Abstract
This prospective observational single-center cohort study aimed to determine an association between cerebrovascular autoregulation (CVAR) and outcomes in hypoxic-ischemic brain injury post-cardiac arrest (CA), and assessed 100 consecutive post-CA patients in Japan between June 2017 and May 2020 who experienced a return of spontaneous circulation. Continuous monitoring was performed for 96 h to determine CVAR presence. A moving Pearson correlation coefficient was calculated from the mean arterial pressure and cerebral regional oxygen saturation. The association between CVAR and outcomes was evaluated using the Cox proportional hazard model; non-CVAR time percent was the time-dependent, age-adjusted covariate. The non-linear effect of target temperature management (TTM) was assessed using a restricted cubic spline. Of the 100 participants, CVAR was detected using the cerebral performance category (CPC) in all patients with a good neurological outcome (CPC 1–2) and in 65 patients (88%) with a poor outcome (CPC 3–5). Survival probability decreased significantly with increasing non-CVAR time percent. The TTM versus the non-TTM group had a significantly lower probability of a poor neurological outcome at 6 months with a non-CVAR time of 18%–37% (p < 0.05). Longer non-CVAR time may be associated with significantly increased mortality in hypoxic-ischemic brain injury post-CA.
Keywords: Cardiac arrest, cerebrovascular autoregulation, near-infrared spectroscopy, hypoxic-ischemic brain injury, single-center cohort study
Introduction
Out-of-hospital cardiac arrest (CA) occurs in approximately 80–100 patients per 100,000 persons/year worldwide.1,2 Despite advances in resuscitation science, many patients are likely to experience poor neurological outcomes after CA.3–5 Amelioration of neurological outcomes is an important research theme. Hypoxic-ischemic brain injury (HIBI) begins with CA, which results in an interruption of cerebral blood flow, neuronal ischemia, and cell death (termed primary brain injury). Subsequently, reperfusion injury, microcirculatory disturbances, and neuronal death can occur as a secondary brain injury within hours to days after the return of spontaneous circulation (ROSC). 6 Secondary brain injury is thought to occur due to reperfusion, microcirculatory dysfunction, impaired cerebrovascular autoregulation (CVAR), anemia, hypoxemia, hyperoxia, hyperthermia, and fluctuations in arterial carbon dioxide.7–14 Cerebral regional oxygen saturation (crSO2) is a near-infrared light-based index of the oxygen supply and demand balance in the brain, which may be useful for neurological management following CA.10,15 However, crSO2 values are affected by impaired CVAR (when observed) and constant optimal levels of mean arterial pressure (MAP) in post-CA patients. 16 Thus, clinical interpretation of crSO2 values at a certain time is difficult in the setting of dynamically changing hemodynamics after ROSC and the use of crSO2 values in clinical practice in neurointensive care is limited.
Regarding a reliable method for crSO2 interpretation and evaluation of blood pressure variations, a previously described approach is to evaluate CVAR and appropriately control blood pressure by determining the correlation coefficient (COx) between MAP per unit time and crSO2.16–18 The analysis of crSO2 changes based on considering hemodynamic variables—rather than the conventional interpretation of crSO2 alone—provides a clinical index for the assessment of CVAR and appropriate blood pressure. In addition, this evaluation may lead to a reliable determination of treatment effects, prognosis, and individualized treatments by establishing a new target population. Several studies have reported impaired CVAR post-CA9,19 and some studies have assessed the association between CVAR and neurological outcome in HIBI.16,20 The sample size in these studies was small and further validation is required.
Our study hypothesis was that a relationship exists between CVAR post-CA and outcomes in HIBI. The study also assessed the effect of therapeutic interventions on neurological outcome caused by the difference of CVAR.
