Abstract
Neurologic outcome from out-of-hospital pediatric cardiac arrest remains poor. Although therapeutic hypothermia has been attempted in this patient population, a beneficial effect has yet to be demonstrated, possibly because of the delay in achieving target temperature. To minimize this delay, we developed a simple technique of transnasal cooling. Air at ambient temperature is passed through standard nasal cannula with an open mouth to produce evaporative cooling of the nasal passages. We evaluated efficacy of brain cooling with different airflows in different size piglets. Brain temperature decreased by 3°C within 25 minutes with nasal airflow rates of 16, 32, and 16 L/min in 1.8-, 4-, and 15-kg piglets, respectively, whereas rectal temperature lagged brain temperature. No substantial spatial temperature gradients were seen along the neuroaxis, suggesting that heat transfer is via blood convection. The evaporative cooling did not reduce nasal turbinate blood flow or sagittal sinus oxygenation. The rapid and selective brain cooling indicates a high humidifying capacity of the nasal turbinates is present early in life. Because of its simplicity, portability, and low cost, transnasal cooling potentially could be deployed in the field for early initiation of brain cooling prior to maintenance with standard surface cooling after pediatric cardiac arrest.
Keywords: Blood flow, high nasal airflow, hypothermia, pediatric, piglet
Introduction
Approximately 16,000 children suffer a cardiac arrest annually in the US, and many suffer permanent neurologic injuries. 1 Improvements in emergency medical team (EMT) response times, access to automatic external defibrillators, and public education programs have led to some increases in resuscitation rates and survival to hospital discharge; however, good neurological outcome occurs in only a small fraction of out-of-hospital arrest victims.2–4 Return of spontaneous circulation (ROSC), hospital discharge rate, and neurologic recovery remain poor, particularly for out-of-hospital pediatric cardiac arrests. For out-of-hospital adult cardiac arrest victims5,6 and newborns with evidence of hypoxic-ischemic (HI) encephalopathy (HIE),7–9 therapeutic hypothermia (HT) has been found to improve neurologic outcome and is currently the only therapy. For pediatric cardiac arrest, a small retrospective study from Taiwan of in- and out-of-hospital arrest reported a significant improvement in mortality with induction of HT. 10 The Therapeutic Hypothermia After Pediatric Cardiac Arrest randomized controlled trial was powered to detect a 20% effect size, and the observed 9% effect on improved 1-year survival and 8% effect on improved behavior outcome was not significant. 11 However, HT was started at a median of 5.9 h (5.2–6.7 IQR) after ROSC and an additional 4 h was needed to achieve a target temperature of 33°C. Even in the positive neonatal and adult HT trials, about half of the survivors had unfavorable neurologic outcome.12,13
Persistent neurologic deficits are due, in part, to the severity and duration of the insult and the delay in initiating HT. The latter is a major and addressable factor influencing outcome. The delay in achieving the target temperature is due to the time for transport to the hospital with or without active cooling, the delay in initiating in-hospital cooling, and the long time-constants for cooling the entire body mass. In HIE newborns, those treated with hypothermia starting within 3 hours of birth had better motor outcome than those in which treatment started between 3–6 hours, 14 and delaying HT beyond 6 hours no longer provided a definitive improvement in outcome. 15 The concept that “time is brain” with regard to HT is also supported by studies in animals that demonstrate greatly diminished neuroprotection when HT is delayed by 3–6 h.16–18 Thus, a critical need exists to find a safe and simple means to induce hypothermia rapidly in the field and emergency room for children who experience cardiac arrest.
A variety of alternative cooling methods, such as those utilizing catheters perfused with cold fluids and intranasal high flow with perfluorocarbon spray, have been investigated in adults.19,20 Chava et al. 21 has devised a new technology for rapid and preferential brain cooling after cardiopulmonary resuscitation (CPR) that is simple to implement in the field by EMTs and paramedics and in the emergency room in adult cardiac arrest victims. The new technique of transnasal cooling involves blowing dry air at ambient temperature through the nostrils with standard nasal cannula and letting the air escape passively through the mouth. This technique can induce selective brain cooling in rats 22 and adult pigs23,24; in human adults, it can decrease esophageal temperature 25 and treat fever in patients with neurologic injury. 26 Work on 30–40-kg pigs shows that evaporative cooling of the nasal epithelia cools the brain faster than the rest of the body and faster than standard whole-body surface cooling.21,23 Accelerating the rate of selective brain cooling, together with rapid initiation in the field, should enhance the efficacy of therapeutic HT, which then can be sustained at later times with standard cooling blankets.
The nasal turbinates possess a high capacity for humidifying air by evaporation, which cools the nasal mucosa and its venous blood drainage.27,28 The admixture of this venous blood with other cephalic venous drainage will result in a lower cephalic venous temperature than incoming arterial blood, setting up a standing temperature gradient for heat exchange from cephalic arteries to cephalic veins, especially at high nasal airflow. 21 If this convective heat loss mechanism plays a role in brain cooling, then we would predict uniform cooling among brain regions. On the other hand, if brain cooling is via heat loss conduction through bone and tissue, brain cooling would be non-uniform.
For application in pediatrics, one needs to consider several developmental factors affecting heat exchange. As the nose enlarges during development, nasal turbinate surface area increases and nasal airway resistance decreases 29 to match the age-dependent increase in basal minute ventilation and the dynamic range required for high levels of exercise as children gain increased mobility. However, neonates and infants do not require such a large dynamic range of humidification capacity. Thus, neonates might have less capacity to humidify as airflow increases. Other developmental factors that could affect the rate of brain cooling are the size of the brain, cerebral blood flow, and cerebral metabolic heat production resulting from developmental changes in energy production. 30 Before application to pediatric CPR, it is important to determine efficacy of transnasal cooling of brain in different age groups in an animal model of comparable mass to neonates, infants and young children who are most vulnerable to cardiac arrest among the pediatric population. 2 We chose piglets as a pediatric animal model because their body mass, brain mass, and cerebral blood flow scales more closely to these parameters in humans during development than rodent models.
The objective of this study was to determine efficacy of selective brain cooling over a range of transnasal airflow with developmental increases in body mass, brain mass, and nasal turbinate surface area. The specific hypotheses tested were: 1) high nasal flow of dry ambient-temperature air through standard nasal cannula in piglets produces sufficient evaporative cooling to cool the brain in an airflow-dose-dependent fashion in each age group, 2) the brain is cooled faster than the body, and 3) the brain is cooled uniformly with no major regional differences in temperature. We also evaluated whether the rate of cooling increases with developmental increases in the nasal turbinate surface area available for humidification and thus evaporative cooling of the nasal blood. Because cooling typically reduces blood flow in many organs, we determined whether nasal cooling decreases blood flow to the turbinates, which could limit the efficacy of the transnasal cooling procedure.
