Abstract
Study design
Prospective, observational.
Objectives
The aim of our study was to assess the amount of reduction in lean muscle mass (LMM) of multifidus muscle (MFM) between conventional open Transforaminal lumbar interbody fusion (CO-TLIF) as compared to Minimally invasive spine Transforaminal lumbar interbody fusion (MIS-TLIF).
Methods
This study was conducted between 2017 and 2020. It included 100 patients divided into two groups, 50 patients treated with CO-TLIF, 50 treated with MIS-TLIF. Only patients undergoing single level, primary lumbar fusion at L4-5 or L5-S1 level for degenerative pathologies were included. All patients were assessed by magnetic resonance imaging (MRI) scans 1-year post surgery. Measurements were performed using ImageJ image processing program.
Results
Mean percentage reduction in LMM in CO-TLIF group was 45.52 ± 12.36% and MIS-TLIF group was 25.83 ± 9.64% [statistically significant (t = 8.78, P < .001)]. Mean percentage reduction in LMM on side of cage insertion was 39.63 ± 15.96% and opposite side was 31.40 ± 15.01% [statistically significant (t = 9.06, P < .001)]. Mean reduction of LMM among males was 29.38 ± 15.23% and females was 40.42 ± 12.67% [statistically significant (t = −3.95, P < .001)].
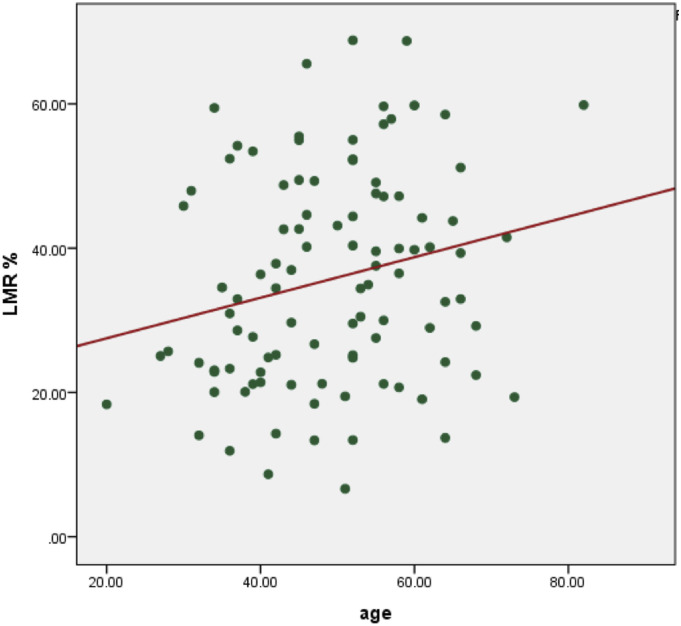
We observed significant but weak degree of correlation between age and percentage reduction of LMM (r = .22, P = .028).
Conclusion
Mean reduction in LMM was greater in CO-TLIF group as compared to MIS-TLIF. There was greater reduction in LMM in females and on side of cage insertion. We also found greater reduction in LMM with increasing age in both groups.
Keywords: transforaminal lumbar interbody fusion, fibrosis, multifidus, lean muscle mass
Clinical trial registry number: CTRI/2017/08/009540.
Introduction
In 1940, the first reported posterior lumbar interbody fusion (PLIF) was performed using spinous process autograft. 1 Transforaminal lumbar interbody fusion (TLIF) was popularized by Harms et al. as an alternative to PLIF. 2 Transforaminal lumbar interbody fusion offers several technical advantages over PLIF. One major advantage is reduced retraction of dural sac lessening risk of postoperative radiculitis.2,3 For decades, open approaches have been considered the gold standard when instrumented fusion is used, with reliable improvement in patient’s quality of life. However, open approaches are associated with significant soft-tissue morbidity. 4 Minimally invasive spine surgery (MIS) has attracted increasing attention in the past few years. 4 The first description of MIS-TLIF appeared in the literature in 2003, and since that time, use of this technique has been gaining popularity. 5 Proponents of MIS fusion suggest that it is associated with reduced blood loss, short hospital stay and lesser postoperative pain. Also, it reduces soft-tissue dissection and maintains structural integrity of paraspinal musculature. 6
Intramuscular pressure has been seen to increase substantially in conventional open posterior spine surgery with use of self retaining retractors. Up to 36.80% of multifidus muscle (MFM) atrophy was seen in patients undergoing conventional open TLIF (CO-TLIF). 7 The denervation and subsequent atrophy of paraspinal muscles, particularly the MFM, leads to disability and increased biomechanical strain. Also, fibrosis can compress neural tissue causing postoperative radiculopathy. 8 This injury to MFM leads to reduction in the lean muscle mass (LMM) which can be assessed on magnetic resonance imaging (MRI) in the post-operative period.8,9 The aim of our study was to assess the amount of reduction in LMM of MFM at the operated level in CO-TLIF as compared to MIS-TLIF. We believe, this quantitative reduction in the LMM of MFM would give an idea about the probable disability the patients will experience in their post-operative period.
