Abstract
Carney-Stratakis syndrome (CSS) is an autosomal dominant rare syndrome, with incomplete penetrance, characterized by the association of paragangliomas and/or pheochromocytomas and gastrointestinal stromal tumors (GISTs). CSS is caused by germline heterozygous loss-of-function pathogenic variants (PVs) in the succinate dehydrogenase subunit genes (SDHB, SDHC, SDHD), with SDHB and SDHD being the most frequent. To date, only 2 germline SDHC PVs (c.43 C > T; c.405 + 1G > A) have been described in 3 patients with CSS. Three patients with CSS and very distinct clinical presentations are reported here: 1 caused by a germline SDHC large deletion and the others with metastatic GIST and negative genetic investigation for SDHx defects. Two cases (1 and 2) presented with pheochromocytoma (case 1 also with abdominal paraganglioma) and metastatic GIST. Although these 2 cases fulfilled the diagnostic criteria for CSS, the genetic investigation for SDHx PVs by next-generation sequencing and multiplex ligation-dependent probe amplification was negative. Case 3 had a large abdominal paraganglioma and a small low-grade GIST not associated with recurrence or metastasis. This case harbored a germline SDHC exon 3 deletion, not previously reported. In conclusion, CSS is a rare and morbid disease with distinct clinical presentations and genetic heterogeneity, which can contribute to underdiagnosis.
Keywords: Carney-Stratakis syndrome, pheochromocytoma, paraganglioma, gastrointestinal stromal tumor, succinate-dehydrogenase subunits
Introduction
Carney-Stratakis syndrome (CSS; OMIM #606864), also reported as “paraganglioma and gastrointestinal stromal tumor syndrome” or Carney-Stratakis dyad, is an autosomal dominant rare syndrome with incomplete penetrance, characterized by the association of paragangliomas (PGLs) and/or pheochromocytomas (PHEOs) and gastrointestinal stromal tumors (GISTs) described initially in 2002 by Carney and Stratakis. It affects both males and females during childhood and adolescence [1].
CSS is caused by germline heterozygous loss-of-function pathogenic variants (PVs) in the succinate dehydrogenase subunit genes (SDHB, SDHC, SDHD), with SDHB and SDHD being the most frequent [2, 3]. Somatic SDHC PVs were previously found in GIST samples from patients without CSS diagnosis [4]. To date, only 2 germline SDHC PVs (c.43 C > T; c.405 + 1G > A) have been described in 3 patients with CSS [2, 5]. The Carney triad (OMIM #604287), characterized by the association of PGL, GIST, and pulmonary chondroma, is not caused by germline SDHx PV, but it has been associated with DNA hypermethylation in the promoter and first exon of SDHC [6].
Here, 3 patients with CSS and distinct clinical presentations are reported, 1 patient with a germline SDHC large deletion and the other 2 with metastatic GIST and negative genetic investigation for SDHx defects. Therefore, the spectrum of the clinical presentation and genetics of CSS was highlighted.
Case Presentation
Case 1
A 59-year-old woman was incidentally diagnosed with 2 different lesions (a retroperitoneal mass and a left adrenal tumor) in a routine ultrasound. She did not present with adrenergic paroxysms, headache, abdominal pain, or intestinal obstipation. The patient was previously diagnosed with severe obesity, stage 2 hypertension, and type 2 diabetes at the age of 35 years. She had a history of cholecystectomy and partial gastrectomy because of a gastric ulcer 20 years ago. She had no family history of GIST, PHEO, or PGL. At physical examination, she presented with a body mass index = 41.2 kg/m2, blood pressure = 150 × 90 mm Hg, heart rate = 90 beats/min, and no stigmas of Cushing syndrome.
Case 2
A 62-year-old woman presented with weight loss (8 kg) and hematemesis (3 episodes in the month before hospital admission). The patient had no history of alcohol consumption. She was previously diagnosed with arterial hypertension. Her father had melanoma and her brother had prostate cancer. She had no family history of GIST, PHEO, or PGL.
Case 3
A 42-year-old male patient was incidentally diagnosed with an expansive retroperitoneal heterogeneous lesion measuring 13.5 cm with a necrotic center. The patient underwent tumor resection in another hospital (before being referred to us). Histopathological analysis and immunohistochemistry confirmed PHEO. He mentioned no information about preoperative α-adrenergic receptor blocker. Histopathological analysis (with paraffin blocks and slides) was reviewed at our institution and confirmed the initial diagnosis of PGL.
