Abstract
Pediocin PA-1 bound to anionic lipid vesicles with saturated or unsaturated fatty acid chains in a lipid concentration-dependent fashion. Little change in binding parameters was observed for zwitterionic lipid vesicles. Decreasing the anionic lipid content of the vesicles gave a higher relative dissociation constant for the peptide-lipid interactions and further supports the electrostatic interaction model of binding.
Pediocin PA-1 is a class IIa bacteriocin that contains a YGNGV consensus motif. It has 44 amino acids with two disulfide bonds, which may account for its broad range of antimicrobial activity (11, 12). Like other bacteriocins from lactic acid bacteria, pediocin PA-1 acts at the cytoplasmic membranes of target cells through a multistep process of binding, insertion, and pore formation (1, 6, 10, 16). How various factors, such as pH, membrane potential, and lipid composition, influence poration is a major focus of research on bacteriocins, especially nisin, a class I bacteriocin (3, 7, 8, 14, 15). Studies which have focused on the charge of the peptide demonstrated that pediocin PA-1 functions in the absence of a protein receptor and that the initial binding step is mediated by electrostatic interactions between positively charged amino acid residues in the peptide and negatively charged phospholipids in the target membrane (4, 5). In this study, we examined the influence of lipid composition on the initial binding step, and we present further evidence to support the electrostatic interaction model.
Homogeneous lipid vesicles were prepared by the extrusion method, as previously described (4). The phospholipids (Avanti Polar Lipids, Alabaster, Ala.) used were dimyristoyl-phosphatidylglycerol (DMPG), dimyristoyl-phosphatidylcholine (DMPC), dioleoyl-phosphatidylglycerol (DOPG), and dioleoyl-phosphatidylcholine (DOPC). Binding of pediocin PA-1 (a kind gift of P. Vandenbergh and J. Henderson, Quest International, Sarasota, Fla.) to lipid vesicles with different compositions was determined as described previously (4). Changes in the fluorescence of tryptophan residues were measured by titrating a fixed concentration of peptide (3.0 or 3.75 μM) with increasing amounts of lipid vesicles. Tryptophan emission spectra were recorded at room temperature with a spectrofluorometer (model F1T11; Spex Industries, Metuchen, N.J.). Each emission spectrum was analyzed to determine the maximum emission wavelength (λmax). A change in λmax (blue shift) upon addition of lipid vesicles was determined as follows: Δλmax = λmaxo − λmax, where the subscript “o” denotes the parameter in the absence of lipid vesicles. In some cases, the maximum intensity increase (I/Io) was determined, where Io and I were the intensity of the spectrum in buffer and in the presence of a saturating amount of lipid vesicles, respectively.
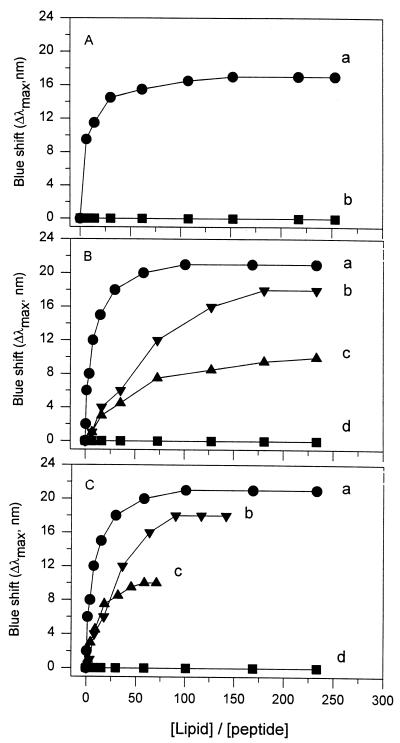
Pediocin PA-1 binding to anionic lipid vesicles.