Materials and methods
Study population
This prospective, observational, cohort study was conducted in Japan in northern Osaka at a single-center affiliated with the Osaka University Graduate School of Medicine. The trauma and acute critical care center is a restricted mixed medical/surgical unit with 20 beds. Multidisciplinary resuscitation treatments, including extracorporeal cardiopulmonary resuscitation, are available at this center. The center receives about 100 patients with out-of-hospital CA each year. Patient data were collected from June 2017 to May 2020.
The study enrolled consecutive adult patients (age ≥18 years) who were brought to the center with CA and who subsequently achieved ROSC. The exclusion criteria were: (i) sustained ROSC achieved but expectation of death within a few hours due to difficulty maintaining circulation (death within 2 h after ROSC, due to the difficulty in determining the presence or absence of CVAR); (ii) confirmation of occupied intracranial lesions by diagnostic imaging; (iii) a history of severe traumatic brain injury, intracranial hemorrhage, or stroke; (iv) confirmation of refusal to continue life-supporting treatment; and (v) significant missing patient data.
Study protocol
Immediately on arrival, near-infrared spectroscopy pads (TOS-OR, TOSTEC, Tokyo, Japan) were affixed to the patient’s forehead bilaterally. An arterial line was inserted for invasive blood pressure measurement, and crSO2 and MAP were continuously monitored for up to 96 h after ROSC. Blood tests, electrocardiography, ultrasonography, and whole-body computed tomography were performed on all patients after ROSC to investigate the cause of CA. If coronary lesions were the suspected cause of CA, coronary angiography was performed with subsequent percutaneous coronary intervention, if necessary. As a rule, extracorporeal cardiopulmonary resuscitation was performed in patients with shockable rhythm detected prehospital, or as initial care and in cases of refractory arrhythmia. In other cases, extracorporeal cardiopulmonary resuscitation was performed if clinicians determined this approach to be effective. Successfully resuscitated patients were considered to have HIBI; consequently, they were admitted to a center for subsequent intensive care.
Regarding adjustments to respiratory parameters, we followed post-cardiac arrest care guidelines.21,22 FiO2 was set to maintain patients' SpO2 within a range of 92%–98%. Ventilator settings were adjusted to maintain a PaCO2 between 35–45 mmHg. For blood pressure management, we ensured that the mean arterial pressure was maintained at ≥65 mmHg, or systolic blood pressure at ≥90 mmHg. Appropriate fluid therapy and catecholamine administration were implemented as required.
In the target temperature management (TTM) protocol, the criterion for indication was the presence of a shockable rhythm during prehospital transit or during initial care after hospital arrival. In addition, patients with coma after ROSC were eligible for TTM. During TTM, sedatives were administered, and the target body temperature was constant (34°C for 24 h), followed by temperature recovery to 36°C over the next 48 h. The temperature setting in the circuit was adjusted in patients with extracorporeal membrane oxygenation; in other patients, TTM was performed using an intravascular cooling catheter. Finally, normothermia at 36°C was used as a rule for post-CA management in patients with non-shockable rhythm or unstable circulation.
For anesthetic administration, propofol and midazolam were used as sedatives and fentanyl as an analgesic. Sedatives were administered when implementing TTM when the patient had strong body movement or anxiety. Intensive care management was performed with a policy of providing sufficient analgesia and administering sedatives as needed.
Variables
All-cause in-hospital death was identified as the primary outcome measure. A reliable assessment of neurological outcome was obtained by cerebral performance category (CPC)23,24 at 6 months. In this study, CPC 1 or 2 was defined as a good neurological outcome and CPC 3–5 as a poor neurological outcome. A patient information document with baseline characteristics, treatment, and prognosis was created for this study. In the case of unwitnessed CA, the duration of CA was calculated from the time of emergency medical service staff contact to ROSC.
