Abstract
Objectives:
The detection of respiratory syncytial virus (RSV) in upper airway samples does not necessarily infer causality of illness. We aimed to calculate the attributable fraction (AF) of RSV in clinical syndromes across age groups.
Methods:
Using unconditional logistic regression models, we estimated the AF of RSV-associated influenza-like illness (ILI) and severe acute respiratory illness (SARI) cases by comparing RSV detection prevalence among ILI and SARI cases to those of healthy controls in South Africa, 2012-2016. The analysis, stratified by HIV serostatus, was conducted in the age categories <1, 1-4, 5-24, 25-44, 45-64, and ≥65 years.
Results:
We included 12,048 individuals: 2687 controls, 5449 ILI cases, and 5449 SARI cases. RSV-AFs for ILI were significant in <1, 1-4, 5-and 24, 25-44-year age groups: 84.9% (95% confidence interval [CI] 69.3-92.6%), 74.6% (95% CI 53.6-86.0%), 60.8% (95% CI 21.4-80.5%) and 64.1% (95% CI 14.9-84.9%), respectively. Similarly, significant RSV-AFs for SARI were 95.3% (95% CI 91.1-97.5) and 83.4% (95% CI 70.9-90.5) in the <1 and 1-4-year age groups respectively. In HIV-infected persons, RSV was significantly associated with ILI cases vs controls in individuals aged 5-44 years.
Conclusion:
High RSV-AFs in young children confirm RSV detection is associated with severe respiratory illness in South African children, specifically infants. These estimates will assist with refining burden estimates and cost-effectiveness models.
Keywords: Attributable fraction, Respiratory syncytial virus, Burden of disease
Background
It is estimated that up to 33.1 million episodes of respiratory syncytial virus (RSV)-associated lower respiratory tract infection (LRTI) occur annually globally in children under-5 years of age, approximately 10% of which result in hospitalization [1]. Of the nearly 120,000 estimated RSV-associated global deaths among children aged <5 years annually, 99% occur in low-and-middle-income countries of Africa and Asia [2]. Data from South Africa confirm a substantial RSV burden in children, with estimated hospitalization rates of 3262/100,000 population in children aged <1 year [3]. HIV-exposed infants under 6 months of age are considered at increased risk of hospitalization with RSV-associated LRTI compared to HIV-unexposed infants (incidence rate ratio [IRR] 1.4; 95% confidence interval [CI] 1.3-1.6) [4].
In addition, there may be a substantial burden of RSV-associated LRTI in adults, with an estimated incidence of hospitalization 30/100,000 in the general South African population aged ≥5 years and from 106 to 390/100,000 population in individuals living with HIV [5]. HIV infection is a known risk factor for severe respiratory illness (SRI) in all age groups [5].
Most recent burden estimates have relied on sensitive polymerase chain reaction (PCR) assays to detect RSV in respiratory samples [1]. Describing the correlation between the detection of RSV on these sensitive assays and the presence of RSV disease is important to refine burden estimates. Accurate burden estimates will be important to advocate for the introduction of new RSV prevention technologies, such as vaccines and new-generation monoclonal antibodies [6,7]. In addition, estimates of the attributable fraction (AF) of RSV (RSV-AF) in LRTI and influenza-like-illness (ILI) will assist in building accurate cost-effectiveness models for the RSV interventions in the pipeline.
While there are some data on RSV-AF in children, there is very little information on RSV-AF in HIV-infected and HIV-exposed uninfected (HEU) children [8,9]. The AF of RSV to disease in adults is also not well characterized. Prior to the introduction of interventions for RSV-associated illness, policymakers need accurate estimates of both in- and outpatient RSV disease burden. Burden estimates collected before introduction will assist with the assessment of vaccine impact.
We aimed to evaluate the AF of RSV detection in individuals of all ages presenting with non-hospitalized ILI or hospitalized severe acute respiratory illness (SARI). Further, we looked specifically at the following groups: HIV-infected and HIV-uninfected children <5 years of age, HEU infants (aged <6 months); and lastly, HIV-infected and HIV-uninfected adults. These analyses were further stratified by age groups < 1 year, 1-4 years, 5-24 years, 25-44 years, 45-64 years, and ≥65 years. We also aimed to estimate the proportion of ILI and SARI cases attributable to RSV infection after adjusting the observed detection rate of RSV for the RSV-AF.
