Abstract
Background:
The purpose of this study was to estimate the number and lifetime medical cost of HIV infections attributable to incident sexually transmitted infections (STIs) in the United States in 2018.
Methods:
We combined data from published models regarding the number or percentage of HIV infections attributable to STIs with updated estimates of the lifetime medical cost per HIV infection. We used two distinct calculation methods. Our first calculation used recent estimates of the percentage of HIV infections in men who have sex with men (MSM) attributable to gonorrhea and chlamydia. Our second calculation, based on older studies, used estimates of the expected number of STI-attributable HIV infections per new STI infection, for gonorrhea, chlamydia, syphilis, and trichomoniasis.
Results:
Our first calculation method suggested that 2,489 (25th–75th percentile: 1,895–3,000) HIV infections in 2018 among MSM could be attributed to gonorrhea and chlamydia, at an estimated lifetime medical cost of $1.05 billion (25th–75th percentile: $0.79–1.26 billion). Our second calculation method suggested that 2,349 (25th–75th percentile: 1,948–2,744) HIV infections in the general population (including MSM) could be attributed to chlamydia, gonorrhea, syphilis, and trichomoniasis acquired in 2018, at an estimated lifetime medical cost of $0.99 billion (25th–75th percentile: $0.80–1.16 billion).
Conclusions:
Despite ambiguity regarding the degree to which STIs affect HIV transmission, our combination of data from published STI/HIV transmission models and an HIV lifetime medical cost model can help to quantify the estimated burden of STI-attributable HIV infections in the United States.
Short Summary
Available modeling evidence suggests about 1,900 to 3,000 HIV infections were attributable to chlamydia, gonorrhea, syphilis, and trichomoniasis in 2018 in the United States, costing about $1 billion.
Introduction
In a sexual partnership of discordant human immunodeficiency virus (HIV) status, the presence of another sexually transmitted infection (STI) in one or both partners can increase the likelihood of HIV transmission.1–5 An STI in a person without HIV can increase susceptibility to HIV through mechanisms such as increasing the number of cells susceptible to HIV, disruption of epithelial barriers in the genital tract, and suppression of the systemic immune response.1,6,7 An STI in a person with HIV can increase HIV infectiousness through mechanisms such as increased concentrations of HIV in genital secretions.1,6,7
An STI-attributable HIV infection can be defined as an HIV infection that would not have occurred without the facilitative effects of STIs on HIV acquisition and transmission. The number of STI-attributable HIV infections cannot be measured using surveillance data, but instead requires some type of mathematical modeling approach. The models used to estimate the number of STI-attributable HIV infections can range from simple algebraic expressions to complex agent-based models of sexual networks with cocirculating HIV and other STIs.3,4,8–11
Regardless of their degree of complexity, models that estimate the number of STI-attributable HIV infections require assumptions regarding the degree to which STIs increase the probability of HIV transmission. For example, if one assumes that the presence of a given STI doubles the probability of HIV transmission, and the per-act probability of HIV transmission is 0.001 in the absence of the given STI, then the per-act probability of HIV transmission is 0.002 if the STI is present. Different adjustments can be applied depending on whether the STI is present in the partner with HIV, the partner without HIV, or both. Modelers have typically estimated the number of HIV infections attributable to a given STI by (a) calculating the number of HIV infections that occur in the model over a specific time period when assuming the presence of the STI increases the probability of HIV transmission by some degree, and (b) subtracting the number of HIV infections that occur in the model over the same time period when assuming the presence of the STI has no effect on the probability of HIV transmission.
Given the availability of model-based estimates of the percentage of HIV infections attributable to STIs,3 estimates of STI incidence in 2018,12 updated estimates of the average lifetime medical cost per HIV infection,13 and other relevant data,8,9,14 the purpose of this study was to estimate the number and lifetime medical cost of HIV infections attributable to four incident STIs (gonorrhea, chlamydia, syphilis, and trichomoniasis) in the United States in 2018. We did not include viral STIs such as genital herpes. Because viral infections can be lifelong, the inclusion of viral STIs acquired in 2018 would have required us to assess the effects of an incident viral STI on lifetime HIV transmission and acquisition, which was beyond the scope of this study.
Methods
We used two distinct calculation methods to estimate the number of HIV infections that could be attributed to STIs acquired in 2018, both based on results from published disease transmission models. The first calculation method was to multiply the estimated number of incident HIV infections in 2018 by the estimated percent of new HIV infections that can be attributed to STIs. The second calculation method was to multiply the estimated number of incident STIs in 2018 by the estimated number of STI-attributable HIV infections per incident STI. These two calculation methods were applied to different populations and different STIs in accordance with data availability, as described in more detail below.
