma is a promising approach through the transfer of neutralizing antibodies specific to SARS-CoV-2 in patients with B-cell immunosuppression and persistent viral shedding.
2. Introduction
Immunocompromised patients treated with B-cell depletion treatment (e.g. anti-CD20 monoclonal antibodies (mAbs)) have an increased susceptibility to develop severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persistent viral shedding [1]. SARS-CoV-2 infection with respiratory presentation cannot be excluded after performing nasopharyngeal SARS-CoV-2 real-time reverse transcription-polymerase chain reaction (RT-PCR) [2]. Treatments for prolonged coronavirus disease 2019 (COVID-19) are rarely described and there are a lack of recommendations [3,4,5].
In this particular population of patients undergoing anti-CD20 therapy, the T-cell response is conserved [6,7,8], present also against variants [9], and detectable by commercial tests [10]. ‘Prolonged’ SARS-CoV-2 infection was defined as confirmed COVID-19 with symptoms persisting for 1 month or more. We reported herein four cases of confirmed prolonged SARS-CoV-2 infection (despite negative nasopharyngeal RT-PCR result) in patients receiving anti-CD20 mAbs who recovered after administration of high-titer post-vaccine COVID-19 convalescent plasma (CCP).
3. Results
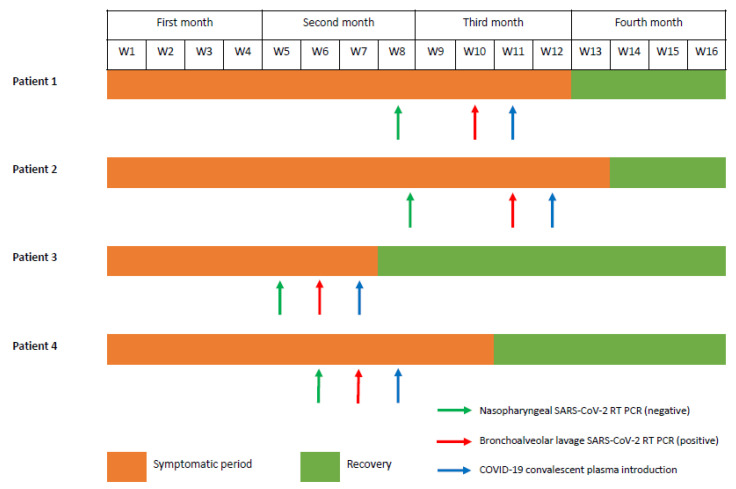
Three of four patients were females with a median age of 54 (43–65) years. All patients were receiving anti-CD20 mAbs, such as ocrelizumab and rituximab, for multiple sclerosis (n = 3) and rheumatoid polyarthritis (n = 1), respectively. Symptoms onset began 48 (33–58) days before hospitalization with fever and upper respiratory symptoms (Figure 1 and Table 1).
Figure 1.
Timeline in patients with prolonged SARS-CoV-2 infection with recovery after COVID-19 convalescent plasma transfusion.
Table 1.
Demographic data, clinical characteristics, laboratory findings, and prescribed treatments in patients with B-cell depletion and prolonged SARS-CoV-2 infection, Nord Franche-Comté Hospital, 2022–2023.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
|---|---|---|---|---|---|
| Patients characteristics | |||||
| Sex | F | F | F | M | |
| Age, y | 43 | 65 | 48 | 61 | |
| Underlying comorbidities | Rheumatoid arthritis | Multiple sclerosis | Multiple sclerosis, diabetes mellitus | Multiple sclerosis | |
| Past history of anti-CD20 mAbs (y) | - | Rituximab (2017–2019) | Rituximab (2017–2019) | Rituximab (2018–2019) | |
| Current anti-CD20 mAbs (y) | Rituximab (since 2016) | Ocrelizumab (since 2019) | Ocrelizumab (since 2019) | Ocrelizumab (since 2019) | |
| Other treatments | Leflunomid, CTC | Sitagliptin | |||
| Clinical features | |||||
| Clinical presentation | Onset date | 15 March 2023 | 28 September 2022 | 25 January 2022 | 28 March 2023 |
| Symptoms | Fever, sweating, weight loss, dyspnea | Fever with ILI, cough, dyspnea | Fever, asthenia, cough, dyspnea, retrosternal pain | Fever, cough, sweating, dyspnea | |
| Sounds heard on pulmonary auscultation | Without abnormalities | Unilateral crackling (left) | Unilateral crackling (left) | Bilateral crackling | |
| Oxygen support | 4 L/min | 0 L/min | 0 L/min | 0 L/min | |
| Laboratory findings and microbiological findings | |||||
| NP SARS-CoV-2 RT-PCR | Days from onset (date) | 56 (10 May 2023) | 58 (26 November 2023) | 33 (27 February 2023) | 46 (13 May 2023) |
| Results | Negative | Negative | Negative | Negative | |
| Serology SARS-CoV-2 | Negative | Negative | Negative | Negative | |