The binding of pediocin PA-1 to DOPG vesicles with negatively charged head groups is shown in Fig. 1A (curve a). An increase in blue shift (or a decrease in λmax) reflects the translocation of a tryptophan residue(s), and thus the binding of a peptide, to a lipid membrane(s) (4, 13, 14, 17). Upon addition of DOPG vesicles to a fixed concentration of pediocin PA-1, there was a blue shift in λmax for the tryptophan emission spectrum. As progressively higher concentrations of DOPG vesicles were added, there was a greater blue shift in λmax. This indicated that progressively more pediocin PA-1 molecules became bound to the vesicles. The binding pattern of pediocin PA-1 for DOPG vesicles was similar to that for DMPG vesicles reported previously (Fig. 1B, curve a). The binding curves reached a plateau as the lipid/pediocin ratio increased.
FIG. 1.
Influence of lipid composition on binding of pediocin PA-1 to lipid vesicles. (A) DOPG (•) and DOPC (■). (B and C) Molar ratios of DMPG to DMPC were 1:0 (•), 1:1 (▾), 1:3 (▴), and 0:1 (■). In panels A and B, the x-axis was calculated as the ratio of total lipid concentration to peptide concentration. In panel C, the x-axis was calculated by using the anionic lipid concentration instead of the total lipid concentration in curves b and c, while curves a and d were the same as curves a and d in panel B.
Pediocin PA-1 binding to zwitterionic lipid vesicles.
There was little blue shift upon addition of PC vesicles with zwitterionic head groups to pediocin PA-1 (Fig. 1A and B, curves d). The spectra of pediocin PA-1 were recorded at room temperature. Figure 1A (curve d) shows the binding of pediocin PA-1 to DOPC vesicles in a liquid crystalline phase (melting temperature [Tm] = −20°C), and Fig. 1B (curve d) shows binding to DMPC vesicles around the transition temperature (Tm = 23°C) (2). Regardless of the saturation state of lipid chains, incubation of pediocin PA-1 and PC vesicles did not change the fluorescence emission maximum for lipid/pediocin molar ratios as high as 250. Although there are no reports for other class IIa bacteriocins, data for the class I bacteriocin nisin show no significant binding to zwitterionic PC vesicles at lipid/nisin ratios up to 50 (9).
Pediocin PA-1 binding to lipid vesicles with mixed head groups.
To further examine the influence of lipid composition on pediocin PA-1–membrane interactions, binding titration curves were determined for lipid vesicles prepared by using a mixture of DMPG and DMPC at PG-to-PC molar ratios of 1:1 (Fig. 1B, curve b) and 1:3 (Fig. 1B, curve c). The final extent of the blue shift decreased as DMPG content decreased. Furthermore, the magnitude of blue shift increased markedly at low lipid concentrations for vesicles containing 100% DMPG and less markedly for vesicles containing 50 and 25% DMPG.
Binding parameters.
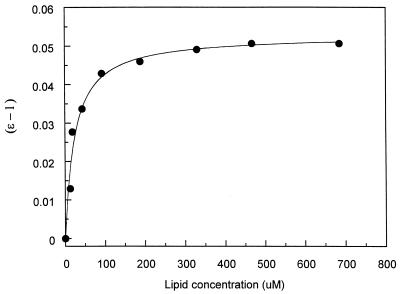
Based on previously described procedures (4, 14), Fig. 2 illustrates the determination of Kd/n (where n is the number of binding sites per lipid molecule), the relative dissociation constant, for DOPG vesicles. The nonlinear plot was analyzed with KaleidaGraph version 3.09 (Synergy Software, Reading, Pa.). Other binding curves were analyzed similarly to obtain Kd/n values.
FIG. 2.
Determination of Kd/n. The datum points derived from Fig. 1A (curve a) were fitted by using the equation (ɛ − 1) = (ɛb − 1)m/(Kd/n + m). ɛ was defined as λmaxo/λmax, and m was the total lipid concentration.
