Abstract
Background: Recent studies have raised concerns about genotoxic effects associated with titanium dioxide nanoparticles (TiO2 NPs), which are commonly used. This meta-analysis aims to investigate the potential genotoxicity of TiO2 NPs and explore influencing factors. Methods: This study systematically searched Chinese and English literature. The literature underwent quality evaluation, including reliability evaluation using the toxicological data reliability assessment method and relevance evaluation using routine evaluation forms. Meta-analysis and subgroup analyses were performed using R software, with the standardized mean difference (SMD) as the combined effect value. Results: A total of 26 studies met the inclusion criteria and passed the quality assessment. Meta-analysis results indicated that the SMD for each genotoxic endpoint was greater than 0. This finding implies a significant association between TiO2 NP treatment and DNA damage and chromosome damage both in vivo and in vitro and gene mutation in vitro. Subgroup analysis revealed that short-term exposure to TiO2 NPs increased DNA damage. Rats and cancer cells exhibited heightened susceptibility to DNA damage triggered by TiO2 NPs (p < 0.05). Conclusions: TiO2 NPs could induce genotoxicity, including DNA damage, chromosomal damage, and in vitro gene mutations. The mechanism of DNA damage response plays a key role in the genotoxicity induced by TiO2 NPs.
Keywords: titanium dioxide, nanoparticles, genotoxicity, hazard evaluation, meta-analysis
1. Introduction
Titanium dioxide nanoparticles (TiO2 NPs) are particles ranging in size from 1 to 100 nm in at least one dimension of three-dimensional space [1]. Compared to coarse TiO2 particles, TiO2 NPs exhibit enhanced conductivity, reactivity, photocatalytic activity, and permeability. These outstanding properties have positioned TiO2 NPs as one of the most extensively used nanomaterials, finding applications in various industries such as cosmetics, toothpaste, and drug carriers [2,3]. They are also widely used as food additives, primarily added to the coatings of dairy and confectionery products [4].
The unique physicochemical properties of nanoparticles bring about both application advantages and safety concerns. One major concern is that the NPs may increase cellular uptake rate and internalization behavior due to their diminutive size and extensive surface area [5]. Once inside a cell, NPs can disrupt normal cellular functions, leading to cell damage [6]. Moreover, nanomaterials have the potential to interact with molecules within organisms, interfering with biochemical reactions and signaling pathways, thereby affecting the entire biological system [7,8]. Additionally, solubility and ionization play important roles in the cellular responses and toxicity induced by NPs at the molecular, cellular, tissue, and systemic levels [9,10]. Therefore, the risk of adverse effects from TiO2 NPs may be amplified when there are additional routes of exposure and high levels of exposure, such as prolonged dermal contact and inhalation.
Evidence suggests TiO2 NPs may be genotoxic, including DNA and chromosomal damage. Based on the risk assessment report in 2021, the European Food Safety Authority (EFSA) updated its opinion that food-grade titanium dioxide (E171) was no longer a safe food additive. New research indicated that up to fifty percent of the NPs in E171 could induce DNA strand breakage and chromosome damage [11]. A meta-analysis focusing on the in vitro genotoxicity of TiO2 NPs revealed significant increases in tail DNA percentage, olive tail moment, and gene mutation rates [12]. Furthermore, Shi et al. [13] conducted a comprehensive review encompassing both in vivo and in vitro studies on TiO2 NPs, which collectively suggested the potential of these NPs to induce genotoxic effects. Both in vivo and in vitro tests confirmed the genotoxic nature of TiO2 NPs, with gene mutation and DNA strand breakage serving as sensitive genetic indicators [14]. Notably, the manifestation of genotoxicity depended not only on the particle surface, size, and exposure pathway but also on the duration and concentration of exposure [15].
This study systematically retrieved the latest literature from Chinese and English databases to evaluate the genotoxic effects of TiO2 NPs in vivo and in vitro; the selection of the eligible literature adhered to predefined inclusion and exclusion criteria. In addition, a comprehensive quality evaluation of the included literature was conducted, including reliability evaluation based on the toxicological data reliability assessment method and relevance evaluation using routine evaluation forms. The meta-analysis was performed separately for different genotoxic endpoints. Subgroup analyses were used to investigate potential influencing factors, such as particle size, experimental subjects, exposure duration, and exposure concentration. The primary objective of this study was to provide an up-to-date and comprehensive reference for assessing TiO2 NPs’ genotoxicity.
2. Materials and Methods
2.1. Search Strategy
This study comprehensively scoured relevant articles from databases, including PubMed, Web of Science (WoS), China National Knowledge Infrastructure (CNKI), and EFSA reports. The search was conducted using a set of keywords, which included “TiO2”, “Titanium dioxide”, “TiO2 NPs”, “genotoxicity”, “genotoxic”, “gene”, “DNA”, “chromosome”, and “mutation”. EFSA’s 2016 report on evaluating titanium dioxide as a food additive and common terminology found in CNKI’s translation assistant influenced the choice of these keywords. All papers in English and Chinese published before 30 June 2022 were considered for inclusion in this study.
2.2. Selection Criteria
The inclusion criteria in the systematic retrieval included (1) experimental research; (2) studies published in either Chinese or English; (3) mammalian cells or mammals as experimental subjects; (4) studies focused on genotoxic effects, such as gene mutation, chromosome aberration, DNA damage, oxidative stress, etc; and (5) the genotoxicity endpoints reported in the study that included the percentage of DNA in tail (T DNA%), tail length (TL), olive tail moment (OTM), mutation frequency (MF), frequency of micronucleus (MN), percentage of chromosomal aberrations (CA), etc.
The exclusion criteria were also established, including (1) non-original research such as case reports, comments, editorials, reviews, letters, or reports; (2) studies on the joint exposure of TiO2 with other substances or ultraviolet rays; (3) studies performed with TiO2 nanofibers, nanocomposites, nanotubes, or non-nanoparticles;(4) in vivo studies on non-oral exposure; (5) only epigenetics of genotoxic effects; and (6) no quantitative results or incomplete data.
2.3. Quality Assessment
Based on the reliability definition of Klimisch, the toxicological data reliability assessment method (TRAM) was developed to evaluate the reliability of toxicological data. The evaluation process incorporated the meticulous assessment of the physicochemical properties of the substances, as well as the conformance to established design standards governing toxicity tests. TRAM was an ideal tool for undertaking safety assessment in China, as it considered the soundness and validity of the research methodology employed in the studies under review [16].
The TRAM evaluation team comprised 18 experts from Jiangsu’s Center for Disease Control and Prevention (CDC). All members were required to possess professional qualifications in the field of toxicology, including a Master’s degree or higher, a minimum of three years of work experience, and intermediate or senior professional titles. Following TRAM training, the experts evaluated studies based on specific evaluation criteria tailored to different types of data. Each criterion was assigned a weighted score, which was then aggregated and converted into a percentage. Studies that received a score below 60% were categorized as having low reliability, those scoring between 60% and 80% were deemed to have medium reliability, and those scoring above 80% were classified as having high reliability. Studies falling into the “low reliability” category were promptly excluded from subsequent analyses to uphold the analytical rigor and integrity of the process.
Routine evaluation forms were used to determine the relevance of toxicological data. Furthermore, 17 experts from the CDC in Beijing were invited to participate in the evaluation process. The outcome of the evaluation was systematically categorized into three distinct classifications, namely “A”, “B”, or “C”, based on the extent of alignment with the research objectives and the applicability for hazard assessment. Notably, data assigned the label “A” signified a robust concurrence with the research objectives, rendering it highly recommended for hazard evaluation. In contrast, data allocated to the designation “B” embodied a moderated degree of correlation and held the potential for inclusion within the assessment framework. Under circumstances where there was a lack of substantial correlation, the corresponding research was assigned the classification of “C” and consequently excised from any further consideration. Furthermore, to indicate the degree of relevance between exposure route, duration, concentration, and risk assessment, a “+” symbol was added to the results.
2.4. Data Extraction
To extract useful information, researchers independently collected and recorded the following contents including (1) basic information, such as the lead author, publication year and country; (2) subject characteristics and interventions, such as species or cells, routes of administration, particle characteristics, treatment time, concentration and sample size (n); and (3) outcome measures, consisting of (a) T DNA%, TL, and OTL in comet assay, (b) MF in gene mutation assay, (c) MN frequency in MN assay, and (d) CA frequency in CA assay. The mean ± standard deviation (SD) was used to describe the outcome variables.
2.5. Statistical Analysis
In assessing the combined genotoxic effects of TiO2 NPs, the standardized mean difference (SMD) and its 95% confidence interval (CI) were employed. An SMD greater than 0 indicated higher genotoxicity in the exposed groups compared to control groups, while an SMD of 0 suggested no difference between the two groups.
Among the included studies, statistical heterogeneity was estimated by I-squared (I2) analysis. The significance of heterogeneity was determined by I2 > 50 or p < 0.05 in the Q-test. In instances where substantial heterogeneity was present among the individual studies, a random-effects model was employed. Conversely, a fixed-effects model was selected for the meta-analysis. Subgroup analysis was performed to identify the potential sources of heterogeneity and to examine the association between treatment variables (e.g., particle size, treatment object, exposure time, and concentration) and the genotoxic effects of TiO2 NPs. The stability and reliability of the meta-analysis results were assessed through sensitivity analysis. Considering the limitation of the included literature, a threshold of at least nine studies was established for conducting funnel plots and Egger test analyses to examine the potential for publication bias. All tests were two-tailed, and a significance level of p < 0.05 was adopted. R-4.2.0 software and the meta package were utilized for all statistical analyses.
3. Results
3.1. Literature Screening
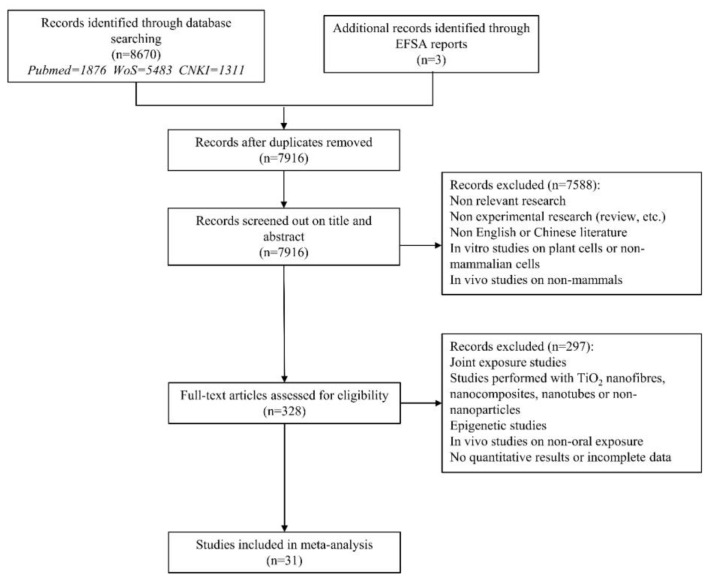
The process of the literature retrieval and screening is depicted in Figure 1. Of the total retrieved articles, 1876 were obtained from PubMed, 5483 from WoS, and 1311 from CNKI, resulting in a cumulative count of 8670 articles. After excluding 944 duplicate studies, the titles and abstracts of the remaining 7916 records were screened. From this initial filtering, 328 articles were retained for further consideration. Finally, a full-text screening identified 31 studies that met the eligibility criteria for inclusion. Among these, 12 studies were conducted in vivo, while the remaining 19 were conducted in vitro. The focal point of these studies was to discern the genotoxic potential of TiO2 NPs.
Figure 1.
Flow diagram of the literature search and screening.
3.2. Basic Characteristics and Quality Assessment
Information from in vivo genotoxicity research of TiO2 NPs is summarized in Table 1. The included studies were classified based on outcome indicators as T DNA% (six studies), TL/μm (three studies), OTM/μm (six studies), MN frequency (two studies), and CA frequency (five studies). The results of quality assessments indicated medium to high reliability, with relevance ratings of “B++” and above.
Table 1.