| White blood cells count (G/L) | 4.70 | 5.15 | 7.67 | 11.29 | |
| C-reactive protein (mg/L) | 57.53 | 13.44 | 146.79 | 50.07 | |
| Peripheral Blood cultures | Negative | NA | Negative | Negative | |
| BAL | Days from onset (date) | 69 (23 May 2023) | 76 (13 December 2023) | 40 (6 March 2023) | 50 (17 May 2023) |
| Culture | Negative | Negative | Negative | Negative | |
| SARS-CoV-2 RT-PCR Results * (CT if available) | Positive (NA) | Positive (E 33.1-RdRP 35.2-N2 37.0) | Positive (NA) | Positive (QS5 27.8- ORF1ab 27.9-S 27.8) |
|
| Imaging findings | |||||
| Chest X-ray | Bronchial thickening | ND | Interstitial lung, left base condensation | Interstitial lung opacities | |
| Pulmonary CT scan | Bilateral interstitial lung disease, bilateral GGO | Bilateral GGO | Bilateral GGO with a basal distribution | Ground glass and consolidation in middle and lower lobes | |
| Treatments | |||||
| Antimicrobial drugs | 3GC/Piperacillin-tazobactam/Levofloxacine/TMP-SMX | NA | 3GC/Amoxicillin clavulanate/Piperacillin-tazobactam/Spiramycine | 3GC/Amoxicillin clavulanate/Piperacillin-tazobactam/Spiramycine | |
| Specific treatments (drugs) | Remdesivir/IL6-receptor antagonists (TCZ)/ CTC (DXM)/CCP ** |
CCP ** | CPP ** | Remsedevir/Nirmatrelvir-ritonavir/CPP ** | |
| Outcomes | |||||
| Clinical | Recovery | Yes 85 (8 June 2023) |
Yes 88 (25 December 2022) |
Yes 52 (18 March 2023) |
Yes 67 (3 June 2023) |
| Resolution of symptoms from onset, in days (date) | |||||
| Follow-up from recovery, in days (last date) | 24 (1 July 2023) | 186 (30 June 2023) | 49 (5 July 2023) | 32 (5 July 2023) | |
| Microbiological NP SARS-CoV-2 RT-PCR 7 days after CPP administration *** |
Negative | Negative | Negative | Negative | |
Abbreviations (alphabetic order): 3GC: third-generation cephalosporin; BAL: Broncho alveolar lavage; CCP: COVID-19 convalescent plasma; CT: cycle threshold, CTC: corticosteroids therapy; CT scan: computed tomography scan; DXM: dexamethasone; E: envelope gene; F: female; GGO: ground glass opacities; G/L: giga per liter; L/min: liter per minute; ILI: influenza-like illness; N: nucleocapsid gene; NA: not applicable; NP: Nasopharyngeal; ND: not done; M: male; mAbs: monoclonal antibodies (mAbs); mg/L: milligram per liter; ORF1ab: specific Open Reading Frame; RdRP: ARN polymerase gene; RT-PCR: reverse transcription polymerase chain reaction; S: protein S gene; TCZ: tocilizumab; TMP-SMX: Trimethoprim-sulfamethoxazole; y: years. * We performed in all our patients DiagCORE ®, Hong Kong, China, Respiratory Panel 2—SAT Dx., which detects viral and bacterial pathogens including human mastadenovirus A-G (formerly adenovirus), primate bocaparvovirus 1 + 2 (formerly bocavirus), human (hMPV), rhinovirus/enterovirus, influenza A virus (as no subtype, subtype H1, H1N1/2009, or H3), influenza B virus, human respirovirus 1 or 3, human orthorubulavirus 2 or 4 (formerly human parainfluenza virus type 1–4), human orthopneumovirus, Mycoplasma pneumoniae, Legionella pneumophilia, Bordetella pertussis and Chlamydia pneumoniae, coronavirus (differentiating HKU1, NL63, OC43, or 229E), human metapneumovirus A/B, and SARS-CoV-2. Amplification curves and cycle threshold (Ct) values were not mentioned in patient 1 and 3. ** We administered four units of high-titer post-vaccine Omicron COVID-19 convalescent plasma in all patients (two units on day 1 and two units on day 2). *** BAL SARS-CoV-2 RT-PCR follow-up after convalescent plasma administration were not performed in any patients regarding the complete recovery.
