Abstract
Rift Valley fever phlebovirus (RVFV) is a zoonotic pathogen that causes Rift Valley fever (RVF) in livestock and humans. Currently, there is no licensed human vaccine or antiviral drug to control RVF. Although multiple species of animals and humans are vulnerable to RVFV infection, host factors affecting susceptibility are not well understood. To identify the host factors or genes essential for RVFV replication, we conducted CRISPR-Cas9 knockout screening in human A549 cells. We then validated the putative genes using siRNA-mediated knock-downs and CRISPR-Cas9-mediated knock-out studies. The role of a candidate gene in the virus replication cycle was assessed by measuring intracellular viral RNA accumulation, and the virus titers were analyzed using plaque assay or TCID50 assay. We identified approximately 900 genes with potential involvement in RVFV infection and replication. Further evaluation of the effect of six genes on viral replication using siRNA-mediated knock-downs revealed that silencing two genes (WDR7 and LRP1) significantly impaired RVFV replication. For further analysis, we focused on the WDR7 gene since the role of the LRP1 gene in RVFV replication was previously described in detail. WDR7 knockout A549 cell lines were generated and used to dissect the effect of WRD7 on a bunyavirus, RVFV, and an orthobunyavirus, La Crosse encephalitis virus (LACV). We observed significant effects of WDR7 knockout cells on both intracellular RVFV RNA levels and viral titers. At the intracellular RNA level, WRD7 affected RVFV replication at a later phase of its replication cycle (24 h) when compared with the LACV replication, which was affected in an earlier replication phase (12 h). In summary, we identified WDR7 as an essential host factor for the replication of two different viruses, RVFV and LACV, both of which belong to the Bunyavirales order. Future studies will investigate the mechanistic role through which WDR7 facilitates phlebovirus replication.
Keywords: RVFV, host factor, WDR7 gene, MP-12, A549 cells, LACV, bunyavirus, phlebovirus
1. Introduction
Rift Valley fever phlebovirus (RVFV) is a mosquito-borne, segmented RNA virus that belongs to the family Phenuiviridae, genus phlebovirus. RVFV was first isolated and characterized in the Rift Valley of Kenya in 1931 [1] and is the causative agent of Rift Valley fever (RVF). It is endemic throughout sub-Saharan Africa [2], the Arabian Peninsula (Saudi Arabia and Yemen), and Mayotte [3,4]. RVFV can be naturally transmitted to and cause disease in several animal species such as cattle, sheep, goats, and camels [5,6,7]. We have recently shown that white-tailed deer are highly susceptible to experimental infection with RVFV [8]. RVF in livestock is characterized by abortion storms in pregnant ewes and pregnant cattle and up to 100% mortality in newborn animals [5,6,7]. In humans, RVFV infection may be subclinical or causes mild flu-like symptoms and sometimes severe disease with hepatitis, retinitis, and encephalitis [9,10] with a small number of cases being lethal [11]. RVFV can infect and replicate in a multitude of cell lines (e.g., neurons, epithelial cells, etc.) from different animal species such as frogs, pigs, elk, mule deer, pronghorn, and reptiles, among others [12,13,14,15,16,17]; this highlights the potential for the virus to infect a wide variety of animals.
RVFV is mainly transmitted by infected mosquitoes (Culex and Aedes), through direct contact with infected animal secretions and exudates [18,19], or through aerosol exposure [20]. Currently, there are no FDA-approved therapeutic drugs or licensed vaccines available to control RVF in humans [20]. There is a real risk of the introduction of arboviruses such as RVFV to non-endemic countries, such as Europe, Asia, and North America [21], where competent vector mosquito species (e.g., Culex and Aedes) are present [18,22,23,24]. Therefore, RVFV poses a global threat to the health of livestock and humans, and to animal trade and commerce [24].
The successful development of antiviral therapies requires detailed knowledge of viral protein function or of host factors that support virus replication [25]. RVFV enters cells via receptor-mediated endocytosis and releases its nucleocapsid after fusion of the virus envelope with endosomal membranes. After the completion of replication, the viral particles assemble and bud from the Golgi apparatus [26]. Like many other RNA viruses, RVFV depends on various host factors to complete its replication cycle [27,28,29]. Several groups have conducted exploratory studies aimed at finding host factors or co-factors that might play a role in RVFV replication [27,29,30,31,32,33,34,35,36,37]. Notably, other researchers have shown that the low-density lipoprotein receptor-related protein 1 (LRP1) [29,37] and heparin sulfate [33] play essential roles in the cell entry of RVFV. Furthermore, the exogenous administration of the LRP1 inhibitor mRAPD3 protected mice from infection with a virulent strain of RVFV [29]. Devignot et al. (2023) reported that a LRP1 gene knockout in Huh cells significantly affected intracellular RVFV RNA accumulation [37]. Bracci et al. (2022) found that UBR4 depletion affects RVFV production and virus titers in mammalian and mosquito cells [36]. Although these studies have identified host factors in mouse cells associated with RVFV replication, none of the host factors were able to completely abolish productive RVFV infection in gene-edited knockout cells. This indicates that RVFV interacts with a multitude of host factors to complete its replication cycle, exploiting multiple redundant cellular pathways. Our studies had the following aims: (1) to identify unique host factors that could significantly affect RVFV infection and replication; (2) to identify host factors that could be used as a potential drug target; and (3) to identify host factors that are conserved between different host species. To this end, a genome-wide CRISPR-Cas9 knockout (GeCKO) screening in human A549 cells infected with the RVFV MP-12 vaccine strain was performed in order to identify the host factors essential for RVFV infection and replication. We identified the WD repeat domain 7 (WDR7) gene as a critical host factor that plays a role in the late phase of RVFV replication. In addition, the WDR7 gene also plays a role in the replication of an orthobunyavirus, the La Crosse encephalitis virus.
2. Materials and Methods
2.1. Cells
A549 cells (ATCC® CCL-185™, American Type Culture Collection, Manassas, VA, USA) were cultured in an F-12 medium (ATCC, Manassas, VA, USA), supplemented with 10% fetal bovine serum (FBS, R&D Systems, Minneapolis, MN, USA) and 1% penicillin–streptomycin solution (ThermoFischer Scientific, Waltham, MA, USA). The Vero-MARU cell line is a clone of the Vero cells derived from African green monkey kidney cells and obtained from the Middle America Research Unit (Corozal, Panama). These cells are susceptible to RVFV infection, and they are the standard cell line used for all our plaque-based titration assays. The Vero-MARU, MRC-5 (ATCC® CCL-171™), and Vero E6 (ATCC® CRL-1586™) cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Corning, New York, NY, USA), supplemented with 5% FBS (R&D Systems, USA) and 1% penicillin–streptomycin solution (ThermoFischer Scientific, USA). All mammalian cells were maintained at 37 °C under a 5% CO2 atmosphere. The Aedes albopictus larva (C6/36, ATCC® CRL-1660™) cells were maintained at 28 °C and cultured in an L-15 medium (ATCC, USA), supplemented with 10% insect cell culture tested FBS (IFBS, Catalog Number: F4135, Sigma-Aldrich, St. Louis, MO, USA), 10% tryptose phosphate broth (TPB, Catalog Number: T9157, Sigma-Aldrich, USA), and 1% penicillin–streptomycin solution (ThermoFischer Scientific, USA).
