Abstract
Background:
We aimed to assess the efficacy of the “fast first and then slowly” steroid-tapering regimen used in managing corticoid-sensitive patients with severe irAE after anti-PD-(L)1 therapy. Corticosteroids are the primary therapy for severe immune-related adverse events (irAEs). Less is known about the standard steroid-tapering regimen for corticoid-sensitive patients.
Methods:
This study was a single-center, retrospective medical record review of patients with severe irAE after anti-PD-(L)1 treatment for cancer from October 13, 2021 to October 20, 2022. The efficacy was assessed by Common Terminology Criteria for Adverse Events (CTCAE) grading system.
Results:
Among the 187 patients with severe irAEs associated with immune checkpoint inhibitors (ICIs), 136 (72.7%) cases were corticoid-sensitive, and 96 (51.3%) cases were scheduled “fast first and then slowly” steroid-tapering regimen. And of these, 87 (90.6%) cases got irAEs solution.
Conclusions:
The “fast first and then slowly” steroid-tapering regimen stayed shorter in the hospital. More studies are needed to confirm this efficacy and find more details about the steroid-tapering regimen.
Keywords: Steroid-tapering regimen, anti-PD-(L)1 therapy, immune-related adverse events
Introduction
Immune checkpoint inhibitors (ICIs) have been broadly used in the treatment of advanced cancers and improved outcomes, which included anti-cytotoxic T lymphocyte–associated antigen (CTLA4), anti-programmed death-1 receptor (PD-1), and anti-its ligand (PD-L1) agents. Recently, 36% of patients with cancer were eligible for ICI therapy annually, 1 and this number will continue to increase as newly approved indications. Despite these successes, T-cell activation may be against self-antigens and lead to immune-related adverse events (irAEs). In clinical trials, approximately 10% to 30% of participants developed severe irAEs (grade ⩾3). 2 According to the National Comprehensive Cancer Network (NCCN) guidelines, 3 patients with severe toxicity (grades 3 or 4) should be managed in a hospital setting and treated with corticosteroids (1-2 mg/kg/d). When corticosteroids were effective, they were suggested to be tapered in 4 to 8 weeks. However, there is little detail about the reduction plan, and there is no standard steroid-tapering regimen, which might be first fast and then slowly, first slowly and then fast, or equal days reduction.
With a large patient population, public hospitals in China must keep their patients’ stay as short as possible to serve more patients. Under these conditions, for corticoid-sensitive patients with severe immunotherapy toxicities, we routinely intravenously infused methylprednisone and tapered fast in the hospital. Then, it was converted to oral prednisone and reduced slowly after discharge.
This retrospective study aims to identify and assess the efficacy of the “fast first and then slowly” steroid-tapering regimen used to manage corticoid-sensitive patients with severe immunotherapy toxicities after anti-PD-(L)1 therapy for cancer.
Methodology
Design and patients
This study was a single-center, retrospective medical record review at the First Affiliated Hospital of Soochow University from October 13, 2021 to October 20, 2022. Patients with cancer who suffered from severe irAEs (grade 3-4 in Common Terminology Criteria for Adverse Events [CTCAE] Version 5.0 grading system) after at least 1 dose of an ICI (anti-PD-1 or anti-PD-L1) and who responded quickly (in 1 week) to steroids were included in this study. Exclusion criteria were as follows: age <18 years old or >75 years old, ECOG PS >2, active autoimmune diseases, or concomitant use of corticosteroids at the time of enrollment (prednisone dose ⩾10 mg or equivalent per day).
“Fast first and then slowly” steroid-tapering regimen
Patients were initially treated with intravenous methylprednisone (1-2 mg/kg/d). If symptoms improved in the first 3 days, methylprednisone was reduced by 25% from day 4 to 6 and 50% from day 7 to 9 in the hospital. Patients at day 10 were discharged and took the oral prednisone from a convert dose of 25% to 35% of initial methylprednisone (an integer multiple of 5 mg [1 tablet]), which was reduced by 5 mg every 5 days. For example, a patient (80 kg) with grade 3 ICI-related pneumonitis was initially treated with intravenous 120 mg methylprednisone from day 1 to 3. If symptoms were improved, intravenous methylprednisone was reduced to 90 mg from day 4 to 6 and 60 mg from day 7 to 9. And then, he was discharged and took 40 mg of oral prednisone from day 10 to day 14, 35 mg from day 15 to 19, 30 mg from day 20 to 24, and so on. The entire taper course of this patient was 49 days (7 weeks). Moreover, these patients had taken calcium and vitamin D, and the patients at higher risk of gastritis were given proton-pump inhibitors and H2 blockers.