Methods
All procedures received prior approval by the Johns Hopkins University Animal Care and Use Committee and followed the NIH guidelines for animal experimentation. Experiments were performed in compliance with the ARRIVE guidelines. Male and female piglets were studied at ages of approximately 3 days (∼1.8 kg body weight; ∼38 g brain weight), 2 weeks (∼4 kg body weight; ∼41 g brain weight), and 6 weeks (∼15 kg body weight; ∼54 g brain weight; adult pig brain ∼110 g). Anesthesia was induced with 5% isoflurane in a 70%/30% nitrous oxide/oxygen mixture delivered via face mask. For the 15-kg piglets, a pre-anesthetic dose of 2 mg/kg ketamine was injected intramuscularly to provide sedation before administering gas anesthesia. Piglets were orally intubated and the lungs were mechanically ventilated with 1.5–2% isoflurane in a 70%/30% nitrous oxide/oxygen mixture. Pulse oximetry and end-tidal CO2 monitoring were used to assure normoxia and normocarbia. A temperature probe was inserted into the rectum and the piglets were placed on a warming blanket to maintain rectal temperature at 38.5°C (normal for swine) during surgery. A catheter was inserted through a femoral artery into the abdominal aorta for monitoring arterial pressure and blood gases. A catheter was inserted through a femoral vein into the inferior vena cava for infusion of a solution of 5% dextrose and 0.45% saline at a rate of 4 mL/kg/h. Fentanyl was infused intravenously with a loading dose of 20 µg/kg and a maintenance dose of 20 µg/kg/h to suppress shivering during induced hypothermia. Blood pH, PCO2, PO2, hemoglobin concentration, and O2 saturation were measured with a blood gas analyzer (Radiometer ABL Flex 800, Brea, California) with values corrected for body temperature.
Effect of nasal airflow on regional brain temperature
To measure regional brain temperature, thermistor probes were placed in five separate regions of the brain through 1-mm drill holes in the skull. The fine-wire probes were inserted with stereotaxic guidance into olfactory bulb, parasagittal parietal cortex, putamen, thalamus, and cerebellum based on the swine brain atlas.31,32 The scalp incisions were closed to restore its thermal insulative properties.
A standard nasal cannula was inserted bilaterally into each nostril of the pig. Tape was wrapped around the cannula to ensure a snug fit in the nostril. The cannula was connected to a pressurized source of air with a flowmeter in the circuit. Baseline measurements were made of rectal temperature, regional brain temperatures, and mean arterial blood pressure (MABP). An arterial blood sample was obtained for measurement of temperature-corrected pH and blood gases. Airflow through the nasal cannula then commenced at a preset rate. The mouth was kept open to provide an exit for airflow. Temperature and MABP measurements were recorded at 15-min interval for the first 2 hours of cooling and then at 30-min intervals for the next 2 hours of cooling. Arterial blood gases were measured hourly. As brain temperature approached 34°C (a drop of approximately 4.5°C), the airflow rate was reduced to maintain a steady state temperature of approximately 33–34°C. At the end of the cooling period, the piglets were euthanized and the positions of the thermistors in the brain were verified.
Piglets in each age cohort were randomized to one of three airflow rates. In the 1.8-kg piglets, airflow rates of 4 L/min (n = 6), 8 L/min (n = 6), and 16 L/min (n = 6) were tested. In the 4-kg piglets, airflow rates of 16 L/min (n = 6), 32 L/min (n = 8), and 48 L/min (n = 6) were tested. In the 15-kg piglets, airflow rates of 16 L/min (n = 7), 32 L/min (n = 7), and 48 L/min (n = 7) were tested. Animals with instrumentation technical failures were excluded; all animals that completed the protocol were included.
Effect of high nasal airflow on nasal turbinate blood flow
Nasal turbinate blood flow was measured with the microsphere technique 33 in which 15 ± 2 µm diameter spheres labeled with four different lanthanide elements were injected into the left atrium at four different time points. To implant a left atrial catheter, mechanical ventilation was employed with 5 cm water positive end-expiratory pressure, and a left thoracotomy was performed at the third interspace. The ribs were retracted, an incision was made in the pericardium, and the appendage of the left atrium was temporarily cross-clamped and incised. A catheter was inserted into the left atrium and the incision was closed with a purse string stitch. The ribs were apposed and closed with suture. A few sigh breaths were administered to assure re-inflation of the lungs, and the muscle and skin were sutured closed in layers. Next, the sagittal sinus was catheterized for obtaining cerebral venous blood samples to measure cerebral O2 extraction. A midline incision was made in the scalp and a drill hole was made in the skull between bregma and lamba landmarks to expose the dura mater above the sagittal sinus. A catheter was inserted caudally into the sagittal sinus to obtain cerebral venous blood samples for measuring O2 content.
For each blood flow measurement, a dose of approximately 1 × 106 spheres in 4-kg piglets and 3 × 106 spheres in 15-kg piglets were injected over approximately 10 s into the left atrium to obtain good mixing within the left ventricle before ejection of well-mixed blood into the aorta. During the microsphere injection and for 1-min after flushing the injection catheter with saline, arterial blood was withdrawn at a constant rate into a glass syringe with a pump to obtain the arterial input function of the microsphere concentration for calibrating tissue blood flow. The blood withdrawal rate was 5.1 ml/min, and the withdrawn volume was replaced with saline. This procedure was performed at baseline and at approximately 15, 30, and 60 min of 32 L/min nasal airflow with microspheres composed of a polystyrene matrix cross-linked with either lanthanum, lutetium, samarium, ytterbium, or gold. Before each sphere injection, blood samples were drawn from the femoral artery and sagittal sinus to measure the arteriovenous O2 content difference for calculating the cerebral metabolic rate of O2 (CMRO2). After the last injection, the pig was euthanized and tissue was harvested, weighed, dried. Samples included the full length of the nasal turbinates, samples from brain (frontal, parietal, temporal and occipital cortex, olfactory bulb, caudate-putamen, thalamus, cerebellum, and brainstem) and left cardiac ventricular muscle. The dried samples were shipped to BioPhysics Assay Laboratory, Inc. (Worcester MA) for neutron activation of the metals to generate stable radioactive isotopes that were then assayed by spectrographic scintillation counting. The ratio of the disintegrations per minute in the tissue sample to the disintegrations per minute in the arterial blood withdrawal sample was calculated and multiplied by the 5.1 ml/min withdrawal rate to obtain tissue blood flow. The blood flow was normalized by the tissue wet weight.
Nasal turbinate surface area and air space volume
At autopsy, the nose of the pig was cut serially in cross-section at 1 cm intervals. The anterior and posterior views of each section was photographed. Using Image J software, the internal perimeter of the nasal turbinate was traced on the anterior and posterior view of each section and averaged. To obtain the nasal turbinate surface area, the average perimeter for each section was multiplied by the section thickness and summed across all sections. To obtain the air space volume, the area within the traced perimeter of the nasal turbinate was measured and averaged from the anterior and posterior views. The average area for each section was multiplied by the section thickness and the air spaced volume was summed across all sections.
Statistical analysis
Analysis of variance (ANOVA) was used on data that passed the Shapiro-Wilk test for normality. To compare temperature among brain regions for a specific nasal airflow in each age group, a 2-way repeated measures ANOVA was performed where brain region and time were within-subject factors. If there was a significant effect of temperature site or a significant interaction of temperature site and time, then post hoc comparisons were made among the 5 brain regions brain at each time point with the Holm-Sidak procedure for multiple comparisons.
To compare the time course of brain temperature with rectal temperature, the temperature readings from the five brain regions were averaged, and a 2-way repeated measures ANOVA was performed where brain/rectal temperature was one within-subject factor and time was a second within-subject factor. If there was a significant effect of temperature site or a significant interaction of temperature site and time, then post hoc comparisons were made among the regional average brain temperature and rectal temperature at each time point with the Holm-Sidak procedure for multiple comparisons.
To compare the average of the regional brain temperatures among the same age groups with different nasal airflow rates, a 2-way ANOVA was performed where airflow rate was a between-subject factor and time was a within-subject factor. If there was a significant effect of airflow rate or a significant interaction of airflow rate and time, then post hoc comparisons were made between airflow rate groups at each time point with the Holm-Sidak procedure for multiple comparisons.