Calculation method 1: Using model-based estimates of the percentage of HIV infections attributable to STIs
When using this first calculation method, our analysis was limited to HIV infections attributable to gonorrhea and chlamydia among MSM, owing to a lack of recent modeling studies examining populations other than MSM or STIs other than gonorrhea and chlamydia for the United States. For this analysis, we used a 2019 modeling study by Jones and colleagues which estimated that 10.2% of incident HIV infections in MSM aged 18 to 40 years in the United States could be attributed to gonorrhea and chlamydia.3 We selected the Jones study to inform our estimates rather than a 2015 modeling study by Beck and colleagues4 primarily because the Beck study is older and focuses on a subgroup of young MSM. Similarly, we selected the Jones study rather than a 2013 modeling study by Xiridou and colleagues11 primarily because the Xiridou model is older, represents MSM in the Netherlands instead of the United States, and considers only one STI (chlamydia).
To estimate the number of HIV infections among MSM attributable to gonorrhea and chlamydia acquired in 2018, we multiplied the estimated number of HIV infections acquired through male-to-male sexual contact (24,400) in 201815 by the percentage of HIV infections among MSM attributable to gonorrhea and chlamydia (10.2%) in the Jones study.3
To calculate the 25th–75th percentiles for the estimated number of HIV infections among MSM attributable to gonorrhea and chlamydia, we generated 10,000 simulations of this estimate, each time drawing a random value for the percentage of HIV infections among MSM attributable to gonorrhea and chlamydia, which we multiplied by a random value of the number of HIV infections acquired through male-to-male sexual contact. The random value for the percentage of HIV infections attributable to gonorrhea and chlamydia was drawn from a beta distribution with α and β shape parameters 8.294 and 73.018, respectively. These distribution parameters were selected such that the resulting distribution would yield values consistent with the mean of 10.2% and with the published interquartile range of 7.9–12.4%.3 The random value for the number of HIV infections in MSM was drawn from a normal distribution with a mean of 24,400 and a standard error of 1,020 (an approximation based on the published 95% confidence interval of 22,400–26,400).15 This probabilistic sensitivity analysis accounted for the combined uncertainties associated with the two components of the estimated number of HIV infections in MSM attributable to gonorrhea and chlamydia: (1) the estimated percent of HIV infections among MSM attributable to gonorrhea and chlamydia and (2) the estimated number of HIV infections acquired through male-to-male sexual contact. We quantified this uncertainty as the 25th and 75th percentiles of the 10,000 simulations.
Calculation method 2: Using model-based estimates of the number of STI-attributable HIV infections per STI infection
We used the second calculation method to estimate the number of HIV infections attributable to gonorrhea, chlamydia, syphilis, and trichomoniasis among the overall US population, including MSM. We assumed that the average number of STI-attributable HIV infections per STI infection was 0.000220 for chlamydia and gonorrhea, 0.004620 for syphilis, and 0.000066 for trichomoniasis (Table 1). These values were calculated as follows. First, we obtained results from simple Bernoulli models of HIV transmission,8,9 which to our knowledge are the only published studies to calculate estimates of the average number of STI-attributable HIV infections per STI. Second, we incorporated downward adjustments to account for factors such as HIV serosorting and partner overlap (e.g., Person C cannot acquire an STI-attributable HIV infection from both Person A and Person B),16–19 as was done in previous studies.14,20 Third, we further decreased the downward-adjusted values by an additional 56% to account for reductions in HIV transmission due to viral suppression among those with HIV and preexposure prophylaxis (PrEP) among those without HIV. This 56% reduction represents the combined effect of an assumed 50% reduction due to viral suppression (based on reports that about 50% of people with HIV in the United States are virally suppressed)21 and an assumed 12% reduction due to PrEP (based on estimated PrEP coverage of 12% among those indicated for PrEP in the United States).22–24 Table 2 provides additional details regarding our derivation of the base case values of the average number of STI-attributable HIV infections per STI infection presented in Table 1.
Table 1.
Estimated number of STI-attributable HIV infections per STI infection, for four STIs: Base case value, range, and distribution parameters used in sensitivity analyses
| STI | Base case value* | Range used in one-way sensitivity analysis† | Distribution used in probabilistic sensitivity analysis‡ |
|---|---|---|---|
| Chlamydia | 0.000220 | 0.000022–0.000418 | beta (4.741, 21,547) |
| Gonorrhea | 0.000220 | 0.000022–0.000418 | beta (4.741, 21,547) |
| Syphilis | 0.004620 | 0.000462–0.008778 | beta (4.716, 1,016) |
| Trichomoniasis | 0.000066 | 0.000007–0.000125 | beta (4.807, 72,826) |
STI=sexually transmitted infection; HIV=human immunodeficiency virus.
We derived the base case values from a range of sources as described in the text and in Table 2.8,9,14,20
The ranges were calculated as the base case value plus or minus 90%, as suggested in previous analyses.14,20 We interpreted these ranges as approximate 95% confidence intervals for the purposes of calculating the distribution parameters listed in the final column.
