Abstract
Delayed cerebral ischemia (DCI) is a devastating complication of aneurysmal subarachnoid hemorrhage (ASAH) causing brain infarction and disability. Cerebral microdialysis (CMD) monitoring is a focal technique that may detect DCI-related neurochemical changes as an advance warning. We conducted retrospective analyses from 44 poor-grade ASAH patients and analyzed glucose, lactate, pyruvate, and glutamate concentrations in control patients without DCI (n = 19), and in patients with DCI whose CMD probe was located within (n = 17) or outside (n = 8) a new infarct. When monitored from within a lesion, DCI was preceded by a decrease in glucose and a surge in glutamate, accompanied by increases in lactate/pyruvate and lactate/glucose ratios whereas these parameters remained stable in control patients. When CMD monitoring was performed outside the lesion, the glutamate surge was absent, but glucose and L/G ratio were still significantly altered. Overall, glucose and L/G ratio were significant biomarkers of DCI (se96.0, spe73.7-68.4). Glucose and L/G predicted DCI 67 h before CT detection of a new infarct. The pathogenesis of DCI therefore induces early metabolic disturbances that can be detected by CMD as an advance warning. Glucose and L/G could provide a trigger for initiating further examination or therapy, earlier than when guided by other monitoring techniques.
Keywords: Brain metabolism, glucose, lactate, cerebral microdialysis, multimodal monitoring
Introduction
Delayed cerebral ischemia (DCI) is the most preventable cause of poor-grade aneurysmal subarachnoid hemorrhage (ASAH) morbidity.1,2 DCI, which occurs 3∼21 days after cranial hemorrhage, has been linked to multiple events, including cerebral vasospasm, microcirculatory constriction, microthrombosis, cortical spreading depression, and delayed cellular apoptosis. 3 It may be diagnosed based on the clinical identification of a new (focal or global) neurological impairment or based on the follow-up neuroimaging finding of a new cerebral infarction that was not apparent immediately after aneurysm occlusion and cannot be attributed to another neurologic or systemic condition.3–5 Clinical examination is unreliable during the period of DCI risk in almost half of patients with poor-grade ASAH. 6 Without direct proof of deterioration, patients are left with a silent cerebral infarction. 7
Several bedside monitoring techniques can provide continuous monitoring of brain pathophysiological parameters, such as intracranial pressure (ICP) or brain tissue oxygen pressure (PbtO2). Although an ICP > 20 mmHg and a PbtO2 < 10 mmHg correlate with poor outcomes, there is not a consensus as to whether these findings allow timely detection of DCI.8–10
Cerebral microdialysis (CMD), a promising technique first implemented in intensive care units (ICUs) in the 1990s,11,12 measures interstitial concentrations of metabolites and neurotransmitters, such as glucose, lactate, pyruvate, and glutamate, on an hourly basis. The onset of cerebral ischemia is characterized by low brain concentrations of glucose, pyruvate and oxygen, anaerobic glycolysis, and lactate elevation. In addition, ischemia eventually leads to uncontrolled glutamate release. A 2014 consensus statement indicated that the following threshold values correlate with poor outcome: glucose <0.2–0.7 mM, lactate >4 mM, and lactate/pyruvate (L/P) ratio >40. 13 More recently, CMD-revealed low glucose concentrations and elevated L/P ratios were shown to be predictive of DCI up to 12 h before hypoperfusion can be seen by computed tomography (CT).7,14 However, because CMD is a focal technique that monitors a small portion of brain parenchyma, CMD findings depend on placement of the probe. CMD probes lose almost all DCI detection power when placed a far distance from an infarction.7,15 There is not yet a consensus regarding whether CMD can detect impending DCI reliably in ASAH patients and provide advance warning to trigger beneficial therapeutic intervention.
In the present retrospective study, we analyzed changes in glucose, lactate, pyruvate, and glutamate concentrations, as well as changes in L/P and lactate/glucose (L/G) ratios, in 44 poor-grade ASAH patients subjected to multimodal monitoring with CMD, ICP, and PbtO2 probes. We focused our analysis on the 84 h (3.5 days) preceding the first demonstrative CT sign of delayed infarction, distinguishing between patients in whom the CMD probe was located within versus outside an infarcted area. We hypothesized that some CMD-monitored biomarkers and pathological changes due to ischemia would affect neighboring areas, allowing sensitivity and specificity appropriate for detection of impending DCI regardless of probe placement. Using these biomarkers and their optimal threshold values, we determined the average advance warning time that CMD could provide for intervention on a patient-by-patient basis.
Materials and methods
Inclusion criteria, demographic and clinical description of the three ASAH patient groups
The study protocol complied with the Declaration of Helsinki and was approved by the ethics committee of the Lyon University Medical Center (French Clinical Research Program HCL99.173) and by the Lyon institutional review board (Comité de Protection des Personnes Sud-Est IV, ref12-135, “Biothèque du service de neurobiologie du centre de Biologie Pathologie Est des Hospices Civils de Lyon”, approved 09/06/2012). Retrospective analyses were conducted with data from patients treated at the neurological ICU at Lyon Neurological Hospital (Hospices Civils de Lyon) since 2000. All conscious patients, or legal representatives of unconscious patients, gave written informed consent. The inclusion criteria were: a CT/angiography-based diagnosis of ASAH; age >18 years; and aneurysm treatment within 72 h of hospitalization. Patients were managed according to standard ASAH management guidelines. 16 DCI prevention included daily oral nimodipine. DCI and vasospasm detection involved clinical examination and transcranial Doppler imaging, as well as ICP, CMD, and PbtO2 monitoring in comatose patients (spontaneous or sedated for IH treatment or respiratory failure).
Among 72 patients with ASAH registered in our database (Figure 1(b)), we identified 51 who developed DCI (70.8%) and 21 controls who did not (29.2%). Of the 51 DCI patients, 26 were excluded due to missing multimodal monitoring data (N = 21), missing imaging data (N = 3), and <2 days of CMD monitoring (N = 2). Of the 25 remaining patients, 8 patients had CMD probes outside the infarcted brain area (7 were <2 cm from the hypodensity boundary defined by the radiologist; 1 was >2 cm away) and 17 had CMD probes within an infarct. Of the 21 control patients, we excluded 2 patients due to a local hematoma near the CMD probe and CMD monitoring for <2 days. The following analyses were therefore performed on 44 patients (25 DCI and 19 controls).
Figure 1.
(a) Time period of interest for retrospective analysis. The period most likely to observe neurochemical and metabolic changes spanned from the last negative scan without evidence of infarction (left) to the first positive scan revealing a new infarcted brain area (right). Data were compared across patients whose CMD probes were within versus outside an infarcted area. ASAH, aneurismal subarachnoid hemorrhage and (b) Patient selection. Of the 72 patient files present in the source database, 21 did not display DCI and provided control monitoring values (2 excluded), and 51 showed DCI with cerebral infarction evidenced by CT (24 excluded). Among the 27 patients with infarction related to DCI, 17 and 10 had a CMD probe placed within and placed outside the infarct, respectively. CMD, cerebral microdialysis; DCI, delayed cerebral ischemia; ASAH, aneurismal subarachnoid hemorrhage; CMD, cerebral microdialysis; DCI, delayed cerebral ischemia.