Methods
Cases were enrolled as part of a prospective, hospital-based, sentinel surveillance program for respiratory illness from June 2012 through May 2016 at two sites in North West (Klerksdorp-Tshepong Hospital Complex [KTHC], Klerksdorp), and KwaZulu-Natal (Edendale Hospital [EDH], Pietermaritzburg) provinces of South Africa and primary health care clinics in the hospital catchment population [10]. Specific descriptions of enrollment are provided below.
Controls and influenza-like illness cases
Study-specific controls were defined as individuals who sought medical care at one of the sentinel clinics for non-respiratory, non-gastrointestinal symptoms and who did not have a recorded temperature or history of fever within the last 14 days. Most of the controls were selected from non-urgent outpatient service visits including dental clinics, family planning, immunization and well-baby visits, voluntary HIV counseling and testing, or acute care for minor injuries. Two controls, one living with and one without HIV, were enrolled weekly in each clinic among age groups: <1 year, 1-4 years, 5-24 years, 25-44 years, 45-64 years, and ≥65 years. ILI cases were enrolled at the same clinics. ILI was defined as illness in an outpatient of any age who had a recorded temperature ≥38°C or a history of fever and cough of ≤10 days duration.
Severe acute respiratory illness surveillance
A SARI case was defined as an illness in a hospitalized individual meeting the following age-specific criteria. A SARI case in children 3 months to <5 years of age was defined as any child with physician-diagnosed acute LRTI with symptom duration ≤10 days, including bronchopneumonia, pneumonia, bronchitis, bronchiolitis, and pleural effusion irrespective of signs and symptoms; we also included infants aged 2 days to <3 months with physician-diagnosed sepsis. A SARI case in individuals aged ≥5 years was defined as any individual with a recorded temperature ≥38 °C or history of fever and cough.
Study procedures
Detailed procedures for the surveillance program have been described [10]. Briefly, dedicated surveillance staff systematically screened potentially eligible outpatients and hospitalized individuals and collected a clinical specimen and detailed history of symptoms and other medical conditions from consenting persons meeting surveillance case definitions. Individuals with ILI who were referred to hospital on the day of enrollment were excluded from the analysis.
Determination of HIV status
HIV status was determined from one of the following sources: (i) in-hospital/clinic voluntary counseling and testing by rapid HIV test according to standard clinical protocols (ii) patient records or (iii) if necessary (with consent), a dried blood spot tested at the National Institute for Communicable Diseases (NICD), Johannesburg. Testing for individuals aged ≥18 months was done by an HIV ELISA, and PCR testing was conducted on samples from children aged <18 months.
HIV-exposed uninfected infants
HEU infants were those aged <6 months born to a mother who was living with HIV, and in whom the HIV infection status of the child was confirmed to be negative. These infants were enrolled at the same surveillance sites as the cases and controls above.
Samples collection and virus detection
Nasopharyngeal aspirates were collected from children aged <5 years and for individuals aged ≥5 years both oropharyngeal and nasopharyngeal swabs were collected. The specimens were placed in universal transport medium (Copan, Murrieta, California, USA) and stored at 4-8°C at the surveillance site before being transported to NICD for testing. Testing was conducted within 72 hours of collection. All specimens were tested for RSV and nine other respiratory viruses (influenza virus A and B, parainfluenza types 1, 2, and 3, adenovirus, human metapneumovirus, enteroviruses, and rhinovirus). Testing was done on a multiplex real-time reverse transcription PCR assay during 2012-2014 [11]. From 2015, a combination of the commercial Fast Track Diagnostics (FTD) Flu/HRSV assay (Fast Track Diagnostics, Luxembourg) and Allplex Respiratory Assay, panels 2 and 3 (Seegene, Seoul, Korea) were used to test for other respiratory viruses.
