Abstract
Study Design: This was a retrospective observational study that assessed the clinical outcome of ageing patients who received ultrasound-guided (USG) mechanical needling with sterile water injection. In addition, the clinical outcome of age-and gender matched patients randomly selected from patients who received needling with sterile water was compared to the patients injected with lidocaine in a 1:1 ratio.
Objective: This present study aimed to explore the clinical effects of USG mechanical needling with sterile water injection for lumbar spinal stenosis (LSS).
Methods: The data was extracted from the medical records of ageing patients with LSS who received USG injection at the lumbosacral spine by the first author. Low back pain or axial pain, and leg pain or radicular pain were assessed by the visual analogue scale, and gait ability with walking distance were obtained at six different time points.
Results: A total of 4328 medical records were examined. Four thousand two hundred and twenty-eight ageing patients received mechanical needling with sterile water injection and found the efficacy lasted up to 6 months. One hundred patients were compared with 100 patients who received lidocaine injection. Those who received lidocaine had pain returned at 3 months and 6 months post-injection.
Conclusions: USG mechanical needling with sterile water injection could help relieve axial and radicular pain for at least 6 months. Removal of calcification and fibrosis as well as reduction of sensitization are all possible mechanisms.
Keywords: mechanical needling, sterile water, lumbar spinal stenosis, pain, calcification, fibrosis
Introduction
Back pain with neurogenic claudication caused by lumbar spinal stenosis (LSS) is a common and rapidly expanding public health problem among the elderly. 1 It’s characterized by bilateral or unilateral back pain and/or lower extremity discomfort, as well as pain, weakness, or heaviness brought on by walking and extended standing, and relieved by stooping forward and sitting.2,3 The underlying cause is mainly age-related osteoarthritic changes in the lumbar intervertebral discs, facets joints, and ligaments, which cause narrowing of the central and/or lateral spinal canals, as well as compression and/or ischemia of the spinal nerves.2,4 In neurogenic claudication, limited walking capacity is the most prevalent symptom and the most common cause for seeking treatment. 5 It is linked to a considerable deterioration in functional status, quality of life, and independence. Facet joint hypertrophy is a condition which the cartilage between the inferior and superior articular processes breaks down and becomes inflamed, causing pain signals to be sent to the innervated medial branch nerve endings.6-11 The L4–L5 region of the lumbar spine is the most prevalent site of LSS.7,9-13 Radicular pain in spinal stenosis is caused by inflammation or compression of the spinal nerve root, with disc herniation being the most prevalent cause.7,9-13
Degenerative disc disease is often the first step in a series of occurrences. As the diffusion of nutrients reduces due to endplate degeneration, it encourages disc degradation, resulting in lower disc heights, altered biomechanics, and the development of facet arthropathy, osteophytes, spondylosis, and degenerative spinal stenosis. Spondylolisthesis and degenerative scoliosis cause spine instability, which is unavoidable as the condition worsens.6-11
There is moderate-quality evidence suggesting a multimodal approach that incorporates manual therapy and exercise, with or without education, is a successful treatment for LSS with neurogenic claudication, and that epidural steroids are ineffective. The quality of evidence for all other non-operative therapies was insufficient to make inferences about their efficacy.4,14
To treat back pain or axial pain, as well as leg pain or radicular pain, injections of corticosteroids, lidocaine, 5% dextrose in water, hyaluronic acid (HA), or autologous platelet rich plasma (PRP) into the facet joints have been advocated.9,11-18 Due to spur growth and degenerative changes, needle entry into the facet joints can be difficult.9,11-18
The aim of this study was to see if using ultrasound-guided (USG) mechanical needling to clear calcification and fibrosis, then cleaning with sterile water around the facet joint, medial branch and nerve root, and multifidus muscles, could help relieve axial and radicular pain in aging with spinal stenosis. A low dose of lidocaine was administered to alleviate the pain of the procedure. In addition, the clinical outcome of age- and gender-matched patients randomly selected from patients who received mechanical needling with sterile water was compared to the patients injected with lidocaine, as the control standard treatment, in a 1:1 ratio. This treatment can be used in aging people with many diseases and polypharmacy because it does not require any chemical substances or drugs. In addition, the active ingredient as HA, PRP which are promising for boosting regeneration, can be simply administered thereafter if needed.
Methods and Materials
Ethical Approval
The Institutional Review Board of the Faculty of Medicine at Chulalongkorn University approved this study (IRB number 426/64). Since this was a retrospective study, written informed consent was waived.
Participants
The researchers analyzed the medical data from patients aged 60– 92 years who had axial and radicular pain from LSS and were given either mechanical needling with sterile water injection or lidocaine injection. The following symptoms were used to diagnose patients with LSS: (1) having leg or buttock pain while walking; (2) flexing forward to relieve symptoms; (3) feeling relief when using a shopping cart or bicycle; (4) having motor or sensory disturbance while walking; (5) the pulses in the foot are present and symmetric; (6) have lower extremity weakness and decreased walking ability; (7) have low back pain. Patients were then selected to be recruited if there were the following signs: (1) Kemp’s sign, (2) induced pain in the articular or transverse apophysis, and (3) sign of facet tension or new lumbar facet sign. Patients were then selected to be recruited if there was magnetic resonance imaging: (1) There was pressure on nerves in the spinal cord at bilateral L4–5 and L5–S1 levels; (2) there was no abnormal mass at the lumbosacral spine level.