2.2. Virus Strains
The RVFV MP-12 vaccine strain provided by the US Army Medical Research Institute for Infectious Diseases (Fort Detrick, MD, USA) [38] was propagated in MRC-5 cells; the RVFV Kenya 128B-15 virulent strain was provided by R. Bowen, Colorado State University (Fort Collins, CO, USA), with authorization from B. Miller, Centers for Disease Control, Fort Collins, CO, USA [39] and was grown in C6/36 cells. La Crosse Encephalitis virus (LACV), NR-540, was obtained from BEI Resources, NIAID, and propagated in Vero E6 cells. RVFV MP-12 and Kenya 128B-15 strains were titered using plaque assay and the LACV by TCID50-CPE assay. We used the attenuated MP-12 vaccine strain, which is classified as a BSL-2 agent, to perform our initial studies, including the pooled CRISPR-KO screening, as well as the siRNA gene knock-down and CRISPR-Cas9 knock-out experiments. Subsequently, we conducted studies with the virulent Kenya 128B-15 strain, which is a wild-type RVFV strain, in a BSL-3+ facility at the Biosecurity Research Institute of Kansas State University (Manhattan, KS, USA) to confirm the critical role of WDR7 in RVFV infections.
2.3. Generation of GeCKO-A549 Cell Line and RVFV Screening
The lentiCRISPRv2 library, which targets 19,000 human genes, was obtained from Addgene (Catalog Number: 1000000048, Addgene, Watertown, MA, USA). The library contains non-target control sgRNAs, sgRNAs targeting miRNAs, and six unique sgRNAs designed to target each individual human gene. To generate GeCKO-A549 cells, a pooled lentivirus library was created using the lentiCRISPRv2 plasmids, following previously described methods [40,41]. A puromycin (Catalog Number: A1113803, Sigma-Aldrich, St. Louis, MO, USA) cytotoxicity curve was performed on A549 cells, and the puromycin concentration used was determined to be 2 μg/mL medium. Then, the transduction efficiency of the lentivirus library on A549 cells was determined as previously described [40,41]. Two independently pooled GeCKO-A549 cell lines were generated and subjected to forward genetic screening. Briefly, 80 million GeCKO-A549 cells were subjected to up to three rounds of cytolytic infection with RVFV MP-12 (1 MOI), and the surviving cells were expanded between each round of infection. The gDNAs were extracted from the round 0 (mock-infected), round 1, and 3 virus infections of GeCKO-A549 cells using a MIDI gDNA Extraction Kit (Qiagen, Germantown, MD, USA). The sgRNA’s DNA copies were PCR-amplified from the extracted gDNAs for next-generation sequencing (Figure 1). Next-generation sequencing was performed using NextSeq (Illumina, San Diego, CA, USA), and the obtained data were analyzed using MAGeCK software version 0.5.9. The ranking of genes was determined using robust ranking aggregation [42].
Figure 1.
Schematics of GeCKO-A549 cells generation, selection, NGS, and data analysis. A549 cells were transduced with the lentivirus-CRISPR-Cas9 library to generate GeCKO-A549 cells. Then, the GeCKO-A549 cells were subjected to three rounds of infection with the RVFV MP-12 (1 MOI) virus. The genomic DNA of round 0 GeCKO-A549 cells, round 1, and round 3 GeCKO-A549cells were sequenced using the Illumina NextSeq 550 platform. The output NGS data were analyzed using the MaGeCK program to generate the list of genes involved in RVFV replication.
2.4. siRNA Transfection
Six genes were selected after NGS analysis of the RVFV-resistant GeCKO-A549 cells for siRNA gene knockdown studies (Table S1). The gene targets for the siRNAs were as follows: siRNAs: NTC-non-target control (Catalog Number: D-001206-14-05); WDR7 (Catalog Number: M-012867-01-0005); LRP1 (Catalog Number: M-004721-01-0005); EXOC4 (Catalog Number: M-013068-01-0005); SLC35B2 (Catalog Number: M-007543-01-0005); CT47A1 (Catalog Number: M-184305-00-0005); and EMC3 (Catalog Number: M-010715-00-0005); they were commercially purchased (Dharmacon, Lafayette, CO, USA). The positive control siRNA-si46N [43] targeting the RVFV nucleoprotein was obtained from Integrated DNA Technologies (Coralville, IA, USA). A549 cells were plated in 96-well plates and incubated overnight. The cells were transfected with siRNAs (50 nM) using lipofectamine RNAimax reagent (ThermoFischer Scientific, USA). Forty-eight hours post-transfection, cells were infected with RVFV MP-12 at 0.1 MOI, and the infected cell supernatant was collected at 24 h post-infection (h pi). The virus titer of the supernatants was determined using plaque assay on Vero-MARU cells.
2.5. RT-qPCR for Host Gene Expression
To confirm gene knockdown, two-step RT-qPCR assays were performed. Briefly, A549 cells were transfected with gene-specific siRNAs at 50 nM, and 48 h later, the total cellular RNA was extracted. The RNA extraction was performed using the RNAqueous Micro Total RNA Isolation Kit (ThermoFischer Scientific, USA) following the manufacturer’s protocol. Prior to cDNA synthesis, residual gDNA was removed from the extracted RNA using the DNAse I enzyme (ThermoFischer Scientific, USA). Then, 400 ng of RNA was used for cDNA synthesis using the Superscript IV First-Strand Synthesis Kit with oligo dT primers (ThermoFischer Scientific, USA) following the manufacturer’s protocol. All RT-qPCR reactions were performed in a CFX96 Real-Time thermocycler (BioRad, Hercules, CA, USA). The standard real-time qPCR assays were performed using Perfecta Fastmix II (Quanta BioSciences, Beverly, MA, USA) with gene-specific primers (Table S2). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control [44]. The percentage gene knockdown was calculated using the 2–∆∆CT method [45].
