Abstract
Objective:
This study examined the associations between edentulism, dental care service utilization, and cognitive functioning trajectories among older adults.
Method:
Longitudinal data from the Health and Retirement Study (2006–2014) were employed to examine individuals aged 51 and older who were identified as having normal cognition at baseline (N = 12,405). Cognitive functioning was measured with a modified version of the Telephone Interview for Cognition Status. Edentulism was selfreported as total tooth loss at baseline. Dental care service utilization was measured by self-report of having visited a dentist at least once during the previous 2 years.
Results:
The results indicated that edentulism and dental care service utilization were independently associated with cognitive decline during the observation period. Findings also showed that dental care service utilization moderated the association between edentulism and cognitive decline.
Discussion:
The findings suggested that providing access to dental services may promote cognitive health and potentially reduce health care expenditures.
Keywords: oral health, TICS, Health and Retirement Study, cognitive impairment, dental visits
Introduction
Estimates indicate that globally 46.8 million people had dementia in 2015, and that by 2050, 131 million people will be living with this condition (Prince et al., 2015). A recent report indicated that the prevalence of dementia and cognitive impairment, not dementia (CIND) among persons aged 65 years and older in the United States were estimated to be 8.8% and 18.8%, respectively (Langa et al., 2017). The financial costs of dementia worldwide were estimated to be more than US$800 billion in 2015, which accounted for more than 1% of global gross domestic product (GDP; Wimo et al., 2017). Dementia researchers often focus on cognitive decline, a marker of preclinical prodrome that often leads to Alzheimer’s disease and related dementia (ADRD; Sperling et al., 2011).
Oral Health and Cognition
Edentulism (i.e., tooth loss) was implicated in a growing number of studies as being associated with cognitive decline, CIND, and dementia (Wu, Fillenbaum, Plassman, & Guo, 2016). Edentulism was a useful marker of oral health because it represents the end result of tooth caries and periodontal disease (Slade, Akinkugbe, & Sanders, 2014). The prevalence of edentulism in the United States was estimated to be 13.7% for persons aged 65 to 74 years old and 24.1% for persons aged 75 years old and older, circa 2009–2012 (Slade et al., 2014). The prevalence of edentulism has been decreasing over time in high income countries, in part because preventive care through dental service visits has increased over time, reducing the likelihood that teeth need to be extracted (Manski & Meyerhoefer, 2017; Wu, Liang, Plassman, Remle, & Luo, 2012).
The present study sought to add to the scientific literature by conducting a longitudinal study using five waves of nationally representative data from the Health and Retirement Study (HRS). We employed a prospective study design to investigate the relationship between edentulism, dental care service utilization, and trajectories of cognitive functioning, identified through a modified version of the Telephone Interview for Cognitive Status (m-TICS). We also evaluated whether dental care service utilization was a moderator of the edentulism–cognition relationship.
Some recent studies and systematic reviews have found associations between poor oral health and cognitive decline and dementia for humans as well as in animal models (Cerutti-Kopplin et al., 2016; Daly et al., 2017; Tonsekar, Jiang, & Yue, 2017; Wu et al., 2016). However, other studies did not find support for this relationship, and thus, the evidence to-date is equivocal. Findings from earlier studies were limited by a number of methodological shortcomings, such as small sample sizes, reliance on community and clinical samples, variability in measures of both cognition and oral health, and cross-sectional study designs.
A limited number of studies have been conducted using longitudinal data; yet, these designs provide the most confidence in the estimated outcomes. However, much of the existing longitudinal research was based on small samples. Exceptions included a study with the English Longitudinal Study of Ageing data, showing that word recall measured as a discrete variable was lower among edentulous persons but this relationship did not remain statistically significant after controlling for socioeconomic status; however, edentulism was consistently related to word recall when measured as a continuous variable (Tsakos, Watt, Rouxel, Oliveira, & Demakakos, 2015). Longitudinal studies with smaller sample sizes did not report a statistically significant relationship between oral health and cognition (Hansson et al., 2014; Naorungroj et al., 2015; Shimazaki et al., 2001).
Role of Dental Care Service Utilization
We found only two studies that included dental care visits in models assessing oral health and cognitive decline and dementia and none that examined the more complex roles that oral health and dental care service utilization may play in cognitive outcomes (Paganini-Hill, White, & Atchison, 2012; Yamamoto et al., 2012). Treatment provided by dentists when caring for dentate and edentulous older adults is different. Dental professionals help dentate patients by treating and remediating dental caries and periodontal disease, ultimately saving teeth from needing to be extracted. In more challenging cases, they may extract teeth. For edentulous persons who wear prosthesis, annual evaluations can detect infections, inflammation, and disease associated with dentures. Dental care utilization patterns are also different; edentate persons are less likely to see a dentist than dentate persons in part because they are less likely to have dental insurance, likely related to the association between socioeconomic status, dental insurance access, and edentulism.
The directional relationship between oral health and cognition is also not settled and the relationship may well be reciprocal (Cerutti-Kopplin et al., 2016; Daly et al., 2017). Most research takes the view that poor oral health is a potential explanation for cognitive decline. However, there are often unobserved confounders and selection processes that influence both oral health and cognition. Research designs that minimize the potential impact of selection processes are preferred, including designs that utilize prospective cohort studies with samples of persons without cognitive impairment at baseline along with the inclusion of appropriate confounders.