Diagnostic Assessment
Case 1
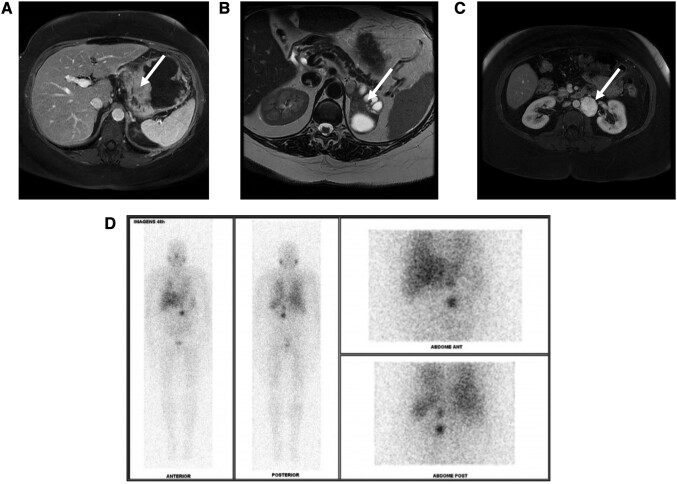
Abdominal magnetic resonance imaging (MRI) scan revealed a left hypervascular para-aortic retroperitoneal mass measuring 4 cm and a solid cystic lesion with 7 cm located in the left adrenal gland topography (Fig. 1). Additionally, the MRI scan also showed a 10-cm mass, with origin in the gastric body and fundus, suggesting a mesenchymal neoplasia (GIST). Plasma metanephrine levels were < 0.2 nmol/L (38.46 pg/mL, normal range <0.5 nmol/L [96.15 pg/mL]), and normetanephrine levels were 1.5 nmol/L (288.45 pg/mL, normal range <0.9 nmol/L [173.07 pg/mL]). A meta-iodine-benzyl-guanidine labeled with iodine 131 scan demonstrated a high uptake in the left para-aortic and adrenal lesions (Fig. 1). After 2 weeks of α-adrenergic receptor blocker treatment, an upper gastric endoscopic ultrasound-guided biopsy revealed a 10-cm lesion with a hypoechoic solid component, homogenous throughout the entire lesion, originating from the muscular layer of the gastric wall. Histopathological analysis and immunohistochemistry were compatible with GIST. Pulmonary chondroma was not detected at the chest computed tomography (CT), ruling out a diagnosis of Carney triad.
Figure 1.
(A) Magnetic resonance imaging (MRI) scan showing a 10-cm GIST with apparent origin in the gastric body and fundus (white arrow). (B) Axial T2-weighted MRI showing a 7-cm left solid cystic pheochromocytoma (white arrow). (C) Axial T2-weighted MRI indicating 4-cm para-aortic paraganglioma (white arrow). (D) Meta-iodine-benzyl-guanidine labeled with iodine 131 (131I-MIBG) scan demonstrating a high uptake in the left para-aortic and adrenal lesions. GIST, gastrointestinal stromal tumor.
Case 2
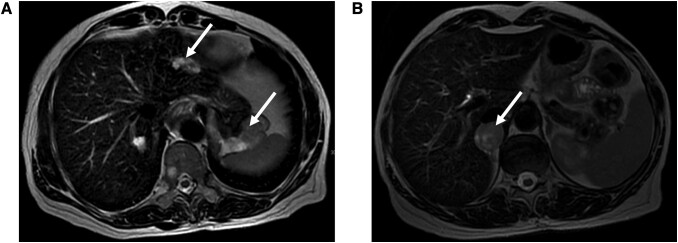
Upper gastrointestinal endoscopy revealed a 6-cm elevated gastric fundus lesion covered by mucosa with an ulcerated region. An upper gastric endoscopic ultrasound-guided biopsy revealed a predominantly hypoechoic, irregular, heterogeneous lesion, measuring 8 cm with anechoic areas compatible with necrosis. The lesion had endophytic and exophytic projections, apparently originating from the muscular layer of the gastric fundus. Histopathology and immunohistochemistry were compatible with GIST. MRI scan detected an 8-cm gastric lesion, a 3-cm tumor in the right adrenal gland, and 3 secondary lesions in the right hepatic lobe, measuring up to 2.1 cm (Fig. 2). Biochemical investigation revealed a plasma normetanephrine level of 5.2 nmol/L (999.96 pg/mL, normal range <0.9 nmol/L [173.07 pg/mL]) and metanephrine levels of 0.5 nmol/L (96.15 pg/mL, normal range <0.5 nmol/L [96.15 pg/mL]), confirming the biochemical diagnosis of pheochromocytoma. Pulmonary chondroma was not detected on the chest CT.
