Abstract
Background
Olinia rochetiana has been used traditionally to cure diarrheal disease. Therefore, this study aimed to investigate the acute toxicity and antidiarrheal effect of O. rochetiana leaf extracts.
Methods
Cold maceration was used to extract plant leaf powder with 80% methanol. The extract’s antidiarrheal action was tested against a castor oil-induced diarrheal model, a charcoal meal test, and enteropooling tests at doses of 100, 200, and 400 mg/kg. Negative controls received the vehicle at 10 mL/kg, while positive controls received loperamide at 3 mg/kg.
Results
From the study, no apparent toxicity was observed when a single dose of 2000 mg/kg was administered. In the castor oil-induced model, the extract delayed the onset of diarrhea, reduced stool frequency, and decreased wet feces weight and number in a dose-dependent manner at 200 mg/kg (p < 0.05) and 400 mg/kg (p < 0.01). The percent reduction in moist feces at 100, 200, and 400 mg/kg was 54.2, 23.97, and 18.26%, respectively, indicating a significant dose-dependent decrease. In a charcoal meal test, the extracts at 200 and 400 mg/kg revealed a peristaltic index of 65 and 46%, respectively, with considerable inhibition of charcoal transport at 23 and 39%. The weight and volume of intestinal contents dropped significantly at a dose of 400 mg/kg (p < 0.01), which is 0.43 mg/kg, in the enteropooling test when compared with the tested dose. The computed in vivo antidiarrheal index revealed diarrheal inhibition values of 46.06 and 71.06% at 200 and 400 mg/kg, respectively.
Conclusion
In the current investigation, O. rochetiana showed significant antidiarrheal activity with no symptoms of toxicity in mice.
Keywords: antidiarrheal activity, castor oil-induced diarrhea, gastrointestinal transit time, enteropooling, Olinia rochetiana
Introduction
Diarrhea is a gastrointestinal illness defined by a change in bowel habits or an increase in the water content, volume, or frequency of stools.1 The WHO defines it as having at least three transits of watery feces in a 24-hour period or more than is customary for that person.2 It is often accompanied by pain, urgency, perianal discomfort, and incontinence.3 Diarrhea is the world’s second largest cause of childhood mortality, with 688 million morbidities and 499,000 fatalities among children under the age of five.4,5 Ethiopia has the fifth-highest fatality rate worldwide.6 The two-week prevalence of diarrhea among children under the age of five was 13%, and it is the second leading cause of death for all ages after lower respiratory illness.7,8
Most cases of self-limited diarrhea have no known cause.9 A virus that infects the bowel is the most prevalent cause of diarrhea.10 Antibiotics can change the balance of bacteria normally found in the gastrointestinal tract. Other potential causes of diarrhea include eating foods that upset the digestive system or having allergies or food intolerances that result in poor absorption.8,11,12 Enteric microorganisms such as viruses, bacteria, and parasites largely cause acute diarrhea, while inflammatory bowel disease and malabsorption disorders commonly cause chronic diarrhea.13,14
The primary goal of diarrhea treatment is to keep the patient hydrated, successfully counter electrolyte and fluid losses, and avoid morbidity and mortality.15 Fluid therapy is the most life-saving treatment measure, and it is given to patients based on their medical history, physical examination, laboratory findings, and grasp of electrolyte and fluid dynamics, with the goal of restoring fluid balance.13,16 Medicines used to treat diarrhea include opiates, synthetic antidiarrheal, anticholinergic and adsorbents. However, all currently available management methods and medicaments have many drawbacks, side effects and allergies, which can limit their use in different conditions.17
O. rochetiana is a tree distributed in tropical Africa and is used to treat a variety of ailments, including diarrhea, stomach ailments, respiratory infections, and wounds. The bark, leaves, and roots of the plant are used in traditional medicine.18 Previous studies on this plant revealed the presence of glycosides, saponins, tannins, steroids, and terpenoids,17 and ethanol extracts of O. rochetiana showed the presence of alkaloids, flavonoids, glycosides, saponins, tannins, terpenoids, steroids and phenol.18 Other reports also indicated antibacterial,19 antifungal,20 antiviral and anticancer,21 and skin disorder activities.19 In Ethiopia, this medicinal plant is used to treat diarrhea, as plant leaves are crushed, soaked, and taken orally. A decoction of this plant is also used by others to treat this ailment. It has been documented and reported that O. rochetiana is used traditionally for the treatment of diseases such as diarrhea in Ethiopia around the Negelle Arsi and Sidama Zones.22 The current study was conducted to evaluate the antidiarrheal activities of the leaf extract of O. rochetiana in experimental laboratory animals.
Materials and Methods
Study Animals
Healthy Swiss albino mice of either sex weighing 25–30 g and aged 8–12 weeks were used during the test. The animals were obtained from the animal house of EPHI (Ethiopian Public Health Institute) and housed in plastic cages at room temperature with free access to pellet food and water on a 12-hour light/dark cycle. One week before the trial, the animals were introduced to the working laboratory environment.
