Abstract
Increasing evidence indicates that deep venous thrombosis (DVT) is a common peripheral vascular disease. This study aims to investigate the mechanisms of thioredoxin-interacting protein (TXNIP) and nod-like receptor protein 3 (NLRP3) inflammasome in deep venous thrombosis (DVT). A total of 66 Sprague–Dawley (SD) rats were employed to conduct DVT model. DVT rat was treated with silenced TXNIP (si-TXNIP) lentivirus and MCC950 (a NLRP3 inhibitor). The thrombosis weight and weight/length ratio, tissue factor, inflammatory factors, superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) were measured. Hematoxylin–eosin (H&E) staining was used to investigate the pathological change. Western blotting was used to determine the protein expression level. The expression level of thioredoxin (TRx) was suppressed, whereas TXNIP and NLRP3 were elevated in DVT rat. Si-TXNIP or MCC950 could reduce the thrombosis weight and weight/length ratio, ameliorate the pathological change, and decrease inflammatory reaction. Mechanistically, si-TXNIP or MCC950 inhibited the expression levels of TXNIP, NLRP3, and interleukin (IL)-1β while elevating the TRx level, thereby suppressing the DVT. Our study indicated that si-TXNIP or MCC950 injection rescued the injury of vein induced by DVT. The possible mechanisms connected with the inhibition of TXNIP and NLRP3. TXNIP is a possible therapeutic target for DVT.
Keywords: Inflammation, oxidative stress, MCC950, thioredoxin, venous thrombosis, pathological change
Impact Statement
Thioredoxin-interacting protein (TXNIP) involves in cell proliferation, differentiation, and apoptosis, which is an oxidative stress regulator. However, the effect of TXNIP on DVT is uncertain. Our study indicated that the inhibition of TXNIP reduced the thrombosis weight and weight/length ratio, and ameliorated the pathological change and inflammatory reaction. The underlying mechanism was related to the inhibition of nod-like receptor protein 3 (NLRP3)/interleukin (IL)-1β. TXNIP may be a novel therapeutic target for DVT.
Introduction
Deep vein thrombosis (DVT) is caused by abnormal coagulation of venous blood, which leads to partial or total blockage of blood vessels by coagulated blood. Venous thromboembolism disease is manifested in two ways at different locations and time periods, namely pulmonary embolism and deep venous thrombosis. 1 Approximately two-thirds of patients diagnosed with deep vein thrombosis and approximately one-third of patients diagnosed with pulmonary embolism are observed among all individuals affected by venous thromboembolism. 2 It can be seen from the survey data that the incidence rate of deep venous thrombosis is 1/1000 in the adults. 3
TXNIP involves in cell proliferation, differentiation, and apoptosis, which is an oxidative stress regulator. 4 TXNIP also inhibits the anti-oxidant activity of endogenous anti-oxidant thioredoxin (TRx) by binding to it. Previous study indicates that both TRx and TXNIP are expressed in cytoplasm and mitochondria. 5 TXNIP participates in oxidative stress and inflammation.6,7
Recently, studies have shown that the injury of venous endothelial cells which is caused by oxidative stress is one of the important factors in the formation of DVT. Reactive oxygen-free radicals can lead to oxidative damage, aging, and apoptosis of endothelial cells, start the procoagulant reaction, and lead to thrombosis. 8 NLRP3 inflammasome is an intracellular protein complex, including NLRP3, apoptosis-associated speck-like protein (ASC), and cysteinyl aspartate-specific protein precursor (pro-caspase-1). After activation, NLRP3 inflammasome can release pro-inflammatory factors IL-1β and IL-18. Recently, studies demonstrate that NLRP3 participates in the venous thrombosis response to hypoxia and cerebral venous sinus thrombosis. 9 However, there is no clear evidence about the effect of TXNIP/NLRP3 on DVT.
Herein, we examined the expression levels of TXNIP and NLRP3 at different times after DVT. Furthermore, we evaluated the inhibition of TXNIP and subsequent influence on NLRP3 inflammasome. We found that the expression level of TXNIP increased after DVT, which further activated NLRP3 inflammasome and promoted the formation of DVT.
Materials and methods
Animal modeling
Specific pathogen-free (SPF) grade male Sprague–Dawley (SD) rats (6 weeks old, weighing 210 ± 10 g) were fed adaptively for one week. The feeding environment was 12 h alternating day and night; the indoor temperature was controlled at 18–22°C; the humidity was controlled at 50–60%. Rats could freely access to food and water. Rats were purchased from Jinan Peng Yue Experimental Animal Breeding Co. Ltd, Certificate No.: SCXK (Lu) – 20180030. The animal use process complied with the National Research Council’s Guide for the Care and Use of Laboratory Animals, and approved by the animal ethics committee of Yantaishan Hospital. The DVT model was prepared by the inferior vena cava (IVC) stenosis as previously described. 10 After the rats were anesthetized with 1% (40 mg/kg, intraperitoneal injection) pentobarbital sodium, the rats were fixed in the supine position, and the median abdominal incision was made for about 3 cm. After the IVC was separated, a #4 suture was used to tie down on the IVC and an associated 30-g needle, and then, the needle was removed, to produce a stenosis of the IVC. The abdominal wall was sutured layer by layer and disinfected. The control group was treated the same as the model group, but without IVC stenosis.