Statistical analysis
We used an unconditional logistic regression to estimate the AF of RSV-associated SARI and ILI by comparing the RSV detection rate observed in our surveillance among ILI and SARI to those of controls [9,12]. These estimates were adjusted for HIV infection and co-infections with the other respiratory viruses tested for, these include influenza, rhinovirus, enterovirus, parainfluenza (1, 2, 3), and metapneumovirus. The analysis was implemented overall (all age groups), and stratified by HIV serostatus in the following age categories: <1 year, 1-4 years, 5-24 years, 25-44 years, 45-64 years, and ≥65 years. HIV infection and age group were also included as covariates in the non-HIV-stratified overall model and the models implemented among children aged <5 (<1 and 1-4 years) and persons aged ≥5 years (5-24, 25-44, 45-64 ≥65 years). Significant associations were considered at P <0.05.
The adjusted odds ratios (OR) obtained from these models were used to estimate the AF by applying the formula: . We also estimated the RSV detection rate associated with illness among individuals with ILI and SARI (PrevIllness) from the observed detection rate (PrevObserved) as follows: adjusted .
The statistical analysis was conducted using STATA version 14.1 (StataCorp, College Station, Texas, USA).
Ethical considerations
Approval for the hospital-based surveillance was obtained from the University of the Witwatersrand Human Research Ethics Committee (HREC) for the Klerksdorp site and from the University of KwaZulu-Natal Human Biomedical Research Ethics Committee (BREC) for the Pietermaritzburg site; protocol numbers M081042 and BF157/08, respectively. The ILI and control enrollment protocol was approved by HREC and BREC (protocol numbers M120133 and BF080/12, respectively). This surveillance was deemed non-research by the US Centers for Disease Control and Prevention (non-research determination number: 2012-6197).
Results
Study population
We enrolled 12,048 individuals of whom 99% (12,008) had RSV test results. This included 2687 controls, 5449 with ILI, and 3912 with SARI.
Overall, the RSV detection positivity was 2% (52/2687) in controls, 5% (303/5449) in ILI cases, and 14% (553/3912) in SARI cases. The highest positivity was in children aged <1 year with SARI (31%; 413/1,328). Among ILI cases, detection was highest in children aged <1 year (13%; 78/621) (Table 1). RSV detection was similar among controls (3%; 12/389 and 3%; 17/552) in age groups <1 year and 1-4 years, respectively. The RSV detection rate in controls was lower in the older age groups; 2% (13/787) in 5-24-year age group and 1% in all other age groups (25-44 years, 6/411; 45-64 years; 3/379 and ≥ 65 years, 1/169). The overall detection rate of influenza was 9% (1080/12,008), rhino 22% (2642/12,008), adenovirus 9% (1080/12,008), paraflu-influenza 4% (480/12,008), both enterovirus and human metapneumovirus 3% (360/12,008).
Table 1.