Plant Materials
Fresh leaves of O. rochetiana were collected from Wondo Genet, which is located southeast of Hawassa district22, and identified with the help of the herbalist Tegenu Mekuria (Shashemene National Herbarium Department). The plant was then identified by a taxonomist and authenticated (a voucher specimen, LT001), which was given and deposited at the National Herbarium of the College of Natural and Computational Sciences at Addis Ababa University for future reference.
Chemicals, Equipment and Materials
For the studies, a rotary vapor (Yamato Sci., Japan), dry oven, deep freezer, mortar and pestle, measuring cylinders, funnel, transparent plastic ruler, beaker, surgical scalpel blade, oral gavage, forceps, and Erlenmeyer flask were used. Castor oil (Amman Pharm., Jordan), loperamide (Daehwa Pharm., Republic of Korea), charcoal meal (Acuro Organics, New Delhi), methanol (Follium Pharm., Ethiopia), and distilled water were the drugs and chemicals employed in the experiment.
Extract Preparation
O. rochetiana leaves were properly cleaned with running tap water to remove dust before drying in the shade at room temperature. Powdered leaves (250 g) were macerated at room temperature for 72 hours with 80% methanol. A rotary evaporator was used to filter and dry the extract. The dried extracts were stored in tightly sealed containers in a deep freezer until they were employed in the experiment.
Grouping and Dosing of Animals
A total of thirty mice were used for each antidiarrheal model and then randomly allocated into five groups of six mice of either sex.23 The experimental antidiarrheal models were castor oil-induced diarrhea, castor oil-induced transit in mice, and the gastrointestinal enter pooling test. The five groups of animals were as follows: group I, negative controls; group II, positive controls; and test groups (groups III, IV, and V). The negative control group, group I, was treated with distilled water at 10 mL/kg, and the positive control group, group II, was administered the standard drug, loperamide, at 3 mg/kg. The test groups received (group III, 100 mg/kg), (group IV, 200 mg/kg), and (group V, 400 mg/kg) each extract orally.12
Phytochemical Screening
Preliminary screening of phytochemicals such as steroids, alkaloids, flavonoids, saponins, tannins, phenols, cardiac glycosides, terpenoids, coumarins, anthraquinones, flavonoids, and anthocyanins as per the testing procedure of secondary metabolites.24 The results were expressed as (+) for the presence and (-) for the absence of phytochemicals.
Oral Acute Toxicity Test
The acute oral toxicity tests for the extract were carried out on five female Swiss albino mice that were in good health, nulliparous, and not pregnant, according to the limit test recommendation of the OECD guideline.25 Mice were acclimatized to laboratory settings for one week before the experiment. On the first day of the test, one female Swiss albino mouse fasted for 4 hours with a normal supply of water. The fasting body weight of the animal was measured, and the dose was calculated based on the weight. Then, the extract was administered orally by oral gavage at a dose of 2000 mg/kg, and food was withheld for 2 hours after administration. The mouse was observed for signs of toxicity, such as behavioral changes in feeding, water intake, locomotor activity, lethargy, grooming, distress, and death, with special attention for the first four hours. Based on the results of the first animals, the next four female mice fasted for an estimated 4 hours, then received a single dose of 2000 mg/kg of extract orally and were followed in the same manner. Mice were observed for any visible toxicity signs in the same way as the first mouse.
Antidiarrheal Activity Determination
Castor Oil-Induced Diarrhea
After being screened with 0.5 mL of castor oil, mice of either sex were fasted for 18 hours with free access to water, randomly allocated, and treated as described in the grouping and dosing sections.26 After 1 hour of administration of the corresponding treatment doses, the mice received 0.5 mL of castor oil orally using oral gavage and were individually placed in a separate transparent cage with a white, non-wetting, transparent paper-lined floor.27 Then, the paper was changed every hour for a total of four hours. During the observational period, the onset of diarrhea, the number and weight of wet stools, and the total number and weight of fecal output were recorded. The onset was identified by the time interval in minutes between the administration of castor oil and the occurrence of the first diarrheal feces.28 Finally, the percentage inhibition of weight, diarrhea, and feces was calculated using the formula described.