Demographic and clinical data are presented in Table 1. DCI-related infarctions were always identified by CT; cerebral infarction was confirmed by diffusion-weighted MRI in 9/25 patients (36%). Six months after ASAH diagnosis, DCI patients had a much higher proportion of unfavorable outcomes (GOS 1–3) than control patients (Table 1). GOS score repartition was not different between the DCI inside and DCI outside groups (p = 0.32).
Table 1.
Demographic and clinical characteristics of included ASAH patients.
| Parameters | Control | DCI, probe inside infarcted area | DCI, probe outside infarcted area | P |
|---|---|---|---|---|
| Sex, N females/males | 11/8 | 11/6 | 3/5 | 0.44 |
| Age, mean ± standard deviation | 49.3 ± 8.3 | 47.2 ± 12.1 | 43.0 ± 15.2 | 0.88 |
| WFNS score, N | ||||
| Grade 1–3 | 1 | 3 | 1 | 0.64 |
| Grade 4 | 7 | 3 | 2 | |
| Grade 5 | 11 | 11 | 5 | |
| Fisher score, N | ||||
| Grade 1–2 | 0 | 1 | 0 | 0.35 |
| Grade 3 | 1 | 3 | 0 | |
| Grade 4 | 18 | 13 | 8 | |
| Aneurysm location, N | ||||
| ACoA | 9 | 8 | 5 | 0.55 |
| MCA | 6 | 2 | 1 | |
| PCoA | 2 | 3 | 0 | |
| Other | 2 | 4 | 2 | |
| Aneurysm treatment, N Coiling/clipping | 14/5 | 14/3 | 7/1 | 0.67 |
| Delay of CMD probe implantation (days post-ASAH), median [25–75th percentile] | 3.1 [1.5–7.2] | 3.3 [2.7–6.1] | 4.8 [2.1–9.7] | 0.62 |
| Duration of CMD monitoring (days), median [25–75th percentile] | 9.9 [6.0–11.6] | 7.0 [6.2–7.8] | 10.4 [7.9–16.3] | 0.08 |
| Delay of DCI from ASAH onset (days), median [25–75th percentile] | – | 10.4 [8.6–12.0] | 9.5 [6.7–13.8] | 0.91 |
| Duration between last negative and first DCI positive CT-scan (hours), median [25–75th percentile] | – | 105 [81–128] | 98 [73–262] | 0.86 |
| GOS score at 6 months, N | ||||
| GOS 1–3 | 5 | 16 | 8 | <0.0001 |
| GOS 4–5 | 14 | 1 | 0 |
P < 0.05, significant (bold). ACoA: anterior communicating artery; CMD: cerebral microdialysis; DCI: delayed cerebral ischemia; GOS: Glasgow outcome scale; MCA: middle cerebral artery; N: number; PCoA: posterior communicating artery; WFNS: World Federation of neurosurgical societies.
CMD implementation
An ICP probe (Sophysa or Codman-Integra Life Sciences, Besançon/Saint Priest, France), PbtO2 probe (LICOX, Integra NeuroSciences, Saint-Priest, France), and CMD catheter (CMA 70; M Dialysis AB, Johanneshov, Sweden; cut-off, 20 kDa; flow rate, 0.3 µL/min) were inserted via a triple-lumen catheter by neuro-intensivists as recommended. 17 Implantation depth was 10 mm for CMD, and 35 mm for PbtO2 probes. Collected samples were analyzed as previously described.15,18 Induced hypertension as well as volume and respiratory optimization were the first line treatment in cases of developing vasospasm, neurological impairment, or PbtO2 < 15 mmHg. CMD monitoring was observational; only patients with >2 days of monitoring and available CT/MRI scans were included in the analyses. Outcomes were assessed with the Glasgow Outcome Scale (GOS), with GOS grade 1–3 representing less favorable outcomes than grade 4–5.
Imaging data and identification of secondary infarction related to DCI
In all patients, CT scans were obtained (1) at admission, (2) after aneurysm treatment, (3) following placement of PtbO2, ICP, and CMD probes or an external ventricular drain (EVD), (4) after any unexplained neurological change, such as sustained ICP elevation, and (5) at the initiative of ICU physicians to evaluate possible delayed brain injury at a distance from the ASAH event. CMD data was not considered for referring patients to the imaging facility. MRI was preferred when a suspicion of DCI existed due to, for example, unexplained neurological deterioration or velocity increases detected by transcranial Doppler. However, MRI scans were often not feasible because of the presence of magnetic materials in indwelling monitoring probes and difficult-to-access MRI facilities in our hospital, thus increasing the risk of critical patient transportation (we should note that MRI access in our clinical center was limited for neurological ICU patients prior to 2006, but has increased since). In our population of mainly comatose patients, infarction from DCI was identified as the presence of a cerebral infarct on CT or MRI scans that was not visible 24∼48 h after aneurysm occlusion, but then appeared within 6 weeks of the initial bleed and could not be attributed to other causes, such as surgical clipping or endovascular treatment. 4 CT and MRI scans were reviewed by an experienced radiologist (blinded to the monitoring data) to detect infarction from DCI. We selected DCI group and control group patients based on follow-up CT/MRI scans. DCI patients were classified according to whether the probe had been implanted within or outside an infarcted area. We identified the first infarct-positive scan and the preceding infarct-negative scan in each patient (Figure 1(a)). Only patients with complete CMD follow-up until the first positive scan were retained. CT scans were available for all included DCI patients.
Analysis of CMD samples
Cerebral dialysate was collected in a microvial hourly and analyzed directly on a microdialysis analyzer (CMA 600 before 2009 and ISCUSFlex thereafter, M Dialysis AB, Stockholm, Sweden). Glucose, lactate, and pyruvate were measured, and then the analyzer computed L/P and L/G ratios. Analytical validation of both microdialysis analyzers was performed according to a previously published quality control program, that included periodic analysis of standards and in-house prepared standard additions into CSF samples. 18
Because early CMD data obtained soon after probe implantation may be unreliable due to transient insertion trauma,13,19 we analyzed CMD data obtained in control patients without DCI to determine the temporal window after which CMD data were stable (Figure 2). Extracellular glutamate concentrations were high (36.5 [19.4–58.4] µM during the first 6 h), then decreased to low micromolar levels (1.7 [1.3–3.2] µM, after 12 h; p = 0.006, Figure 2(a1)). It took 33 [27–54] h for glutamate to remain at stable intermediate values (33rd–66th percentile of total monitoring data). All other CMD biomarkers were stable soon after probe implantation (Figure 2). Interestingly, there was no difference in the initial pattern of CMD data in patients whose probes were placed <48 h (N = 8) versus >100 h (N = 7) after the initial bleed (Figure 2(a2)), indicating that early elevation of glutamate was related to local implantation injury rather than to primary hemorrhagic injury. To avoid confounding effects resulting from these local perturbations, we discarded the first 24 h of monitoring data obtained in control patients. In patients with DCI, we discarded CMD data before the last negative scan. This last negative scan was obtained >24 h and <24 h after probe implantation in 21 and 4 patients, respectively (data from the latter were excluded like in control patients).