Basic characteristics and quality evaluation of the included studies on in vivo genotoxicity of TiO2 NPs 1.
| Included Studies | Country | Test Animals and Exposure Methods | TiO2-NP Characteristics | Dose (mg/kg bw) |
Exposure | Control | Reliability Evaluation | Correlation Evaluation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | Size (nm) |
Purity (%) |
n | Mean ± SD | n | Mean ± SD | ||||||
| Outcomes were described as T DNA% | ||||||||||||
| Shukla R. K. 2014 [17] | India | Male Swiss albino mice (continuous gavage for 14 d) | Anatase | 20–50 | 99.7 | 10 | 5 | 17.72 ± 0.72 | 5 | 14.29 ± 0.67 | high | A+++ |
| 50 | 5 | 18.98 ± 1.21 | 5 | 14.29 ± 0.67 | ||||||||
| 100 | 5 | 20.28 ± 1.11 | 5 | 14.29 ± 0.67 | ||||||||
| Martins A. D. C., Jr. 2017 [18] | Brazil | Male Wistar rats (continuous gavage for 45 d) | NA | 41.99 ± 1.63 | NA | 0.5 | 6 | 4.64 ± 0.82 | 6 | 3.6 ± 0.35 | medium | B+++ |
| Fadda L. M. 2018 [19] | Saudi Arabia | Male Wistar Albino rats (continuous gavage for 21 d) | Anatase | 60 ± 10 | NA | 1000 | 10 | 4.32 ± 0.24 | 10 | 2.26 ± 0.31 | medium | B+++ |
| Chakrabarti S. 2019 [20] | India | Female/male Swiss-Albino mice (oral for 90 d) | NA | 58.25 ± 8.11 | NA | 200 | 10 | 0.07 ± 0.012 (liver) 0.085 ± 0.009 (kidney) |
10 | 0.068 ± 0.007 (liver) 0.084 ± 0.004 (kidney) |
high | A+++ |
| 500 | 10 | 0.236 ± 0.066 (liver) 0.27 ± 0.075 (kidney) |
10 | 0.068 ± 0.007 (liver) 0.084 ± 0.004 (kidney) |
||||||||
| Sallam M. F. 2022 [21] | Egypt | Male SD rats (continuous gavage for 21 d) | NA | 50 ± 2.4 | NA | 50 | 10 | 19.25 ± 0.86 | 10 | 9.05 ± 0.25 | medium | B++ |
| Sallam M. F. 2022 [22] | Egypt | Male SD rats (continuous gavage for 21 d) | NA | 28 | NA | 50 | 10 | 18.74 ± 1.77 | 10 | 9.77 ± 1.24 | medium | B++ |
| Outcomes were described as TL (μm) | ||||||||||||
| Hassanein K. M. 2016 [23] | Egypt | Adult male SD rats (continuous gavage for 90 d) | NA | 21 | NA | 150 | 10 | 20.39 ± 1.6 | 10 | 10.57 ± 1.3 | medium | A+++ |
| Fadda L. M. 2018 [19] | Saudi Arabia | Male Wistar Albino rats (continuous gavage for 21 d) | NA | 60 ± 10 | NA | 1000 | 10 | 4.27 ± 0.10 | 10 | 1.14 ± 0.13 | medium | B+++ |
| Chakrabarti S. 2019 [20] | India | Female/male Swiss-Albino mice (oral for 90 d) | NA | 58.25 ± 8.11 | NA | 200 | 10 | 0.579 ± 0.041 (liver) 0.655 ± 0.009 (kidney) |
10 | 0.575 ± 0.028 (liver) 0.651 ± 0.007 (kidney) |
high | A+++ |
| 500 | 10 | 2.213 ± 0.059 (liver) 1.858 ± 0.041 (kidney) |
10 | 0.575 ± 0.028 (liver) 0.651 ± 0.007 (kidney) |
||||||||
| Outcomes were described as OTM (μm) | ||||||||||||
| Shukla R. K. 2014 [17] | India | Male Swiss albino mice (continuous gavage for 14 d) | Anatase | 20–50 | 99.7 | 10 | 5 | 2.71 ± 0.25 | 5 | 1.93 ± 0.14 | high | A+++ |
| 50 | 5 | 2.98 ± 0.22 | 5 | 1.93 ± 0.14 | ||||||||
| 100 | 5 | 3.76 ± 0.23 | 5 | 1.93 ± 0.14 | ||||||||
| Mohamed H. R. 2015 [24] | Egypt | Male Swiss Webster mice (continuous gavage for 5 d) | Anatase/Rutile | 46.23 ± 3.45 | 99.5 | 5 | 5 | 3.01 ± 0.36 | 5 | 1.86 ± 0.26 | medium | B+++ |
| 50 | 5 | 3.43 ± 0.71 | 5 | 1.86 ± 0.26 | ||||||||
| 500 | 5 | 5.78 ± 2.02 | 5 | 1.86 ± 0.26 | ||||||||
| Shi Z. 2015 [25] | China | Female/male wild-type ICR mice, Nrf2(-/-) ICR mice (continuous gavage for 7 d) | Anatase | 10–25 | 99.7 | 500 | 8 | 1.43 ± 0.15 (liver) 2.06 ± 0.28 (kidney) |
8 | 0.84 ± 0.30 (liver) 0.61 ± 0.24 (kidney) |
high | A+++ |
| 1000 | 8 | 3.29 ± 0.21 (liver) 4.33 ± 0.36 (kidney) |
8 | 0.84 ± 0.30 (liver) 0.61 ± 0.24 (kidney) |
||||||||
| 2000 | 8 | 8.59 ± 2.67 (liver) 8.07 ± 2.91 (kidney) |
8 | 0.84 ± 0.30 (liver) 0.61 ± 0.24 (kidney) |
||||||||
| Chakrabarti S. 2019 [20] | India | Female/male Swiss-Albino mice (oral for 90 d) | NA | 58.25 ± 8.11 | NA | 200 | 10 | 0.546 ± 0.041 (liver) 0.554 ± 0.01 (kidney) |
10 | 0.523 ± 0.025 (liver) 0.549 ± 0.007 (kidney) |
high | A+++ |
| 500 | 10 | 0.835 ± 0.074 (liver) 0.758 ± 0.026 (kidney) |
10 | 0.523 ± 0.025 (liver) 0.549 ± 0.007 (kidney) |
||||||||
| Sallam M. F. 2022 [21] | Egypt | Male SD rats (continuous gavage for 21 d) | NA | 50 ± 2.4 | NA | 50 | 10 | 2.74 ± 0.17 | 10 | 1.08 ± 0.04 | medium | B++ |
| Sallam M. F. 2022 [22] | Egypt | Male SD rats (continuous gavage for 21 d) | NA | 28 | NA | 50 | 10 | 3.57 ± 0.14 | 10 | 1.12 ± 0.02 | medium | B++ |
| Outcomes were described as MN frequency (MN/1000 PCEs) | ||||||||||||
| Shukla R. K. 2014 [17] | India | Male Swiss albino mice (continuous gavage for 14 d) | Anatase | 20–50 | 99.7 | 10 | 5 | 1.50 ± 0.51 | 5 | 1.20 ± 0.20 | high | A+++ |
| 50 | 5 | 2.25 ± 0.49 | 5 | 1.20 ± 0.20 | ||||||||
| 100 | 5 | 3.0 ± 0.68 | 5 | 1.20 ± 0.20 | ||||||||
| Chakrabarti S. 2019 [20] | India | Female/male Swiss-Albino mice (oral for 90 d) | NA | 58.25 ± 8.11 | NA | 200 | 10 | 5.83 ± 0.75 | 10 | 0.16 ± 0.40 | high | A+++ |
| 500 | 10 | 7.16 ± 0.75 | 10 | 0.16 ± 0.40 | ||||||||
| Outcomes were described as CA frequency | ||||||||||||
| Ali S. A. 2019 [26] | Egypt | Male Swiss albino mice (continuous oral for 5 d) | NA | 21 | NA | 50 | 15 | 13.30 ± 0.98 | 15 | 4.72 ± 0.24 | medium | A+++ |
| 250 | 15 | 15.80 ± 0.34 | 15 | 4.72 ± 0.24 | ||||||||
| 500 | 15 | 31.70 ± 0.67 | 15 | 4.72 ± 0.24 | ||||||||
| Ali S. A. 2019 [26] | Egypt | Male Swiss albino mice (continuous oral for 5 d) | NA | 80 | NA | 50 | 15 | 12.00 ± 0.66 | 15 | 4.72 ± 0.24 | medium | A+++ |
| 250 | 15 | 15.00 ± 0.69 | 15 | 4.72 ± 0.24 | ||||||||
| 500 | 15 | 24.00 ± 1.67 | 15 | 4.72 ± 0.24 | ||||||||
| Manivannan J. 2019 [27] | India | Male Swiss albino mice (continuous gavage for 28 d) | Rutile | 25.074 ± 3.593 | NA | 0.2 | 5 | 0.05 ± 0.04 | 5 | 0.01 ± 0.01 | high | B+++ |
| 0.4 | 5 | 0.14 ± 0.04 | 5 | 0.01 ± 0.01 | ||||||||
| 0.8 | 5 | 0.19 ± 0.03 | 5 | 0.01 ± 0.01 | ||||||||
| Chakrabarti S. 2019 [20] | India | Female/male Swiss-Albino mice (oral for 90 d) | NA | 58.25 ± 8.11 | NA | 200 | 10 | 0.83 ± 0.23 | 10 | 0.76 ± 0.29 | high | A+++ |
| 500 | 10 | 1.9 ± 0.20 | 10 | 0.76 ± 0.29 | ||||||||
| Salman A. S. 2021 [28] | Germany | Male Balb/c mice (continuous gavage for 21 d) | NA | 28.9 | NA | 25 | 6 | 13.2 ± 0.35 | 6 | 1.6 ± 0.2 | high | A+++ |
1 NA: not applicable; n: sample size; SD: standard deviation; T DNA%: the percentage of DNA in tail; TL: tail length; OTM: olive tail moment; MF: mutation frequency; MN/1000 PCEs: no. of micronucleus/1000 polychromatic erythrocytes; CA frequency: percentage of cells exhibiting chromosomal aberrations.
Table 2 provides information on in vitro genotoxicity studies of TiO2 NPs. An article with a determined reliability assessment of “low” and four articles exhibiting a correlation evaluation result of “C” were excluded from the meta-analysis. Outcome indicators classified the included studies as T DNA% (nine studies), TL/μm (two studies), OTM/μm (six studies), MF (three studies), MN frequency (seven studies), and CA frequency (two studies).
Table 2.
Basic characteristics and quality evaluation of the included studies on in vitro genotoxicity of TiO2 NPs 1.
| Included Studies | Country | Test Cells and Exposure Methods | TiO2-NP Characteristics | Concentration (μg/mL) |
Exposure | Control | Reliability Evaluation | Correlation Evaluation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | Size (nm) |
Purity (%) |
n | Mean ± SD | n | Mean ± SD | ||||||
| Outcomes were described as T DNA% | ||||||||||||
| Shukla R. K. 2011 [29] | India | Human epidermal cells line A431, exposed for 6 h | Anatase | 50 | 99.7 | 0.008 | 3 | 9.72 ± 0.78 | 3 | 9.36 ± 0.69 | high | B |
| 0.08 | 3 | 9.76 ± 0.40 | 3 | 9.36 ± 0.69 | ||||||||
| 0.8 | 3 | 11.79 ± 0.94 | 3 | 9.36 ± 0.69 | ||||||||
| 8 | 3 | 2.35 ± 0.43 | 3 | 9.36 ± 0.69 | ||||||||
| 80 | 3 | 12.89 ± 0.47 | 3 | 9.36 ± 0.69 | ||||||||
| Hong L. 2011 [30] | China | Human lung adenocarcinoma cells, exposed for 6 h | NA | 5–10 | >99.9 | 25 | 25 | 9.94 ± 6.72 | 25 | 5.53 ± 3.70 | medium | A+++ |
| 50 | 25 | 14.26 ± 13.67 | 25 | 5.53 ± 3.70 | ||||||||
| 100 | 25 | 12.37 ± 5.16 | 25 | 5.53 ± 3.70 | ||||||||
| 200 | 25 | 9.47 ± 4.97 | 25 | 5.53 ± 3.70 | ||||||||
| Shukla R. K. 2013 [31] | India | HepG2 human hepatocellular carcinoma cells, exposed for 6 h | Anatase | 30–70 | 99.7 | 1 | 3 | 8.61 ± 0.67 | 3 | 7.75 ± 0.36 | high | B |
| 10 | 3 | 9.13 ± 0.54 | 3 | 7.75 ± 0.36 | ||||||||
| 20 | 3 | 10.53 ± 0.49 | 3 | 7.75 ± 0.36 | ||||||||
| 40 | 3 | 11.61 ± 0.38 | 3 | 7.75 ± 0.36 | ||||||||
| 80 | 3 | 13.55 ± 0.43 | 3 | 7.75 ± 0.36 | ||||||||