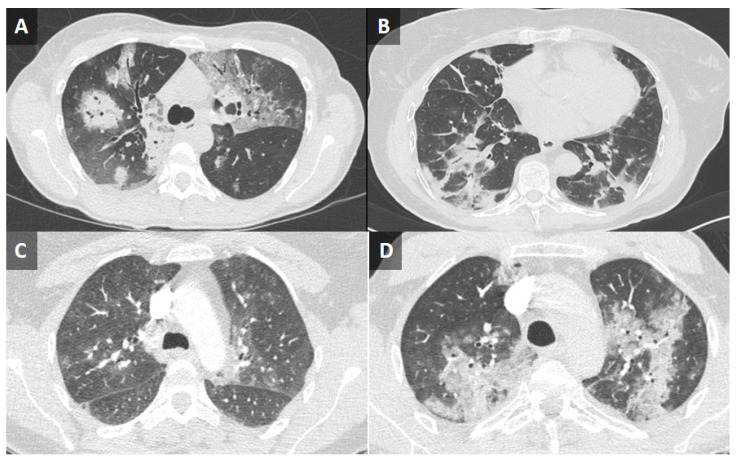
Real-time reverse transcription-polymerase chain reaction (RT-PCR) on nasopharyngeal swab specimen (TaqPath™ COVID-19 CE-IVD RT-PCR Kit, ThermoFisher Scientific, Waltham, MA, USA) and SARS-CoV-2 serologies (chimiluminescence, Liaison® XL) for IgG (anti-RBD, anti-NTD, anti-Interface) were negative in all patients. Pulmonary computed tomography (CT) scans showed bilateral ground-glass opacities in all patients (Figure 2). SARS-CoV-2 infection was confirmed from bronchoalveolar lavage (BAL) fluid by performing DiagCORE (Maryland, USA) ® Respiratory Panel 2—SAT Dx. CCP was administered in all patients (a total of four units) with a complete recovery without relapse after a mean of 73 days of follow-up.
Figure 2.
Thoracic computed tomography scan in patients with B-cell depletion and prolonged SARS-CoV-2 infection. (A) Ground-glass interstitial syndrome with a crazy paving, predominant on the left upper lobe, and alveolar condensation in the right lower lobe with pleural effusion (Patient 1). (B) Bilateral ground-glass opacities with sequelae lesions (Patient 2). (C) Subpleural ground-glass opacities with a basal distribution (Patient 3). (D) Multiple bilateral foci of ground-glass opacities, affecting all lobes except the right upper lobe (Patient 4).
3.1. Case 1
A 43-year-old female with a past history of rheumatoid arthritis treated with rituximab presented with daily fever for two months, chills, and respiratory symptoms such as dypnea. Respiratory and neurologic examinations were normal and RT-PCR SARS-CoV-2 in a nasopharyngeal sample was negative. Antimicrobial empiric treatment was started intravenously with no clinical response. On day 5, the patient developed hypoxemia which needed oxygen therapy flow at 4 L/min and was transferred to an intensive care unit. Given concern for prolonged SARS-CoV-2 infection, induced BAL was performed and COVID-19 was diagnosed from results of a RT-PCR panel. Treatment began with oral steroids (dexamethasone, 6 mg daily), then intravenous (IV) tocilizumab (one injection of 8 mg) and remdesivir IV (200 mg was administered, followed by 100 mg daily for a total of five days) with no response and the persistence of hypoxemia. A high-titer of Omicron CCP (four units) was administered with total regression of symptoms.
3.2. Case 2
A 65-year-old female with a past history of multiple sclerosis treated with ocrelizumab sought care for a persistent non-productive cough and dyspnea four months after a SARS-CoV-2 infection. Pulmonary auscultation found unilateral crackling sounds, confirmed on imaging findings. Although RT-SARS-CoV-2 PCR on nasopharyngeal swab was negative, positive PCR results were obtained from the BAL sample, with persistent presence of the SARS-CoV-2 in the lower respiratory tract. She received only IV high-titer Omicron CCP (>8000 IU). A significant clinical improvement was observed, followed by complete resolution of the cough and dyspnea without relapse after 6 months of follow-up.