Figure 2.
Initial stabilization of CMD markers in control patients. Evolution of glutamate (a), glucose (b), lactate (c), pyruvate concentrations (d), as well as L/P (e) and L/G (f) ratios during the first 140 h (5.8 days) following probe implantation. Extracellular glutamate concentrations were elevated in the first 6 h of monitoring, then decreased gradually to a low micromolar range (a1). There was no difference in the initial pattern of CMD data in patients whose probes were placed <48 h (N = 8) versus >100 h (N = 7) after the initial bleed (a2), indicating that early elevation of glutamate was related to local implantation injury rather than to primary hemorrhagic injury. Data are represented as median (interquartile range).
In control patients, CMD data from the last 84 h before probe removal, excluding the first 24 h after probe implantation (see Results), were collated for analyses. In DCI patients, we analyzed CMD, PbtO2, and ICP data obtained between the last infarct-negative scan and the first infarct-positive scan. When the two scans were separated by more than 84 h (3.5 days), we focused on the last 84 h preceding the positive scan (Figure 1(a)). This temporal window corresponds to the occurrence of cerebral infarction from DCI and is most likely to reveal biomarker changes. We determined optimal cutoff values (using the Youden index) by way of receiver operating characteristic (ROC) analyses of maximum/minimum biomarker values for each patient during the temporal window. We determined when each biomarker passed its cutoff value and calculated the delay before the first positive scan. For each CMD parameter, we calculated the time interval between passing the cutoff and the first positive scan to estimate precocity relative to DCI occurrence.
Statistics
CMD and PbtO2 data were compared longitudinally across groups. We compared control group data with data obtained in DCI patients hourly before the first positive scan (N = 8–14 for each hour). Longitudinal comparisons between control patients and DCI patients probe inside or outside infarct were performed using a linear regression mixed effect regression model analysis for each biomarker. We investigated the effect of the interaction between the group (control, DCI inside or DCI outside) and the sample time (from the onset of DCI in the DCI groups or the end of the monitoring in the control group, and going back 84 h in time) with the subject as random effect. The control group was considered the reference level of the group effect. We used the Mann-Whitney test for post-hoc analyses to compare the control and the DCI or the outside group with a Bonferroni correction for multiple analyses, if there was a significant effect of the interaction between time and group level. We also performed a second analysis using the DCI group as the reference level to investigate whether there was a significant interaction effect of time and group between the DCI and outside levels. Data were analyzed with the R software (R Foundation for Statistical Computing, Vienna, Austria, v, version 4.1.2) using a linear regression mixed effect model (lmer function from the lme4 library) with the interaction between the time in hours (before DCI or probe removal) and the group (Control, DCI probe inside, DCI probe outside) as fixed effects and a random intercept between subjects.
We determined optimal cutoff values (using the Youden index) by way of receiver operating characteristic (ROC) analyses of maximum/minimum biomarker values for each patient. Only parameters that reached an area under the curve (AUC) > 0.8 were considered significant for predicting DCI occurrence.
Categorical and continuous clinical data were analyzed with χ2 tests and with Mann-Whitney U- (non-parametric) or Student's t- (parametric) tests, respectively. (Non-)parametric distribution was determined with Kolmogorov-Smirnov tests. Parametric data are expressed as means ± standard deviations; non-parametric data are expressed as medians [25th–75th percentile]. Differences were considered significant at p < 0.05. Coded/anonymized clinical data will be made available upon request to the corresponding author.
Results
After the initial 24 h stabilization period, glucose, lactate, pyruvate, glutamate concentrations and L/G and L/P ratios remained essentially stable in control patients, until they were considered out of risk for DCI and the probe was removed.
Changes in metabolite and glutamate concentrations inside the infarct
With the probe in an infarcted area, CMD should detect ischemia-induced neurochemical changes. We thus first compared metabolite and glutamate concentrations when the probe was located within an infarcted area (Figure 3). We compared extracellular concentrations measured within infarcted areas during the 84 h (3.5 days) preceding the first positive CT scan revealing an infarct to the total set of monitoring data observed in control patients. We performed ROC analyses of glutamate, glucose, lactate, and pyruvate concentrations, and of L/G and L/P ratios across groups to assess their value as DCI-related infarction biomarkers.
Figure 3.
Evolution of glutamate, glucose, lactate, and pyruvate concentrations, as well as lactate/pyruvate and lactate/glucose ratios before the first positive scan displaying brain infarction in patients whose CMD probe was placed within an infarct. (a–f). Median (interquartile range) concentrations/ratios measured during the 84-h period preceding the first scan showing evidence of DCI with a probe located within an infarct (red) and the control group (black): glutamate (a.), glucose (b.), lactate (c.), and pyruvate (d.) concentrations; lactate/pyruvate (L/P) (e.) and lactate/glucose (L/G) (f.) ratios. The optimal cutoff values obtained were >16.7 µmol/L glutamate, <0.1 mM glucose, >6.3 mM lactate, <19 µM pyruvate, L/P ratio > 73.1, and L/G ratio > 35.2 (blue line). Insets: ROC curve analysis with maximum values of each patient. *p < 0.05, **p < 0.01, ***p < 0.001, linear regression mixed effect model with Man-Whitney post-hoc tests.
Glutamate
Extracellular glutamate concentration increased dramatically from a basal level of 2.5 [1.5–4.3] µM 84 h pre-infarct CT detection to 108 [36–195] µM immediately before infarct detection in the DCI group. By comparison, median glutamate concentration in control patients was 1.6 [1.0–2.6] µM and evolved in a 5–95% confidence interval (CI) of 0.5–7.6 µM. Glutamate concentration was significantly elevated in the DCI group compared to controls 7 h before the first positive scan. ROC analysis revealed that glutamate was an excellent biomarker of DCI-associated infarction (AUC = 0.92). The optimal cutoff (16.7 μM) yielded 92.9% sensitivity (Se) and 93.3% specificity (Sp) for DCI detection (Figure 3(a), Table S1).
Glucose
Extracellular glucose concentration collapsed to almost undetectable levels (detection limit, 0.1 mM) before DCI. It fell from 0.66 [0.28–1.13] mM at 84 h to 0.1 [0.1–0.3] mM immediately before an infarct was observed by CT. Glucose concentration in control patients was 1.1 [0.6–1.5] mM and evolved in a 5–95% CI of 0.2–2.5 mM. Glucose concentration was significantly reduced compared to controls as early as 56 h before the first positive scan (numerical values are shown in Table S3). ROC analysis indicated that glucose was an excellent biomarker of cerebral infarction from DCI (AUC = 0.94) with an optimal cutoff of 0.18 mM (Se: 91.4%, Sp: 84.2%; Figure 3(b), Table S1).