Within each age group, the time at which temperature decreased 3°C from baseline was compared among groups with different nasal airflow rate by the Kruskal-Wallis analysis of ranks and the Dunn’s test for multiple comparisons.
For the microsphere-determined blood flow and blood gas measurements, a one-way repeated measures ANOVA was performed. If a significant effect was found, measurements during transnasal airflow were compared to baseline with the two-tail Dunnett’s test.
Results
Nasal turbinate development
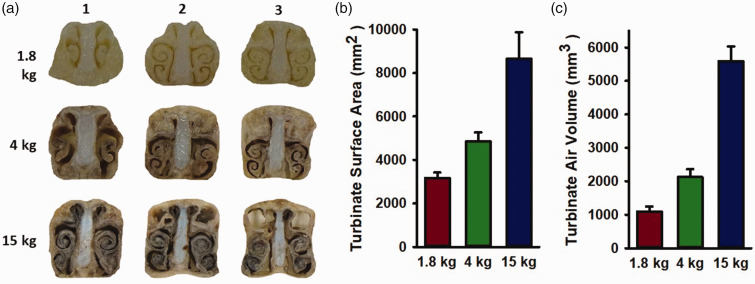
The structure of nasal turbinate increased in complexity during porcine development, as evident by the increase in the extent of spiral turns in the cross-section view (Figure 1(a)). The length of the air-turbinate interface and the cross-sectional area of the air space were traced on each 1 cm-thick cross-section and summed for all cross-sections to calculate the surface area of the air-turbinate interface and the volume of the air space, respectively. As shown in Figure 1(b), both the surface area and air volume increased progressively with body weight.
Figure 1.
Nasal turbinates increase in complexity with age, resulting in increased surface area and air volume for humidification. (a) Examples of turbinate cross-sections along the nasal axis of a 1.8, 4, and 15-kg piglet and (b, c) Turbinate surface area and air space volume derived from tracing the perimeter of the turbinate and the area within the perimeter summed across consecutive 1-cm cross sections. Values are means ± SD; n = 5 per group.
Regional brain temperature during transnasal airflow
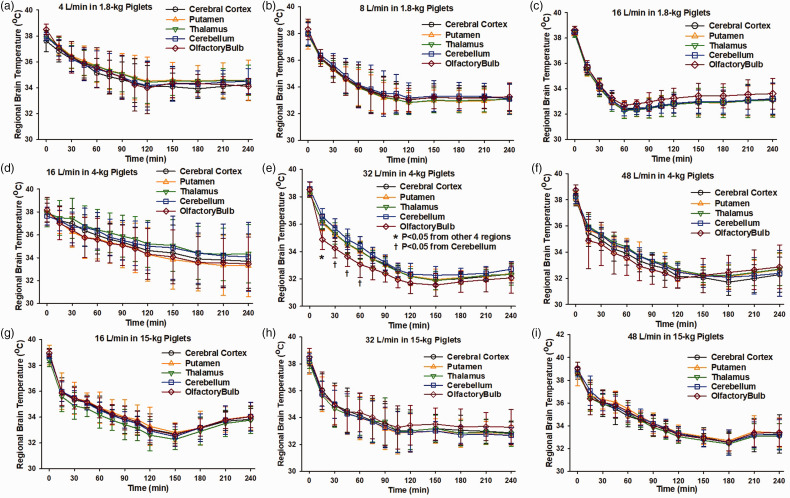
Brain temperature was recorded in parietal cerebral cortex, putamen, thalamus, cerebellum, and olfactory bulb to determine whether a temperature gradient developed during high transnasal airflow. Temperature rapidly decreased in all five well-separated brain regions in all age groups (Figure 2). Furthermore, for each airflow setting the temporal trajectory essentially overlapped among the five regions. The only exception was in the case of 32 L/min in the 4-kg group, where two-way ANOVA revealed significant interactions between brain region and duration of nasal airflow and the Holm-Sidak procedure indicated a lower temperature in the olfactory bulb during the first hour of airflow (Figure 2(e)).
Figure 2.
At a given airflow, temperature fell at similar rates among 5 brain regions in each age group, indicating no spatial gradient. Temperature was measured in parietal cortex, putamen, thalamus, cerebellum, and olfactory bulb during high nasal airflow of 4 L/min (a, n = 6), 8 L/min (b, n = 6), and 16 L/min (c, n = 6) in 1.8-kg piglets, of 16 L/min (d, n = 6), 32 L/min (e, n = 8), and 48 L/min (f, n = 6) L/min in 4-kg piglets, and 16 L/min (g, n = 7), 32 L/min (h, n = 7), 48 L/min (i, n = 7) in 15-kg piglets. Values are means ± SD; *P < 0.05 between olfactory bulb and other 4 regions, †P < 0.05 between olfactory bulb and cerebellum by repeated measures ANOVA and the Holm-Sidak procedure for multiple comparisons at each time point.
Effect of nasal airflow rate on brain cooling
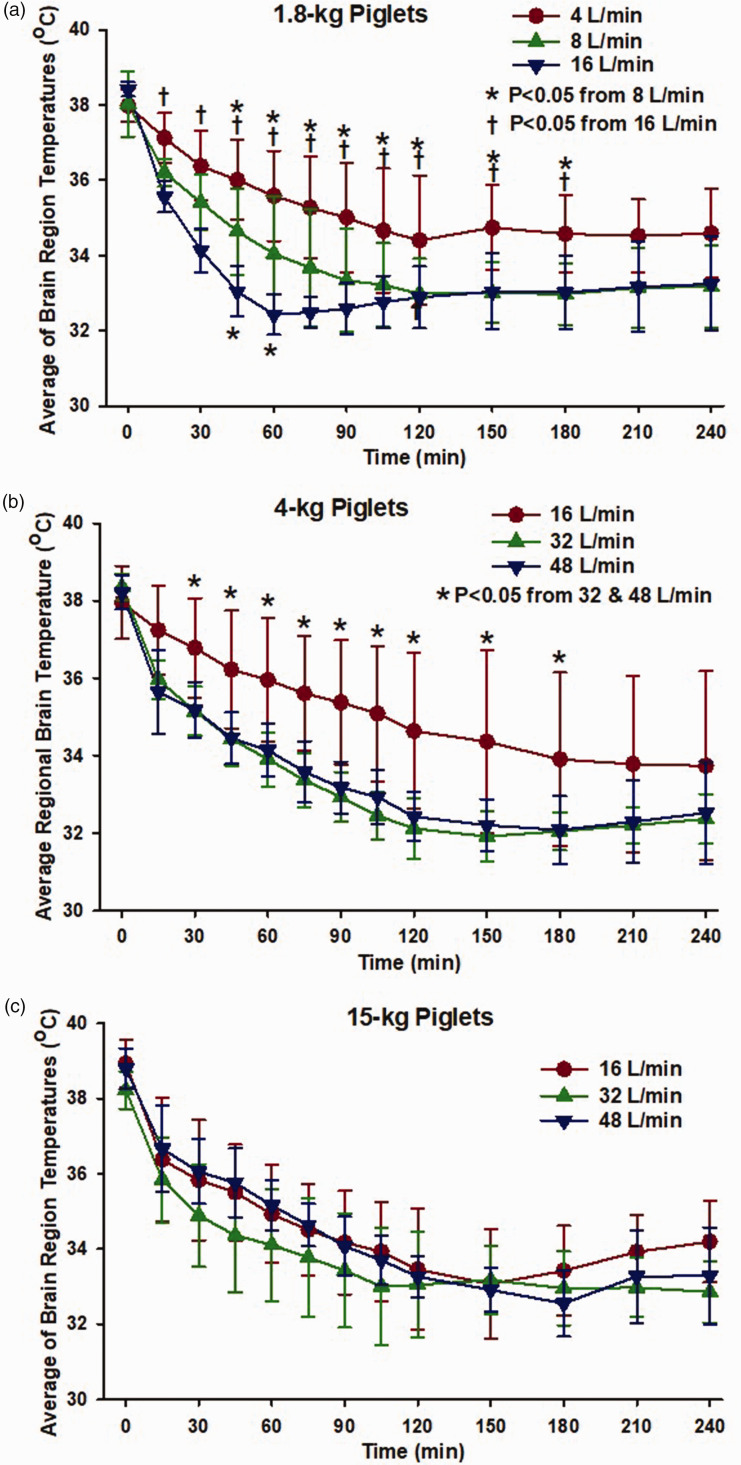
Because no substantial regional differences in brain temperature were evident, the temperatures of the five regions were averaged for further analysis of effects of airflow rate. In the 1.8-kg piglets, airflow rates of 4, 8, and 16 L/min all produced reductions in the average brain temperature (Figure 3(a)). An airflow rate of 8 L/min produced lower brain temperature than a rate of 4 L/min, with significant differences attained by 45 minutes of nasal airflow. Increasing the airflow rate to 16 L/min further enhanced the early drop in brain temperature; significant differences from the 4 L/min group were attained by 15 minutes and significant differences from the 8 L/min group were attained by 45 minutes of nasal airflow.