Figure 2.
(A) Axial T2-weighted magnetic resonance imaging (MRI) scan indicating an 8-cm GIST and a metastatic GIST liver lesion (white arrows). (B) Axial T2-weighted magnetic MRI scan showing a 3-cm right pheochromocytoma (white arrow). GIST, gastrointestinal stromal tumor.
Case 3
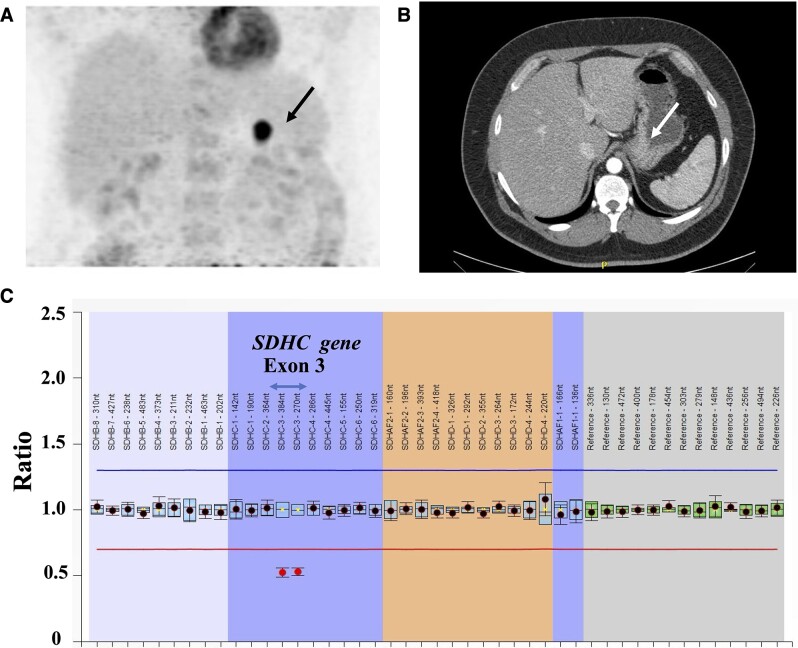
During the patient follow-up, a positron emission tomography CT imaging with 2-[(18)F]fluoro-2-deoxy-D-glucose revealed an undetermined nodular lesion in the posterior gastric body wall, suggestive of a neoplastic process (Fig. 3). A subsequent upper gastrointestinal endoscopy indicated a gastric elevated subepithelial lesion, and the histopathology with immunohistochemistry was compatible with GIST. An abdominal CT scan showed a 1.9-cm lesion in the gastric body. Chest CT did not indicate pulmonary chondroma.
Figure 3.
(A) Positron emission tomography (PET)-CT imaging with 2-[(18)F]fluoro-2-deoxy-D-glucose (FDG) revealing an undetermined nodular lesion in the posterior gastric body wall, suggestive of a neoplastic process (black arrow). (B) CT scan revealed a lesion in the gastric body, measuring 1.9 cm (white arrow). (C) Genetic investigation revealed a germline SDHC exon 3 deletion detected using multiplex ligation-dependent probe amplification (MLPA). CT, computed tomography; GIST, gastrointestinal stromal tumor.
Treatment
Case 1
The patient started perioperative preparation with α-adrenergic receptor blocker (doxazosin 2 mg twice per day) and a high-sodium diet (3-5 g/d). After a week of α-adrenergic blocker, a ß-adrenergic receptor blocker was initiated to control reflex tachycardia. Blood pressure remained well controlled (120 × 80 mm Hg) without postural hypotension. She successfully underwent a subtotal gastrectomy, left adrenalectomy, and resection of the para-aortic lesion. Six peritoneal lymph nodes were also resected. Histopathological analysis revealed PHEO (Pheochromocytoma of the Adrenal gland Scaled Score [PASS] score = 4, Ki67% < 1%, chromogranin positive, synaptophysin positive) and PGL (Ki67% 1%-2%, chromogranin positive, synaptophysin positive). A metastatic abdominal lymph node was detected with positive staining for chromogranin and synaptophysin. Histopathological analysis of the gastric lesion evidenced an epithelioid GIST (mitotic count: >3/5 mm2) positive for C-KIT (CD117), CD34, and DOG1. Therefore, the diagnosis of CSS was confirmed.