Animal grouping
A total of 36 rats were first divided into two groups: control group (n = 6) and DVT group (n = 30). Next, another 30 rats were divided into five groups, including control (rats received the same surgical procedure without IVC stenosis) group, DVT group, si-NC group, silenced TXNIP (si-TXNIP) (1 µg/µL, intravenous injection 100 μL, GenePharma, Shanghai, China) group, and MCC950 (a NLRP3 inhibitor, gavaged 20 mg/kg body weight every day, MCE, USA) group. TXNIP siRNA was administrated two days before operation and one day after operation. Rats were sacrificed on day 3 after operation.
Enzyme-linked immunosorbent assay
The levels of D2D, TF, IL-18, and IL-1β were detected by enzyme-linked immunosorbent assay (ELISA) kit according to manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Hematoxylin–eosin (H&E) staining and Immunohistochemistry
The rats were anesthetized with 1% (40 mg/kg, intraperitoneal injection) pentobarbital sodium and fixed on the 37°C constant temperature table on the back. Blood samples were collected from the heart until the rats reached the point of euthanasia. The IVC vessels were stripped, fixed with Bouin’s solution, embedded in paraffin, and sliced about 4 μm. After dehydration and dewaxing with xylene and gradient alcohol, hematoxylin staining for 5 min, 1% hydrochloric acid (HCl) ethanol differentiation for 20 s, 1% ammonia back blue for 15 s, 1% eosin staining for 2 min, gradient alcohol dehydration, and sealing. The numbers of neutrophils, monocytes in the vein wall, and thrombus were counted in five distinct fields (×400) according to previous study. 11 For IVC vessel, tissues from rats were prepared for immunohistochemistry (IHC), as described in previous study. 12 The tissues were incubated with anti-TXNIP (1:100) and anti-NLRP3 (1:200) antibodies. An optical microscope (Leica, Germany) was used to observe pathological changes and positive cells in IVC.
Analysis of oxidant stress
The levels of superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) were detected with responding kits according to manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Western blotting
The collected samples were added with protein buffer for routine protein extraction, and the quantitative analysis was carried out by diquinoline formic acid (BCA, Solarbio, Beijing, China) method. Fifty-microgram protein samples were loaded with 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF, Millipore, USA) membrane. The membrane was completely immersed in Tris HCl buffer (TBST) with 5% skimmed milk powder and then incubated at room temperature for 1 h. Primary antibodies anti-TXNIP (Bioss antibodies, Beijing, China), anti-NLRP3 (Thermo Fisher Scientific, USA), anti-TRx (Abcam, USA), pro-caspase-1 (ABclonal, Wuhan, China), and pro-IL-1β (ABclonal, Wuhan, China) were incubated overnight at 4°C, then added horseradish peroxidase (HRP)-labeled goat antirabbit IgG secondary antibody (Bioss antibodies, Beijing, China), and incubated at 37°C for 1 h. After incubation, the blots were washed with TBST for three times, then added electrochemiluminescence (ECL) solution, and exposed. The gray value of protein bands was analyzed by Image J software (NIH, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal reference.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses between two groups were evaluated based on Student’s t-test. For experiments involving three or more groups, data were evaluated using one-way analysis of variance (ANOVA) with Tukey’s post hoc test with the GraphPad Prism version 8.0 (GraphPad Software Inc., USA). P < 0.05 was considered significant.