Figure 3.
Dependence of rate of brain cooling on nasal airflow rate. (a) In 1.8-kg piglets, brain temperature (averaged across 5 regions) fell more rapidly by increasing the airflow rate from 4 L/min (n = 6) to 8 L/min (n = 6) and from 8 L/min to 16 L/min (n = 6); *P < 0.05 from 8 L/min, †P < 0.05 from 16 L/min. (b) In 4-kg piglets, brain temperature fell more rapidly with airflow rates of 32 L/min (n = 8) or 48 L/min (n = 6) than with 16 L/min (n = 6); *P < 0.05 from 32 and 48 L/min and (c) In 15-kg piglets, airflow had no differential effect between 16, 32 and 48 L/min (n = 7 per group). Values are means ± SD; data analyzed with two-way ANOVA and Holm-Sidak post hoc comparisons.
In the 4-kg piglets, nasal airflow rates of 16, 32, and 48 L/min were tested (Figure 3(b)). With a rate of 32 L/min, average brain temperature fell more quickly than with a rate of 16 L/min, and significant differences were attained by 30 minutes of nasal airflow. However, increasing the rate to 48 L/min did not further accelerate the rate of brain cooling.
In the 15-kg piglets, increasing the nasal airflow rate from 16 to 32 L/min tended to accelerate brain cooling, but the differences in brain temperature did not attain statistical significance at any time point. Moreover, further increasing airflow rate to 48 L/min did not provide any additional enhancement of brain cooling.
Comparison of brain and rectal temperatures
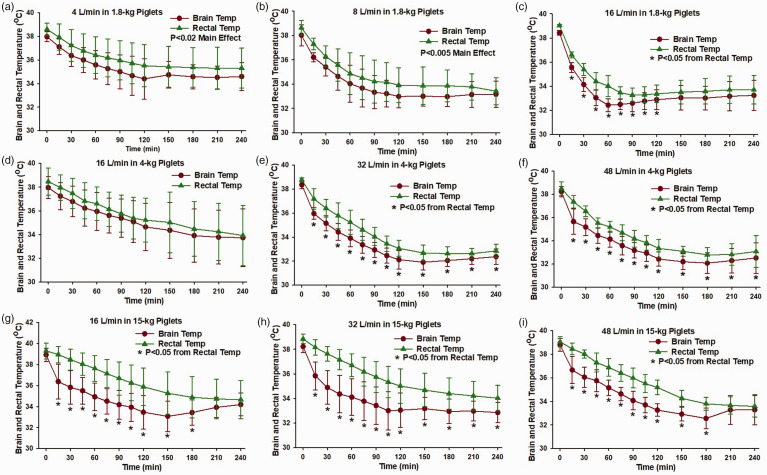
Figure 4 compares the time course of brain and rectal temperatures at each of the nasal airflow rates in each size group. In the 1.8-kg piglets, 2-way repeated measures ANOVA indicated that brain temperature was significantly lower than rectal temperature throughout the course of nasal airflow at a rate of 16 L/min. In 4-kg piglets, brain temperature was significantly lower than rectal temperature with airflow rates of 32 and 48 L/min. In 15-kg piglets, brain temperature was significantly lower than rectal temperature with airflow rates of 16, 32, and 48 L/min. Thus, the airflow rates that produced the most rapid rates of brain cooling in each age group were the rates that produced significant differences between brain and rectal temperatures during the cooling process.
Figure 4.
Comparison of brain and rectal temperature. Brain temperature (averaged across 5 regions) fell faster than rectal temperature in 1.8-kg piglets with 16 L/min nasal airflow (c. n = 6), in 4-kg piglets with 32 L/min (e. n = 8) and 48 L/min (f. n = 6) nasal airflow, and in 15-kg piglets with 16 L/min (g. n = 7), 32 L/min (h. n = 7), and 48 L/min (i. n = 7) nasal airflow. Differences between brain and rectal temperature were not significant in 1.8-kg piglets with 4 L/min (a. n = 6) or 8 L/min (b. n = 6) nasal airflow or in 4-kg piglets with 16 L/min (D. n = 6) nasal airflow. *P < 0.05 from rectal temperature at each time point by paired analysis with two-way repeated measures ANOVA and Holm-Sidak post hoc comparisons; Values are means ± SD.
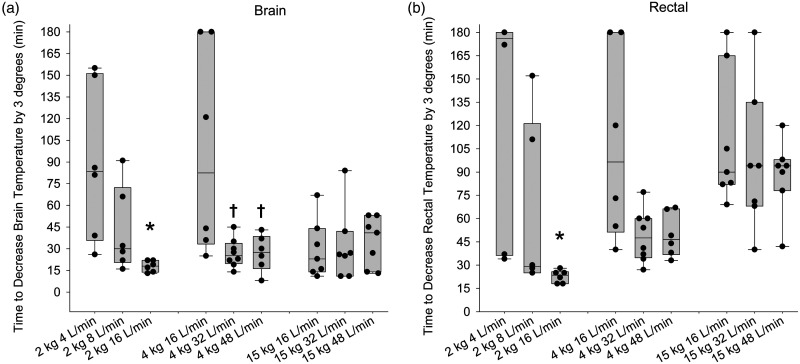
To better quantitate the rate of cooling, we measured the time required to decrease the average regional brain temperature and rectal temperature by 3°C from the baseline value for each piglet. Individual data together with medians and quartiles are shown for each group in Figure 5. In the 1.8 kg piglets, the time to reduce brain and rectal temperature by 3°C was significantly less at an airflow of 16 L/min than the respective times at 4 L/min. In the 4-kg piglets, the time to cool the brain by 3°C was significantly quicker at 32 L/min than at 16 L/min, and the time at 48 L/min did not differ from that at 32 L/min. The median time to decrease rectal temperature by 3°C was numerically less with 32 and 48 L/min than with 16 L/min, although the differences were not statistically different. In the 15-kg piglets, no significant differences were seen in the brain cooling time with different airflow rates, and the times were similar to the optimal cooling times obtained in the 1.8-kg and 4-kg groups. The rectal temperature cooling times also did not differ with different airflow rates in the 15-kg piglets, although the cooling times were more than doubled the time to cool the brain by 3°C. This body size-dependent difference between brain and rectal temperature cooling rates was not unexpected because body mass increases disproportionately faster than brain mass at this stage of development (58-g brain in 15-kg piglets vs. 41-g brain in 4-kg-piglets).