Methods
This single center, prospective observational study was conducted at a tertiary care spine institute in India. The study duration was from August 2017 to July 2020. Approval from the Institutional Ethics committee of Stavya Spine Hospital and Research Institution (Number-SSHRI/ CS/ NS/ Fibro/ DD/ 18/ 052016) and CTRI registration was done prior to commencement. This study was a prospective study of 100 operated patients of TLIF, 50 being MIS-TLIF and 50 being CO-TLIF. Only patients who underwent single level, primary lumbar fusion at L4-5 or L5-S1 level for degenerative pathologies were included. Informed consent was taken from those patients who were willing to participate in study for use of clinical data including images. The enrolled study subjects were followed up for 1 year postoperatively. Patients undergoing revision lumbar surgery, decompression only procedures, multi-level fusion surgeries, underlying diagnosis other than degenerative pathologies (infection/ tumor, etc.), prior history of radiation were excluded from the study.
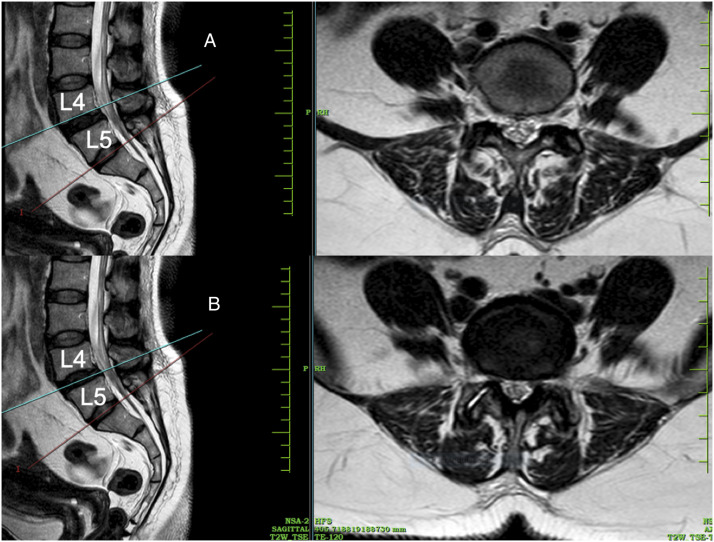
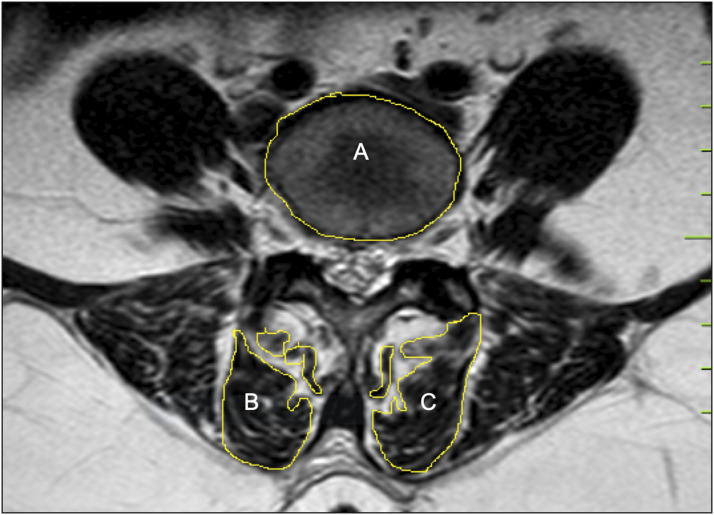
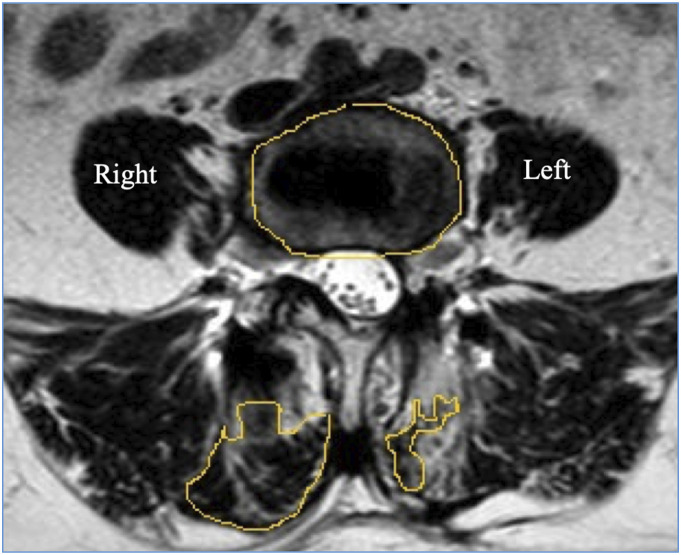
All surgical procedures had been performed by the same team of surgeons. All patients were assessed by MRI scans performed 1-year post surgery (Model: 1.5 T, Multiva, Phillips Equip ID: 68003991) by the same radiologist with > 10 years’ experience in the field of musculoskeletal imaging. Few of the preoperative MRI scans were performed at our center and the rest being scans from outside. Though this is a potential limitation, all scans were 1.5 T and were assessed by the same image processing program, which makes them fairly uniform. Image of Axial T2W MRI was taken at the Vertebral End Plate Level superior and adjacent to the level of TLIF. For example, for TLIF at the L4-L5 level, Axial T2W image at L4 lower end plate level was taken (Figure 1). Both preoperative and postoperative images were taken at the same level. To avoid disparity in measurement scales due to different sizes of images and not having calibrated MRI scans, we used a ratio for calculating the fibrosis where in the total area of bilateral MFM was divided by area of the vertebral body (Figure 2). This was the reason behind taking the image at lower end plate level of L4 as an accurate denominator of the vertebral body area cannot be taken at the disc level. Also, the metal artifact at end plate level is lesser than the disc level in the postoperative MRI. Thus, we got 2 values of MFM area through which we calculated the loss of LMM between the 2 groups. We also calculated the MFM area within the groups, for the side through which the cage was inserted and compared that with the opposite side in the MIS-TLIF group. All measurements were independently performed by 2 orthopedic spine surgeons and reviewed by the same radiologist who performed the MRI scan using ImageJ image10,11 processing program and a consensus value was reached for every measurement. In case of any conflict, the mean value was taken.