AF and AF-adjusted prevalence of RSV among individuals in a study of the AF of RSV detection in mild and severe illness, Klerksdorp and Edendale, South Africa, June 2012 to May 2016.
| All enrolled participants | ||||||
|---|---|---|---|---|---|---|
| ILI |
SARI |
|||||
| Age group | Observed RSV detection rate n/N (%) |
AF % (95% CI) | AF-adjusted prevalence % |
Observed RSV detection rate n/N (%) |
AF, % (95% CI) | AF- adjusted prevalence % |
| <1 | 78/621 (13) | 84.9 (69.3-92.6) | 11 | 413/1328 (31) | 95.3 (91.1-97.5) | 30 |
| 1-4 | 116/1101 (11) | 74.6 (53.6-86.0) | 8 | 101/786 (13) | 83.4 (70.9-90.5) | 11 |
| 5-24 | 43/1531 (3) | 60.8 (21.4-80.5) | 2 | 6/287 (2) | 25.6 (0-73.8) | 1 |
| 25-44 | 49/1532 (3) | 64.1 (14.9-84.9) | 2 | 16/842 (1) | 26.1 (0-72.6) | <1 |
| 45-64 | 15/561 (3) | 69.6 (0-90.8) | 2 | 11/492 (2) | 52.6 (0-87.9) | 1 |
| 65+ | 2/103 (2) | 48.0 (0-94.7) | 1 | 6/177 (3) | 80.6 (0-96.8) | 2 |
| Individuals living with HIV | ||||||
| Observed RSV detection rate | ILI | Observed RSV detection rate | SARI | |||
| AF, % (95% CI) | AF-adjusted prevalence, % |
AF, % (95% CI) | AF-adjusted prevalence, % |
|||
| <1 | 0/8 (0) | 0 (0-92.5) | ND | 3/20 (15) | 0 (0-71.9) | ND |
| 1-4 | 1/19 (5) | 75.8 (0-96.5) | 4 | 9/91 (10) | 72.1 (0-95.9) | 7 |
| 5-24 | 12/230 (5) | 78.1 (36.5-92.5) | 4 | 5/114 (3) | 55.4 (0-87.7) | 3 |
| 25-44 | 29/865 (3) | 76.6 (13.6-93.7) | 2 | 16/722 (2) | 61.8 (0-90.0) | 1 |
| 45-64 | 6/227 (3) | 75.7 (0-96.1) | 2 | 8/299 (3) | 80.4 (0-97.6) | 2 |
| 65+ | 0/10 (0) | No RSV | NA | 3/33 (9) | 87.4 (0-99.4) | 8 |
| HIV-uninfected individuals | ||||||
| ILI | SARI | |||||
| Observed RSV detection rate | AF, % (95% CI) | AF-adjusted prevalence, % |
Observed RSV detection rate | AF, % (95% CI) | AF-adjusted prevalence, % |
|
| <1 | 68/574 (12) | 87.5 (72.2-94.4) | 11 | 371/1109 (33) | 96.7 (93.0-98.5) | 32 |
| 1-4 | 112/1038 (11) | 77.7 (56.6-88.5) | 9 | 91/656 (14) | 84.6 (70.3-92.0) | 12 |
| 5-24 | 27/1158 (2) | 39.0 (0-74.1) | 1 | 1/123 (1) | 0 (0-78.7) | ND |
| 25-44 | 18/588 (3) | 30.9 (0-76.0) | 1 | 0/93 (0) | 0(0-53.5) | ND |
| 45-64 | 7/301 (2) | 60.8 (0-91.0) | 1 | 0/162 (0) | 0 (0-91.3) | ND |
| 65+ | 1/84 (1) | 46.0 (0-94.5) | 0.5 | 3/129 (2) | 64.3 (0-94.8) | 1 |
AF, Attributable fraction; CI, confidence interval; ILI, influenza-like illness; ND, not determined; RSV, respiratory syncytial virus; SARI, severe acute respiratory illness.
HIV results were available for 95% (11,445/12,048) of enrolled participants.
Attributable fractions
Influenza-like illness
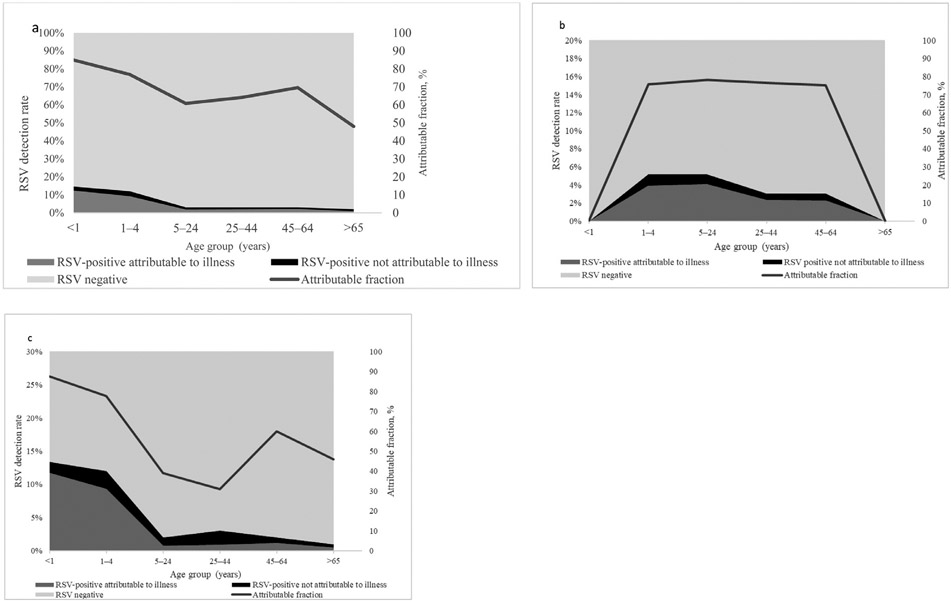
RSV-AFs in ILI cases were significantly different from zero in the following age groups: <1 year (84.9%, 95% CI 69.3-92.6%), 1-4 years (74.6%, 95% CI 53.6-86.0%), 5-24 years (60.8%, 95% CI 21.4-80.5%) and 25-44 years (64.1%, 95% CI 14.9-84.9%). In older age groups, the detection of RSV in ILI cases was not significantly attributable to disease (45-64 years, 69.6%, 95% CI 0-90.8% and ≥65 years, 48.0%, 95% CI 0-94.7%) (Table 1, Figure 1a-c).