| Chen Z. 2014 [14] | China | V79 cells, exposed for 6 h, 24 h | Anatase | 75 ± 15 | 99.90 | 5 | 3 | 12.863 ± 11.00(6 h) 7.557 ± 6.846(24 h) |
3 | 11.836 ± 6.073(6 h) 6.000 ± 6.866(24 h) |
high | A+++ |
| 20 | 3 | 11.470 ± 8.074(6 h) 9.007 ± 10.417(24 h) |
3 | 11.836 ± 6.073(6 h) 6.000 ± 6.866(24 h) |
||||||||
| 100 | 3 | 12.094 ± 7.677(6 h) 9.005 ± 7.177(24 h) |
3 | 11.836 ± 6.073(6 h) 6.000 ± 6.866(24 h) |
||||||||
| Frenzilli G. 2014 [32] | Italy | Human fibroblast (HuDE), exposed for 4 h, 24 h and 48 h | Anatase | 20–50 | 99.7 | 20 | 2 | 16.5 ± 1.9(4 h) 14.0 ± 3.7(24 h) 20.3 ± 5.3(48 h) |
2 | 12.1 ± 1.8(4 h) 13.7 ± 2.3(24 h) 20.3 ± 6.6(48 h) |
medium | B+ |
| 50 | 2 | 18.6 ± 3.3(4 h) 16.3 ± 5.7(24 h) 20.9 ± 1.7(48 h) |
2 | 12.1 ± 1.8(4 h) 13.7 ± 2.3(24 h) 20.3 ± 6.6(48 h) |
||||||||
| 100 | 2 | 23.4 ± 4.7(4 h) 17.3 ± 2.9(24 h) 21.0 ± 4.1(48 h) |
2 | 12.1 ± 1.8(4 h) 13.7 ± 2.3(24 h) 20.3 ± 6.6(48 h) |
||||||||
| 150 | 2 | 25.0 ± 2.6(4 h) 16.4 ± 8.6(24 h) 20.8 ± 6.7(48 h) |
2 | 12.1 ± 1.8(4 h) 13.7 ± 2.3(24 h) 20.3 ± 6.6(48 h) |
||||||||
| Frenzilli G. 2014 [32] | Italy | Bottlenose dolphin fibroblast (BDF), exposed for 4 h, 24 h and 48 h | Anatase | 20–50 | 99.7 | 20 | 2 | 34.6 ± 10.5(4 h) 38.4 ± 2.5(24 h) 32.7 ± 14.8(48 h) |
2 | 22.6 ± 6.5(4 h) 17.6 ± 2.1(24 h) 13.5 ± 5.2(48 h) |
medium | B+ |
| 50 | 2 | 31.1 ± 8.0(4 h) 25.6 ± 5.1(24 h) 27.3 ± 9.3(48 h) |
2 | 22.6 ± 6.5(4 h) 17.6 ± 2.1(24 h) 13.5 ± 5.2(48 h) |
||||||||
| 100 | 2 | 34.8 ± 7.2(4 h) 21.9 ± 1.9(24 h) 25.2 ± 2.4(48 h) |
2 | 22.6 ± 6.5(4 h) 17.6 ± 2.1(24 h) 13.5 ± 5.2(48 h) |
||||||||
| 150 | 2 | 21.2 ± 9.6(4 h) 25.0 ± 0.1(24 h) 25.9 ± 7.6(48 h) |
2 | 22.6 ± 6.5(4 h) 17.6 ± 2.1(24 h) 13.5 ± 5.2(48 h) |
||||||||
| Frenzilli G. 2014 [32] | Italy | Mouse fibroblast (3 T3), exposed for 4 h, 24 h and 48 h | Anatase | 20–50 | 99.7 | 20 | 2 | 24.4 ± 3.1(4 h) 21.4 ± 14.9(24 h) 18.3 ± 5.1(48 h) |
2 | 17.2 ± 4.2(4 h) 14.5 ± 2.7(24 h) 22.1 ± 5.3(48 h) |
medium | B+ |
| 50 | 2 | 21.8 ± 4.3(4 h) 26.0 ± 9.1(24 h) 28.3 ± 10.1(48 h) |
2 | 17.2 ± 4.2(4 h) 14.5 ± 2.7(24 h) 22.1 ± 5.3(48 h) |
||||||||
| 100 | 2 | 13.8 ± 2.7(4 h) 14.5 ± 4.8(24 h) 21.0 ± 3.9(48 h) |
2 | 17.2 ± 4.2(4 h) 14.5 ± 2.7(24 h) 22.1 ± 5.3(48 h) |
||||||||
| 150 | 2 | 18.8 ± 2.0(4 h) 15.9 ± 1.8(24 h) 26.3 ± 4.9(48 h) |
2 | 17.2 ± 4.2(4 h) 14.5 ± 2.7(24 h) 22.1 ± 5.3(48 h) |
||||||||
| Frenzilli G. 2014 [32] | Italy | Human leukocytes (HL), exposed for 4 h, 24 h and 48 h | Anatase | 20–50 | 99.7 | 20 | 2 | 10.6 ± 4.5(4 h) 14.6 ± 5.9(24 h) 14.7 ± 3.2(48 h) |
2 | 8.3 ± 2.3(4 h) 11.8 ± 3.2(24 h) 10.0 ± 2.1(48 h) |
medium | B+ |
| 50 | 2 | 12.3 ± 4.4(4 h) 11.2 ± 2.5(24 h) 14.4 ± 7.8(48 h) |
2 | 8.3 ± 2.3(4 h) 11.8 ± 3.2(24 h) 10.0 ± 2.1(48 h) |
||||||||
| 100 | 2 | 13.2 ± 4.8(4 h) 13.1 ± 2.5(24 h) 12.6 ± 5.1(48 h) |
2 | 8.3 ± 2.3(4 h) 11.8 ± 3.2(24 h) 10.0 ± 2.1(48 h) |
||||||||
| Frenzilli G. 2014 [32] | Italy | Bottlenose dolphin leukocytes (BDL), exposed for4 h, 24 h and 48 h | Anatase | 20–50 | 99.7 | 20 | 2 | 33.8 ± 15.1(4 h) 44.5 ± 22.6(24 h) 29.5 ± 9.7(48 h) |
2 | 25.5 ± 10.6(4 h) 35.2 ± 19.5(24 h) 36.1 ± 14.3(48 h) |
medium | B+ |
| 50 | 2 | 27.8 ± 7.8(4 h) 50.4 ± 19.4(24 h) 44.9 ± 18.8(48 h) |
2 | 25.5 ± 10.6(4 h) 35.2 ± 19.5(24 h) 36.1 ± 14.3(48 h) |
||||||||
| 100 | 2 | 35.3 ± 15.9(4 h) 47.5 ± 16.2(24 h) 43.9 ± 12.1(48 h) |
2 | 25.5 ± 10.6(4 h) 35.2 ± 19.5(24 h) 36.1 ± 14.3(48 h) |
||||||||
| Demir E. 2015 [33] | Spain | Human embryonic kidney cells (HEK293), cultured for 1 h | Rutile | 21 | ≥99.5 | 10 | 4 | 14.11 ± 0.21 | 4 | 11.31 ± 0.67 | high | A |
| 100 | 4 | 15.11 ± 0.22 | 4 | 11.31 ± 0.67 | ||||||||
| 1000 | 4 | 32.21 ± 0.77 | 4 | 11.31 ± 0.67 | ||||||||
| 50 | ≥98 | 10 | 4 | 12.89 ± 0.75 | 4 | 11.31 ± 0.67 | ||||||
| 100 | 4 | 13.88 ± 0.65 | 4 | 11.31 ± 0.67 | ||||||||
| 1000 | 4 | 30.29 ± 0.67 | 4 | 11.31 ± 0.67 | ||||||||
| Demir E. 2015 [33] | Spain | Mouse embryonic kidney cells (NIH/3 T3), cultured for 1 h | Rutile | 21 | ≥99.5 | 10 | 4 | 14.10 ± 0.27 | 4 | 12.31 ± 0.17 | high | A |
| 100 | 4 | 15.41 ± 0.29 | 4 | 12.31 ± 0.17 | ||||||||
| 1000 | 4 | 35.91 ± 0.57 | 4 | 12.31 ± 0.17 | ||||||||
| 50 | ≥98 | 10 | 4 | 12.10 ± 0.78 | 4 | 12.31 ± 0.17 | ||||||
| 100 | 4 | 13.59 ± 0.73 | 4 | 12.31 ± 0.17 | ||||||||
| 1000 | 4 | 31.77 ± 0.60 | 4 | 12.31 ± 0.17 | ||||||||
| Kansara K. 2015 [34] | India | Human lung cancer cell line (A549), exposed for 6 h | Rutile | 4–8 | 99.7 | 25 | 3 | 5.14 ± 0.12 | 3 | 4.48 ± 0.11 | medium | B |
| 50 | 3 | 6.06 ± 0.15 | 3 | 4.48 ± 0.11 | ||||||||
| 75 | 3 | 8.25 ± 0.24 | 3 | 4.48 ± 0.11 | ||||||||
| 100 | 3 | 9.49 ± 0.25 | 3 | 4.48 ± 0.11 | ||||||||
| Andreoli C. 2018 [35] | Italy | Peripheral blood monocytes, exposed for 24 h | Anatase | 20–60 | >99.5 | 10 | 4 | 1.14 ± 0.23 | 4 | 0.52 ± 0.12 | medium | A |
| 50 | 4 | 1.62 ± 0.47 | 4 | 0.52 ± 0.12 | ||||||||
| 100 | 4 | 2.01 ± 0.66 | 4 | 0.52 ± 0.12 | ||||||||
| 200 | 4 | 1.54 ± 0.52 | 4 | 0.52 ± 0.12 | ||||||||
| Andreoli C. 2018 [35] | Italy | Peripheral blood monocytes, exposed for 24 h | Rutile | 30 × 100 | >99.5 | 10 | 4 | 1.19 ± 0.19 | 4 | 0.44 ± 0.05 | medium | A |
| 50 | 4 | 2.33 ± 0.68 | 4 | 0.44 ± 0.05 | ||||||||
| 100 | 4 | 2.62 ± 0.54 | 4 | 0.44 ± 0.05 | ||||||||
| 200 | 4 | 3.48 ± 1.59 | 4 | 0.44 ± 0.05 | ||||||||
| Andreoli C. 2018 [35] | Italy | Peripheral blood monocytes, exposed for 24 h | Anatase/Rutile | 45–262 | >99.5 | 10 | 4 | 1.30 ± 0.04 | 4 | 0.34 ± 0.01 | medium | A |
| 50 | 4 | 2.51 ± 0.96 | 4 | 0.34 ± 0.01 | ||||||||
| 100 | 4 | 4.44 ± 0.18 | 4 | 0.34 ± 0.01 | ||||||||
| 200 | 4 | 4.45 ± 0.09 | 4 | 0.34 ± 0.01 | ||||||||
| Osman I. F. 2018 [36] | UK | Lymphocytes from patients with respiratory diseases, exposed for 72 h | Anatase | 40–70 | 99.7 | 10 | 40 | 17.7 ± 5.4 | 40 | 15.4 ± 5.3 | high | B |
| 30 | 40 | 19.0 ± 5.5 | 40 | 15.4 ± 5.3 | ||||||||
| 50 | 40 | 23.3 ± 6.5 | 40 | 15.4 ± 5.3 | ||||||||
| Osman I. F. 2018 [36] | UK | Lymphocytes from healthy people, exposed for 72 h | Anatase | 40–70 | 99.7 | 10 | 12 | 12.4 ± 6.1 | 12 | 10.2 ± 4.7 | high | B |
| 30 | 12 | 13.8 ± 5.5 | 12 | 10.2 ± 4.7 | ||||||||
| 50 | 12 | 15.3 ± 6.3 | 12 | 10.2 ± 4.7 | ||||||||
| Outcomes were described as TL (μm) | ||||||||||||
| Hong L. 2011 [30] | China | Human lung adenocarcinoma cells, exposed for 6 h | NA | 5–10 | >99.9 | 25 | 25 | 65.23 ± 26.86 | 25 | 37.50 ± 15.35 | medium | A+++ |
| 50 | 25 | 78.19 ± 37.43 | 25 | 37.50 ± 15.35 | ||||||||
| 100 | 25 | 69.54 ± 20.61 | 25 | 37.50 ± 15.35 | ||||||||
| 200 | 25 | 66.18 ± 17.87 | 25 | 37.50 ± 15.35 | ||||||||
| Ünal F. 2021 [37] | Turkey | Human lymphocytes, exposed for 30 min | NA | <100 | NA | 20 | 3 | 51.60 ± 0.64 | 3 | 52.70 ± 0.55 | medium | A+++ |
| 40 | 3 | 53.49 ± 0.68 | 3 | 52.70 ± 0.55 | ||||||||
| 60 | 3 | 54.29 ± 0.70 | 3 | 52.70 ± 0.55 | ||||||||
| 80 | 3 | 54.38 ± 0.63 | 3 | 52.70 ± 0.55 | ||||||||
| 100 | 3 | 57.59 ± 1.02 | 3 | 52.70 ± 0.55 | ||||||||
| Outcomes were described as OTM (μm) | ||||||||||||
| Shi Y. 2010 [38] | China | Human fetal liver L-02 cells, exposed for 24 h | Anatase/Rutile | 30–50 | NA | 0.01 | 9 | 0.91 ± 0.75 | 9 | 0.79 ± 0.74 | high | C |
| 0.1 | 9 | 1.28 ± 0.96 | 9 | 0.79 ± 0.74 | ||||||||
| 1 | 9 | 1.30 ± 1.01 | 9 | 0.79 ± 0.74 | ||||||||
| Du H. 2012 [39] | China | Human fetal liver L-02 cells, exposed for 24 h | NA | 25–50 | >99.5 | 0.001 | 3 | 0.67 ± 0.09 | 3 | 0.65 ± 0.06 | median | C |
| 0.01 | 3 | 0.68 ± 0.10 | 3 | 0.65 ± 0.06 | ||||||||
| 0.1 | 3 | 0.71 ± 0.08 | 3 | 0.65 ± 0.06 | ||||||||
| 1 | 3 | 0.73 ± 0.09 | 3 | 0.65 ± 0.06 | ||||||||
| 10 | 3 | 0.76 ± 0.09 | 3 | 0.65 ± 0.06 | ||||||||
| Shukla R. K. 2011 [29] | India | Human epidermal cell line A431, exposed for 6 h | Anatase | 50 | 99.7 | 0.008 | 3 | 1.27 ± 0.05 | 3 | 1.20 ± 0.01 | high | B |
| 0.08 | 3 | 1.30 ± 0.03 | 3 | 1.20 ± 0.01 | ||||||||
| 0.8 | 3 | 1.43 ± 0.09 | 3 | 1.20 ± 0.01 | ||||||||
| 8 | 3 | 1.79 ± 0.08 | 3 | 1.20 ± 0.01 | ||||||||
| 80 | 3 | 1.91 ± 0.04 | 3 | 1.20 ± 0.01 | ||||||||
| Hong L. 2011 [30] | China | Human lung adenocarcinoma cells, exposed for 6 h | NA | 5–10 | >99.9 | 25 | 25 | 12.08 ± 8.45 | 25 | 4.27 ± 2.76 | medium | A+++ |
| 50 | 25 | 12.43 ± 10.79 | 25 | 4.27 ± 2.76 | ||||||||
| 100 | 25 | 12.48 ± 2.71 | 25 | 4.27 ± 2.76 | ||||||||
| 200 | 25 | 8.46 ± 4.73 | 25 | 4.27 ± 2.76 | ||||||||
| Shukla R. K. 2013 [31] | India | HepG2 human hepatocellular hepatoma cells, exposed for 6 h | Anatase | 30–70 | 99.7 | 1 | 3 | 1.13 ± 0,06 | 3 | 0.94 ± 0.06 | high | B |
| 10 | 3 | 1.20 ± 0.05 | 3 | 0.94 ± 0.06 | ||||||||