Lactate and pyruvate
Lactate and pyruvate displayed modest concentration changes. Lactate concentration increased from a baseline of 3.5 [2.7–6.4] mM at 84 h to 5.1 [3.4–6.3] mM before the first positive scan, compared to a control 5–95% CI of 1.5–6.2 mM (median [inter-quartile range] = 3.1 [2.3–41] mM). Pyruvate decreased from 114 [93–157] µM to 28 [19–104] µM before the first positive scan (detection limit, 19 µM). By contrast, pyruvate concentration in control patients was within a 5–95% CI of 47–219 µM. Lactate and pyruvate concentrations were significantly different from control levels 9 h and 3 h, respectively, before the first positive scan. ROC analyses revealed that neither lactate (AUC = 0.73) nor pyruvate (AUC = 0.72) were significant biomarkers of infarction (Figure 3(c) and (d), Table S1).
L/P And L/G ratios
L/P increased dramatically from 36 [27–41] to 143 [64–202], compared to control values at 29 [23–34] (5–95% range of 18–46). Similarly, L/G increased from 4.9 [2.8–13.7] to 31.1 [25.3–55.5], compared to control values at 3.2 [1.8–5.7] (5–95% range of 0.9–16). The ratios in DCI patients differed significantly from those measured in control patients 20 h and 56 h before the first positive scan, respectively. ROC analyses revealed that L/P (AUC = 0.88) and L/G (AUC = 0.97) were excellent biomarkers of infarction from DCI. Optimal thresholds were 73.1 for L/P (Se: 82.4%, Sp: 89.4%) and 35.2 for L/G (Se: 88.2%, Sp: 94.7%) (Figure 3(e) and (f), Table S1).
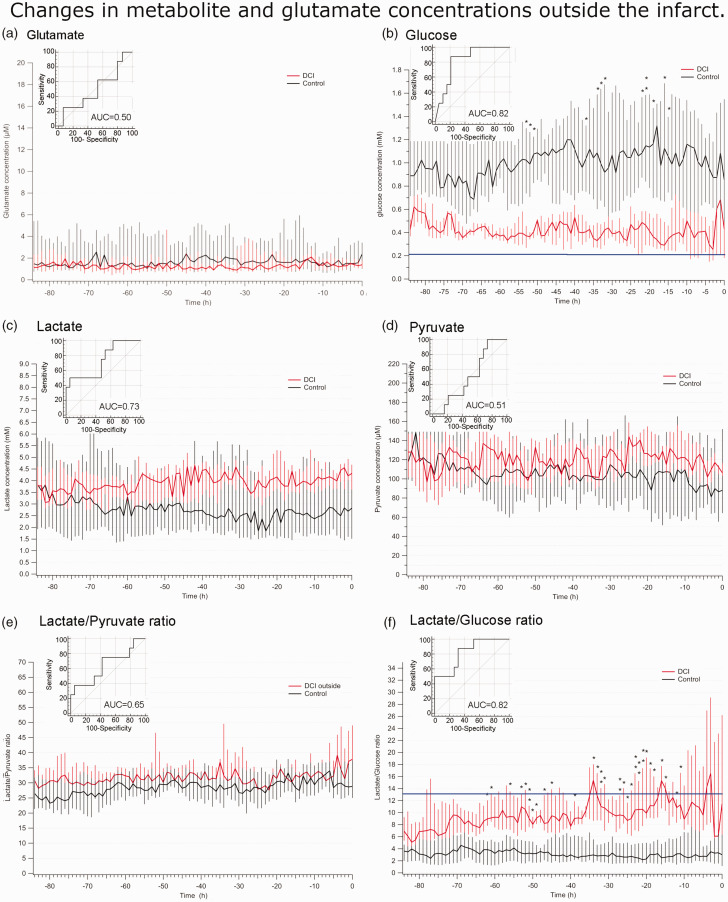
Changes in metabolite and glutamate concentrations outside the infarct
Only glucose and L/G ratio displayed significant changes in the eight patients in whom the CMD probe was located outside an infarcted area. No significant differences in glutamate, lactate, pyruvate and L/P ratio concentrations were detected (Figure 4(a), (c) to (e)). Glucose decreased from 0.61 [0.41–0.94) mM to 0.43 [0.15–0.83] mM, and there was a significant decrease in glucose concentration between control and DCI patients 53 h before the first positive scan (Figure 4(b), Table S3). The L/G ratio increased from 6.4 [4.4–10.4] to 16.9 [5.7–26.6], which differed significantly from control values 62 h before the first positive scan (Figure 4(f)). The mixed model statistical analysis with the DCI inside group taken as the reference group revealed that there was no significant difference in glucose (p = 0.968), lactate (p = 0.128) or pyruvate (p = 0.06) between the DCI inside and the DCI outside groups. By contrast, glutamate (p < 0.01), L/P (p < 0.01) and L/G (p = 0.01) displayed significantly different changes in concentration over time between the DCI inside and DCI outside groups (post-hoc comparisons between concentrations inside and outside the infarcts are shown in figure S1). Therefore, probe placement made a significant difference in glutamate, L/P, and L/G monitoring, but not for glucose, lactate and pyruvate.
Figure 4.
Evolution of glutamate, glucose, lactate and pyruvate concentrations, as well as lactate/pyruvate and lactate/glucose ratios before the first positive scan displaying brain infarction in patients whose CMD probe was placed outside the infarct. (a–f) Median (interquartile range) glutamate concentrations/ratios measured during the 84-h period preceding the first brain scan showing evidence of DCI with a probe located outside the infarct (red) and the control group (black): glutamate (a.), glucose (b.), lactate (c.), and pyruvate (d.) concentrations; lactate/pyruvate (L/P) (e.) and lactate/glucose (L/G) (f.) ratios. Inset: ROC curve analysis with maximum glutamate values of each patient. *p < 0.05, **p < 0.01, ***p < 0.001, linear regression mixed effect model with Man-Whitney post-hoc tests.
ROC curve analysis (Figure 4) demonstrated significance for glucose (AUC = 0.82) and L/G ratio (AUC = 0.82), but not for glutamate (AUC = 0.50), lactate (AUC = 0.73), pyruvate (AUC = 0.51), and L/P ratio (AUC = 0.65, table S2). Therefore, most metabolic changes within lesions were dampened in non-infarcted areas, except for glucose and L/G ratio, which displayed large alterations well before infarction was observed. Optimal thresholds for detecting DCI with a probe outside the infarcted area were 0.21 mM glucose and an L/G ratio of 13.3.
Predictive value of CMD biomarkers in all patients
When all patients were analyzed together (controls, DCI inside or outside), only glucose and L/G ratio displayed significant AUCs (Table 2; Figure 5(a1) and (a2)). L/G had an optimal cutoff value at 13.3 according to the Youden index, with an AUC of 0.92 (Se 96%, sp 68.4%), while glucose had a cutoff concentration of 0.26 mM with an AUC of 0.91 (Se 96%, Sp 76.7%). L/P was marginally significant with a cutoff value of 87.1 and an AUC of 0.80 (Se 64%, Sp 94.7%). By contrast, glutamate was no longer a significant biomarker of DCI, with an AUC of 0.77 (Se 68.2%, Sp 93.3%). Lactate and pyruvate displayed non-significant AUCs of 0.73 and 0.65 respectively (Table 2).