In sum, we posed the following research questions for this study:
Research Question 1: Is edentulism associated with cognitive decline?
Research Question 2: Is dental care service utilization associated with cognitive decline?
Research Question 3: Is the association between edentulism and cognitive decline moderated by dental care service use?
Method
Data and Study Sample
This study utilized data from the HRS, a biennial panel study of middle-aged and older persons in the United States (Sonnega et al., 2014). The HRS initially interviewed a national probability sample of persons aged 51 to 61 in 1992, subsequently adding earlier and later birth cohorts, comprising what is now a nationally representative sample of adults aged 51 years old and older. African Americans and Hispanics were oversampled in the HRS (Sonnega et al., 2014).
For this study, five waves of data from 2006 to 2014 were analyzed; information regarding edentulism was not available priortothe 2006 wave. In2006,16,188 non-proxyparticipants who were part of the targeted HRS cohorts were interviewed. To partly account for the potential bi-directional relationship between edentulism and cognitive decline, we excluded from the study sample participants whose cognitive functioning at baseline was identified as below normal cognition, as assessed with the m-TICS (n = 3,461, see below; Crimmins, Kim, Langa, & Weir, 2011). Of the remaining sample (n = 12,727), approximately 2.5% participants had missing information on at least one of the study variables at baseline; these participants were excluded from the sample (n = 322). The final study sample included 12,405 participants.
Although sample attrition occurred throughout the 8-year observation period due to reasons common to panel studies (e.g., death, illness, lost to survey, institutionalization), the multilevel modeling approach employed here allowed us to retain study participants in the analyses as long as they provided complete information for all study variables at a given follow-up wave. Accounting for attrition during the study period, the 12,405 participants contributed to 52,026 person-wave observations (average of 4.2 observations) out of a potential maximum of 62,025 observations (83.9%). Compared with participants who remained in the study for less than five waves, those who provided complete information for all five waves were more likely to be female and have higher levels of education; at baseline, they were younger, less likely to be edentulous but more likely to use dental services, and more likely to be married and to engage in regular exercise. They were also less likely to smoke and to be in the normal weight category, and less likely to have physician diagnosed heart problems, stroke, and diabetes, compared with their counterparts (differences were all significant at p < .01).
Measures
Cognitive functioning at each wave was assessed with the m-TICS. The measure is comprised of (a) an immediate and delayed 10-noun recall (range = 0–20); (b) a serial seven subtraction task (range = 0–5); and (c) a backward counting task (range = 0–2 points) devised to capture short-term memory, working memory, and speed of processing, respectively. The total score for m-TICS ranges from 0 to 27, with higher scores indicating better cognitive functioning. The study sample included participants who were identified as cognitively healthy at baseline. We used an approach developed and validated based on the HRS Aging, Demographics, and Memory Study (ADAMS), where participants with baseline m-TICS scores of 12 and higher were considered to have normal cognitive functioning; participants whose m-TICS scores were below 12 were excluded from the sample (Crimmins et al., 2011).
We used self-reported edentulism (i.e., complete tooth loss) at baseline as the marker of oral health; information on edentulism was not consistently assessed in follow-up waves of the HRS. Participants were asked “Have you lost all of your upper and lower natural permanent teeth?” Based on responses to this question, participants were categorized as 1 = edentate and 0 = dentate.
Self-report of dental visits at each wave was utilized as a measure of dental care service utilization, which was assessed with a single item, “In the past 2 years, have you seen a dentist for dental care, including dentures?” Responses to this question were coded dichotomously (1 = yes, 0 = no).
We included in the analyses a set of covariates identified in the literature that potentially confounds the associations between oral health, dental care service utilization, and cognitive functioning (Baumgart et al., 2015; Rocca et al., 2011; Wu et al., 2016). Time-invariant covariates included age (in years), gender (1 = female), race and ethnic status (non-Hispanic White [reference group], non-Hispanic Black, non-Hispanic “other race,” and Hispanic), and years of education. Time-varying covariates included marital status (1 = married, 0 = not married), household wealth (assets minus debits, inverse hyperbole sine-transformed; Friedline, Masa, & Chowa, 2015), and insurance coverage (1 = insured through any source, 0 = not insured). Health behavior characteristics included physical exercise (1 = vigorous or moderate weekly exercise; 0 = vigorous or moderate physical activity less than weekly), smoking (1 = current smoker; 0 = former/nonsmoker), alcohol consumption (1 = 3+ drinks per day; 0 = others), and body mass index (BMI—kg/m2) (1 = normal weight [BMI=18.5–24.9]; 0 = other weight categories); health conditions included self-reported physician diagnosed heart problems, stroke, and diabetes (separate indicators for each condition; 1 = ever diagnosed; 0 = never diagnosed), and depressive symptoms assessed with the eight-item version of the Center for Epidemiologic Studies Depression scale (range = 0–8).
Statistical Analysis
We first examined sample characteristics at baseline, including differences between participants with and without any natural teeth (Table 1). We also examined study participants’ cognitive functioning and dental visits at each wave, stratified by edentulism status at baseline (Table 2). The research questions were addressed using multilevel (two-level) linear regression models, where time of observation (Level 1) was nested within persons (Level 2). Specifically, we used a within-between random effects model approach to decompose information contained in each time-varying variable, including the key independent variable (i.e., dental care service utilization), into between-person (BP; Level 2; person-mean for the time-varying predictors) and within-person (WP; deviation from the person-mean at each wave) components (Allison, 2009; Bell & Jones, 2015). In the context of this study, the BP component (i.e., proportion of waves in which participants made a dental visit) compares the cognitive functioning of persons who made dental visits more often during the course of the study period with others who made dental visits less often; the WP component compares cognitive functioning of a person who made a dental visit at one time with the same person at a different time when the person did not make a dental visit (because edentulism was only measured at baseline, a WP analysis for absence of teeth was not possible).