Patients were excluded if there were any of the following: (1) red flag sign, (2) discogenic pain from acute disc herniation by positive straight leg raising test, (3) had low back pain from infection, inflammation, or tumor, (4) had incomplete medical records, and (5) had been treated with any nonsteroidal anti-inflammatory drugs (NSAIDs), analgesic drugs, rehabilitation, or other lumbar interventions at the same time, (6) had previous back surgery. The data was extracted from the medical records of patients with LSS who were treated by the first author at the outpatient clinic of the Department of Rehabilitation Medicine at King Chulalongkorn Memorial Hospital in Bangkok, Thailand, between January 1, 2019, and December 31, 2020, for USG injections at the lumbosacral spine.
Study Designs
This retrospective cohort study assessed the changes of clinical outcome of patients who received USG mechanical needling with sterile water injection. The clinical outcome of the procedure was assessed at six time points. The study also compared the clinical outcome of patients received USG mechanical needling with sterile water who age-and gender matched to the patients who received lidocaine in ratio 1:1.
Measurements
Self-reported Visual Analogue Scale (VAS) was used to assess the severity of low back pain or axial pain, leg pain, or radicular pain after injection. The gait ability with a walking distance before calf pain was assessed after injection. The symptoms and satisfaction details of all patients were analyzed at 6 time points: pre-injection (T0), immediately after injection (T1), 1 week after injection (T2), 1 month after injection (T3), 3 months after injection (T4), and 6 months after injection (T5). The clinical outcomes at the 6 - time points were compared.
Study Procedures
The innovative technique was previously published 19 and was used in all patients in this study. The USG injections were administered at the facet joint, the medial branch of the dorsal rami that innervated the facet joint, and the multifidus muscle with a single needle. The injections were bilaterally administered at L4–5 and L5–S1, which are the most common sites where degeneration occurs.
The sterile water group received USG mechanical needling with 2.5 mL of sterile water plus .5 mL of 1% lidocaine without adrenaline per area, 10 mL of totally sterile water plus 2 mL of 1% lidocaine, 1 mL at the medial branch, 1 mL at the facet joint, and 1 mL at the multifidus muscles, 3 mL at each level. Ultrasound was used by the injector (the first author) to look for calcification, spurs, and fibrosis around the facet joint, medial branch, and multifidus. The needle was used to physically remove the calcification and fibrosis, and the area was washed with a considerable amount of sterile water. To make the procedure less painful, a small amount of lidocaine was given. The total time of treatment procedures per area was only 1 minute. The procedure was repeated once a week for 4 weeks with the same technique, volume, and region to gradually eliminate the calcification and fibrosis while avoiding harm to any tissue. The lidocaine group received 12 mL of 1% lidocaine without adrenaline injection at the facet joint, medial branch, and multifidus without mechanical needling. The patients were informed that they received treatment for spinal stenosis.
Statistical Analysis
The characteristics of the cohort were presented as mean, standard deviation, and frequency. Changes in clinical outcomes at 6-time points were analyzed by using repeated measures ANOVA. In case of broken sphericity assumption, the Greenhouse-Geisser correction was used. Student’s t-test or Mann–Whitney U tests were used for continuous variables (depending on normality) and chi-square tests for categorical data were used where appropriate. The significant level was P < .05.
Results
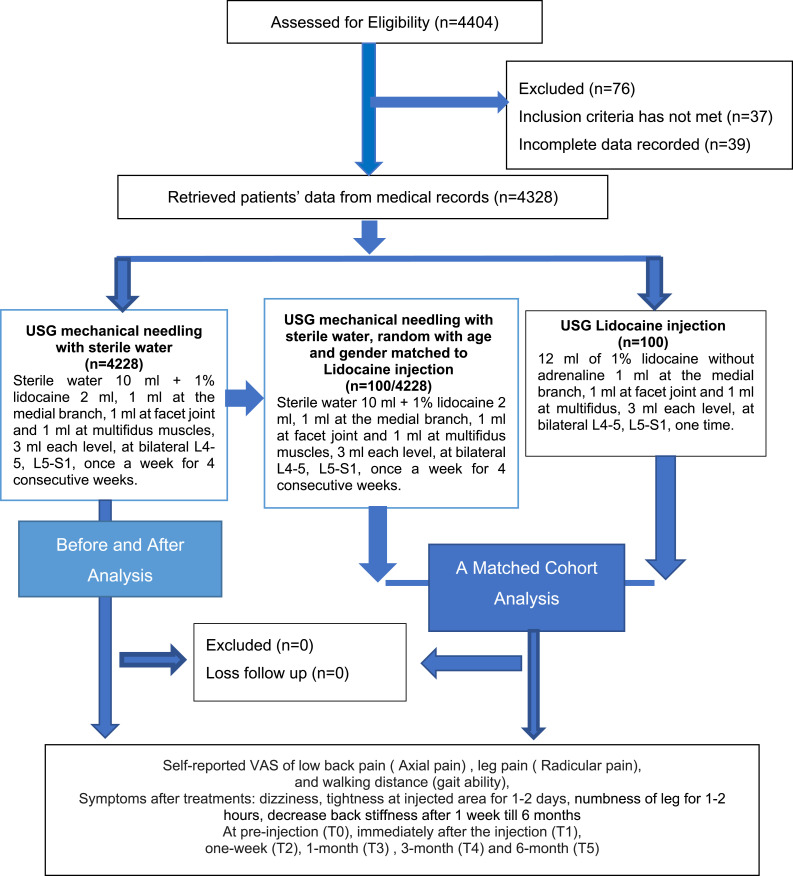
The medical records of 4404 LSS patients with axial and radicular pain were evaluated for eligibility. The STROBE flowchart, which represents the Strengthening the Reporting of Observational Studies in Epidemiology, is shown in Figure 1. Seventy-six medical records were omitted because they did not meet the inclusion criteria (n = 37) or had inadequate data (n = 39). There were 4228 medical records of patients who received mechanical needling with sterile water, 100 of the patients received lidocaine, resulting in a total of 4328 patients that were analyzed. In a 1:1 comparison, 100 patients were randomly selected from the mechanical needling sterile water group and were age-and gender matched to 100 patients who received lidocaine. No patients were lost to follow-up at all time points (T1–T5). The baseline characteristics of the patients are shown in Table 1.
