Abstract
Study Design
Retrospective Cohort Study.
Objective
The purpose of this study was to determine the effect of low bone mineral density (BMD), as assessed by preoperative Dual-energy X-ray Absorptiometry (DEXA) scans, on intraoperative blood loss following adult spinal deformity (ASD) surgery.
Methods
Patients who received spinal fusion for ASD (>5 levels fused) at a single academic center from 2010-2018 were included in this study. The lowest preoperative T-score was recorded for patients who had preoperative DEXA scans within a year of surgery. Patients with liver/kidney disease or on prescription anticoagulant medication were excluded. Major blood loss was a binary variable defined as above or below the 90th percentile of our cohort. Binomial regression was performed controlling for age, number of vertebrae fused, 3-column osteotomy, primary vs. revision surgery, preoperative platelet count, and if the patient was taking medication for osteoporosis.
Results
91 patients were identified in the cohort. Mean age was 63 ± 11.6 years, 81% female. 56 (62%) of cases included revision of previous instrumentation. Patients had a mean SVA of 9.6 ± 8.6 cm and median of 9 vertebrae fused (range 5-22). The average T-score was -1.2 ± 1.0. Each point lower T-score was associated with significantly higher odds of major blood loss (OR 2.5, 95% CI 1.0 – 5.9) when controlling for age, number of vertebrae fused, 3-column osteotomy, preoperative platelet count and primary vs. revision surgery.
Conclusions
Preoperative T-score is independently associated with increased odds of major blood loss in ASD surgery.
Keywords: adult spinal deformity, bone mineral density, blood loss, dual-energy x-ray absorptiometr scan, osteoporosis
Introduction
Surgical treatment of patients with adult spinal deformity (ASD) has been shown to lead to improvements in pain and functional outcomes for appropriately selected patients.1-3 With the ultimate goal of restoring spinal alignment, these procedures commonly involve a combination of multi-level vertebral instrumentation, placement of interbody grafts, pelvic fixation, and osteotomies. 4 Given the inherent invasiveness of ASD surgery, especially for complex deformity and revision surgery, intraoperative blood loss is an unfortunately common perioperative complication.4-7 The relationship between intraoperative blood loss and adverse postoperative outcomes is well known, and patients with increased blood loss following ASD surgery have been shown to be at higher risk of both spinal complications as well as non-spinal adverse events such as postoperative delirium.8,9 As such, it is crucial to determine modifiable risk factors for perioperative blood loss following ASD surgery and ultimately rectify these predisposing factors in patients needing surgery.
One risk factor for adverse events following spine surgery is bone mineral density (BMD), which is a measurement of the amount of bone mineral within bone tissue. 10 At present, dual-energy X-ray absorptiometry (DEXA) scans are the gold-standard imaging tool used for measuring BMD. 11 Regardless of operative treatment, low BMD has been shown to worsen the natural course of spinal deformity. 12 In ASD patients who undergo spinal arthrodesis, low BMD has also been associated with severe postoperative complications such as proximal junction failure.13-16 Although an association between low preoperative BMD and suboptimal outcomes following spinal surgery is evident, additional research is needed to determine the effects of low BMD and specific outcomes such as intraoperative blood loss in patients undergoing ASD surgery.
Previous studies have reported on several risk factors for blood loss following ASD surgery, including increased invasiveness of surgery, malalignment in both coronal and sagittal planes, and osteoporosis. 4 However, we are not aware of any studies that have directly assessed the relationship between preoperative BMD and postoperative blood loss following ASD surgeries. Thus, the purpose of this study was to determine the effect of low BMD, as assessed by preoperative DEXA scans, on intraoperative blood loss following surgical correction of ASD.
Methods
Patient Sample
This study was approved by our medical center’s institutional review board under IRB00135145, including a waiver of HIPAA privacy authorization allowing for retrospective chart review of patient records without patient consent. We retrospectively reviewed patients >18 years of age with a diagnosis of ASD who underwent posterior spinal fusion surgery with ≥5 vertebral levels fused from 2010 to 2018 at a single institution’s orthopaedic spine surgical practice. Patients were included from our multi-provider group and were treated by one of three fellowship-trained spinal surgeons with between 7-20 years of experience following orthopaedic surgery residency and spinal surgery fellowship. All patients were consecutively reviewed from 2010-2018 for potential inclusion into this study. We included patients who had a preoperative DEXA scan within a year prior to surgery. Patients with liver/kidney disease or on prescription anticoagulant medication were excluded. To eliminate the potential confounding effect of patients taking prescription anticoagulant medications on our primary outcome of intraoperative blood loss, patients were excluded who were taking prescription anticoagulant medications prior to surgery. Since patients undergoing ASD surgeries at our institution routinely have DEXA scans performed at outside hospitals, 205 of the 296 patients initially deemed eligible for inclusion did not have DEXA scans within a year of surgery on retrospective chart review. Exclusion of these patients yielded 91 patients in the cohort.