2.6. Generation of WDR7 Knock-Out (KO) Cells
Two WDR7 knockout (KO) cell lines and a control non-KO cell line were generated as previously described [41]. WDR7-gene targeting sgRNAs (sgRNA 1: 5′GTGACATCCTGTTACGATCG3′ and sgRNA 5: 5′AAGATGGCAAGATCGATGCT’3) were applied to generate two WDR7 KO cell lines: the WDR7 KO cell line 1 (WDR7 KO 1) and 2 (WDR7 KO 2). The non-KO control cell line (CT) was generated via the transduction of the lentiCRISPRv2 vector with the Cas9 backbone without sgRNAs. LentiCRISPRv2 plasmids 1 and 5 containing sgRNAs specific for the WDR7 gene were purchased from Genescript, USA. The control and WDR7 sgRNA plasmids were packaged into lentivirus, and the A549 cells were transduced with 0.5 MOI of lentivirus. The transduced cells were kept under puromycin selection and passed three times prior to testing. The gDNA of the two WRD7 KO cell lines were extracted using the DNAeasy kit (Qiagen, Germantown, MD, USA), and the gDNA was PCR-amplified for NGS analysis. Sequencing was performed using a MiSeq (Illumina, USA). The indel percentage of the KO cell lines was calculated using a Python script [41].
2.7. Western Blot Analysis
A549 cells, CT cells, and WDR7 KO 1 and 2 cells at passage 3 were used for Western blot analyses. The cell lysates were prepared as previously described [46]. Cell lysates containing 55.0 µg total protein were loaded onto 4–12% Bis-Tris polyacrylamide gels (ThermoFischer Scientific, USA) and transferred onto a polyvinylidene difluoride (PVDF) membrane using a Trans-Blot Turbo Transfer Pack (BioRad, USA). The membrane was blocked using 5% skim milk and then incubated with a primary polyclonal antibody against WDR7 (diluted 1:500, Catalog Number: sab2109026, Sigma-Aldrich, USA) or β-actin (diluted 1:5000, Catalog Number: ab20272, Abcam, Waltham, MA, USA) for 1 h at room temperature. The membrane was then incubated with horseradish peroxidase (HRP)-conjugated polyclonal goat anti-rabbit immunoglobulin (diluted 1:1000, Catalog Number: 31460, ThermoFischer Scientific, USA). The target proteins were detected using Super Signal West Femto Maximum Sensitivity Substrate according to the manufacturer’s protocol (Catalog Number: 34095, ThermoFischer Scientific, USA). The images were taken using a ChemiDoc MP Imaging System (BioRad, USA).
2.8. Testing of WDR7 KO Cells for Virus Replication
The non-knockout control (CT) and WDR7 KO cell lines were seeded onto 96-well plates and allowed to incubate overnight. Afterward, the cells were infected with either RVFV MP-12, RVFV Kenya 128B-15, or La Crosse encephalitis virus (LACV) at 0.1 MOI, and the cell supernatants were collected at 6, 12, 24, or 48 h pi. The titer of collected supernatants was determined using the plaque assay (RVFV) or the TCID50-CPE (LACV) assay.
2.9. Intracellular Viral RNA Accumulation Assay
The viral RNA accumulation was determined at various time points (0, 2, 5, and 24h pi) as previously described [37,47]. The CT and WDR7 KO 1 cells were plated in 6-well plates. Twenty-four hours later, cells were infected with RVFV MP-12 or LACV at a MOI of 0.1 for 1 h at 0 °C to allow for virus attachment and entry. For the 0 h infection, immediately after infection, the cells were washed three times with 1× phosphate-buffered saline (PBS (pH = 7.2–7.6), Catalog Number: P4417, Sigma-Aldrich, St. Louis, MO, USA), lysed in 350 μL RLT buffer (Qiagen, Germantown, MD, USA), and then stored at −80 °C for subsequent use. For the post-infection time points, the cells were washed once with 1× PBS after the initial 1 h of incubation and then incubated with 2 mL of pre-warmed fresh medium. At 2 h pi, cells were first trypsinized and collected into microcentrifuge tubes. Then, the trypsinized cells were washed three times with 1× PBS via centrifugation at 10,000× g for 5 min. The cell pellets were lysed in RLT buffer and stored at −80 °C till further use. For the 5 and 24 h time points, the cells were washed once with 1× PBS and lysed in RLT buffer for 10 min prior to storage at −80 °C for future analysis. The total cellular RNA was extracted using an RNeasy Mini Kit (Qiagen, Germantown, MD, USA). One-step RT-qPCR assays were performed using q-script XLT (2×) Master mix (Quanta BioSciences, Beverly, MA, USA) with virus gene-specific primers and probes (Table S3); the phosphoglycerate kinase (PGK1) gene was used as an internal housekeeping control gene [44]. Respective gene expressions were calculated using the 2–∆∆CT method [48].
2.10. Plaque Assay
Vero-MARU cells were seeded in 12- or 24-well plates and incubated at 37 °C and 5% CO2 overnight. After overnight incubation, the cells were infected with RVFV for one hour, and then the medium was replaced with an overlay of 1% methylcellulose-1× MEM (ThermoFischer Scientific, USA), 5% FBS, 1% antibiotics/antimycotic. The cells were incubated for 5–7 days and then stained and fixed with a 5% crystal violet fixative solution. The plaques were counted, and the titer was expressed as pfu/mL.
2.11. TCID50-CPE Assay
Vero E6 cells were seeded in 96-well plates one day prior to infection. Ten-fold serial dilutions of LACV were prepared in 96-well plates in DMEM supplemented with 5%FBS and 1% antibiotic–antimycotic solution. The diluted viral suspensions were then added to Vero E6 cells. Three to four days after infection, the cells were visually observed under a microscope for CPE, and the titer was calculated using the Spearman–Karber method [49].
2.12. Statistical Analysis
The statistical analyses performed in this study are described in the figure legends. All the statistical tests were carried out using GraphPad Prism version 9.3.0.3.
3. Results
3.1. Identification of Host Factors Involved in RVFV Replication
To identify the genes potentially involved in RVFV replication, we performed CRISPR-Cas9 knockout screening in A549 cells. The A549 type II alveolar human cell line was selected for the screening because it is susceptible to RVFV and can be easily transduced with the human GeCKO library. The GeCKO-A549 cells were subjected to three rounds of infection with the RVFV MP-12 vaccine strain to select for resistance to RVFV infection and to identify the key host factors that are required for virus replication. Extensive cytopathic effect (CPE) was observed during the first round of infection. Surviving cells were reinfected, and the CPE was much less extensive during the second and third rounds of infection. To assess the susceptibility of GeCKO-A549 cells after three rounds of RVFV infection, virus growth kinetic assays were performed. A significant difference in MP-12 virus titers was observed between round 0 and round 3 GeCKO-A549 cells at 24 and 48 h post-infection (h pi) (Supplementary Figure S1), indicating that the round 3 GeCKO-A549 cells were less permissive to RVFV infection. Next, the putative genes involved in RVFV replication were determined by analyzing the NGS data from round 0, round 1, and round 3 GeCKO-A549 cells. Our analysis of round 3 GeCKO-A549 cells revealed that 907 genes (p-value < 0.05) seem to be involved in RVFV MP-12 replication (Supplementary File S1). For further analysis, we selected the six top genes significantly enriched in round 3 GeCKO-A549 cells: LRP1, SLC35B2, EMC3, WDR7, EXOC4, and CT47A1 (Supplementary File S1). We did not investigate the other top two genes, ART3 and CEBPD (Supplementary File S1), as they were associated with essential cellular functions.