Figure 1.
Image of Axial T2W MRI at Vertebral End Plate Level superior adjacent to the level of TLIF (e.g., For TLIF of L4-L5: Axial T2W image at L4 lower end plate level) is taken. Both preoperative and postoperative images were taken at the same level. A- Axial section at vertebral end plate level, B- Axial section at disc level.
Figure 2.
To avoid disparity in measurement scales due to different sizes of images and not having a scale mentioned in the MRI we have selected the ratio as follows- Total area of bilateral multifidus/ Area of vertebral body (B+C/A).
Statistical analysis: SPSS software version 23 (IBM SPSS, IBM Corp, 2017) 12 was used for statistical analysis. The data was represented in terms of mean and standard deviation. Difference between 2 groups of continuous variables was assessed using t-test. 13 A P value of less than .05 was considered statistically significant. Pearson correlation coefficient 14 was used to see association between 2 continuous variables and data was represented as a scatter plot.
Results
We had a total of 100 patients in our series. There were 43 males and 57 female patients. The mean age of patients was 49.06 ± 11.61 (Range 20-82) years. The mean age in CO-TLIF group was 50.74 ± 10.48 years. The mean age in MIS-TLIF group was 47.38 ± 12.52 years. The most commonly operated surgical level was L5-S1 level in CO-TLIF group and L4-5 level in the MIS-TLIF group. The mean percentage reduction in LMM in the CO-TLIF group was 45.52 ± 12.36%. The mean percentage reduction in LMM in the MIS-TLIF group was 25.83 ± 9.64%. On applying, the independent t-test, the difference in mean reduction of LMM was statistically significant (t = 8.78, P < .001) (Figure 3). The mean percentage reduction in LMM on side of cage insertion in was 39.63 ± 15.96% and on the opposite side was 31.40 ± 15.01% (Figure 4). On applying the paired t-test, the difference was found to be statistically significant (t = 9.06, P < .001). We also observed a significant but weak degree of correlation between age and percentage reduction of the LMM (r = .22, P = .028). Increasing age was associated with greater percentage reduction in LMM with r value of .22 (Figure 5). The mean reduction of LMM among males was 29.38 ± 15.23% and females was 40.42 ± 12.67%. On applying, independent t-test, the difference in mean LMM among both sexes was statistically significant (t = −3.95, P < .001) (Figure 6).