Analog arterial blood pressure signals were observed using a hemodynamic monitor; data processing was performed with a DT9800 data acquisition module (Data Translation, Marlboro, MA, USA). The signals were filtered as non-overlapping 15-s average values that were time-integrated. This procedure allowed the research team to obtain a moving average filter with a 15 s time window and resampling at 0.067 Hz, with the removal of high-frequency noise caused by respiration and pulse waveforms (detection of oscillations and transients with a frequency <0.13 Hz was allowed). 25 A moving Pearson correlation coefficient between MAP modifications and crSO2 was obtained, and the variable COx values approaching 0 highlighted functional autoregulation (i.e., crSO2 and MAP were not correlated), whereas impaired autoregulation was evident in COx values close to 1. The MAP and mean crSO2 values were determined in a 15-s window and collected as 20 data points. The correlation coefficient was calculated in a 300-s window to monitor COx values. The mean of the right side and left side crSO2 was used as the crSO2 value for each patient, and the COx value was obtained with a correlation analysis using ICM software (Cambridge Enterprise, Cambridge, United Kingdom). Both crSO2 and MAP were monitored continuously for a maximum of 96 h after ROSC, and the presence or absence of CVAR was determined in the time window every 12 h (96/12 h = 8 periods). The presence or absence of CVAR was evaluated using a Cox value of 0.3 as a threshold. 18 The presence of CVAR was defined preserved if the COx value was <0.3 for more than half of each period.
Statistical analysis
Statistical data for the patient demographic factors were expressed as number, percentage, and median with interquartile range (IQR), when appropriate. The Mann–Whitney U test was used to compare continuous data, whereas the Pearson’s chi-square test was performed for categorical data. In the subgroups, according to CVAR status, basic characteristics were evaluated in the first period up to 12 h after ROSC to evaluate the bias of factors that could affect CVAR. In the heatmap representation, CVAR was depicted for each time period as present, absent, missing, or death; MAP (average over 12 hours) was depicted as ≥60 mmHg, <60 mmHg, missing, or death. Patients were sorted according to their CPC at 6 months when constructing the heatmap. The association between non-CVAR and mortality was evaluated using the Cox proportional hazard (PH) model in which a variable indicating CVAR or non-CVAR was treated as a time-dependent covariate and patient age as a time-fixed covariate. A 12-h unit was used for the variable to indicate time after ROSC. For each 12-h time interval, the time-dependent presence of CVAR was defined when COx was obtained for more than 2 h. The analysis ignored any CVAR that was considered missing. Hazard ratios (HRs) obtained from the Cox regression analysis together with a 95% confidence interval (CI) are presented. Age-adjusted survival curves were depicted based on Cox PH regression. Additionally, a sensitivity analysis was performed by excluding CVAR data for cases with a MAP <60 mmHg and performing a survival analysis.
As a secondary analysis, the duration of non-CVAR was defined as a proportion of time within 96 h during which COx was greater than 0.3. Logistic regression was used to evaluate whether the effect on poor neurological outcome of the duration of non-CVAR was modified by the presence or absence of TTM (defined as a binary variable of the 6-month CPC greater than 2), with adjustment for age using a cross-product term between the two variables. The non-linear effect of the non-CVAR time percent was estimated using a restricted cubic spline in the logistic regression.
A two-tailed p-value less than 0.05 was considered significant for all statistical inferences. All analyses were performed using R software version 4.0.2. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines 26 and the principles of the Declaration of Helsinki. Formal approval for the study was obtained from the Institutional Review Board of Osaka University Hospital (approval number: 17207). Given that the population of this study were patients with CA, consent for participation was obtained from their families, and when the patients’ awareness improved, the patients themselves were informed and consented to participate in the study.