The anionic lipid content of the vesicles influenced the relative dissociation constant, Kd/n, and the maximum blue shift, Δλmax (Table 1). A higher Δλmax indicates a more hydrophobic environment for the tryptophan residues, which reflects a deeper insertion of the peptide into the membrane; meanwhile, a smaller Kd/n indicates a stronger affinity of the peptide for the membrane (4, 13, 14). Binding of pediocin PA-1 to the vesicles with different lipid compositions correlates well with the amount of PG in the vesicles: the maximum blue shift was highest for vesicles containing 100% DMPG, and as DMPG content decreased to 50 and 25%, there was a progressively smaller maximum blue shift. There was no change in Δλmax upon addition of zwitterionic lipid vesicles, which strongly suggests that anionic lipids are essential to induce the fluorescence parameter changes associated with binding in pediocin PA-1. The influence of anionic lipid content on tryptophan fluorescence intensity followed the same pattern, where the amount of intensity increase was lower for the vesicles with a smaller molar ratio of DMPG to DMPC, and little change was observed upon addition of PC vesicles (data not shown).
TABLE 1.
Binding parameters for pediocin PA-1 interaction with lipid vesicles of different compositiona
| Lipid composition (ratio) | Δλmax (nm)b |
Kd/n (mM) for:
|
|
|---|---|---|---|
| Total lipidsc | Anionic lipidsd | ||
| DMPG | 20.5 | 0.027 | 0.027 |
| DMPG-DMPC (1:1) | 17.8 | 0.313 | 0.167 |
| DMPG-DMPC (1:3) | 10.0 | 0.140 | 0.038 |
| DMPC | 0 | NAe | NA |
| DOPG | 16.8 | 0.027 | 0.027 |
| DOPC | 0 | NA | NA |
Data are the averages of two or three independent experiments. For Kd/n determination by using the equation (ɛ − 1) = (ɛb − 1)m/(Kd/n + m), ɛ was defined as λmaxo/λmax, and m was lipid concentration.
Maximum blue shift in λmax in the presence of a saturating amount of lipid vesicles.
The values for Kd/n were calculated based on titration curves in Fig. 1A and B; i.e., total lipids were used in the calculation of m.
The values for Kd/n were calculated based on titration curves in Fig. 1A and C; i.e., only anionic lipids were used in the calculation of m.
NA, the equation could not be applied to an ɛ value of 1 (Δλmax = 0).
The Kd/n for vesicles with 50% DMPG was more than an order of magnitude higher than that for the 100% DMPG vesicles (Table 1, column 3). On the other hand, the Kd/n for vesicles with 25% DMPG was fivefold higher than that for 100% DMPG vesicles. This suggests that pediocin PA-1’s affinity for the vesicles with 25% DMPG is weaker than that for pure DMPG vesicles but stronger than that for vesicles with 50% DMPG. These results were obtained based on the premise that m is conventionally defined as total lipid concentration. However, the binding titration curves in Fig. 1A and B clearly indicate that the zwitterionic lipids could not induce a blue shift. Therefore, it may be more appropriate to use PG alone, instead of the combination of PG and PC, as the lipid concentration.
The titration curves in Fig. 1C, where lipid concentrations included only anionic lipid, show binding patterns similar to those in Fig. 1B, except that saturation of binding occurred at a lower ratio of lipid concentration to peptide concentration. Based on the curves in Fig. 1C, the Kd/n for the vesicles containing 50% DMPG was about sixfold higher than that for 100% DMPG vesicles, while the constant for vesicles with 25% DMPG was only slightly increased (Table 1, column 4). These results suggest that the presence of a small amount of DMPG contributed significantly to the binding of pediocin PA-1 to mixed DMPG-DMPC vesicles. While a larger amount of DMPG in these vesicles might cause better insertion of pediocin PA-1 into the lipid bilayer (a bigger Δλmax), the affinity of pediocin PA-1 for the 50% DMPG vesicles was weaker than that for pure DMPG vesicles. The increase in the Kd/n value when DMPG content increased from 25 to 50% might reflect a change in the parameter n rather than the dissociation constant, Kd, per se. In addition, vesicles with different lipid compositions might have heterogeneous surfaces, due to phase segregation, that could also account for the trend in Kd/n. Although these data clearly indicate that anionic lipid is required for the binding of pediocin PA-1 to lipid vesicles as measured by changes in tryptophan fluorescence parameters, there is a complex aspect of the peptide-membrane interaction which remains elusive.