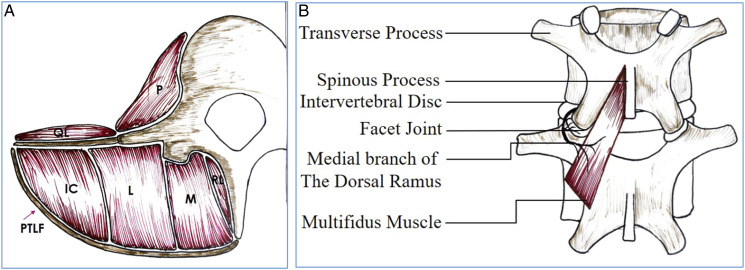
Figure 6.
Schematic diagram showing the location of the MFM (A) and its innervation by the medial branch of dorsal ramus (B), with no inter-segmental nerve supply as in the other paraspinal muscles [Longissimus (L) iliocostal muscles (IC) multifidus muscle (M) rotators lumborum muscles (RL) posterior thoraco-lumbar fascia (PTLF) psoas muscle (P) quadratus lumborum muscle (QL)].
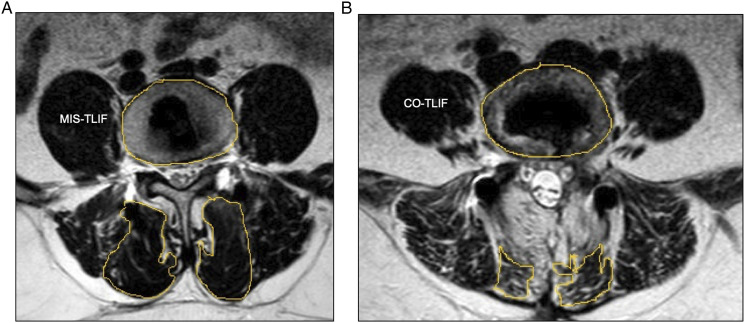
Figure 3.
The mean percentage reduction in LMM in the CO-TLIF group was 45.52 ± 12.36 and the MIS-TLIF group was 25.83 ± 9.64 %. On applying, the independent t-test, the difference in mean reduction of LMM was statistically significant. Representative images showing greater postoperative MFM fibrosis in CO-TLIF (B) as compared to MIS-TLIF (A).
Figure 4.
The mean reduction in LMM on side of cage insertion was greater than the opposite side as seen in the representative figure above where cage is inserted from left with greater MFM atrophy on left side.
Figure 5.
We observed significant but weak degree of correlation between age and LMR% (r = .22, P = .028). Increasing age was associated with greater percentage reduction in LMM.
Discussion
Minimally invasive spine was developed to reduce the degree of iatrogenic muscle injury while still accomplishing the goals of open procedures. 15 Preliminary results suggested that the MIS posterior lumbar fusion procedures hold the promise of decreased iatrogenic muscle injury and approach-related morbidity, while achieving the same surgical goals as CO-TLIF. 16 Our study was performed to objectively assess the amount of reduction in LMM of the MFM at the operated level in CO-TLIF as compared to MIS-TLIF using MRI scans.