The values in parentheses for the beta distributions are the α and β shape parameters. These distribution parameters were calculated such that the distribution’s mean value would be consistent with the base case value and that about 95% of the values from the distribution would fall within the range shown in the middle column.30
Table 2.
Derivation of base case values for the number of STI-attributable HIV infections per STI infection, for four STIs
| Source of estimates | Estimated number of STI-attributable HIV infections per STI infection | |||
|---|---|---|---|---|
| Chlamydia | Gonorrhea | Syphilis | Trichomoniasis | |
| Estimates published in 2000 based on Bernoulli model8 | 0.00108 | 0.00066 | 0.02386 | NA* |
| Estimates suggested in 2006,20 based on 2000 estimates from previous row † | Heterosexual: 0.00081 MSM: 0.00014 |
Heterosexual: 0.00050 MSM: 0.00008 |
Heterosexual: 0.01790 MSM: 0.00298 |
NA* |
| Estimates published in 2016,14 based on 2006 estimates from previous row‡ | 0.00050 | 0.00050 | 0.01050 | NA* |
| Base case values applied in this study, based on 2016 estimates from previous row, except for trichomoniasis§ | 0.000220 | 0.000220 | 0.004620 | 0.000066¶ |
STI=sexually transmitted infection; HIV=human immunodeficiency virus; NA=not available; MSM=men who have sex with men
The studies referenced in the first three rows did not provide an estimate for trichomoniasis.
The 2006 study recommended reducing the original 2000 estimates by 25% for heterosexuals and 50% for MSM to account for partner overlap. Partner overlap adjustments have been suggested when applying Bernoulli models in cost-effectiveness analyses of HIV prevention interventions. For example, in previous studies an adjustment of 25% has been applied for high-risk men and women16 and an adjustment of 50% has been applied for MSM;17 however, the need for empirical data to quantify this adjustment has been noted.17 The 2006 study also recommended an additional 75% reduction for MSM to account for factors such as HIV serosorting and for the residual lifetime risk of HIV acquisition (i.e., the idea that one who acquires an STI-attributable HIV infection might have otherwise acquired a non-STI-attributable HIV infection at a later point in time). The 2006 study did not explain how the 75% adjustment was derived, but instead cited two studies: one suggesting the plausibility of reductions of 65% or more in unprotected anal intercourse with a partner known to have HIV among MSM without HIV as a result of serosorting;18 and one suggesting that up to 10% of infections estimated to be “averted” by an HIV prevention intervention might actually have been merely delayed if the model had accounted for the remaining lifetime risk of acquiring HIV.19
The 2016 study calculated the average of the heterosexual and MSM estimates from the 2006 study and rounding to the nearest multiple of 0.0005.
For this study, we reduced the values from the 2016 study by an additional 56% to account for reductions in HIV transmission due to viral suppression and HIV preexposure prophylaxis (PrEP) as described in the text. The 56% reduction is the product of the 50% reduction and the 12% reduction; i.e., (1–0.5)*(1–0.12) ~ (1–0.56).
The study on which the trichomoniasis estimate was based9 did not specifically provide the estimated number of STI-attributable HIV infections per infection for trichomoniasis. Instead, we inferred a value of 0.0002 (calculated as 746/3,700,000) based on the number of women with trichomoniasis (3.7 million) and the number of HIV infections attributable to trichomoniasis (746) in that study. Similar to our approach for the other STIs, we reduced this estimate by 25% to account for partner overlap and then by 56% to account for the effects of viral suppression and PrEP. Specifically, we obtained the value 0.000066 as the product of these three terms: 0.0002, (1–0.25), and (1–0.56).
For each of the four STIs, we calculated the number of STI-attributable HIV infections by multiplying the estimated number of incident infections for the given STI in 2018 by the estimated number of STI-attributable HIV infections per STI for the given STI (Table 1). To calculate the 25th–75th percentiles for the estimated number of HIV infections attributable to these four STIs, we generated 10,000 simulations of this estimate, each time drawing a random value for the estimated number of incident infections for the given STI in 2018, which we multiplied by a random value for the estimated number of STI-attributable HIV infections per STI for the given STI. For the number of incident STIs, we applied 10,000 values from the Monte Carlo simulations described by Kreisel et al.,12 which we obtained from Ian Spicknall via personal communication on August 13, 2020 and January 6, 2021. For the number of STI-attributable HIV infections per incident STI, we obtained 10,000 values for each STI using the beta distributions described in Table 1.