Results
Changes of thrombus weight at different time points
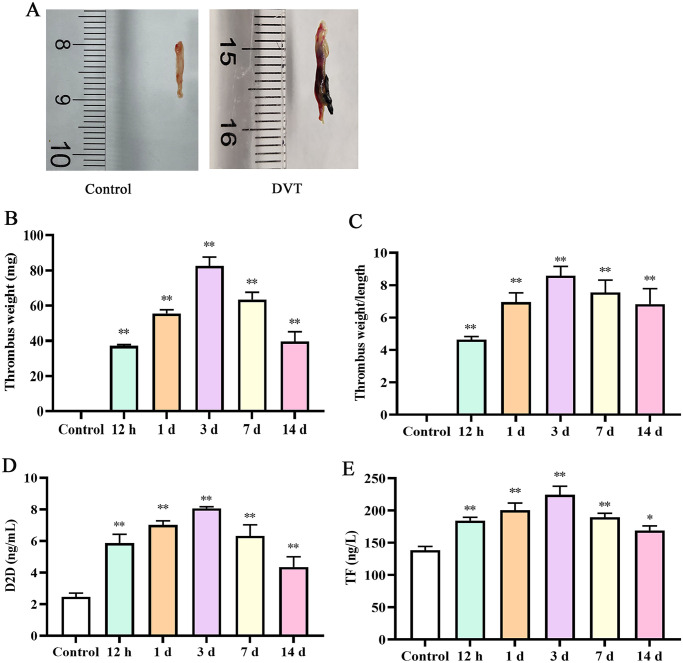
We first investigated the changes of thrombus weight at different time point. As presented in Figure 1, thrombus weight was increased after DVT. On the third day, the thrombus weight was the highest (P < 0.05). However, there were no significantly changes after three days. In addition, we investigated the weight/length ratio, and the result indicated that thrombosis was notably stronger in different times, with the highest on day 3. The results of ELISA presented that the levels of D2D and tissue factor (TF) increased after DVT. In addition, the levels peaked on day 3. After day 3, the levels of D2D and TF exhibited a downward trend; nevertheless, there was no significantly difference between day 7 and day 14.
Figure 1.
Deep vein thrombosis was induced in rats: (A) Schematic images of normal blood vessel (control) and deep vein thrombosis (DVT); (B) thrombus weight; (C) thrombus weight/length ratio (mg/mm); (D) the content of D2D in IVC; (E) the content of TF in IVC. IVC: inferior vena cava.
*P < 0.05 and **P < 0.01 compared with the control group.
Pathological changes of deep vein after DVT
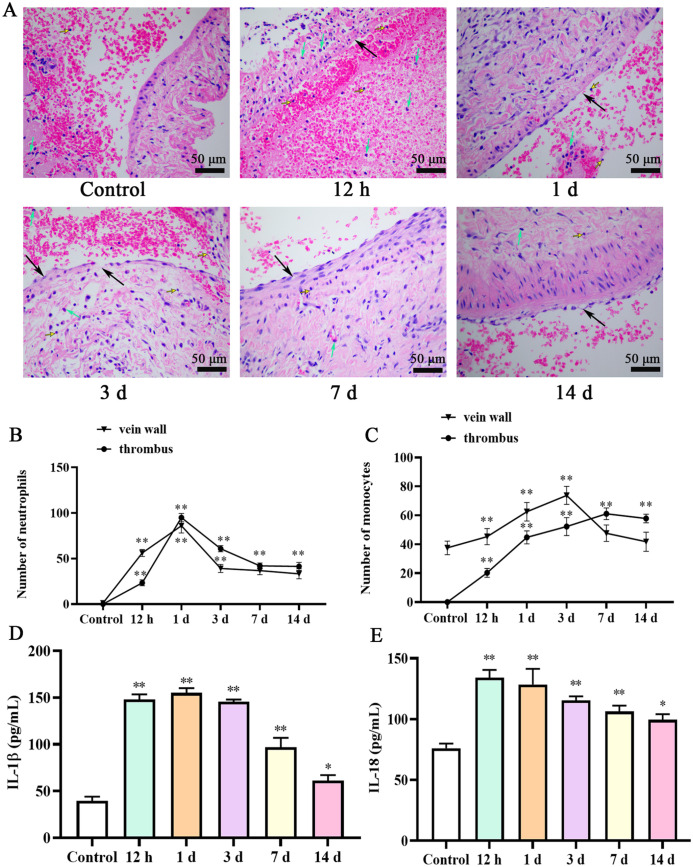
The IVC lumen was not significantly dilated in the control group, and there was no substantial thrombosis in the lumen. Normal venous lumen could be seen. The venous structure and intima were intact, and no endothelial cell damage or necrosis was found. There was no inflammatory cell infiltration around the vein wall (Figure 2(A)). In DVT group, there were a lot of thrombosis, and the color was dark red. The results showed that most of the sites of venous thrombosis were red blood cell aggregation, loose vascular wall tissue, edema, and thickening, and irregular vascular intima and inflammatory cell infiltration can be seen around the vein wall. However, the most serious pathological changes were on day 3. After day 3, the inflammatory cell was reduced on days 7 and 14. The number of neutrophils reached a maximum at one day in the vein wall and thrombus. Monocytes were significantly increased throughout 12 h to day 14 in thrombus. In addition, the results of ELISA presented that the levels of IL-18 and IL-1β decreased on day 7 and day 14, but still higher than those in the control group (P < 0.05; Figure 2(B)).
Figure 2.
Pathological structure changes in IVC of rat at different time points after operation: (A) H&E staining presented the thrombosed in the vein wall is shown (50 μm). Number of neutrophils (B) and monocytes (C) in the vein wall and thrombus. IL-1β (D) and IL-18 (E) levels in serum were determined by ELISA. Block arrows indicate vascular wall tissues become loose, edema, or thickening; green arrows indicate neutrophils; yellow arrows indicate monocytes. IVC: inferior vena cava; ELISA: enzyme-linked immunosorbent assay; IL: interleukin.