Figure 1.
Strengthening the reporting of observational studies in epidemiology flow chart.
Table 1.
General Characteristics.
| Total (4328) | Needling With Sterile Water (4228) | Needling With Sterile Water (100/4228) | Lidocaine (100/100) | P-value | |
|---|---|---|---|---|---|
| Female (%) | 2923 (68.36%) | 2889 (68.33%) | 67 (67%) | 67 (67%) | |
| Age (mean ± SD) (years) | 71.45 ± 8.23 | 71.31 ± 8.19 | 69.21 ± 5.41 | 69.21 ± 5.41 | |
| Range (min-max) | 60-92 | 60–92 | 60–89 | 60–89 | |
| BMI (kg/m2) | 24.35 ± 3.12 | 24.61 ± 3.09 | 23.64 ± 3.24 | 23.64 ± 3.24 | .889 |
| (19.5-32.5) | (19.5-32.5) | (20.1-29.1) | (20.1-29.1) | ||
| Duration of LBP (years) | 12.09 ± 6.11 | 12.41 ± 6.27 | 9.79 ± 4.73 | 9.45 ± 5.01 | .882 |
| Range (min-max) | 5-30 | 6–30 | 5–25 | 5–25 |
The comparison between random sterile water (n = 100) and lidocaine (n = 100) were using Mann–Whitney U test, significant difference at P < .05.
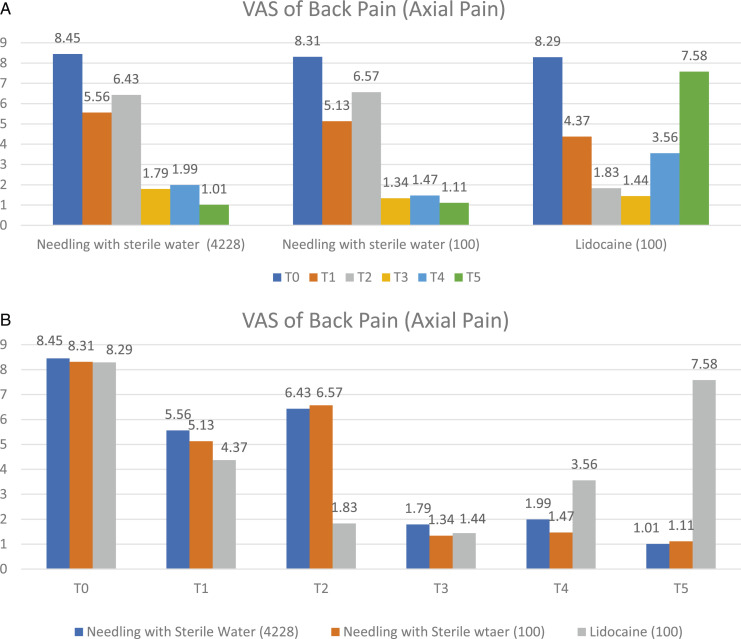
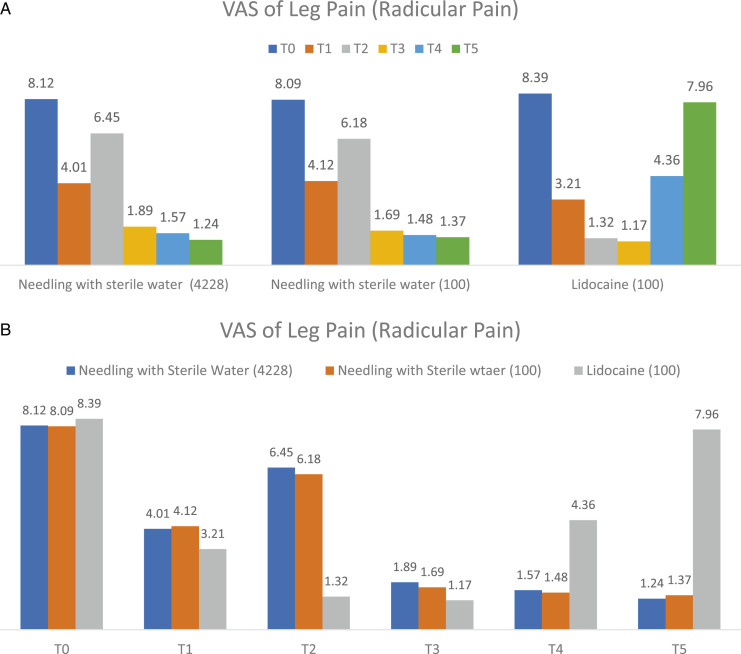
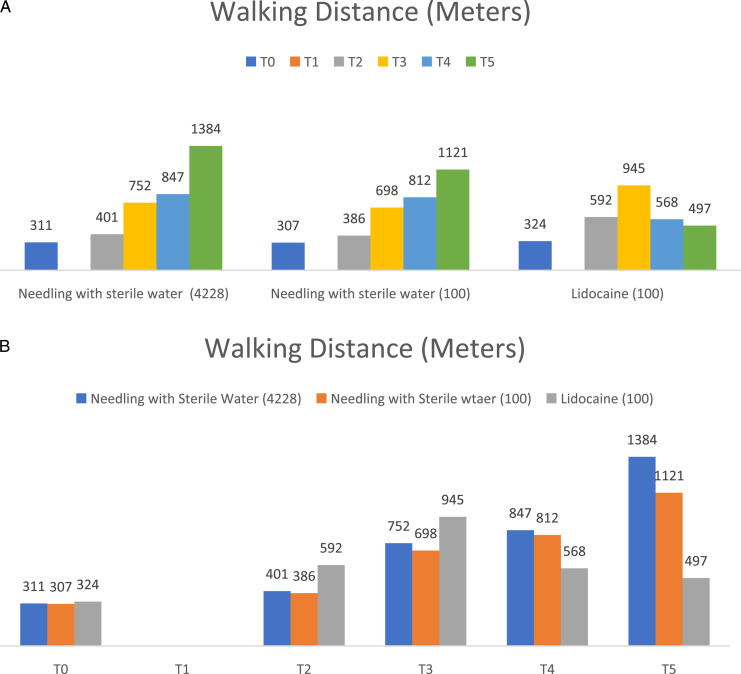
Within the group analysis of 4228 patients who received mechanical needling with sterile water injection, the VAS for back pain at T1, T3, T4, and T5 were significantly better than T0 at P < .05, as shown in Figure 2A and Table 2. Moreover, the VAS for leg pain at T1, T3, T4, and T5 were significantly better than T0 at P < .05, as shown in Figure 3A and Table 2. In addition, the walking distances at T3, T4, and T5 were considerably better than T0 at P < .05, as shown in Figure 4A and Table 2.
Figure 2.
The comparison within group (A) and between groups (B) of visual analogue scale for back pain (axial pain) at 6-time points as pre-injection (T0), immediately (T1), one week (T2), 1-month post-injection (T3), 3-month (T4), and 6-month (T5).
Table 2.
Outcome of Treatments.
| Needling With Sterile Water (n = 4228) | P-value* | Needling With Sterile Water (n = 100/4228) | P-value* | Lidocaine (n = 100) | P-value* | P-value** | ||
|---|---|---|---|---|---|---|---|---|