Results
Patient characteristics
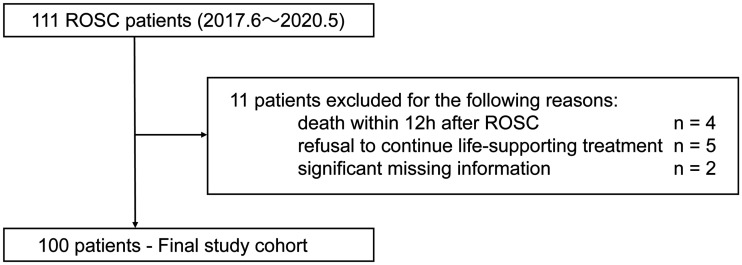
A total of 100 patients participated (Figure 1). Their demographic features are presented in Supplemental Table 1. The median age of all patients was 72.0 years (IQR 58.8–81.0) and 36 participants were women. The median monitoring time of crSO2 was 72.0 h (IQR 23.5–90.0). Of all 100 participants, 73 with CA had witnesses, bystander cardiopulmonary resuscitation was performed in 64, and shockable rhythm was detected in 41. The median duration of CA was 33.5 minutes (IQR 17.0–48.0). The median crSO2 at hospital arrival was 48.9% (IQR: 43.2%–59.2%). With regard to treatments, TTM was performed in 27 patients, percutaneous coronary angioplasty in 16, and extracorporeal cardiopulmonary resuscitation in 24. Of the 100 patients, 24 had a good neurological outcome and 76 had a poor neurological outcome. Additionally, patient characteristics up to 12 h after ROSC are presented in Supplemental Table 2. CVAR was detectable in 73 patients from ROSC to the first 12 h, with 18 patients having no CVAR and 9 patients having missing data. The CVAR detection group had a significantly higher MAP, milder lactic acidosis, less frequent use of vasopressors, and more frequent use of sedative drugs. No significant differences were identified for age, sex, cardiac arrest time, crSO2, body temperature, PaCO2, PaO2, bicarbonate, hemoglobin, TTM, or ECPR.
Figure 1.
Patient flow diagram.
ROSC: return of spontaneous circulation.
Trend of CVAR and MAP after ROSC
The trend of CVAR and MAP in each patient over time is shown in Figure 2. In all patients with a good neurological outcome within 96 h after ROSC, CVAR was detected. In contrast, CVAR was detected in 67 (88%) patients with a poor neurological outcome. Discharge due to death occurred in nine patients with undetected CVAR. A total of 36 patients had a period with undetectable CVAR after CA, and 4 patients had non-CVAR periods despite maintaining blood pressure at some time (patients 2, 4, 15, and 18), including those with good neurological outcomes. In some cases, CVAR appeared and disappeared repeatedly. The condition of CVAR changed over time in several patients.
Figure 2.
Trend of cerebrovascular autoregulation and mean arterial pressure after return of spontaneous circulation (ROSC). One band represents one patient; the horizontal axis represents the elapsed time after ROSC, and the presence or absence of cerebrovascular autoregulation (CVAR) every 12 h is indicated by color-coding. The light blue color indicates when CVAR was detected, red indicates when no CVAR was detected, and white indicates missing data. Finally, black means death. Patients were classified and sorted by cerebral performance category (CPC) 6 months after cardiac arrest (left figure). The right figure shows the temporal changes in mean arterial pressure (MAP) after ROSC. The mean MAP at 12 h is represented in light blue for cases >60 mmHg, red for cases <60 mmHg, white for missing data, and black for deceased patients. Patients were classified and sorted by CPC 6 months after cardiac arrest.
Survival analysis with non-CVAR time as a time-dependent covariate
Non-CVAR time was significantly associated with mortality (crude HR 4.01; 95% CI, 1.84–8.73; p < 0.001; adjusted HR 4.02; 95% CI, 1.82–8.85; p < 0.001). Figure 3 shows survival curves after ROSC by CVAR status. The survival probability significantly decreased as the exposure time of non-CVAR increased. In a sensitivity analysis excluding hypotensive CVAR data, non-CVAR time was significantly associated with mortality (crude HR 8.89; 95% CI, 2.53–31.2; p < 0.001; adjusted HR 8.81; 95% CI, 2.41–32.3; p = 0.001, Supplemental Figure 1).
Figure 3.