*P < 0.05 and **P < 0.01 compared with the control group.
Evidence for TXNIP participated in the DVT
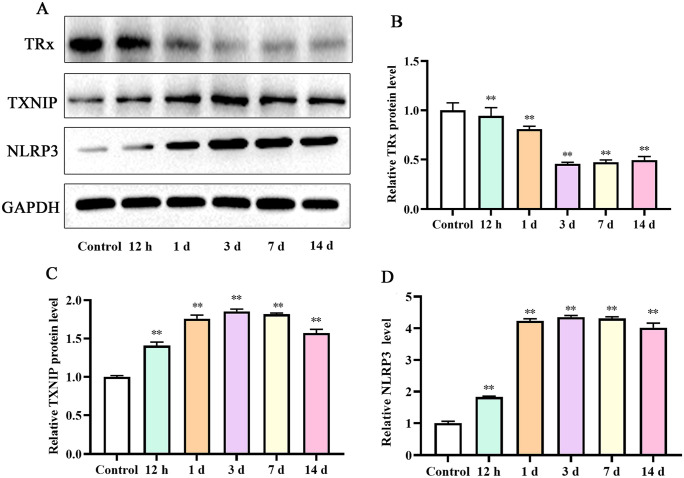
We found that the expression level of TXNIP increased after DVT (P < 0.05). TXNIP expression level increased over time. Furthermore, TXNIP expression level peaked on day 3. After day 7, the expression level of TXNIP presented a downward trend. Interestingly, the change of NLRP3 was consistent with the trends of TXNIP. Importantly, the expression trend of TRx was opposite to that of TXNIP and NLRP3 (Figure 3).
Figure 3.
Expression of proteins in IVC at various time points: (A) Western blot assay for the TRx, TXNIP, and NLRP3 protein detection; relative TRx (B), TXNIP (C), and NLRP3 (D) protein densities have been normalized against the control group. IVC: inferior vena cava; TXNIP: thioredoxin-interacting protein; TRx: thioredoxin.
**P < 0.01 compared with the control group.
Downregulation of TXNIP inhibited the DVT formation
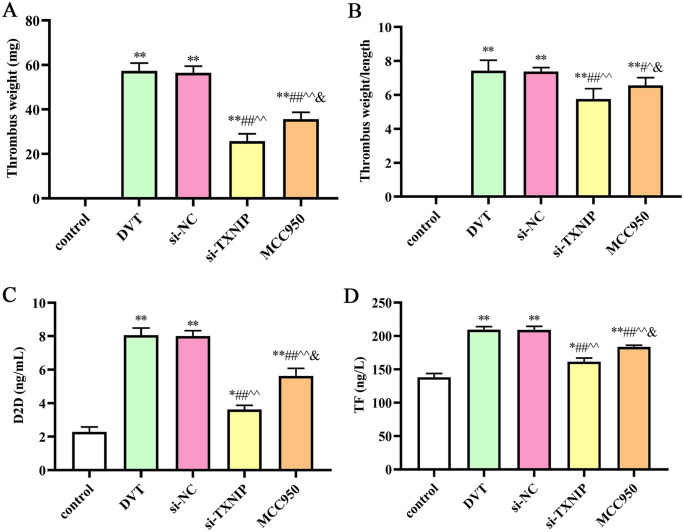
To evaluate the effect of TXNIP on DVT formation, TXNIP small interfering RNA (siRNA) was applied to downregulate the expression level of TXNIP, and MCC950 was used to inhibit the expression of NLRP3. After TXNIP siRNA or MCC950 treatment, the thrombus weight was decreased (P < 0.05), and the weight/length ratio was also decreased compared with the DVT or small interfering RNA negative control (si-NC) group. Furthermore, the levels of D2D and TF decreased after TXNIP siRNA or MCC950 treatment (P < 0.05; Figure 4).
Figure 4.
Deep vein thrombosis was induced in rats after different treatment: (A) Thrombus weight; (B) thrombus weight/length ratio (mg/mm); (C) the content of D2D in IVC; (D) the content of TF in IVC. IVC: inferior vena cava.
**P < 0.01 compared with the control group; ##P < 0.01 compared with the DVT group; ^^P < 0.01 compared with the si-NC group; &P < 0.05 compared with the si-TXNIP group.