Table 2.
ROC curve analysis for all patients, including patients a cerebral infarct from DCI (probe within or outside) and control patients.
| Parameter | AUC (95%CI) | Cutoffs | Sensitivity (95%CI) | Specificity (95%CI) |
|---|---|---|---|---|
| L/G ratio | 0.92 (0.80–0.98) | >7.5 | 100.0 (86.3–100.0) | 47.4 (24.4–71.1) |
| >13.3 | 96.0 (79.6–99.9) | 68.4 (43.4–87.4) | ||
| >37.1 | 72.0 (50.6–87.9) | 100.0 (82.4–100.0) | ||
| Glucose | 0.91 (0.78–0.97) | ≤0.35 mM | 96.0 (79.6–99.9) | 52.6 (28.9–75.6) |
| ≤0.26 mM | 96.0 (79.6–99.9) | 73.7 (48.8–90.9) | ||
| ≤0.21 mM | 92.0 (74.0–99.0) | 79.0 (54.4–93.9) | ||
| ICP | 0.89 (0.75–0.97) | >26 mmHg | 95.5 (77.2–99.9) | 58.8 (32.9–81.6) |
| >40 mmHg | 77.3 (54.6–92.2) | 94.1 (71.3–99.9) | ||
| CPP | 0.83 (0.67–0.93) | ≤66 mmHg | 86.4 (65.1–97.1) | 52.9 (27.8–77.0) |
| ≤55 mmHg | 77.3 (54.6–92.2) | 94.1 (71.3–99.9) | ||
| PbtO2 | 0.83 (0.67–0.93) | ≤11 mmHg | 90.5 (69.6–98.8) | 50.0 (24.7–75.3) |
| ≤6 mmHg | 81.0 (58.1–94.6) | 81.3 (54.4–96.0) | ||
| L/P ratio | 0.80 (0.65–0.91) | >47.2 | 84.0 (63.9–95.5) | 57.9 (33.5–79.7) |
| >87.1 | 64.0 (42.5–82.0) | 94.7 (74.0–99.9) | ||
| Glutamate | 0.77 (0.60–0.89) | >16.7 µM | 68.2 (45.1–86.1) | 93.3 (68.1–99.8) |
| >33.1 µM | 54.6 (33.2–75.6) | 100.0 (78.2–100.0) | ||
| Lactate | 0.73 (0.58–0.85) | >6.3 mM | 60.0 (38.7–75.6) | 84.2 (60.4–96.6) |
| >7.0 mM | 56.0 (34.9–75.6) | 89.5 (74.0–99.9) | ||
| Pyruvate | 0.65 (0.49–0.79) | ≤56.7 µM | 68.0 (46.5–85.1) | 63.2 (38.4–83.7) |
Cut-off values (defined according to the Youden index) are in bold. Non-bolded cutoff values had high sensitivity and acceptable specificity in association algorithm (>40%). AUC: area under the curve; CI: confident interval; CPP: cerebral perfusion pressure; ICP: intracranial pressure; L/G: lactate/glucose; L/P: lactate/pyruvate; PbtO2: brain tissue oxygen tension.
Figure 5.
Comparison of most efficient biomarkers of DCI. a. ROC analysis of minimal glucose (a1), maximal lactate/glucose ratio ICP (a2), minimal PbtO2 (a3) and maximal ICP (a4) in all patients with or without DCI (irrespective of probe placement). b and c. Evolution of PbtO2 values in patients with a probe located within (b.) or outside (c.) an infarct area (inset: ROC analysis of maximal PbtO2 within or outside infarct zone). d and e. Evolution of intracranial pressure (ICP) in patients with a probe located within (d.) or outside (e.) an infarct area (inset: ROC analysis of maximal ICP within or outside infarct zones). f and g. Box and whisker plot of time intervals between L/G, glucose, PbtO2, and ICP crossing their respective cutoff values and the first evidence of cerebral infarction from DCI on a positive brain scan. Boxes show medians and interquartile values, while whiskers show 10–90%tiles (individual dots are extreme values). Cutoff values were defined as the optimal threshold according to the Youden index (f.) or as the threshold providing maximal sensitivity (g.). All biomarkers crossed their cutoffs with significantly advanced timing compared to imaging, and L/G was significantly earlier than PbtO2 with both threshold definitions (ANOVA followed by Tukey’s multiple comparisons, p = 0.04).
Comparison to ICP and PbtO2
Because ICP and PbtO2 are key bedside monitoring measures in patients with ASAH, we analyzed their variations along with CMD. With the CMD, ICP, and PbtO2 probes inserted via the same burr hole, the three probes had a similar placement relative to the infarct (with a 25 mm difference in depth between the tip of the CMD and PbtO2 probes). ICP increased from 10.5 [8.2–15.0] mmHg at 84 h to 40.0 [21.5–82.0] mmHg immediately before infarct detection by CT (Figure 5(d)). In control patients without DCI, ICP was 12.5 [8.0–17.0] mmHg. ICP elevation was significant 25 h before infarct detection, illustrating a high prevalence of IH among patients with DCI, consistent with a similar population described by Zoerle et al. 20 For the subset of patients with a probe located outside an infarcted area, the increase in ICP was not significant. ICP immediately before infarct detection was 18.0 [15.0–34.0] mmHg (Figure 5(e)). Although IH was highly prevalent in patients with the probe within (14/15 patients) or outside (8/8 patients) an infarct, ICP was less elevated in the latter. It is possible that those patients with the probe outside the lesion tended to have less severe infarctions and/or smaller infarcted areas than those with a probe within the infarct.
PbtO2 fell to almost undetectable levels before the onset of DCI. When detected from within the infarct, it decreased from 26.7 [22.5–35.4] mm Hg at 84 h to 1.4 [0–17.0] mmHg immediately pre-CT (Figure 5(b)). The decrease in PbtO2 observed before infarct detection was less pronounced when the probe was outside the infarct, reaching 14.1 [9.3–18.0] mmHg (Figure 5(c)). In control patients without DCI, PbtO2 was 28.0 [21.0–35.2] mmHg. However, these changes in PbtO2 did not reach significance, neither inside nor outside infarcted brain areas.
Time intervals between cutoff values crossing and CT infarct detection
Biomarker cutoff values, defined as optimal thresholds according to the Youden index, were: L/G > 13.3, glucose <0.26 mM, PbtO2 < 6 mmHg, and ICP > 40 mmHg. The four biomarkers crossed these cutoff values at the following time intervals prior to imaging infarct detection: L/G, 43 [21–110] h; glucose, 42 [20–92] h; PbtO2, 21 [6–42] h; and ICP, 25 [1–46] h. The L/G cutoff was reached significantly earlier than the PbtO2 cutoff (p = 0.04, Figure 5(f)).