| 20 | 3 | 1.40 ± 0.02 | 3 | 0.94 ± 0.06 | ||||||||
| 40 | 3 | 1.55 ± 0.07 | 3 | 0.94 ± 0.06 | ||||||||
| 80 | 3 | 1.76 ± 0.09 | 3 | 0.94 ± 0.06 | ||||||||
| Chen Z. 2014 [14] | China | V79 cells, exposed for 6 h, 24 h | Anatase | 75 ± 15 | 99.90 | 5 | 3 | 5.857 ± 6.198(6 h) 3.113 ± 4.285(24 h) |
3 | 4.698 ± 3.375(6 h) 2.576 ± 3.928(24 h) |
high | A+++ |
| 20 | 3 | 5.086 ± 4.700(6 h) 4.174 ± 7.453(24 h) |
3 | 4.698 ± 3.375(6 h) 2.576 ± 3.928(24 h) |
||||||||
| 100 | 3 | 4.999 ± 4.594(6 h) 3.870 ± 4.116(24 h) |
3 | 4.698 ± 3.375(6 h) 2.576 ± 3.928(24 h) |
||||||||
| Ryu A. R. 2016 [40] | Korea | Peripheral blood lymphocytes of rats, exposed for 30 min | NA | NA | NA | 60 | 6 | 23.08 ± 0.52 | 6 | 8.79 ± 2.18 | low | B |
| 80 | 6 | 25.66 ± 6.11 | 6 | 8.79 ± 2.18 | ||||||||
| Osman I. F. 2018 [36] | UK | Lymphocytes from patients with respiratory diseases, exposed for 72 h | Anatase | 40–70 | 99.7 | 10 | 40 | 4.3 ± 1.6 | 40 | 3..7 ± 1.5 | high | B |
| 30 | 40 | 5.0 ± 2.0 | 40 | 3..7 ± 1.5 | ||||||||
| 50 | 40 | 6.2 ± 2.2 | 40 | 3..7 ± 1.5 | ||||||||
| Osman I. F. 2018 [36] | UK | Lymphocytes from healthy people, exposed for 72 h | Anatase | 40–70 | 99.7 | 10 | 12 | 2.3 ± 1.0 | 12 | 1.8 ± 0.7 | high | B |
| 30 | 12 | 2.7 ± 1.0 | 12 | 1.8 ± 0.7 | ||||||||
| 50 | 12 | 3.2 ± 1.2 | 12 | 1.8 ± 0.7 | ||||||||
| Ünal F. 2021 [37] | Turkey | Human lymphocytes, exposed for 30 min | NA | <100 | NA | 20 | 3 | 1.01 ± 0.11 | 3 | 1.03 ± 0.09 | medium | A+++ |
| 40 | 3 | 1.59 ± 0.29 | 3 | 1.03 ± 0.09 | ||||||||
| 60 | 3 | 1.73 ± 0.36 | 3 | 1.03 ± 0.09 | ||||||||
| 80 | 3 | 1.49 ± 0.25 | 3 | 1.03 ± 0.09 | ||||||||
| 100 | 3 | 1.90 ± 0.41 | 3 | 1.03 ± 0.09 | ||||||||
| Outcomes were described as MF | ||||||||||||
| Xu A. 2009 [41] | US | Primary embryonic fibroblasts of transgenic mice, incubated in medium for 24 h | Anatase | 5 | 99.7 | 0.1 | 3 | 12.52 ± 4.11 | 3 | 5.69 ± 1.87 | medium | B |
| Chen Z. 2014 [14] | China | V79 cells, exposed for 24 h | Anatase | 75 ± 15 | 99.9 | 100 | 3 | 22.7 ± 3.0 | 3 | 8.7 ± 1.2 | high | A+++ |
| Jain A. K. 2017 [42] | India | Chinese hamster lung fibroblasts (V-79), exposed for 6 h | Anatase | 12–25 | 99.7 | 100 | 3 | 23.0 ± 2.6 | 3 | 7.7 ± 2.1 | medium | A++ |
| Outcomes were described as MN frequency (BiMN) | ||||||||||||
| Shi Y. 2010 [38] | China | Human fetal liver L-02 cells, exposed for 24 h | Anatase/Rutile | 30–50 | NA | 0.01 | 9 | 0.91 ± 0.75 | 9 | 0.79 ± 0.74 | high | C |
| 0.1 | 9 | 1.28 ± 0.96 | 9 | 0.79 ± 0.74 | ||||||||
| 1 | 9 | 1.30 ± 1.01 | 9 | 0.79 ± 0.74 | ||||||||
| Kang S. J. 2008 [43] | South Korea | Peripheral blood lymphocytes, exposed for 20 h | Anatase/Rutile | 25 | NA | 20 | 3 | 15.00 ± 1.00 | 3 | 9.33 ± 1.52 | median | C |
| 50 | 3 | 18.33 ± 2.08 | 3 | 9.33 ± 1.52 | ||||||||
| 100 | 3 | 23.67 ± 0.58 | 3 | 9.33 ± 1.52 | ||||||||
| Reis É.deM 2016 [44] | Brazil | V79 cells, exposed for 3 h | Anatase | 3.4 | 99.7 | 30 | 3 | 6.67 ± 1.15 | 3 | 7.00 ± 1.00 | high | C |
| 60 | 3 | 12.00 ± 1.00 | 3 | 7.00 ± 1.00 | ||||||||
| 120 | 3 | 14.67 ± 2.06 | 3 | 7.00 ± 1.00 | ||||||||
| Reis É.deM 2016 [44] | Brazil | V79 cells, exposed for 3 h | Anatase | 6.2 | 99.7 | 30 | 3 | 11.33 ± 2.31 | 3 | 7.00 ± 1.00 | high | C |
| 60 | 3 | 8.33 ± 1.15 | 3 | 7.00 ± 1.00 | ||||||||
| 120 | 3 | 10.00 ± 2.00 | 3 | 7.00 ± 1.00 | ||||||||
| Reis É.deM 2016 [44] | Brazil | V79 cells, exposed for 3 h | Anatase | 78 | 99.7 | 30 | 3 | 5.33 ± 1.53 | 3 | 7.00 ± 1.00 | high | C |
| 60 | 3 | 7.67 ± 1.15 | 3 | 7.00 ± 1.00 | ||||||||
| 120 | 3 | 12.33 ± 2.52 | 3 | 7.00 ± 1.00 | ||||||||
| Shukla R. K. 2011 [29] | India | Human epidermal cell line A431, exposed for 6 h | Anatase | 50 | 99.7 | 0.008 | 3 | 11.67 ± 1.20 | 3 | 9.33 ± 1.00 | high | B |
| 0.08 | 3 | 12.67 ± 0.88 | 3 | 9.33 ± 1.00 | ||||||||
| 0.8 | 3 | 14.67 ± 1.20 | 3 | 9.33 ± 1.00 | ||||||||
| 8 | 3 | 15.67 ± 0.88 | 3 | 9.33 ± 1.00 | ||||||||
| 80 | 3 | 16.00 ± 0.58 | 3 | 9.33 ± 1.00 | ||||||||
| Srivastava R. K. 2013 [45] | India | Human lung cancer cell line (A549), exposed for 24 h | Anatase | <25 | NA | 10 | 3 | 12.66 ± 0.33 | 3 | 5.33 ± 0.33 | medium | B |
| 50 | 3 | 17.33 ± 0.33 | 3 | 5.33 ± 0.33 | ||||||||
| Shukla R. K. 2013 [31] | India | HepG2 human hepatocellular carcinoma cells, exposed for 6 h | Anatase | 30–70 | 99.7 | 1 | 3 | 8.00 ± 1.15 | 3 | 7.00 ± 0.58 | high | B |
| 10 | 3 | 11.00 ± 1.53 | 3 | 7.00 ± 0.58 | ||||||||
| 20 | 3 | 15.00 ± 0.58 | 3 | 7.00 ± 0.58 | ||||||||
| 40 | 3 | 12.33 ± 0.33 | 3 | 7.00 ± 0.58 | ||||||||
| 80 | 3 | 10.67 ± 0.88 | 3 | 7.00 ± 0.58 | ||||||||
| Kansara K. 2015 [34] | India | Human lung cancer cell line (A549), exposed for 6 h | Anatase | 4–8 | 99.7 | 25 | 3 | 7.33 ± 1.20 | 3 | 6.00 ± 2.80 | medium | B |
| 50 | 3 | 9.66 ± 2.84 | 3 | 6.00 ± 2.80 | ||||||||
| 75 | 3 | 12.33 ± 2.96 | 3 | 6.00 ± 2.80 | ||||||||
| 100 | 3 | 14.66 ± 2.33 | 3 | 6.00 ± 2.80 | ||||||||
| Andreoli C. 2018 [35] | Italy | Peripheral blood monocytes, exposed for 24 h | Anatase | 20–60 | >99.5 | 50 | 2 | 9.0 ± 1.41 | 2 | 8.5 ± 0.71 | medium | A |
| 100 | 2 | 10.0 ± 4.24 | 2 | 8.5 ± 0.71 | ||||||||
| Andreoli C. 2018 [35] | Italy | Peripheral blood monocytes, exposed for 24 h | Rutile | 30 × 100 | >99.5 | 50 | 2 | 9.0 ± 2.83 | 2 | 7.5 ± 3.54 | medium | A |
| 100 | 2 | 7.0 ± 2.83 | 2 | 7.5 ± 3.54 | ||||||||
| 200 | 2 | 8.0 ± 1.41 | 2 | 7.5 ± 3.54 | ||||||||
| Andreoli C. 2018 [35] | Italy | Peripheral blood monocytes, exposed for 24 h | Anatase/Rutile | 45–262 | >99.5 | 50 | 2 | 9.5 ± 0.71 | 2 | 9.5 ± 0.71 | medium | A |
| 100 | 2 | 8.0 ± 4.24 | 2 | 9.5 ± 0.71 | ||||||||
| 200 | 2 | 5.5 ± 2.12 | 2 | 9.5 ± 0.71 | ||||||||
| Osman I. F. 2018 [36] | UK | Lymphocytes from patients with respiratory diseases, exposed for 72 h | Anatase | 40–70 | 99.7 | 5 | 40 | 8.29 ± 1.55 | 40 | 8.54 ± 1.40 | high | B |
| 10 | 40 | 11.03 ± 1.70 | 40 | 8.54 ± 1.40 | ||||||||
| Osman I. F. 2018 [36] | UK | Lymphocytes from healthy people, exposed for 72 h | Anatase | 40–70 | 99.7 | 5 | 12 | 4.47 ± 2.39 | 12 | 1.87 ± 1.63 | high | B |
| 10 | 12 | 7.21 ± 1.69 | 12 | 1.87 ± 1.63 | ||||||||
| Ünal F. 2021 [37] | Turkey | Human lymphocytes, exposed for 48 h | NA | <100 | NA | 20 | 3 | 0.30 ± 0.099 | 3 | 0.13 ± 0.066 | medium | A+++ |
| 40 | 3 | 0.30 ± 0.099 | 3 | 0.13 ± 0.066 | ||||||||
| 60 | 3 | 0.30 ± 0.099 | 3 | 0.13 ± 0.066 | ||||||||
| 80 | 3 | 0.17 ± 0.075 | 3 | 0.13 ± 0.066 | ||||||||
| 100 | 3 | 0.13 ± 0.066 | 3 | 0.13 ± 0.066 | ||||||||
| Outcomes were described as CA frequency | ||||||||||||
| Catalán J. 2011 [46] | Finland | Human lymphocytes, exposed for 24 h, 48 h and 72 h | Anatase | <25 | 99.7 | 6.25 | 2 | 1.25 ± 1.26(24 h) 0.50 ± 0.58(48 h) 0.25 ± 0.50(72 h) |
2 | 0.75 ± 0.96(24 h) 0.00 ± 0.00(48 h) 0.50 ± 1.00(72 h) |
high | A++ |
| 12.5 | 2 | 0.50 ± 0.58(24 h) 0.50 ± 0.58(48 h) 1.25 ± 0.96(72 h) |
2 | 0.75 ± 0.96(24 h) 0.00 ± 0.00(48 h) 0.50 ± 1.00(72 h) |
||||||||
| 25 | 2 | 0.00 ± 0.00(24 h) 0.25 ± 0.50(48 h) 0.25 ± 0.50(72 h) |
2 | 0.75 ± 0.96(24 h) 0.00 ± 0.00(48 h) 0.50 ± 1.00(72 h) |
||||||||
| 50 | 2 | 0.50 ± 0.58(24 h) 0.25 ± 0.50(48 h) 0.50 ± 1.00(72 h) |
2 | 0.75 ± 0.96(24 h) 0.00 ± 0.00(48 h) 0.50 ± 1.00(72 h) |
||||||||
| 100 | 2 | 0.00 ± 0.00(24 h) 1.00 ± 0.82(48 h) 0.75 ± 0.96(72 h) |
2 | 0.75 ± 0.96(24 h) 0.00 ± 0.00(48 h) 0.50 ± 1.00(72 h) |
||||||||
| 150 | 2 | 0.25 ± 0.50(24 h) 1.25 ± 0.50(48 h) 0.50 ± 0.58(72 h) |
2 | 0.75 ± 0.96(24 h) 0.00 ± 0.00(48 h) 0.50 ± 1.00(72 h) |
||||||||
| 300 | 2 | 1.00 ± 1.15(24 h) 1.00 ± 0.82(48 h) 0.50 ± 0.58(72 h) |
2 | 0.75 ± 0.96(24 h) 0.00 ± 0.00(48 h) 0.50 ± 1.00(72 h) |
||||||||
| Ünal F. 2021 [37] | Turkey | Human lymphocytes, exposed for 24 h, 48 h | NA | <100 | NA | 20 | 3 | 6.00 ± 1.37(24 h) 5.33 ± 1.30(48 h) |
3 | 1.33 ± 0.66(24 h) 1.33 ± 0.66(48 h) |
medium | A+++ |
| 40 | 3 | 6.67 ± 1.44(24 h) 3.00 ± 0.98(48 h) |
3 | 1.33 ± 0.66(24 h) 1.33 ± 0.66(48 h) |
||||||||
| 60 | 3 | 4.33 ± 1.17(24 h) 3.33 ± 1.03(48 h) |
3 | 1.33 ± 0.66(24 h) 1.33 ± 0.66(48 h) |
||||||||
| 80 | 3 | 5.00 ± 1.26(24 h) 3.33 ± 1.03(48 h) |
3 | 1.33 ± 0.66(24 h) 1.33 ± 0.66(48 h) |
||||||||
| 100 | 3 | 6.00 ± 1.37(24 h) 4.00 ± 1.13(48 h) |
3 | 1.33 ± 0.66(24 h) 1.33 ± 0.66(48 h) |
||||||||
1 NA: not applicable; n: sample size; SD: standard deviation; T DNA%: the percentage of DNA in tail; TL: tail length; OTM: olive tail moment; MF: mutation frequency; BiMN: no. of micronucleus/1000 binucleated cells; CA frequency: percentage of cells exhibiting chromosomal aberrations.