Downregulation of TXNIP ameliorated pathological changes of IVC
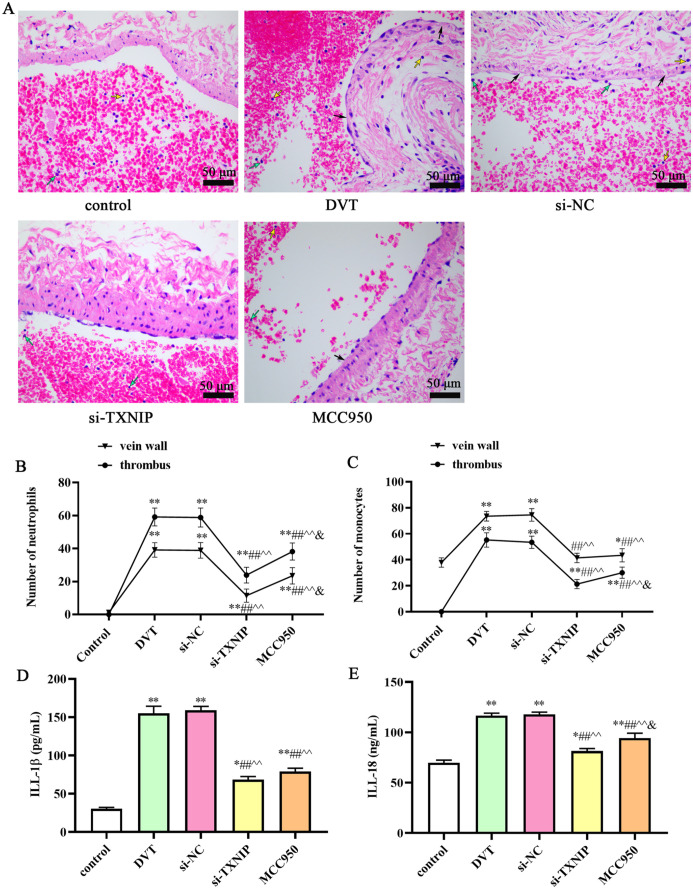
As shown in Figure 5, in the si-TXNIP group or MCC950 group, the lumen of the IVC was slightly dilated, a small amount of substantial thrombosis was formed in the lumen, the vein wall tissue was loose, the vascular intima was irregular, and no inflammatory cell infiltration was found around the IVC wall. The number of neutrophils and monocytes was significantly increased in the vein wall and thrombus after DVT. After si-TXNIP or MCC950 treatment, the number of neutrophils and monocytes was decreased compared with the DVT and si-NC groups (P < 0.05). However, the number of neutrophils and monocytes after treatment is still higher than that in the control group. Furthermore, the levels of IL-18 and IL-1β decreased in si-TXNIP and MCC950 groups compared with DVT group (P < 0.05).
Figure 5.
Pathological structure changes in IVC of rat in different groups: (A) H&E staining presented the thrombosed in the vein wall is shown (scale bar: 50 μm). Number of neutrophils (B) and monocytes (C) in the vein wall and thrombus. IL-1β (D) and IL-18 (E) levels in serum were determined by ELISA. Block arrows indicate vascular wall tissues become loose, edema, or thickening; green arrows indicate neutrophils; yellow arrows indicate monocytes.
*P < 0.05 and **P < 0.01 compared with the control group; #P < 0.05 and ##P < 0.01 compared with the DVT group; ^P < 0.05 and ^^P < 0.01 compared with the si-NC group; &P < 0.05 compared with the si-TXNIP group. IVC: inferior vena cava; H&E: hematoxylin–eosin; IL: interleukin; ELISA: enzyme-linked immunosorbent assay.
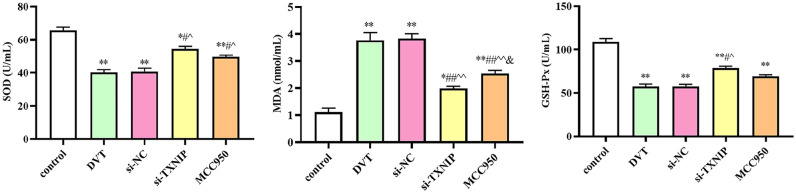
Downregulation of TXNIP improved oxidative stress injury
We detected the levels of SOD, MDA, and GSH-Px. As presented in Figure 6, the contents of SOD and GSH-Px decreased in DVT group, while the levels increased in si-TXNIP and MCC950 groups (P < 0.05). In addition, the content of MDA significantly increased in the DVT group, while the content decreased in si-TXNIP and MCC950 groups (P < 0.05; Figure 6). These results indicated that oxidative stress was involved during the DVT.
Figure 6.
The levels of SOD, MDA, and GSH-Px. SOD: superoxide dismutase; MDA: malondialdehyde; GSH-Px: glutathione peroxidase.
*P < 0.05 and **P < 0.01 compared with the control group; #P < 0.05 and ##P < 0.01 compared with the DVT group; ^P < 0.05 and ^^P < 0.01 compared with the si-NC group; &P < 0.05 compared with the si-TXNIP group.