Clinical Data
Medical records were reviewed for covariates that may have influenced operative blood loss, including age, previous surgical history, preoperative platelet count, and hemoglobin. DEXA scan results can include a variety of different measurements, usually at the femoral neck, lumbar spine, and distal radius. Given the heterogeneity of reporting these measurements, and that all DEXA scan reports do not include every standard measurement, the patient’s lowest T-score was used as the measure of bone mineral density for this study. For patients with prior lumbar spinal fusion surgery, the lumbar spine measurement was not used. Medical records were also reviewed for the use of medications intended to increase bone mineral density (bisphosphonates and parathyroid hormone analogues). Our primary outcome measure of intraoperative blood loss was quantified by reviewing anesthesiology records. Operative time was recorded from incision to skin closure, also as reported in the anesthesia medical record.
Radiographic measurements were recorded from the patient’s most recent standing scoliosis radiographs prior to surgery. For descriptive purposes, radiographic measurements described in this study included sagittal vertical axis (SVA), pelvic tilt (PT), coronal Cobb angle, and thoracic kyphosis (TK).
Statistical Analysis
Descriptive statistics were reported as either means ± standard deviation (SD) or N (%), unless stated otherwise. “Major” blood loss was characterized as a binary variable, using the 90th percentile level of blood loss according to the population of patients in our study cohort.
In order to examine the relationship between bone mineral density and major blood loss, binomial logistic regression was used, with predetermined covariates that were selected by the principal investigator. Covariates were selected based on clinical relevance and were limited to reduce collinearity and overfitting. These covariates included age, the number of vertebrae fused, if the patient had a 3-column osteotomy, primary vs. revision surgery, preoperative platelet count, and if the patient was taking medication for the purpose of augmenting bone mineral density.
Patients were also classified as being osteoporotic (lowest T score <-2.5), osteopenic (lowest T score ≥-2.5 and ≤-1) or normal (lowest T score >-1) in an additional analysis. Student t-test was used to compare those with normal bone mineral density to those with osteoporotic bone mineral density. Additional analysis compared blood loss between those with and without treatment with medications to increase bone density.
Significance was set at P < .05 for all statistical tests.
Results
Patient Characteristics
Ninety-one patients were identified in the cohort. Mean age was 63 ± 11.6 years, 81% female. 56/91 (62%) of cases included revision of previous instrumentation. The average T-score was -1.2 ± 1.0. Mean EBL was 1698 ± 798 mL, and 27 (30%) of patients had a 3-column osteotomy. 55 (66%) patients had previously performed spinal surgery with a median of 9 vertebrae fused (range 5-22). The mean BMI was 29 ± 6.6, mean platelet count was 248 ± 82, and preoperative hemoglobin was 13 ± 2.0 (Table 1). Patients had a mean SVA of 9.6 ± 8.6 cm, pelvic tilt of 30 ± 11°, coronal cobb angle of 33 ± 14°, thoracic kyphosis of 34 ± 13°, pelvic incidence of 63 ± 18°, lumbar lordosis of 36 ± 27°, and sacral slope of 33 ± 17.
Table 1.
Demographic and Surgical Characteristics.
| Characteristics | N (%) |
|---|---|
| vaAge | 63 ± 12 |
| Gender, (% female) | 73 (80) |
| Race | |
| Caucasian | 71 (77) |
| African American | 7 (7.7) |
| Asian | 2 (2.2) |
| Other | 11 (12) |
| T Score (lowest) | −1.2 ± 1.03 |
| EBL | 1698 ± 798 |
| 3-Column osteotomy | 27 (30) |
| Previous spine surgery | 56 (66) |
| Number of levels fused a | 9 (5, 22) |
| Body Mass index | 29 ± 6.6 |
| Preoperative platelet count | 248 ± 82 |
| Preoperative hemoglobin | 13 ± 2.0 |
Abbreviations: EBL indicates estimated blood loss; SVA, sagittal vertical axis; PT, pelvic tilt; TK, Thoracic Kyphosis
*Data presented as mean ± standard deviation.
aData presented as median (range).