3.2. Validation of Genes from the Pooled GeCKO-A549 Cell Screening
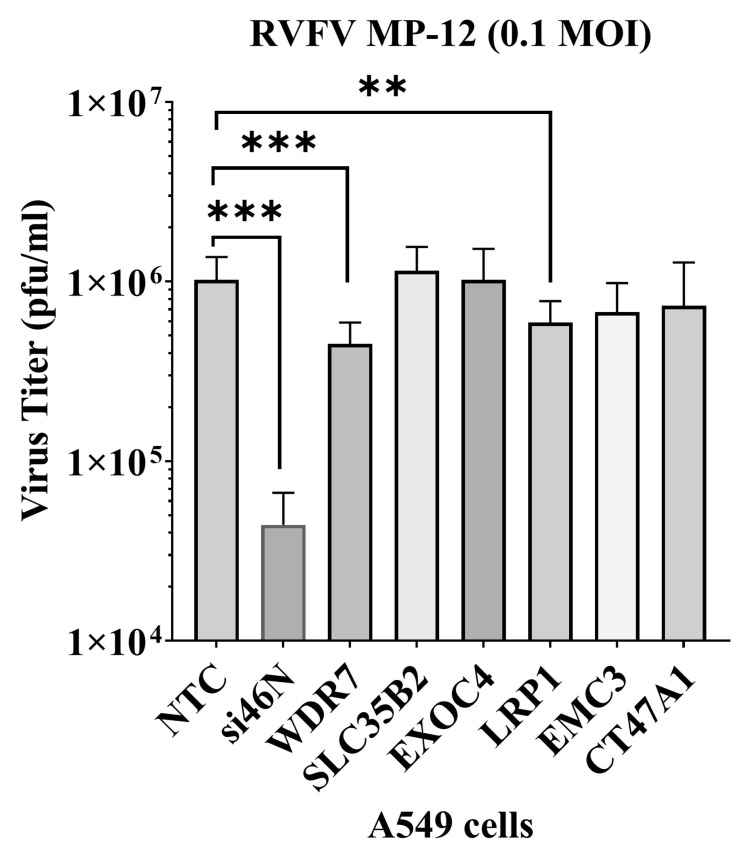
To assess the effect of the six top genes enriched in round 3 GeCKO-A549 cells on RVFV replication, we used siRNA-mediated gene silencing (gene knockdown) in A549 cells. Gene knockdown was confirmed via respective RT-qPCR assays and the average reduction in gene expression ranged from approximately 55% to 90% (Supplementary Figure S2). After gene knockdown, the cells were infected with RVFV MP-12 virus at 0.1 MOI for 24 h, supernatants were harvested, and extracellular virus titer was determined using plaque assay. There was an average of 56% or 42% reduction in virus titer upon WDR7 and LRP1 knockdown, respectively, compared with non-target control (NTC) siRNA targeting the firefly luciferase mRNA (Figure 2). The positive control siRNA, siRNA-si46N, targeted the N protein gene of RVFV and caused a reduction of approximately 96% in virus titer compared with the negative control group. We observed no significant effect on virus titers following the knockdown of the other four selected top genes, namely EXOC4, CT47A1, EMC3, and SLC35B2 (Figure 2). These results demonstrate that the knockdown of WDR7 and LRP1 significantly impaired RVFV replication. Given that the role of the LPR1 gene in RVFV replication has been recently demonstrated [29,37], we focused our further analysis on the newly discovered putative RVFV host factor WDR7.
Figure 2.
Validation of gene hits via siRNA gene knockdown study. A549 cells were transfected with 50 nM of siRNAs. At 48 h post-transfection, the cells were infected with RVFV MP-12 virus at 0.1 MOI. At 24 h post-infection, the supernatant was collected and titered using plaque assay. NTC-non-target control siRNA; si46N-anti-RVFV siRNA; and WDR7, SLC35B2, EXOC4, LRP1, EMC3, CT47A1 gene-specific siRNAs were transfected. Each bar represents the average virus titer (pfu/mL) along with the corresponding standard deviation. Statistical analysis was performed on two independent experiments with four replicates for each, using the Mann–Whitney U test and independent Student’s t-test (** p-value < 0.005, *** p-value < 0.001).
3.3. Generation and Characterization of Knockout Cells
To investigate the role of WRD7 in the RVFV replication cycle, we employed highly enriched sgRNAs targeting the WDR7 gene to generate two knockout (KO) A 549 cell lines: WDR7 KO line 1 and WDR7 KO line 2. The established WRD7 knockout cells were analyzed via NGS sequencing, which confirmed indels in nearly 100% of the WDR7 KO cells (99% and 98%, respectively, for the two WDR7 KO cell lines 1 and 2, (Supplementary Table S1)). There was also a significant decrease in WDR7 protein expression in the WDR7 KO cell lines as compared to the control and non-transduced A549 cells (Figure 3A). However, we noted the presence of a faint WDR7 band in both WDR7 KO cells.
Figure 3.
Effect of WDR7 gene knockout (KO) on virus production of bunyaviruses: (A) A549 cells CT (non-knockout control) cells and WDR7 gene KO cell lines 1 and 2 were analyzed for WDR7 protein expression via Western blot using a WDR7-specific polyclonal antibody. (B–E) CT cells and WDR7 KO A549 cells were infected with RVFV MP-12 vaccine strain (B), with the wild-type RVFV Kenya 128B-15 strain (C), or with La Crosse encephalitis virus (D,E) at 0.1 MOI. The supernatant was collected at 6, 12 or 24 h post-infection (h pi) and titered using plaque assay (RVFV) or TCID50-CPE assay (LACV). RVFV MP-12 testing on A549 CT cells and WDR7 KO lines 1 or 2, involved three to five independent experiments with three to four technical replicates each. RVFV Kenya 128B-15 testing involved independent experiments with three technical replicates each. LACV testing was performed in two independent experiments with eight technical replicates each. Statistical analysis was performed using the Mann–Whitney U test and independent Student’s t-test (* p-value < 0.05, ** p-value < 0.005, *** p-value < 0.001).
