Abstract
Study design
Retrospective Cohort Study
Objective
The purpose of this study was to investigate the prognosis of respiratory function in elderly patients with cervical spinal cord injury (SCI) and to identify predictive factors.
Methods
We included 1353 cases of elderly cervical SCI patients collected from 78 institutions in Japan. Patients who required early tracheostomy and ventilator management and those who developed respiratory complications were defined as the respiratory disability group. Patients’ background characteristics, injury mechanism, injury form, neurological disability, complications, and treatment methods were compared between the disability and non-disability groups. Multiple logistic regression analysis was used to examine the independent factors. Patients who required respiratory management for 6 months or longer after injury and those who died of respiratory complications were classified into the severe disability group and were compared with minor cases who were weaned off the respirator.
Results
A total of 104 patients (7.8%) had impaired respiratory function. Comparisons between the disabled and non-disabled groups and between the severe and mild injury groups yielded distinct trends. In multiple logistic regression analysis, age, blood glucose level, presence of ossification of posterior longitudinal ligament (OPLL), anterior vertebral hematoma, and critical paralysis were selected as independent risk factors.
Conclusion
Age, OPLL, severe paralysis, anterior vertebral hematoma, hypoalbuminemia, and blood glucose level at the time of injury were independent factors for respiratory failure. Hyperglycemia may have a negative effect on respiratory function in this condition.
Keywords: cervical spinal cord injury, respiratory dysfunction, risk factor, hyperglycemia
Introduction
In the early stage of spinal cord injury (SCI), spinal cord shock causes loss of reflexes below the level of injury and flaccid paralysis. In acute cervical SCI with residual diaphragmatic function, contraction of the diaphragm pulls in the relaxed upper thorax, resulting in ectopic respiration. Because of the poor ventilatory efficiency of ectopic respiration, tracheostomy should be considered if respiratory status does not improve with temporary ventilator management and long-term intubation is necessary. In the autonomic nervous system, the sympathetic nervous system is paralyzed and the parasympathetic nervous system becomes dominant, resulting in increased airway secretions and pulmonary edema. As a result, in acute cases, even if the paralytic level is below the C4 level and the diaphragm is functional and positive pressure ventilation with a ventilator is unnecessary, respiratory complications due to sputum accumulation may occur due to paralysis of the abdominal and intercostal muscles, which play an important role in expelling lung secretions. In addition, edema of the injured spinal cord may cause paralysis to ascend, resulting in temporary worsening of respiratory muscle paralysis. Under these circumstances, respiratory function may also deteriorate owing to respiratory muscle fatigue. Because of these mechanisms, the respiratory condition may deteriorate within a few days, although respiration is maintained immediately after the injury. Therefore, cases have been observed where patients who had no breathing problems for approximately 2 days after receiving the injury, gradually developed breathing problems and required tracheostomy and ventilator management. In recent years, the number of injuries due to minor trauma in the elderly has been increasing.1,2 Elderly patients with SCI are at a high risk of developing pneumonia or atelectasis, which can be fatal due to decreased respiratory, cardiopulmonary, and swallowing functions, all directly related to increased mortality. In particular, respiratory failure can occur even with good respiratory conditions at the time of admission. Early prediction and intervention in such cases are directly related to treatment outcomes. In this study, we examined cases of cervical SCI in elderly patients who required tracheostomy and ventilator management due to respiratory failure and investigated the risk factors for requiring tracheostomy. Furthermore, this study investigated the risk factors in patients with severe respiratory failure who cannot be taken off the ventilator for long and need to be managed.
Materials and Methods
This study retrospectively analyzed multicenter registry data collected by the Japan Association of Spine Surgeons with Ambition (JASA). The institutional review board of a representative facility reviewed and approved this study (No. 3352-1).
Informed Consent
Because this was a retrospective study, informed consent was not required for submission. On the contrary, the optout of this study was posted on the website (and title https://web.sapmed.ac.jp/orsurg/guide/hj0g2h00000007ax-att/pgsps60000000g3l.pdf and https://web.sapmed.ac.jp/orsurg/guide/hj0g2h00000007ax-att/pgsps60000000g3l.pdf) and we did not receive any inquiries.
Patient Population
Participants included patients aged 65 years and above with traumatic cervical SCI and/or traumatic cervical fracture, who were treated conservatively or surgically from 2010 to 2020 at an institution registered in the JASA and monitored for at least 3 months after the injury. Patients with cervical metastasis, chest injury, thoracolumbar injury, and missing data were excluded. In total, 1353 patients from 78 institutions were enrolled in this study (average age: 75.9± 6.9 years; 906 males and 447 females). The definition of cervical SCI without bone injury was SCI with no evidence of spinal fracture or dislocation on plain radiography or computed tomography (CT).
We divided subjects into 2 groups based on respiratory status after injury. We defined respiratory dysfunction as cases that required tracheostomy early after injury, or cases in which respiratory complications such as pneumonia or atelectasis appeared. Furthermore, among the respiratory dysfunction cases, those who were weaned from respiratory management at 6 months after injury were classified as the mild respiratory insufficiency group, and those who were not weaned from respiratory management or died due to respiratory insufficiency were classified as the severe respiratory insufficiency group.
Additional to the analysis of all cases, we extracted only cases without bone injury and analyzed them through similar classifications.
Collected Data
Patients’ characteristics, age, sex, height, weight, body mass index, and history of smoking were investigated to determine the endogenous factors affecting the outcomes. Laboratory data on admission included the levels of hemoglobin (Hb) (g/dL), total protein (g/dL), albumin (Alb) (g/dL), blood glucose level (mg/dL), and HbA1c (%), which included the presence of a cervical fracture and cervical ossification of the posterior longitudinal ligament (OPLL) as detected by plain radiography and/or CT. In addition, data regarding signal changes in the spinal cord and anterior vertebral hematoma on T2-weighted magnetic resonance imaging were collected.