In the context of multimodal monitoring, defining a cutoff value as the value that provides maximal sensitivity (with specificity > 40%) may give more advance warning, and trigger an imaging referral. Applying maximally sensitive cutoff values (L/G > 7.5; glucose < 0.35 mM; PbtO2 < 14 mmHg; and ICP > 26 mmHg), the intervals between each threshold crossing and CT infarct detection were increased to 79 [40–115] h for ICP, 67 [33–112] h for L/G, 64 [27–107] h for glucose, and 31 [12–64] h for PbtO2. L/G alterations were evident earlier than PbtO2 alterations (p = 0.04, Figure 4(g)).
Because CT detection of new infarcts is a late marker of DCI, we looked for an earlier sign of DCI, in the absence of clinical neurological signs in comatose patients. In patients with a CMD probe located within an infarct, glutamate concentration surged rapidly in relation to DCI, probably reflecting uncontrolled glutamate release when neuronal cells lost their transmembrane ion gradients. This is usually considered an early phenomenon in the pathophysiology of ischemia. 21 Glutamate exceeded its 16.7 µM threshold value 15.5 [3.5–22.75] h before infarct detection using CT scan, whereas L/G exceeded its 7.5 threshold value 51.5 [28–91.75] h before the observation of the infarct. Therefore, when the glutamate surge was detectable (probe inside infarct), L/G > 7.5 significantly preceded glutamate by nearly 1.5 day (p = 0.003). Therefore, L/G (and perhaps glucose and/or ICP) appears to be a promising early warning of infarction risk, that not only preceded infarct detection using CT scan, but also the glutamate surge indicative of ischemia.
In this cohort of 44 ASAH patients, indication for examination after observing L/G > 7.5 would have sent 31 patients to brain imaging (25 of whom developed DCI within the next 84 h), while leaving 13 control patients undisturbed. False alarms were infrequent. The glucose < 0.35 mM and L/G > 7.5 thresholds were crossed only once [1–2] and twice [1–2] respectively, within the 84 h preceding the first positive CT scan, and were crossed zero times [0–1] and once [0–3.5] respectively, in control patients. Thus, timely evaluations based on CMD monitoring could enable earlier diagnosis of impaired brain perfusion and DCI.
Discussion
CMD is a focal technique that samples the interstitial fluid in a small volume, within a few millimeters of the probe along its length.17,22 In control patients without DCI, after an initial 24-h period of elevated glutamate likely from probe-insertion trauma, we observed stability of glucose, lactate, pyruvate, and glutamate concentrations over 7∼10 days, a typical monitoring period for patients with severe ASAH. Similarly, Schulz et al. 23 observed initially elevated glutamate concentrations. Andelius et al. (2019) observed stabilization of glucose, lactate, and pyruvate over 12 h; they did not measure glutamate levels. Thus, we favor allowing a 24-h stabilization period for CMD to exclude this initial period of high glutamate.
The major novelty in our approach consists firstly in focusing on measurements performed between the last negative scan and the first positive scan showing evidence of DCI. This focus led us to discard about 60% of the CMD data available from patients who developed DCI. The remaining CMD data were aligned on the last measurement performed immediately before the positive scan. This approach likely enhanced statistical power relative to analyzing all CMD values regardless of DCI occurrence timing. It also allowed us to visualize changes during the 84-h period preceding infarct detection. When we measured from within the lesion, there was a clear ischemic pattern characterized by drops in glucose and oxygen, an increase in lactate, and a massive glutamate surge. These observations made it possible to identify the metabolic biomarkers that were most predictive of DCI.
The focal nature of CMD enables differentiation of monitoring data measured from within the lesion—where dramatic DCI-related biochemical changes are expected—from data measured outside the lesion. Here, CMD probes were implanted in the territories of an affected parent vessel, usually the anterior cerebral artery or middle cerebral artery.13,17 The data were acquired before recent publications proposing slightly more efficient implantation algorithms.15,24 The CMD probes implanted in this study fell within infarcted brain areas in 68% of patients (8/25), which was slightly lower than the 71% and 79% rates in Ulrich et al. 24 and Tholance et al. 15 respectively. In fact, probes missing infarcted areas constituted the main reason for failures to detect the development of DCI in advance.
Glutamate has been consistently reported to increase in association with DCI.23,25–29 Here, glutamate increased dramatically before DCI only when monitored from infarcted brain areas. This lack of overflow limited the utility of glutamate as a biomarker of DCI, and decreased its sensitivity to only 68.2%. In addition, even when the probe was correctly placed within an infarct, the increase in glutamate interstitial concentration appeared several hours later than changes in glucose of L/G. It is likely that glutamate was released, when neuronal cells lose their ability to maintain transmembrane ion gradients, and release their neurotransmitter content. Overall, glutamate monitoring showed little interest as an advanced warning for DCI in our study. Glucose, L/P, and L/G had the potential to give advance warning of infarct occurrence by 18 h, 20 h, and 56 h, respectively, when measured from within the lesion. The advance warning was reduced to 4 h, 1 h, and 35 h, respectively, when the probe was located outside infarct zones. Patients in whom the CMD probe missed the infarcted area had a less elevated ICP than those with the probe located in the lesion. It is possible that those patients with the probe outside the lesion tended to have less severe infarctions and/or smaller infarcted areas than those with a probe within the infarct. If so, it would have contributed to smaller metabolic changes detected by probes outside the lesion.
We identified glucose and L/G ratio as biomarkers within as well as outside lesions. This spillover into neighboring areas could reflect diffusion of glucose and lactate over astrocytic networks supplying ischemic brain areas from interstitial sources in remote healthy areas. 30 Alternatively, cortical spreading depolarizations, which are often detected in poor-grade ASAH patients, could leave glucose decrements and augmented lactate in their wake as they travel away from infarcted areas.31,32 In most cases, probes located outside lesions missed the infarct by less than 2 cm. Therefore, glucose and L/G alterations did not need to extend far to enable detection. Conversely, pyruvate and lactate, and therefore L/P ratio, were poor biomarkers. Pyruvate decreased only in a subset of patients with pathological IH and low CPP, and lactate displayed only modest increases before DCI.
The L/P ratio is widely used to estimate anaerobic glycolysis in the brain, and has been proposed as a biomarker of ischemic secondary injury in ASAH patients.7,12,13,33–38 Although L/P increases are well-documented before DCI7,34,37 and during episodes of hypoperfusion,14,38 the potential value of L/P as a biomarker of DCI has seldom been quantified. Previous studies found that L/P ratio had 100% sensitivity and specificity in 5 patients, 34 and a 70–75% positive predictive value in 20 patients. 14 In this study, including CMD values both within and outside infarcted areas, L/P ratio had only 50% sensitivity and 100% specificity, which was not sufficient for use as a DCI biomarker. Helbok et al. 7 attributed changes in L/P preceding DCI mostly to variations in lactate concentration. Moreover, Jacobsen et al. 36 identified a subset of patients presenting anomalously high lactate and glutamate, but normal pyruvate levels, reflecting a state of mitochondrial dysfunction that sometimes evolved into DCI. Here, pyruvate measured before DCI decreased only in a subset of patients showing pathological IH (ICP > 20 mmHg and CPP < 50 mmHg), but remained stable in patients with CPP > 50 mmHg. Overall, pyruvate, and hence, the L/P ratio appear to correlate poorly with later DCI occurrence.