Estimation of the lifetime medical cost of HIV infections attributable to STI incident infections in 2018
We used the health care sector perspective and included all direct medical costs of HIV treatment and care without regard to who paid the costs. We calculated the lifetime medical cost of STI-attributable HIV infections by multiplying the estimated number of HIV infections attributable to STIs by the estimated lifetime medical cost per HIV infection. The lifetime medical cost per HIV infection we applied was $420,285 (range: $326,411–$490,045).13 This point estimate of $420,285 was obtained from the base case scenario examined in the source study, which included a 3-year median diagnosis delay and a 3% monthly probability of dropping out of care. The values of $326,411 and $490,045 were obtained from a least-favorable scenario (a 5% dropout rate, 5-year median diagnosis delay) and a most-favorable scenario (1% dropout rate, a 1-year median diagnosis delay), respectively.13
To calculate the 25th–75th percentiles for the lifetime medical cost of STI-attributable HIV infections in 2018, we performed 10,000 simulations in which we multiplied the number of STI-attributable HIV infections by the lifetime medical cost per HIV infection, each time applying a random value for each of these two inputs. In doing so, we applied the 10,000 estimates for the number of STI-attributable HIV infections described above (for calculation method 1, and again for calculation method 2). For the lifetime medical cost per HIV infection, we obtained 10,000 values using a lognormal distribution with mean and standard deviation parameters μ and σ of 12.944 and 0.099, respectively. These distribution parameters were selected such that the mean of the distribution would be consistent with the base case value of $420,285 and that about 95% of the draws from the distribution would fall within the range of $326,411–$490,045.25
We also conducted one-way sensitivity analyses to examine how the estimated lifetime medical cost of STI-attributable HIV infections changed when varying one parameter value at a time. For calculation method 1, we varied the following parameter values: the number of HIV infections acquired through male-to-male sexual contact in 2018 (range: 22,400–26,400, from the 95% confidence interval described above); the percentage of incident HIV infections attributed to gonorrhea and chlamydia (range: 4.6% to 17.7%, based on the 2.5th and 97.5th percentiles of the beta distribution described above); and the lifetime medical cost per HIV infection (range: $326,411–$490,045, from the range described above). For calculation method 2, we varied the following parameter values: the number of incident STIs (range: 3,401,000–4,719,000 for chlamydia, 1,236,000–2,107,000 for gonorrhea, 108,000–197,000 for syphilis, 5,036,000–9,062,000 for trichomoniasis, based on the 2.5th and 97.5th percentiles of 10,000 estimates described above); the number of STI-attributable HIV infections per incident STI (using the ranges listed in Table 1); and the lifetime medical cost per HIV infection (range: $326,411–$490,045).
Results
Our first calculation method suggested that 2,489 (25th–75th percentile: 1,895–3,000) HIV infections in 2018 among MSM could be attributed to gonorrhea and chlamydia, at an estimated lifetime medical cost of $1.05 billion (25th–75th percentile: $0.79–1.26 billion) (Table 3). Our second calculation method suggested that 2,349 (25th–75th percentile: 1,948–2,744) HIV infections in the general population (including MSM) could be attributed to chlamydia, gonorrhea, syphilis, and trichomoniasis acquired in 2018, at an estimated lifetime medical cost of $0.99 billion (25th–75th percentile: $0.80–1.16 billion) (Table 4). Of these 2,349 HIV infections, 1,221 were attributed to gonorrhea and chlamydia.
Table 3.
Estimates of the number of HIV infections acquired through male-to-male sexual contact in 2018 and the percent, number, and lifetime medical costs of HIV infections among men who have sex with men (MSM) attributable to gonorrhea and chlamydia in the United States
| Item estimated | Source of estimate | Estimate | |
|---|---|---|---|
| Base case | 25th–75th percentiles* | ||
| Number of HIV infections acquired through male-to-male sexual contact in 2018 | HIV surveillance report15 | 24,400 | 23,700–25,100 |
| Percent of HIV infections among MSM attributable to gonorrhea and chlamydia | Jones et al. (2019)3 | 10.20% | 7.8%–12.2% |
| Lifetime medical cost per HIV infection | Bingham et al. (2021)13 | $420,285 | $391,325–$447,196 |
| Number of HIV infections among MSM attributable to gonorrhea and chlamydia | Calculated† | 2,489 | 1,895–3,000 |
| Cost of HIV infections among MSM attributable to gonorrhea and chlamydia | Calculated‡ | $1.05 billion | $0.79–$1.26 billion |
HIV=human immunodeficiency virus.
Costs are in 2019 US dollars. Lifetime HIV costs have been discounted at 3% annually to the time of infection.
The 25th–75th percentiles represent the results from our 10,000 simulations. In these 10,000 simulations, a random value was chosen for each of the first three parameters listed in this table, and the number and lifetime medical cost of HIV infections among MSM attributable to gonorrhea and chlamydia was calculated using these three random values. For the number of HIV infections acquired through male-to-male sexual contact in 2018, we assumed a normal distribution with a mean equal to the base case value and a standard error of 1,020, which we approximated based on the published 95% confidence interval of 22,400–26,400. For the percent of HIV infections among MSM attributable to gonorrhea and chlamydia, we assumed a beta distribution with α and β shape parameters 8.294 and 73.018. For the lifetime medical cost per HIV infection, we assumed a lognormal distribution with mean and standard deviation parameters μ and σ of 12.944 and 0.099, respectively. See text for additional details.