As a neurological impairment scale to determine the presence of SCI, the ASIA Impairment Scale (AIS) 3 was used. Complications at injury, including head injury, abdominal organ damage, pelvic fracture, and vertebral artery injury were estimated. Data on concomitant disease, cerebrovascular disease, dementia, heart disease, respiratory illness, and diabetes mellitus were collected. The initial response, steroid, and early post-injury surgery (<24 h after injury) were estimated. Regarding treatment, presence of surgical treatment, surgical procedure, operation time, and amount of blood loss were assessed.
Statistical Analysis
Continuous variables were evaluated using Student’s t-tests. Non-continuous variables were evaluated using the χ2 test and Fisher’s exact test. For each cohort, all variables were included in the multivariate analysis as explanatory variables. Furthermore, to identify predictors of respiratory dysfunction, we performed multivariate logistic regression analysis with stepwise variable selection using all of the survey items before respiratory dysfunction. The adjusted odds ratios (aORs) and 95% confidence intervals (CIs) of the dependent variables were calculated. All analyses were performed using SPSS software (version 23; SPSS, Chicago, IL, USA). Statistical significance was set at P < .05.
Results
Patient Characteristics
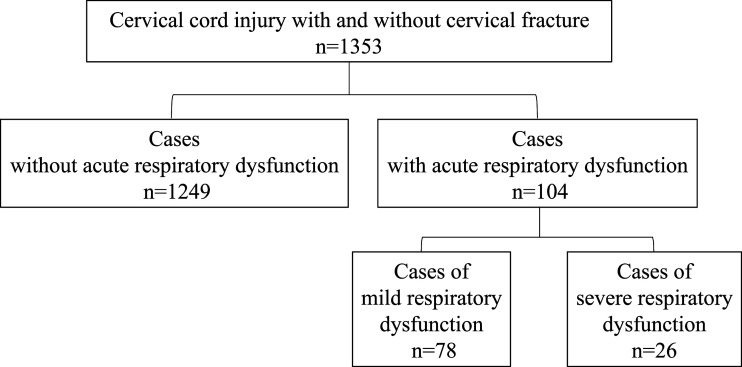
This study involved 104 patients (7.8% of entire cohort) with early respiratory dysfunction who required early tracheostomy or had respiratory complications after injury. In addition, 26 of the 104 patients with severe disability required respiratory management for up to 6 months after injury and died of respiratory complications, while 78 patients with mild disability were successfully weaned from the ventilator (Figure 1).
Figure 1.
Group Comparisons: Entire Cohort
The comparison between the 2 groups with regard to acute respiratory dysfunction in the entire cohort showed that the incapacitated patients were older (P = .006), more likely male (P < .001), taller (P = .033), blood tests for total lower protein (P < .001), lower Alb (P < .001), lower Hb (P = .003), and higher blood glucose level at the time of injury (P < .001). In radiologic data, respiratory dysfunction group demonstrated a prevalence of OPLL (P < .001), magnetic resonance imaging (MRI) intramedullary signal change (P < .001), anterior vertebral hematoma (P = .002), rate of neurological symptoms (P < .001), severity of paralysis (P < .001), abdominal injury (P = .023), and vertebral artery injury (P = .003).There were significantly more cases of previous heart disease (P = .006), respiratory disease (P = .011), and early surgery (P = .001) (Table 1).
Table 1.
Univariate Analysis of Demographics and Clinical Data of the Influence on Acute Respiratory Dysfunction in all Patients.
| All cases | Item | Respiratory dysfunction - | Respiratory dysfunction + | P | |
|---|---|---|---|---|---|
| n = 1249 | n = 104 | ||||
| Patients’ background | Age at injury | 75.7 ± 6.9 | 77.7 ± 7.1 | 0.006 | |
| Sex: Male (%) | 65.5 | 84.6 | <0.001 | ||
| Height (cm) | 159.2 ± 9.2 | 161.3 ± 9.8 | 0.033 | ||
| Weight (kg) | 56.1 ± 11.2 | 57.2 ± 11.4 | 0.363 | ||
| Body mass index (kg/m2) | 22.1 ± 3.5 | 22.1 ± 4.4 | 0.995 | ||
| Smoking history | 18.7 | 23.1 | 0.341 | ||
| Laboratory data | TP (g/dL) | 6.7 ± 0.7 | 6.3 ± 0.7 | <0.001 | |
| Alb (g/dL) | 3.8 ± 0.6 | 3.4 ± 0.5 | <0.001 | ||
| Hb (g/dL) | 12.8 ± 1.9 | 12.2 ± 1.8 | 0.003 | ||
| Blood glucose level(mg/dL) | 138.4 ± 50.4 | 165.5 ± 84.5 | <0.001 | ||
| HbA1c (%) | 6.2 ± 1.0 | 6.0 ± 1.1 | 0.353 | ||
| Radiologic data | Cervical OPLL (%) | 11.7 | 31.7 | <0.001 | |
| Intramedullary signal change (%) | 57.1 | 78.8 | <0.001 | ||
| Level of intramedullary signal change (%) | C1 | 3.4 | 1.0 | 0.345 | |
| C2 | 5.9 | 11.3 | |||
| C3 | 37.8 | 38.9 | |||
| C4 | 25.6 | 17.5 | |||
| C5 | 20.5 | 15.5 | |||
| C6 | 7.9 | 13.4 | |||
| C7 | 0.9 | 2.4 | |||
| Anterior vertebral hematoma (%) | 50.3 | 66.3 | 0.002 | ||
| Neurological impairment scale | Spinal cord injury (%) | 69.7 | 92.3 | <0.001 | |
| AIS A | 7.4 | 50.9 | <0.001 | ||
| B | 5.5 | 12.3 | |||
| C | 32.0 | 28.1 | |||
| D | 55.1 | 8.7 | |||
| Complications (%) | Complicated | 28.6 | 35.1 | 0.162 | |