3.3. Meta-Analysis for In Vivo Genotoxicity of TiO2 NPs
3.3.1. Heterogeneity Test and Meta-Analysis
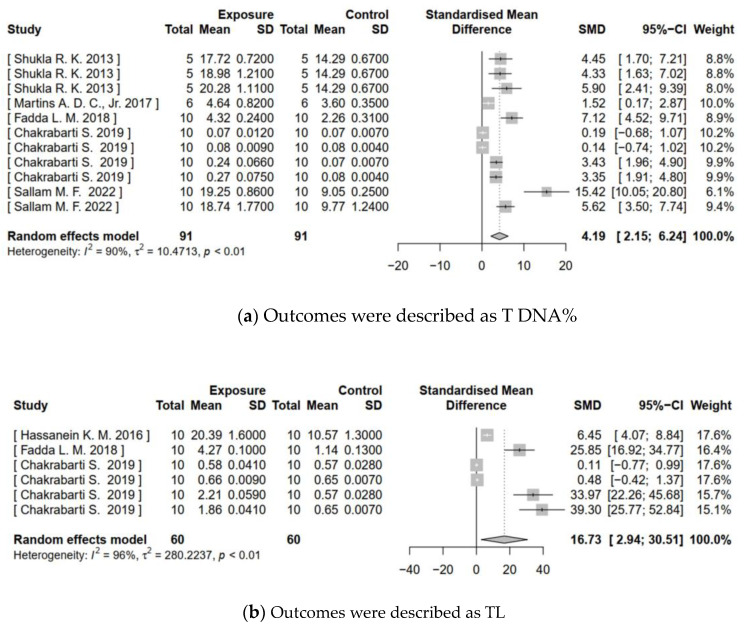
The results of the I2 analysis for different genotoxic endpoints showed significant heterogeneity (p < 0.01, I2 ≥ 50%). Consequently, the random-effects model was employed to estimate the combined effects.
Meta-analysis of in vivo genotoxicity of TiO2 NPs summarized the SMDs of five categories of genotoxicity endpoints (as shown in Figure 2). The forest plots illustrated significant increases in T DNA% (Z = 4.02, p < 0.0001), TL (Z = 2.38, p = 0.0174), and OTM (Z = 5.44, p < 0.0001). The SMDs and 95%CIs were 4.19 (2.15–6.24), 16.73 (2.94–30.51), and 5.62 (3.59–7.64), respectively, indicating that treatment with TiO2 NPs could cause DNA damage. Similarly, MN frequency (Z = 2.59, p = 0.0097) and CA frequency (Z = 3.58, p = 0.0003) in the exposed group also significantly increased, with SMDs and 95%CIs of 5.07 (1.23–8.91) and 15.81 (7.16–24.45). This evidence suggested that TiO2 NPs may induce chromosome damage.
Figure 2.
Meta-analysis for in vivo genotoxicity of TiO2 NPs. (a–e) Show the forest plots for genotoxicity endpoints of T DNA%, TL, OTM, MN frequency, and CA frequency, respectively. ‘Total’ is the sample size; ‘SD’ is the standard deviation; ‘SMD’ is the standardized mean difference; ‘95%CI’ is the 95% confidence interval; ‘I2′ is Higgins’s inconsistency statistic; ‘τ2′ is the estimate of between-study variance. Significance is at p < 0.05.
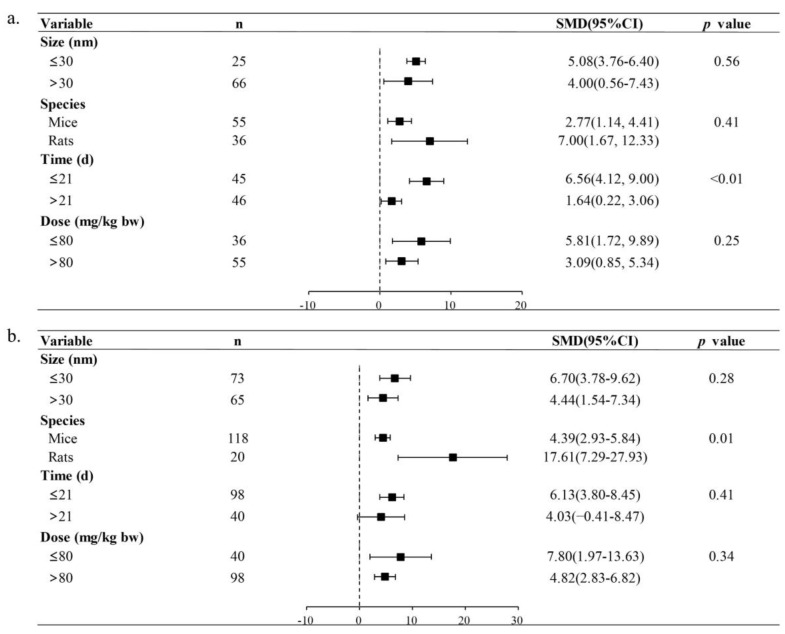
3.3.2. Subgroup Analysis
Given the limited available literature on the in vivo genotoxicity of TiO2 NPs, our subgroup analysis focused on T DNA% and OTM data. Figure 3 depicts that the observed heterogeneity in the results may be attributed to the exposure time (p < 0.01) and the species used in experiments (p = 0.01). Specifically, the TiO2 NPs-treated group exhibited significantly higher T DNA% in short-term exposures (≤ 21 days) (SMD = 6.56, 95%CI: 4.12–9.00) compared to long-term exposures (>21 days) (SMD = 1.64, 95%CI: 0.22–3.06). Additionally, OTM was significantly higher in rats (SMD = 17.61, 95%CI: 7.29–27.93) than in mice (SMD = 4.39, 95%CI: 2.93–5.84). However, no statistically significant results were observed when considering particle size and treatment dose for T DNA% or OTM. These findings suggested that short-term exposure could potentially contribute to in vivo DNA damage caused by TiO2 NPs. Furthermore, rats seem more sensitive to the genotoxic impacts of TiO2 NP-induced DNA damage than mice.
Figure 3.
Subgroup analyses of TiO2 NPs genotoxicity on in vivo T DNA% (a) and OTM (b). ’n’ is the sample size; ’SMD’ is the standardized mean difference; ’95%CI’ is the 95% confidence interval; and ‘p value’ represents the heterogeneity between subgroups. Significant heterogeneity between subgroups is at p < 0.05.
3.3.3. Sensitivity Analysis and Publication Bias
The presence of heterogeneity among in vivo studies focusing on various genotoxic endpoints was noted. This analysis did not reveal any significant differences in the study outcomes, as indicated by the SMD and its 95%CI. Considering the relatively limited number of included studies for each genotoxicity endpoint, no publication bias test was performed.
3.4. Meta-Analysis for In Vitro Genotoxicity of TiO2 NPs
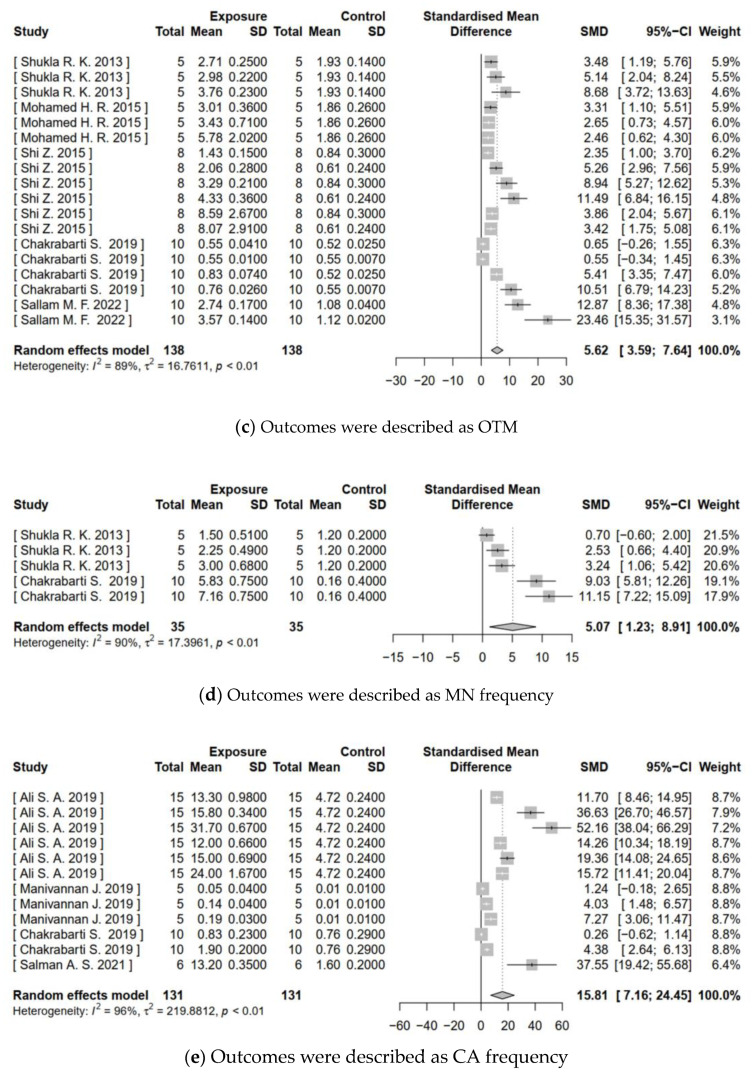
3.4.1. Heterogeneity Test and Meta-Analysis
Due to the observed heterogeneity among in vitro studies with outcome indicators of T DNA%, OTM, and MN frequency (p < 0.01), the random-effects model was utilized to analyze the combined effects. Conversely, for the outcome indicators of TL, MF, and CA frequency, which showed no significant heterogeneity, the fixed-effects model was considered appropriate.