The number of HIV infections among MSM attributable to gonorrhea and chlamydia (row 4) was estimated by multiplying row 1 (the number of HIV infections acquired through male-to-male sexual contact in 2018) by row 2 (the percent of HIV infections among MSM attributable to gonorrhea and chlamydia). The 25th–75th percentiles were derived from the results of these 10,000 multiplications of row 1 by row 2.
The lifetime medical costs of HIV infections among MSM attributable to gonorrhea and chlamydia (row 5) was estimated by multiplying row 4 (the number of HIV infections among MSM attributable to gonorrhea and chlamydia) by row 3 (the lifetime medical cost per HIV infection). The 25th–75th percentiles were derived from the results of these 10,000 multiplications of row 4 by row 3.
Table 4.
Number of Incident Sexually Transmitted Infections (STIs) in 2018, Number of STI-Attributable HIV Infections per Incident STI, and Number and Lifetime Medical Cost of HIV Infections Attributable to Incident STIs in 2018 in the United States
| Item estimated | Source of estimate | Sexually transmitted infection (STI) | ||||
|---|---|---|---|---|---|---|
| Chlamydia | Gonorrhea | Syphilis | Trichomoniasis | All 4 STIs | ||
| No. of incident STIs in 2018 | Kreisel 202112 | |||||
| Base case value | 3,983,000 | 1,568,000 | 146,000 | 6,861,000 | 12,600,000 | |
| 25th percentile* | 3,770,000 | 1,438,000 | 126,000 | 6,209,000 | 11,891,000 | |
| 75th percentile* | 4,223,000 | 1,722,000 | 170,000 | 7,563,000 | 13,378,000 | |
| No. of STI-attributable HIV infections per incident STI | Table 1 | |||||
| Base case value | 0.000220 | 0.000220 | 0.004620 | 0.000066 | Not applicable | |
| 25th percentile* | 0.000146 | 0.000145 | 0.003051 | 0.000045 | Not applicable | |
| 75th percentile* | 0.000278 | 0.000275 | 0.005814 | 0.000084 | Not applicable | |
| No. of STI-attributable HIV infections | Calculated† | |||||
| Base case result | 876 | 345 | 675 | 453 | 2,349 | |
| 25th percentile* | 578 | 229 | 437 | 300 | 1,948 | |
| 75th percentile* | 1,113 | 440 | 875 | 581 | 2,744 | |
| Cost of STI-attributable HIV infections | Calculated‡ | |||||
| Base case result | $368,300,000 | $145,000,000 | $283,500,000 | $190,300,000 | $987,100,000 | |
| 25th percentile* | $240,700,000 | $94,800,000 | $181,200,000 | $123,100,000 | $803,000,000 | |
| 75th percentile* | $468,600,000 | $185,700,000 | $365,700,000 | $244,900,000 | $1,160,900,000 | |
HIV: human immunodeficiency virus.
Costs are in 2019 US dollars. Lifetime HIV costs have been discounted at 3% annually to the time of infection.
The 25th 75th percentiles represent the results from 10,000 simulations. In these 10,000 simulations, for each STI (chlamydia, gonorrhea, syphilis and trichomoniasis), a random value was chosen for (1) the number of incident STIs in 2018, (2) the number of STI-attributable HIV infections per incident STI, and (3) the lifetime medical cost per HIV infection. For the number of incident STIs, we applied 10,000 values from the Monte Carlo simulations described by Kreisel et al. (2021),12 which we obtained from Ian Spicknall via personal communication on August 13, 2020 and January 6, 2021. For the number of STI-attributable HIV infections per incident STI, we obtained 10,000 values for each STI using the beta distributions described in Table 1. For the lifetime medical cost per HIV infection, we obtained 10,000 values using the lognormal distribution with mean and standard deviation parameters μ and σ of 12.944 and 0.099.
For a given STI (chlamydia, gonorrhea, syphilis and trichomoniasis), the number of HIV infections attributable to the STI was calculated by multiplying the number of incident infections of the given STI by the estimated number of STI-attributable HIV infections per incident STI. The 25th 75th percentiles were derived from the results of these 10,000 multiplications.
The lifetime medical costs of HIV infections attributable to each STI (chlamydia, gonorrhea, syphilis and trichomoniasis) was estimated by multiplying the number of HIV infections attributable to the STI by the lifetime medical cost per HIV infection. The 25th 75th percentiles were derived from the results of these 10,000 multiplications. As described in Table 3, the lifetime medical cost per HIV infection was $420,285 in the base case.