Assessments
The efficacy of the “fast first and then slowly” steroid-tapering regimen was defined as a return to grade 0 or 1 toxicity according to the CTCAE Version 5.0 grading system. These effective patients did not relapse irAEs during corticosteroids treatment and lasted 3 months or until ICI rechallenge. This study also evaluated the incidence of corticoid-sensitive patients with severe immunotherapy toxicities after anti-PD-(L)1 therapy for cancer.
Statistical analysis
Collected data were analyzed with Prism software (Version 7.0a). The primary analysis of “fast first and then slowly” taper regimen efficacy, and the incidence of corticoid-sensitive patients with severe immunotherapy toxicities after anti-PD-(L)1 therapy for cancer, were conducted using descriptive statistics.
Results
Patient characteristics
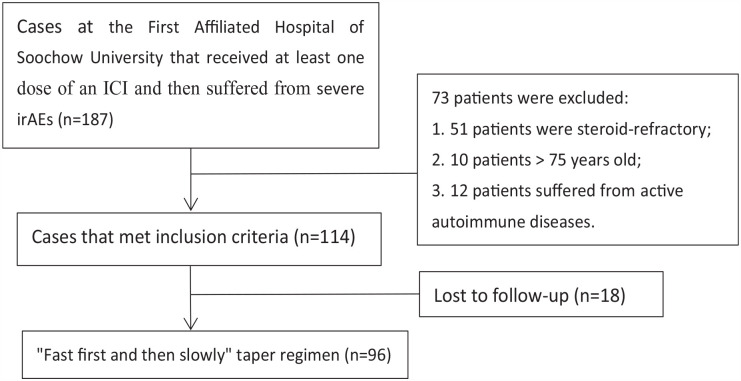
A total of 187 cases were identified with severe irAEs associated with anti-PD-(L)1 agents between October 13, 2021 and October 20, 2022. Among the 187 patients, 51 cases were refractory to corticosteroids (no improvement in 1 week), 10 cases were older than 75 years, 12 cases were suffering from active autoimmune diseases (4 Graves’s diseases, 3 Crohn’s diseases, 3 Rheumatoid arthritis, 1 Ulcerative colitis, 1 Systemic lupus erythematosus), and 114 cases met the inclusion criteria. Out of the 114 patients, 18 were lost to follow-up and excluded, and 96 patients were finally analyzed for the efficacy of the “fast first and then slowly” steroid-tapering regimen (Figure 1).
Figure 1.
Study enrollment. ICI indicates immune checkpoint inhibitors.
Of these 96 recorded patients, 70.8% of cases were male, and most commonly occurred in lung cancer and esophageal carcinoma (Table 1). The median time from the first dose of ICIs to severe irAEs was 65 days (26-231 days). Of total, 36.5% of cases suffered from severe immune pneumonia, 15.6% of cases from severe immune hepatitis, 12.5% of patients from severe immune endocrine diseases (thyroiditis or hypophysitis), 11.5% of cases from severe ICIs-associated diarrhea and colitis, and less than 10% of cases from severe immune nephritis, dermatitis, and cardiotoxicity. Most patients (59.4%) were treated with anti-PD-1 therapy (sintilimab, tislelizumab, pembrolizumab, or nivolumab), the others (40.6%) were treated with anti-PD-L1 therapy (atezolizumab, durvalumab, or envafolimab).
Table 1.
Patient characteristics.
| Sex, n (%) | |
| Male | 68 (70.8) |
| Female | 28 (29.2) |
| Median time from the first dose of ICIs to severe irAEs, days (range) | 65 (26-231) |
| Primary cancer diagnosis, n (%) | |
| Lung cancer | 43 (44.8) |
| Esophageal carcinoma | 25 (26.0) |
| Hepatocellular cancer | 12 (12.5) |
| Renal cell carcinoma | 11 (11.5) |
| Melanoma | 5 (5.2) |
| Severe irAE, n (%) | |
| Pulmonary | 35 (36.5) |
| Hepatic toxicity | 15 (15.6) |
| Endocrine | 12 (12.5) |
| Diarrhea/colitis | 11 (11.5) |
| Renal | 9 (9.4) |
| Skin | 9 (9.4) |
| Cardiovascular | 5 (5.2) |
| ICI, n (%) | |
| Anti-PD-1 agents | 57 (59.4) |
| Anti-PD-L1 agents | 39 (40.6) |
Abbreviations: anti-PD-1, anti-programmed death-1; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event.