A total of 24 patients (26%) were treated for osteoporosis preoperatively, 22 (24%) patients were treated with teriparatide, 1 patient was treated with alendronate, and 1 was treated with raloxifene. Of the patients treated with osteoporosis medications, treatment duration was a mean of 7.3 ± 5.3 months. The difference in estimated blood loss between those taking (mean 1870±752 mL) or not taking (mean 1701±808 mL) osteoporosis medications was not statistically significant (P = .38).
Association Between Bone Mineral Density and Intraoperative Blood Loss
Each point lower T-score was associated with significantly higher odds of major blood loss (OR 2.5, 95% CI 1.0 – 5.9) when controlling for age, the number of vertebrae fused, 3-column osteotomy, preoperative platelet count and primary vs. revision surgery (Table 2).
Table 2.
Univariate and Multivariate Analysis of Factors Associated with Major Blood Loss During Adult Spinal Deformity surgery.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Characteristics | Odds Ratio | P Value | Odds Ratio | P Value |
| T-Score (lowest) | 1.79 [1.05, 3.06] | .032 | 2.47 [1.03, 5.89] | .042 |
| Age | 1.01 [.97, 1.05] | .595 | 1.03 [.98, 1.09] | .288 |
| Number of levels fused | .87 [.76, 1.02] | .085 | .83 [.64, 1.00] | .046 |
| 3-Column osteotomy | .55 [.19, 1.55] | .256 | .46 [.11, 1.93] | .288 |
| Previous spine surgery | 1.46 [.55, 3.87] | .446 | 1.04 [.23, 4.61] | .962 |
| Preoperative platelet count | 1.01 [1.00, 1.02] | .010 | 1.01 [1.00, 1.02] | .085 |
| Taking medication for osteoporosis | .89 [.29, 2.69] | .832 | 2.63 [.39, 17.7] | .321 |
Significance was set to P < .05 for all statistical tests including those bolded in this table.
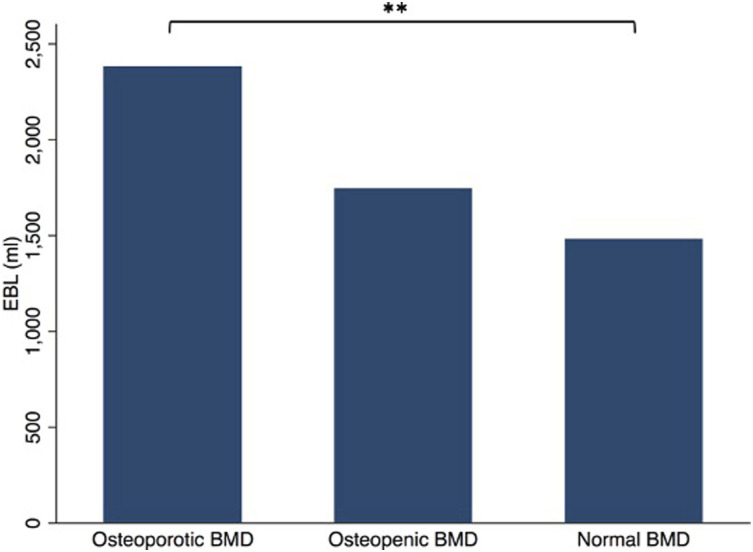
In the osteoporotic group, mean EBL was 2383 ± 1,019 mL. In the osteopenic group, mean EBL was 1744 ± 816 mL. In the normal BMD group, mean EBL was 1482 ± 622 mL. The patients with normal bone mineral density had significantly less blood loss than patients with osteoporotic bone mineral density (P<.01; Figure 1). The difference between osteoporotic and osteopenic groups was not significantly different (P > .05; Figure 1).
Figure 1.
Estimated Blood Loss During Adult Spinal Deformity Surgery Stratified by Preoperative Bone Mineral Density
Discussion
Since blood loss has been shown to be a major source of postoperative morbidity in ASD patients, understanding risk factors for major intraoperative blood loss is crucial for preoperative risk stratification and intraoperative planning. 8 The present study demonstrates that lower preoperative bone mineral density is an independent predictor of major intraoperative blood loss in ASD patients undergoing surgery. Specifically, each standard deviation of the T score lower the mean was associated with 2.5 times greater odds of having major intraoperative blood loss.