Survival curves analysis with non-cerebrovascular autoregulation time as a time-dependent covariate. The dotted red line indicates the group with non-CVAR exposure, and the solid blue line indicates the group with preserved CVAR. The thin area shows the 95% confidence interval. The horizontal axis shows the time after the ROSC (hours). Survival curves adjusted by age are shown (median age, 72 years).
Evaluation of interaction effect of TTM
The interaction between the intervention (TTM or non-TTM) and the non-CVAR time percent was evaluated. The results showed that the interaction with non-CVAR time was not statistically significant (p = 0.820). In addition, the predicted probability of poor neurological outcome was significantly lower in the TTM group than in the non-TTM group at non-CVAR time percentages of 18%–37%. Otherwise, there was no statistically significant difference (Figure 4).
Figure 4.
Association between non-cerebrovascular autoregulation time and in-hospital mortality. Regression line for each treatment group was defined by using a restricted cubic spline model with an interaction term between target temperature management (TTM) and non-CVAR time. Spline curves adjusted by age are shown (median age, 72 years). The lines and thin areas indicate the survival probability and 95% confidence intervals, respectively. The solid blue and dotted red lines represent patients in the TTM and non-TTM groups, respectively..
Discussion
This study assessed CVAR after ROSC using the Cox PH model and examined its impact on outcomes. Analysis showed that mortality increased with increasing non-CVAR time after ROSC. In addition, the neurological outcome at 6 months could worsen with increasing non-CVAR time within 96 h after ROSC. Moreover, there may be no significant difference on the impact on in-hospital mortality between the TTM and non-TTM groups for cases in which the non-CVAR time percentage was exceeded by 37% after ROSC. These findings can be incorporated into the early management of post-CA patients to help predict outcomes. Moreover, these results can be useful for creating a new index for post-CA management based on cerebral circulation.
The prediction of neurological outcomes using crSO2 has been attempted with averaged crSO2 values at a certain time point or period.27–29 In the present study, the change in crSO2 for MAP was interpreted using a moving correlation coefficient, which allowed the research team to use all monitored crSO2 data and consider the effect of blood pressure. Thus, this study is novel in that it is the largest to capture these time series data comprehensively and to examine the relationship between CVAR and outcomes after resuscitation in post-CA patients. Furthermore, in this study, the effect of TTM became smaller as the exposure time of non-CVAR increased as shown in the non-linear analysis. A significant difference was observed in neurological outcome between non-TTM and TTM groups when non-CVAR time percentages were between 18% and 37%. To the best of our knowledge, no report has evaluated the effectiveness of TTM based on the presence or absence of CVAR. These results can help clinicians make treatment choices because findings are based on data in the post-CA early phase. These choices are often difficult to determine in post-CA patients in clinical practice and our findings can help clinicians avoid unnecessary withholding of treatment.
In this study, four patients with a good neurological outcome were found to have a phase when CVAR was not observed. This fact may be a reversible and transient CVAR disorder because blood pressure was maintained appropriately during that time. Consequently, several patients in the poor neurological outcome group did not have CVAR several hours before death. It may be assumed that these patients could not maintain circulation and had blood pressure below CVAR. The time course of CVAR after ROSC is unknown. Stroke research reports that impaired CVAR occurs during the acute phase of ischemia, whereas CVAR improves with time through the subacute phase. 29 Resuscitation research reports that CVAR improves over time in patients with improved consciousness. 30 This study suggests that non-CVAR causes two types of change: reversible change (such as “stunning”) and irreversible change (impairment). If future research can clearly distinguish between these two types of change, then earlier therapeutic intervention will be possible.