Efficacy
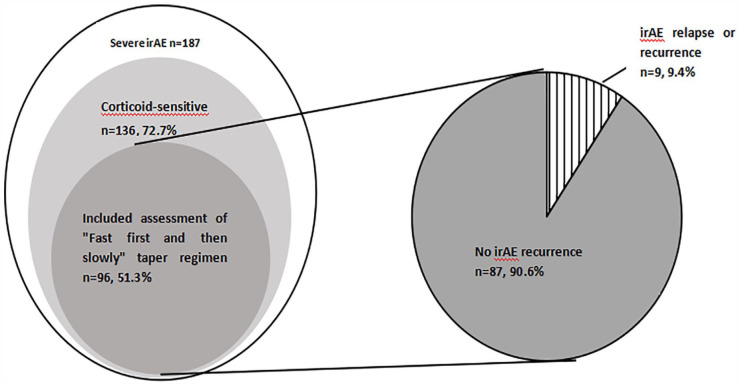
Among the 187 patients with severe irAEs associated with ICIs, 136 (72.7%) cases were corticoid-sensitive and 96 (51.3%) cases were enrolled to assess the efficacy of “fast first and then slowly” steroid-tapering regimen. Treatment with a “fast first and then slowly” steroid-tapering regimen for patients with severe irAEs of ICIs, who were sensitive to corticoids, demonstrated a high response rate, with 90.6% of cases getting irAEs solution (Figure 2). Six patients developed worsening irAEs following a brief improvement, and 3 got irAEs relapse in 1 month after the entire course of corticoid therapy. The median initiating doses of methylprednisone was 80 mg (40-200 mg), and the median corticoid course was 29 (19-59) days.
Figure 2.
Corticoid-sensitive patients and “fast first and then slowly” steroid-tapering regimen rates and irAE relapse or recurrence rates. irAE indicates immune-related adverse events.
Discussion
Immune checkpoint inhibitors mediated activation of the immune system and might cause unintended effects in any organ system, which were irAEs. Severe irAEs (grade ⩾3) were life-threatening and should be admitted inpatient and mainly treated with corticosteroids (methylprednisolone or prednisone). As high doses and extended usage of corticosteroids will suppress the hypothalamic-pituitary-adrenal axis (HPA axis), corticosteroids should be tapered gradually. 4 Although major oncologic societies, such as the European Society of Medical Oncology (ESMO), NCCN, American Society of Clinical Oncology (ASCO), and the Society for Immunotherapy of Cancer (SITC), have all published clinical guidelines or textbooks on irAEs management,2,3,5,6 the detail about taper arrangement of corticosteroids is scarce. For other immune diseases, reported steroid-tapering regimens are based on very low-quality evidence. 7
In this study, we originally scheduled the “fast first and then slowly” steroid-tapering regimen, and it was effective in most corticoid-sensitive patients (90.6%), which can provide a step-wise approach for corticosteroids reduction in corticoid-sensitive patients with severe immunotherapy toxicities after anti-PD-(L)1 therapy for cancer. With this regimen, most patients with severe irAEs could stay less than 10 days in the hospital when they are sensitive to corticosteroid therapy. And shorter hospital stays may save more medical costs and improve bed turnover.
Nine cases got relapse or recurrence during or shortly after corticosteroid therapy, which may be followed by a too-fast reduction rate initially. However, there is no report about the efficacy of other steroid-tapering regimens. Therefore, there is no confirmed answer to whether slow reduction at the beginning is more effective. Other possibilities may include tumor progression, too low onset dose, other infections, and so on.