The deflection of muscle from the spinal processes, and subsequent prolonged wide retraction of conventional lumbar fusion may lead to ischemia and denervation of the paraspinal musculature. This in turn may be associated with postoperative muscle atrophy and pain. 17 The MFM represents the deepest muscle group of the lumbar spine. Principal action of the MFM is rotation in sagittal plane. Force exerted by paraspinal muscles stiffens the functional lumbar spinal unit, with MFM having the highest influence. 18 Wilke et al. 19 examined action of simulated muscle force of MFM on motion segment stiffness. Compared with sacrospinalis and psoas, MFM contributed two-thirds of the increased stiffness imparted by the simulated contraction of paraspinal muscles. Therefore, wasting of this muscle would be expected to have direct effects on lumbar segmental stability, and thus predispose to further damage. The MFM is most vulnerable to damage during posterior spinal surgery, as its innervated only by medial branch of dorsal ramus 20 and has no inter-segmental nerve supply unlike other paraspinals. The medial branch of dorsal ramus is vulnerable to compression during lateral displacement of muscle mass at surgery, particularly where the nerve is relatively fixed as it runs under the fibro-osseous mamillo-accessory ligament.20,21 Patients with postoperative failed back syndrome have dorsal ramus lesions in 1 or more segments covered by fibrosis with local paraspinal muscle atrophy at corresponding segments. Dysfunction of MFM may contribute to “fusion disease.” This term is used for patients having successful fusion on radiographs but clinical outcome is poor. 22
Histologic, enzymatic, and radiologic evidences of paraspinal muscle injury in lumbar surgery have described by several authors. Kawaguchi et al 23 analyzed effects of retractor blade pressure on paraspinal muscles during spinal surgery. They determined that elevated serum levels of creatine phosphokinase MM isoenzyme, an indicator of muscle injury is directly related to retraction pressure and duration. Gejo et al 24 examined postoperative MRIs and trunk muscle strength in 80 patients who had undergone lumbar surgery. They concluded that injury to lumbar musculature was directly proportional to intra-operative retraction time. Furthermore, incidence of low back pain was significantly increased in patients with longer muscle retraction times. Kahanovitz et al demonstrated a 30% decrease 25 in lumbar isokinetic strength on flexion test, even after short microdiscectomy procedures in young, healthy males. Rantanen et al 26 saw persistent pathologic changes in paraspinal muscles in patients with poor outcomes after lumbar spine surgery. Previous literature has reported that dissection/ retraction of MFM can lead to denervation and atrophy. This in turn may lead to increased risk of failed back surgery syndrome.27,28 Minimally invasive spine was developed as a potential solution to the above-mentioned problems by reducing the amount of iatrogenic soft-tissue injury while still accomplishing the traditional goals of the open procedure.29,30
We found that the mean reduction in LMM in the MIS-TLIF group was significantly less than that in the CO-TLIF group. This correlates with some of the findings in previously published literature (Table 1) where authors have compared the MFM damage between CO-TLIF and MIS-TLIF utilizing various modalities. All measurements in our study were performed using the ImageJ image processing program. ImageJ10,11is a Java-based image processing program developed at the National Institutes of Health and the Laboratory for Optical and Computational Instrumentation (LOCI, University of Wisconsin). It can read multiple image file formats, including JPEG. 31 It can calculate area and pixel value statistics of user-defined selections and intensity-threshold objects, which is what we utilized. The image format used was JPEG.10,11,31 In our study, some images were direct from PACS while others were clicked with a camera which is one of the potential limitations of the study.
Table 1.
Review of Literature Showing Previously Published Articles Comparing Paraspinal Muscle Damage in MIS vs CO-TLIF Group.
| Sr. No | Author | Sample Size | Outcome Measures | Conclusions |
|---|---|---|---|---|
| 1 | Fan et al 1 | 59 | VAS, ODI score, creatine kinase level, CSA of lean MFM, and the T2 signal intensity ratio of multifidus to psoas muscle | MIS caused less change in MFM, less postoperative back pain and functional disability than conventional open approach. |
| 2 | Stevens et al 8 | 8 | Maximum IMP generated using an ultra-miniature pressure Transducer. MRI: Edema and atrophy within multifidus, with T2 mapping and diffusion-weighted imaging |
Peak IMP generated by MIS retractor was significantly less than with CO. Postoperatively, less muscle edema was demonstrated after MIS lumbar spinal fusion. |
| 3 | Hyun et al 41 | 29 | CSA, thickness, and width of the MFM were measured by CT. |
The degree of postoperative paraspinal muscle atrophy was significantly greater on the MA side than on the contralateral PIA side (−20.7% and −4.8%, respectively, P < .01). |
| 4 | Kim et al 17 | 19 | CSA and T2-weighted signal intensity of MFM was measured by MRI and trunk extension muscle strength [Medx back extension machine (Ocala, FL)]. | Percutaneous pedicle screw fixation caused less paraspinal muscle damage than CO pedicle screw fixation and had positive effects on postoperative Trunk muscle performance. |
| 5 | Min et al 30 | 48 | Quantitative analysis of the change in fat infiltration percentage and the change in CSA of paraspinal muscle, before and after surgery using the pseudo coloring technique | Both single and multi-segment fusion showed less change in fat infiltration percentage and cross-sectional area in the MI-TLIF as compared to CO-TLIF |
[Abbreviations: VAS- visual analogue scale, ODI- Oswestry Disability Index, CSA- Cross-Sectional Area, MIS- minimally invasive spine, MFM- Multifidus muscle, IMP- Intra Muscular Pressure, MRI- Magnetic Resonance Imaging, CT- Computed Tomography, CO- Conventional Open, TLIF- Transforaminal Lumbar Interbody Fusion, MA- Midline Approach, PIA- Paramedian Interfascial Approach].