In the present context of multimodal monitoring, PbtO2 and ICP also provided advance warning for DCI. Similar to CMD markers, PbtO2 lost most of its advance warning power when the probe was located outside the lesion. Although our small sample size did not allow us to differentiate the predictive value of these four biomarkers, there was a significantly longer interval to infarct detection on CT from L/G change than from PbtO2 change. Therefore L/G appears to be a particularly efficient parameter for prediction of DCI. ICP has the advantage of being uniform throughout the cranium. We could not detect any significant difference between ICP and glucose or L/G in terms of either biomarker sensitivity or specificity, or temporal delay between cutoff value crossing and infarct detection. Larger sample larger clinical trials would be required to further determine the strengths and weaknesses of CMD markers over ICP.
Perfusion CT and CT angiography can reveal areas of hypoperfusion and thus indicate which regions are most at risk of DCI.39–43 However, repeated CT scans require frequent intra-hospital transports, which may cause adverse effects that increase risk of delayed injury.44,45 Bedside online monitoring methods have the potential to indicate when more powerful imaging proof of hypoperfusion is warranted, while minimizing repeated imaging. We propose that a criterion of L/G > 7.5 for imaging can provide optimally sensitive advance warning of DCI threat. In our hands, this threshold provided 100% sensitivity, allowing the 70% of patients most at risk for DCI to be selected for timely brain imaging within 3 days before a possible infarct.
Determination of the exact date of infarct formation is difficult. CT scan is usually considered a poorly sensitive method to detect brain infarcts, and it is likely that our CT scan observations of DCI-related lesions came later than their actual development in patients. By contrast, uncontrolled glutamate release is considered an immediate consequence of energy failure leading to the loss of transmembrane ion gradients, and therefore, an early step in lesion development. It is likely that infarcts developed during the time period between the glutamate surge and its detection by CT scan, which in our hands, represented less than one day. Therefore, even considering the possible delay between infarct formation and its observation with CT scan, LG > 7.5, and possibly, glucose or ICP, offered significant advance warning of DCI occurrence. Application of this recommendation could facilitate DCI detection risk in severe ASAH patients, allowing for more timely therapeutic intervention with treatments such a nimodipine, balloon angioplasty, or intra-arterial vasodilators. 46
Study limitations
This study employed retrospective analysis of multimodal monitoring data obtained at a single center with a limited cohort size. Hence, our results require generalization to a larger population at other centers. Additionally, identifying probe location as inside or outside the infarcted area from CT images can be complicated, especially retrospectively, although this distinction has been determined by expert radiologists. CMD probe location was determined based on the gold tip of the CMD probe, but PbtO2 probes were placed 25 mm deeper in the tissue. In addition, because PbtO2 and ICP data are instantaneously sampled, transient fluctuations (<1 h) in these parameters could have been missed.
Finally, we could not reconstruct the volume of the brain infarcts detected by CT scan. It is possible that in patients with a CMD probe outside the infarct, the volume of the brain infarct could have been smaller in average than in patients with a CMD probe inside the infarct (this possibility is supported by a smaller increase in ICP when the CMD probe was outside the infarct). If infarct volume was significantly smaller in the probe outside group compared to the probe inside group it would be possible that biomarker changes would also be smaller in this group.
Conclusion
This study demonstrated that, despite its local nature, CMD represents a powerful method for investigating the brain metabolic disturbances preceding DCI in severe ASAH patients. CMD data were predictive of DCI more than 2 days before infarctions became apparent. L/G ratio and glucose emerged as the most reliable predictive biomarkers of DCI with their variations being detectable both within and nearby infarcted areas. We propose that L/G > 7.5 be used as a criterion to order imaging for confirmation of hypoperfusion likely to evolve towards DCI. This approach could allow more timely intervention and thus improve patient outcomes.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231193661 for Early brain metabolic disturbances associated with delayed cerebral ischemia in patients with severe subarachnoid hemorrhage by Yannick Tholance, Sami Aboudhiaf, Baptiste Balança, Gleicy Keli Barcelos, Sebastien Grousson, Romain Carrillon, Thomas Lieutaud, Armand Perret-Liaudet, Frédéric Dailler and Stéphane Marinesco in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors thank the entire team who supported and directed care for the patient in the neurological ICU of Lyon, including physicians, nurses, laboratory personnel, environmental services, and hospital administrators.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SM, SA, and BB were supported by the Lyon Neuroscience Research Center, Inserm U1028, CNRS UMR5292 and Université Claude Bernard Lyon I, and Fondation des Gueules Cassées (grant FGC 31-2018).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: YT designed the study, performed CMD measurements, analyzed data, wrote the manuscript; SA analyzed the data and discussed the manuscript; BB performed statistical analyses and discussed the manuscript; GKB, SG, RC, TL, and FD selected patients, provided patient care, analyzed the data and discussed the manuscript; A.P-L. analyzed data and discussed the manuscript; SM designed the study, analyzed data and wrote the manuscript.
ORCID iD: Baptiste Balança https://orcid.org/0000-0001-7917-9645
Supplementary material: Supplemental material for this article is available online.