In HIBI, MAP and crSO2 have been reported to vary among patients, 31 suggesting that the optimal MAP for cerebral perfusion varies among patients and that MAP and crSO2 alone may be insufficient to assess cerebral oxygenation. The evaluation of CVAR using the Cox PH model may be a better index for assessing cerebral circulation post-CA because it considers two factors, MAP and crSO2, and it can also be monitored non-invasively and continuously. Sundgreen et al reported that CVAR was absent or shifted to the right side in most patients in the acute phase after CA. 9 In our study, 36% of the patients had a phase with undetectable CVAR after CA, which was similar to that found in previous studies. 16 The absence of CVAR in this study may indicate that the blood pressure was outside the optimal MAP range or that CVAR ability was lost. In either case, this absence reflects an inability to maintain optimal cerebral perfusion. Therefore, it makes sense that the higher the temporal exposure to undetectable CVAR, the poorer the neurological outcome, from a pathophysiological perspective. In line with the results of the current study, undetectable CVAR has been shown to correlate with unfavorable outcomes after traumatic brain damage 32 and subarachnoid hemorrhage. 33
In considering neurological outcome after ROSC, subgrouping based on the assessment of CVAR or non-CVAR may be reasonable in that it is based on cerebral circulation rather than solely on clinical information such as duration of CA or initial waveform. New subgrouping can contribute to the identification of latent target populations with potential efficacy for TTM. If CVAR can be assessed in a shorter period and earlier phases, CVAR assessment may be helpful when considering indications for TTM. This study suggests that as non-CVAR time increases, mortality may increase, and the effect of TTM may decrease. Future studies must verify the clinical efficacy of aggressive management within ideal blood pressure ranges to reduce secondary disability and improve outcomes after CA.
This study has several limitations. First, this investigation is designed as observational research, and some limitations are generally inherent in this type of study. Because of methodological limitations in observational studies that may generate bias and confounders, inferences of causality are not reliable. Second, due to differences in patient characteristics and clinical protocols, the results of this study may not be completely generalizable to other medical practices. Third, factors such as underlying diseases and age may have led to the decline in cerebrovascular autoregulation function in some patients before cardiac arrest. However, this study targeted patients admitted after out-of-hospital cardiac arrest, and it was impossible to confirm the state of cerebrovascular autoregulation before cardiac arrest. Fourth, the study did not account for the potential influence of TTM and CO2 on cerebral circulation. Regarding CVAR during hypothermia therapy, different results have been reported in several studies, and there is no consensus. Some studies have reported that CVAR does not change during hypothermia therapy because changes in PaCO2 during therapy trigger a response that maintains cerebral perfusion.1–3 Conversely, other studies have reported that hypothermia therapy affects cerebral circulation. 4 Therefore, hypothermia therapy and PaCO2 may have influenced the study results. Fifth, the COx variable was calculated in a 300-s window, and the presence of CVAR was assessed at 12-h intervals; therefore, results would differ if the time window for COx or CVAR assessment is changed or redefined.
Sixth, due to the lack of evaluation of factors such as cerebral metabolic rate, the interpretation of the results of this study could potentially change if real-time monitoring that takes these factors into account becomes possible in the future. Finally, this study determines the presence or absence of CVAR based solely on COx. Although there is no established gold standard for evaluating the presence or absence of CVAR, it is desirable to assess it using multiple modalities. If the cases in this study were analyzed using different evaluation methods, the results might differ from our conclusions.