To ensure the authenticity of the A549 control cells, we sequenced the WDR7 gene at the target site and found that the WDR7 gene was not mutated in the CT cells (Supplementary File S2); also, the WDR7 protein expression in CT cells was at a similar level to the level in the non-transduced A549 cells (Figure 3A). Moreover, the CT cells showed comparable levels of virus replication to the non-transduced wild-type A549 cells (Supplementary Figure S3E). Additionally, cell viability did not differ significantly between the WDR7 KO cell lines 1 and 2, and the CT cell line, neither prior to nor after RVFV MP-12 infection (Supplementary Figure S3A,D).
3.4. Effect of WDR7 Gene Knockout on RVFV and LACV Infection
Next, we infected the two WDR7 KO cell lines with the RVFV MP-12 strain at 0.1 MOI and determined the extracellular virus titers using plaque assay. The WRD7 gene KO resulted in a significant reduction of approximately 74% in virus titer compared with CT cells at 24 h post-infection (Figure 3B), while no difference in virus titers was observed at 48 h post-infection (Supplementary Figure S4A). We then evaluated the effect of the WDR7 gene KO on the virulent RVFV strain Kenya 128B-15. Our results showed an average reduction of 66% and 75% in RVFV Kenya 128B-15 titers using WDR7 KO cell lines 1 and 2, respectively, when compared to the CT cells (Figure 3C). Taken together, these findings support the results obtained using the WDR7 siRNA knockdown experiments, and confirm the critical role of the WDR7 gene in the RVFV replication cycle.
In addition, we evaluated if the WDR7 gene plays a role in the replication cycle of an orthobunyavirus. For this purpose, we used the La Crosse encephalitis virus (LACV) and infected the CT cells and WDR7 KO cell 1 line with the LACV; the cell supernatant was collected at various time points post-infection, and the virus titer was determined using TCID50-CPE assay. The results showed an average reduction in LACV titer of 57% and 77% at 6 h pi and 12 h pi, respectively, in the WDR7 KO 1 cell line compared with the control CT cells (Figure 3D,E). At 24 h pi, the reduction in virus titer was approximately 39% which was statistically not significant (Supplementary Figure S4B). Overall, these findings highlight the importance of the WDR7 gene in LACV replication.
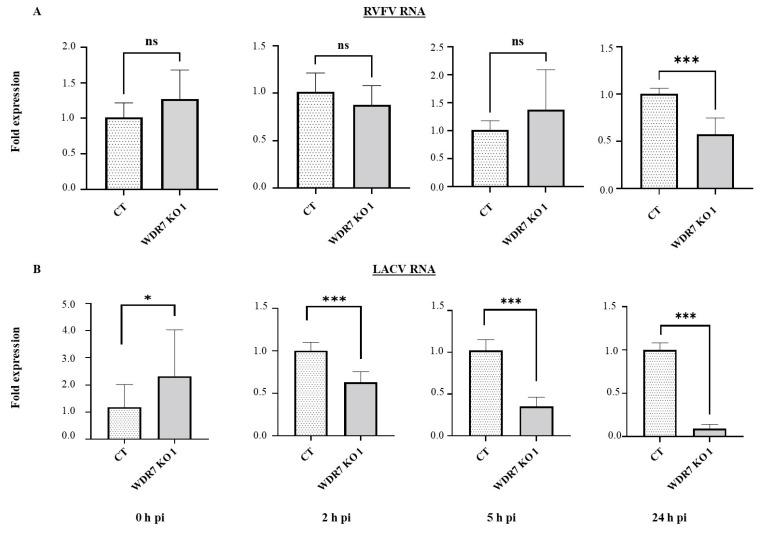
3.5. WDR7 Gene Knock-Out Impairs RVFV and LACV Intracellular RNA Accumulation
To investigate the role of WDR7 in the RVFV and LACV replication cycle, we quantified intracellular viral RNA accumulation at 0 h pi (attachment phase), 2 h pi (entry phase), 5 h pi (replication phase), and 24 h pi (late phase of replication) using previously established protocols [37,47]. At 0, 2, and 5 h pi, there was no significant difference in RVFV RNA accumulation between the CT control cells and the WDR7 KO cell line 1 (Figure 4A). However, at 24 h pi, we observed a significant reduction in virus RNA accumulation between the WDR7 KO and CT control cells (Figure 4A). When we infected the WDR7 KO cell line 1 and CT control cells with the LACV, we found that the WDR7 KO cells had higher levels of LACV RNA accumulation at 0 h pi, i.e. in the attachment phase, compared with the CT control cells (Figure 4B). However, at early time points, 2 and 5 h pi, and up to 24 h pi, we observed a significant reduction in LACV RNA accumulation in WDR7 KO 1 cells when compared to the CT control cells (Figure 4B). These results suggest that WDR7 gene disruption affects intracellular viral RNA accumulation primarily at the late phase of the RVFV replication cycle and at the early phase of the LACV replication cycle.
Figure 4.
Viral RNA accumulation at various time points post-infection in WDR7 knockout (KO) cells. CT and WDR7 KO 1 cells were infected with (A) RVFV MP-12 vaccine strain or (B) LACV, both at 0.1 MOI. Total cellular RNA was harvested at various hours post-infection (h pi). One-step RT-qPCR was performed to detect the level of viral RNA using the PGK1 gene as an internal control. CT and WDR7 KO 1 cells were utilized. Each bar graph represents the average fold change in viral RNA expression, along with the corresponding standard deviation. Statistical analysis was performed on three independent experiments with two to three technical replicates for each, using the Mann–Whitney U test and independent Student’s t-test (* p-value < 0.05, *** p-value < 0.001, ns, non-significant).
4. Discussion
RVFV has a broad cell tropism and is reported to infect several animal and mosquito species. RVFV interacts with different host factors in a variety of cell types [29,30,31,32,33,34,36,37]. We identified WDR7, a member of the WD repeat protein family, as a host factor important in the lifecycle of RVFV and LACV bunyaviruses. We confirmed WDR7 gene knock-out through NGS and indel analysis; Western blotting detected a faint band corresponding to the WDR7 protein in WDR7 knock-out cells. This minimal expression could be due to a single guide sgRNA inducing minor double-stranded DNA breaks that resulted in the production of some non-functional protein. Importantly, we demonstrated that the disruption of the WDR7 gene impairs viral RNA accumulation and infectious virus production of two bunyaviruses, RVFV and LACV. However, the exact role of WDR7 in the replication cycle of these viruses needs further investigation. Previous studies have shown that WDR7 also plays a significant role in the replication cycle of other RNA viruses such as the dengue virus, Zika virus, West Nile virus [50], and influenza A virus [51].