| Head injury | 12.2 | 5.8 | 0.073 | ||
| Abdominal organ damage | 0.5 | 2.9 | 0.023 | ||
| Pelvic fracture | 0.8 | 1.0 | 1.000 | ||
| Vertebral artery injury | 2.7 | 8.7 | 0.003 | ||
| Concomitant diseases (%) | Cerebrovascular disease | 10.4 | 5.8 | 0.180 | |
| Dementia | 6.2 | 4.8 | 0.731 | ||
| Heart disease | 14.3 | 25.0 | 0.006 | ||
| Respiratory disease | 5.0 | 11.5 | 0.011 | ||
| Diabetes mellitus | 22.1 | 25.8 | 0.384 | ||
| Initial response (%) | Steroid | 14.0 | 20.2 | 0.115 | |
| Early post-injury surgery | 5.1 | 13.5 | 0.001 | ||
| Halo-vest | 6.7 | 7.7 | 0.862 | ||
| Surgical treatment | Surgical treatment (%) | 60.3 | 67.3 | 0.192 | |
| Post. decomp | 30.6 | 25.9 | 0.349 | ||
| Post. fusion | 38.0 | 38.3 | |||
| Post. decomp and fusion | 21.3 | 27.1 | |||
| Ant. fusion | 5.3 | 2.5 | |||
| Ant. decomp and fusion | 2.0 | 6.2 | |||
| Ant. and Post. | 0.7 | 0.0 | |||
| Post. and Ant. | 2.1 | 0.0 | |||
| Operation time (min) | 160.4 ± 68.2 | 176.1 ± 70.8 | 0.074 | ||
| Amount of blood loss (mL) | 206.8 ± 312.7 | 271.2 ± 354.0 | 0.121 | ||
Abbreviations: TP, total protein; Alb, albumin; Hb, hemoglobin; OPLL, ossification of posterior longitudinal ligament; AIS, ASIA Impairment Scale; Post., posterior; Ant., anterior.
Comparison of severe respiratory dysfunction between the 2 groups in the entire cohort showed that intramedullary signal changes (P = .027), neurological symptoms (P < .001), and heart disease history (P = .036) were significantly higher in the severe group (Table 2).
Table 2.
Results of Univariate Analysis of Demographic and Clinical Data of Respiratory Worsening Severity in all Patients.
| All cases | Item | Mild respiratory dysfunction | Severe respiratory dysfunction | P | |
|---|---|---|---|---|---|
| n = 78 | n = 26 | ||||
| Patients’ background | Age at injury | 77.0 ± 7.1 | 79.6 ± 7.4 | 0.108 | |
| Sex: Male (%) | 80.8 | 96.2 | 0.117 | ||
| Height (cm) | 160.6 ± 9.8 | 163.5 ± 6.8 | 0.222 | ||
| Weight (kg) | 57.9 ± 11.4 | 55.1 ± 11.9 | 0.305 | ||
| Body mass index (kg/m2) | 22.6 ± 4.4 | 20.6 ± 4.7 | 0.064 | ||
| Smoking history | 25.6 | 15.4 | 0.420 | ||
| Laboratory data | TP (g/dL) | 6.4 ± 0.7 | 6.1 ± 0.7 | 0.173 | |
| Alb (g/dL) | 3.5 ± 0.5 | 3.3 ± 0.5 | 0.062 | ||
| Hb (g/dL) | 12.3 ± 1.8 | 11.9 ± 1.6 | 0.337 | ||
| Blood glucose level (mg/dL) | 175.2 ± 84.5 | 137.3 ± 52.9 | 0.069 | ||
| HbA1c (%) | 6.0 ± 1.1 | 6.4 ± 1.5 | 0.292 | ||
| Radiologic data | Cervical OPLL (%) | 34.6 | 23.1 | 0.395 | |
| Intramedullary signal change (%) | 61.5 | 84.6 | 0.027 | ||
| Level of intramedullary signal change (%) | C1 | 1.3 | 0.0 | 0.345 | |
| C2 | 11.8 | 9.5 | |||
| C3 | 41.4 | 28.0 | |||
| C4 | 15.8 | 23.8 | |||
| C5 | 14.5 | 19.0 | |||
| C6 | 11.8 | 18.9 | |||
| C7 | 3.4 | 0.8 | |||
| Anterior vertebral hematoma | 71.8 | 50.0 | 0.072 | ||
| Neurological impairment scale | Spinal cord injury | 73.1 | 96.2 | <0.001 | |
| AIS A | 49.5 | 56.5 | 0.202 | ||
| B | 14.1 | 4.6 | |||
| C | 28.6 | 26.1 | |||
| D | 7.8 | 12.8 | |||
| Complications (%) | Complicated | 30.0 | 42.5 | 0.244 | |
| Head injury | 3.8 | 11.5 | 0.331 | ||
| Abdominal organ damage | 3.8 | 0.0 | 0.735 | ||
| Pelvic fracture | 0.0 | 3.8 | 0.562 | ||
| Vertebral artery injury | 7.7 | 11.5 | 0.840 | ||
| Concomitant diseases (%) | Cerebrovascular disease | 6.4 | 3.8 | 1.000 | |
| Dementia | 3.8 | 7.7 | 0.791 | ||
| Heart disease | 19.2 | 42.3 | 0.036 | ||
| Respiratory illness | 11.5 | 11.5 | 1.000 | ||
| Diabetes mellitus | 26.9 | 23.3 | 0.485 | ||
| Initial response (%) | Steroid | 21.8 | 15.4 | 0.672 | |
| Early post-injury surgery | 14.1 | 11.5 | 1.000 | ||
| Halo-vest | 7.7 | 7.7 | 1.000 | ||
| Surgical treatment | Surgical treatment (%) | 71.8 | 53.8 | 0.148 | |
| Post. decomp | 26.2 | 25.0 | 0.755 | ||
| Post. fusion | 33.8 | 56.3 | |||
| Post. decomp and fusion | 32.3 | 12.5 | |||
| Ant. fusion | 1.5 | 6.3 | |||
| Ant. decomp and fusion | 6.2 | 0.0 | |||
| Ant. and Post. | 0.0 | 0.0 | |||
| Post. and Ant. | 0.0 | 0.0 | |||
| Operation time (min) | 176.8 ± 70.8 | 173.0 ± 50.5 | 0.869 | ||
| Amount of blood loss | 281.5 ± 354.0 | 226.3 ± 272.1 | 0.630 | ||
Abbreviations: TP, total protein; Alb, albumin; Hb, hemoglobin; OPLL, ossification of posterior longitudinal ligament; AIS, ASIA Impairment Scale; Post., posterior; Ant., anterior.