Conclusions
In HIBI, we found that mortality increased significantly with longer non-CVAR time within 96 h after ROSC. The results obtained from this study may be helpful for the creation of a new index for post-CA management based on cerebral circulation. This line of research would be promising because of the long-term advantages for patients who experience CA and consequent events.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231185658 for Association between time-dependent changes in cerebrovascular autoregulation after cardiac arrest and outcomes: A prospective cohort study by Jotaro Tachino, Yuta Nonomiya, Satsuki Taniuchi, Ayumi Shintani, Shunichiro Nakao, Ryosuke Takegawa, Tomoya Hirose, Tomohiko Sakai, Mitsuo Ohnishi, Takeshi Shimazu and Tadahiko Shiozaki in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors appreciate the technical advice provided by Haruo Yamamura, the developer of the near-infrared spectroscopy system (TOS-OR) used in this study. Moreover, the authors appreciate the contributions of the paramedics, nurses, and emergency physicians involved and the patients who cooperated in this study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI Grant (numbers JP20K17893 and JP19H03758).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: JT conceived and designed this study; he contributed to the acquisition, analysis, and interpretation of the data; further, he was responsible for drafting, editing, and submitting the manuscript. YN and ST played a major role in cleaning the data and interpreting the results. AS contributed to the study design, acquisition, analysis, and interpretation of the data, as well as the drafting of the manuscript. SN and RT played a significant role in the analysis of the data and helped to draft the manuscript. TH, TS, and MO had an influence on the interpretation of the data. TS provided general support for the study and helped interpret the results of the data. TS had a major influence on the interpretation of the data and critical appraisal of the manuscript. All authors contributed to the acquisition of data and reviewed, discussed, and approved the final manuscript.
ORCID iDs: Jotaro Tachino https://orcid.org/0000-0002-0423-7713
Yuta Nonomiya https://orcid.org/0000-0003-0511-0244
Supplementary material: Supplemental material for this article is available online.
References
- 1.Berdowski J, Berg RA, Tijssen JG, et al. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010; 81: 1479–1487. [DOI] [PubMed] [Google Scholar]
- 2.Grasner JT, Lefering R, Koster RW, et al. EuReCa ONE-27 nations, Europe ONE. ONE Registry: a Prospective One Month Analysis of out-of-Hospital Cardiac Arrest Outcomes in 27 Countries in Europe. Resuscitation 2016; 105: 188–195. [DOI] [PubMed] [Google Scholar]
- 3.Girotra S, van Diepen S, Nallamothu BK, et al. Regional variation in out-of-hospital cardiac arrest survival in the United States. Circulation 2016; 133: 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okubo M, Gibo K, Wallace DJ, et al. Regional variation in functional outcome after out-of-hospital cardiac arrest across 47 prefectures in Japan. Resuscitation 2018; 124: 21–28. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics – 2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 6.Lipton P. Ischemic cell death in brain neurons. Physiol Rev 1999; 79: 1431–1568. [DOI] [PubMed] [Google Scholar]
- 7.Madathil RJ, Hira RS, Stoeckl M, et al. Ischemia reperfusion injury as a modifiable therapeutic target for cardioprotection or neuroprotection in patients undergoing cardiopulmonary resuscitation. Resuscitation 2016; 105: 85–91. [DOI] [PubMed] [Google Scholar]
- 8.Bro-Jeppesen J, Johansson PI, Hassager C, et al. Endothelial activation/injury and associations with severity of post-cardiac arrest syndrome and mortality after out-of-hospital cardiac arrest. Resuscitation 2016; 107: 71–79. [DOI] [PubMed] [Google Scholar]
- 9.Sundgreen C, Larsen FS, Herzog TM, et al. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 2001; 32: 128–132. [DOI] [PubMed] [Google Scholar]
- 10.Ameloot K, Genbrugge C, Meex I, et al. Low hemoglobin levels are associated with lower cerebral saturations and poor outcome after cardiac arrest. Resuscitation 2015; 96: 280–286. [DOI] [PubMed] [Google Scholar]