Osteoporosis, the underlying pathology quantified by BMD, is an important consideration for patients undergoing any orthopaedic procedure.17-19 In 2014, over 50 million Americans had low bone mass or osteoporotic bone, and this number has likely increased in the past few years with the population’s expanding age. 20 Given the increasing focus of surgeons on perioperative risk stratification, osteoporosis is becoming increasingly recognized as a risk factor for complications following spinal surgery, such as nonunion, instrumentation failure, and fracture. 20 This paradigm is consistent with adult spinal deformity surgeries as well, as several studies have identified osteoporosis as a risk factor for proximal junctional kyphosis in spinal deformity patients undergoing surgical correction. 13
Although there is a paucity of literature examining the specific relationship between BMD and blood loss, previous studies have investigated other risk factors for increased intraoperative blood loss in patients undergoing spine surgery.4-6,21 In a previously published retrospective study of 176 ASD patients conducted at our institution, we demonstrated that osteoporosis was an independent risk factor for major blood loss in ASD patients (OR = 2.4), with additional independent risk factors being three-column osteotomy (OR = 4.1), arthrodesis of 11 or more levels (OR = 3.2), and malalignment in both coronal and sagittal planes (OR = 3.2). Other previously established risk factors for blood loss in ASD surgeries include age of 65-years or older, American Society of Anesthesiologists (ASA) classification of 3 or greater, cardiac comorbidities, bleeding disorder, and longer operative time.6,22,23
The present study thus adds to existing literature by identifying low BMD T-score as an independent risk factor for intraoperative blood loss. Furthermore, we quantify the extent to which low BMD T-score is associated with intraoperative blood loss. Interestingly, Cao, et al conducted a single-institution retrospective review of 122 patients who underwent kyphoplasty for osteoporotic vertebral compression fractures and found no significant relationship between BMD and blood loss. 24 Instead, operative time, percentage of vertebral height loss, and percentage of vertebral height restoration were significant predictors of blood loss in their investigation. This may be because kyphoplasty is a percutaneous surgery which inherently has lower risk of blood loss compared to more extensive spine surgeries such as ASD surgery. Since ASD surgeries are among the most invasive types of spine surgeries, this discrepancy may suggest that the degree of impact that BMD has on intraoperative blood loss may be somewhat influenced by surgical invasiveness. In other words, lower BMD may potentially be associated with a greater degree of blood loss in more invasive surgeries such as ASD correction, compared to less invasive spinal procedures. Increased blood loss may lead to unnecessary transfusions, anemia, and increased length of stay.4,5,7
It is important to note that preoperative BMD is potentially modifiable through medications like bisphosphonates or parathyroid hormone analogs.13,25-27 In a prospective case series, Yagi, et al demonstrated that treatment of osteoporosis with teriparatide led to decreased incidence of proximal junctional kyphosis, suggesting that it may be beneficial to medically optimize osteoporotic patients prior to deformity surgery to reduce postoperative spinal complications. 13 Since the present study establishes a direct relationship between low BMD and blood loss, an additional benefit of addressing low bone mineral density in ASD patients may be to minimize intraoperative blood loss and associated postoperative morbidity. Further, our findings also have implications for preoperative patient counseling. Patients who have osteoporosis may be counseled by their surgeons that their risk of blood loss may potentially be higher than that of the general ASD population. With this information, patients and spine surgeons can collectively identify the level of risk ASD surgery poses for the patient, assess if any preoperative measures to improve BMD may be effective, and ultimately determine the best approach for optimizing surgical outcomes.
The conclusions of this study should be interpreted in the context of its limitations. First, we recognize that there are many potentially confounding factors that contribute to major blood loss in ASD patients. To address this potential limitation, our study controlled for age, surgical invasiveness, preoperative platelet count, and preoperative osteoporosis medications. However, there were other factors, including preoperative coagulation laboratory values (i.e., INR/PT/PTT), the use of antifibrinolytics, which we were unable to control for. Second, due to the retrospective nature of our study, we describe an association between low BMD and blood loss without establishing causality. Third, some studies have reported decreased sensitivity of DEXA scan for identifying osteoporosis in ASD patients compared to the general population. 14 At present, DEXA is still the gold-standard for determining BMD. Furthermore, DEXA scans were utilized in this study to directly quantify BMD, and not to make a designation of osteoporosis. Future investigations should seek to determine if there is a causal relationship between treatment of osteoporosis and intraoperative blood loss in ASD patients. Further, 80% of the patients in this cohort were female, which may limit the generalizability of our findings to males. It is important to note that both adult spinal deformity and DEXA scans are most prevalent in females. In addition, further work should be done to determine the optimal mean arterial blood pressure and TXA dosing regimen for simultaneously reducing blood loss and optimizing outcomes in ASD patients with low BMD. Finally, data on postoperative hemoglobin and transfusion rates was not available at the time we performed this study.