Figure 1.
Attributable fraction of respiratory syncytial virus (RSV) and proportion of RSV-positive cases attributable to illness and RSV-positive cases not attributable to illness in individuals with influenza-like illness (ILI) (1a: Overall, 1b: HIV-infected and 1c: HIV uninfected), in two provinces South Africa 2012-2016.
Severe acute respiratory illness
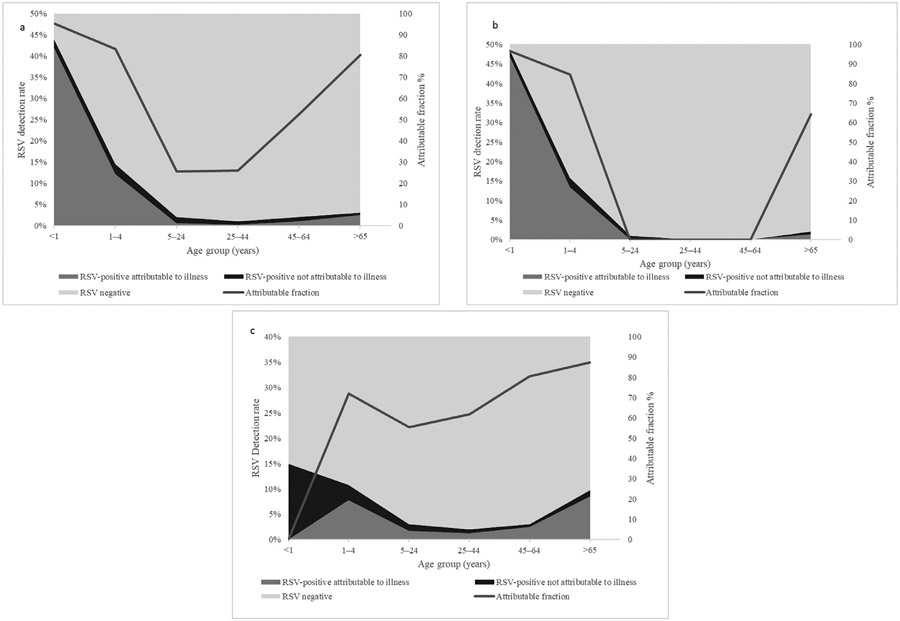
The RSV-AFs for SARI were significantly different from zero in the age groups <1 year (95.2%, 95% CI 90.9-97.5) and 1-4 years (82.9%, 95% CI 70.2-90.1) (Table 1, Figure 2a). This association was also seen in HIV-uninfected individuals with similar RSV-AFs observed in HIV-uninfected infants aged <1 year with SARI (96.7%, 95% CI, 93.0-98.5%) (Table 1, Figure 2a-c). The RSV-AF was not significantly different from zero in the age group ≥65years irrespective of HIV status (80.6%, 95% CI, 0-96.6%) and similarly in the age groups 5-24 years, 25-44 years, and 45-64 years (25.6%, 95% CI 0-73.8%; 26.1%, 95% CI 0-72.6% and 52.6%, 95% CI, 0-87.9%, respectively) (Table 1, Figure 2a-c).
Figure 2.
Attributable fraction of RSV and proportion of RSV-positive cases attributable to illness and RSV-positive cases not attributable to illness in individuals with severe acute respiratory illness (2a: Overall, 2b: HIV-infected and 2c: HIV-uninfected), in two provinces South Africa 2012-2016. (Footnote RSF-attributable fraction in age groups 5-24, 25-44, and 45-54 were not estimated for HIV-uninfected individuals as no cases were detected in these age groups). RSV, respiratory syncytial virus.