Risk Factors: Entire Cohort
In the multivariate analysis with early disability as the dependent variable, age at injury (P = .033; odds ratio [OR] 1.055), Alb at injury (P = .037; OR .509), blood glucose level (P = .022; OR 1.006), presence of cervical OPLL (P < .001; OR 4.152), anterior vertebral hematoma (P = .005; OR 3.226), and severe neurological symptoms (AIS A or B) (P < .001 OR 9.364) were independent risk factors (Table 3).
Table 3.
Logistic Regression Analysis of Risk Factors for Acute Respiratory Dysfunction in the Entire Cohort.
| Partial regression coefficient | Standard error | Wald | P-value | Odds ratio | Confidence interval | ||
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Age at injury | 0.054 | 0.025 | 4.536 | 0.033 | 1.055 | 1.004 | 1.109 |
| Alb (g/dL) | −0.676 | 0.323 | 4.369 | 0.037 | 0.509 | 0.270 | 0.959 |
| Blood glucose level | 0.006 | 0.002 | 5.239 | 0.022 | 1.006 | 1.001 | 1.011 |
| Cervical OPLL | 1.424 | 0.371 | 14.736 | <0.001 | 4.152 | 2.007 | 8.588 |
| Anterior vertebral hematoma | 1.171 | 0.418 | 7.854 | 0.005 | 3.226 | 1.422 | 7.318 |
| AIS A or B | 2.237 | 0.342 | 42.817 | <0.001 | 9.364 | 4.792 | 18.300 |
Abbreviations: Alb, albumin; OPLL, ossification of posterior longitudinal ligament; AIS, ASIA Impairment Scale.
By contrast, in the multivariate analysis with severe respiratory dysfunction as the dependent variable, Alb at injury (P = .038; OR .228), and head injury (P = .048; OR 11.045) were identified as independent risk factors (Table 4).
Table 4.
Logistic Regression Analysis of Risk Factors for Worsening of Respiratory Dysfunction in the Entire Cohort.
| Partial regression coefficient | Standard error | Wald | P-value | Odds ratio | Confidence interval | ||
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Alb (g/dL) | −1.479 | 0.779 | 3.603 | 0.038 | 0.228 | 0.050 | 1.049 |
| Head injury | 2.402 | 1.316 | 3.334 | 0.048 | 11.045 | 0.838 | 145.537 |
Abbreviations: Alb, albumin.
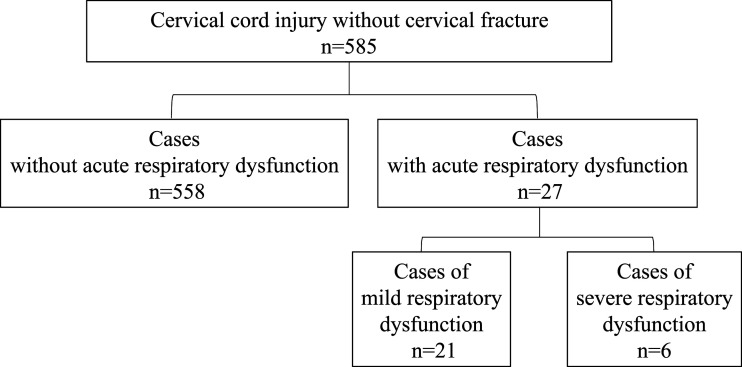
There was a total of 585 cases of cervical SCI without bone injury, of which 27 (4.6% of those analyzed) had early respiratory dysfunction. Of these 27 patients, 6 with severe disability required respiratory management for up to 6 months after injury and died of respiratory complications, and 21 patients had mild dysfunction who were successfully weaned from the ventilator (Figure 2).
Figure 2.
Group Comparisons: Cohort Without Bone Injury
The comparison between the 2 groups with regard to acute respiratory dysfunction in the cohort without bone injury showed older age (P < .001); higher percentage of males (P = .025); lower total protein (P = .037) and Alb (P = .001); and higher incidence of severe paralysis (P < .001), prevalence of heart disease (P = .042), and percentage of early surgical cases (P < .001) (Table 5). There were no significant differences between severely injured and mild cases (Table 6).
Table 5.
Univariate Analysis of Demographics and Clinical Data of the Influence on Acute Respiratory Dysfunction in Patients without Bone Injury.