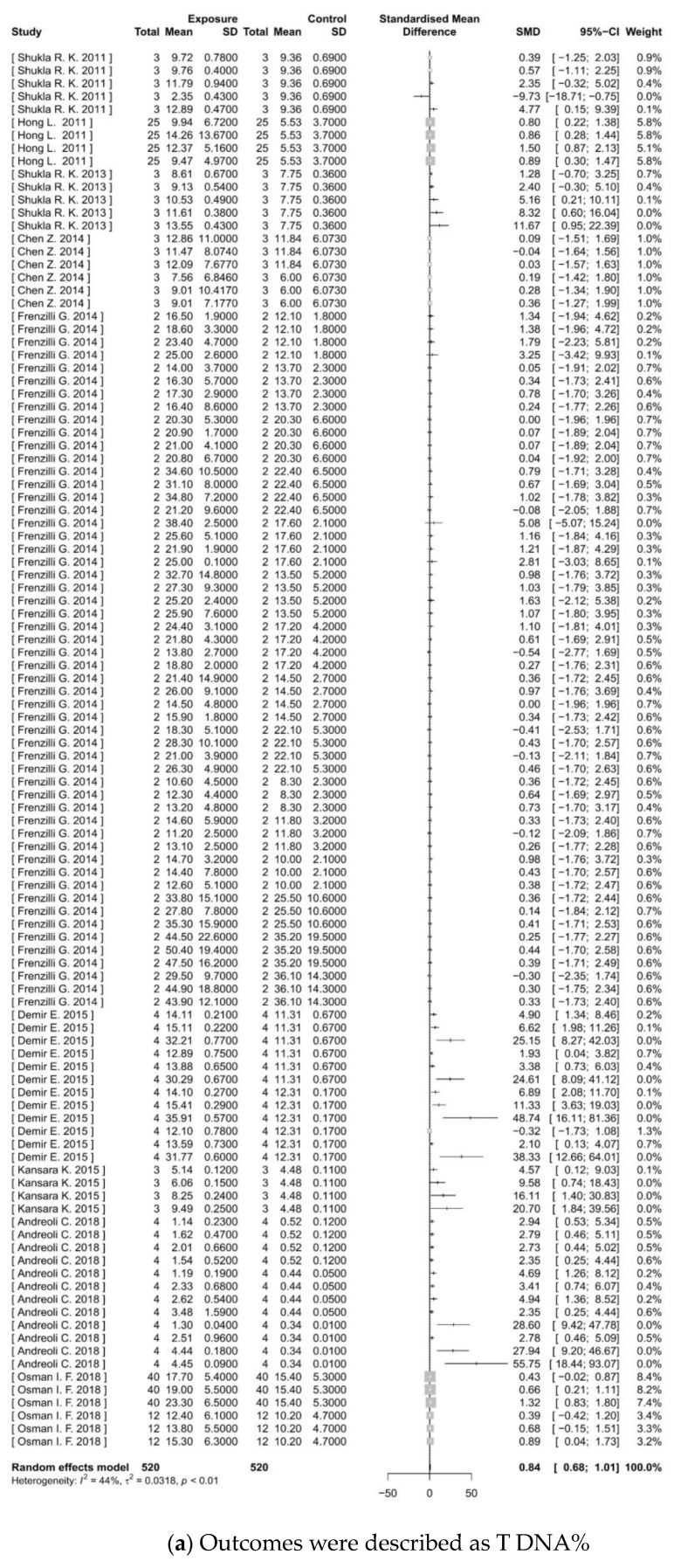
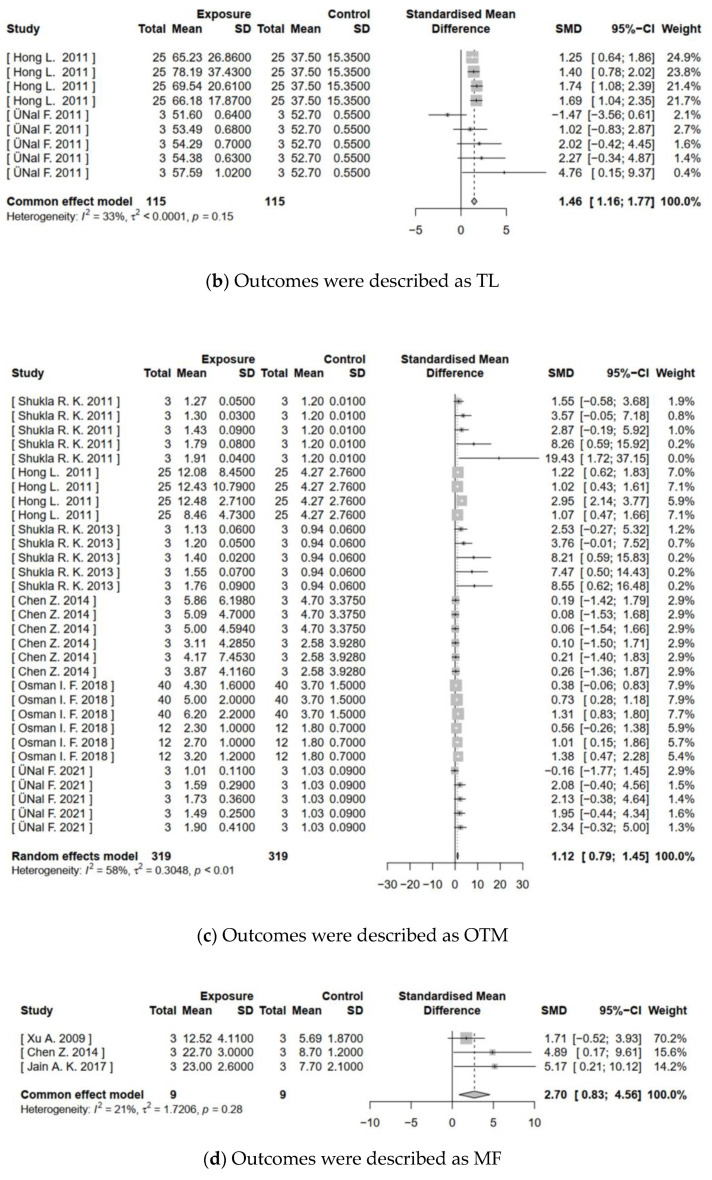
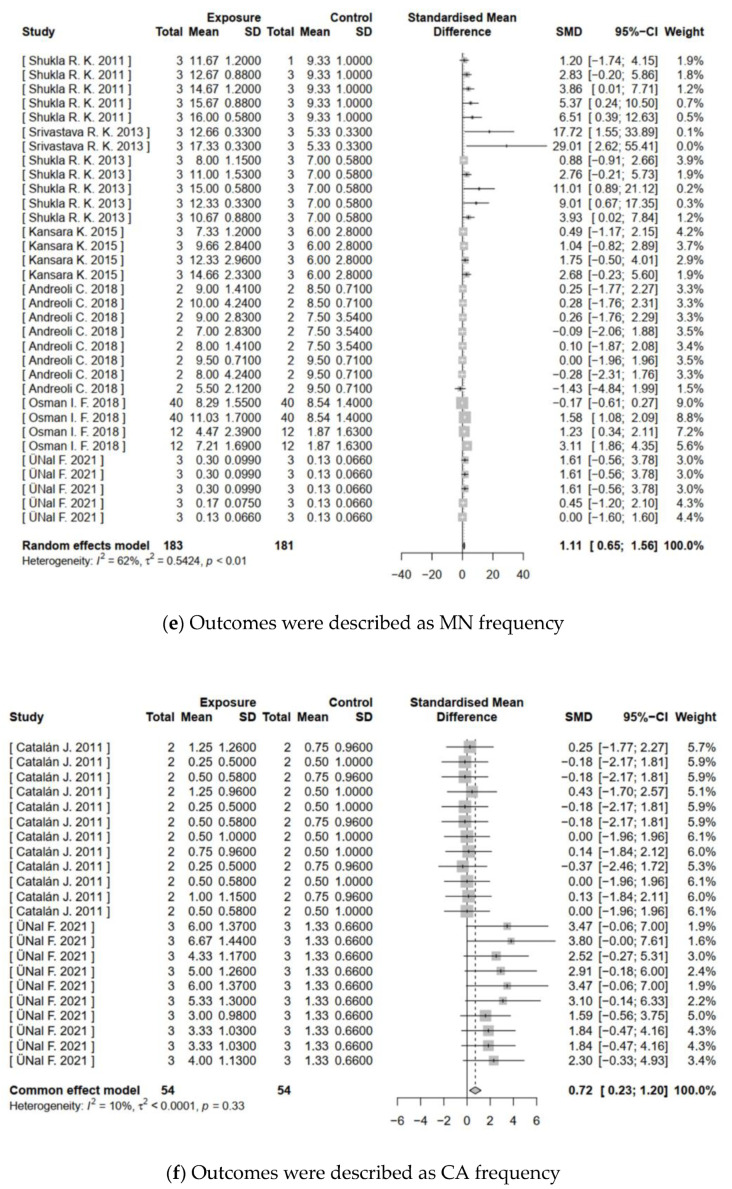
The meta-analysis of in vitro genotoxicity of TiO2 NPs revealed significant findings across six categories of outcome indicators (as shown in Figure 4). The results from the forest plots indicate that the experimental group exposed to TiO2 NPs has significantly higher levels of T DNA% (Z = 10.12, p < 0.0001), TL (Z = 9.42, p < 0.0001), and OTM (Z = 7.09, p < 0.0001) than controls. The SMDs and 95%CIs were 0.84 (0.68–1.01), 1.46 (1.16–1.77), and 1.12 (0.79–1.45), respectively. These findings suggested that TiO2 NP treatment could cause DNA damage. There was a significant increase in MF (Z = 2.83, p = 0.0046) with a result of 2.70 (0.83–4.56), indicating the potential of TiO2 NPs to induce gene mutations. Moreover, significant increases were observed in MN frequency (Z = 5.68, p < 0.0001) and CA frequency (Z = 2.90, p = 0.0037). The SMDs and 95%CIs were 1.11 (0.65–1.56) and 0.72 (0.23–1.20), respectively, suggesting chromosomal damage effects.
Figure 4.
Meta-analysis for in vitro genotoxicity of TiO2 NPs. (a–f) Show the forest plots for genotoxicity endpoints of T DNA%, TL, OTM, MF, MN frequency, and CA frequency, respectively. ‘SD’ is the standard deviation; ‘SMD’ is the standardized mean difference; ‘95%CI’ is the 95% confidence interval; ‘I2′ is Higgins’s inconsistency statistic; and ‘τ2′ is the estimate of between-study variance. Significance is at p < 0.05.
3.4.2. Subgroup Analysis
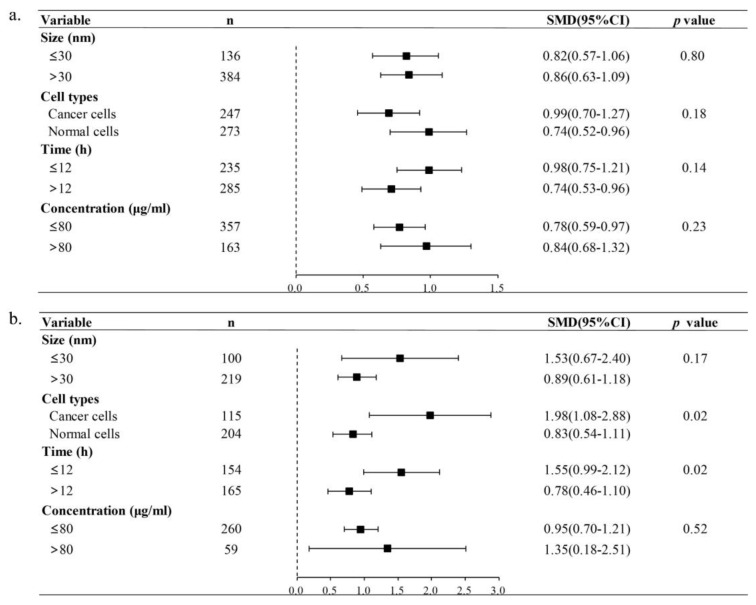
In the subgroup analysis conducted on in vitro studies, a specific focus was placed on the examination of T DNA% and OTM data. As illustrated in Figure 5, the potential origins of heterogeneity were identified as the exposure time and the type of experimental cells (p = 0.02). For the TiO2 NPs-treated group, the results of subgroup analysis revealed that OTM was significantly higher during short-term exposure (≤12 h) (SMD = 1.55, 95%CI: 0.99–2.12) compared to long-term exposure (>12 h) (SMD = 0.78, 95%CI: 0.46–1.10). Furthermore, the OTM value for cancer cells (SMD = 1.98, 95%CI: 1.08–2.88) was significantly higher than that of normal cells (SMD = 0.83, 95%CI: 0.54–1.11). Nevertheless, neither particle size nor exposure concentration exhibited statistically significant differences in relation to T DNA% and OTM. In summation, brief periods of exposure to TiO2 NPs may potentially result in DNA damage in vitro. Additionally, cancer cells were discerned to manifest a heightened sensitivity to in vitro DNA damage elicited by TiO2 NPs.
Figure 5.
Subgroup analyses of TiO2-NPs genotoxicity on in vitro T DNA% (a) and OTM (b). ’n’ is the sample size; ’SMD’ is the standardized mean difference; ’95%CI’ is the 95% confidence interval; and ‘p value’ represents heterogeneity between subgroups. Significant heterogeneity between subgroups is at p < 0.05.
3.4.3. Sensitivity Analysis and Publication Bias
The sensitivity analysis of the data from the in vitro assay indicated that no single study significantly impacted the overall results. Furthermore, the merged effect values remained consistent, suggesting that the original results of forest plots were statistically reliable and robust.
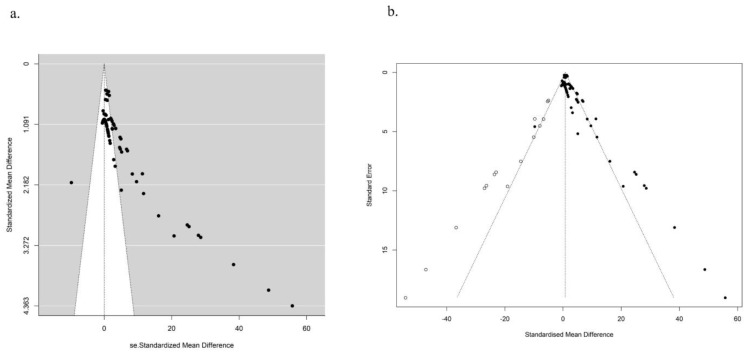
A publication bias test was performed specifically for the studies with a genotoxicity endpoint of T DNA%. The funnel plot in Figure 6a revealed an uneven distribution of points representing the effect values for each study. A significant proportion of these points were positioned to the right of the combined effect value and lay outside the associated confidence interval. In addition, the p-value of the Egger test was found to be less than 0.05. These findings collectively suggested the presence of publication bias, which possibly affected the accuracy of meta-analysis. To eliminate publication bias, an additional 15 studies were needed, as indicated by hollow origin in Figure 6b.
Figure 6.
Egger funnel diagram of in vitro T DNA% before (a) and after (b) publication bias correction by the trim and fill method. The middle line shows the overall estimated standard mean difference. Black dots represent the original studies included, and white dots indicate studies that need supplementation.