 |
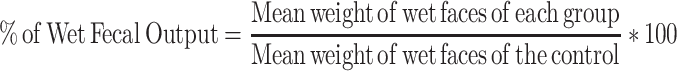 |
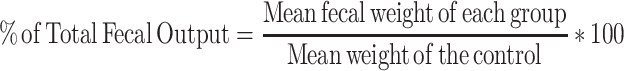 |
Gastrointestinal Transit Time
The study was carried out by adapting the method explained by Sisay et al.16 The mice were fasted for eighty hours with water ad libitum. One hour after treatment with vehicle, extract, and reference drug, each mouse received 0.5 mL of castor oil. One hour after castor oil administration, all mice received 1 mL of 5% activated charcoal suspension.29 The mice were then sacrificed sixty minutes after the administration of charcoal meal. The abdomen of each mouse was opened, and the small intestine was immediately removed.5 The length of the small intestine from the pylorus to the cecum and the length transversed by the charcoal marker were measured.30 The peristaltic index for each mouse was calculated and expressed as a percentage of the distance transversed by the charcoal meal relative to the total length of the small intestine. The percentage inhibition relative to the control was also calculated as follows:
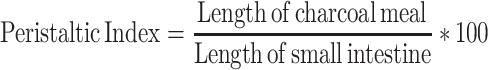 |
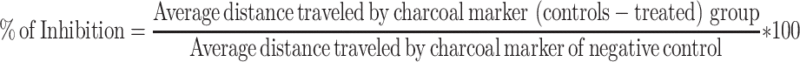 |
Castor Oil-Induced Enteropooling
Animals were fasted for 18 hours before being grouped and treated according to the grouping and dosage section. The mice were slaughtered by cervical dislocation one hour after being given castor oil. Each mouse’s abdomen was opened, and the intestine was ligated, dissected, and carefully removed from the pylorus to the cecum. The contents of the small intestines were collected by milking into a graduated tube and quantifying each volume.31 The percentage reduction in intestinal secretion and weight of intestinal contents were determined by using the following formulas:
 |
where MVICC is the mean volume of the intestinal content of the negative control group and MVICT is the mean volume of the intestinal content of the test group.
 |
where C is the mean weight of the intestinal content of the control and T/D is the mean weight of the intestinal content of the test/drug group.
In vivo-Antidiarrheal Index
The in vivo anti-diarrheal index (ADI) was expressed according to the following formula developed by Aye-than et al.31
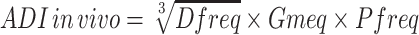 |
where Dfreq is the delay in defecation time or diarrheal onset (as a % of control), Gmeq is the gut meal travel reduction (as a % of control), and Pfreq is the purging frequency or reduction in the number of stools (as a % of control).
Each of these parameters was calculated using the formula below:
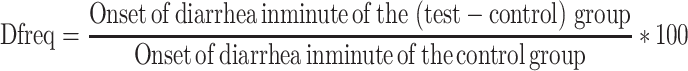 |
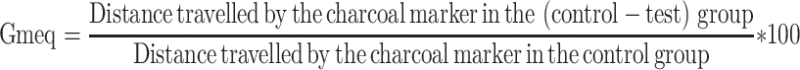 |
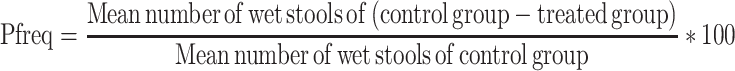 |
Statistical Analysis
All data were entered and analyzed using SPSS version 26. One-way ANOVA was used to determine significant differences among groups, followed by Tukey post hoc multiple comparison tests. The results are presented as the mean ± standard error of the mean (SEM). P-values below 0.05 were considered statistically significant.
Ethical Consideration
The experimental procedure of the study was granted permission by the Institutional Review Board of the College of Medicine and Health Science, Hawassa University with “Protocol version No. 1, Ref. No. IRB/204/14.” All tests were conducted in a peaceful laboratory setting resembling an animal house. All investigations were carried out in compliance with the Guide for the Care and Use of Laboratory Animals.32
Results
The Percentage Yield of Extraction
Out of 250 grams of leaf powder, 45 grams of dried 80% methanol extract was obtained, and the total percentage yield was 18%.
Oral Acute Toxicity Test
The findings indicated that the oral LD50 is greater than 2 g/kg because mice treated with crude methanol extracts of O. rochetiana leaves up to 2 g/kg did not die within the first 24 hours or for the next 14 days. The experimental mice underwent thorough physical and behavioral inspections, but no apparent acute poisoning signs, such as vomiting, diarrhea, or loss of appetite, were found.
Phytochemical Screening
A phytochemical screening test showed the presence of alkaloids, tannins, and phenols in crude extracts of O. rochetiana, and steroids were absent (Table 1).
Table 1.
Preliminary Phytochemical Screening Results of O. rochetiana Leaf Extracts
| S.No | Secondary Metabolites | Availability |
|---|---|---|
| 1 | Flavonoid | + |
| 2 | Phenols | + |
| 3 | Alkaloids | + |
| 4 | Terpenoids | + |
| 5 | Tannins | + |
| 6 | Steroids | – |
| 7 | Saponins | + |
Notes: (+), present; (-), absent.
Effects of O. rochetiana on Castor Oil-Induced Diarrhea in Mice
The crude leaf extract of O. rochetiana significantly prolonged the time of onset of diarrhea and total number of fecal drops compared to the negative control. At 400 mg/kg, the test extract exhibited a high degree of delay in diarrheal onset (109.05 ± 1.65). The effects of the extract on the total number of fecal drops were also statistically significant at 100 mg/kg (p < 0.05), 200 mg/kg (p < 0.01), and 400 mg/kg (p < 0.001) when compared to the negative control. The number of wet feces was decreased at 200 mg/kg (p < 0.05) and 400 mg/kg (p < 0.001) compared to the negative control. The percent inhibition of defecation was 18.97%, 51.05%, and 69.93% at doses of 100 mg/kg, 200 mg/kg and 400 mg/kg, respectively. The weights of wet and total fresh feces were also significantly decreased by 22% and 78% at 200 mg/kg (p < 0.05) and 400 mg/kg (p < 0.001), respectively (Table 2).