References
- 1.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58. [DOI] [PubMed] [Google Scholar]
- 2.Charpentier C, Audibert G, Guillemin F, et al. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke 1999; 30: 1402–1408. [DOI] [PubMed] [Google Scholar]
- 3.Suarez JI. Diagnosis and management of subarachnoid hemorrhage. Continuum (Minneap Minn) 2015; 21: 1263–1287. [DOI] [PubMed] [Google Scholar]
- 4.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41: 2391–2395. [DOI] [PubMed] [Google Scholar]
- 5.Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care 2016; 20: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balanca B, Ritzenthaler T, Gobert F, et al. Significance and diagnostic accuracy of early S100B serum concentration after aneurysmal subarachnoid hemorrhage. J Clin Med 2020; 9: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helbok R, Madineni RC, Schmidt MJ, et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit Care 2011; 14: 162–167. [DOI] [PubMed] [Google Scholar]
- 8.Helbok R, Olson DM, Le Roux PD, et al. Intracranial pressure and cerebral perfusion pressure monitoring in non-TBI patients: special considerations. Neurocrit Care 2014; 21 Suppl 2: S85–94. [DOI] [PubMed] [Google Scholar]
- 9.Hani L, Ropelato MD, Wagner F, et al. Individualized brain tissue oxygen-monitoring probe placement helps to guide therapy and optimizes outcome in neurocritical care. Neurocrit Care 2021; 35: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma 2007; 24 Suppl 1: S55–58. [DOI] [PubMed] [Google Scholar]
- 11.Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg 1992; 76: 72–80. [DOI] [PubMed] [Google Scholar]
- 12.Persson L, Valtysson J, Enblad P, et al. Neurochemical monitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg 1996; 84: 606–616. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson PJ, Jalloh I, Helmy A, et al. Consensus statement from the 2014 international microdialysis forum. Intensive Care Med 2015; 41: 1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patet C, Quintard H, Zerlauth JB, et al. Bedside cerebral microdialysis monitoring of delayed cerebral hypoperfusion in comatose patients with poor grade aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2017; 88: 332–338. [DOI] [PubMed] [Google Scholar]
- 15.Tholance Y, Barcelos GK, Perret-Liaudet A, et al. Placing intracerebral probes to optimise detection of delayed cerebral ischemia and allow for the prediction of patient outcome in aneurysmal subarachnoid haemorrhage. J Cereb Blood Flow Metab 2017; 37: 2820–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diringer MN, Bleck TP, Claude Hemphill J, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society's multidisciplinary consensus conference. Neurocrit Care 2011; 15: 211–240. [DOI] [PubMed] [Google Scholar]
- 17.Bellander BM, Cantais E, Enblad P, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med 2004; 30: 2166–2169. [DOI] [PubMed] [Google Scholar]
- 18.Tholance Y, Barcelos G, Quadrio I, et al. Analytical validation of microdialysis analyzer for monitoring glucose, lactate and pyruvate in cerebral microdialysates. Clin Chim Acta 2011; 412: 647–654. [DOI] [PubMed] [Google Scholar]
- 19.Andelius TCK, Pedersen MV, Bogh N, et al. Consequence of insertion trauma – effect on early measurements when using intracerebral devices. Sci Rep 2019; 9: 10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoerle T, Lombardo A, Colombo A, et al. Intracranial pressure after subarachnoid hemorrhage. Crit Care Med 2015; 43: 168–176. [DOI] [PubMed] [Google Scholar]
- 21.Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci 2001; 69: 369–381. [DOI] [PubMed] [Google Scholar]
- 22.Bungay PM, Sumbria RK, Bickel U. Unifying the mathematical modeling of in vivo and in vitro microdialysis. J Pharm Biomed Anal 2011; 55: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz MK, Wang LP, Tange M, et al. Cerebral microdialysis monitoring: determination of normal and ischemic cerebral metabolisms in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 2000; 93: 808–814. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich CT, Fung C, Vatter H, et al. Occurrence of vasospasm and infarction in relation to a focal monitoring sensor in patients after SAH: placing a bet when placing a probe? PloS One 2013; 8: e62754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakowitz OW, Sarrafzadeh AS, Benndorf G, et al. On-line microdialysis following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl 2001; 77: 141–144. [DOI] [PubMed] [Google Scholar]
- 26.Sarrafzadeh A, Haux D, Plotkin M, et al. Bedside microdialysis reflects dysfunction of cerebral energy metabolism in patients with aneurysmal subarachnoid hemorrhage as confirmed by 15 O-H2 O-PET and 18 F-FDG-PET. J Neuroradiol 2005; 32: 348–351. [DOI] [PubMed] [Google Scholar]
- 27.Sarrafzadeh AS, Haux D, Ludemann L, et al. Cerebral ischemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis-PET study. Stroke 2004; 35: 638–643. [DOI] [PubMed] [Google Scholar]
- 28.Sarrafzadeh AS, Sakowitz OW, Kiening KL, et al. Bedside microdialysis: a tool to monitor cerebral metabolism in subarachnoid hemorrhage patients? Crit Care Med 2002; 30: 1062–1070. [DOI] [PubMed] [Google Scholar]
- 29.Unterberg AW, Sakowitz OW, Sarrafzadeh AS, et al. Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 2001; 94: 740–749. [DOI] [PubMed] [Google Scholar]
- 30.Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 2008; 322: 1551–1555. [DOI] [PubMed] [Google Scholar]
- 31.Balança B, Meiller A, Bezin L, et al. Altered hypermetabolic response to cortical spreading depolarizations after traumatic brain injury in rats. J Cereb Blood Flow Metab 2017; 37: 1670–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: Review and recommendations of the COSBID research group. J Cereb Blood Flow Metab 2017; 37: 1595–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Micheli E, Pinna G, Piovan E, et al. Monitoring subtle neurometabolic changes in subarachnoid hemorrhage patients using microdialysis: a study on 16 cases. Acta Neurochir Suppl 2001; 77: 149–153. [DOI] [PubMed] [Google Scholar]
- 34.Enblad P, Valtysson J, Andersson J, et al. Simultaneous intracerebral microdialysis and positron emission tomography in the detection of ischemia in patients with subarachnoid hemorrhage. J Cereb Blood Flow Metab 1996; 16: 637–644. [DOI] [PubMed] [Google Scholar]
- 35.Helbok R, Kofler M, Schiefecker AJ, et al. Clinical use of cerebral microdialysis in patients with aneurysmal subarachnoid hemorrhage – state of the art. Front Neurol 2017; 8: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobsen A, Nielsen TH, Nilsson O, et al. Bedside diagnosis of mitochondrial dysfunction in aneurysmal subarachnoid hemorrhage. Acta Neurol Scand 2014; 130: 156–163. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson OG, Brandt L, Ungerstedt U, et al. Bedside detection of brain ischemia using intracerebral microdialysis: subarachnoid hemorrhage and delayed ischemic deterioration. Neurosurgery 1999; 45: 1176–1184. discussion 1184-1175. [DOI] [PubMed] [Google Scholar]
- 38.Rostami E, Engquist H, Johnson U, et al. Monitoring of cerebral blood flow and metabolism bedside in patients with subarachnoid hemorrhage – a xenon-CT and microdialysis study. Front Neurol 2014; 5: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dankbaar JW, de Rooij NK, Rijsdijk M, et al. Diagnostic threshold values of cerebral perfusion measured with computed tomography for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 2010; 41: 1927–1932. [DOI] [PubMed] [Google Scholar]
- 40.Dankbaar JW, de Rooij NK, Velthuis BK, et al. Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke 2009; 40: 3493–3498. [DOI] [PubMed] [Google Scholar]
- 41.Mir DI, Gupta A, Dunning A, et al. CT perfusion for detection of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2014; 35: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanelli PC, Pandya A, Segal AZ, et al. Cost-effectiveness of CT angiography and perfusion imaging for delayed cerebral ischemia and vasospasm in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2014; 35: 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cremers CH, van der Schaaf IC, Wensink E, et al. CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2014; 34: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuchler J, Tronnier F, Smith E, et al. The impact of intrahospital transports on brain tissue metabolism in patients with acute brain injury. Neurocrit Care 2019; 30: 216–223. [DOI] [PubMed] [Google Scholar]
- 45.Hosmann A, Angelmayr C, Hopf A, et al. Detrimental effects of intrahospital transport on cerebral metabolism in patients suffering severe aneurysmal subarachnoid hemorrhage. J Neurosurg 2021; 1–8. [DOI] [PubMed] [Google Scholar]
- 46.Neifert SN, Chapman EK, Martini ML, et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res 2021; 12: 428–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231193661 for Early brain metabolic disturbances associated with delayed cerebral ischemia in patients with severe subarachnoid hemorrhage by Yannick Tholance, Sami Aboudhiaf, Baptiste Balança, Gleicy Keli Barcelos, Sebastien Grousson, Romain Carrillon, Thomas Lieutaud, Armand Perret-Liaudet, Frédéric Dailler and Stéphane Marinesco in Journal of Cerebral Blood Flow & Metabolism