WDR7 has been associated with V-ATPase, which mediates intracellular vesicle acidification in mouse kidney cells [52], suggesting that WDR7 could play a role in endocytosis or secretory pathways within the virus replication cycle. Here, we demonstrated that WDR7 affects the late phase of the RVFV replication cycle, as shown by the reduction in intracellular viral RNA in WDR7 KO cells compared with non-KO CT cells at 24 h pi. Combined with the significant lower levels of infectious RVFV in WDR7 KO cell supernatants compared with CT control cells, this might suggest that WDR7 impacts virus egress and release. In contrast, for the LACV, WDR7 seems to affect early stages of virus replication, since a significant reduction in both intracellular viral RNA and infectious virus production was found at an early time point post-infectionin WDR7 KO cells compared with CT control cells. This could be due to the fact that the WDR7 gene KO might affect the expression or function of other host factors involved in virus attachment and/or virus entry, or that the WDR7 gene knock-out affects the conformation or expression of cell surface molecules needed for attachment and/or entry. While both RVFV and LACV belong to the order Bunyavirales, they belong to different genera: RVFV is a phlebovirus and LACV is a orthobunyavirus. This could be one explanation for the WDR7 effect at different replication stages for RVFV and LACV.
Interestingly, the effect of the knock-out of the WDR7 gene in A549 cells on virus replication appeared to diminish at later replication time points for both RVFV and LACV. This pattern is consistent with the findings reported by Bracci et al. (2022), who observed a similar trend in RVFV replication in UBR4 knockout cells, with a significant reduction at 24 h pi but no significant effect at 48 h pi [36]. This suggests that the RVFV and LACV have the ability to utilize multiple alternative host factors and pathways to complete their replication cycle. We also observed a significant reduction in the LACV viral RNA at later time points but not in infectious virus production. This result could be attributed to various factors such as a gene knock-out effect on late RNA synthesis, increased RNA degradation, or decreased RNA stability in the absence of WDR7; all these could affect viral RNA synthesis or RNA stability, while virus release or egress was unaffected.
Overall, this study highlights the importance of the WDR7 gene in the replication of two different viruses from the Bunyavirales order: RVFV phlebovirus and LACV orthobunyavirus. It also suggests that WDR7 could be a potential target for the development of antiviral therapies. Further research, including in vivo studies including WDR7 gene knock-out mouse models, is needed to fully elucidate the role of WDR7 in bunyavirus replication.
Acknowledgments
We are grateful to the staff of KSU Biosecurity Research Institute. We also thank Cassidy Keating for assistance with cell culture and RT-PCR assays. Dane Jasperson, USDA, ARS Center for Grain and Animal Health Research, Insect and Cell Culture Laboratory, has provided C6/36 cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15112251/s1, Figure S1: RVFV growth kinetics on wild-type and round 3 GeCKO-A549 cells; Figure S2: Confirmation of WDR7 gene knock-down; Figure S3: Cell viability and virus titers of wild-type, CT, and WDR7 gene knock-out A549 cell lines; Figure S4: Effect of WDR7 gene KO cell lines 1 and 2 on virus replication of RVFV and LACV at 48 or 24 hpi, respectively. Table S1: Location of the indels in the WDR7 gene of knock-out A549 cells; Table S2: Primers used for RT-qPCR, NGS and Sanger sequencing; Table S3: Primer and probes for the detection of viral RNA by RT-qPCR; Supplementary File S1: List of genes enriched in round 1 and round 3 virus-infected GeCKO A549 cells; Supplementary File S2: I. Predicted WDR7 gene sequences of wild-type and WDR7 knock-out A549 cells; II. WDR7 gene sequences of wild-type and WDR7 knock-out A549 cells obtained by Sanger sequencing.
Author Contributions
Conceptualization, investigation, methodology, validation, formal analysis, writing—original draft preparation, writing—review and editing, V.B.; conceptualization, investigation, methodology, writing—review and editing, S.V.I.; investigation, methodology, writing—review and editing, Y.L., D.A.M., L.U.M.R.J., H.L., M.P.H., J.A.S.-N., N.N.G., P.R.H. and F.W.; conceptualization, funding acquisition, project administration, resources, supervision, writing—review and editing, W.C.W. and J.A.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be obtained from the authors upon request.
Conflicts of Interest
All authors declare no conflicts of interest. The JAR laboratory received support from Tonix Pharmaceuticals, Xing Technologies, Esperovax, and Zoetis, outside of the reported work. JAR is the inventor of patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections, owned by Kansas State University, KS.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The conclusions in this report are those of the authors and do not necessarily represent the views of the USDA. The USDA is an equal opportunity provider and employer.