Case 2
The patient started perioperative preparation with α-adrenergic receptor blocker (doxazosin 2 mg twice per day) and a high-sodium diet (3-5 g/d). After a week of α-adrenergic blocker, a ß-adrenergic receptor blocker was initiated to control reflex tachycardia. Blood pressure remained well controlled (110 × 70 mm Hg) without postural hypotension. The patient underwent a right laparoscopic adrenalectomy. After a month of adrenalectomy, the patient underwent GIST resection. Histopathological analysis confirmed PHEO (PASS = 0, Ki67% < 1%, chromogranin positive, synaptophysin positive) and metastatic GIST (spindle cell 80% and epithelioid 20%; mitotic count: 11/5 mm2) positive for C-KIT (CD117), CD34, and DOG1. The association between PHEO and GIST fulfilled the diagnostic criteria for CSS.
Case 3
This patient underwent a segmental gastrectomy, which removed the lesion in the posterior wall of the stomach. Histopathological analysis confirmed PGL (Ki67% < 1%, chromogranin positive, synaptophysin positive) and epithelioid GIST (mitotic count: 3/5 mm2) positive for C-KIT (CD117), CD34, and DOG1.
Outcome and Follow-up
Case 1
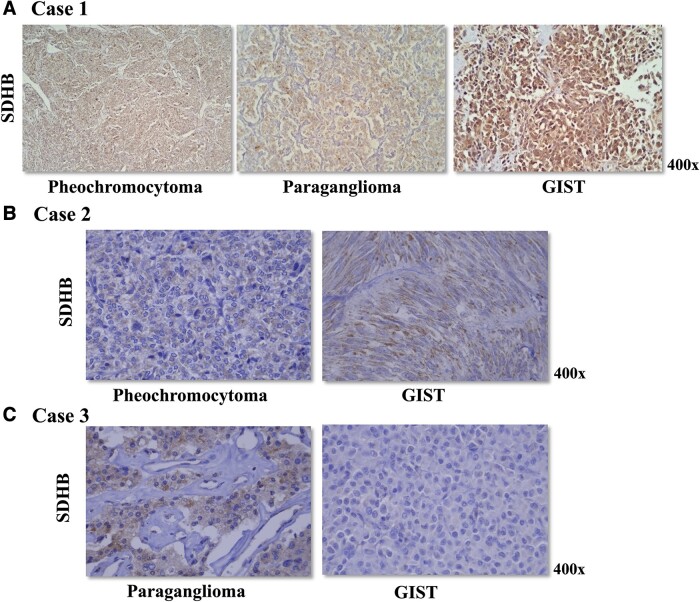
After surgical treatment, the patient’s plasma normetanephrine normalized and MIBG uptake turned negative. After 2 years of follow-up, no recurrence of paraganglioma and pheochromocytoma was detected, but she exhibited progression of peritoneal lymph node metastases from GIST. Genetic analysis of SDHB, SDHD, SDHC, SDHA, and SDHAF2 genes in DNA from peripheral blood lymphocytes using next-generation sequencing and multiplex ligation-dependent probe amplification (MLPA) was negative. GIST did not harbor somatic c-KIT and PDGFRA PVs. Immunohistochemistry for SDHB was positive in the pheochromocytoma, paraganglioma, and GIST (Fig. 4A).
Figure 4.
(A) Immunohistochemistry for SDHB was positive in the pheochromocytoma, paraganglioma, and GIST of case 1. Genetic investigation for SDHx pathogenic variants and large deletions was negative in the germline DNA and all 3 tumors samples from case 1. (B) Pheochromocytoma and GIST of case 2 displayed a positive staining for SDHB. Case 2 did not harbor germline pathogenic variants or large deletions in the SDHx genes. (C) Case 3 harbored a germline SDHC exon 3 deletion. Immunohistochemistry for SDHB was positive in the paraganglioma. GIST displayed a negative staining for SDHB, suggesting a second somatic event in the SDHC (such as promoter hypermethylation).
Case 2
After adrenalectomy, the patient’s plasma normetanephrine and metanephrine levels normalized. Imatinib treatment was initiated for GIST liver metastasis. Genetic analysis of SDHB, SDHD, SDHC, SDHA, and SDHAF2 genes in DNA from peripheral blood lymphocytes using next-generation sequencing and MLPA was negative. Somatic c-KIT and PDGFRA PVs were not detected in the GIST tumor sample. PHEO and GIST showed a positive staining for SDHB (Fig. 4B).