Table 2.
The Effect of O. rochetiana on the Castor Oil-Induced Diarrhea Model
| Groups | OD (min) | MWWS | MWTS | %WFO | %TFO | %R |
|---|---|---|---|---|---|---|
| DW10 | 46.82±2.03 | 0.145±0.018 | 0.90±0.107 | – | – | – |
| Lop3 | 117.05±6.42a***b**c* | 0.017±0.01a***b***c*d* | 0.30±0.042a***b**c* | 11.00 | 22.78 | 86.30 |
| MEOR100 | 56.00±1.37a** | 0.072±0.014a*** | 0.52±0.036a*** | 54.20 | 67.92 | 18.97 |
| MEOR200 | 79.65±1.52a***b* | 0.0376±0.09a**b* | 0.42±0.004a**b*** | 23.97 | 47.02 | 51.05 |
| MEOR400 | 109.05±1.65a***b**c* | 0.026±0.00a***b**c** | 0.36±0.01a***b*c* | 18.26 | 39.94 | 69.93 |
Notes: All values are expressed as the mean ± standard error of the mean (SEM) (n = 6). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test; a, compared to DW10; b, compared to MEOR100; c, compared to MEOR200; d, compared to MEOR400; *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: DW10, distilled water 10 mg/kg; Lop3, loperamide 3 mg/kg; MEOR, methanol extract of O. rochetiana; TFO, total fecal output; WFO, wet fecal output; MWTS, mean weight of total stool; MWWS, mean weight of wet stool; OD, onset of diarrhea; %R, percent reduction.
Effects of the O. rochetiana Extract on Gastro-Intestinal Motility
All three respective doses of the extract significantly reduced the distance traveled by the 100 mg/kg (21%, p < 0.01), 200 mg/kg (47%, p < 0.001), and 400 mg/kg (65.7%, p < 0.001) markers compared to the negative control. The extract produced a percentage inhibition of 15.46%, 23%, and 49.17% for 100, 200 and 400 mg/kg, respectively, in comparison to the negative control (Table 3).
Table 3.
Effects of O. rochetiana on Gastrointestinal Transit in Castor Oil-Induced Diarrhea
| Groups | MSIL (cm) | MDTCM (cm) | PI (%) | % Inhibition |
|---|---|---|---|---|
| DW10 | 52.2±0.60 | 51.33±0.74 | 86±0.13 | _ |
| Lop3 | 54.7±0.08 | 24.4±0.2a***b**c*d* | 43±0.49a**b**c* | 60.67 |
| MEOR100 | 52.3±0.15 | 49.23±0.6a** | 82±0.71a* | 15.46 |
| MEOR200 | 54.8±1.20 | 41.8±0.3a***b** | 65±0.77a**b** | 23.00 |
| MEOR400 | 53.0±0.40 | 31.5±0.26a***b***c** | 46.28±0.44a***b***c** | 49.17 |
Notes: All values are expressed as the mean ± standard error of the mean (SEM) (n = 6). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test; a, compared to DW10; b, compared to MEOR100; c, compared to MEOR200; d, compared to MEOR400; *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: DW10, distilled water 10 mg/kg; Lop3, loperamide 3 mg/kg; MEOR, methanol extract of O. rochetiana; MDTCM, mean distance traveled by charcoal meal; MSIL, mean small intestine length; PI, peristaltic index.
Effects of the O. rochetiana on Castor Oil-Induced Enteropooling
The test extracts significantly reduced the intraluminal volume of fluid accumulation at 200 mg/kg (p < 0.05) and 400 mg/kg (p < 0.001) compared to the negative control. The percentage inhibition in the weight of intestinal content was calculated to be 39.92% (p < 0.05) and 50.65% (p < 0.01) for 200 mg/kg and 400 mg/kg, respectively (Table 4).
Table 4.
The Effects of O. rochetiana Leaf Extract on Castor Oil-Induced Enteropooling
| Groups | MVICC (mL) | MWICC (g) |
|---|---|---|
| DW10 | 0.73±0.02 | 0.87±0.07 |
| Lop3 | 0.33±0.008a***b***c**d* | 0.39±0.02a***b***c* |
| MEOR100 | 0.57±0.11a* | 0.86±0.03 |
| MEOR200 | 0.50±0.02a** | 0.54±0.019a***b*** |
| MEOR400 | 0.43±0.031a***b**c* | 0.45±0.022a***b***c* |
Notes: All values are expressed as the mean ± standard error of the mean (SEM) (n = 6). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test; a, compared to DW10; b, compared to MEOR100; c, compared to MEOR200; d, compared to MEOR400; *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: DW10, distilled water 10 mg/kg; Lop3, loperamide 3 mg/kg; MEOR, methanol extract of O. rochetiana; MVICC, mean volume of intestinal contents; MWICC, mean weight of small intestinal content.