Figure 5.
Dependence of rate of cooling on nasal airflow rate. Box-whisker plots display the duration of nasal airflow at which brain temperature (a) and rectal temperature (b) decreased by 3°C from baseline in 1.8-kg piglets with airflows of 4, 8, or 16 L/min and in 4-kg and 15-kg piglets with airflows of 16, 32, or 48 L/min. *P < 0.05 from 1.8-kg piglets with 4 L/min; †P < 0.05 from 4-kg piglets with 16 L/min airflow by analysis of ranks.
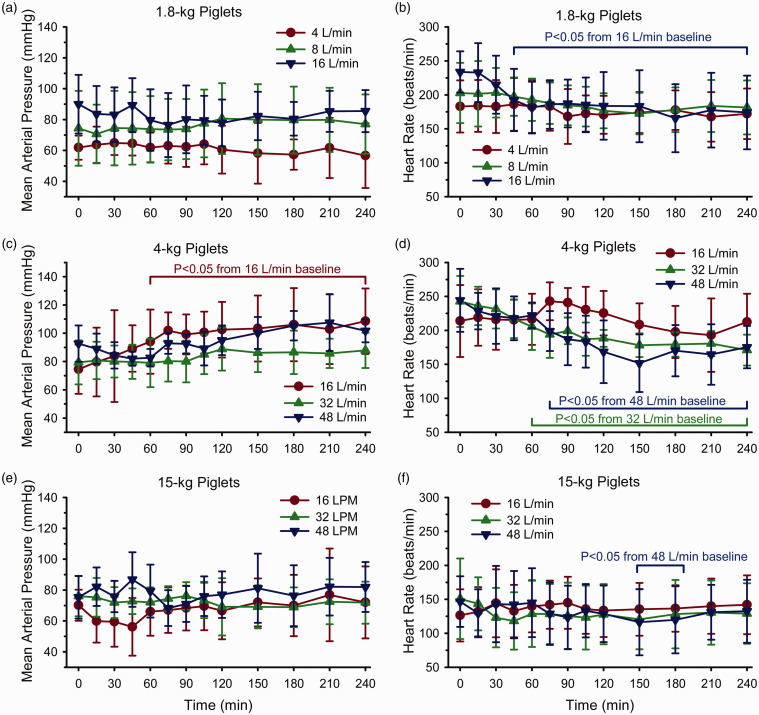
Arterial pH and PCO2 remained in the physiologic range during high nasal airflow (Supplemental Table 1). Arterial PO2 remained above 100 mmHg with the use of approximately 30% inspired O2 (balance N2O). In most groups, mean arterial blood pressure did not significantly change from baseline during the 4-hour period of cooling (Figure 6). The exception was in the 4-kg piglet group where blood pressure significantly increased after 60 minutes of cooling. We do not have an explanation for the approximately 20 mmHg increase in this group. Several groups had persistent decreases in heart rate after 45–75 minutes of cooling, namely the 1.8-kg piglets with 16 L/min airflow and the 4-kg piglets with 32 and 48 L/min airflow. In 15-kg piglets, significant decreases in heart rate occurred only at 150–180 minutes of cooling with 48 L/min. It is noteworthy that these 4 groups had the most consistent rapid rate of cooling of rectal temperature (Figure 5), suggesting that cardiac deceleration was related to the decrease in core and myocardial temperature.
Figure 6.
Mean arterial pressure (Left) and heart rate (Right) in 1.8-kg piglets (a, b), 4-kg-piglets (c, d), and 15-kg piglets (e, f) during the 4-hour cooling period. Brackets show range of times when values differed significantly from baseline by one-way repeated measures ANOVA and the Dunnett’s test for multiple comparison to baseline. Values are means ± SD.
Blood flow
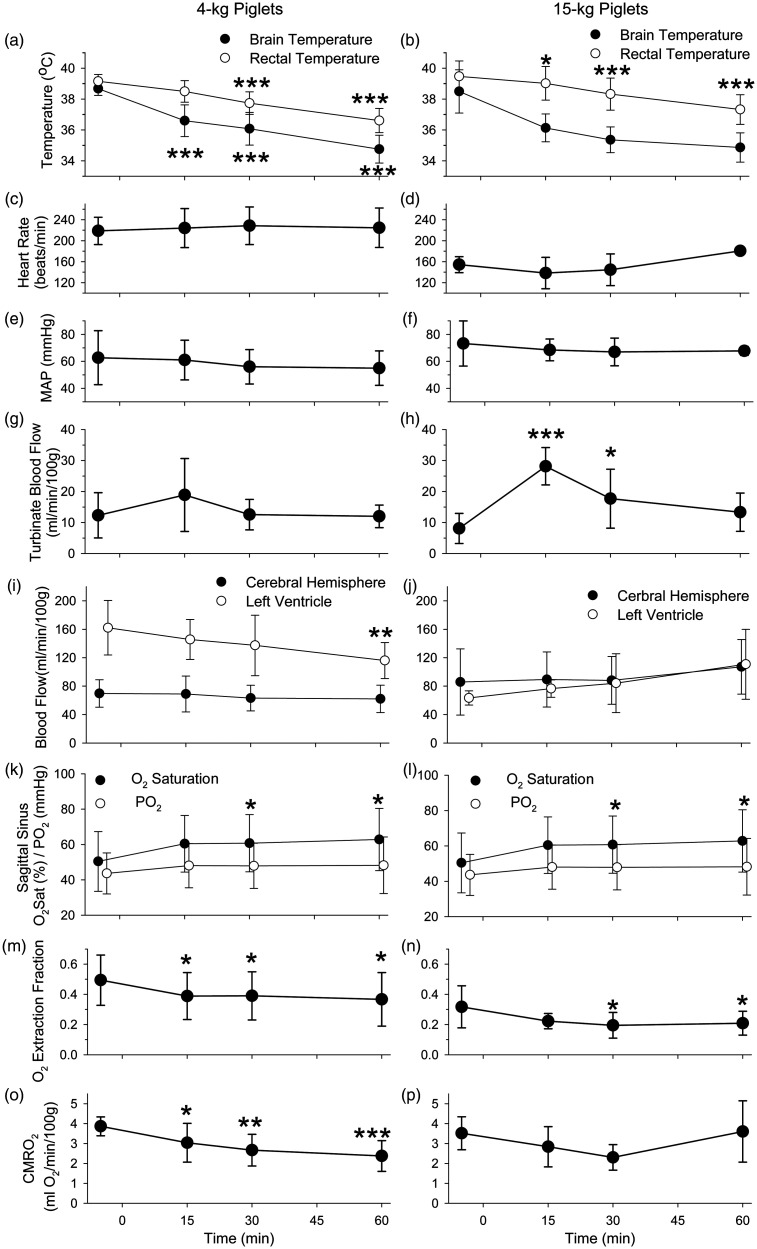
Regional blood flow was measured with left atrial injection of microspheres at baseline and at 15, 30, and 60 minutes of induction of nasal airflow at a rate of 32 L/min in 4-kg and 15-kg piglets. These piglets, which had earlier undergone a thoracotomy to implant a left atrial catheter and then had their chest wall closed, were well oxygenated during the blood flow measurements. Arterial PCO2 was unchanged during the high nasal airflow and was accompanied by a small degree of acidosis (Supplemental Table 2). Similar to the previous cohorts, these piglets exhibited rapid drops in brain temperature and slower decreases in rectal temperature during high nasal flow (Figure 7(a) and (b)). Heart rate and mean arterial blood pressure remained unchanged over the 60-minute period of nasal airflow (Figure 7(c) to (f)). Baseline blood flow to the nasal turbinates averaged 12 ± 7 ml/min/100 g of tissue in the 4-kg piglets (n = 5) and 8 ± 5 ml/min/100 g in the 15-kg piglets (n = 5). During high nasal flow, turbinate blood flow did not significantly change in the 4-kg piglets but transiently tripled in the 15-kg (Figure 7(g) and (h)). In neither group did turbinate blood flow decrease below baseline at any time point during transnasal cooling.