HIV-infected individuals
Among HIV-infected individuals, the RSV-AFs for ILI were significantly different from zero in the 5-24 and 25-44-year age groups only 78.1%, 95% CI, 36.5-92.5% and 76.6%, 95% CI 13.6-93.7%, respectively). We were not able to calculate the RSV-AFs for ILI and SARI in the <1-year age groups due to no RSV observations occurring in HIV-infected infants with ILI and SARI (Table 1, Figures 1b and 2b).
HIV-exposed infants
The RSV-AF, for HEU <6 months of age with SARI, was significantly greater than zero (89.9%, 95% CI 69.2-96.7%). The RSV-AF for ILI in HEU <6 months of age was not significantly different from zero (64.2%, 95% CI 0-90.1%). The RSV-AF for HIV-unexposed-uninfected (HUU) infants aged <6 months was significantly different from zero across the clinical syndromes (ILI 88.0%, 95% CI 54.2-96.8%, and SARI 97.0%, 95% CI 86.0-99.3%) (Table 2).
Table 2.
AF and AF-adjusted prevalence of RSV among HIV exposed-uninfected, HIV-infected and HIV-unexposed uninfected infants aged <6 months, Klerksdorp and Edendale, June 2012 to May 2016.
| Influenza-like illness |
Severe acute respiratory illness |
|||||
|---|---|---|---|---|---|---|
| Observed detection rate of RSV |
AF % (95% CI) | AF-adjusted prevalence % |
Observed detection rate of RSV |
AF, % (95% CI) | AF-adjusted prevalence % |
|
| HIV-exposed-uninfected | 10/77 (13) | 64.2 (0-90.1) | 8 | 5/16 (3) | 89.6 (50.1-97.8) | 2 |
| HIV-infected | 0/3 (0) | 71.0 (0-99.2) | Not determined | 2/10 (20) | 87.3 (0-98.8) | 17 |
| HIV-unexposed-uninfected | 20/82 (11) | 88.0 (54.2-96.8) | 10 | 9/27 (33) | 97.0 (86.0-99.3) | 32 |
AF, Attributable fraction; CI, confidence interval; RSV, respiratory syncytial virus.
Discussion
We were able to describe RSV-AFs that were significantly different from zero in ILI in all age groups except for the older adult age groups (45-64 years and ≥65 years). We confirmed the high RSV-AF in children with SARI with significant RSV-AF in children aged <1 year. In addition, we documented no RSV-AFs in adults with RSV-associated SARI which were significantly different from zero. These associations were similar for adults living with HIV. In HIV-uninfected individuals aged ≥5 years, the detection of RSV was not significantly associated with illness. The RSV-AFs were lower in HEU infants aged <6 months, as compared to the HUU, but higher than HIV-infected infants aged <6 months. The strengths of our study include 5 years of observed RSV infection.
In children, the RSV-AF was high in all age groups, specifically in the children <1 year presenting with SARI confirming the findings in other studies [3, 13]. Detection of RSV was significantly associated with illness in the children presenting with ILI aged <1 year and 1-4 years. Several studies in children confirm a strong association between the detection of RSV and disease that we describe in this analysis. Although there are many different study designs and case definitions of SARI the results are remarkably similar across studies [9,10,13-16]. An interim analysis of a subset of data presented in this study showed similar results to our analysis for those aged <5 years [9]. In the Drakenstein study, a case-control study, conducted in South Africa, of the association between the detection of respiratory viruses and pneumonia, RSV detection was associated with pneumonia (OR 8.5, 95% CI; 4.2-15.4 and very strongly associated with very severe illness OR 25.3, 95% CI 3.3-191.6) [9]. A study in western Kenya described an association between RSV detection and disease in children aged <5 years with pneumonia (adjusted OR 3.0, 95% CI 1.1-8.2) [16]. The PERCH multicentre study reported that viruses account for almost two-thirds of pneumonia cases (61.4%, 95% credible interval (CrI) 57.3-65.6. In addition, this study reported RSV as having the greatest aetiological fraction 31.3 95% CrI, 28.4-34.2) of all pathogens, very similar to our results [15]. Additional evidence is presented in a systematic review and meta-analysis by Shi et al where the authors assign a causal attribution OR of RSV in children of 9.8, 95% CI 4.9-19.3, and an AF of 90%, 95% CI 85-95% [13].