Case 3
The patient’s plasma normetanephrine and metanephrine levels remained normal during the follow-up. He did not present with GIST recurrence after 18 months of follow-up. Genetic investigation of CSS in DNA from peripheral blood lymphocytes revealed a germline SDHC exon 3 deletion detected using MLPA and next-generation sequencing (Fig. 3). Somatic c-KIT and PDGFRA PVs were not detected in the GIST tumor sample. Immunohistochemistry for SDHB was positive in the paraganglioma. GIST showed negative staining for SDHB (Fig. 4C).
Discussion
CSS is a rare syndrome characterized by 2 types of tumors, PHEO/PGL and GIST. In contrast to the Carney triad, which has not been so far associated with germline defects, CSS is mainly caused by germline PVs in SDHB and SDHD [2]. SDHC loss-of-function PVs were rarely reported in CSS [2]. To date, only 2 germline SDHC PVs (c.43 C > T; c.405 + 1G > A) have been described in 3 patients with CSS [2, 5]. Therefore, a germline SDHC exon 3 deletion was first reported here as a cause of CSS in a patient with GIST and abdominal PGL (case 3). Case 3 had a small, low-grade GIST not associated with recurrence or metastasis.
The other 2 cases presented with PHEO (case 1 also with PGL) and metastatic GIST. Although these 2 cases fulfilled the diagnostic criteria for CSS, the genetic investigation for SDHx genetic defects by next-generation sequencing and MLPA was negative. SDHB immunohistochemistry is a reliable tool to identify patients with genetic defects in the SDHB, SDHD, or SDHC genes, although 10% of the SDH-deficient tumors can present SDHB immunoreactivity [7]. In our study, immunohistochemistry for SDHB was positive in the PHEO and PGL of the 3 cases. GIST from cases 1 and 2 (not harboring germline SDHx genetic defects) presented a positive immunoreactivity for SDHB. Only the GIST from case 3 (harboring a germline SDHC exon 3 deletion) was negative for SDHB staining, suggesting a second somatic event in the SDHC gene (such as promoter hypermethylation). All 3 cases have not exhibited pulmonary chondromas so far. Boikos et al reported SDHC promoter hypermethylation (SDH-epimutant) in 6 of 11 GIST from patients with Carney triad [8]. Interestingly, SDH-epimutant GIST was also identified in a patient with CSS [8]. Non-SDH-deficient GISTs are caused by germline KIT/PDGFRA and are rarely associated with lymph node metastasis [1]. However, 2 very aggressive metastatic non-SDH-deficient GIST associated with CSS are described here. Our cases support the concept that CSS is a genetically heterogeneous disease, not only caused by germline SDHx defects.
Very rare patients with CSS but without SDHB, SDHD, or SDHC PVs have been reported [2, 9]. However, these cases were not properly investigated using next-generation sequencing and/or MLPA to exclude large deletions, as shown by 1 of our cases. Therefore, the first 2 fully investigated cases of CSS without a defined genetic etiology are presented here. Although SDHx defects have not been identified in the Carney triad, SDHC-specific hypermethylation is considered the molecular signature of GIST associated with the Carney triad and wild-type-GIST [6, 10].
In addition to genetic heterogeneity, 3 cases of CSS with very distinct clinical presentation were reported. Case 1 had PHEO, PGL, and metastatic GIST, whereas case 2 had a predominance of GIST-related symptoms and a clinically silent PHEO (despite the fact normetanephrine levels were highly elevated). Interestingly, both cases had metastatic GIST. Different from these 2 cases, case 3 had an incidental diagnosis of a large abdominal PGL (data not available about function) and a subsequent diagnosis of a small low-grade GIST during follow-up. Case 3 harbored a germline SDHC exon 3 deletion, as previously mentioned.
In conclusion, CSS is a rare and morbid disease with distinct clinical presentations and genetic heterogeneity, which can contribute to underdiagnosis. In this manuscript, a large germline SDHC deletion was first described as a cause of CSS, and 2 cases of CSS with metastatic GIST not associated with germline SDHx genetic defects were reported.
Learning Points
Carney-Stratakis syndrome (CSS), characterized by the association of pheochromocytoma/paraganglioma and gastrointestinal stromal tumor), is a rare and morbid condition, but its recognition and diagnosis are associated with greater clinical benefits for the patients.
The distinct patterns of clinical presentation in CSS patients might contribute to underdiagnosis of this disease.
CSS is mostly caused by germline genetic defects in SDHB, SDHD, and SDHC genes.