Conclusion
Preoperative T-score is independently associated with increased odds of major blood loss in ASD surgery. As such, T-score may be a useful metric for risk stratifying patients undergoing ASD surgery who are at increased risk of major blood loss.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Kevin Mo https://orcid.org/0000-0002-7728-0093
Khaled M. Kebaish https://orcid.org/0000-0002-1235-6202
References
- 1.Arzeno AH, Koltsov J, Alamin TF, Cheng I, Wood KB, Hu SS. Short-Term outcomes of staged versus same-day surgery for adult spinal deformity correction. Spine Deform. 2019;7:796-803. [DOI] [PubMed] [Google Scholar]
- 2.Kanter AS, Shaffrey CI, Mummaneni P, Wang MY, Uribe JS. Introduction Adult spinal deformity : pathophysiology and corrective. 2014;36:14112. DOI: 10.3171/2014.3.FOCUS14112.Neurosurg 10.3171/2014.3.FOCUS14112.Neurosurg. 10.3171/2014.3.FOCUS14112.Neurosurg [DOI] [PubMed] [Google Scholar]
- 3.Neuman BJ, Ailon T, Scheer JK, et al. Development and validation of a novel adult spinal deformity surgical invasiveness score: Analysis of 464 patients. Neurosurgery. 2018;82:847-853. DOI: 10.1093/neuros/nyx303 10.1093/neuros/nyx303. [DOI] [PubMed] [Google Scholar]
- 4.Raad M, Amin R, Jain A, Frank SM, Kebaish KM. Multilevel arthrodesis for adult spinal deformity: When should we anticipate major blood loss? Spine deformity. 2018;7:141-145. [DOI] [PubMed] [Google Scholar]
- 5.Soroceanu A, Burton DC, Oren JH, et al. Medical complications after adult spinal deformity surgery incidence, risk factors, and clinical impact. Spine. 2016;41:1718-1723. DOI: 10.1097/BRS.0000000000001636 10.1097/BRS.0000000000001636. [DOI] [PubMed] [Google Scholar]
- 6.White SJW, Cheung ZB, Ye I, et al. Risk factors for perioperative blood transfusions in adult spinal deformity surgery. World Neurosurgery. 2018;115:e731-e737. DOI: 10.1016/j.wneu.2018.04.152 10.1016/j.wneu.2018.04.152. [DOI] [PubMed] [Google Scholar]
- 7.Puvanesarajah V, Rao SS, Hassanzadeh H, Kebaish KM. Determinants of perioperative transfusion risk in patients with adult spinal deformity. J Neurosurg Spine. 2018;28:429-435. DOI: 10.3171/2017.10.SPINE17884 10.3171/2017.10.SPINE17884. [DOI] [PubMed] [Google Scholar]
- 8.Thomas K, Wong KH, Steelman SC, Rodriguez A. Surgical risk assessment and prevention in elderly spinal deformity patients. Geriatr Orthop Surg Rehabil. 2019;10:215145931985168. DOI: 10.1177/2151459319851681 10.1177/2151459319851681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okubadejo GO, Bridwell KH, Lenke LG, et al. Aprotinin may decrease blood loss in complex adult spinal deformity surgery, but it may also increase the risk of acute renal failure. Spine. 2007;32:2265-2271. DOI: 10.1097/BRS.0b013e31814ce9b0 10.1097/BRS.0b013e31814ce9b0. [DOI] [PubMed] [Google Scholar]
- 10.Pray C, Feroz NI, Haroon N. Bone Mineral Density and Fracture Risk in Ankylosing Spondylitis: A Meta-Analysis. Calcif Tissue Int. 2017;101:182-192. [DOI] [PubMed] [Google Scholar]
- 11.Makhdoom A, Rahopoto M, Awan S, et al. Bone mineral density by dual energy X-ray absorptiometry in rheumatoid arthritis. Japanese J Clin Immunol. 1991;14:353-357. DOI: 10.2177/jsci.14.353 10.2177/jsci.14.353. [DOI] [Google Scholar]
- 12.Song XX, Jin LY, Li XF, et al. Effects of low bone mineral status on biomechanical characteristics in idiopathic scoliotic spinal deformity. World Neurosurg. 2018;110:e321-e329. DOI: 10.1016/j.wneu.2017.10.177 10.1016/j.wneu.2017.10.177. [DOI] [PubMed] [Google Scholar]