| All cases | Item | No respiratory dysfunction | Respiratory dysfunction | P | |
|---|---|---|---|---|---|
| n = 558 | n = 27 | ||||
| Patients’ background | Age at injury | 75.2 ± 6.7 | 79.8 ± 7.1 | <0.001 | |
| Sex: Male (%) | 70.8 | 92.6 | 0.025 | ||
| Height (cm) | 160.4 ± 8.8 | 161.4 ± 9.4 | 0.614 | ||
| Weight (kg) | 57.9 ± 11.0 | 57.7 ± 11.8 | 0.922 | ||
| Body mass index (kg/m2) | 22.4 ± 3.5 | 22.5 ± 5.0 | 0.929 | ||
| Smoking history | 22.6 | 22.2 | 1.000 | ||
| Laboratory data | TP (g/dL) | 6.7 ± 0.7 | 6.4 ± 0.7 | 0.037 | |
| Alb (g/dL) | 3.8 ± 0.7 | 3.3 ± 0.5 | 0.001 | ||
| Hb (g/dL) | 13.0 ± 1.9 | 12.6 ± 1.9 | 0.281 | ||
| Blood glucose level (mg/dL) | 139.1 ± 53.1 | 159.4 ± 55.7 | 0.097 | ||
| HbA1c (%) | 6.4 ± 1.1 | 5.5 ± 0.9 | 0.056 | ||
| Radiologic data | Cervical OPLL (%) | 33.7 | 48.1 | 0.181 | |
| Intramedullary signal change (%) | 81.9 | 88.9 | 0.503 | ||
| Level of intramedullary signal change (%) | C1 | 2.9 | 4.0 | 0.213 | |
| C2 | 2.9 | 8.0 | |||
| C3 | 45.1 | 56.0 | |||
| C4 | 28.9 | 28.0 | |||
| C5 | 17.4 | 4.0 | |||
| C6 | 2.3 | 0.0 | |||
| C7 | 0.4 | 0.0 | |||
| Anterior vertebral hematoma (%) | 42.8 | 33.3 | 0.438 | ||
| Neurological impairment scale | Spinal cord injury (%) | 100 | 100 | — | |
| AIS A | 4.6 | 32.1 | <0.001 | ||
| B | 5.3 | 28.6 | |||
| C | 33.4 | 35.7 | |||
| D | 56.7 | 3.6 | |||
| Complications (%) | Complicated | 17.7 | 17.9 | 1.0 | |
| Head injury | 8.6 | 14.8 | 0.446 | ||
| Abdominal organ damage | 0.0 | 0.0 | — | ||
| Pelvic fracture | 0.2 | 3.7 | 0.169 | ||
| Vertebral artery injury | 0.2 | 0.0 | 1.000 | ||
| Concomitant diseases (%) | Cerebrovascular disease | 9.0 | 7.4 | 1.000 | |
| Dementia | 4.3 | 7.4 | 0.774 | ||
| Heart disease | 13.6 | 29.6 | 0.042 | ||
| Respiratory illness | 4.5 | 7.4 | 0.812 | ||
| Diabetes mellitus | 28.0 | 28.6 | 1.0 | ||
| Initial response (%) | Steroid | 21.5 | 25.9 | 0.760 | |
| Early post-injury surgery | 2.3 | 18.5 | <0.001 | ||
| Halo-vest | 0.5 | 3.7 | 0.451 | ||
| Surgical treatment | Surgical treatment (%) | 50.3 | 46.4 | 0.883 | |
| Post. decomp | 78.9 | 92.3 | 0.667 | ||
| Post. fusion | 3.4 | 0.0 | |||
| Post. decomp and fusion | 13.9 | 0.0 | |||
| Ant. fusion | 1.0 | 0.0 | |||
| Ant. decomp and fusion | 2.4 | 7.7 | |||
| Ant. and Post. | 0.3 | 0.0 | |||
| Post. and Ant. | 0.0 | 0.0 | |||
| Operation time (min) | 139.9 ± 56.5 | 127.6 ± 44.5 | 0.439 | ||
| Amount of blood loss | 135.4 ± 201.8 | 183.2 ± 171.8 | 0.402 | ||
Abbreviations: TP, total protein; Alb, albumin; Hb, hemoglobin; OPLL, ossification of posterior longitudinal ligament; AIS, ASIA Impairment Scale; Post., posterior; Ant., anterior.
Table 6.
Univariate Analysis of Demographics and Clinical Data on Respiratory Dysfunction Worsening in Patients without Bone Injury.
| All cases | Item | Mild respiratory dysfunction | Severe respiratory dysfunction | P | |
|---|---|---|---|---|---|
| n = 21 | n = 6 | ||||
| Patient background | Age at injury | 78.7 ± 6.2 | 83.7 ± 9.0 | 0.133 | |
| Sex: Male (%) | 90.5 | 100 | 1.000 | ||
| Height (cm) | 160.9 ± 12.4 | 163.3 ± 9.7 | 0.699 | ||
| Weight (kg) | 60.0 ± 10.8 | 49.2 ± 10.7 | 0.059 | ||
| Body mass index (kg/m2) | 23.7 ± 6.4 | 18.4 ± 3.3 | 0.094 | ||
| Smoking history | 23.8 | 16.7 | 1.000 | ||
| Laboratory data | TP (g/dL) | 6.4 ± 0.7 | 6.3 ± 0.4 | 0.633 | |
| Alb (g/dL) | 3.3 ± 0.6 | 3.3 ± 0.3 | 0.946 | ||
| Hb (g/dL) | 12.7 ± 2.1 | 12.3 ± 1.5 | 0.640 | ||
| Blood glucose level (mg/dL) | 166.2 ± 86.8 | 118.7 ± 19.7 | 0.367 | ||
| HbA1c (%) | 5.8 ± 1.0 | 4.8 ± 1.2 | 0.263 | ||
| Radiologic data | Cervical OPLL (%) | 52.4 | 33.3 | 0.719 | |
| Intramedullary signal change (%) | 90.5 | 83.3 | 1.000 | ||
| Level of intramedullary signal change (%) | C1 | 0.0 | 16.7 | 0.513 | |
| C2 | 10.5 | 0.0 | |||
| C3 | 57.9 | 50.0 | |||
| C4 | 26.3 | 0.0 | |||
| C5 | 5.3 | 0.0 | |||
| C6 | 0.0 | 0.0 | |||
| C7 | 0.0 | 0.0 | |||
| Anterior vertebral hematoma (%) | 33.3 | 33.3 | 1.000 | ||
| Neurological impairment scale | Spinal cord injury (%) | 100.0 | 100.0 | — | |
| AIS A | 33.3 | 33.3 | 0.320 | ||
| B | 33.3 | 0 | |||
| C | 33.3 | 33.3 | |||
| D | 0.0 | 33.3 | |||
| Complications (%) | Complicated | 19.0 | 0.0 | 0.612 | |
| Head injury | 19.0 | 0.0 | 0.612 | ||