Downregulation of TXNIP inhibited the protein expression
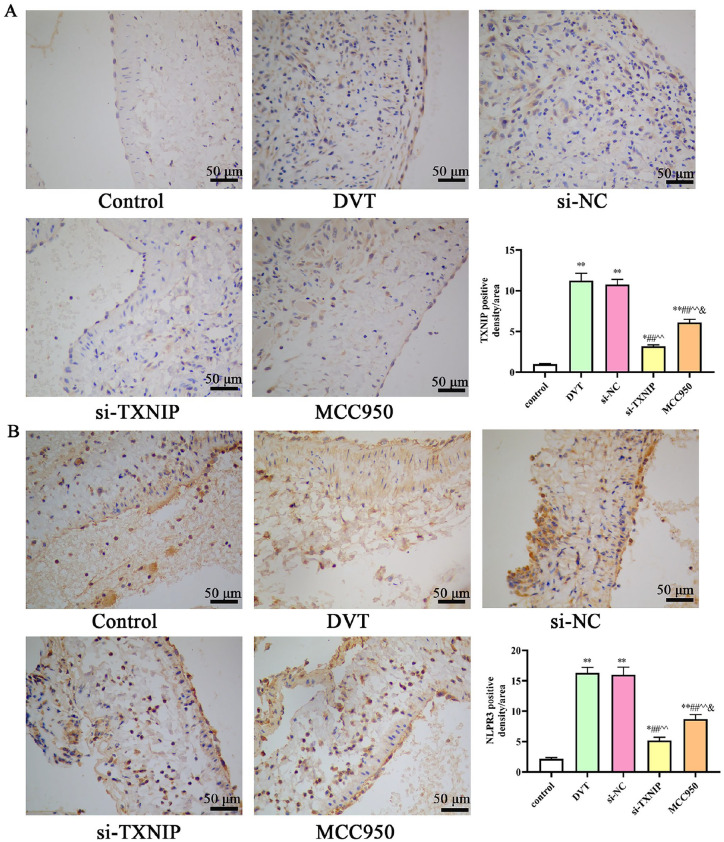
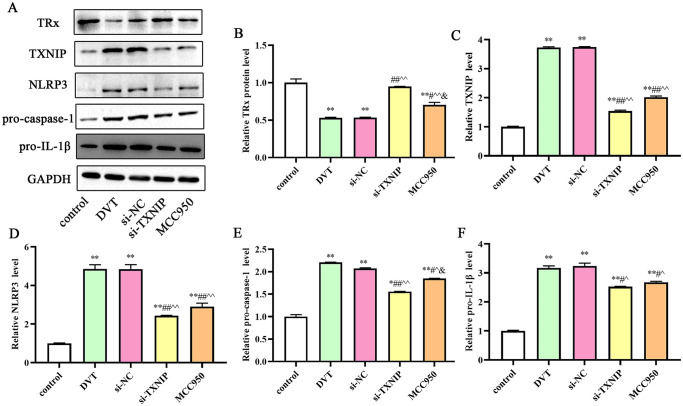
We first investigated the expression levels TXNIP and NLRP3 in IVC by IHC. The results presented that the positive expression of TXNIP and NLRP3 increased in the DVT group. After si-TXNIP and MCC950 treatment, the positive expression of TXNIP and NLRP3 significantly decreased compared with the DVT group (Figure 7). We further detected the protein expression by western blotting. As shown in Figure 8, the expression level of TRx protein decreased, whereas the expression levels of TXNIP, NLRP3, pro-caspase-1, and pro-IL-1β significantly increased in the DVT group (P < 0.05). However, the expression level of TRx protein increased whereas the expression levels of TXNIP, NLRP3, cleaved caspase-1, and cleaved-IL-1β significantly decreased after si-TXNIP or MCC950 treatment (P < 0.05).
Figure 7.
Immunohistochemical staining of TXNIP and NLRP3 in the thrombosed IVC: (A) Representative IHC staining of TXNIP (scale bar: 50 μm); (B) representative IHC staining of NLRP3 (scale bar: 50 μm). TXNIP: thioredoxin-interacting protein; IVC: inferior vena cava; IHC: immunohistochemistry.
*P < 0.05 and **P < 0.01 compared with the control group; #P < 0.05 and ##P < 0.01 compared with the DVT group; ^P < 0.05 and ^^P < 0.01 compared with the si-NC group; &P < 0.05 compared with the si-TXNIP group.
Figure 8.
Expression of proteins in IVC after TXNIP siRNA treatment: (A) Western blot assay for the TRx, TXNIP, NLRP3, pro-caspase-1, and pri-IL-1β protein detection; relative TRx (B), TXNIP (C), NLRP3 (D), pro-caspase-1 (E), and pri-IL-1β (F) protein densities have been normalized against the control group. TXNIP: thioredoxin-interacting protein; IVC: inferior vena cava; TRx: thioredoxin; IL: interleukin.
*P < 0.05 and **P < 0.01 compared with the control group; #P < 0.05 and ##P < 0.01 compared with the DVT group; ^P < 0.05 and ^^P < 0.01 compared with the si-NC group; &P < 0.05 compared with the si-TXNIP group.