| VAS of back pain | Pre-injection (T0) | 8.45 ± .99 | 8.63 ± .41 | 8.72 ± .34 | .751 | |||
| (mean ± SD) | Immediately after the first injection (T1) | 5.56 ± 1.56 | .041* | 5.79 ± 1.56 | .043* | 5.33 ± 1.28 | .035* | .794 |
| 1-week after the first injection (T2) | 6.43 ± 1.91 | .312 | 6.01 ± 1.46 | .256 | 5.89 ± 1.41 | .164 | .212 | |
| 1-month after the first injection (T3) | 1.79 ± 1.14 | .000* | 1.57 ± 1.35 | .000* | 1.64 ± 1.13 | .042* | .351 | |
| 3-month after the first injection (T4) | 1.99 ± 1.25 | .000* | 1.47 ± 1.12 | .000* | 4.35 ± 2.12 | .046* | .014** | |
| 6-month after the first injection (T5) | 1.01 ± 1.56 | .000* | 1.11 ± 1.21 | .000* | 6.44 ± 1.67 | .783 | .000** | |
| VAS of leg pain (mean ± SD) | Pre-injection (T0) | 8.12 ± 1.19 | 8.21 ± 1.24 | 8.18 ± 1.11 | .825 | |||
| Immediately after the first injection (T1) | 4.01 ± 1.31 | .048* | 4.23 ± 1.13 | .046* | 4.99 ± 1.24 | .036* | .592 | |
| 1-week after the first injection (T2) | 6.45 ± 1.03 | .741 | 5.13 ± 1.24 | .526 | 5.15 ± 1.13 | .211 | .311 | |
| 1-month after the first injection (T3) | 1.89 ± 1.21 | .000* | 1.69 ± 1.09 | .000* | 2.11 ± 1.12 | .025* | .544 | |
| 3-month after the first injection (T4) | 1.57 ± 1.12 | .000* | 1.48 ± 1.03 | .000* | 4.84 ± 1.12 | .042* | .000** | |
| 6-month after the first injection (T5) | 1.24 ± 1.36 | .000* | 1.37 ± 1.14 | .000* | 7.47 ± 1.63 | .648 | .000** | |
| Walking distance (meters) | Pre-injection (T0) | 311 ± 37 | 345 ± 78 | 373 ± 62 | .835 | |||
| Immediately after the first injection (T1) | — | — | — | |||||
| 1-week after the first injection (T2) | 401 ± 47 | .812 | 369 ± 53 | .639 | 395 ± 73 | .738 | .749 | |
| 1-month after the first injection (T3) | 752 ± 56 | .000* | 657 ± 46 | .000* | 621 ± 47 | .000* | .628 | |
| 3-month after the first injection (T4) | 847 ± 79 | .000* | 847 ± 46 | .000* | 593 ± 46 | .014* | .047** | |
| 6-month after the first injection (T5) | 1384 ± 92 | .000* | 1121 ± 71 | .000* | 392 ± 36 | .846 | .000** | |
Abbreviation: VAS, visual analogue scale.