Figure 7.
Turbinate blood flow measured with microspheres did not decrease during evaporative cooling with nasal airflow of 32 L/min. Physiological parameters displayed for 4-kg piglets (Left) and 15-kg piglets (Right) (means ± SD; n = 5) include brain and rectal temperature (a, b), heart rate (c, d), mean arterial pressure (MAP; e, f), turbinate blood flow (g, h), blood flow to the cerebral hemispheres and left cardiac ventricle (i, j), sagittal sinus O2 saturation and PO2 (k, l), cerebral oxygen extraction fraction (m, n), and CMRO2 (o, p). *P < 0.05, **P < 0.01, ***P < 0.001 from baseline by one-way repeated measures ANOVA and the Dunnett’s test for multiple comparison to baseline.
Left ventricular myocardial blood flow decreased at 60 minutes of high nasal airflow in the 4-kg piglets but was not significantly altered in the 15-kg group (Figure 7(i) and (j)). Interestingly, cerebral blood flow (CBF) was not significantly changed in either age group despite brain cooling. Blood hemoglobin concentration did not change and arterial PO2 remained >100 mmHg (Supplemental Table 2), but cerebral venous O2 saturation, measured in sagittal sinus blood samples, increased during cooling in both groups (Figure 7(k) and (l)). However, the increase in venous O2 saturation was not associated with a significant increase in venous PO2 (Figure 7(k) and (l)), presumably because the decrease in temperature increased the affinity of O2 with hemoglobin (leftward shift of the oxyhemoglobin dissociation curve). As a result of the increase in venous O2 saturation, the arterial–venous O2 content difference across the brain significantly decreased (Supplemental Table 2) as did the O2 extraction fraction (Figure 7(m) and (n)). In the case of the 4-kg piglets, the calculated CMRO2 significantly decreased during brain cooling, whereas the decrease in the 15-kg piglets did not attain statistical significance (Figure 7(o) and (p)).
Discussion
Previous work in adult animals and humans demonstrated the capability of implementing high nasal airflow to rapidly induce brain cooling.21,23,25 However, the feasibility of applying this technique in pediatrics has not been explored. Here, we used three different size piglets that scale from human newborns to toddlers. The major findings are 1) high nasal airflow is capable of rapidly decreasing brain temperature by 3°C within 30 minutes at rates of 16 L/min in 1.8-kg piglets, 32–48 L/min in 4-kg piglets, and 16–32 L/min in 15-kg piglets, 2) the rate of brain cooling exceeded the rate of body cooling at nasal airflow rates of 16, 32, and 16 L/min in the 1.8-, 4-, and 15-kg piglets, respectively, 3) significant temperature gradients were not evident along the rostral-caudal axis of the brain, and 4) high nasal airflow did not reduce blood flow to the nasal turbinates, thereby sustaining the return of cooled nasal venous blood.
The lack of substantial temperature gradients between cerebral cortex and subcortical structures and between olfactory bulb and cerebellum is consistent with heat loss mediated by convection through blood flow rather than by conduction across the base of the skull. With head cooling caps that are sometimes used for neonatal HIE, 8 in contrast, cooling of subcortical structures lags behind cooling of cerebral cortex. 34 The mechanism of transnasal cooling is based on humidification of inflowing air that produces evaporative cooling of the nasal mucosa and nasal mucosa venous blood. Nasal airflow-dependent decreases in jugular venous blood and carotid arterial blood can be detected in 30-kg pigs, 21 supporting the concept that the drainage of cooled nasal mucosa blood into the jugular vein cools the surrounding tissue and the nearby carotid artery that then cools the brain faster than non-cephalic regions.
The rate of cooling of nasal turbinate venous blood will depend on the air flow rate, the nasal mucosa surface area, and the blood flow through the nasal mucosa. Physiologically, the capacity to humidify the air extends beyond basal levels of ventilation to maintain humidification during exercise.27,35 In the case of swine development, piglets are fairly sedentary during the first few weeks of nursing after birth and then become more physically active during the period of weaning (approximately 4 weeks of age; 6 kg in Yorkshire piglets). Hence, an increase in humidification capacity is anticipated to match the increased ventilatory drive during exercise. In agreement with this concept, the observed age-dependent increase in nasal turbinate surface area allows for a greater humidification capacity. Moreover, the age-dependent increase in airspace volume and associated lumen cross-sectional area lowers airway resistance, accommodating increased airflow rates. Thus, we expected brain cooling to increase over a wide range of airflow well above basal ventilatory airflow rates. This expectation held in the 1.8-kg piglets where increasing airflow from 4 to 8 to 16 L/min produced progressive increases in the rate of brain cooling. In 4-kg piglets, the optimal airflow rate was 32 L/min, with 48 L/min producing no further benefit. The rate of cooling at 32 L/min in the 4-kg piglets was similar to that with 16 L/min in the 1.8 kg piglets (Figure 5). The 70% greater turbinate surface are in 4-kg piglets compared to 1.8-kg piglets roughly matches the doubling of the most effective airflow rate. However, the lack of difference in the rate of brain cooling with airflow rates ranging from 16 to 48 L/min in the 15-kg piglets was unexpected because previous work found that increasing nasal airflow from 20 to 40 to 80 L/min produced stepwise increases in the rate of brain cooling in 30-kg pigs. 21 Other developmental factors affecting heat transfer from the brain may come into play, such as heat exchange through a countercurrent exchange mechanism between cephalic arteries and veins, the pathway of venous drainage from the turbinates, and the ratio of cerebral blood flow to cerebral heat production. In humans, postnatal increases in cerebral blood flow are matched with postnatal increases in energy metabolism and presumably heat production, and this principle holds in other species during development. 30 Perhaps factors such as the distance between cephalic arteries and veins and their blood flow may become rate-limiting for heat transfer in the 15-kg piglets.
In 1.8-kg piglets, brain cooling was maximal at 16 L/min. We did not test whether higher airflow rates would produce faster brain cooling. In clinical practice, nasal airflow with supplemental oxygen rarely exceeds 4 L/min in neonates because very high flow rates might increase pharyngeal airway pressure and distend the stomach. With high nasal airflow, it is important to keep the mouth open and to not let the tongue obstruct the exit of airflow. It is noteworthy that increased pressure in the nasal airways has not been found to reduce humidification, 36 thereby permitting transnasal cooling to operate at the higher intranasal pressure induced by the high flow. Other potential adverse effects, such as rhinorrhea or hemorrhage were not observed.