Effect of the Test Extract on the in vivo Antidiarrheal Index
The in vivo antidiarrheal index (ADI) values at test doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg were 20.08%, 43.06%, and 71.06%, respectively. These findings revealed that the plant extract exhibited significant antidiarrheal activity, with 400 mg/kg demonstrating the highest antidiarrheal activity (Table 5).
Table 5.
In vivo Anti-Diarrheal Index of O. rochetiana Methanol Extracts
| Treatment | DIDT (min) | GMTD | PFNWS | ADI |
|---|---|---|---|---|
| DW10 | – | – | – | – |
| Lop3 | 148 | 60.67 | 86.30 | 90.80 |
| MEOR100 | 20 | 15.46 | 18.00 | 20.08 |
| MEOR200 | 68 | 23.00 | 51.05 | 43.06 |
| MEOR400 | 131 | 39.17 | 69.93 | 71.06 |
Abbreviations: ADI, antidiarrheal index; DW10, distilled water 10 mg/kg; Lop3, loperamide 3 mg/kg; MEOR, methanol extract of O. rochetiana; DIDT, delay in defecation time; GMTD, gut meal travel distance; PFNWS, purging frequency in number of wet stools.
Discussion
The antidiarrheal efficacy of an 80% methanol leaf extract of O. rochetiana in tests for gastrointestinal motility, enteropooling, and motility following castor oil-induced diarrhea was investigated in this study. The plant extract has antidiarrheal capabilities in animal models, according to the study’s findings. Methanol was chosen as an extraction solvent because it is naturally extremely water soluble; a hydromethanol solvent mixture can be used to extract a wide range of compounds with various polarities. Methanol has the ability to suppress the growth of pathogenic microorganisms, which helps to preserve the extract from contamination.33
The percentage yield after methanol extraction of the leaf part obtained was 18%, which is higher than the ethanol extract studied by Mekuriaw et al.17 The highest yield of 15.0% and the lowest yield of 8.0% were obtained from ethanol extracts of O. rochetiana leaf and stem bark, respectively, indicating that the leaf contains more polar chemicals than the stem bark.
In the current study, before proceeding to the main experiment, animals were screened to respond to castor oil by inducing watery diarrhea. A small-scale study (pilot test) was performed using this model with ten mice of either sex, and the results obtained showed a significant increase in wet defecation and a delay in defecation in the treated groups compared with the negative control groups. Then, the doses were selected based on the oral toxicity study. The solvent used for sample preparation was distilled water because the plants were easily dissolved and mixed well, showing that the plant contains water-soluble constituents. According to the parameters observed and the results assessed, there was strong anti-diarrheal activity.
The results showed that the leaf extract of O. rochetiana castor oil-induced diarrhea significantly delayed the onset of diarrhea in a dose-dependent manner. The maximum delay was seen at the highest dose of 400 mg/kg (p < 0.01) (R2= 0.97), 109.5 minutes. Low doses of test extract were devoid of significant prolongation. Several reports stated that this might be associated with the smallest dose of test extract not having sufficient ability to prolong the onset of diarrhea; similarly, low-dose reports show less significance on delay of defecations and percent inhibition.34
The castor oil-treated control mice showed a significant increase in weight and a number of fecal matter when compared to the extract-tested animals. There was a significant difference between the lowest dose of methanol extract and the highest dose, but the low dose did not significantly reduce the defecation number and weight compared with the test-treated group. While assessing the percentage inhibition of diarrhea, loperamide (3 mg/kg) was the highest of all doses of both extracts, with 0.36 for total stool weight and 2.2 for total stool number, p < 0.001. The finding obtained from this model agrees with reports elsewhere where methanol extracts of Calpurnia aurea leaves were used.35
According to Sisay et al’s study, a decrease in stool frequency was associated with the antisecretory and antimotility mechanisms of the plant extract, which decrease the number and weight of wet stools.33 The test plant result expressed a dose-dependent delay in defecation, and the percent reduction of wet feces as 400 mg/kg showed the highest reduction compared to the negative control (p < 0.01, R2 = 0.98); an 80% reduction was noted, and it is thought the extract may contain phytochemicals such as tannins and flavonoids.24
The percentage inhibition was based on the reduction in the frequency of wet fecal output, as the highest recorded was for 400 mg/kg of the extract, even though all doses of extract showed significant reductions (51.05%, p < 0.01 and 69.93%, p < 0.001) at 200 and 400 mg/kg, respectively. The mechanism may be thought to block castor oil-induced prostaglandin synthesis. A study by Mekonin et al indicated that castor oil induces diarrhea by releasing nitric oxide and thereby increasing the permeability of the gastrointestinal membrane for calcium, stimulating prostaglandin synthesis and thereby increasing fluid and electrolytes into the lumen of the bowel and increasing peristalsis.20
The charcoal meal hyperperistalsis model was used to investigate the antidiarrheal activity of distance traveled by charcoal meal parameters to find the peristalsis index.36 It was observed that the O. rochetiana extract significantly suppressed the propulsion of charcoal meal at all tested doses. This finding suggests that this extract has the ability to influence the peristaltic movement of the intestine, thereby indicating the presence of ant motility activity. The standard drug (loperamide) used in this study acts by activating the μ receptor to inhibit the release of acetylcholine to enhance phasic colonic segmentation and inhibit peristalsis, thus increasing intestinal transit time.37
The mean distance moved by the charcoal marker showed a decrease as the dose reached a maximum for the MEOR extract at doses of 200 mg/kg and 400 mg/kg (42 cm and 31 cm), but the activity was lower than that of the standard drug. The combined effect of antidiarrheal agents was generally investigated by calculating the antidiarrheal index (ADI). ADI values showed the dose-dependent nature of each parameter. The highest dose of the crude extract showed the highest ADI compared with the corresponding doses but was not better than the standard drug. This might be due to its better potential for prolonging the onset of diarrhea, decreasing peristaltic movement, and halting purging frequency in the gastrointestinal system compared with the standard drug and its respective doses.