In our assessment, among individuals living with HIV, we describe that RSV detection is significantly associated with illness in individuals aged 5-24 years and 25-44 years for ILI. In HIV-infected children aged <5 years we did not see an association between the detection of RSV and illness, this is likely due to the very low prevalence of HIV in this age group, limiting our ability to detect significant associations. We did not detect any cases of RSV-associated ILI or SARI in HIV-infected individuals <1 year. HEU infants are at increased risk of LRTI and hospitalization with RSV-associated LRTI, the lower RSV-AF in this group compared to HIV-unexposed uninfected infants suggests that other pathogens are contributing proportionately more to the disease in this group of infants [4].
In individuals aged ≥5 years with SARI, the point estimates of RSV-AF suggest that RSV may be associated with illness however in many age groups the RSV-AFs were not significantly higher in cases compared to controls. Although older age (≥65 years) is considered a risk factor for RSV-associated SARI, we do not demonstrate a significant RSV-AF in this group of individuals [17]. This may be due to the low number of older individuals presenting at healthcare facilities in our setting or because there is not truly an association in this age group.
When our RSV-AF data are displayed graphically by age group, U-shaped distributions are seen among SARI cases, with high RSV-AF in young children, dropping in younger adults, and rising again in the elderly (although point estimates of AF were not significant in some groups). Similar to the shape of the distribution for the incidence of hospitalization with RSV-associated SARI [5,18,19]. By comparison, the influenza-AF curve among individuals with SARI has lower estimates for children <1 year and higher estimates for the ≥65-year age group [19]. Conversely, the RSV-AF in individuals with ILI forms a more bell-like shape with the highest AF in the age groups 25-64 years. Data on the causal association of viral detection in LRTI in adults are sparse. In a study conducted in the United States of America, the incidence of RSV-associated pneumonia in adults was estimated at 7/100,000 population 95% CI, 5-9%, lower than the estimate in our setting but without AF estimation, therefore the numbers are not comparable to our study [5,18].
Strengths of our study
The strengths of our study include the estimation of AF-RSV in infants, children, and older age groups by enrolling controls to match these important age groups. Specifically, the difference in AF-RSV between infants and older children. We were also able to document the AF-RSV in HIV-infected individuals and in HIV-exposed infants, an important risk group for SRI. We also observed RSV detection in cases and control over a five-year period including the RSV seasons in these years, in a methodology nested in a sentinel surveillance program.
Limitations of our study
Our case definition of ILI included fever, including fever may have led to an underestimation of the AF-RSV, specifically in the extremes of age. For SARI cases in children <5 we enrolled all children admitted to hospital with physician-diagnosed LRTI, irrespective of symptoms, however individuals over the age of 5 years we enrolled on a case definition that included fever, which again may have led to the underestimation of the attractable fraction in the older age groups.
Although RSV is detected throughout the year in South Africa, there is a seasonal increase in circulation between February and May each year. We did not adjust for seasonal variation in our AF-RSV estimates, which may have affected the point estimates, and possibly the significance of these results.
Conclusion
RSV is strongly associated with respiratory illness, particularly in young South African children. While point estimates suggested an elevated AF for RSV-associated SARI among the elderly, we were not able to conclusively demonstrate an association between RSV detection and illness. More data are needed on the RSV-AF to illness among the elderly. These data are of interest to clinicians and policymakers. In addition, the calculation of the AF will improve the estimates of burden and cost-effectiveness models. These estimates of AF could be used to motivate the introduction of vaccines and new-generation monoclonal antibodies [7].
Funding source
The data collection for this manuscript was funded through a grant to the NICD from the Centers for Disease Control (CDC), Atlanta Georgia. Collaborative Research on Influenza, Coronavirus Diseases 2019 (COVID-19), and Other Respiratory Pathogens in South Africa (Cooperative Agreement Number: 1U01IP001160).
Footnotes
Declarations of competing interest
Prof Cheryl Cohen and Anne von Gottberg have received institutional funding support from US Centers for Disease Control (CDC) related to the present work and also received funding from Bill and Melinda Gates Foundation (BMGF), PATH, Sanofi, Wellcome Trust, and South African Medical Research Council of South Africa (MRC) outside of the submitted work. Dr Moyes received institutional funding from Sanofi and PATH for work outside of this present work. Dr Dawood reports personal fees from Sanofi-South Africa. In addition, she reports workshop attendance sponsorship from Biomieruex-South Africa.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies.