We observed a significant but weak degree of correlation between age and percentage reduction of LMM. Increasing age was associated with greater percentage reduction in LMM. As per our knowledge, none of the previously published papers have specifically assessed the impact of age and MFM atrophy. This assumes significance with changing demographics both in India and world over.32,33 India’s elderly population (aged 60 and above) is projected to touch 194 million in 2031 as compared to 138 million in 2021. This would be a steep 41% increase in,34-36 as per the National Statistical Office (NSO)’s Elderly in India 2021 report. With changing demographics, sarcopenia has been the focus of current research in orthopedics and spine surgery. In 1989, Rosenberg proposed the term “sarcopenia” (Greek “sarx” or flesh + “penia” or loss)37,38 to describe this age-related decrease of muscle mass. Sarcopenia is defined as a loss of skeletal muscle mass and function primarily associated with aging. 39 Yolcu et al investigated operative and postoperative outcomes associated with CO and MIS techniques in elderly patients. 40 This study included 107 patients with age more than 65. The authors found that intra-operative blood loss, length of hospital stay, rate of complications, readmissions (no readmissions in MIS group), reoperations, and pain improvement all favored the MIS group. Apart from these previously published advantages, our study found 1 more factor favoring MIS, given this age group also has associated sarcopenia. With greater postoperative fibrosis in CO-TLIF patients with aging, MIS can be a better option in elderly particularly to avoid MFM atrophy.
We had a greater reduction in LMM in female patients as compared to males and the difference was statistically significant. This correlates with previously published literature that has assessed gender differences in paraspinal muscle structure and function. Hyun et al 41 compared the paramedian interfascial approach (PIA) to traditional midline approach (MA) for lumbar fusion to assess paraspinal muscle damage. They found that the cross-sectional area (CSA) and thickness of the paraspinal muscles did not change significantly after the surgery on side of PIA, but reduced remarkably on that of MA, especially in female patients. Chua et al 42 assessed gender differences in fatty infiltration and sarcopenia in patients with lumbar spinal stenosis. They retrospectively analyzed 63 patients with lumbar spinal stenosis at L3/4 or L4/5 who underwent MIS decompression. They concluded that female patients develop more severe and functionally significant MFM atrophy. This in turn has worse clinical outcomes and higher functional disability. Though we do not know the exact reason behind this phenomenon, there have been studies performed previously that looked at gender differences in paraspinal muscle architecture. To investigate the effects of gender on the dimensions of spinal and paraspinal structures, Cooper et al 43 made anthropometric assessments on 92 patients (39 females and 53 males, aged 20-55 years) suffering from low back pain. CSA of L4 and paraspinal/ psoas muscles were measured at level of upper table of L4. They found greater CSA of all structures in males.
The mean percentage reduction in LMM on side of cage insertion was greater than the opposite side in both MIS and CO-TLIF groups and this difference was statistically significant. This can be explained by the fact that there was greater amount of surgical work done on the side of cage insertion as compared to the contra-lateral side. Also, the cage is usually inserted from the side with greater severity of symptoms. Chon et.al. 44 observed that CSA of paraspinal muscles at L4-5 and L5–S1 levels was less on affected side as compared to uninvolved side in patients with unilateral radiculopathy for more than 3 months. This asymmetric atrophy of paraspinal muscles in patients with chronic unilateral lumbar radiculopathy may have led to the greater degree of MFM atrophy on the side of cage insertion.