Moreover, 72.7% of patients with severe irAEs were corticoid-sensitive, slightly higher than the data in other reports.8 -10 One explanation is that current surface receptor and ligand targets for ICIs include CTLA-4, PD-1, and PD-L1. However, ipilimumab, the only present available anti-CTLA4 agent in China, was very expensive and not covered by most medical insurance. Very few patients could afford it and be treated with it, so there is no patient with an anti-CTLA4 agent in this study. And more severe irAEs with the anti-CTLA4 agents were steroid-refractory.11,12
In addition to the efficacy of the steroid-tapering regimen, steroid effect on T-cell function and tumor progression should also be considered. As high-dose and long-term corticosteroids may interfere with the therapeutic effects of ICIs and impact the results of the efficacy of steroid-tapering regimen, 13 this study excluded patients with active autoimmune diseases or concomitant use of corticosteroids at the time of enrollment (prednisone dose ⩾10 mg or equivalent per day). Presently, main immunosuppressive treatment of severe irAEs in all clinical guidelines or textbooks was methylprednisolone or prednisone in that prednisone in contrast to dexamethasone (another steroid for managing cancer-associated symptoms, particularly nausea, and pain) did not compromise T-cell cytokine production 14 and may lessen the impact on tumor progression. Nevertheless, the effect factors of corticosteroids on tumor prognosis were mixed, such as the time of anti-tumor therapy discontinued, different proliferation rates of tumors themselves, and the relationship between irAE and improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy. 15 Although the impact of corticosteroids on tumor prognosis is beyond the scope of our topic, it is interesting and meaningful. Presently, the impact of corticosteroids on tumor prognosis is still a controversial clinical issue. Further studies are needed on this issue.
One limitation of this study is that this is a small-size retrospective analysis, which may lead to reporting biases. Thus, more lager size and randomized controlled studies are needed to find more details about the steroid-tapering regimens for corticoid-sensitive patients with severe irAEs after anti-PD-(L)1 therapy for cancer. The other limitation is lack of comparison groups of other steroid-tapering regimens.
Conclusions
Little details could be found in current clinical guidelines or textbooks on irAEs management. We originally scheduled the “fast first and then slowly” steroid-tapering regimen for corticoid-sensitive patients with severe irAEs after anti-PD-(L)1 therapy for cancer. With this regimen, most patients were effective, although hospital stay was short. More comparative research works are needed to decipher the most effective steroid-tapering regimen in irAE resolution and focus on the impact of corticosteroids on tumor prognosis.
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Qi Gui  https://orcid.org/0000-0001-7702-1887
https://orcid.org/0000-0001-7702-1887
Declarations
Ethics Approval and Consent to Participate: Ethical approval was obtained from the Medical Ethics Committee of the First Affiliated Hospital of Soochow University (approval no. [2021]EA306) on September 6, 2021. Consent to participate is not applicable.
Consent for publication: Not applicable.
Author Contributions: Chengcheng Xu: Conception of the research, design of the research, and critically revised the manuscript and approved the final manuscript.
Qi Gui: Design of the research, acquisition of the data, and critically revised the manuscript and approved the final manuscript.
Liping Xie: Acquisition of the data and analysis, interpretation of results, and critically revised the manuscript and approved the final manuscript.
Shaoqi Cheng: Analysis and interpretation of results and critically revised the manuscript and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (grant nos 81502275 and 81702254).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: None.
References
- 1. Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3:e200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264-iv266. [DOI] [PubMed] [Google Scholar]
- 3. Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:387-405. [DOI] [PubMed] [Google Scholar]
- 4. Prete A, Bancos I. Glucocorticoid induced adrenal insufficiency. BMJ. 2021;374:n1380. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prete A, Paragliola RM, Bottiglieri F, et al. Factors predicting the duration of adrenal insufficiency in patients successfully treated for Cushing disease and nonmalignant primary adrenal Cushing syndrome. Endocrine. 2017;55:969-980. [DOI] [PubMed] [Google Scholar]
- 8. Luo J, Beattie JA, Fuentes P, et al. Beyond steroids: immunosuppressants in steroid-refractory or resistant immune-related adverse events. J Thorac Oncol. 2021;16:1759-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xing H, Wang Y, Qu B, et al. The current status of steroid-refractory immune-checkpoint-inhibitor-related hepatotoxicity. Transl Oncol. 2023;28:101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer. 2021;9:e001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghanem P, Marrone K, Shanbhag S, et al. Current challenges of hematologic complications due to immune checkpoint blockade: a comprehensive review. Ann Hematol. 2022;101:1-10. [DOI] [PubMed] [Google Scholar]
- 12. Abu-Sbeih H, Ali FS, Wang Y. Immune-checkpoint inhibitors induced diarrhea and colitis: a review of incidence, pathogenesis and management. Curr Opin Gastroenterol. 2020;36:25-32. [DOI] [PubMed] [Google Scholar]
- 13. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872-2878. [DOI] [PubMed] [Google Scholar]
- 14. Okoye IS, Xu L, Walker J, et al. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol Immunother. 2020;69:1423-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shafqat H, Gourdin T, Sion A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy. Semin Oncol. 2018;45:156-163. [DOI] [PubMed] [Google Scholar]