**The comparison between random needling with sterile water (n = 100) and lidocaine (n = 100) were using Mann-Whitney U tests, significant difference at P < .05.
*The comparison within each group were using repeated measure ANOVA, significant difference at P < .05.
Figure 3.
The comparison within group (A) and between groups (B) of visual analogue scale for leg pain (radicular pain) at 6-time points as pre-injection (T0), immediately (T1), one week (T2), 1-month (T3), 3-month (T4), and 6-month (T5).
Figure 4.
The comparison within group (A) and between groups (B) of walking distance (metres) at 6-time points as pre-injection (T0), immediately (T1), one week (T2), 1-month (T3), 3-month (T4), and 6-month (T5).
Within the group analysis of 100 patients who received mechanical needling with sterile water injection whose age and gender matched those who received lidocaine injection had significantly better VAS for back pain and leg pain at T1, T3, T4, and T5 compared to T0 at P < .05, as shown in Figures 2A and 3A, and Table 2. In addition, the walking distances at T3, T4, and T5 were significantly better than T0 at P < .05, as shown in Figure 4A, and Table 2.
Within the group analysis of 100 patients who received lidocaine injection, the VAS of back pain, leg pain and walking distance was significantly better at T3, and T4 compared to T0 at P < .05, as shown in Figures 2A, 3A and 4A, and Table 2. In addition,VAS for back pain and leg pain at T1 were significantly better than T0 at P < .05, as shown in Figures 2A, 3A and Table 2.
An age-and-gender-matched subset randomly selected from the sterile water injection group was compared to the lidocaine injection group (ratio 1:1). The VAS of back pain, leg pain, and walking distance in the patients who received mechanical needling with sterile water were significantly better than those who received lidocaine at T5 and T6 at P < .05, as shown in Figure 2B, 3B, 4B, and Table 2.
There were considerably fewer incidences of dizziness after mechanical needling with sterile water injection for 1–2 hours and numbness of leg for 1–2 hours, decrease back stiffness after 1 week till 6 months, significantly at P < .05, as shown in Table 3
Table 3.
Symptoms After Injections.
| Needling With Sterile Water (4228) | Needling With Sterile Water (100) | Lidocaine (100) | P-value | |
|---|---|---|---|---|
| Dizziness after injection for 1–2 hours | 150 (3.5%) | 6 (6%) | 65 (65%) | .000* |
| Tightness of injected area for 1–2 days | 1528 (35.63%) | 40 (40%) | 39 (39%) | .672 |
| Numbness of leg for 1–2 hours | 16 (3.73%) | 3 (3%) | 68 (68%) | .000* |
| Decrease back stiffness after 1 week till 6 months | 4122 (96.13%) | 96 (96%) | 26 (26%) | .000* |
The comparison between random needling with sterile water (n = 100) and lidocaine (n = 100) were using chi-square, significant difference at P < .05.
Discussion
A total of 4328 medical records of patients with LSS were analyzed retrospectively; 4228 patients received mechanical needling with further sterile water washout the calcification and fibrosis, and 100 patients received lidocaine injections. One hundred patients were chosen at random from the mechanical needling with sterile water group to age and gender match the lidocaine group.
In this study, 4328 patients received an innovative technique that used USG injections at the facet joint, the medial branch of the dorsal rami that innervates the facet joint, and the multifidus muscle with a single needle, as previously described. 19 In 4228 patients, mechanical needling was used to scrape off the calcification and fibrosis, and sterile water injection induced substantially more breakdown. There were statistically significantly reductions in back pain or axial pain, and leg pain or radicular pain immediately after needling and sterile water injection at 1month post-injection, 3 months post-injection, and 6 months post-injection compared to pre-injection or at baseline (P < .05). There were statistically significantly reduction of back pain or axial pain, leg pain or radicular pain, and improvement in walking distance at 3 months and 6 months in 100 patients who received mechanical needling with sterile water compared to the 100 patients who received lidocaine. In the lidocaine group, the back and leg pain returned at 3 months and 6 months post-injection. No needling was used to scrape off the calcification or fibrosis; only lidocaine was injected at the facet joint, medial branch, and multifidus. We found that mechanical needling with sterile water was useful in reducing axial and radicular pain for at least 6 months. Immediately after injection, 1 week, and at 1 month post-injection, the efficacy of mechanical needling with sterile water was comparable to lidocaine.