There are some limitations in the present study that deserve mention. Firstly, this study was a single centre analysis and was not randomized or controlled. It was a radiological study only with no clinical correlation or functional evaluation of MFM. The study though prospective in nature, did not include consecutive patients. Only those patients who consented for participation and a follow-up MRI evaluation were included. Despite these limitations, our study is the largest one in terms of sample size, comparing MFM fibrosis in CO vs MIS-TLIF, in currently published literature. It will provide a baseline for future research on the subject including gender differences in LMM reduction and role of MFM atrophy and correlation with adjacent segment degeneration.
Conclusion
The mean reduction in LMM is greater in the CO-TLIF group as compared to MIS-TLIF. There was a greater reduction in LMM in females as compared to males and on the side of cage insertion. We also found significant but weak degree of correlation between greater reduction in LMM with increasing age in both the groups.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Informed consent taken from all the study participants for use of their clinical data as well as images.
ORCID iDs
Nandan Marathe https://orcid.org/0000-0002-8939-2690
Shivanand Mayi https://orcid.org/0000-0003-3753-8712
Devanand Degulmadi https://orcid.org/0000-0002-7263-9706
Ravi Ranjan Rai https://orcid.org/0000-0002-0873-6907
Kirit Jadav https://orcid.org/0000-0002-1340-3963
Prarthan Amin https://orcid.org/0000-0002-2431-8019
Ajay Krishnan https://orcid.org/0000-0001-9808-2371
References
- 1.Fenton-White HA. Trailblazing: The historical development of the posterior lumbar interbody fusion (PLIF). Spine J. 2021. [DOI] [PubMed] [Google Scholar]
- 2.Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Current Rev Musculoskeletal Med. 2009;2(2):118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian SG, Sonone S, Dahapute AA, et al. A comparative prospective study of clinical and radiological outcomes between open and minimally invasive transforaminal lumbar interbody fusion. Indian Spine Journal. 2019;2(2):138. [Google Scholar]
- 5.Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: A historical review. Neurosurg Focus. 2009;27(3):E9. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Shakya A, Singh V, et al. Incidence of dural tears in open versus minimally invasive spine surgery: A single-center prospective study. As Spine J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: Minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens KJ, Spenciner DB, Griffiths KL, et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. Clin Spine Surg. 2006;19(2):77-86. [DOI] [PubMed] [Google Scholar]
- 9.Chen KY, Tseng KY, Hueng DY, Chang TS, Pang CY. Clinical outcome and multifidus muscle changes of transforaminal lumbar interbody fusion: Minimally invasive procedure versus conventional open approach. Formosan J Surg. 2021;54(4):135. [Google Scholar]
- 10.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The imageJ ecosystem: An open platform for biomedical image analysis. Mol Reprod Dev. 2015;82(7-8):518-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform.2017;18(1):1-26. Yockey RD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SPSS® Demystified: A Simple Guide and Reference. New York: Routledge; 2017. [Google Scholar]
- 13.Lowry R. Concepts and Applications of Inferential Statistics.
- 14.Sedgwick P. Pearson’s correlation coefficient. BMJ. 2012:345:e4483. [Google Scholar]
- 15.Krishnan A, Barot MP, Dave BR, Bang P, Devanand D, Patel D, et al. Percutaneous transforaminal endoscopic decompression and cageless percutaneous bone graft transforaminal lumbar interbody fusion: A feasibility study. J Orthopaed Allied Sci. 2018;6(3):21. [Google Scholar]
- 16.McClelland S, III, Goldstein JA. Minimally invasive versus open spine surgery: What does the best evidence tell us? J Neurosci Rural Pract. 2017;08(02):194-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine. 2005;30(1):123-129. [PubMed] [Google Scholar]
- 18.Ward SR, Kim CW, Eng CM, et al. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Jt Surg Am Vol. 2009;91(1):176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine. 1995;20(2):192-197. [DOI] [PubMed] [Google Scholar]