Table 1.
Study Sample Characteristics at Baseline.
| Dentate (n = 10,495) | Edentulous (n = 1,910) | |||||
|---|---|---|---|---|---|---|
| Variables | M | (SE) | M | (SE) | t or χ2 | |
|
| ||||||
| m-TICS score (0–27) | 17.05 | (3.12) | 16.06 | (2.92) | 12.95 | *** |
| Dental visitsa | 77.29 | 22.09 | 2,300.00 | *** | ||
| Covariates | ||||||
| Age (51–100) | 66.49 | (9.25) | 70.48 | (8.93) | −17.42 | *** |
| Female | 57.94 | 61.88 | 10.35 | ** | ||
| Married | 66.92 | 53.82 | 121.65 | *** | ||
| Race/ethnicity (%) | ||||||
| Non-Hispanic White | 80.94 | 79.16 | 14.20 | ** | ||
| Non-Hispanic Black | 10.02 | 12.77 | ||||
| Non-Hispanic Other | 1.98 | 1.88 | ||||
| Hispanic | 7.05 | 6.18 | ||||
| Education in years (0–17) | 13.35 | (2.70) | 11.69 | (2.55) | 25.04 | *** |
| Wealth (IHS-transformed) | 5.69 | (2.29) | 4.48 | (2.62) | 20.75 | *** |
| Median wealth (in US$1,000) | 237.00 | 98.00 | ||||
| Insured (%) | 95.11 | 95.97 | 2.62 | ** | ||
| Physical exerciseb (%) | 64.80 | 49.95 | 152.16 | *** | ||
| Smoking (current smoker) (%) | 11.20 | 24.08 | 234.56 | *** | ||
| Drinking (3+ drinks/day) (%) | 2.52 | 2.57 | 0.02 | |||
| BMI (normal weight ≥ 18.5, <25) (%) | 29.06 | 28.38 | 0.37 | |||
| Heart problems (%) | 20.71 | 30.79 | 94.89 | *** | ||
| Stroke (%) | 5.42 | 9.53 | 47.93 | *** | ||
| Diabetes (%) | 16.14 | 23.56 | 62.22 | *** | ||
| Depressive symptoms (0–8) | 1.25 | (1.83) | 1.65 | (2.05) | −8.69 | *** |
Note. Sample N = 12,405. Participants with baseline scores of 12 and higher were included in the sample. Group differences were tested using t tests for continuous variables and the chi-square statistics for categorical variables. m-TICS = modified version of Telephone Interview for Cognition Status; IHS = inverse hyperbole sine; BMI = body mass index.
Measured with “In the last 2 years, have you seen a dentist for dental care, including dentures?”
Vigorous or moderate physical exercise at least once a week (=1).
p < .01.
p < .001.
Table 2.
Cognitive Functioning and Dental Visits at Each Wave.
| Total | 2006 | 2008 | 2010 | 2012 | 2014 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | |
|
| ||||||||||||
| Dentate (n = 10,495) | ||||||||||||
| m-TICS | 16.21 | (3.78) | 17.05 | (3.12) | 16.42 | (3.74) | 15.96 | (3.88) | 15.66 | (3.96) | 15.67 | (4.13) |
| Dental visits (%) | 76.09 | 77.29 | 77.04 | 77.43 | 74.54 | 73.31 | ||||||
| n | 44,708 | 10,495 | 9,626 | 8,823 | 8,249 | 7,515 | ||||||
| Edentulous (n = 1,910) | ||||||||||||
| m-TICS | 14.63 | (3.83) | 16.06 | (2.92) | 14.72 | (3.77) | 14.24 | (3.97) | 13.57 | (4.00) | 13.66 | (4.17) |
| Dental visits (%) | 19.65 | 22.09 | 18.26 | 21.26 | 17.32 | 18.02 | ||||||
| n | 7,318 | 1,910 | 1,687 | 1,425 | 1,253 | 1,043 | ||||||
Note. Full sample N = 12,405. Range of m-TICS was from 12 to 27 in 2006, and from 0 to 27 in all subsequent waves. m-TICS = modified version of Telephone Interview for Cognition Status.
To model trajectories of cognitive functioning, we used a parameterization approach that captured longitudinal changes in cognitive functioning (i.e., individual change, assessed with time from baseline) while accounting for cross-sectional differences in cognitive functioning between those with different ages (assessed with age at baseline; Mendes De Leon, 2007; Morrell, Brant, & Ferrucci, 2009). This approach provided a more accurate modeling approach compared with approaches that used age as the sole time metric, as the latter approach relies on the often inaccurate assumption that cross-sectional and longitudinal effects of aging are equivalent. The distinction between longitudinal and cross-sectional time is especially important when the study sample includes a wide range of age cohorts, as is the case with the HRS (Mendes De Leon, 2007; Morrell et al., 2009). We included quadratic terms for time and age to capture possible curvilinear associations with cognition, and also added interaction term between age at baseline and time to capture potential age-differences in longitudinal changes in cognitive decline (for a detailed discussion of this modeling approach, see Mendes De Leon, 2007; Morrell et al., 2009).