Funding Statement
This work was funded in part by the Department of Homeland Security Center of Excellence for Emerging and Zoonotic Animal Diseases (CEEZAD), Grant No. 2010-ST061-AG0001, and NBAF Transition Funds from the State of Kansas. Funding for this study was also partially provided through the AMP and MCB Cores of the Center of Emerging and Zoonotic Infectious Diseases (CEZID) from the National Institute of General Medical Sciences (NIGMS) under Award Number P20GM130448. F.W. was funded by the Swedish Research Council (VR; No. 2018-05766). W.W. was funded by the USDA, Agricultural Research Service.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Daubney R., Hudson J.R., Garnham P.C. Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from east africa. J. Pathol. Bacteriol. 1931;34:545–579. doi: 10.1002/path.1700340418. [DOI] [Google Scholar]
- 2.Morvan J., Saluzzo J.-F., Fontenille D., Rollin P.E., Coulanges P. Rift valley fever on the east coast of Madagascar. Res. Virol. 1991;142:475–482. doi: 10.1016/0923-2516(91)90070-J. [DOI] [PubMed] [Google Scholar]
- 3.Shoemaker T., Boulianne C., Vincent M.J., Pezzanite L., Al-Qahtani M.M., Al-Mazrou Y., Khan A.S., Rollin P.E., Swanepoel R., Ksiazek T.G., et al. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–2001. Emerg. Infect. Dis. 2002;8:1415–1420. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sissoko D., Giry C., Gabrie P., Tarantola A., Pettinelli F., Collet L., D’ortenzio E., Renault P., Pierre V. Rift valley fever, mayotte, 2007–2008. Emerg. Infect. Dis. 2009;15:568–570. doi: 10.3201/eid1504.081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coetzer J.A. Natural Cases in New-Born Lambs. Onderstepoort J.Vet.Res. 1977;44:205–211. [PubMed] [Google Scholar]
- 6.Coetzer J.A. The pathology of Rift Valley fever. II. Lesions occurring in field cases in adult cattle, calves and aborted foetuses. Onderstepoort J. Vet. Res. 1982;49:11–17. [PubMed] [Google Scholar]
- 7.Pepin M., Bouloy M., Bird B.H., Kemp A., Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010;41:61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson W.C., Kim I.J., Trujillo J.D., Sunwoo S.Y., Noronha L.E., Urbaniak K., McVey D.S., Drolet B.S., Morozov I., Faburay B., et al. Susceptibility of white-tailed deer to Rift valley fever virus. Emerg. Infect. Dis. 2018;24:1717–1719. doi: 10.3201/eid2409.180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boushab B.M., Fall-Malick F.Z., Baba S.E.W.O., Salem M.L.O., Belizaire M.R.D., Ledib H., Ahmed M.M.O.B., Basco L.K., Ba H. Severe human illness caused by rift valley fever virus in Mauritania, 2015. Open Forum Infect. Dis. 2016;3:2–5. doi: 10.1093/ofid/ofw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikegami T., Makino S. The pathogenesis of rift valley fever. Viruses. 2011;3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linthicum K.J., Davies F.G., Kairo A., Bailey C.L. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an inter-epizootic period in Kenya. J. Hyg. 1985;95:197–209. doi: 10.1017/S0022172400062434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gommet C., Billecocq A., Jouvion G., Hasan M., do Valle T.Z., Guillemot L., Blanchet C., van Rooijen N., Montagutelli X., Bouloy M., et al. Tissue tropism and target cells of NSs-deleted rift valley fever virus in live immunodeficient mice. PLoS Negl. Trop. Dis. 2011;5:e1421. doi: 10.1371/journal.pntd.0001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman A.L., Powell D.S., Bethel L.M., Caroline A.L., Schmid R.J., Oury T., Reed D.S. Aerosolized Rift Valley Fever Virus Causes Fatal Encephalitis in African Green Monkeys and Common Marmosets. J. Virol. 2014;88:2235–2245. doi: 10.1128/JVI.02341-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scharton D., Van Wettere A.J., Bailey K.W., Vest Z., Westover J.B., Siddharthan V., Gowen B.B. Rift valley fever virus infection in golden Syrian hamsters. PLoS ONE. 2015;10:e0116722. doi: 10.1371/journal.pone.0116722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudreault N.N., Indran S.V., Bryant P.K., Richt J.A., Wilson W.C. Comparison of Rift Valley fever virus replication in North American livestock and wildlife cell lines. Front. Microbiol. 2015;6:664. doi: 10.3389/fmicb.2015.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odendaal L., Davis A.S., Fosgate G.T., Clift S.J. Lesions and Cellular Tropism of Natural Rift Valley Fever Virus Infection in Young Lambs. Vet. Pathol. 2020;57:66–81. doi: 10.1177/0300985819882633. [DOI] [PubMed] [Google Scholar]
- 17.Rissmann M., Lenk M., Stoek F., Szentiks C.A., Eiden M., Groschup M.H. Replication of rift valley fever virus in amphibian and reptile-derived cell lines. Pathogens. 2021;10:681. doi: 10.3390/pathogens10060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumley S., Horton D.L., Hernandez-Triana L.L.M., Johnson N., Fooks A.R., Hewson R. Rift valley fever virus: Strategies for maintenance, survival and vertical transmission in mosquitoes. J. Gen. Virol. 2017;98:875–887. doi: 10.1099/jgv.0.000765. [DOI] [PubMed] [Google Scholar]
- 19.Wichgers Schreur P.J., Vloet R.P.M., Kant J., van Keulen L., Gonzales J.L., Visser T.M., Koenraadt C.J.M., Vogels C.B.F., Kortekaas J. Reproducing the Rift Valley fever virus mosquito-lamb-mosquito transmission cycle. Sci. Rep. 2021;11:1477. doi: 10.1038/s41598-020-79267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caroline A.L., Powell D.S., Bethel L.M., Oury T.D., Reed D.S., Hartman A.L. Broad Spectrum Antiviral Activity of Favipiravir (T-705): Protection from Highly Lethal Inhalational Rift Valley Fever. PLoS Negl. Trop. Dis. 2014;8:2–9. doi: 10.1371/journal.pntd.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwaśnik M., Rożek W., Rola J. Rift Valley fever—A growing threat to humans and animals. J. Vet. Res. 2021;65:7–14. doi: 10.2478/jvetres-2021-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turell M.J., Wilson W.C., Bennett K.E. Potential for North American mosquitoes (Diptera: Culicidae) to transmit rift Valley fever virus. J. Med. Entomol. 2010;47:884–889. doi: 10.1093/jmedent/47.5.884. [DOI] [PubMed] [Google Scholar]
- 23.Brustolin M., Talavera S., NuÑez A., SantamarÍa C., Rivas R., Pujol N., Valle M., Verdún M., Brun A., Pagès N., et al. Rift Valley fever virus and European mosquitoes: Vector competence of Culex pipiens and Stegomyia albopicta (=Aedes albopictus) Med. Vet. Entomol. 2017;31:365–372. doi: 10.1111/mve.12254. [DOI] [PubMed] [Google Scholar]
- 24.Hartman D.A., Rice L.M., DeMaria J., Borland E.M., Bergren N.A., Fagre A.C., Robb L.L., Webb C.T., Kading R.C. Entomological risk factors for potential transmission of Rift Valley fever virus around concentrations of livestock in Colorado. Transbound. Emerg. Dis. 2019;66:1709–1717. doi: 10.1111/tbed.13206. [DOI] [PubMed] [Google Scholar]
- 25.Cakir M., Obernier K., Forget A., Krogan N.J. Target Discovery for Host-Directed Antiviral Therapies: Application of Proteomics Approaches. mSystems. 2021;6:e0038821. doi: 10.1128/mSystems.00388-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson G.W., Slone T.W., Peters C.J. Pathogenesis of Rift Valley fever virus (RVFV) in inbred rats. Microb. Pathog. 1987;2:283–293. doi: 10.1016/0882-4010(87)90126-4. [DOI] [PubMed] [Google Scholar]