- 13.Yagi M, Fujita N, Tsuji O, et al. Low bone-mineral density is a significant risk for proximal junctional failure after surgical correction of adult spinal deformity. Spine. 2018;43:485-491. DOI: 10.1097/BRS.0000000000002355 10.1097/BRS.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Upadhyaya S, Patel A, et al. DEXA sensitivity analysis in patients with adult spinal deformity. Spine J. 2020;20:174-180. DOI: 10.1016/j.spinee.2019.08.011 10.1016/j.spinee.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Noh SH, Ha Y, Obeid I, et al. Modified global alignment and proportion scoring with body mass index and bone mineral density (GAPB) for improving predictions of mechanical complications after adult spinal deformity surgery. Spine J. 2020;20:776-784. DOI: 10.1016/j.spinee.2019.11.006 10.1016/j.spinee.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Yagi M, Ohne H, Konomi T, et al. Teriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformity. Osteoporos Int. 2016;27:3495-3502. DOI: 10.1007/s00198-016-3676-6 10.1007/s00198-016-3676-6. [DOI] [PubMed] [Google Scholar]
- 17.Spencer SJ, Blyth MJ, Lovell F, Holt G. Does bone mineral density affect hip fracture severity? Orthopedics. 2012;35:945-950. DOI: 10.3928/01477447-20120525-34 10.3928/01477447-20120525-34. [DOI] [PubMed] [Google Scholar]
- 18.Hansen RL, Langdahl BL, Jørgensen PH, Petersen KK, Søballe K, Stilling M. Changes in periprosthetic bone mineral density and bone turnover markers after osseointegrated implant surgery: A cohort study of 20 transfemoral amputees with 30-month follow-up. Prosthet Orthot Int. 2019;43:508-518. DOI: 10.1177/0309364619866599 10.1177/0309364619866599. [DOI] [PubMed] [Google Scholar]
- 19.Kates ST, Ackert-Bicknell C. How do bisphosphonates affect fracture healing. Physiol Behav. 2017;176:139-148. DOI: 10.1016/j.physbeh.2017.03.040 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassanzadeh H, Puvanesarajah V, Dalkin AC. Medical management of osteoporosis for elective spine surgery. Clin Spine Surg. 2016;29:134-140. DOI: 10.1097/BSD.0000000000000376 10.1097/BSD.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 21.McCunniff PT, Young ES, Ahmadinia K, Ahn UM, Ahn NU. Smoking is associated with increased blood loss and transfusion use after lumbar spinal surgery. Clin Orthop Relat Res. 2016;474:1019-1025. DOI: 10.1007/s11999-015-4650-x 10.1007/s11999-015-4650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YH, Ou CY. Significant blood loss in lumbar fusion surgery for degenerative spine. World Neurosurg. 2015;84:780-785. DOI: 10.1016/j.wneu.2015.05.007 10.1016/j.wneu.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Bible JE, Mirza M, Knaub MA. Blood-loss management in spine surgery. J Am Acad Orthop Surg. 2018;26:35-44. DOI: 10.5435/JAAOS-D-16-00184 10.5435/JAAOS-D-16-00184. [DOI] [PubMed] [Google Scholar]
- 24.Cao D, Zhang S, Yang F, Shen K, Tan Z. Hidden blood loss and its influencing factors after percutaneous kyphoplasty surgery. Med (United States). 2018;97:e0435. DOI: 10.1097/MD.0000000000010435 10.1097/MD.0000000000010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cs A, Jhs Y, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis (Review). Cochrane Libr CD001347; 2016. DOI: 10.1002/14651858.CD001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434-1441. DOI: 10.1056/NEJM200105103441904 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 27.Nayak S, Greenspan S. A systematic review and meta-analysis of the effect of bisphosphonate drug holidays on bone mineral density and osteoporotic fracture risk. Osteoporos Int. 2019;30:705-720. DOI: 10.1007/s00198-019-05113-4 10.1007/s00198-019-05113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]