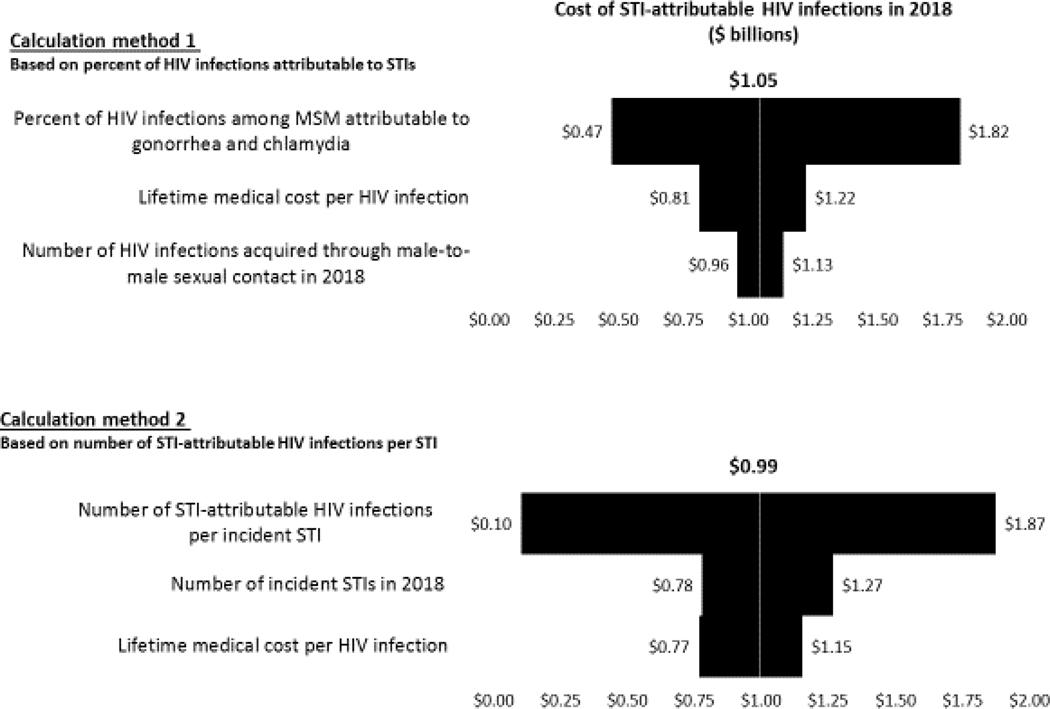
In the one-way sensitivity analysis (Figure 1) of our first calculation method, the estimated lifetime medical cost of HIV infections attributable to STIs acquired in 2018 was most sensitive to assumptions regarding the percent of HIV infections among MSM attributable to gonorrhea and chlamydia. For the second calculation method, our results were most sensitive to the number of STI-attributable HIV infections per incident STI.
Figure 1. Tornado diagram showing results of the one-way sensitivity analyses of the estimated lifetime medical cost of HIV infections attributable to STIs acquired in 2018 for calculation method 1 (top) and calculation method 2 (bottom).
This diagram shows how the estimated lifetime medical cost of HIV infections attributable to STIs acquired in 2018 changed from the base case result of $1.05 billion (calculation method 1) and $0.99 billion (calculation method 2) when one parameter value was varied at a time and all other parameters were kept at their base case values. For example, for calculation method 1, the estimated cost of STI-attributable HIV infections varied from $0.47 billion to $1.82 billion when the percent of HIV infections attributable to gonorrhea and chlamydia was varied from its 2.5th percentile value of 4.6% to its 97.5th percentile value of 17.7%. For calculation method 2, when an STI-specific parameter value was varied, it was varied for all four STIs (e.g., when varying the number of incident STIs in 2018, we first calculated the cost of HIV infections attributable to STIs acquired in 2018 when setting all four STIs to their respective lower bound incidence values, and then when setting all four STIs to their respective upper bound incidence values).
Discussion
We used two distinct approaches to approximate the number and lifetime medical cost of STI-attributable HIV infections in the United States in 2018. The first approach suggested that 2,489 HIV infections in 2018 in MSM could be attributable to gonorrhea and chlamydia. The second approach suggested that 2,349 HIV infections in the overall population (including MSM) could be attributable to gonorrhea, chlamydia, syphilis, and trichomoniasis acquired in 2018. Chlamydia accounted for more of these HIV infections than gonorrhea (876 and 345, respectively) because of the higher estimated incidence for chlamydia. Despite its relatively low estimated incidence, syphilis accounted for the second-highest number (675) of HIV infections, because the assumed probability of an STI-attributable HIV infection was notably higher for syphilis than for the other STIs.
The results of the first approach (2,489 estimated HIV infections attributable to gonorrhea and chlamydia in MSM) differ from the results of the second approach (1,221 HIV infections attributable to gonorrhea and chlamydia in the overall population, including MSM), and should be interpreted in the context of the modeling studies that informed these two approaches. Our first approach was informed by a dynamic transmission model of STIs and HIV, which accounted for potential HIV acquisition in the partners of those with STI-attributable HIV infections, and in their partners’ partners, and so on. In contrast, our second approach was based on simpler models that focused only on HIV infections acquired over the course of the STI, without considering the potential for HIV transmission to future partners after the resolution of the STI. Another difference to consider is that the Jones model did not explicitly account for the effects of PrEP on HIV incidence. As PrEP coverage among MSM increases, the number of STI-attributable HIV infections might be biased when applying the results of the Jones model, but the potential direction and magnitude of this bias are difficult to predict.