Another factor that could limit heat transfer is blood flow through the nasal turbinates. In many tissues, decreases in temperature will reduce blood flow. Moreover, high airflow rates will increase nasal airway pressure and could compress nasal mucosal veins. However, the microsphere-based measurements of nasal turbinate blood flow did not show a reduction in blood flow. Indeed, the 15-kg piglets displayed an early increase in blood flow to the nasal turbinates, which may have contributed to the rapid decrease in brain temperature. In interpreting the lack of significant changes in blood flow in the 1.8-kg piglets, one needs to consider possible sources of error. The number of spheres summed over the entire nasal turbinate structure and in the reference blood sample was in the order of 104, which would contribute only ∼2% error based on the coefficient of variation for a Poisson distribution. 37 The blood withdrawal rate of 5.1 ml/min should be adequate to overcome any effect of microsphere axial streaming at the catheter tip withdrawal site. 38 Microsphere streaming within the turbinate microcirculation should not be a major factor since the entire turbinate structure was counted. Thus, much of the variability in the measurement is likely the result of biologic variability. The turbinates are highly vascularized with venous sinusoids whose drainage is controlled by downstream smooth muscle. 39 One factor contributing to biologic variability is the physiological status of arterio-venous shunts feeding into the venous sinusoids. 35 The fraction of 15 µm spheres passing through these shunts leads to an underestimation of the true blood flow. How this shunt fraction would change in the current protocol with high nasal airflow and cooling is unknown. Another factor is that the turbinate resistance and capacitance vessels are regulated by adrenergic, cholinergic, and peptidergic receptors, but how these systems are affected by high airflow and temperature is not well-defined. 27 With regard transnasal cooling implementation after resuscitation, the α-adrenergic agonists typically used during resuscitation could constrict nasal arterioles and thus could limit the efficacy of brain cooling by high nasal airflow. However, catecholamines are rapidly metabolized and nasal blood flow would likely be restored as long as exogenous catecholamine administration is avoided after return of spontaneous circulation.
Left ventricular myocardial blood flow was found to decrease by 60 minutes of high nasal airflow in the 4-kg piglets, at which time rectal temperature had decreased by approximately 2.5°C. The decrease in blood flow may have been coupled to a decrease myocardial O2 consumption associated with mild hypothermia. If there was a decrease in myocardial O2 consumption, it was not due to bradycardia or arterial hypotension, as heart rate and mean arterial pressure were unchanged over the 60-minute measurement period in this set of piglets. In the case of the 15-kg piglets, however, we do not have an explanation of why a similar drop in core temperature did not produce a decrease in left ventricular blood flow. Nevertheless, decreases in heart rate were observed in cohorts with a 4-hour measurement period as rectal temperature continued to fall, and a decrease in myocardial blood flow would be anticipated beyond 60 minutes.
Hypothermia decreases CMRO2, 40 and the associated decrease in mitochondrial electron transport rate is considered one of the factors involved in hypothermic neuroprotection. 41 Here, we found a 39% decrease in CMRO2 by 60 minutes of nasal airflow in the 4-kg piglets, at which time brain temperature had decreased by 4°C. A similarly large decrement of 40–50% in CMRO2 has been reported with 4°C cooling in newborn piglets. 42 In the 15-kg piglet cohort, we did not obtain a statistically significant reduction in CMRO2, but the decrease in the arterial–venous O2 content difference without a significant change in cerebral blood flow suggests that a true decrease in CMRO2 would have been detectable with a larger sample size in this age group.
The decrease in CMRO2 was not coupled with a decrease in CBF. Instead, brain cooling increased the sagittal sinus O2 saturation without increasing the sagittal sinus PO2. The lack of a concurrent increase in PO2 could be attributed to the leftward shift of the O2-dissociation curve and decrease in the P50 (the PO2 at 50% oxyhemoglobin saturation) that occurs with decreasing temperature. Previous work has shown that the cerebral O2 extraction fraction, which is equivalent to the ratio of CMRO2/(CaO2 X CBF), varies directly with the P50.43,44 Under normothermic conditions, a decrease in P50 will increase CBF and result in a decrease in the O2 extraction fraction without a change in CMRO2. The increase in CBF serves to mitigate the decrease in capillary PO2 that would normally occur with increased O2 affinity of hemoglobin (decreased P50). However, under hypothermic conditions, the decrease in CMRO2 drives the decrease in O2 extraction fraction such that an increase in CBF is not required to prevent a decrease in capillary PO2. It should also be noted that deeper levels of hypothermia with greater reductions in CMRO2 are likely to result in reductions in CBF. Moreover, piglets are born with low levels of hemoglobin, which gradually increase during development, as evident by the higher levels in the 15-kg piglets than in the 4-kg piglets. The level of hemoglobin and other developmental factors may influence the regulation of CBF during mild hypothermia.
One potential limitation of using swine for studying cephalic heat exchange is the presence of a rete mirabile in this species. In pigs, blood flows from the common carotid artery into the ascending pharyngeal artery, which divides into a network of small arterioles surrounded by cavernous sinus venous blood, before reemerging into a short internal carotid artery that feeds the Circle of Willis.45,46 This contact of venous blood with the arterial vessel network allows for heat exchange that could be important for brain temperature regulation. Thus, it is possible that the rete mirabile augments brain cooling induced by high nasal airflow. However, selective brain cooling has been observed in rats, which do not have a rete mirabile. 22 As the internal maxillary vein communicates with the cavernous sinus venous plexus, nasal venous drainage into the internal maxillary vein could cool the internal carotid artery passing through the cavernous sinus in humans and enhance selective brain cooling in humans in addition to arterial-venous heat exchange in the neck.
High nasal flow with humidified oxygen-enriched gas is commonly used in critically ill neonates and children in the intensive care unit to avoid chronic tracheal intubation. The differences in the brain cooling technique used here are the use of normal humidity gas and a higher range of flow rates. Indeed, the effect of high nasal flow on brain cooling is lost with highly humidified air. 21 Here we show that transnasal airflow with ambient air can effectively rapidly cool the brain to temperatures typically targeted in therapeutic hypothermia within 30 minutes, whereas the rate of cooling of the body becomes progressively delayed as body size increases. Transnasal cooling could be a simple and effective technique to deploy in the field by emergency medical personnel to optimize the benefit of early brain cooling in pediatric patients.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231189463 for Rapid, selective and homogeneous brain cooling with transnasal flow of ambient air for pediatric resuscitation by Raymond C Koehler, Michael Reyes, C Danielle Hopkins, Jillian S Armstrong, Suyi Cao, Ewa Kulikowicz, Jennifer K Lee and Harikrishna Tandri in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Institutes of Health R21 NS095036 and R01 NS060703.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Harikrishna Tandri is the founder of CoolTech Inc, which is developing a transnasal device for hypothermia. Other authors declare that they have no conflict of interest.
Authors’ contributions: Study Design – RCK, JKL, HT
Data Collection – MR, CDH, JSA, SC, EK
Data Analysis – RCK
Manuscript preparation – RCK, JKL, CDH, HT
ORCID iD: Raymond C Koehler https://orcid.org/0000-0002-5890-9992
Supplementary material: Supplemental material for this article is available online.