4. Discussion
In this paper, a comprehensive analysis of 12 in vivo and 14 in vitro studies was conducted to assess the genotoxic effects of TiO2 NPs. These studies were selected based on meeting the reliability and relevance assessment criteria. The meta-analysis results showed that the SMD for each genotoxic endpoint was greater than 0, suggesting that TiO2 NPs significantly induced DNA damage and chromosome damage both in vivo and in vitro. Furthermore, there was a significant association between TiO2 NP treatment and gene mutation in vitro. These findings confirmed the potential risks of genotoxicity associated with human exposure to TiO2 NPs. Evidently, the duration of exposure and experimental subjects emerged as significant variables influencing DNA damage in the TiO2 NPs-treated group. Short-term exposure to TiO2 NPs displayed a higher likelihood of inducing DNA damage. The in vivo comet assay revealed that rats exhibited greater sensitivity to DNA damage induced by TiO2 NPs than mice. Furthermore, the in vitro comet assay demonstrated that cancer cells exhibited heightened susceptibility to DNA damage induced by TiO2 NPs than normal cells. However, it was essential to be cautious about the potential influence of publication bias on the accuracy of the meta-analysis results.
Currently, three mechanisms have been proposed for the genotoxicity of TiO2 NPs. The first mechanism involves direct interaction with DNA. The second one refers to an indirect mechanism in which TiO2 NPs interact with other molecules and affect the genetic material. Finally, reactive oxygen species (ROS) are generated due to the catalytic potential of the particles [46]. However, the available evidence questions the direct effect of TiO2 NPs on DNA and favors the role of the latter two mechanisms. According to the French Agency for Food, Environmental, and Occupational Health and Safety, there was no evidence of direct interaction between TiO2 NPs and DNA or the mitotic apparatus. However, they suggested that direct effects on molecules interacting with genetic material could not be completely excluded [47]. A comprehensive weight of evidence assessment suggested that observed genotoxic effects of TiO2 (nano and other forms) were secondary to physiological stress rather than direct DNA damage [48]. Nanoparticle-induced oxidative stress was viewed as a signal transducer for further physiological effects, including genotoxicity and cytotoxicity [49,50]. EFSA concluded that the relative contribution of different molecular mechanisms triggered by TiO2 NPs remained unknown [11].
Extensive research has demonstrated that TiO2 NP exposure is associated with increased occurrence of DNA damage. This propensity for DNA damage appears to be particularly pronounced following short-term exposure to TiO2 NPs. This conclusion is substantiated by the collective findings of all in vivo comet assays and the majority of in vitro comet assays encompassed within this meta-analysis. This aligned with the findings of Ling et al. [12], who also observed severe DNA damage following brief exposure to TiO2 NPs. This phenomenon can likely be attributed to the insufficient time for effective DNA repair due to the constricted exposure window. Additionally, comet assay studies showed a correlation between longer exposure periods and reduced DNA damage [51,52]. This implied that TiO2 NPs possibly cause early and reversible DNA damage, but cells adapt to the TiO2 NPs environment and initiate repair mechanisms during prolonged exposures. The potential impact of genotoxicity includes influencing cellular responses like DNA repair, cell cycle arrest, and apoptosis. Inadequate DNA repair before or during damaged DNA replication could potentially trigger mutagenic and oncogenic events [53].
In comet assay, rats and cancer cells subjected to TiO2 NP exposure exhibited a pronounced susceptibility to DNA damage, as evidenced by their significantly higher OTM than mice and normal cells. This observed discrepancy most likely depended on the inherent capacity of DNA damage response (DDR). Cancer cells showed a broad spectrum of mutations and abnormal gene expressions within the domain of DNA repair responses, which set in motion a state of genome instability [54,55]. The frequent compromise of certain DDR pathways in cancer cells facilitated the accumulation of genomic instability. As a result, the loss of functional DDR pathways rendered cancer cells more prone to DNA damage and additional defects within the DDR network [56]. Conversely, the meticulously controlled replication observed in normal cells acted as a buffer against the onset of a hyperactivated DDR [57]. This observation was validated by evidence that the incidence of DNA lesions within cancer cell lines was elevated compared to primary cells cultivated under controlled laboratory conditions [58]. Close attention must be paid to the risks of cancer treatments based on TiO2 NP drug delivery systems [59].Studies have shown that exposure to TiO2 NPs of high concentrations or small size is usually associated with higher genotoxicity. A literature review concluded that genotoxicity exhibited an increasing trend with decreasing particle size and increasing concentrations of TiO2 NPs [13]. Moreover, Dubey et al. [60] observed a dose-dependent escalation in DNA damage, lipid peroxidation, and protein carbonylation as concentrations of exposed nanoparticles increased. In this study, no difference in DNA damage induced by TiO2 NPs was observed under varying particle sizes and exposure concentrations. More high-quality literature is needed to be included in the comprehensive analysis.
The impact of TiO2 NPs on gene mutation and chromosome aberrations has been extensively studied. Jain et al. [43] reported a linear correlation between the mutation rates and the exposure levels of TiO2 NPs. Moreover, the mutagenic potential of TiO2 NPs in V-79 cells was evaluated via mammalian HGPRT gene forward mutation assay, showing a 2.98-fold increase in 6TGR HGPRT mutant frequency [42]. The presence of heightened levels of ROS could interact with cellular components, including DNA bases or the deoxyribosyl backbone of DNA, resulting in the formation of damaged bases or strand breaks. Certain oxidative DNA lesions, which might not be fully repaired, could act as precursors to mutagenesis. This phenomenon is particularly relevant to mismatch repair or incomplete repair mechanisms, which can give rise to specific mutational events [42,61]. The study employing transmission electron microscopy yielded evidence suggesting that the internalization of TiO2 NPs by cells is observable within cytoplasmic vesicles and close to and inside the nucleus. Notably, larger agglomerates of TiO2 NPs were believed to possess the capacity to disrupt or damage chromosomal structures, potentially leading to chromosome aberrations [62]. This meta-analysis incorporated the most recent studies of in vivo and in vitro genotoxicity and underwent rigorous quality assessments to enable quantitative analysis. However, there were still some limitations. The available data were primarily limited as only the Chinese and English literature was included in the screening process. However, the high reliability and relevance of the included literature increased confidence in the results. Secondly, it is suggested that future studies pay closer attention to the substance characterization of TiO2 NPs, such as shape, size, and charge. The association between these important characteristics and genotoxicity is worth discussing in depth. Finally, more high-quality genotoxicity studies on TiO2 NPs are needed to help minimize the impact of publication bias.
Moving forward, there are several key aspects that researchers should focus on in future studies concerning TiO2 NPs and genotoxicity. Long-term animal studies would be valuable to explore the underlying molecular mechanisms of genotoxicity induced by TiO2 NPs further. Researchers should also investigate the catabolism of TiO2 NPs once they enter the human body. Study results will provide valuable insights into the internal exposure dose of nanoparticles within target organs or cells. Furthermore, establishing a cut-off value for TiO2 particle size in relation to genotoxicity is an important area of research. Establishing stringent regulations and guidelines for the judicious application of TiO2 NPs is essential to mitigate their potential genotoxic effects, thus ensuring effective protection of public health.
5. Conclusions
This meta-analysis has provided evidence that TiO2 NPs could induce genotoxicity, including DNA damage and chromosomal damage both in vivo and in vitro, as well as in vitro gene mutations. Short-term exposure to TiO2 NPs would lead to increased DNA damage. Rats were more sensitive to TiO2 NPs-induced DNA damage in vivo than mice, and cancer cells exhibited heightened susceptibility to in vitro DNA damage induced by TiO2 NPs than normal cells. The interaction between TiO2 NPs and DNA, along with the activation of ROS, influenced the DNA repair response and induced genotoxicity. Therefore, it is necessary to raise public awareness about the potential risks associated with using TiO2 NPs, particularly in products intended for consumption as food and drugs.
Abbreviations
| TiO2 NPs | Nanoparticles Titanium Dioxide Nanoparticles |
| EFSA | European Food Safety Authority |
| WoS | Web of Science |
| CNKI | China National Knowledge Infrastructure |
| T DNA% | the percentage of DNA in tail |
| TL | tail length |
| OTM | olive tail moment |
| MF | mutation frequency |
| MN | micronucleus |
| CA | chromosomal aberrations |
| TRAM | toxicological data reliability assessment method |
| CDC | Center for Disease Control and Prevention |
| SMD | standardized mean difference |
| CI | confidence interval |
| ROS | reactive oxygen species |
| DDR | DNA damage response |
Author Contributions
Conceptualization, Y.C. and J.C.; methodology, Y.C. and Y.S.; software, Y.C.; validation, Q.B. and J.N.; formal analysis, L.Y. and T.O.; writing—original draft preparation, Y.C.; writing—review and editing, Y.S. and S.W.; visualization, Y.C.; project administration, Y.S.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The research data can be found in the figures and tables within this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by [Risk Assessment Project of Dietary Intake of Titanium Dioxide in China] grant number [2022-CP-05].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sungur Ş. Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications, Titanium Dioxide Nanoparticles. Springer International Publishing; Berlin/Heidelberg, Germany: 2020. pp. 1–18. [Google Scholar]
- 2.Adam V., Loyaux-Lawniczak S., Quaranta G. Characterization of engineered TiO₂ nanomaterials in a life cycle and risk assessments perspective. Environ. Sci. Pollut. Res. Int. 2015;22:11175–11192. doi: 10.1007/s11356-015-4661-x. [DOI] [PubMed] [Google Scholar]
- 3.Ziental D., Czarczynska-Goslinska B., Mlynarczyk D.T., Glowacka-Sobotta A., Stanisz B., Goslinski T., Sobotta L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials. 2020;10:387. doi: 10.3390/nano10020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachler G., von Goetz N., Hungerbuhler K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2015;9:373–380. doi: 10.3109/17435390.2014.940404. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X., Ma G., Wei W. Simulation of nanoparticles interacting with a cell membrane: Probing the structural basis and potential biomedical application. NPG Asia Mater. 2021;13:52. doi: 10.1038/s41427-021-00320-0. [DOI] [Google Scholar]
- 6.Augustine R., Hasan A., Primavera R., Wilson R.J., Thakor A.S., Kevadiya B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020;25:101692. doi: 10.1016/j.mtcomm.2020.101692. [DOI] [Google Scholar]
- 7.Kumar V., Sharma N., Maitra S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017;7:243–256. doi: 10.1007/s40089-017-0221-3. [DOI] [Google Scholar]
- 8.Oberdorster G., Oberdorster E., Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung I., Rekha K., Venkidasamy B., Thiruvengadam M. Effect of Copper Oxide Nanoparticles on the Physiology, Bioactive Molecules, and Transcriptional Changes in Brassica rapa ssp. rapa Seedlings. Water Air Soil. Pollut. 2019;230:48. doi: 10.1007/s11270-019-4084-2. [DOI] [Google Scholar]
- 10.Horie M., Stowe M., Tabei M., Kuroda E. Metal Ion Release of Manufactured Metal Oxide Nanoparticles Is Involved in the Allergic Response to Inhaled Ovalbumin in Mice. Occup. Dis. Environ. Med. 2016;4:17–26. doi: 10.4236/odem.2016.42003. [DOI] [Google Scholar]
- 11.EFSA Panel on Food Additives and Flavourings (FAF) Younes M., Aquilina G., Castle L., Engel K.H., Fowler P., Frutos Fernandez M.J., Fürst P., Gundert-Remy U., Gürtler R., et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021;19:e06585. doi: 10.2903/j.efsa.2021.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling C., An H., Li L., Wang J., Lu T., Wang H., Hu Y., Song G., Liu S. Genotoxicity Evaluation of Titanium Dioxide Nanoparticles in Vitro: A Systematic Review of the Literature and Meta-analysis. Biol. Trace Elem. Res. 2021;199:2057–2076. doi: 10.1007/s12011-020-02311-8. [DOI] [PubMed] [Google Scholar]