3.3. Case 3
A 48-year-old woman with a history of multiple sclerosis treated with ocrelizumab sought care for a fever which had persisted for 3 months and respiratory symptoms such as a dry cough and dyspnea. Supportive treatment was initiated (analgesics, antitussives) with empirical antimicrobial drugs (amoxicillin/clavulanate, 3 g by IV infusion per day) with no recovery. On admission, she was febrile and had unilateral crackling sounds on pulmonary auscultation. Laboratory findings revealed a high C-reactive protein (CRP) level of 146 mg/L. Nasopharyngeal PCR and serological assay for SARS-CoV-2 were negative. However, we performed a PCR respiratory panel on BAL which detected SARS-CoV-2 RNA. Administrations of four units of high-titer CCP (>8000 IU) were carried out, with a total symptom disappearance and CRP control decrease to 13 mg/L, with no evidence of relapse afterwards.
3.4. Case 4
A 61-year-old male, receiving ocrelizumab for multiple sclerosis for 6 months, presented to the emergency room with an approximate 2 months history of daily fever and cough. He received two empiric courses of antibiotics (spiramycine once and amoxicillin-clavulanate twice) for presumed community-acquired pneumonia. A nasopharyngeal swab for SARS-CoV-2 was negative. COVID-19 was diagnosed from the results of RT-PCR on BAL, and CT thoracic imaging (Figure 2). After CCP, oral nirmatrelvir/ritonavir for five days and IV remdesevir (200 mg on the first day, then 100 mg/day for two days) administration outcome was favorable with rapid improvement of general condition and respiratory symptoms.
4. Discussion
This case series suggested (i) the use of lower respiratory samples such as bronchoalveolar lavage (BAL) is required in patients with anti-CD20 agents, in case of clinical examination and imaging finding suggesting SARS-CoV-2 infection and (ii) using CCP is a good alternative treatment for patients receiving anti-CD20 agents (such as ocrelizumab and rituxumab) who experience prolonged SARS-CoV-2 infection.
In our patients, repeated nasopharyngeal swabs were negative for SARS-CoV2 RT-PCR, but these negative results on nasopharyngeal swabs are clearly insufficient to rule out COVID-19 diagnosis [2]. We strongly recommend the use of lower respiratory tract specimen testing for the direct detection of SARS-CoV-2 infection in immunosuppressed patients, in case of clinical and/or radiological suspicion [11]. Morandi et al. evaluated the additional value of lower respiratory tract sampling in the diagnosis of COVID-19 in patients with clinically suspected SARS-CoV-2 infection with available chest CT scan and at least two negative nasopharyngeal swab RT-PCR tests for SARS-CoV-2 [12]. A correlation was found between SARS-CoV2 detection on the lower respiratory tract and the presence of a cough as well as with typical CT features. The Fab domains of anti-CD20 mAbs, such as ocrelizumab and rituxumab, target CD20+ B lymphocytes, causing selective depletion of circulating B cells through natural killer cell-mediated, antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and antibody-triggered apoptosis. In all our patients who received B-cell depletion therapy, prolonged SARS-CoV-2 viral shedding was reported with a mean of 48 days, which was consistent with medical literature data [1]. Recently, remdesivir and nirmatrelvir/ritonavir combined with sotrovimab were suggested as treatment in several cases of COVID-19 patients with prolonged clinical symptoms and viral shedding [13,14,15], but also in pre- and post-exposure prophylaxis of COVID-19 [16]. The efficacy of CCP to treat long-standing COVID-19 in patients with B-Cell depletion was discussed [4,17]. In our paper, patient 2 and 3 were treated exclusively with high-titer post-vaccine CCP with a complete recovery. Scott et al. suggested that spike-protein proteolytic antibodies in CCP contribute to SARS-CoV-2 neutralization [18]. Cognasse et al. also demonstrated that CCP exhibited moderately increased inflammatory markers on endothelial cells, neutralizing auto-Abs to type I IFNs compared to the control plasma with no discernible differences in ex vivo bioactivity [3]. CCP therapy with robust Fc-effector antiviral functions can serve as secondary defense when neutralization is compromised [19]. Given the increasingly recognized role of T-cell responses in protection against severe COVID-19, the circulation of SARS-CoV-2 variants, and the potential implementation of novel vaccines, it becomes imperative to continuously monitor T-cell responses [20,21].