The model used in the Jones study was designed for advanced scientific research,26 was fit to a range of current data regarding the natural history of HIV as well as STI and HIV prevalence in MSM, and incorporated sexual network data to simulate main, casual, and one-time MSM partnerships.3 Of note, the Jones study finding that 10.2% of HIV infections among MSM can be attributed to chlamydia and gonorrhea is reasonably consistent with findings from a 2015 modeling study by Beck and colleagues,4 which suggested that 14.6% (95% confidence interval 14.1–15.2%) of HIV infections among young MSM in the United States were attributable to chlamydia and gonorrhea. Both of these modeling studies are consistent with a longitudinal cohort study of MSM in Atlanta that estimated that 14.6% (95% confidence interval: 6.8–31.4%) of incident HIV could be attributable to rectal chlamydia, gonorrhea, and syphilis.27 All three of these studies yielded much more conservative estimates than a 2013 modeling study by Xiridou and colleagues11 which suggested that 15% of new HIV infections among MSM in the Netherlands could be attributed to chlamydia. Given the numerous strengths of the Jones study and its consistency with the findings from the Beck model and the longitudinal cohort study, we think our estimates of the number and lifetime medical cost of STI-attributable HIV infections based on the Jones study are more reliable than our estimates based on the older, simpler models.8,9
Our estimation of the number and lifetime medical cost of STI-attributable HIV infections in 2018 is part of a larger project to estimate the incidence, prevalence, and lifetime medical cost of STIs acquired in 2018.12 In estimating the number of HIV infections attributable to STIs acquired in a single year like 2018, we inherently assumed a steady state in which the annual number of STIs and the annual number of HIV infections attributable to STIs are constant from one year to the next. This assumption of a steady state is particularly important in our application of the results of the Jones model, whose estimate of the percentage of HIV infections attributable to STIs in a single year in part reflects STI and HIV interactions in preceding years.
Another important caveat of using estimates from the Jones model is that it focuses on HIV infections among MSM attributable to just two STIs: chlamydia and gonorrhea. If the Jones model were expanded to include additional STIs like syphilis and trichomoniasis and to include heterosexual populations, the estimated number of STI-attributable HIV infections would likely be substantially higher. Of note, the number of HIV infections attributable to STIs when using our second calculation method was 1,221 when limited to gonorrhea and chlamydia, but 2,349 when also including syphilis and trichomoniasis, which illustrates the relative impact of including additional STIs.
The models on which our estimates were based did not explicitly consider the potential effects of antimicrobial drug resistance, which could have caused us to underestimate the number of STI-attributable HIV infections. For example, gonococcal resistance to treatment can increase the average duration of infection, thereby increasing not only the incidence and prevalence of gonorrhea, but also the number of gonorrhea-attributable HIV infections.28
We did not include estimates of HIV infections attributable to viral STIs such as genital herpes. Given the substantial incidence and prevalence of genital herpes in the United States,12 the number and lifetime medical cost of HIV infections attributable to viral STIs could equal or surpass that of non-viral STIs.29
Models that estimate the number or percentage of STI-attributable HIV infections must apply assumptions about the effect of STIs on HIV transmission. Unfortunately, limited data exist to inform these assumptions.5 The effects of STIs on HIV transmission are difficult to ascertain for numerous reasons, most importantly behavioral confounding in which behaviors such as having multiple sex partners and condomless sex can put one at risk for HIV and other STIs.3,5 Because of behavioral confounding, even if STIs had no biological effect on the risk of HIV acquisition and transmission, we would expect to observe higher HIV prevalence among those with other STIs than among those without other STIs. A key limitation of the models from which we obtained our data, and thus a key limitation of our study, is that the models are based on imprecise estimates of the effect of STIs on HIV transmission.
To account for ambiguity regarding the effects of STIs on HIV transmission and other limitations of the models on which our calculations are based, we calculated ranges for our estimate of the number and lifetime medical cost of STI-attributable HIV infections. This approach, which incorporated the ranges of the model-based estimates of the number or percentage of HIV infections attributable to STIs, helped to quantify the impact and magnitude of the uncertainty in these models. Still, the ranges we calculated do not capture the full range of uncertainty associated with these estimates. For example, if there is in fact no biological effect of STIs on HIV transmission, then the number and lifetime medical cost of STI-attributable HIV infections would both be 0.