- 13.Shi J., Han S., Zhang J., Liu Y., Chen Z., Jia G. Advances in genotoxicity of titanium dioxide nanoparticles in vivo and in vitro. NanoImpact. 2022;25:100377. doi: 10.1016/j.impact.2021.100377. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Wang Y., Ba T., Li Y., Pu J., Chen T., Song Y., Gu Y., Qian Q., Yang J., et al. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicol. Lett. 2014;226:314–319. doi: 10.1016/j.toxlet.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Shabbir S., Kulyar M.F., Bhutta Z.A., Boruah P., Asif M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. Bionanoscience. 2021;11:621–632. doi: 10.1007/s12668-021-00836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bian Q., Ping Y., Jun W., Lyu Z., Song Y., Zhang L., Liu Z. A new method to evaluate toxicological data reliability in risk assessments. Toxicol. Lett. 2019;311:125–132. doi: 10.1016/j.toxlet.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Shukla R.K., Kumar A., Vallabani N.V., Pandey A.K., Dhawan A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine. 2014;9:1423–1434. doi: 10.2217/nnm.13.100. [DOI] [PubMed] [Google Scholar]
- 18.Martins A.D.C., Jr., Azevedo L.F., de Souza Rocha C.C., Carneiro M.F.H., Venancio V.P., de Almeida M.R., Antunes L.M.G., de Carvalho Hott R., Rodrigues J.L., Ogunjimi A.T., et al. Evaluation of distribution, redox parameters, and genotoxicity in Wistar rats co-exposed to silver and titanium dioxide nanoparticles. J. Toxicol. Environ. Health A. 2017;80:1156–1165. doi: 10.1080/15287394.2017.1357376. [DOI] [PubMed] [Google Scholar]
- 19.Fadda L.M., Hagar H., Mohamed A.M., Ali H.M. Quercetin and Idebenone Ameliorate Oxidative Stress, Inflammation, DNA damage, and Apoptosis Induced by Titanium Dioxide Nanoparticles in Rat Liver. Dose Response. 2018;16:1559325818812188. doi: 10.1177/1559325818812188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakrabarti S., Goyary D., Karmakar S., Chattopadhyay P. Exploration of cytotoxic and genotoxic endpoints following sub-chronic oral exposure to titanium dioxide nanoparticles. Toxicol. Ind. Health. 2019;35:577–592. doi: 10.1177/0748233719879611. [DOI] [PubMed] [Google Scholar]
- 21.Sallam M.F., Ahmed H.M.S., Diab K.A., El-Nekeety A.A., Abdel-Aziem S.H., Sharaf H.A., Abdel-Wahhab M.A. Improvement of the antioxidant activity of thyme essential oil against biosynthesized titanium dioxide nanoparticles-induced oxidative stress, DNA damage, and disturbances in gene expression in vivo. J. Trace Elem. Med. Biol. 2022;73:127024. doi: 10.1016/j.jtemb.2022.127024. [DOI] [PubMed] [Google Scholar]
- 22.Sallam M.F., Ahmed H.M.S., El-Nekeety A.A., Diab K.A., Abdel-Aziem S.H., Sharaf H.A., Abdel-Wahhab M.A. Assessment of the Oxidative Damage and Genotoxicity of Titanium Dioxide Nanoparticles and Exploring the Protective Role of Holy Basil Oil Nanoemulsions in Rats. Biol. Trace Elem. Res. 2022;201:1301–1316. doi: 10.1007/s12011-022-03228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassanein K.M., El-Amir Y.O. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathol. Res. Pract. 2017;213:13–22. doi: 10.1016/j.prp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed H.R. Estimation of TiO2 nanoparticle-induced genotoxicity persistence and possible chronic gastritis-induction in mice. Food Chem. Toxicol. 2015;83:76–83. doi: 10.1016/j.fct.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z., Niu Y., Wang Q., Shi L., Guo H., Liu Y., Zhu Y., Liu S., Liu C., Chen X., et al. Reduction of DNA damage induced by titanium dioxide nanoparticles through Nrf2 in vitro and in vivo. J. Hazard. Mater. 2015;298:310–319. doi: 10.1016/j.jhazmat.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Ali S.A., Rizk M.Z., Hamed M.A., Aboul-Ela E.I., El-Rigal N.S., Aly H.F., Abdel-Hamid A.Z. Assessment of titanium dioxide nanoparticles toxicity via oral exposure in mice: Effect of dose and particle size. Biomarkers. 2019;24:492–498. doi: 10.1080/1354750X.2019.1620336. [DOI] [PubMed] [Google Scholar]
- 27.Manivannan J., Banerjee R., Mukherjee A. Genotoxicity analysis of rutile titanium dioxide nanoparticles in mice after 28 days of repeated oral administration. Nucleus. 2019;63:17–24. doi: 10.1007/s13237-019-00277-0. [DOI] [Google Scholar]
- 28.Salman A.S., Al-Shaikh T.M., Hamza Z.K., El-Nekeety A.A., Bawazir S.S., Hassan N.S., Abdel-Wahhab M.A. Matlodextrin-cinnamon essential oil nanoformulation as a potent protective against titanium nanoparticles-induced oxidative stress, genotoxicity, and reproductive disturbances in male mice. Environ. Sci. Pollut. Res. Int. 2021;28:39035–39051. doi: 10.1007/s11356-021-13518-0. [DOI] [PubMed] [Google Scholar]
- 29.Shukla R.K., Sharma V., Pandey A.K., Singh S., Sultana S., Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In Vitro. 2011;25:231–241. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Hong L., Ding S., Zhu J., Zhu Y., Zhang T. Comparative Study of Cytotoxicity and DNA Damage Induced by Nano- and Micro-TiO2 Particles on A549 Cells in Vitro. J. Environ. Occup. Med. 2011;28:393–397. (In Chinese) [Google Scholar]
- 31.Shukla R.K., Kumar A., Gurbani D., Pandey A.K., Singh S., Dhawan A. TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology. 2013;7:48–60. doi: 10.3109/17435390.2011.629747. [DOI] [PubMed] [Google Scholar]
- 32.Frenzilli G., Bernardeschi M., Guidi P., Scarcelli V., Lucchesi P., Marsili L., Fossi M.C., Brunelli A., Pojana G., Marcomini A., et al. Effects of in vitro exposure to titanium dioxide on DNA integrity of bottlenose dolphin (Tursiops truncatus) fibroblasts and leukocytes. Mar. Environ. Res. 2014;100:68–73. doi: 10.1016/j.marenvres.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Demir E., Akca H., Turna F., Aksakal S., Burgucu D., Kaya B., Tokgün O., Vales G., Creus A., Marcos R. Genotoxic and cell-transforming effects of titanium dioxide nanoparticles. Environ. Res. 2015;136:300–308. doi: 10.1016/j.envres.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Kansara K., Patel P., Shah D., Shukla R.K., Singh S., Kumar A., Dhawan A. TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ. Mol. Mutagen. 2015;56:204–217. doi: 10.1002/em.21925. [DOI] [PubMed] [Google Scholar]
- 35.Andreoli C., Leter G., De Berardis B., Degan P., De Angelis I., Pacchierotti F., Crebelli R., Barone F., Zijno A. Critical issues in genotoxicity assessment of TiO2 nanoparticles by human peripheral blood mononuclear cells. J. Appl. Toxicol. 2018;38:1471–1482. doi: 10.1002/jat.3650. [DOI] [PubMed] [Google Scholar]
- 36.Osman I.F., Najafzadeh M., Sharma V., Shukla R.K., Jacob B.K., Dhawan A., Anderson D. TiO2 NPs Induce DNA Damage in Lymphocytes from Healthy Individuals and Patients with Respiratory Diseases—An ex Vivo/in Vitro Study. J. Nanosci. Nanotechnol. 2018;18:544–555. doi: 10.1166/jnn.2018.15236. [DOI] [PubMed] [Google Scholar]
- 37.Ünal F., Korkmaz F.D., Suludere Z., Erol Ö., Yüzbaşıoğlu D. Genotoxicity of Two Nanoparticles: Titanium Dioxide and Zinc Oxide. Gazi Univ. J. Sci. 2021;34:948–958. doi: 10.35378/gujs.826911. [DOI] [Google Scholar]
- 38.Shi Y., Zhang J.H., Jiang M., Zhu L.H., Tan H.Q., Lu B. Synergistic genotoxicity caused by low concentration of titanium dioxide nanoparticles and p,p’-DDT in human hepatocytes. Environ. Mol. Mutagen. 2010;51:192–204. doi: 10.1002/em.20527. [DOI] [PubMed] [Google Scholar]
- 39.Du H., Zhou Y., Zhu X. DNA damage and OGG1 expression induced by a combined effect of titanium dioxide nanopartices and lead acetate in human hepatocytes. J. Environ. Health. 2012;29:403. doi: 10.1002/tox.20682. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 40.Ryu A.R., Bang I.C., Lee S.A., Lee M.Y. The protective role of phytochemicals on TiO₂ nanoparticles-induced DNA damage in lymphocytes. J. Environ. Biol. 2016;37:913–917. [PubMed] [Google Scholar]
- 41.Xu A., Chai Y., Nohmi T., Hei T.K. Genotoxic responses to titanium dioxide nanoparticles and fullerene in gpt delta transgenic MEF cells. Part. Fibre Toxicol. 2009;6:3. doi: 10.1186/1743-8977-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain A.K., Senapati V.A., Singh D., Dubey K., Maurya R., Pandey A.K. Impact of anatase titanium dioxide nanoparticles on mutagenic and genotoxic response in Chinese hamster lung fibroblast cells (V-79): The role of cellular uptake. Food Chem. Toxicol. 2017;105:127–139. doi: 10.1016/j.fct.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Kang S.J., Kim B.M., Lee Y.J., Chung H.W. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ. Mol. Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- 44.Reis Ede M., Rezende A.A., Oliveira P.F., Nicolella H.D., Tavares D.C., Silva A.C., Dantas N.O., Spanó M.A. Evaluation of titanium dioxide nanocrystal-induced genotoxicity by the cytokinesis-block micronucleus assay and the Drosophila wing spot test. Food Chem. Toxicol. 2016;96:309–319. doi: 10.1016/j.fct.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava R.K., Rahman Q., Kashyap M.P., Singh A.K., Jain G., Jahan S., Lohani M., Lantow M., Pant A.B. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum. Exp. Toxicol. 2013;32:153–166. doi: 10.1177/0960327112462725. [DOI] [PubMed] [Google Scholar]
- 46.Catalan J., Jarventaus H., Vippola M., Savolainen K., Norppa H. Induction of chromosomal aberrations by carbon nanotubes and titanium dioxide nanoparticles in human lymphocytes in vitro. Nanotoxicology. 2012;6:825–836. doi: 10.3109/17435390.2011.625130. [DOI] [PubMed] [Google Scholar]
- 47.Magdolenova Z., Collins A., Kumar A., Dhawan A., Stone V., Dusinska M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8:233–278. doi: 10.3109/17435390.2013.773464. [DOI] [PubMed] [Google Scholar]
- 48.ANSES OPINION of the French Agency for Food, Environmental and Occupational Health & Safety on the risks associated with ingestion of the food additive E171. ANSES J. 2019:40. [Google Scholar]
- 49.Kirkland D., Aardema M.J., Battersby R.V., Beevers C., Burnett K., Burzlaff A., Czich A., Donner E.M., Fowler P., Johnston H.J., et al. A weight of evidence review of the genotoxicity of titanium dioxide (TiO2) Regul. Toxicol. Pharmacol. 2022;136:105263. doi: 10.1016/j.yrtph.2022.105263. [DOI] [PubMed] [Google Scholar]
- 50.El-Ghor A.A., Noshy M.M., Galal A., Mohamed H.R. Normalization of nano-sized TiO2-induced clastogenicity, genotoxicity and mutagenicity by chlorophyllin administration in mice brain, liver, and bone marrow cells. Toxicol. Sci. 2014;142:21–32. doi: 10.1093/toxsci/kfu157. [DOI] [PubMed] [Google Scholar]
- 51.Hanot-Roy M., Tubeuf E., Guilbert A., Bado-Nilles A., Vigneron P., Trouiller B., Braun A., Lacroix G. Oxidative stress pathways involved in cytotoxicity and genotoxicity of titanium dioxide (TiO2) nanoparticles on cells constitutive of alveolo-capillary barrier in vitro. Toxicol. In Vitro. 2016;33:125–135. doi: 10.1016/j.tiv.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Armand L., Tarantini A., Beal D., Biola-Clier M., Bobyk L., Sorieul S., Pernet-Gallay K., Marie-Desvergne C., Lynch I., Herlin-Boime N., et al. Long-term exposure of A549 cells to titanium dioxide nanoparticles induces DNA damage and sensitizes cells towards genotoxic agents. Nanotoxicology. 2016;10:913–923. doi: 10.3109/17435390.2016.1141338. [DOI] [PubMed] [Google Scholar]
- 53.El Yamani N., Collins A.R., Runden-Pran E., Fjellsbø L.M., Shaposhnikov S., Zienolddiny S., Dusinska M. In vitro genotoxicity testing of four reference metal nanomaterials, titanium dioxide, zinc oxide, cerium oxide and silver: Towards reliable hazard assessment. Mutagenesis. 2017;32:117–126. doi: 10.1093/mutage/gew060. [DOI] [PubMed] [Google Scholar]
- 54.de Almeida L.C., Calil F.A., Machado-Neto J.A., Costa-Lotufo L.V. DNA damaging agents and DNA repair: From carcinogenesis to cancer therapy. Cancer Genet. 2021;252–253:6–24. doi: 10.1016/j.cancergen.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Motegi A., Masutani M., Yoshioka K.I., Bessho T. Aberrations in DNA repair pathways in cancer and therapeutic significances. Semin. Cancer Biol. 2019;58:29–46. doi: 10.1016/j.semcancer.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Hopkins J.L., Lan L., Zou L. DNA repair defects in cancer and therapeutic opportunities. Genes. Dev. 2022;36:278–293. doi: 10.1101/gad.349431.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puigvert J.C., Sanjiv K., Helleday T. Targeting DNA repair, DNA metabolism and replication stress as anti-cancer strategies. FEBS J. 2016;283:232–245. doi: 10.1111/febs.13574. [DOI] [PubMed] [Google Scholar]
- 58.Rothkamm K., Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc. Natl. Acad. Sci. USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raja G., Cao S., Kim D.H., Kim T.J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;107:110303. doi: 10.1016/j.msec.2019.110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dubey A., Goswami M., Yadav K., Chaudhary D. Oxidative Stress and Nano-Toxicity Induced by TiO2 and ZnO on WAG Cell Line. PLoS ONE. 2015;10:e127493. doi: 10.1371/journal.pone.0127493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kryston T.B., Georgiev A.B., Pissis P., Georgakilas A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 62.El Yamani N., Rubio L., García-Rodríguez A., Kažimírová A., Rundén-Pran E., Magdalena B., Marcos R., Dusinska M. Lack of mutagenicity of TiO2 nanoparticles in vitro despite cellular and nuclear uptake. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022;882:503545. doi: 10.1016/j.mrgentox.2022.503545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data can be found in the figures and tables within this article.