- 11.Spindelboeck W, Schindler O, Moser A, et al. Increasing arterial oxygen partial pressure during cardiopulmonary resuscitation is associated with improved rates of hospital admission. Resuscitation 2013; 84: 770–775. [DOI] [PubMed] [Google Scholar]
- 12.Dell'Anna AM, Lamanna I, Vincent J-L, et al. How much oxygen adult card arrest? Crit Care 2014; 18: 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37: S186–S202. [DOI] [PubMed] [Google Scholar]
- 14.Roberts BW, Kilgannon JH, Chansky ME, et al. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation 2013; 127: 2107–2113. [DOI] [PubMed] [Google Scholar]
- 15.Genbrugge C, Eertmans W, Meex I, et al. What is the value of regional cerebral saturation in post-cardiac arrest patients? A prospective observational study. Crit Care 2016; 20: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 2007; 38: 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010; 41: 1951–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekhon MS, Smielewski P, Bhate TD, et al. Using the relationship between brain tissue regional saturation of oxygen and mean arterial pressure to determine the optimal mean arterial pressure in patients following cardiac arrest: a pilot proof-of-concept study. Resuscitation 2016; 106: 120–125. [DOI] [PubMed] [Google Scholar]
- 19.Van den Brule JMD, Van Der Hoeven JG, Hoedemaekers CWE. Cerebral perfusion and cerebral autoregulation after cardiac arrest. BioMed Res Int 2018; 2018: 4143636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham P, Bindra J, Chuan A, et al. Are changes in cerebrovascular autoregulation following cardiac arrest associated with neurological outcome? Results of a pilot study. Resuscitation 2015; 96: 192–198. [DOI] [PubMed] [Google Scholar]
- 21.Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020; 142: S366–S468. [DOI] [PubMed] [Google Scholar]
- 22.Soar J, Böttiger BW, Carli P, et al. European resuscitation council guidelines 2021: adult advanced life support. Resuscitation 2021; 161: 115–151. [DOI] [PubMed] [Google Scholar]
- 23.Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet 1975; 1: 480–484. [DOI] [PubMed] [Google Scholar]
- 24.Brain Resuscitation Clinical Trial I Study Group. Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. N Engl J Med 1986; 314: 397–403. [DOI] [PubMed] [Google Scholar]
- 25.Ono M, Zheng Y, Joshi B, et al. Validation of a stand-alone near-infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery. Anesth Analg 2013; 116: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 27.Ito N, Nishiyama K, Callaway CW, et al. Noninvasive regional cerebral oxygen saturation for neurological prognostication of patients with out-of-hospital cardiac arrest: a prospective multicenter observational study. Resuscitation 2014; 85: 778–784. [DOI] [PubMed] [Google Scholar]
- 28.Storm C, Leithner C, Krannich A, et al. Regional cerebral oxygen saturation after cardiac arrest in 60 patients – a prospective outcome study. Resuscitation 2014; 85: 1037–1041. [DOI] [PubMed] [Google Scholar]
- 29.Petersen NH, Ortega-Gutierrez S, Reccius A, et al. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc Dis 2015; 39: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balu R, Rajagopalan S, Baghshomali S, et al. Cerebrovascular pressure reactivity and intracranial pressure are associated with neurologic outcome after hypoxic-ischemic brain injury. Resuscitation 2021; 164: 114–121. [DOI] [PubMed] [Google Scholar]
- 31.Sekhon MS, Gooderham P, Menon DK, et al. The burden of brain hypoxia and optimal mean arterial pressure in patients with hypoxic ischemic brain injury after cardiac arrest. Crit Care Med 2019; 47: 960–969. [DOI] [PubMed] [Google Scholar]
- 32.Czosnyka M, Smielewski P, Kirkpatrick P, et al. Monitoring of cerebral autoregulation in head-injured patients. Stroke 1996; 27: 1829–1834. [DOI] [PubMed] [Google Scholar]
- 33.Soehle M, Czosnyka M, Pickard JD, et al. Continuous assessment of cerebral autoregulation in subarachnoid hemorrhage. Anesth Analg 2004; 98: 1133–1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231185658 for Association between time-dependent changes in cerebrovascular autoregulation after cardiac arrest and outcomes: A prospective cohort study by Jotaro Tachino, Yuta Nonomiya, Satsuki Taniuchi, Ayumi Shintani, Shunichiro Nakao, Ryosuke Takegawa, Tomoya Hirose, Tomohiko Sakai, Mitsuo Ohnishi, Takeshi Shimazu and Tadahiko Shiozaki in Journal of Cerebral Blood Flow & Metabolism