- 27.Kainulainen M., Lau S., Samuel C.E., Hornung V., Weber F. NSs Virulence Factor of Rift Valley Fever Virus Engages the F-Box Proteins FBXW11 and β-TRCP1 To Degrade the Antiviral Protein Kinase PKR. J. Virol. 2016;90:6140–6147. doi: 10.1128/JVI.00016-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman A. Rift Valley Fever virus. Physiol. Behav. 2017;176:139–148. doi: 10.1016/j.cll.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganaie S.S., Schwarz M.M., McMillen C.M., Price D.A., Feng A.X., Albe J.R., Wang W., Miersch S., Orvedahl A., Cole A.R., et al. Lrp1 is a host entry factor for Rift Valley fever virus. Cell. 2021;184:5163–5178.e24. doi: 10.1016/j.cell.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Léger P., Tetard M., Youness B., Cordes N., Rouxel R.N., Flamand M., Lozach P. Differential Use of the C-Type Lectins L-SIGN and DC-SIGN for Phlebovirus Endocytosis. Traffic. 2016;17:639–656. doi: 10.1111/tra.12393. [DOI] [PubMed] [Google Scholar]
- 31.Lozach P.Y., Kühbacher A., Meier R., Mancini R., Bitto D., Bouloy M., Helenius A. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe. 2011;10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Phoenix I., Lokugamage N., Nishiyama S., Ikegami T. Mutational analysis of the rift valley fever virus glycoprotein precursor proteins for Gn protein expression. Viruses. 2016;8:151. doi: 10.3390/v8060151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riblett A.M., Blomen V.A., Jae L.T., Altamura L.A., Doms R.W., Brummelkamp T.R., Wojcechowskyj J.A. A Haploid Genetic Screen Identifies Heparan Sulfate Proteoglycans Supporting Rift Valley Fever Virus Infection. J. Virol. 2016;90:1414–1423. doi: 10.1128/JVI.02055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Boer S.M., Kortekaas J., de Haan C.A.M., Rottier P.J.M., Moormann R.J.M., Bosch B.J. Heparan Sulfate Facilitates Rift Valley Fever Virus Entry into the Cell. J. Virol. 2012;86:13767–13771. doi: 10.1128/JVI.01364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann H., Li X., Zhang X., Liu W., Kühl A., Kaup F., Soldan S.S., González-Scarano F., Weber F., He Y., et al. Severe Fever with Thrombocytopenia Virus Glycoproteins Are Targeted by Neutralizing Antibodies and Can Use DC-SIGN as a Receptor for pH-Dependent Entry into Human and Animal Cell Lines. J. Virol. 2013;87:4384–4394. doi: 10.1128/JVI.02628-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bracci N., de la Fuente C., Saleem S., Pinkham C., Narayanan A., García-Sastre A., Balaraman V., Richt J.A., Wilson W., Kehn-Hall K. Rift Valley fever virus Gn V5-epitope tagged virus enables identification of UBR4 as a Gn interacting protein that facilitates Rift Valley fever virus production. Virology. 2022;567:65–76. doi: 10.1016/j.virol.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devignot S., Sha T.W., Burkard T., Schmerer P., Hagelkruys A., Mirazimi A., Elling U., Penninger J.M., Weber F. Low Density Lipoprotein Receptor-Related Protein 1 (LRP1) as an auxiliary host factor for RNA viruses. Life Sci. Alliance. 2023;6:e202302005. doi: 10.26508/lsa.202302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caplen H., Peters C.J., Bishop D.H.L. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J. Gen. Virol. 1985;66:2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- 39.Shivanna V., McDowell C., Wilson W.C., Richt J.A. Complete Genome Sequence of Two Rift Valley Fever Virus Strains Isolated from Outbreaks in Saudi Arabia (2000) and Kenya (2006 to 2007) Genome Announc. 2016;4:e00926-16. doi: 10.1128/genomeA.00926-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanjana N.E., Shalem O., Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joung J., Konermann S., Gootenberg J.S., Abudayyeh O.O., Platt R.J., Brigham M.D., E Sanjana N., Zhang F. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat. Protoc. 2017;12:828–863. doi: 10.1038/nprot.2017.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang B., Wang M., Zhang W., Xiao T., Chen C., Wu A., Wu F., Traugh N., Wang X., Li Z., et al. Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nat. Protoc. 2019;14:756–780. doi: 10.1038/s41596-018-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faburay B., Richt J.A. Short interfering RNA inhibits rift valley fever virus replication and degradation of protein kinase R in human cells. Front. Microbiol. 2016;7:1889. doi: 10.3389/fmicb.2016.01889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali H., Du Z., Li X., Yang Q., Zhang Y.C., Wu M., Li Y., Zhang G. Identification of suitable reference genes for gene expression studies using quantitative polymerase chain reaction in lung cancer in vitro. Mol. Med. Rep. 2015;11:3767–3773. doi: 10.3892/mmr.2015.3159. [DOI] [PubMed] [Google Scholar]
- 45.Haimes J., Kelley M. Demonstration of a ΔΔCq Calculation Method to Compute Relative Gene Expression from qPCR Data. Joshua GE HealthCare. 2010. [(accessed on 1 March 2022)]. Available online: https://horizondiscovery.com/-/media/Files/Horizon/resources/Technical-Manuals/delta-cq-solaris-technote.pdf.
- 46.Lee J., Yu H., Li Y., Ma J., Lang Y., Duff M., Henningson J., Liu Q., Li Y., Nagy A., et al. Impacts of different expressions of PA-X protein on 2009 pandemic H1N1 virus replication, pathogenicity and host immune responses. Virology. 2017;504:25–35. doi: 10.1016/j.virol.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habjan M., Penski N., Wagner V., Spiegel M., Överby A.K., Kochs G., Huiskonen J.T., Weber F. Efficient production of Rift Valley fever virus-like particles: The antiviral protein MxA can inhibit primary transcription of bunyaviruses. Virology. 2009;385:400–408. doi: 10.1016/j.virol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Hierholzer J.C., Killington R.A. Virology Methods Manual. Elsevier; Amsterdam, The Netherlands: 1996. Virus isolation and quantitation; pp. 25–46. [DOI] [Google Scholar]
- 50.Savidis G., McDougall W.M., Meraner P., Perreira J.M., Portmann J.M., Trincucci G., John S.P., Aker A.M., Renzette N., Robbins D.R., et al. Identification of Zika Virus and Dengue Virus Dependency Factors using Functional Genomics. Cell Rep. 2016;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 51.Li B., Clohisey S.M., Chia B.S., Wang B., Cui A., Eisenhaure T., Schweitzer L.D., Hoover P., Parkinson N.J., Nachshon A., et al. Genome-wide CRISPR screen identifies host dependency factors for influenza A virus infection. Nat. Commun. 2020;11:164. doi: 10.1038/s41467-019-13965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merkulova M., Paunescu T.G., Azroyan A., Marshansky V., Breton S., Brown D. Mapping the H+ (V)-ATPase interactome: Identification of proteins involved in trafficking, folding, assembly and phosphorylation. Sci. Rep. 2015;5:14827. doi: 10.1038/srep14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be obtained from the authors upon request.