References
- [1].Shi T, McAllister DA, O’Brien KL, Simoes EA, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390:946–58. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McMorrow ML, Tempia S, Walaza S, Treurnicht FK, Moyes J, Cohen AL, et al. The role of Human Immunodeficiency Virus in influenza- and respiratory syncytial virus-associated hospitalizations in South African children, 2011–2016. Clin Infect Dis 2019;68:773–80. doi: 10.1093/cid/ciy532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cohen C, Moyes J, Tempia S, Groome M, Walaza S, Pretorius M, et al. Epidemiology of acute lower respiratory tract infection in HIV-exposed uninfected infants. Pediatrics 2016;137:e20153272. doi: 10.1542/peds.2015-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moyes J, Walaza S, Pretorius M, Groome M, von Gottberg A, Wolter N, et al. Respiratory syncytial virus in adults with severe acute respiratory illness in a high HIV prevalence setting. J Infect 2017;75:346–55. doi: 10.1016/j.jinf.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vekemans J, Moorthy V, Giersing B, Friede M, Hombach J, Arora N, et al. Respiratory syncytial virus vaccine research and development: World Health Organization technological roadmap and preferred product characteristics. Vaccine 2019;37:7394–5. doi: 10.1016/j.vaccine.2017.09.092. [DOI] [PubMed] [Google Scholar]
- [7].Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018;18:e295–311. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- [8].Pretorius MA, Tempia S, Walaza S, Cohen AL, Moyes J, Variava E, et al. The role of influenza, RSV and other common respiratory viruses in severe acute respiratory infections and influenza-like illness in a population with a high HIV sero-prevalence, South Africa 2012–2015. J Clin Virol 2016;75:21–6. doi: 10.1016/j.jcv.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med 2016;4:463–72. doi: 10.1016/S2213-2600(16)00096-5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cohen C, Moyes J, Tempia S, Groom M, Walaza S, Pretorius M, et al. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis 2013;19:1766–74. doi: 10.3201/eid1911.130546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pretorius MA, Madhi SA, Cohen C, Naidoo D, Groome M, Moyes J, et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness–South Africa, 2009–2010. J Infect Dis 2012;206:S159–65. doi: 10.1093/infdis/jis538. [DOI] [PubMed] [Google Scholar]
- [12].Tempia S, Walaza S, Moyes J, Cohen AL, Von Mollendorf C, McMorrow ML, et al. Attributable fraction of influenza virus detection to mild and severe respiratory illnesses in HIV-infected and HIV-uninfected patients, South Africa, 2012–2016. Emerg Infect Dis 2017;23:1124–32. doi: 10.3201/eid2307.161959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta–analysis. J Glob Health 2015;5:010408. doi: 10.7189/jogh.05.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gilani Z, Kwong YD, Levine OS, Deloria-Knoll M, Scott JA, O’Brien KL, et al. A literature review and survey of childhood pneumonia etiology studies: 2000–2010. Clin Infect Dis 2012;54:S102–8. doi: 10.1093/cid/cir1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pneumonia Etiology Research for Child Health (PERCH) Study GroupCauses of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019;394:757–79. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hammitt LL, Kazungu S, Morpeth SC, Gibson DG, Mvera B, Brent AJ, et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis 2012;54(Suppl. 2):S190–9. doi: 10.1093/cid/cir1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004;189:233–8. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- [18].Pastula ST, Hackett J, Coalson J, Jiang X, Villafana T, Ambrose C, et al. Hospitalizations for respiratory syncytial virus among adults in the United States, 1997–2012. Open Forum Infect Dis 2017;4:ofw270. doi: 10.1093/ofid/ofw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tempia S, Walaza S, Moyes J, Cohen AL, Von Mollendorf C, McMorrow ML, et al. The effects of the attributable fraction and the duration of symptoms on burden estimates of influenza-associated respiratory illnesses in a high HIV prevalence setting, South Africa, 2013–2015. Influenza Other Respir Viruses 2018;12:360–73. doi: 10.1111/irv.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]