Different mechanisms are hypothesized as mechanisms for the antidiarrheal effect of O. rochetiana constituents inhibiting prostaglandin inhibition, which has the ability to prevent diarrhea. O. rochetiana different extracts were found to be highly active against some antimicrobial strains, which can be taken as a supporting study for its antidiarrheal activity.38 The percent inhibition estimated at a high dose (400 mg/kg) showed a significant decrease in intestinal fluid (p < 0.01, R2= 0.97), showing a dose dependency on decreasing the volume and weight of intestinal content.
Castor oil-induced enteropooling results showed a reduction in the weight of intraluminal contents by 39.92% (p < 0.05) % and 50% (p < 0.01) at 200 mg/kg and 400 mg/kg, respectively. These findings imply that the antidiarrheal properties of O. rochetiana leaves can prevent prostaglandins, promote gastrointestinal motility, and secrete water and electrolytes under these circumstances. There was a dose-dependent reduction in both the average weight and volume of intestinal contents at all test doses compared with the negative control. Even though the maximum effect was seen with the standard drug, intestinal fluid secretions secondary to castor oil administration are related to ricinoleic acid, which activates the nitric oxide pathway and induces nitric oxide-dependent gut secretion along with prostaglandin synthesis.
The effectiveness of treating diarrhea increases with increasing ADI values. The plant extract produced a maximum ADI, R2=0.98 (71.06), at a dose of 400 mg/kg, indicating that it is highly effective at treating diarrhea at this level. Generally, the ADI value indicates a measure of how effective an extract is in treating diarrhea.39 The ADI increased with dose, suggesting the dose-dependent nature of the parameter. The 80% methanol extract of O. rochetiana showed the highest ADI value among the high doses administered, reinforcing the notion that this extract is endowed with better antidiarrheal activity. The increased effect is most likely due to the presence of a significant quantity of phytochemical components such as alkaloids, tannins and phenols, which may be responsible for the antidiarrheal activity.
Conclusion
The current study revealed that an 80% methanol extract of O. rochetiana leaves had significant anti-diarrheal effects. The degree of the anti-motility activity of the three dosages varied, with 400 mg/kg being the most active. The overall antidiarrheal action of the crude extract was linked to its combined inhibitory effects on castor oil-induced gastrointestinal motility and fluid secretion. The antidiarrheal effects could be due to the presence of bioactive secondary metabolites, which can act singularly or in combination to generate the overall antidiarrheal effect, and this research supports the medicinal plant’s traditional use as an antidiarrheal treatment agent.
Acknowledgments
We gratefully acknowledge the Ethiopian Public Health Institute (EPHI) for providing laboratory space, animals, and laboratory sets for this work, as well as Hawassa University’s College of Medicine and Health Science School of Pharmacy for financial support. This manuscript’s full thesis is available on the Hawassa University repository at this link: http://etd.hu.edu.et//handle/123456789/3651.
Funding Statement
This study was funded by the School of Pharmacy, College of Medicine and Health Science, Hawassa University.
Abbreviations
ADI: Antidiarrheal Index, ANOVA: Analysis of Variance, IRB: Institutional Review Board, MVICC: Mean volume of the intestinal content of control group, MVICT: Mean volume of the intestinal content of test group, OECD: Organization for Economic Co-operation and Development, WHO: World Health Organization.
Data Sharing Statement
The corresponding author will provide all the datasets used and analyzed during the current work upon reasonable request.
Ethical Approval
Ethical clearance and approval were obtained from the institutional review board (IRB) with Ref No: IRB/204/14 from Hawassa University, College of Medicine and Health Sciences.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare they have no competing interests in this work.