Discussion
The occurrence factors of deep venous thrombosis are complex and diverse. At present, there are no accurate and sensitive specific observation indicators for DVT clinical prediction and diagnosis. TXNIP is a potential biomarker in cardiovascular and ischemic diseases. 13 An increasing number of studies have indicated that TXNIP participated in the formation of thrombosis.14,15 However, there is no clear evidence presents the change of TXNIP in DVT. In our study, we first investigated the changes of TXNIP and NLRP3 levels at different times after DVT. As presented in our study, thrombus weight and weight/length ratio were increased over time. At the third day, the thrombus weight and weight/length ratio were the highest. After day 3, the weight and weight/length ratio of thrombus had no significantly difference between day 7 and day 14. Interestingly, the amounts of neutrophils and monocytes increased after DVT. As the time gradually increases, the amounts of neutrophils and monocytes gradually decreased. In addition, the trends of D2D, TF, IL-18, and IL-1β were consistent with the changes of thrombus weight and weight/length ratio. Furthermore, the level of TRx was downregulated while the levels of TXNIP and NLRP3 were upregulated in DVT model. TXNIP expression level peaked on day 3. Ding et al. reported that the expression of TXNIP increased sharply 6 h after cerebral venous sinus thrombosis, peaked three days, and decreased gradually. 14 Feng et al. reported that TXNIP expression was elevated in DVT mice. 15 Our result was similar to their research. However, there is still no clear evidence about TXNIP interact with NLRP3 in DVT formation.
TRx is a protein of choice for fighting oxidative stress in the vessel. TRx activity could be negatively modulated by TXNIP. 16 In this study, the level of TRx decreased after DVT; the TRx level was the lowest on day 3 and then slightly increased on day 7 and day 14. The result was consistent with previous conclusion. We postulated that the inhibition of TXNIP or NLRP3 could suppress the formation of DVT. Hence, we employed siRNAs specifically targeting TXNIP. MCC950 is a first-in-class, highly potent, and selective small molecule inhibitor of NLRP3. 17 MCC950 reduces liver inflammation and fibrosis in experimental non-alcoholic steatohepatitis in mice. 18 In addition, MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia, and prevents aortic aneurysms and dissections in mice 19,20 The increasing evidences indicated that MCC950 can effectively inhibit NLRP3 activity. In this study, we employed si-TXNIP and MCC950 to administrate the DVT rat. The results showed that thrombus weight and weight/length ratio were decreased, and the levels of D2D, TF, IL-18, and IL-1β also decreased after si-TXNIP or MCC950 treatment. We also investigated the number of neutrophils and monocytes. Our results presented that the amounts of neutrophils and monocytes in the si-TXNIP and MCC950 decreased. Interestingly, we found the levels of SOD and GSH-Px increased while the level of MDA decreased. The most obvious finding to emerge from the analysis was that the level of TRx increased after TXNIP inhibited. TRx/TXNIP system has been suggested to be an important contributor to oxidative stress. 21 Our results confirmed this point.
Previously published article reported that TXNIP is an upstream partner to NLRP3 and that these two proteins’ interaction was necessary for NLRP3 inflammasome activation.22,23 Our results indicated that si-TXNIP treatment decreased the expression levels of TXNIP and NLRP3 inflammasome. In addition, the MCC950 treatment also suppressed the expression of NLRP3 inflammasome and TXNIP. However, the effect of si-TXNIP had no significant difference with MCC950. The result may be explained by the fact that possible mechanism of TXNIP on DVT was connected with NLRP3 inflammasome. These results were consistent with previous studies.22,23 Importantly, both si-TXNIP and MCC950 treatment could ameliorate the inflammatory infiltration of vein wall.
Our results supported that the inhibition of TXNIP attenuated the formation of DVT, the possible mechanism connecting with NLRP3 inflammasome. Nevertheless, this study is not without limitations, and the downstream of TXNIP is complex, probably not only the NLRP3 inflammasome. Hence, the probability of other factors involved in TXNIP on DVT needs further observation.
Conclusions
In conclusions, our study indicates that TXNIP/NLRP3 inflammasome system plays a crucial role in DVT. We provide a theoretical basis for TXNIP as a promising target of the DVT treatment.
Footnotes
Authors’ Contributions: JF and JJ contributed to conception and design. JF contributed to administrative support. JF, HW, JH, XZ, HM, XQ, CY, and JJ contributed to provision of study materials or patients. JF, HW, JH, and XZ contributed to collection and assembly of data. JF, HW, JH, XZ, HM, XQ, and CY contributed to data analysis and interpretation. All authors contributed to article writing. All authors contributed to final approval of article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Key Research and Development Plan of Yantai City (grant no. 2018SFGY096).