We first estimated a model with edentulism, dental care service utilization, and all control variables (Table 3, Model 1). The first research question regarding whether edentulism at baseline was associated with cognitive decline over time was addressed with the inclusion of an interaction term involving time and edentulism at baseline (Model 2). The second research question regarding whether differential use of dental care service over time was associated with cognitive decline was evaluated with an interaction term between time and the BP component for dental visits. The third research question regarding whether the linkage between edentulism and cognitive decline varied depending on the frequent (vs. less frequent) use of dental care services over time was addressed with a three-way interaction term including time, edentulism, and the BP component of dental care service utilization (Model 3). Finally, as the cross-level interaction terms involving time (in Models 2 and 3) also included WP and BP components, BP effects for the interaction terms were also controlled in the models (Schunck, 2013). All analyses were performed using the MIXED procedure in Stata (Version 15.1).
Table 3.
Edentulism, Dental Visits, and Cognitive Functioning.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| b | (SE) | p | b | (SE) | p | b | (SE) | p | |
|
| |||||||||
| Intercept | 15.38 | (0.21) | <.001 | 15.47 | (0.27) | <.001 | 15.44 | (0.28) | <.001 |
| Linear time | −0.88 | (0.03) | <.001 | −1.10 | (0.07) | <.001 | −1.16 | (0.08) | <.001 |
| Quadratic time | 0.09 | (0.01) | <.001 | 0.12 | (0.02) | <.001 | 0.13 | (0.02) | <.001 |
| Edentulous | −0.06 | (0.07) | .397 | 0.37 | (0.27) | .168 | 0.45 | (0.32) | .160 |
| Dental visits: WP | 0.09 | (0.04) | .031 | 0.08 | (0.04) | .056 | 0.08 | (0.04) | .069 |
| Dental visits: BP | 0.42 | (0.07) | <.001 | 0.16 | (0.23) | .491 | 0.22 | (0.26) | .404 |
| 2-way interaction | |||||||||
| Edentulous × Linear Time | −0.28 | (0.09) | .003 | −0.12 | (0.12) | .322 | |||
| Edentulous × Quadratic Time | 0.05 | (0.02) | .051 | 0.02 | (0.03) | .518 | |||
| Dental Visits: BP × Linear Time | 0.39 | (0.09) | <.001 | 0.45 | (0.09) | <.001 | |||
| Dental Visits: BP × Quadratic Time | −0.05 | (0.02) | .014 | −0.06 | (0.02) | .004 | |||
| Edentulous × Dental Visits: BP | −0.29 | (0.61) | .632 | ||||||
| 3-way interaction | |||||||||
| Edentulous × Dental Visits: BP × Linear Time | −0.64 | (0.28) | .023 | ||||||
| Edentulous × Dental Visits: BP × Quadratic Time | 0.10 | (0.07) | .138 | ||||||
| WP effects (Level 1) | |||||||||
| Married | −0.01 | (0.07) | .883 | −0.03 | (0.07) | .636 | −0.03 | (0.07) | .645 |
| Wealtha | 0.17 | (0.03) | <.001 | 0.16 | (0.03) | <.001 | 0.16 | (0.03) | <.001 |
| Insured | −0.01 | (0.10) | .945 | −0.06 | (0.10) | .504 | −0.06 | (0.10) | .508 |
| Physical exercise | 0.25 | (0.12) | .039 | 0.24 | (0.12) | .054 | 0.23 | (0.12) | .055 |
| Smoking | −0.10 | (0.05) | .056 | −0.09 | (0.05) | .085 | −0.09 | (0.05) | .087 |
| Drinking | −0.14 | (0.07) | .059 | −0.13 | (0.07) | .077 | −0.13 | (0.07) | .079 |
| BMI | −0.93 | (0.11) | <.001 | −0.88 | (0.11) | <.001 | −0.88 | (0.11) | <.001 |
| Heart problems | 0.06 | (0.08) | .485 | 0.07 | (0.08) | .402 | 0.07 | (0.08) | .391 |
| Stroke | −0.04 | (0.01) | <.001 | −0.04 | (0.01) | <.001 | −0.04 | (0.01) | <.001 |
| Diabetes | 0.03 | (0.01) | .001 | 0.03 | (0.01) | .002 | 0.03 | (0.01) | .002 |
| Depressive symptoms | −0.01 | (0.09) | .950 | 0.03 | (0.09) | .762 | 0.03 | (0.09) | .736 |
| BP effects (Level 2) | |||||||||
| Linear age | −0.08 | (0.01) | <.001 | −0.09 | (0.01) | <.001 | −0.09 | (0.01) | <.001 |
| Linear Age × Time | −0.00 | (0.00) | <.001 | −0.00 | (0.00) | <.001 | −0.00 | (0.00) | <.001 |
| Quadratic age | −0.03 | (0.00) | <.001 | −0.03 | (0.00) | <.001 | −0.03 | (0.00) | <.001 |
| Female | 0.85 | (0.05) | <.001 | 0.84 | (0.05) | <.001 | 0.84 | (0.05) | <.001 |
| Race/ethnicity (ref: Non-Hispanic White) | |||||||||
| Non-Hispanic Black | −1.57 | (0.08) | <.001 | −1.57 | (0.08) | <.001 | −1.56 | (0.08) | <.001 |
| Non-Hispanic Other | −0.95 | (0.16) | <.001 | −0.94 | (0.16) | <.001 | −0.94 | (0.16) | <.001 |
| Hispanic | −0.56 | (0.09) | <.001 | −0.55 | (0.09) | <.001 | −0.55 | (0.09) | <.001 |
| Education | 0.30 | (0.01) | <.001 | 0.30 | (0.01) | <.001 | 0.30 | (0.01) | <.001 |
| Married | −0.13 | (0.05) | .014 | −0.13 | (0.05) | .016 | −0.13 | (0.05) | .016 |
| Wealtha | 0.32 | (0.07) | <.001 | 0.32 | (0.07) | <.001 | 0.32 | (0.07) | <.001 |
| Insured | −0.03 | (0.08) | .745 | −0.02 | (0.08) | .765 | −0.02 | (0.08) | .774 |
| Physical exercise | 0.35 | (0.18) | .058 | 0.34 | (0.18) | .062 | 0.35 | (0.18) | .058 |