| Abdominal organ damage | 0.0 | 0.0 | — | ||
| Pelvic fracture | 4.8 | 0.0 | 1.000 | ||
| Vertebral artery injury | 0.0 | 0.0 | — | ||
| Concomitant diseases (%) | Cerebrovascular disease | 9.5 | 0.0 | 1.000 | |
| Dementia | 4.8 | 16.7 | 0.922 | ||
| Heart disease | 28.6 | 33.3 | 1.000 | ||
| Respiratory illness | 9.5 | 0.0 | 1.000 | ||
| Diabetes mellitus | 23.8 | 33.3 | 0.844 | ||
| Initial response (%) | Steroid | 23.8 | 33.3 | 0.633 | |
| Early post-injury surgery | 23.8 | 0.0 | 0.555 | ||
| Halo-vest | 4.8 | 0.0 | 1.000 | ||
| Surgical treatment | Surgical treatment (%) | 57.1 | 16.7 | 0.198 | |
| Post. decomp | 91.7 | 100 | 1.000 | ||
| Post. fusion | 0.0 | 0.0 | |||
| Post. decomp and fusion | 0.0 | 0.0 | |||
| Ant. fusion | 0.0 | 0.0 | |||
| Ant. decomp and fusion | 8.3 | 0.0 | |||
| Ant. and Post. | 0.0 | 0.0 | |||
| Post. and Ant. | 0.0 | 0.0 | |||
| Operation time (min) | 134.2 ± 39.4 | 49.0 | — | ||
| Amount of blood loss | 196.8 ± 171.9 | 20.0 | — | ||
Abbreviations: TP, total protein; Alb, albumin; Hb, hemoglobin; OPLL, ossification of posterior longitudinal ligament; AIS, ASIA Impairment Scale; Post., posterior; Ant., anterior.
Risk Factors: Cohort Without Bone Injury
In the multivariate analysis with early disability as the dependent variable, Alb at injury (P = .013; OR .236) and severe neurological symptoms (AIS A or B) (P < .001; OR 11.504) were independent risk factors (Table 7).
Table 7.
Logistic Regression Analysis of Risk Factors for Acute Respiratory Dysfunction in Patients without Bone Injury.
| Partial regression coefficient | Standard error | Wald | P-value | Odds ratio | Confidence interval | ||
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Alb (g/dL) | −1.443 | 0.581 | 6.169 | 0.013 | 0.236 | 0.076 | 0.738 |
| AIS A or B | 2.443 | 0.676 | 13.055 | <0.001 | 11.504 | 3.058 | 43.285 |
Abbreviations: Alb, albumin; AIS, ASIA Impairment Scale.
Multivariate analysis with severe respiratory dysfunction as the dependent variable did not reveal any independent risk factors.
Discussion
Various complications can occur after a cervical SCI, although the most frequent are respiratory complications.4-8 Typical complications include atelectasis, pneumonia, and ventilatory failure. These complications often occur in the acute phase within 5 days of injury. 1 If the period of unstable respiratory status in the acute–subacute phase of injury can be weathered, then the respiratory status will subsequently stabilize. Thus, predicting the risk factors for intubation or tracheostomy in patients with cervical SCI is important. 9 In this study, 7.8% of the total patients were classified as the respiratory insufficiency group early after injury. These figures range from 15.2% to 81%, and recent studies have noted that a relatively high percentage of these patients required tracheostomy.1,10-13 The differing results may be due to the fact that the present study included approximately 30% of all fracture cases without neurological symptoms. The incidence of neurological disability was significantly lower in patients without neurological symptoms. The results indicated that in the entire cohort, the risk factors were age at injury, lower Alb, blood glucose level, presence of cervical OPLL, anterior vertebral hematoma, and severe neurological symptoms (AIS A or B). In addition, lower Alb, and head injury were selected as independent risk factors for long-term respiratory dysfunction. When the analysis of factors was limited to cervical SCI cases without cervical fracture, in addition to the aforementioned items, lower Alb and severe neurological symptoms were independent risk factors. Risk factors for respiratory dysfunction did not differ significantly between patients with and without bone injury.
Numerous studies have reported that complete paralysis is a risk factor for intubation or tracheostomy.10,11 In this study, AIS A-B, complete motor paralysis was also an independent risk factor for respiratory dysfunction. Yugue et al 10 reported that 18.8% of patients with AIS A upon admission had an improved level of paralysis (grade other than A) at the final follow-up, suggesting that the degree of paralysis at the time of injury may be useful in predicting respiratory failure.