ORCID iD: Junjie Jiang  https://orcid.org/0000-0003-0636-9629
https://orcid.org/0000-0003-0636-9629
References
- 1. Hagan KA, Harrington LB, Kim J, Lindström S, Camargo CA, Jr, Grodstein F, Kabrhel C. Adiposity throughout the life course and risk of venous thromboembolism. Thromb Res 2018;172:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gutmann C, Siow R, Gwozdz AM, Saha P, Smith A. Reactive oxygen species in venous thrombosis. Int J Mol Sci 2020;21:1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosendaal FR. Causes of venous thrombosis. Thromb J 2016;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han Y, Xu X, Tang C, Gao P, Chen X, Xiong X, Yang M, Yang S, Zhu X, Yuan S, Liu F, Xiao L, Kanwar YS, Sun L. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol 2018;16:32–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, Yodoi J. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol 2014;4:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brocker CN, Kim D, Melia T, Karri K, Velenosi TJ, Takahashi S, Aibara D, Bonzo JA, Levi M, Waxman DJ, Gonzalez FJ. Long non-coding RNA Gm15441 attenuates hepatic inflammasome activation in response to PPARA agonism and fasting. Nat Commun 2020;11:5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yumnamcha T, Guerra M, Singh LP, Ibrahim AS. Metabolic dysregulation and neurovascular dysfunction in diabetic retinopathy. Antioxidants (Basel, Switzerland) 2020;9:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Q, Zennadi R. Oxidative stress and thrombosis during aging: the roles of oxidative stress in RBCs in venous thrombosis. Int J Mol Sci 2020;21:4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, Ashraf MZ. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci USA 2017;114:4763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao X, Chen W, Liu J, Liu H, Zhan JY, Guan S, Lu Z, Tang P, Li P, Lin B. Deep vein thrombosis is modulated by inflammation regulated via sirtuin 1/NF-κB signalling pathway in a rat model. Thromb Haemost 2019;119:421–30 [DOI] [PubMed] [Google Scholar]
- 11. Nosaka M, Ishida Y, Kimura A, Kondo T. Time-dependent appearance of intrathrombus neutrophils and macrophages in a stasis-induced deep vein thrombosis model and its application to thrombus age determination. Int J Legal Med 2009;123:235–40 [DOI] [PubMed] [Google Scholar]
- 12. Yao X, Chen W, Liu J, Liu H, Zhan JY, Guan S, Lu Z, Tang P, Li P, Lin B. Deep vein thrombosis is modulated by inflammation regulated via sirtuin 1/NF-kappaB signalling pathway in a rat model. Thromb Haemost 2019;119:421–30 [DOI] [PubMed] [Google Scholar]
- 13. Domingues A, Jolibois J, Marquet de Rougé P, Nivet-Antoine V. The emerging role of TXNIP in ischemic and cardiovascular diseases; a novel marker and therapeutic target. Int J Mol Sci 2021;22:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding R, Ou W, Chen C, Liu Y, Li H, Zhang X, Chai H, Ding X, Wang Q. Endoplasmic reticulum stress and oxidative stress contribute to neuronal pyroptosis caused by cerebral venous sinus thrombosis in rats: involvement of TXNIP/peroxynitrite-NLRP3 inflammasome activation. Neurochem Int 2020;141:104856. [DOI] [PubMed] [Google Scholar]
- 15. Feng Y, Lei B, Zhang H, Niu L, Li X, Luo X, Zhang F. MicroRNA-136-5p from endothelial progenitor cells-released extracellular vesicles mediates TXNIP to promote the dissolution of deep venous thrombosis. Shock (Augusta, Ga) 2022;57:714–21 [DOI] [PubMed] [Google Scholar]
- 16. Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol (Baltimore, Md: 1950) 2000;164:6287–95 [DOI] [PubMed] [Google Scholar]
- 17. Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015;21:248–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017;66:1037–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ward R, Li W, Abdul Y, Jackson L, Dong G, Jamil S, Filosa J, Fagan SC, Ergul A. NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol Res 2019;142:237–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren P, Wu D, Appel R, Zhang L, Zhang C, Luo W, Robertson AAB, Cooper MA, Coselli JS, Milewicz DM, Shen YH, LeMaire SA. Targeting the NLRP3 inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J Am Heart Assoc 2020;9:e014044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu H, Guo W, Guo H, Zhao L, Yue L, Li X, Feng D, Luo J, Wu X, Cui W, Qu Y. Bakuchiol attenuates oxidative stress and neuron damage by regulating Trx1/TXNIP and the phosphorylation of AMPK after subarachnoid hemorrhage in mice. Front Pharmacol 2020;11:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dai X, Liao R, Liu C, Liu S, Huang H, Liu J, Jin T, Guo H, Zheng Z, Xia M, Ling W, Xiao Y. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol 2021;45:102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010;11:136–40 [DOI] [PubMed] [Google Scholar]