Since the large amount of liquid generated pressure at the facet joints, medial branches, and multifidus muscles, 40% of those who received mechanical needling with sterile water and 39% of those who received lidocaine had back discomfort. However, within 1–2 days, the liquids in the interstitial tissues dissolved and return to the blood vessel. The gliding of the facet joints and muscles considerably improved after calcification and fibrosis were eliminated from the facet joints and muscles in 95% of patients who underwent mechanical needling with sterile water and 26% of those who received lidocaine. This could be attributed to the revascularization of the facet joints and nerve regeneration. The function of the blood vessels at the joint, nerves, and muscles is improved since there is less calcification or fibrosis blockage. Additional research, however, is needed to validate this. Dizziness was 65% in the lidocaine group immediately after injection and relief within 1–2 hours of injection, compared to 6% in the mechanical needling with sterile water group. Mechanical needling with sterile water injection resulted in a 96% reduction in back stiffness after 1 week through 6 months, compared to 26% in the lidocaine group. Back stiffness was reduced as calcification and fibrosis surrounding the facet joints, nerves, and multifidus muscles were eliminated, allowing the facet joints to move more freely and improving the contract-relax characteristics of the muscles. Mechanical needling with sterile water injection had both mechanical disruption and a water jet effect, while lidocaine injection had only a water jet effect. It is a possible solution effect of sterile water. Sterile water molecules are rapidly distributed in the total body water, resulting in less hypervolemia. 20 Sterile water had pH 7.0, while 1% lidocaine had a pH of 6.09 ± .16. 21 The acidic effect of lidocaine might cause periarticular soft tissue inflammation led to fibrosis, inhibit osteoblast function. Acidosis has a direct impact on bone cells as well. In vitro, small pH reductions within the pathophysiological range result in substantial increases in osteoclast resorptive activity. 22 In primary cultures of osteoblasts, acidosis (pH 6.9) also suppresses the production of mineralized bone nodules. Part of this inhibition can be due to the physicochemical dissolution of hydroxyapatite 23 ; nevertheless, in acidic circumstances, osteoblast expression and activity of tissue non-specific alkaline phosphatase is also dramatically reduced. 24
Facet joint injections (FJI) have been demonstrated to be effective in the treatment of LSS because they provide direct access to the facet joints via the paraspinal muscles and have fewer side effects than intraspinal injections.4,25-27 There were some variation in intra-articular FJI practices. Early trials failed to demonstrate a significant benefit of the active substance over a placebo of normal saline, resulting in a lack of evidence to support the use of intra-articular FJI in guidelines. However, intra-articular FJI use in real-world practices is increasing. Despite a lack of evidence that local anesthesia or corticosteroids have a greater benefit than normal saline in intra-articular FJI, the trials used a combination of corticosteroid and local anesthesia 28 or corticosteroid alone 4 as a comparator of a novel injected substance such as HA or autologous PRP rather than normal saline as a control group. From these findings, it might be assumed that intra-articular FJI with any solution could improve axial and radicular pain in spinal stenosis. However, injecting the facet joint successfully, especially in the elderly, might be challenging because of spurs and cartilaginous metaplasia.9,11-18 The blocking of medial branch of facet joint was increasing popular since the less satisfaction of FJI in spinal stenosis.4,11-14,29-31 However, the outcome was still debate.4,11-14,29-31
Mechanical needling was employed to scrape away the calcification and fibrosis, and sterile water injection resulted in significantly more disintegration, as well as changes in the neurochemistry of deeper tissue structures,32-37 possibly improving the analgesic effect.32-37 Mechanical needling may diminish both peripheral and central sensitization by reducing the source of peripheral nociception, such as the trigger point (TrP) region, facet joint calcification, and fibrosis, modifying spinal dorsal horn activity, and activating the central inhibitory pain pathway. When a needle is inserted into the body, it causes a variety of natural neurophysiological responses, including stimulation of the A and C fibers and activation of cortical brain areas.32-37 The water jet procedure can help eliminate calcification and fibrosis from the facet joint, nerves, and muscles by using sterile water. The procedure’s efficacy was seen to last up to 6 months in this investigation. Because it could reduce the source of mobility restriction, inflammation, pain, and neurovascular compression, mechanical needling with sterile water injection was more successful and produced faster results than lidocaine injection. Walking distance was also increased as a result of the outcomes. The cause is often considered to be nerve compression in the spinal canal. The treatment in this study mechanically alleviated this compression, which should result in joint vasculature and nerve regeneration after calcification and fibrosis removal. The mechanical needling with sterile water is cost-effective, chemical-free, and does not necessitate the use of several medical facilities. Any pharmacological injection, on the other hand, could be utilized subsequently to help with joint regeneration.
Study Strengths and Limitations
The study’s strengths were the large sample size and the fact that it evaluated the clinical outcomes after USG mechanical needling with sterile water injection at six distinct time points. Second, in this trial, a fraction of randomized participants were age and gender matched to the lidocaine group in a ratio of 1:1. However, this is a retrospective data analysis study with only one injector and one institution involved. Third, the benefits should not be due to the placebo effect because all patients were informed that the procedures they received were the treatments of choice for spinal stenosis.
Additional prospective RCT studies should be conducted in an international, multi-center setting with a large sample size to assess sensitization through quantitative sensory testing and ultrasonography to detect hyperechoic areas such as dense connective tissue, fibrosis, and calcification at baseline, during, and after treatment completion, with a longer follow-up period. More research is needed to confirm if greater facet joint gliding following calcification and fibrosis removal leads to joint revascularization and nerve regeneration. In addition, the randomization into three groups as dry mechanical needling, mechanical needling with sterile water injection, and lidocaine injection could be done for biochemical analysis of lidocaine and sterile water on periarticular tissues and for physical and anatomical analysis of mechanical needling.
Conclusions
USG mechanical needling with sterile water injections at the lumbar facet joints, medial branch of the facet joint, and multifidus muscles can alleviate pain for at least 6 months. Reduced sensitization, resulting in analgesia, the removal of calcification, and the reduction of fibrosis are all plausible mechanisms that aid joint and nerve regeneration by gliding with the vasculature. Because no chemicals or pharmaceuticals are used, the method is both affordable and extremely safe.