References
- 1.Hao M, Song J, Zhai X, et al. Improvement of loperamide-hydrochloride-induced intestinal motility disturbance by Platycodon grandiflorum polysaccharides through effects on gut microbes and colonic serotonin. Front Cell Infect Microbiol. 2023;13:1–15. doi: 10.3389/fcimb.2023.1105272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Diarrheal disease fact sheet [Internet]. World Health Organization Media Center; 2022:1–4. Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease. Accessed September 4, 2023. [Google Scholar]
- 3.Andargie Y, Sisay W, Molla M, et al. Evaluation of In vivo antidiarrheal activity of hydro-methanolic extract of the root of Rumex nepalensis in Swiss Albino mice. Metab Open. 2022;15:100197. doi: 10.1016/j.metop.2022.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao YF, Guo XJ, Zhang ZS, et al. Epidemiology of functional diarrhea and comparison with diarrhea-predominant irritable bowel syndrome: a population-based survey in China. PLoS One. 20227. doi: 10.1371/journal.pone.0043749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amuzat A, Ndatsu Y, Adisa M, et al. Anti-diarrhoeal Effects of Aqueous Extract of Vitex doniana Stem Bark in Castor Oil-induced Wistar Rats. Tanzania J Sci. 2020;46:723–732. [Google Scholar]
- 6.Meresa A, Gemechu W, Basha H, et al. Herbal Medicines for the Management of Diabetic Mellitus in Ethiopia and Eretria including their Phytochemical Constituents. Am J Adv Drug Deliv. 2017;05:040–058. [Google Scholar]
- 7.Woldeyohannes MG, Eshete GT, Abiye AA, et al. Antidiarrheal and Antisecretory Effect of 80% Hydromethanolic Leaf Extract of Moringa stenopetala Baker f. in Mice. Biochem Res Int. 2022;2022:1–7. doi: 10.1155/2022/5768805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dereje B, Yibabie S, Keno Z, et al. Antibiotic utilization pattern in treatment of acute diarrheal diseases: the case of Hiwot Fana Specialized University Hospital, Harar, Ethiopia. J Pharm Policy Pract. 2023;16(1):1–10. doi: 10.1186/s40545-023-00568-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang ZD, DuPont HL. Etiology of travellers’ diarrhea. J Travel Med. 2017;24:S13–S16. doi: 10.1093/jtm/tax003 [DOI] [PubMed] [Google Scholar]
- 10.Thielman NM, Guerrant RL. Clinical Practice. Acute Infectious Diarrhea. N Engl J Med. 2004;350(1):38–47. doi: 10.1056/NEJMcp031534 [DOI] [PubMed] [Google Scholar]
- 11.Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med. 2017;24:S57–S74. doi: 10.1093/jtm/tax060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayalew M, Bekele A, Mengistie MG, et al. Evaluation of the antidiarrheal activity of 80% methanol extract and solvent fractions of the leaf of Bersama abyssinica fresen (Melianthaceae) in mice. BMC Complement Med Ther. 2022;22(1):8. doi: 10.1186/s12906-021-03498-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvao TF, Thees MFR, Pontes S, et al. Zinc supplementation for treating diarrhea in children: a systematic review and meta-analysis. Rev Panam Salud Publica. 2013;33:370–377. doi: 10.1590/S1020-49892013000500009 [DOI] [PubMed] [Google Scholar]
- 14.Megersa A, Dereje B, Adugna M, et al. Evaluation of Anti-Diarrheal Activities of the 80% Methanol Extract and Solvent Fractions of Maesa lanceolata Forssk (Myrsinaceae) Leaves in Mice. J Exp Pharmacol. 2023;15:391–405. doi: 10.2147/JEP.S429403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaman S, Aamir K, Hanson L, et al. High doses of Antisecretory Factor stop diarrhea fast without recurrence for six weeks post treatment. Int J Infect Dis. 2018;71:48–52. doi: 10.1016/j.ijid.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 16.Sisay M, Bussa N, Gashaw T, et al. Investigating In Vitro Antibacterial Activities of Medicinal Plants Having Folkloric Repute in Ethiopian Traditional Medicine. J Evidence-Based Integr Med. 2019;24:1–9. doi: 10.1177/2515690X19886276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekuriaw E, Mengistu E, Erdedo A, et al. In Vitro Antibacterial Activity, Preliminary Phytochemical Screening Profile, and in Vivo Toxicity of Seven Traditional Medicinal Plants in Ethiopia. Tradit Integr Med. 2021;6:398–414. [Google Scholar]
- 18.Kebede M, Yirdaw E, Luukkanen O, et al. Plant community analysis and effect of environmental factors on the diversity of woody species in the moist Afromontane forest of Wondo Genet, South Central Ethiopia. Biodivers Res Conserv. 2013;29:63–80. doi: 10.2478/biorc-2013-0003 [DOI] [Google Scholar]
- 19.Nigussie D, Davey G, Tufa TB, et al. Antibacterial and Antifungal Activities of Ethiopian Medicinal Plants: a Systematic Review. Front Pharmacol. 2021;12:1–17. doi: 10.3389/fphar.2021.633921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usman H, Oluchukwu B, Abubakar F. Levels of Castor Oil-Induced Diarrhoea in Rats Treated with Leaf Extract of Corchorus olitorius Linn and Aerial Part Extract of Scoporia dulcis Linn. Adv J Chem A. 2020;3:1–8. [Google Scholar]