The Kd/n values for DMPG and DOPG vesicles were the same. This suggests that pediocin PA-1 has a similarly strong affinity for both types of anionic lipid vesicles. Compared to the changes caused by lipid head groups, the saturation state of the fatty acid chains has minor, if any, influence on pediocin PA-1 binding. The blue shift in the presence of saturating amount of DMPG was higher than that with DOPG (Table 1). In addition, the binding of pediocin PA-1 to saturating amount of DMPG vesicles caused a 30.3% increase in fluorescence intensity, while for DOPG vesicles the increase was 17.2%. These results indicated a less hydrophobic environment for the tryptophan residue(s) of DOPG-bound pediocin molecules than for those of DMPG-bound molecules (4, 13, 17). The matrix of DOPG fatty acid chains may provide a less hydrophobic environment than that of DMPG due to a higher fluidity (Tm of DOPG is lower than that of DMPG), which allowed water molecules to diffuse further into the matrix. In addition, double bonds in oleoyl chains (18:1c9; DOPG) are less hydrophobic than their single bond counterparts in myristoyl chains (14:0; DMPG).
We reported previously that the net positive charge of pediocin PA-1’s N-terminal fragments parallels their binding to DMPG vesicles (4). Altering the membrane vesicle composition modifies the charge properties of the target, instead of the properties of the peptides. Both types of manipulation give similar information about how electrostatic interactions modulate bacteriocin binding to membranes. Results from the present study demonstrated that binding of pediocin PA-1 to the membrane strongly depended on the negative charge on the membrane surface. Similar findings have been reported for other bacteriocins. The binding of the cationic class I bacteriocin epilancin K7 to DOPG vesicles involves electrostatic interactions (8). Nisin binds strongly to dipalmitoyl-phosphatidylglycerol and DOPG vesicles but very weakly to PC vesicles with the same fatty acid (9). Furthermore, the proportion of anionic phospholipids in the membrane is a major determinant for nisin insertion and pore formation. Nisin penetrates more strongly into monolayers of anionic phospholipids than those of zwitterionic phospholipids (7). In lipid vesicles with varying ratios of anionic and zwitterionic phospholipids, nisin-induced release of vesicular contents increases with an increasing proportion of anionic phospholipid (3). Results from this study provide the first evidence that the initial binding step for pediocin PA-1, a class IIa bacteriocin, displays a similar anionic lipid dependency.
In conclusion, the lipid composition of the target membrane plays an important role in modulating pediocin PA-1 action. A higher content of negatively charged phospholipids increases the affinity of pediocin PA-1 for the membrane. Together with previous evidence obtained by altering the charge properties of the pediocin molecules, this makes a compelling case that electrostatic interactions are responsible for the initial binding of pediocin PA-1 to target membranes.
Acknowledgments
Research in the authors’ laboratory and preparation of the manuscript were supported by state appropriations, U.S. Hatch Act Funds, and the U.S.-Israel Binational Agricultural Research and Development Fund (no. US-2113-92).
We thank K. Schaich for the use of instruments in her laboratory and Z. Rajfur for assistance with spectrofluorometer operations.
Footnotes
Report D-10131-1-98 of the New Jersey Agricultural Experiment Station.