Currently, the use of CCP is permitted in France as an off-label indication, and it implies authorization by ‘The National Reference Multidisciplinary Team’. CCP therapy seems to be a promising approach through the transfer of neutralizing antibodies specific to SARS-CoV-2 in patients with B-cell immunosuppression and prolonged COVID-19. Further studies are required to clarify therapeutic management strategies for immunocompromised patients receiving B-cell depletion therapy who experience prolonged COVID-19.
Author Contributions
Conceptualization, T.K. and S.Z.; validation, K.L. and V.G.; investigation, L.D.S., R.N., N.S., T.L., A.Q., L.T. and J.C.; resources, S.Z., L.D.S., T.K. and J.C.; writing—original draft preparation, S.Z., L.D.S., T.K. and J.C.; writing—review and editing, K.L., Q.R. and V.G.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The treatment with COVID-19 convalescent plasma was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of ‘The National Reference Multidisciplinary Team’. Sorbonne University, INSERM, Pierre Louis Institute of Epidemiology and Public Health (IPLESP) and Infectious Diseases Department, Saint Antoine Hospital, Paris.
Informed Consent Statement
Informed consent was obtained from all patients regarding publication of this paper.
Data Availability Statement
Data available on request due to privacy restrictions. The data presented in this case study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gibson E.G., Pender M., Angerbauer M., Cook C., Jones B., Spivak A.M., Spivak E.S., Swaminathan S. Prolonged SARS-CoV-2 Illness in a Patient Receiving Ocrelizumab for Multiple Sclerosis. Open Forum Infect. Dis. 2021;8:ofab176. doi: 10.1093/ofid/ofab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winichakoon P., Chaiwarith R., Liwsrisakun C., Salee P., Goonna A., Limsukon A., Kaewpoowat Q. Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19. J. Clin. Microbiol. 2020;58:e00297-20. doi: 10.1128/JCM.00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cognasse F., Hamzeh-Cognasse H., Rosa M., Corseaux D., Bonneaudeau B., Pierre C., Huet J., Arthaud C.A., Eyraud M.A., Prier A., et al. Inflammatory markers and auto-Abs to type I IFNs in COVID-19 convalescent plasma cohort study. EBioMedicine. 2023;87:104414. doi: 10.1016/j.ebiom.2022.104414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomisti L., Angelotti F., Lenzi M., Amadori F., Sarteschi G., Porcu A., Capria A.-L., Bertacca G., Lombardi S., Bianchini G., et al. Efficacy of Convalescent Plasma to Treat Long-Standing COVID-19 in Patients with B-Cell Depletion. Life. 2023;13:1266. doi: 10.3390/life13061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Abramo A., Vita S., Maffongelli G., Mariano A., Agrati C., Castilletti C., Goletti D., Ippolito G., Nicastri E. Prolonged and severe SARS-CoV-2 infection in patients under B-cell-depleting drug successfully treated: A tailored approach. Int. J. Infect. Dis. 2021;107:247–250. doi: 10.1016/j.ijid.2021.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tortorella C., Aiello A., Gasperini C., Agrati C., Castilletti C., Ruggieri S., Meschi S., Matusali G., Colavita F., Farroni C., et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with MS Using Different Disease-Modifying Therapies. Neurology. 2022;98:e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello A., Coppola A., Ruggieri S., Farroni C., Altera A.M.G., Salmi A., Vanini V., Cuzzi G., Petrone L., Meschi S., et al. Longitudinal characterisation of B and T-cell immune responses after the booster dose of COVID-19 mRNA-vaccine in people with multiple sclerosis using different dis-ease-modifying therapies. J. Neurol. Neurosurg. Psychiatry. 2023;94:290–299. doi: 10.1136/jnnp-2022-330175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dynamic Evolution of Humoral and T-Cell Specific Immune Response to COVID-19 mRNA Vaccine in Patients with Multiple Sclerosis Followed until the Booster Dose—PubMed. [(accessed on 27 October 2023)]; doi: 10.3390/ijms24108525. Available online: https://pubmed.ncbi.nlm.nih.gov/37239872/ [DOI] [PMC free article] [PubMed]