Acknowledgments
The authors are grateful to the data analysts and research management team at King Chulalongkorn Memorial Hospital, Bangkok, Thailand who were pivotal in curating and linking the data to inform the analysis presented.
Footnotes
Author Contributions: All authors, Areerat Suputtitada, Carl PC Chen and Krit Pongpirul, have read and approved the final version of the manuscript. Study design was developed by Areerat Suputtitada and Krit Pongpirul. Acquisiton of data was performed by Areerat Suputtitada and Krit Pongpirul. Interpretation of the data was performed by Areerat Suputtitada and Carl PC Chen. Literature search was performed by Areerat Suputtitada and Carl Chen. Manuscript preparation and drafting was done by Areerat Suputtitada. Manuscript editing was done by Areerat Suputtitada and Carl PC Chen.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Authors’ Note: The research was performed as part of the first author’s employment at King Chulalongkorn Memorial Hospital, Bangkok, Thailand. This article is selected to be presented on the auspicious occasions of His Majesty King Bhumibol Adulyadej The Great’s Birthday Anniversary, Father’s Day, National Day of Thailand, and World Soil Day. The two beloved Kings: His Majesty King Bhumibol Adulyadej The Great, and His Majesty King Maha Vajiralongkorn Phra Vajiraklaochaoyuhua, were honored with a Special Tribute Ceremony and Forum entitled ‘The New World’ on December 5, 2021.
Ethical Approval: This study was performed in accordance with the principles of the Declaration of Helsinki and was also approved by the Institutional Review Board of the Faculty of Medicine at the Chulalongkorn University in Thailand approved this study (IRB number 426/64). Since this was a retrospective study, written informed consent was waived.
ORCID iD
Areerat Suputtitada https://orcid.org/0000-0002-9920-7188
References
- 1.Jensen RK, Jensen TS, Koes B, Hartvigsen J. Prevalence of lumbar spinal stenosis in general and clinical populations: A systematic review and meta-analysis. Eur Spine J. 2020;29(9):2143-2163. doi: 10.1007/s00586-020-06339-1. [DOI] [PubMed] [Google Scholar]
- 2.Bays A, Stieger A, Held U, et al. The influence of comorbidities on the treatment outcome in symptomatic lumbar spinal stenosis: A systematic review and meta-analysis. N Am Spine Soc J. 2021;6:100072. doi: 10.1016/j.xnsj.2021.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houle M, Bonneau JD, Marchand AA, Descarreaux M. Physical and psychological factors associated with walking capacity in patients with lumbar spinal stenosis with neurogenic claudication: A systematic scoping review. Front Neurol. 2021;12:720662. doi: 10.3389/fneur.2021.720662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammendolia C, Hofkirchner C, Plener J, et al. Non-operative treatment for lumbar spinal stenosis with neurogenic claudication: An updated systematic review. BMJ Open. 2022;12(1):e057724. doi: 10.1136/bmjopen-2021-057724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlesso C, Piva SR, Smith C, Ammendolia C, Schneider MJ. Responsiveness of outcome measures in nonsurgical patients with lumbar spinal stenosis: A secondary analysis from a randomized controlled trial. Spine. 2021;46(12):788-795. doi: 10.1097/BRS.0000000000003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Güneş M, Özmen T, Güler TM. The association between pain, balance, fall, and disability in patients with lumbar spinal stenosis with vascular claudication. Korean J Pain. 2021;34(4):471-478. doi: 10.3344/kjp.2021.34.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron R, Binder A, Attal N, Casale R, Dickenson AH, Treede RD. Neuropathic low back pain in clinical practice. Eur J Pain. 2016;20(6):861-873. doi: 10.1002/ejp.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurdle M-FB. Ultrasound-guided spinal procedures for pain: A review. Phys Med Rehabil Clin N Am. 2016;27(3):673-686. doi: 10.1016/j.pmr.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Karkucak M, Batmaz İ, Kerimoglu S, Ayar A. Comparison of clinical outcomes of ultrasonography-guided and blind local injections in facet syndrome: A 6-week randomized controlled trial. J Back Musculoskelet Rehabil. 2020;33(3):431-436. doi: 10.3233/BMR-181447. [DOI] [PubMed] [Google Scholar]
- 10.Sanders ARJ, de Wit NJ, Zuithoff NPA, van Dulmen S. The effect of shared decision-making on recovery from non-chronic aspecific low back pain in primary care; A post-hoc analysis from the patient, physician and observer perspectives. BMC Prim Care. 2022;23(1):22. doi: 10.1186/s12875-022-01624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manchikanti L, Kaye AD, Boswell MV, et al. A systematic review and best evidence synthesis of the effectiveness of therapeutic facet joint interventions in managing chronic spinal pain. Pain Physician. 2015;18(4):E535-E582. [PubMed] [Google Scholar]
- 12.Manchikanti L, Kaye AD, Soin A, et al. Comprehensive evidence-based guidelines for facet joint interventions in the management of chronic spinal pain: American society of interventional pain physicians (ASIPP) guidelines facet joint interventions 2020 guidelines. Pain Physician. 2020;23(3S):S1-S127. [PubMed] [Google Scholar]