CSS seems to be a genetic heterogeneous disease not only caused by germline SDHx pathogenic variants. Other genetic etiologies remain to be defined.
Contributors
All authors made individual contributions to authorship. ECL and GFCF: was involved in the review of medical records, preparation of images and manuscript writing. FF-C and LSS: were involved in the genetic investigation and preparation of images; ICS: was involved in the anatomopathological analysis and immunohistochemistry; MQA: was involved in the diagnosis and management of the patients, conception and critical review of the manuscript. All authors reviewed and approved the final draft.
Abbreviations
- CSS
Carney-Stratakis syndrome
- CT
computed tomography
- GIST
gastrointestinal stromal tumor
- MLPA
multiplex ligation-dependent probe amplification
- MRI
magnetic resonance imaging
- PGL
paraganglioma
- PHEO
pheochromocytoma
- PV
pathogenic variant
Contributor Information
Eduardo C Lobato, Unidade de Adrenal, Laboratório de Endocrinologia Molecular e Celular LIM/25, Divisão de Endocrinologia e Metabologia, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo 01246-903, Brasil.
Felipe F Castro, Unidade de Adrenal, Laboratório de Endocrinologia Molecular e Celular LIM/25, Divisão de Endocrinologia e Metabologia, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo 01246-903, Brasil.
Lucas S Santana, Unidade de Adrenal, Laboratório de Endocrinologia Molecular e Celular LIM/25, Divisão de Endocrinologia e Metabologia, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo 01246-903, Brasil.
Ibere C Soares, Divisão de Patologia, Instituto do Câncer do Estado de São Paulo (ICESP), Faculdade de Medicina da Universidade de São Paulo, São Paulo 01246-000, Brasil.
Gustavo F C Fagundes, Unidade de Adrenal, Laboratório de Endocrinologia Molecular e Celular LIM/25, Divisão de Endocrinologia e Metabologia, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo 01246-903, Brasil.
Madson Q Almeida, Unidade de Adrenal, Laboratório de Endocrinologia Molecular e Celular LIM/25, Divisão de Endocrinologia e Metabologia, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo 01246-903, Brasil; Divisão de Oncologia Clínica, Instituto do Câncer do Estado de São Paulo (ICESP), Faculdade de Medicina da Universidade de São Paulo, São Paulo 01246-000, Brasil.
Funding
This work was supported by Sao Paulo Research Foundation (FAPESP) grant 2019/15873-6 (to M.Q.A.), FAPESP post-doctoral fellowship 2021/11240-9 (to F.F.-C.), FAPESP post-doctoral fellowship 2021/10363-0 (to L.S.S.), and FAPESP fellowship 2022/08560-4 (to E.C.L.). M.Q.A. was also supported by National Council for Scientific and Technological Development (CNPq) 304091/2021-9.
Disclosures
MQA is a board member of JCEM Case Reports. The other authors have nothing to disclose.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patients.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Pitsava G, Settas N, Faucz FR, Stratakis CA. Carney triad, Carney-Stratakis syndrome, 3PAS and other tumors due to SDH deficiency. Front Endocrinol (Lausanne). 2021;12:680609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16(1):79‐88. [DOI] [PubMed] [Google Scholar]
- 3. McWhinney SR, Pasini B, Stratakis CA. Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med. 2007;357(10):1054‐1056. [DOI] [PubMed] [Google Scholar]
- 4. Strajina V, Dy BM, Farley DR, et al. Surgical treatment of malignant pheochromocytoma and paraganglioma: retrospective case series. Ann Surg Oncol. 2017;24(6):1546‐1550. [DOI] [PubMed] [Google Scholar]
- 5. Szarek E, Ball ER, Imperiale A, et al. Carney triad, SDH-deficient tumors, and Sdhb+/- mice share abnormal mitochondria. Endocr Relat Cancer. 2015;22(3):345‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haller F, Moskalev EA, Faucz FR, et al. Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr Relat Cancer. 2014;21(4):567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papathomas TG, Oudijk L, Persu A, et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol. 2015;28(6):807‐821. [DOI] [PubMed] [Google Scholar]
- 8. Boikos SA, Pappo AS, Killian JK, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2(7):922‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perry CG, Young WF Jr, McWhinney SR, et al. Functioning paraganglioma and gastrointestinal stromal tumor of the jejunum in three women: syndrome or coincidence. Am J Surg Pathol. 2006;30(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 10. Killian JK, Miettinen M, Walker RL, et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med. 2014;6(268):268ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.