- 9.Petrone L., Tortorella C., Aiello A., Farroni C., Ruggieri S., Castilletti C., Meschi S., Cuzzi G., Vanini V., Palmieri F., et al. Humoral and Cellular Response to Spike of Delta SARS-CoV-2 Variant in Vaccinated Patients with Multiple Sclerosis. Front. Neurol. 2022;13:881988. doi: 10.3389/fneur.2022.881988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiello A., Coppola A., Vanini V., Petrone L., Cuzzi G., Salmi A., Altera A.M.G., Tortorella C., Gualano G., Gasperini C., et al. Accuracy of QuantiFERON SARS-CoV-2 research use only assay and characterization of the CD4+ and CD8+ T cell-SARS-CoV-2 response: Comparison with a homemade interferon-γ release assay. Int. J. Infect. Dis. 2022;122:841–849. doi: 10.1016/j.ijid.2022.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puyskens A., Michel J., Stoliaroff-Pepin A., Bayram F., Sesver A., Wichmann O., Harder T., Schaade L., Nitsche A., Peine C. Direct comparison of clinical diagnostic sensitivity of saliva from buccal swabs versus combined oro-/nasopharyngeal swabs in the detection of SARS-CoV-2 B.1.1.529 Omicron. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2023;165:105496. doi: 10.1016/j.jcv.2023.105496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morandi L., Torsani F., Forini G., Tamburrini M., Carnevale A., Pecorelli A., Giganti M., Piattella M., Guzzinati I., Papi A., et al. The Additional Value of Lower Respiratory Tract Sampling in the Diagnosis of COVID-19: A Real-Life Observational Study. Diagnostics. 2022;12:2372. doi: 10.3390/diagnostics12102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K., Sugiyama M., Ishizuka H., Sasajima T., Minakawa Y., Sato H., Miyazawa M., Kitakawa K., Fujita S., Saito N., et al. Prolonged infective SARS-CoV-2 omicron variant shedding in a patient with diffuse large B cell lymphoma successfully cleared after three courses of remdesivir. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2023;29:820–824. doi: 10.1016/j.jiac.2023.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzafame M., Gottardi M., Guella L., Collini L., Costa G., Guella A., Vento S. Successful treatment of persistent SARS-CoV-2 infection with nirmatrelvir/ritonavir plus sotrovimab in four immunocompromised patients. J. Chemother. 2023;35:623–626. doi: 10.1080/1120009X.2023.2196917. [DOI] [PubMed] [Google Scholar]
- 15.Baldi F., Dentone C., Mikulska M., Fenoglio D., Mirabella M., Magnè F., Portunato F., Altosole T., Sepulcri C., Giacobbe D.R., et al. Case report: Sotrovimab, remdesivir and nirmatrelvir/ritonavir combination as salvage treatment option in two immunocompromised patients hospitalized for COVID-19. Front. Med. 2022;9:1062450. doi: 10.3389/fmed.2022.1062450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vita S., Rosati S., Ascoli Bartoli T., Beccacece A., D’Abramo A., Mariano A., Scorzolini L., Goletti D., Nicastri E. Monoclonal Antibodies for Pre- and Postex-posure Prophylaxis of COVID-19: Review of the Literature. Pathogens. 2022;11:882. doi: 10.3390/pathogens11080882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueso T., Godron A.-S., Lanoy E., Pacanowski J., Levi L.I., Gras E., Surgers L., Guemriche A., Meynard J.-L., Pirenne F., et al. Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: A longitudinal cohort and propensity score analysis. Leukemia. 2022;36:1025–1034. doi: 10.1038/s41375-022-01511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McConnell S.A., Sachithanandham J., Mudrak N.J., Zhu X., Farhang P.A., Cordero R.J.B., Wear M.P., Shapiro J.R., Park H.-S., Klein S.L., et al. Spike-protein proteolytic antibodies in COVID-19 convalescent plasma contribute to SARS-CoV-2 neutralization. Cell Chem. Biol. 2023;30:726–738.E4. doi: 10.1016/j.chembiol.2023.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullah I., Beaudoin-Bussières G., Symmes K., Cloutier M., Ducas E., Tauzin A., Laumaea A., Grunst M.W., Dionne K., Richard J., et al. The Fc-effector function of COVID-19 con-valescent plasma contributes to SARS-CoV-2 treatment efficacy in mice. Cell Rep. Med. 2023;4:100893. doi: 10.1016/j.xcrm.2022.100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goletti D., Petrone L., Manissero D., Bertoletti A., Rao S., Ndunda N., Sette A., Nikolayevskyy V. The potential clinical utility of measuring severe acute respiratory syndrome coronavirus 2-specific T-cell responses. Clin. Microbiol. Infect. 2021;27:1784–1789. doi: 10.1016/j.cmi.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrone L., Sette A., de Vries R.D., Goletti D. The Importance of Measuring SARS-CoV-2-Specific T-Cell Responses in an Ongoing Pandemic. Pathogens. 2023;12:862. doi: 10.3390/pathogens12070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy restrictions. The data presented in this case study are available on request from the corresponding author.