Laboratory data suggest that blood glucose and Alb are useful predictors of respiratory impairment. Kobayakawa et al 14 investigated the influence of hyperglycemia in acute SCI and revealed that hyperglycemia was associated with a worse functional outcome and was identified as a potential poor prognostic factor for SCI, irrespective of whether the subjects had a history of diabetes mellitus; hyperglycemia also resulted in a worse prognosis for motor function. The current study showed that hyperglycemia immediately after SCI may affect respiratory status and suggested that glycemic control is important for the prognosis of respiratory function in SCI, regardless of whether or not the patient has a history of diabetes. Diabetes mellitus and chronic obstructive pulmonary disease are both systemic inflammatory diseases, and the 2 conditions are related. Lawlor reported that lung function measures were inversely associated with insulin resistance and type 2 diabetes mellitus. 15 Hypoalbuminemia has traditionally been used as a marker of poor health and nutritional status. Patients with cervical cord injury are undernourished due to the release of inflammatory cytokines and inflammatory agonists, in addition to impaired feeding and swallowing function. In hypoalbuminemia, pleural effusion may affect respiratory status. Respiratory failure due to anterior vertebral hematoma is a complication that should be considered in the event of damage to the cervical spine, regardless of the presence of neurological symptoms. In particular, the posterior pharyngeal gap, which is the space between the posterior wall of the pharynx and the vertebral body, 16 can cause airway obstruction by spreading extensively and draining the anterior trachea once bleeding occurs. 17 The causes of posterior pharyngeal gap hematoma include tears of the longissimus dorsi muscle and anterior longitudinal ligament due to hyperextension of the cervical spine, and damage to the vertebral artery branches.18,19 Many elderly patients are taking antithrombotic drugs, which can increase the size of the hematoma, reported to occur even with minor trauma. 20 Airway obstruction by hematoma can be fatal; thus, it is important to secure the airway by tracheostomy or other methods. Because hematoma takes 2-4 weeks to disappear, 17 it is necessary to maintain a secure airway. Cervical OPLL is associated with a high rate of ossification of the anterior longitudinal ligament or yellow ligament in the cervical or thoracolumbar spine. According to a report on the prevalence of spinal ligament ossification using CT, 21 the prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in healthy subjects is 12%. On the contrary, DISH was found in 48.7% of patients with cervical OPLL, 22 which is a higher rate than that in healthy subjects. DISH is an independent vital prognostic factor in patients with cervical SCI, and the main cause of death in patients with DISH is pneumonia. 23 Although the presence of thoracic spine DISH was not evaluated in this study, it is possible that thoracic spine DISH was included in the OPLL cases, which may have resulted in respiratory dysfunction. In SCI, the respiratory muscles are paralyzed, resulting in decreased ventilatory capacity and decreased ability to clear the upper airway. In complete paralysis, lung capacity reduces to 40% of normal at the C4 injury level, 50% at C5, 60% at C6, and 70% at C7, resulting in decreased ventilatory capacity. 24 In the present study, of the cases with intramedullary signal changes, the area where the signal changes were most cephalad was evaluated but was not found to be a significant risk factor for respiratory dysfunction. In the acute phase of SCI, blurred hyperintensity on T2-weighted MRI is indicative of spinal cord contusion and edema, which is believed to indicate SCI.25-27 However, Tanaka et al 9 reported that the level of injury on MRI and the neurological level of injury did not necessarily coincide, suggesting it is an unsuitable assessment method. Tanaka et al 15 also indicated that severe SCI and T2-weighted images showing hypointensity within hyperintensity can suggest “hematoma-like change” and a significant risk factor for tracheotomy. Similar signal changes need to be investigated in the future.
The efficacy of early surgery has been controversial, especially in cervical SCI without cervical fracture.28-30 In this study, there were significantly more cases of surgery in the early respiratory failure group, although it was not an independent risk factor in all cases or in those without bone injury. This may be due to the fact that some patients were difficult to extubate after intraoperative tracheal intubation or were not extubated to avoid risk.
Limitation
This study had several limitations. First, this was a retrospective study, and detailed imaging evaluation was not available. Second, no clear criteria were set for tracheostomy eligibility, which can vary between institutions and physicians. Third, the study did not consider the patients’ original respiratory status and other details of their history, and not all long-term cases could be monitored for several years.
Conclusion
This large-scale study involved 78 participating institutions and over 1300 patients in Japan. Significantly, older age, hypoalbuminemia, blood glucose level, cervical OPLL, anterior vertebral hematoma, and severe neurological symptoms were identified as independent risk factors, and we believe that these characteristics are important indicators when considering early tracheostomy and ventilator management.
Acknowledgments
The submitted manuscript does not contain any information about medical drugs.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Ryosuke Hirota https://orcid.org/0000-0002-2210-0497
Takeshi Sasagawa https://orcid.org/0000-0002-3849-0178
Kei Ando https://orcid.org/0000-0002-1088-2903
Hiroaki Nakashima https://orcid.org/0000-0002-0039-9678
Naoki Segi https://orcid.org/0000-0001-9681-2422
Kota Watanabe https://orcid.org/0000-0002-4830-4690
Tomohiro Yamada https://orcid.org/0000-0002-7220-7321
Hidenori Suzuki https://orcid.org/0000-0002-3156-0591
Yasuaki Imajo https://orcid.org/0000-0003-1291-745X
Shota Ikegami https://orcid.org/0000-0001-6404-5249
Masashi Uehara https://orcid.org/0000-0003-0718-6357
Koji Tamai https://orcid.org/0000-0003-1467-2599
Gen Inoue https://orcid.org/0000-0001-6500-9004
Yasushi Oshima https://orcid.org/0000-0003-4696-1846
Toshitaka Yoshii https://orcid.org/0000-0003-3511-9020