| Smoking | −0.27 | (0.06) | <.001 | −0.27 | (0.06) | <.001 | −0.27 | (0.06) | <.001 |
| Drinking | 0.05 | (0.06) | .398 | 0.05 | (0.06) | .387 | 0.05 | (0.06) | .384 |
| BMI | −0.45 | (0.09) | <.001 | −0.45 | (0.09) | <.001 | −0.45 | (0.09) | <.001 |
| Heart problems | −0.30 | (0.06) | <.001 | −0.30 | (0.06) | <.001 | −0.30 | (0.06) | <.001 |
| Stroke | −0.21 | (0.02) | <.001 | −0.21 | (0.02) | <.001 | −0.21 | (0.02) | <.001 |
| Diabetes | 0.10 | (0.01) | <.001 | 0.09 | (0.01) | <.001 | 0.09 | (0.01) | <.001 |
| Depressive symptoms | 0.35 | (0.16) | .028 | 0.33 | (0.16) | .045 | 0.32 | (0.16) | .048 |
| Random effects | |||||||||
| Intercept variance | −0.21 | (0.02) | <.001 | −0.21 | (0.02) | <.001 | −0.21 | (0.02) | <.001 |
| Residual variance | −0.21 | (0.02) | <.001 | −0.21 | (0.02) | <.001 | −0.21 | (0.02) | <.001 |
| Model fit | |||||||||
| −2 log-likelihood | 260,703.2 | 260,570.6 | 260,562.0 | ||||||
| AIC | 260,785.1 | 260,660.7 | 260,658.0 | ||||||
Note. Person N = 12,405; person-wave observation N = 52,026. Models are adjusted for between-effects of time and all cross-level interaction terms. WP = within-person; BP = between-person; BMI = body mass index; AIC = Akaike information criterion.
Wealth transformed by the inverse hyperbole sine function.
Results
Descriptive sample characteristics, stratified by edentulism at baseline, are presented in Table 1. Of the 12,405 participants in the study sample, about 15% were identified as edentulous (n = 1,910). On average, dentate participants had higher cognitive functioning at baseline, as measured by m-TICS, compared with edentulous participants (p < .001). At baseline, more than three quarters of dentate participants used dental care services over the previous 2 years, whereas less than one quarter of edentulous participants made dental visits. As shown on Table 2, the differences in cognitive functioning and dental visits between dentate and edentulous samples were observed throughout the study period.
Results from the multilevel models are presented in Table 3. Model 1 examined the time trend in cognitive decline, as well as the association between levels of cognitive functioning and all study variables. Participants, on average, showed significant cognitive decline (indicated by the statistically significant linear time coefficient; b = −0.88, p < .001), but the decline was attenuated over time (indicated by the statistically significant quadratic coefficient; b = 0.09, p < .001). Controlling for the other study variables, edentulism was not significantly associated with cognitive functioning (b = 0.06, p = .397). However, both WP and BP components of dental service utilization were associated with cognitive functioning, such that individuals showed higher cognitive functioning on waves they reported using dental care service compared with other waves when they did not use dental services (i.e., WP effect; b = 0.09, p < .031). Furthermore, individuals who made dental visits more consistently during the study period showed higher levels of cognitive functioning compared with their counterparts who did not make dental visits as often (i.e., BP effect; b = 0.42, p < .001).
The first two research questions were addressed in Model 2. First, we examined whether the rate of cognitive decline was different for participants with and without any natural teeth, with the inclusion of an interaction term for time (both linear and quadratic) and edentulism. Second, we examined whether participants who reported more consistent use of dental care services during the observation period showed different cognitive trajectories compared with those who used dental services less often. The second question was addressed with an interaction term between both components of time and BP effects of dental visits. As expected, edentulous participants showed more rapid cognitive decline compared with their dentate counterparts, as indicated by significant interaction term between edentulism and linear time(b = −0.28,p = .003). Also, dental care service utilization was associated with cognitive decline, as participants who more consistently reported dental visits showed slower cognitive decline compared with their counterparts who made dental visits less often during the study period, as indicated by the significant interaction term between BP effects of dental visits and linear time (b = 0.39, p < .001); however, the attenuation of cognitive decline was also less pronounced for those with more frequent dental visits, as indicated by the interaction term involving quadratic time (b = −0.05, p = .14).