- 13.Perolat R, Kastler A, Nicot B, et al. Facet joint syndrome: From diagnosis to interventional management. Insights Imaging. 2018;9(5):773-789. doi: 10.1007/s13244-018-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davison MA, Lilly DT, Moreno J, Bagley C, Adogwa O. A comparison of successful versus failed nonoperative treatment approaches in patients with degenerative conditions of the lumbar spine. J Clin Neurosci. 2021;86:71-78. doi: 10.1016/j.jocn.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Song L, Ying S, Wang Z, Yuan X. Ultrasound-guided lumbar multifidus block provides effective postoperative analgesia for lumbar spine surgery. J Clin Anesth. 2018;46:116-117. doi: 10.1016/j.jclinane.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Zhou J, Liu C, et al. A prospective study comparing platelet-rich plasma and local anesthetic (LA)/corticosteroid in intra-articular injection for the treatment of lumbar facet joint syndrome. Pain Pract. 2017;17(7):914-924. doi: 10.1111/papr.12544. [DOI] [PubMed] [Google Scholar]
- 17.Annaswamy TM, Armstead C, Carlson L, Elkins NJ, Kocak D, Bierner SM. Intra-articular triamcinolone versus hyaluronate injections for low back pain with symptoms suggestive of lumbar zygapophyseal joint arthropathy: A pragmatic, double-blind randomized controlled trial. Am J Phys Med Rehabil. 2018;97(4):278-284. doi: 10.1097/PHM.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 18.Dagenais S, Yelland MJ, Del Mar C, Schoene ML. Prolotherapy injections for chronic low-back pain. Cochrane Database Syst Rev. 2007;2007(2):CD004059. doi: 10.1002/14651858.CD004059.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CPC, Chen JL, Ho CS, Suputtitada A. Ultrasound-guided medial branch blocks, facet joint, and multifidus mInjections: How it is done under one needle insertion point. Anesthesiology. 2020;132(3):582-583. doi: 10.1097/ALN.0000000000003043. [DOI] [PubMed] [Google Scholar]
- 20.Aghamir SM, Alizadeh F, Meysamie A, Assefi Rad S, Edrisi L. Sterile water versus isotonic saline solution as irrigation fluid in percutaneous nephrolithotomy. Urol J. 2009;6(4):249-253. [PubMed] [Google Scholar]
- 21.Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20(2):71-73. doi: 10.1177/229255031202000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnett TR, Dempster DW. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology. 1986;119(1):119-124. doi: 10.1210/endo-119-1-119. [DOI] [PubMed] [Google Scholar]
- 23.Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005;77(3):167-174. doi: 10.1007/s00223-004-0285-8. [DOI] [PubMed] [Google Scholar]
- 24.Di Pompo G, Cortini M, Baldini N, Avnet S. Acid microenvironment in bone sarcomas. Cancers. 2021;13(15):3848. doi: 10.3390/cancers13153848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SY, Lee JW, Lee GY, Kang HS. Lumbar facet joint injection: feasibility as an alternative method in high-risk patients. Eur Radiol. 2013;23(11):3153-3160. doi: 10.1007/s00330-013-2921-z. [DOI] [PubMed] [Google Scholar]
- 26.Fornari M, Robertson SC, Pereira P, et al. Conservative treatment and percutaneous pain relief techniques in patients with lumbar spinal stenosis: WFNS spine committee recommendations. World Neurosurg X. 2020;7:100079. doi: 10.1016/j.wnsx.2020.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C-C, Jaw F-S, Young Y-H. Radiological and functional assessment in patients with lumbar spinal stenosis. BMC Musculoskelet Disord. 2022;23(1):137. doi: 10.1186/s12891-022-05053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreiner DS, Shaffer WO, Baisden JL, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 2013;13(7):734-743. doi: 10.1016/j.spinee.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Friedly JL, Comstock BA, Turner JA, et al. Long-Term Effects of Repeated Injections of Local Anesthetic With or Without Corticosteroid for Lumbar Spinal Stenosis: A Randomized Trial. Arch Phys Med Rehabil. 2017;98(8):1499-1507.e2. doi: 10.1016/j.apmr.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Won HS, Yang M, Kim YD. Facet joint injections for management of low back pain: a clinically focused review. Anesth Pain Med (Seoul). 2020;15(1):8-18. doi: 10.17085/apm.2020.15.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CPC, Chen C-K, Chen J-L, Chen HM, Suputtitada A. Ultrasound-guided lumbar spine medial branch blocks for the treatment of low back pain. Am J Phys Med Rehabil. 2021;100(5):e73-e74. doi: 10.1097/PHM.0000000000001591. [DOI] [PubMed] [Google Scholar]
- 32.Chae Y, Chang D-S, Lee S-H, et al. Inserting needles into the body: A meta-analysis of brain activity associated with acupuncture needle stimulation. J Pain. 2013;14(3):215-222. doi: 10.1016/j.jpain.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh Y-L, Chou L-W, Joe Y-S, Hong CZ. Spinal cord mechanism involving the remote effects of dry needling on the irritability of myofascial trigger spots in rabbit skeletal muscle. Arch Phys Med Rehabil. 2011;92(7):1098-1105. doi: 10.1016/j.apmr.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Huang Q-M, Liu Q-G, et al. Evidence for dry needling in the management of myofascial trigger points associated with low back pain: A systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99(1):144-152. doi: 10.1016/j.apmr.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16-23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Srbely JZ, Dickey JP, Lee D, Lowerison M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med. 2010;42(5):463-468. doi: 10.2340/16501977-0535. [DOI] [PubMed] [Google Scholar]
- 37.Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber L. Myofascial trigger points then and now: A historical and scientific perspective. PM R. 2015;7(7):746-761. doi: 10.1016/j.pmrj.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]