- 20.Kay AG. An extensive literature review of the lumbar multifidus: Anatomy. J Man Manip Ther. 2000;8(3):102-114. [Google Scholar]
- 21.Macintosh JE, Valencia F, Bogduk N, Munro RR. The morphology of the human lumbar multifidus. Clin Biomech. 1986;1(4):196-204. [DOI] [PubMed] [Google Scholar]
- 22.Hung CW, Wu MF, Hong RT, Weng MJ, Yu GF, Kao CH. Comparison of multifidus muscle atrophy after posterior lumbar interbody fusion with conventional and cortical bone trajectory. Clin Neurol Neurosurg. 2016;145:41-45. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: A histologic and enzymatic analysis. Spine. 1996;21(8):941-944. [DOI] [PubMed] [Google Scholar]
- 24.Gejo R, Kawaguchi Y, Kondoh T, et al. Magnetic resonance imaging and histologic evidence of postoperative back muscle injury in rats. Spine. 2000;25(8):941-946. [DOI] [PubMed] [Google Scholar]
- 25.Kahanovitz N, Viola K, Gallagher M. Long-term strength assessment of postoperative diskectomy patients. Spine. 1989;14(4):402-403. [DOI] [PubMed] [Google Scholar]
- 26.Rantanen J, Hurme M, Falck B, et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine. 1993;18(5):568-574. [DOI] [PubMed] [Google Scholar]
- 27.David HG, Stekas ND, Varlotta CG, et al. Comparative analysis of two transforaminal lumbar interbody fusion techniques: Open TLIF: Versus: Wiltse MIS TLIF. Spine. 2019;44(9):E555-E560. [DOI] [PubMed] [Google Scholar]
- 28.Tan JH, Liu G, Ng R, Kumar N, Wong HK, Liu G. Is MIS-TLIF superior to open TLIF in obese patients?: A systematic review and meta-analysis. Eur Spine J. 2018;27(8):1877-1886. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Shakya A, Singh V, et al. Does a high BMI affect the outcome of minimally invasive TLIF? A retrospective study of 207 patients. Eur Spine J. 2021;30(12):3746-3754. [DOI] [PubMed] [Google Scholar]
- 30.Min SH, Kim MH, Seo JB, Lee JY, Lee DH. The quantitative analysis of back muscle degeneration after posterior lumbar fusion: Comparison of minimally invasive and conventional open surgery. As Spine J. 2009;3(2):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017;18(1):529-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuster V. Changing demographics: a new approach to global health care due to the aging population. J Am Coll Cardiol. 2017;69(24):3002-3005. [DOI] [PubMed] [Google Scholar]
- 33.Halaweish I, Alam HB. Changing demographics of the American population. Surg Clin. 2015;95(1):1-10. [DOI] [PubMed] [Google Scholar]
- 34.Visaria P. Demographics of ageing in India. Econ Polit Wkly. 2001;36:1967-1975. [Google Scholar]
- 35.Bloom DE. Population Dynamics in India and Implications for Economic Growth. St. Gallen, Switzerland: WDA-Forum, University of St. Gallen. [Google Scholar]
- 36.Dey S, Nambiar D, Lakshmi JK, Sheikh K, Reddy KS. Health of the elderly in India: challenges of access and affordability. In: Aging in Asia: Findings from New and Emerging Data Initiatives 2012. Washington, DC: National Academies Press (US), 2012 [PubMed] [Google Scholar]
- 37.Rosenberg IH. Sarcopenia: Origins and clinical relevance. J Nutr. 1997;127(5):990S-991S. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27(3):337-339. [DOI] [PubMed] [Google Scholar]
- 39.Janssen I. The epidemiology of sarcopenia. Clin Geriatr Med. 2011;27(3):355-363. [DOI] [PubMed] [Google Scholar]
- 40.Yolcu YU, Helal A, Alexander AY, et al. Minimally invasive versus open surgery for degenerative spine disorders for elderly patients: Experiences from a single institution. World Neurosurg. 2021;146:e1262-e1269. [DOI] [PubMed] [Google Scholar]
- 41.Hyun SJ, Kim YB, Kim YS, et al. Postoperative changes in paraspinal muscle volume: comparison between paramedian interfascial and midline approaches for lumbar fusion. J Kor Med Sci. 2007;22(4):646-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chua M, Hochberg U, Regev G, et al. Gender differences in multifidus fatty infiltration, sarcopenia and association with preoperative pain and functional disability in patients with lumbar spinal stenosis. Spine J. 2022;22(1):58-63. [DOI] [PubMed] [Google Scholar]
- 43.Cooper RG, Holli S, Jayson MI. Gender variation of human spinal and paraspinal structures. Clin BioMech. 1992;7(2):120-124. [DOI] [PubMed] [Google Scholar]
- 44.Chon J, Kim HS, Lee JH, et al. Asymmetric atrophy of paraspinal muscles in patients with chronic unilateral lumbar radiculopathy. Annals Rehabilitation Med. 2017;41(5):801. [DOI] [PMC free article] [PubMed] [Google Scholar]