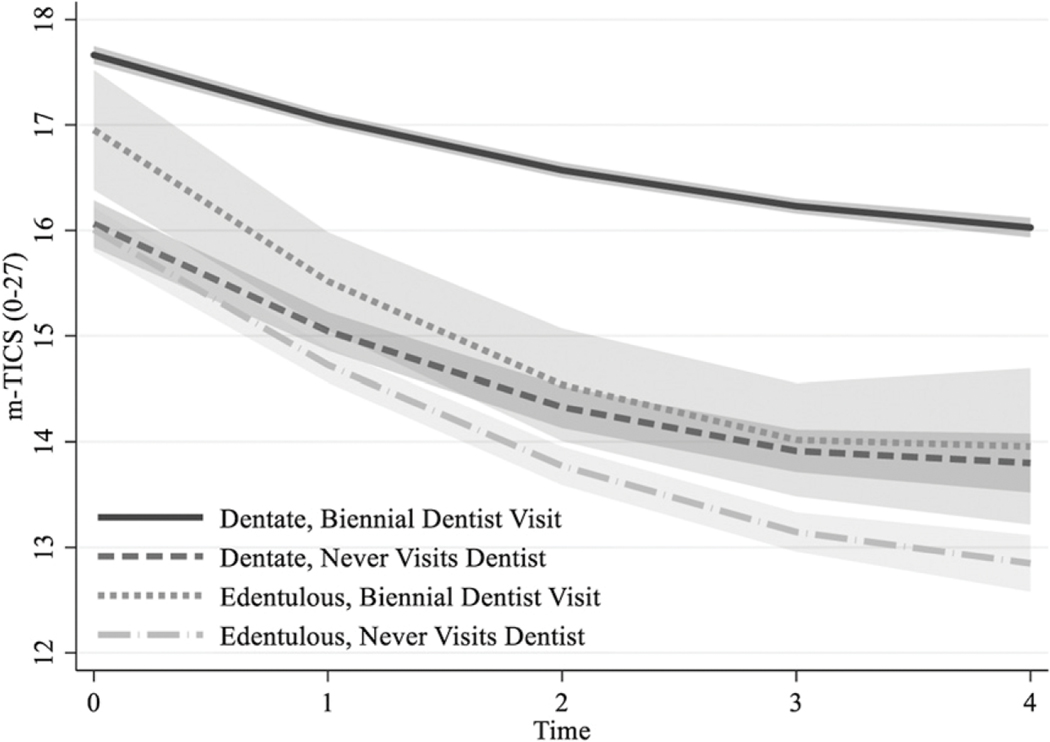
The third research question regarding whether the association between oral health and cognitive decline was moderated by dental care service use was addressed in Model 3, with a three-way interaction term involving time, edentulism, and the BP effect of dental visits. The three-way interaction effect involving linear time (but not quadratic time) was statistically significant but was in an unexpected direction (b = −0.64, p = .023). That is, more consistent dental care service use did not protect against cognitive decline for participants missing all of their teeth; rather, the results suggested that both being dentate at baseline and a consistent use of dental care services over time was a necessary condition for slower cognitive decline. This finding was more clearly depicted in the graphical representation of cognitive trajectories plotted for four hypothetical subgroups (Figure 1). Hypothetical dentate persons who consistently made dental visits during the study period showed slower cognitive decline compared with their counterparts who were dentate but did not visit the dentist, as well as hypothetical subsamples who were edentulous at baseline, irrespective of dental care utilization patterns.
Figure 1.
Cognitive functioning trajectories for hypothetical patterns of dentist visits (i.e., consistent biennial dentist visits vs. no dentist visit during the observation period) and edentulism status at baseline.
Note. Shaded area indicates 95% intervals. Plots are adjusted for all study variables as listed in Table 3. m-TICS = modified version of Telephone Interview for Cognition Status.
Discussion
The results of this study showed support for a statistical association between edentulism and cognitive decline in a large national sample of persons aged 51 and older. Our findings were generally consistent with the relatively few earlier studies that used prospective cohort designs with large samples to examine edentulism and cognitive function, one with a non-probability sample design (Batty et al., 2013) and two based on probability sample designs (Tsakos et al., 2015; Yamamoto et al., 2012). Two of the studies employed proportional hazard techniques to analyze the data and one used logistic regression approach, all of which treated the cognitive outcome measure as a dichotomous measure (e.g., 1 = dementia onset, 0 = no dementia; Batty et al., 2013; Yamamoto et al., 2012; but also see Tsakos et al., 2015). Our results were also consistent with a study of Hispanics and a sample from a retirement community in California (Paganini-Hill et al., 2012; Reyes-Ortiz, Luque, Eriksson, & Soto, 2013), but our results were not consistent with a study based on a sample from one city in Sweden (Hansson et al., 2014).
Our study also showed that less frequent use of dental care services over time was related to cognitive decline. Very few studies employing the research design characteristics of the present study have been reported. One study did not find a statistically significant relationship between dental service use and dementia risk for participants who wore dentures and for those who did not wear dentures (Paganini-Hill et al., 2012). In contrast, another study found a statistically significant effect for dental service use and dementia (Yamamoto et al., 2012). In both cases, these studies focused on a dichotomous measure of dementia and not general cognitive decline; thus, the results were not directly comparable. It is possible that focusing on a continuous measure of cognitive functioning in this study allowed us to better capture the linkages between edentulism, dental care services, and cognition, as compared with earlier studies.