REFERENCES
- 1.Abee T, Krockel L, Hill C. Bacteriocins: modes of action and potentials in food preservation and control of food poisoning. Int J Food Microbiol. 1995;28:169–185. doi: 10.1016/0168-1605(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 2.Avanti Polar Lipids, Inc. Avanti Polar Lipids catalog. Alabaster, Ala: Avanti Polar Lipids, Inc.; 1993. Physical properties; pp. A1–A10. [Google Scholar]
- 3.Breukink E, van Kraaij C, Demel R D, Siezen R J, Kuipers O P, de Kruijff B. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry. 1997;36:6968–6976. doi: 10.1021/bi970008u. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Ludescher R D, Montville T J. Electrostatic interactions, but not the YGNGV consensus motif, govern the binding of pediocin PA-1 and its fragments to phospholipid vesicles. Appl Environ Microbiol. 1997;63:4770–4777. doi: 10.1128/aem.63.12.4770-4777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Shapira R, Eisenstein M, Montville T J. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl Environ Microbiol. 1997;63:524–531. doi: 10.1128/aem.63.2.524-531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chikindas M L, García-Garcerá M J, Driessen A J M, Ledeboer A M, Nissen-Meyer J, Nes I F, Abee T, Konings W N, Venema G. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol. 1993;59:3577–3584. doi: 10.1128/aem.59.11.3577-3584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demel R A, Peelen T, Siezen R J, de Kruijff B, Kuipers O P. Nisin Z, mutant nisin Z and lacticin 481 interactions with anionic lipids correlate with antimicrobial activity, a monolayer study. Eur J Biochem. 1996;235:267–274. doi: 10.1111/j.1432-1033.1996.00267.x. [DOI] [PubMed] [Google Scholar]
- 8.Driessen A J M, van den Hooven H W, Kuiper W, van de Kamp M, Sahl H-G, Konings R N H, Konings W N. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry. 1995;34:1606–1614. doi: 10.1021/bi00005a017. [DOI] [PubMed] [Google Scholar]
- 9.El-Jastimi R, Lafleur M. Structural characterization of free and membrane-bound nisin by infrared spectroscopy. Biochim Biophys Acta. 1997;1324:151–158. doi: 10.1016/s0005-2736(96)00221-0. [DOI] [PubMed] [Google Scholar]
- 10.García-Garcerá M J, Elfernic M G L, Driessen A J M, Konings W N. In vitro pore-forming activity of the lantibiotic nisin: role of the proton motive force and lipid composition. Eur J Biochem. 1993;212:417–422. doi: 10.1111/j.1432-1033.1993.tb17677.x. [DOI] [PubMed] [Google Scholar]
- 11.Henderson J T, Chopko A L, van Wassenaar P D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch Biochem Biophys. 1992;295:5–12. doi: 10.1016/0003-9861(92)90480-k. [DOI] [PubMed] [Google Scholar]
- 12.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Iwata T, Oyagi H, Aoyagi H, Ohno M, Anzai K, Kirino Y, Sugihara G. Effect of salts on conformational change of basic amphipathic peptides from β-structure to α-helix in the presence of phospholipid liposomes and their channel-forming ability. Biochim Biophys Acta. 1993;1151:76–82. doi: 10.1016/0005-2736(93)90073-9. [DOI] [PubMed] [Google Scholar]
- 14.Martin I, Ruysschaert J-M, Sanders D, Giffard C J. Interaction of the lantibiotic nisin with membranes revealed by fluorescence quenching of an introduced tryptophan. Eur J Biochem. 1996;239:156–164. doi: 10.1111/j.1432-1033.1996.0156u.x. [DOI] [PubMed] [Google Scholar]
- 15.Moll G N, Clark J, Chan W C, Bycroft B W, Roberts G C K, Konings W N, Driessen A J M. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J Bacteriol. 1997;179:135–140. doi: 10.1128/jb.179.1.135-140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montville T J, Winkowski K, Ludescher R D. Models and mechanisms for bacteriocin actions and application. Int Dairy J. 1995;5:797–814. [Google Scholar]
- 17.Strasburg G M, Ludescher R D. Theory and applications of fluorescence spectroscopy in food research. Trends Food Sci Technol. 1995;6:69–75. [Google Scholar]