Tetsuro Ohba https://orcid.org/0000-0003-3411-1692
Masayuki Ishihara https://orcid.org/0000-0001-6062-6767
Shiro Imagama https://orcid.org/0000-0002-6951-8575
References
- 1.Miyakoshi N, Suda K, Kudo D, et al. A nationwide survey on the incidence and characteristics of traumatic spinal cord injury in Japan in 2018. Spinal Cord. 2021;59(6):626-634. doi: 10.1038/s41393-020-00533-0. [DOI] [PubMed] [Google Scholar]
- 2.Arul K, Ge L, Ikpeze T, Baldwin A, Mesfin A. Traumatic spinal cord injuries in geriatric population: etiology, management, and complications. J Spine Surg. 2019;5(1):38-45. doi: 10.21037/jss.2019.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535-546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagen EM, Lie SA, Rekand T, Gilhus NE, Gronning M. Mortality after traumatic spinal cord injury: 50 years of follow-up. J Neurol Neurosurg Psychiatry. 2010;81(4):368-373. doi: 10.1136/jnnp.2009.178798. [DOI] [PubMed] [Google Scholar]
- 5.Reines HD, Harris RC. Pulmonary complications of acute spinal cord injuries. Neurosurgery. 1987;21(2):193-196. doi: 10.1227/00006123-198708000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82(10):803-814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima A, Honda S, Yoshimura S, Ono Y, Kawamura J, Moriai N. The disease pattern and causes of death of spinal cord injured patients in Japan. Paraplegia. 1989;27(3):163-171. doi: 10.1038/sc.1989.25. [DOI] [PubMed] [Google Scholar]
- 8.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(suppl 24):S2-S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka J, Yugue I, Shiba K, Maeyama A, Naito M. A study of risk factors for tracheostomy in patients with a cervical spinal cord injury. Spine. 2016;41(9):764-771. doi: 10.1097/BRS.0000000000001317. [DOI] [PubMed] [Google Scholar]
- 10.Yugué I, Okada S, Ueta T, et al. Analysis of the risk factors for tracheostomy in traumatic cervical spinal cord injury. Spine. 2012;37(26):E1633-E1638. doi: 10.1097/BRS.0b013e31827417f1. [DOI] [PubMed] [Google Scholar]
- 11.Branco BC, Plurad D, Green DJ, et al. Incidence and clinical predictors for tracheostomy after cervical spinal cord injury: a national trauma databank review. J Trauma. 2011;70(1):111-115. doi: 10.1097/TA.0b013e3181d9a559. [DOI] [PubMed] [Google Scholar]
- 12.Berney SC, Gordon IR, Opdam HI, Denehy L. A classification and regression tree to assist clinical decision making in airway management for patients with cervical spinal cord injury. Spinal Cord. 2011;49(2):244-250. doi: 10.1038/sc.2010.97. [DOI] [PubMed] [Google Scholar]
- 13.Leelapattana P, Fleming JC, Gurr KR, Bailey SI, Parry N, Bailey CS. Predicting the need for tracheostomy in patients with cervical spinal cord injury. J Trauma Acute Care Surg. 2012;73(4):880-884. doi: 10.1097/TA.0b013e318251fb34. [DOI] [PubMed] [Google Scholar]
- 14.Kobayakawa K, Kumamaru H, Saiwai H, et al. Acute hyperglycemia impairs functional improvement after spinal cord injury in mice and humans. Sci Transl Med. 2014;6(256):256ra137. doi: 10.1126/scitranslmed.3009430. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia. 2004;47(2):195-203. doi: 10.1007/s00125-003-1310-6. [DOI] [PubMed] [Google Scholar]
- 16.Wong YK, Novotny GM. Retropharyngeal space—a review of anatomy, pathology, and clinical presentation. J Otolaryngol. 1978;7(6):528-536. [PubMed] [Google Scholar]
- 17.Myssiorek D, Shalmi C. Traumatic retropharyngeal hematoma. Arch Otolaryngol Head Neck Surg. 1989;115(9):1130-1132. doi: 10.1001/archotol.1989.01860330120032. [DOI] [PubMed] [Google Scholar]
- 18.Macnab I. The “whiplash syndrome”. Orthop Clin North Am. 1971;2(2):389-403. doi: 10.1016/S0030-5898(20)31114-7. [DOI] [PubMed] [Google Scholar]
- 19.Penning L. Prevertebral hematoma in cervical spine injury: incidence and etiologic significance. AJR Am J Roentgenol. 1981;136(3):553-561. doi: 10.2214/ajr.136.3.553. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill lV, Toomey 1M, Snyder GG. Retropharyngeal hematoma secondary to minor blunt Traum a in the Elderly patient. J Otolaryngol. 1977;6:43-47. [PubMed] [Google Scholar]
- 21.Fujimori T, Watabe T, Iwamoto Y, Hamada S, Iwasaki M, Oda T. Prevalence, concomitance, and distribution of ossification of the spinal Ligaments: results of whole Spine CT Scans in 1500 Japanese patients. Spine. 2016;41(21):1668-1676. doi: 10.1097/BRS.0000000000001643. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura S, Nagoshi N, Iwanami A, et al. Prevalence and distribution of diffuse idiopathic skeletal hyperostosis on whole-spine computed tomography in patients with cervical ossification of the posterior longitudinal ligament: a multicenter study. Clin Spine Surg. 2018;31(9):E460-E465. doi: 10.1097/BSD.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 23.Sawakami K, Watanabe K, Sato T, et al. Rib hyperostosis as a risk factor for poor prognosis in cervical spine injury patients with diffuse idiopathic skeletal hyperostosis. Spine. 2020;45(5):300-308. doi: 10.1097/BRS.0000000000003252. [DOI] [PubMed] [Google Scholar]
- 24.Anke A, Aksnes AK, Stanghelle JK, Hjeltnes N. Lung volumes in tetraplegic patients according to cervical spinal cord injury level. Scand J Rehabil Med. 1993;25(2):73-77. [PubMed] [Google Scholar]
- 25.Fujii H, Yone K, Sakou T. Magnetic resonance imaging study of experimental acute spinal cord injury. Spine. 1993;18(14):2030-2034. doi: 10.1097/00007632-199310001-00017. [DOI] [PubMed] [Google Scholar]
- 26.Ohshio I, Hatayama A, Kaneda K, Takahara M, Nagashima K. Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine. 1993;18(9):1140-1149. doi: 10.1097/00007632-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Machino M, Yukawa Y, Ito K, et al. Can magnetic resonance imaging reflect the prognosis in patients of cervical spinal cord injury without radiographic abnormality? Spine. 2011;36(24):E1568-E1572. doi: 10.1097/BRS.0b013e31821273c0. [DOI] [PubMed] [Google Scholar]
- 28.Takao T, Okada S, Morishita Y, et al. Clinical influence of cervical spinal canal stenosis on neurological outcome after traumatic cervical spinal cord injury without major fracture or dislocation. Asian Spine J. 2016;10(3):536-542. doi: 10.4184/asj.2016.10.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badhiwala JH, Wilson JR, Witiw CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20(2):117-126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 30.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One. 2012;7(2):e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]