We also found that for dentate participants, utilizing dental care services during the observation period was related to a reduction in cognitive decline. For dentate participants who made less use of dental services overtime and for all edentulous participants, cognition declined at a faster rate during the observation period. To the best of our knowledge, no other study has been reported that demonstrates a moderation effect of dental care service utilization for the relationship between edentulism and cognitive decline.
The current study did not investigate the pathways through which edentulism may be related to cognitive decline. The etiology of the edentulism and cognition relationship is composed of three primary mechanisms. First, older people without teeth (or with few teeth) have trouble chewing food (mastication), sometimes even when they wear dentures. The act of chewing has been shown to be related to cerebral blood flow, which leads to better brain health (Weijenberg, Scherder, & Lobbezoo, 2011). Second, edentulism is directly associated with periodontal disease. Periodontal disease leads to systemic inflammation, which is associated with risk factors and disease outcomes (e.g., cardiovascular disease) known to influence cognitive decline (Beck, Slade, & Offenbacher, 2000). Inflammation can also transfer from blood stream to the brain, subsequently causing cognitive decline (Watts, Crimmins, & Gatz, 2008). Third, older people without teeth, or with few teeth, have difficulty eating a wide range of healthy foods, thus compromising their nutrition, which is also related to cognitive decline. Future research should investigate these possibilities.
Implications
Dental education often does not include instruction on how to deal with full edentulism due to the reduction in this problem in recent years. Yet, millions of older people globally have this condition. Training for treating edentulism is still necessary, especially for older persons. Furthermore, both persons with cognitive impairment and their caregivers should be made aware of the importance of proper dental hygiene and professional care. Cognitive impairment is sometimes reversible, so the findings from this study beg the question of whether increased use of dental care services may actually help some older persons improve their cognitive impairment. More research is needed here.
Limitations
Identification of cognitive functioning was not based on neuropsychological examinations. TICS is a screening measure of cognitive functioning, and thus, clinical ramifications of the study findings with regard to dementia cannot be made. Edentulism was not clinically confirmed, although self-reports of tooth loss are more reliable than other oral health conditions; also, edentulism was not evaluated as a time-varying variable due to data limitations. No information on the use of dentures was available in the HRS. Although the rich data from the HRS provided the opportunity to control for many potential confounders to reduce concerns about omitted variable bias, other unmeasured factors (e.g., health characteristics before observation, dental insurance) as well as sample attrition may have introduced bias regarding the estimated associations. Despite the use of longitudinal data and the sample selection based on normal cognitive functioning at baseline, the observational nature of the study did not allow us to make causal statements about the empirical relationships reported herein.
Contributions
Based on a prospective cohort design with a large sample of middle-aged and older persons, we demonstrated that edentulism and dental care service utilization were independently associated with cognitive decline, and also showed a moderation effect for use of dental services. Access to dental care services may be a modifiable risk factor that may help reduce the decline of cognitive functioning during the aging process.
Acknowledgments
The authors would like to thank Changmin Peng (Department of Gerontology, University of Massachusetts Boston) for her assistance with the literature review.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is partially supported by NIH (1 U01 DE027512-01; Wu). The HRS is sponsored by the National Institute on Aging (Grant NIA U01AG009740) and is conducted by the University of Michigan.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Allison PD (2009). Fixed effects regression models. Thousand Oaks, CA: SAGE. [Google Scholar]
- Batty GD, Li Q, Huxley R, Zoungas S, Taylor BA, Neal B, & Chalmers J. (2013). Oral disease in relation to future risk of dementia and cognitive decline: Prospective cohort study based on the action in diabetes and vascular disease: Preterax and diamicron modified-release controlled evaluation (advance) trial. European Psychiatry, 28, 49–52. doi: 10.1016/j.eurpsy.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, & Johns H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia, 11, 718–726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Beck JD, Slade G, & Offenbacher S. (2000). Oral disease, cardiovascular disease and systemic inflammation. Periodontology, 23, 110–120. doi: 10.1034/j.1600-0757.2000.2230111.x [DOI] [PubMed] [Google Scholar]
- Bell A, & Jones K. (2015). Explaining fixed effects: Random effects modeling of time-series cross-sectional and panel data. Political ScienceResearchandMethods,3,133–153.doi: 10.1017/psrm.2014.7 [DOI] [Google Scholar]
- Cerutti-Kopplin D, Feine J, Padilha DM, De Souza RF, Ahmadi M, Rompre P, . . . Emami E. (2016). Tooth losś increases the risk of diminished cognitive function: A systematic review and meta-analysis. JDR Clinical & Translational Research, 1, 10–19. doi: 10.1177/2380084416633102 [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Langa KM, & Weir DR (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 66, i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly B, Thompsell A, Sharpling J, Rooney YM, Hillman L, Wanyonyi KL, . . . Gallagher JE (2017). Evidence summary: The relationship between oral health and dementia. British Dental Journal, 223, 846–853. doi: 10.1038/sj.bdj.2017.992 [DOI] [PubMed] [Google Scholar]