Despite ambiguity regarding the degree to which STIs affect the per-act or per-partnership probability of HIV transmission, mathematical models have been developed to provide useful estimates of the number or percentage of HIV infections that can be attributable to the facilitative effects of STIs on HIV transmission. We combined data from such models with updated data regarding the lifetime medical cost per HIV infection in order to estimate the number and lifetime medical cost of HIV infections attributable to STIs acquired in 2018 in the United States. Our results can help to inform estimates of the overall burden of STIs acquired in 2018 and can help to quantify the degree to which STI prevention programs like screening and treatment can reduce the health and medical cost burden of HIV in the United States.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Dis 1999;75:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. JAIDS 2010;53:537–543. [DOI] [PubMed] [Google Scholar]
- 3.Jones J, Weiss K, Mermin J, et al. Proportion of incident human immunodeficiency virus cases among men who have sex with men attributable to gonorrhea and chlamydia: A modeling analysis. Sex Trans Dis 2019;46:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck EC, Birkett M, Armbruster B, et al. A data-driven simulation of HIV spread among young men who have sex with men: role of age and race mixing and STIs. JAIDS 2015;70:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Røttingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis 2001;28:579–597. [DOI] [PubMed] [Google Scholar]
- 6.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2(1):33–42. [DOI] [PubMed] [Google Scholar]
- 7.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 1992;19(2):61–77. [PubMed] [Google Scholar]
- 8.Chesson HW, Pinkerton SD. Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. JAIDS 2000;24:48–56. [DOI] [PubMed] [Google Scholar]
- 9.Chesson HW, Blandford JM, Pinkerton SD. Estimates of the annual number and cost of new HIV infections among women attributable to trichomoniasis in the United States. Sex Transm Dis 2004;31:547–551. [DOI] [PubMed] [Google Scholar]
- 10.Boily MC, Brunham RC. The impact of HIV and other STDs on human populations. Are predictions possible? Infect Dis Clin North Ame 1993;7:771–792. [PubMed] [Google Scholar]
- 11.Xiridou M, Vriend HJ, Lugner AK, et al. Modelling the impact of chlamydia screening on the transmission of HIV among men who have sex with men. BMC Infect Dis 2013;13:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Special Issue Sex Transm Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingham A, Shrestha R, Khurana N, et al. Estimated lifetime HIV-related medical costs in the United States. Special Issue Sex Transm Dis 2021. [DOI] [PubMed] [Google Scholar]
- 14.Chesson HW, Ludovic JA, Berruti AA, et al. Methods for sexually transmitted disease prevention programs to estimate the health and medical cost impact of changes in their budget. Sex Transm Dis 2018;45:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2014–2018. HIV Surveill Supplemental Rep 2020:25(No. 1). Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed July 25, 2020. [Google Scholar]
- 16.Pinkerton SD, Holtgrave DR, Johnson-Masotti AP, et al. Cost-effectiveness of the NIMH multisite HIV prevention intervention. AIDS Behav 2002;6(1):83–96. [Google Scholar]
- 17.Holtgrave DR, Kelly JA. Cost-effectiveness of an HIV/AIDS prevention intervention for gay men. AIDS Behav 1997;1(3):173–180. [Google Scholar]
- 18.Truong HM, Kellogg T, Klausner JD, et al. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex Transm Dis 2006;82(6):461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinkerton SD, Chesson HW, Holtgrave DR, et al. When is an HIV infection prevented and when is it merely delayed? Eval Rev 2000;24(3):251–271. [DOI] [PubMed] [Google Scholar]
- 20.Chesson HW, Collins D, Koski K. Formulas for estimating the costs averted by sexually transmitted infection (STI) prevention programs in the United States. Cost Eff Resour Alloc 2008;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Purcell DW, Sansom SL, et al. Vital signs: HIV transmission along the continuum of care - United States, 2016. MMWR Morb Mortal Wkly Rep 2019;68:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoots BE, Finlayson T, Nerlander L, et al. Willingness to take, use of, and indications for pre-exposure prophylaxis among men who have sex with men-20 US cities, 2014. Clin Infect Dis 2016;63(5):672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan PS, Mouhanna F, Mera R, et al. Methods for county-level estimation of pre-exposure prophylaxis coverage and application to the U.S. Ending the HIV Epidemic jurisdictions. Ann Epidemiol 2020;44:16–30. [DOI] [PubMed] [Google Scholar]
- 24.Harris NS, Johnson AS, Huang YA, et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis - United States, 2013–2018. MMWR Morb Mortal Wkly Rep 2019;68(48):1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chesson H, Spicknall IH, Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Special Issue of Sex Transm Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenness SM, Goodreau SM, Morris M. EpiModel: An R package for mathematical modeling of infectious disease over networks. J Stat Softw 2018;84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley CF, Vaughan AS, Luisi N, et al. The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia. AIDS 2015;31(6):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chesson HW, Kirkcaldy RD, Gift TL, et al. An illustration of the potential health and economic benefits of combating antibiotic-resistant gonorrhea. Sex Transm Dis 2018;45(4):250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Looker KJ, Welton NJ, Sabin KM, et al. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data. Lancet Infect Dis 2020;20(2):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010;28:6858–6867. [DOI] [PubMed] [Google Scholar]