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium epistry-cardiac arrest. Circulation 2009; 119: 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink EL, Prince DK, Kaltman JR, et al. Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation 2016; 107: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray JM, Kramer ME, Suskauer SJ, et al. Functional recovery during inpatient rehabilitation in children with anoxic or hypoxic brain injury. Arch Phys Med Rehabil 2023; 104: 918–924. [DOI] [PubMed] [Google Scholar]
- 4.Hickson MR, Winters M, Thomas NH, et al. Long-term function, quality of life and healthcare utilization among survivors of pediatric out-of-hospital cardiac arrest. Resuscitation 2023; 187: 109768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346: 549–556. [DOI] [PubMed] [Google Scholar]
- 6.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346: 557–563. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353: 1574–1584. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005; 365: 663–670. [DOI] [PubMed] [Google Scholar]
- 9.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009; 361: 1349–1358. [DOI] [PubMed] [Google Scholar]
- 10.Lin JJ, Hsia SH, Wang HS, et al. Therapeutic hypothermia associated with increased survival after resuscitation in children. Pediatr Neurol 2013; 48: 285–290. [DOI] [PubMed] [Google Scholar]
- 11.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med 2015; 372: 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 2014; 371: 140–149. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012; 366: 2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013; 104: 228–233. [DOI] [PubMed] [Google Scholar]
- 15.Laptook AR, Shankaran S, Tyson JE, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 2017; 318: 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni X, Yang ZJ, Wang B, et al. Early antioxidant treatment and delayed hypothermia after hypoxia-ischemia have no additive neuroprotection in newborn pigs. Anesth Analg 2012; 115: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Wang B, Lee JH, et al. Additive neuroprotection of a 20-HETE inhibitor with delayed therapeutic hypothermia after hypoxia-ischemia in neonatal piglets. Dev Neurosci 2015; 37: 376–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson M, Tooley JR, Satas S, et al. Delayed hypothermia as selective head cooling or whole body cooling does not protect brain or body in newborn pig subjected to hypoxia-ischemia. Pediatr Res 2008; 64: 74–78. [DOI] [PubMed] [Google Scholar]
- 19.Assis FR, Narasimhan B, Ziai W, et al. From systemic to selective brain cooling – methods in review. Brain Circ 2019; 5: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abou-Chebl A, Sung G, Barbut D, et al. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke 2011; 42: 2164–2169. [DOI] [PubMed] [Google Scholar]
- 21.Chava R, Zviman M, Raghavan MS, et al. Rapid induction of therapeutic hypothermia using transnasal high flow dry air. Ther Hypothermia Temp Manag 2017; 7: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Einer-Jensen N, Khorooshi MH. Cooling of the brain through oxygen flushing of the nasal cavities in intubated rats: an alternative model for treatment of brain injury. Exp Brain Res 2000; 130: 244–247. [DOI] [PubMed] [Google Scholar]
- 23.Assis FR, Bigelow MEG, Chava R, et al. Efficacy and safety of transnasal CoolStat cooling device to induce and maintain hypothermia. Ther Hypothermia Temp Manag 2019; 9: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einer-Jensen N, Khorooshi MH, Petersen MB, et al. Rapid brain cooling in intubated pigs through nasal flushing with oxygen: prevention of brain hyperthermia. Acta Vet Scand 2001; 42: 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chava R, Zviman M, Assis FR, et al. Effect of high flow transnasal dry air on core body temperature in intubated human subjects. Resuscitation 2019; 134: 49–54. [DOI] [PubMed] [Google Scholar]
- 26.Ziai WC, Shah D, Assis FR, et al. Feasibility and safety of transnasal high flow air to reduce core body temperature in febrile neurocritical care patients: a pilot study. Neurocrit Care 2019; 31: 280–287. [DOI] [PubMed] [Google Scholar]
- 27.Cole P. Physiology of the nose and paranasal sinuses. Clin Rev Allergy Immunol 1998; 16: 25–54. [DOI] [PubMed] [Google Scholar]
- 28.Rouadi P, Baroody FM, Abbott D, et al. A technique to measure the ability of the human nose to warm and humidify air. J Appl Physiol (1985) 1999; 87: 400–406. [DOI] [PubMed] [Google Scholar]
- 29.Becquemin MH, Swift DL, Bouchikhi A, et al. Particle deposition and resistance in the noses of adults and children. Eur Respir J 1991; 4: 694–702. [PubMed] [Google Scholar]
- 30.Koehler RC. Regulation of the cerebral circulation during development. Compr Physiol 2021; 11: 2371–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salinas-Zeballlos M-EZ, GA, Gootman PM. A stereotaxic atlas of the developing swine (Sus scrofa) forebrain. In: Tumbleson ME. (ed.) Swine in biomedical research. vol. 2. Plenum Press: New York, 1986, pp. 887–906. [Google Scholar]
- 32.Felix B, Leger ME, Albe-Fessard D, et al. Stereotaxic atlas of the pig brain. Brain Res Bull 1999; 49: 1–137. [DOI] [PubMed] [Google Scholar]
- 33.Rose VC, Shaffner DH, Gleason CA, et al. Somatosensory evoked potential and brain water content in post-asphyxic immature piglets. Pediatr Res 1995; 37: 661–666. [DOI] [PubMed] [Google Scholar]
- 34.Thoresen M, Simmonds M, Satas S, et al. Effective selective head cooling during posthypoxic hypothermia in newborn piglets. Pediatr Res 2001; 49: 594–599. [DOI] [PubMed] [Google Scholar]
- 35.Sahin-Yilmaz A, Naclerio RM. Anatomy and physiology of the upper airway. Proc Am Thorac Soc 2011; 8: 31–39. [DOI] [PubMed] [Google Scholar]
- 36.Sahin-Yilmaz A, Baroody FM, DeTineo M, et al. Effect of changing airway pressure on the ability of the human nose to warm and humidify air. Ann Otol Rhinol Laryngol 2008; 117: 501–505. [DOI] [PubMed] [Google Scholar]
- 37.Heymann MA, Payne BD, Hoffman JI, et al. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 1977; 20: 55–79. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg AA, Jones MD, Jr, Koehler RC, et al. Precautions for measuring blood flow during anemia with the microsphere technique. Am J Physiol 1983; 244: H308–H311. [DOI] [PubMed] [Google Scholar]
- 39.Jones N. The nose and paranasal sinuses physiology and anatomy. Adv Drug Deliv Rev 2001; 51: 5–19. [DOI] [PubMed] [Google Scholar]
- 40.Steen PA, Newberg L, Milde JH, et al. Hypothermia and barbiturates: individual and combined effects on canine cerebral oxygen consumption. Anesthesiology 1983; 58: 527–532. [PubMed] [Google Scholar]
- 41.Gonzalez-Ibarra FP, Varon J, Lopez-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol 2011; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busija DW, Leffler CW. Hypothermia reduces cerebral metabolic rate and cerebral blood flow in newborn pigs. Am J Physiol 1987; 253: H869–H873. [DOI] [PubMed] [Google Scholar]
- 43.Koehler RC, Traystman RJ, Rosenberg AA, et al. Role of O2-hemoglobin affinity on cerebrovascular response to carbon monoxide hypoxia. Am J Physiol 1983; 245: H1019–H1023. [DOI] [PubMed] [Google Scholar]
- 44.Koehler RC, Traystman RJ, Jones MD., Jr. Influence of reduced oxygemoglobin affinity on cerebrovascular response to hypoxic hypoxia. Am J Physiol 1986; 251: H756–H763. [DOI] [PubMed] [Google Scholar]
- 45.Gillilan LA. Blood supply to brains of ungulates with and without a rete mirabile caroticum. J Comp Neurol 1974; 153: 275–290. [DOI] [PubMed] [Google Scholar]
- 46.Eliyas JK, Niekrasz M, Wardrip C, et al. Focused post mortem dissection technique for harvest of rete mirabile in domestic swine (Sus scrofa). J Neurointerv Surg 2016; 8: 973–976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231189463 for Rapid, selective and homogeneous brain cooling with transnasal flow of ambient air for pediatric resuscitation by Raymond C Koehler, Michael Reyes, C Danielle Hopkins, Jillian S Armstrong, Suyi Cao, Ewa Kulikowicz, Jennifer K Lee and Harikrishna Tandri in Journal of Cerebral Blood Flow & Metabolism