- 21.Deyou T, Woo JH, Choi JH, et al. A new natural product from the leaves of Olinia usambarensis and evaluation of its constituents for cytotoxicity against human ovarian cancer cells. South African J Bot. 2017;113:182–185. doi: 10.1016/j.sajb.2017.08.011 [DOI] [Google Scholar]
- 22.Banchiamlak NT. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;7:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadesse E, Engidawork E, Nedi T, et al. Evaluation of the anti-diarrheal activity of the aqueous stem extract of Lantana camara Linn (Verbenaceae) in mice. BMC Complement Altern Med. 2017;17(1):1–8. doi: 10.1186/s12906-017-1696-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palombo EA, Wiley J. Phytochemicals from Traditional Medicinal Plants used in the Treatment of Diarrhoea: modes of Action and Effects on Intestinal Function. Phytother Res. 2006;724:717–724. doi: 10.1002/ptr.1907 [DOI] [PubMed] [Google Scholar]
- 25.OECD. Organization of economic corporation and development‟s Guideline for the testing of chemical No; 425. Manual. 2008;3:27. [Google Scholar]
- 26.Rawat P, Singh PK, Kumar V. Evidence based traditional anti-diarrheal medicinal plants and their phytocompounds. Biomed Pharmacother. 2017;96:1453–1464. doi: 10.1016/j.biopha.2017.11.147 [DOI] [PubMed] [Google Scholar]
- 27.Degu A, Engidawork E, Shibeshi W. Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del. (Euphorbiaceae) in mice model. BMC Complement Altern Med. 2016;16(1):379. doi: 10.1186/s12906-016-1357-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Lu M, Pan Q, et al. Berberine improves intestinal motility and visceral pain in the mouse models mimicking diarrhea-predominant irritable bowel syndrome (IBS-D) symptoms in an opioid-receptor dependent manner. PLoS One. 2015;10:e0145556. doi: 10.1371/journal.pone.0145556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sammari H, Jedidi S, Selmi H, et al. Protective effects of Crataegus azarolus L. berries aqueous extract against castor oil-induced diarrhea, oxidative stress, and inflammation in rat. Neurogastroenterol Motil. 2021;33:e14065. doi: 10.1111/nmo.14065 [DOI] [PubMed] [Google Scholar]
- 30.Lee KJ. Pharmacologic Agents for Chronic Diarrhea. Intest Res. 2015;13(4):306. doi: 10.5217/ir.2015.13.4.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayele TM, Abebe EC, Muche ZT, et al. In vivo antidiarrheal activity of the crude extract and solvent fractions of Rhamnus prinoides (Rhamnaceae) leaves. Heliyon. 2023;9(6):e16654. doi: 10.1016/j.heliyon.2023.e16654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panel AC, Cohen BJ, Ph D. Guide for Laboratory Animal Facilities and Care. ILAR J. 2021;62:345–358. [DOI] [PubMed] [Google Scholar]
- 33.Sisay M, Engidawork E, Shibeshi W. Evaluation of the antidiarrheal activity of the leaf extracts of Myrtus communis Linn (Myrtaceae) in mice model. BMC Complement Altern Med. 2017;17(1):103. doi: 10.1186/s12906-017-1625-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zewdie KA, Bhoumik D, Wondafrash DZ, et al. Evaluation of in-vivo antidiarrhoeal and in-vitro antibacterial activities of the root extract of Brucea antidysenterica J. F. Mill (Simaroubaceae). BMC Complement Med Ther. 2020;20(1):201. doi: 10.1186/s12906-020-03001-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan IA, Varkey TC, Akram M, et al. Evaluation of In Vivo Anti-Diarrheal Activity of Selected Medicinal Plants Traditionally Prescribed for the Management of Diarrhea. Aswan Univ J Environ Stud. 2023;4:34–46. [Google Scholar]
- 36.Ali BH, Mousa HM, Al-Mougy SA, Mousa HM. Gastrointestinal transit in mice treated with various extracts of date (Phoenix dactylifera L.). Food Chem Toxicol. 2003;41:37–39. doi: 10.1016/S0278-6915(02)00203-X [DOI] [PubMed] [Google Scholar]
- 37.Abubakar K, Abubakar MR, Ugwah–Oguejiofor JC, et al. Antidiarrhoel activity of the saponin and flavonoid fractions of Anarcadium occidentale leaves in albino rats. Adv Med Plant Res. 2015;3:23–28. [Google Scholar]
- 38.Tadeg H, Mohammed E, Asres K, et al. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol. 2005;100:168–175. doi: 10.1016/j.jep.2005.02.031 [DOI] [PubMed] [Google Scholar]
- 39.Andleeb R, Aslam N, Saeed MA, et al. Anti-diarrheal, antipyretic and phytochemical investigation of methanolic extract of Ficus lyrata leaves. Pak J Pharm Sci. 2023;281–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author will provide all the datasets used and analyzed during the current work upon reasonable request.