- Friedline T, Masa RD, & Chowa GAN (2015). Transforming wealth: Using the inverse hyperbolic sine (IHS) and splines to predict youth’s math achievement. Social Science Research, 49, 264–287. doi: 10.1016/j.ssresearch.2014.08.018 [DOI] [PubMed] [Google Scholar]
- Hansson P, Eriksson Sorman D, Bergdahl J, Bergdahl M,¨ Nyberg L, Adolfsson R, & Nilsson L-G (2014). Dental status is unrelated to risk of dementia: A 20-year prospective study. Journal of the American Geriatrics Society, 62, 979–981. doi: 10.1111/jgs.12814 [DOI] [PubMed] [Google Scholar]
- Langa KM, Larson EB, Crimmins EM, Crimmins EM, Faul JD, Levine DA, . . . Weir, D. R. (2017). A comparison of the prevalence of dementia in the united states in 2000 and 2012. JAMA Internal Medicine, 177, 51–58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manski RJ, & Meyerhoefer CD (2017). Projecting the demand for dental care in 2040. Journal of Dental Education, 81, eS133–eS145. doi: 10.21815/jde.017.020 [DOI] [PubMed] [Google Scholar]
- Mendes De Leon CF (2007). Aging and the elapse of time: A comment on the analysis of change. The Journals of Gerontology, Series B: Biological Sciences & Medical Sciences, 62, S198–S202. doi: 10.1093/geronb/62.3.S198 [DOI] [PubMed] [Google Scholar]
- Morrell CH, Brant LJ, & Ferrucci L. (2009). Model choice can obscure results in longitudinal studies. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 64, 215–222. doi: 10.1093/gerona/gln024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naorungroj S, Schoenbach VJ, Wruck L, Mosley TH, Gottesman RF,Alonso A, . . . Slade GD (2015).Toothloss, periodontal disease, and cognitive decline in the atherosclerosis risk in communities (ARIC) study. Community Dentistry and Oral Epidemiology, 43, 47–57. doi: 10.1111/cdoe.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini-Hill A, White SC, & Atchison KA (2012). Dentition, dental health habits, and dementia: The leisure world cohort study. Journal of the American Geriatrics Society, 60, 1556–1563. doi: 10.1111/j.1532-5415.2012.04064.x [DOI] [PubMed] [Google Scholar]
- Prince M, Wimo A, Guerchet M, Ali G, Wu Y, & Prina M. (2015). World Alzheimer report 2015: The global impact of dementia. Retrieved from https://www.alz.co.uk/research/worldreport-2015
- Reyes-Ortiz CA, Luque JS, Eriksson CK, & Soto L. (2013). Self-reported tooth loss and cognitive function: Data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly (Hispanic EPESE). Colombia Medica´ , 44, 139–145. [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, . . . White LR (2011). Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia, 7, 80–93. doi: 10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunck R. (2013). Within and between estimates in random-effects models: Advantages and drawbacks of correlated random effects and hybrid model. The Stata Journal, 13, 65–76. [Google Scholar]
- Shimazaki Y, Soh I, Saito T, Yamashita Y, Koga T, Miyazaki H, & Takehara T. (2001). Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. Journal of Dental Research, 80, 340–345. doi: 10.1177/00220345010800010801 [DOI] [PubMed] [Google Scholar]
- Slade GD, Akinkugbe AA, & Sanders AE (2014). Projections of US edentulism prevalence following 5 decades of decline. Journal of Dental Research, 93, 959–965. doi: 10.1177/0022034514546165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, & Weir DR (2014). Cohort profile: The Health and Retirement Study (HRS). International Journal of Epidemiology, 43, 576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, . . . Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 280–292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonsekar PP, Jiang SS, & Yue G. (2017). Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology, 34, 151–163. doi: 10.1111/ger.12261 [DOI] [PubMed] [Google Scholar]
- Tsakos G, Watt RG, Rouxel PL, Oliveira C, & Demakakos P. (2015). Tooth loss associated with physical and cognitive decline in older adults. Journal of the American Geriatrics Society, 63, 91–99. doi: 10.1111/jgs.13190 [DOI] [PubMed] [Google Scholar]
- Watts A, Crimmins EM, & Gatz M. (2008). Inflammation as a potential mediator for the association between periodontal disease and Alzheimer’s disease. Neuropsychiatric Disease and Treatment, 4, 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijenberg RA, Scherder EJ, & Lobbezoo F. (2011). Mastication for the mind—The relationship between mastication and cognition in ageing and dementia. Neuroscience & Biobehavioral Reviews, 35, 483–497. doi: 10.1016/j.neubiorev.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina AM, Winblad B, . . . Prince, M. (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s & Dementia, 13, 1–7. doi: 10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Fillenbaum GG, Plassman BL, & Guo L. (2016). Association between oral health and cognitive status: A systematic review. Journal of the American Geriatrics Society, 64, 739–751. doi: 10.1111/jgs.14036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Liang J, Plassman BL, Remle C, & Luo X. (2012). Edentulism trends among middle-aged and older adults in the United States: Comparison of five racial/ethnic groups. Community Dentistry and Oral Epidemiology, 40, 145–153. doi: 10.1111/j.1600-0528.2011.00640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, & Hirata Y. (2012). Association between self-reported dental health status and onset of dementia: A 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological Evaluation Study (AGES) project. Psychosomatic Medicine, 74, 241–248. doi: 10.1097/PSY.0b013e318246dffb [DOI] [PubMed] [Google Scholar]